TLR4 regulates inflammatory cytokine production from thymic- and peripherally-derived Treg cells during intestinal inflammation.
Keywords: colitis, LPS, MyD88, SOCS3
Abstract
Tregs play a crucial role in the maintenance of intestinal immune homeostasis. However, significant numbers of Foxp3+ Tregs accumulate in the inflamed lesions in experimental colitis and in IBD patients. Treg production of the proinflammatory cytokines IFN-γ and/or IL-17 may arguably explain their ineffectiveness in suppressing intestinal inflammation. However, it remains unknown whether iTreg and tTreg produce proinflammatory cytokines and how TLR signaling regulates this process. Here, we found that Foxp3+Tregs were increased in the intestines of B6.TLR4−/− and B6.IL-10−/− mice when compared with WT B6 mice. TLR4−/− and IL-10−/− resulted in more Tregs within inflamed intestines. The majority of Foxp3+ Tregs in the spleen was Helios+Nrp1+, whereas most Foxp3+ Tregs in the intestinal LP were Helios−Nrp1−. More Helios+Nrp1+ Tregs expressed IFN-γ and/or IL-17 than did Helios−Nrp1− Tregs in the spleen and intestine, which was increased with TLR4−/−. TLR4 signaling in T cells and APCs inhibited Foxp3+ induction via MyD88-dependent, TRIF-independent pathways, which was negatively regulated by SOCS3. Collectively, these data demonstrate Helios+Nrp1+ tTregs and Helios−Nrp1− iTregs produce proinflammatory cytokines in the intestines during inflammation, which was regulated by TLR4 signaling.
Introduction
The gastrointestinal tract provides a major entry for dietary and microbial antigens into the body. Among the substantive microbial population, both beneficial commensal microbiota and potential pathogens reside in the gut. A key mechanism of regulation in the intestinal tract lies with Tregs, capable of surveying a wide array of immune responses to maintain intestinal homeostasis and protect the intestine from inflammation. The majority of Tregs is defined by expression of the transcription factor Foxp3, which is critical for Treg development and stability [1]. The catastrophic inflammation observed in the absence of Foxp3 [1, 2] demonstrates that Tregs are vital for immune regulation and restraint of the inflammatory effects of effector T cells. Foxp3+ Tregs arise in two separate origins, differing in location and TCR repertoire [3]. CD4+ T cells with TCR affinities toward self-antigens become Foxp3+ Tregs in the thymus and are denoted tTregs, whereas Foxp3+ Tregs can also be induced in the periphery after antigen presentation and are denoted iTregs [3]. Tregs−/− results in chronic intestinal inflammation, and colitis can be prevented and cured by transfer of Tregs, showcasing a key role for Tregs in the maintenance of intestine homeostasis [4]. Paradoxically, however, Foxp3+ Tregs are increased in inflamed intestinal tissues in animal models and patients with IBD [5–7], and most of them express inflammatory cytokines, such as IFN-γ and IL-17 [5, 8, 9]. Anti-TNF-α treatment suppresses inflammation in a majority of patients with IBD with a concomitant decrease in local mucosal Tregs and increase in peripheral Tregs [10, 11], indicating that inflammation drives Treg expansion and subsequently promotes accumulation in inflamed lesions. Treg expression of a number of factors has been shown to contribute to Treg function and stability [12–15]. However, how TLR signaling regulates Treg expression of proinflammatory cytokines in inflamed intestine is still not understood completely.
Although a majority of Tregs continue to express high levels of Foxp3 after transfer into noninflamed hosts, many reports demonstrate the plasticity of the Treg differentiation and maintenance program [16, 17]. Foxp3+ Tregs can fully convert into Th1, Th17, and/or follicular Th cells in the intestine, especially during inflammation [5, 18]. This is manifested by the loss of Foxp3 and acquisition of T-bet (Th1), retinoic acid receptor-related orphan receptor-γt (Th17), or B cell lymphoma 6 (follicular Th) by Tregs [18, 19]. However, it is unclear whether tTreg or iTregs have different propensities for acquiring proinflammatory function and how microbial exposure regulates this process. The interaction of the commensal microbiota with TLRs plays an important role in innate immune responses, e.g., the activation of TLRs on DCs and macrophages results in production of inflammatory cytokines and chemokines. Conversely, the intestinal microbiota can promote immune regulation through the activation of TLRs on CD4+ T cells, independent of APCs [20, 21]. TLR4−/− or TLR9−/− in vivo, coupled with IL-10−/−, result in spontaneous colitis that is significantly worse than that seen in IL-10−/− mice, demonstrating that TLR4 and TLR9 have regulatory roles in CD4+ T cells [22, 23]. Notably, Tregs in TLR4−/−IL-10−/− mice express very high levels of inflammatory cytokines, including GM-CSF, IFN-γ, and IL-17, and have decreased suppressive capability [23]. Activation of MyD88, a downstream signaling molecule of most TLRs, has also been shown to be important for Tregs to control intestinal inflammation in vivo [24]. In this report, we demonstrate that LPS influences the generation and expansion of Tregs through TLR4 signaling on APCs and CD4+ T cells in a MyD88-dependent but TRIF-independent manner. Helios+Nrp1+ tTregs and Helios−Nrp1− iTregs produce proinflammatory cytokines in the intestines. TLR4 signaling influences Tregs expressing proinflammatory cytokines; however, it does not appear to favor either Treg subset. Furthermore, Treg expansion under intestinal inflammation occurs in the intestine but not in the periphery.
MATERIALS AND METHODS
Mice
B6, B6.TRIFLPS2, and B6.RAG1−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). CBir1 Tg mice, B6.MyD88−/−, B6.TLR4−/−, B6.IL-10−/−, B6.TLR4−/−IL-10−/− [23], and LysMCre-SOCS3fl/fl mice [25] were maintained in the animal facilities at UTMB, The University of Chicago, and The University of Alabama at Birmingham. Age-matched and cohoused mice of 8–12 weeks were used in these experiments. All experiments were reviewed and approved by the Institutional Animal Care and Use Committees of UTMB, The University of Chicago, and The University of Alabama at Birmingham.
Antibodies and reagents
Fluorochrome-conjugated anti-mouse CD4 (RM4-5), Helios (22F6), IL-17A (TC11-18H10), and IFN-γ (XMG1.2) antibodies and rTGF-β were purchased from BioLegend (San Diego, CA, USA). Anti-mouse Nrp1 was purchased from R&D Systems (Minneapolis, MN, USA). Anti-mouse Foxp3 (FJK-16s) was purchased from eBioscience (San Diego, CA, USA). Live/Dead Fixable Dead Cell stain kits were used to gate on live lymphocytes (Life Technologies, Carlsbad, CA, USA). LPS (0111:B4) was purchased from Sigma-Aldrich (St. Louis, MO, USA). FTY720 was purchased from Cayman Chemical (Ann Arbor, MI, USA).
Isolation of LP cells
As described previously [5], large intestines were removed, sliced, and digested by collagenase IV. The cells were resuspended in 40% Percoll and overlaid carefully onto 70% Percoll. The interface containing the LP lymphocytes was collected. Lymphocytes were restimulated with PMA (50 ng/ml), ionomycin (750 ng/ml), and monensin for 5 h before labeling with antibodies for flow cytometry.
CD4+ T cell purification, in vitro polarization of Tregs, and BMDC generation
CD4+ T cells were isolated by using anti-mouse CD4 magnetic beads (BD Biosciences, San Diego, CA, USA). Naive CD62L+CD4+ T cells were sorted by FACSAria (BD Biosciences), based on CD62L and CD4 coexpression. CD4+ T cells from B6, MyD88−/−, or TRIFLPS2 mice were cultured with plate-bound αCD3 (1 μg/ml) and αCD28 under Treg-polarizing conditions (5 ng/ml TGF-β) or in the absence of polarizing cytokines. Irradiated splenocytes from B6, MyD88−/−, or TRIF−/− mice were cultured with CD4+ T cells from B6 mice with soluble αCD3 (1 μg/ml), with or without Treg polarization, or with CD4+ T cells from CBir1 Tg mice with CBir1 flagellin peptide under Treg polarization.
BMDCs were generated from B6 or SOCS3 conditional knockout femurs. BM cells were cultured with GM-CSF (BioLegend) for 8 days and cultured with CD4+ T cells from CBir1 Tg mice with CBir1 flagellin peptide under Treg polarization.
Statistical analysis
Levels of significance were determined by Student's t-test in Prism 5.0 (GraphPad Software, La Jolla, CA, USA). P < 0.05 was considered to be statistically significant.
RESULTS
TLR4 regulates expansion and inflammatory cytokine expression by Foxp3+ Tregs in the intestines of colitic mice
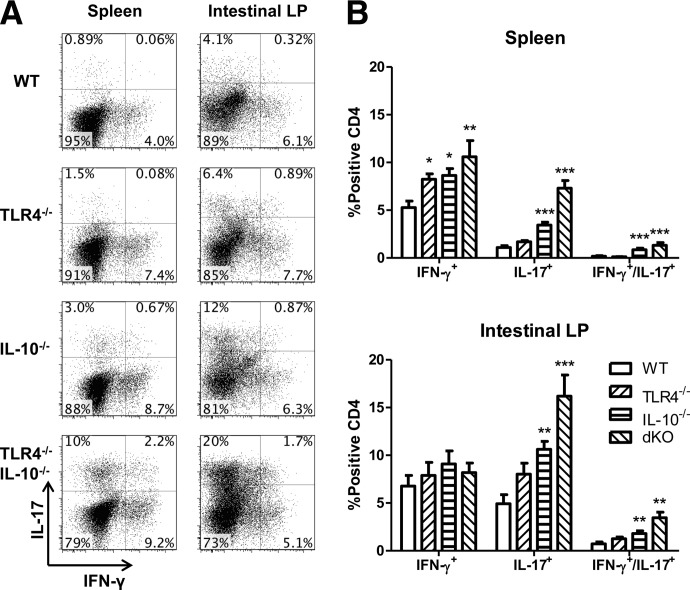
Our previous studies demonstrated that during intestinal inflammation, Foxp3+ Tregs accumulated in the inflamed lesions, and substantial numbers of such Tregs expressed the proinflammatory cytokines IFN-γ and IL-17 in the inflamed colon [5, 23]. However, the factors responsible for driving Treg expansion and expression of these proinflammatory cytokines and whether tTregs or iTregs are capable of producing these cytokines remain unclear, as recent reports have offered conflicting results [17, 26]. We have shown previously that IL-10−/− and TLR4−/− resulted in severely aggravated intestinal inflammation, thereby suggesting a critical role of TLR signaling in controlling proinflammatory activities [23]. To assess how TLR4 regulates tTreg and iTreg expression of proinflammatory cytokines in the presence and absence of inflammation, we analyzed Foxp3+ Treg expression of proinflammatory cytokines from WT B6, TLR4−/−, IL-10−/−, and TLR4−/−IL-10−/− mice. We have reported previously that TLR4−/−IL-10−/− mice developed more severe colitis compared with IL-10−/− mice, and there was no inflammation in the intestines of WT and TLR4−/− mice [23]. Examination of cytokine production by CD4+ T cells showed that IFN-γ-producing Th1 cells and IL-17-producing Th17 cells were increased in spleen of colitic IL-10−/− mice compared with that of WT mice and were amplified further in the intestinal LP (Fig. 1). TLR4−/− alone did not affect CD4 T cell cytokine production in WT mice. There was, however, a considerable increase in the number of cells that produced IFN-γ and IL-17 in colitic IL-10−/− mice, which was increased further in TLR4−/−IL-10−/− mice.
Figure 1. The absence of TLR4 increases CD4 cell inflammatory cytokine production.
(A) Total spleen CD4 cells from TLR4−/−, IL-10−/−, TLR4−/−IL-10−/− [double-knockout (dKO)], and WT mice were analyzed for IFN-γ and IL-17 production. (B) IFN-γ and IL-17 expression from total CD4+ T cells in the spleen and intestine. Bar charts reflect mean ± sem. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with WT mice. Data are representative of three or more experiments of two or more mice/group with similar results. FACS plots are gated upon live CD4+ cells.
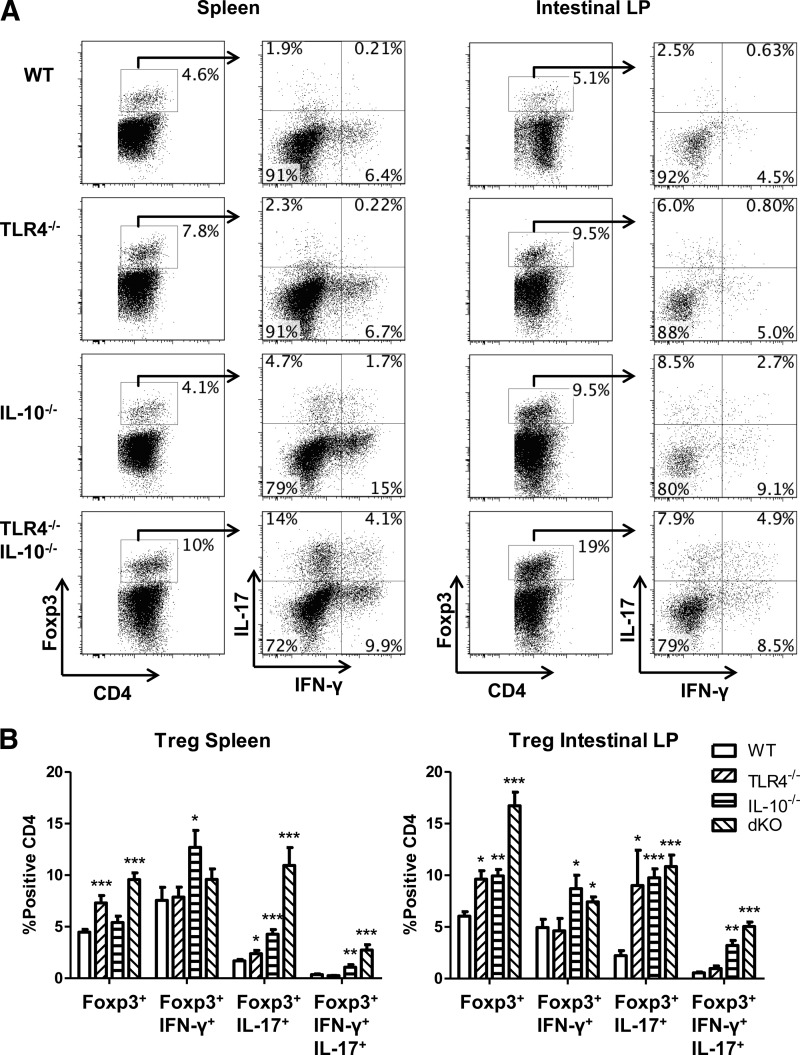
Interestingly, Foxp3+ Tregs were increased in intestinal LP but not in the spleen of colitic IL-10−/− mice compared with WT mice (Fig. 2). However, the presence of Foxp3+ Tregs was increased in spleen and LP of TLR4−/− mice compared with that of WT mice, which was increased further in TLR4−/−IL-10−/− mice. These data are consistent with the hypothesis that TLR4 signaling inhibits Treg expansion. In addition to the increase of Foxp3+ Tregs, there was an increase in Foxp3+ Tregs that produce IL-17, but not IFN-γ, in the intestines of TLR4−/− mice. Interestingly, there were more Foxp3+ Tregs expressing IFN-γ and IL-17 in the intestines of IL-10−/− mice, and TLR4 appeared to play a role in controlling Foxp3+ Treg cytokine production, as the levels of Foxp3+ Tregs expressing IFN-γ and IL-17 are increased further in TLR4−/−IL-10−/− mice compared with those in IL-10−/− mice (Fig. 2).
Figure 2. The absence of TLR4 increases intestinal Treg proinflammatory cytokine production.
(A) CD4 T cells were isolated from the spleens and intestines of TLR4−/−, IL-10−/−, TLR4−/−IL-10−/−, and WT mice and analyzed for IFN-γ and IL-17 production by flow cytometry gated on Foxp3+ cells. (B) IFN-γ and IL-17 expression from total CD4+Foxp3+ T cells in the spleen and intestine. Bar charts of Foxp3+IFN-γ+, Foxp3+IL-17+, and Foxp3+IFN-γ+IL-17+ are calculated based on total CD4+Foxp3+ T cells and reflect mean ± sem. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with WT B6 mice. Data are representative of three or more experiments of two or more mice/group with similar results. FACS plots are gated upon live CD4+ cells.
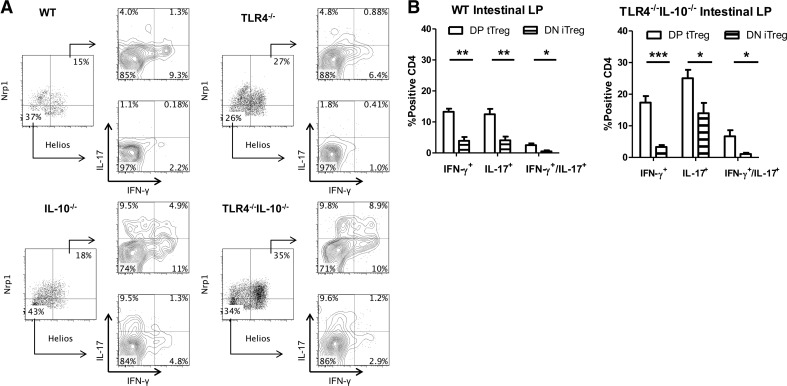
Helios+Nrp1+ and Helios−Nrp1− Foxp3+ Tregs produce IFN-γ and IL-17 in the intestines of colitic mice
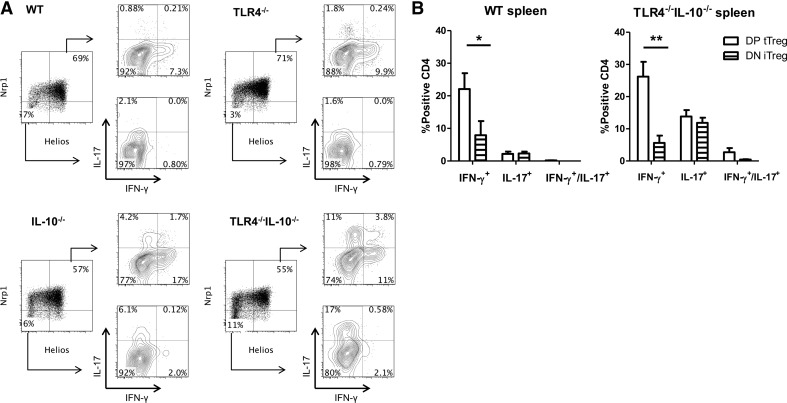
Although it has been shown that Tregs can convert into proinflammatory cytokine-producing T cells [5], it is unclear whether tTreg and iTreg or only iTregs produce proinflammatory cytokines, as tTregs are thought to be relatively stable [26]. As Helios and Nrp1 have been reported to be expressed predominantly, although not absolutely, by tTregs [27, 28], we chose the combined expression of Helios and Nrp1, which is the best identifier for tTregs to investigate the tTregs that produce proinflammatory cytokines. Consequently, the absence of Helios and Nrp1 represents peripherally iTregs [29]. We assessed the difference in proinflammatory cytokine production from Foxp3+ Tregs between Helios+Nrp1+ tTregs and Helios−Nrp1− iTregs in the context of TLR4−/− and inflammation. We found that Helios+Nrp1+ and Helios−Nrp1− Tregs produced IL-17 and IFN-γ in the spleen and LP under steady-state conditions (Figs. 3–6). Analysis of intestinal Tregs in WT mice revealed a higher presence of Helios−Nrp1− Tregs versus Helios+Nrp1+ Tregs than was found in the spleen. Although TLR4−/−IL-10−/− mice had higher frequencies of Tregs in the intestines, the ratio of Helios−Nrp1− Tregs to Helios+Nrp1+ Tregs was conserved between strains (Figs. 3A, 4B, 5A, and 6B). In WT mice, Helios+Nrp1+ Tregs had a higher propensity to produce IFN-γ or IL-17 in the intestines, as opposed to the spleen, whereas Helios+Nrp1+ Tregs only produced IFN-γ with little IL-17. Helios+Nrp1+ Tregs produced higher levels of IFN-γ compared with that of Helios−Nrp1− Tregs in the spleen and LP, and LP Helios+Nrp1+ Tregs produced more IL-17 than did Helios−Nrp1− Tregs (Fig. 5B), whereas Helios−Nrp1− Tregs and Helios+Nrp1+ Tregs produced IL-17 at similar levels in the spleen (Fig. 3B). In IL-10−/− mice, IFN-γ and IL-17 production by Helios−Nrp1− Tregs and Helios+Nrp1+ Tregs in the spleens and LP was increased compared with that in WT mice; however, the pattern remained the same; i.e. Helios+Nrp1+ Tregs produced a higher level of IFN-γ compared with Helios−Nrp1− Tregs, and Helios−Nrp1− Tregs and Helios+Nrp1+ Tregs produced IL-17 at similar levels (Figs. 3–6). Although IL-17 and IFN-γ production by Helios−Nrp1− Tregs and Helios+Nrp1+ Tregs was not different from WT in TLR4−/− mice, TLR4−/− in IL-10−/− mice significantly increased production of IL-17 and IFN-γ by Helios−Nrp1− Tregs and Helios+Nrp1+ Tregs. Notably, the Foxp3+ Tregs expressing IFN-γ and IL-17 were predominantly Helios+Nrp1+ Tregs in the TLR4−/−IL-10−/− mice. Collectively, these data demonstrated that whereas the absence of TLR4 signaling does not affect Treg proinflammatory cytokine production alone, it augments Treg proinflammatory cytokine production during intestinal inflammation in the absence of IL-10.
Figure 3. Helios+Nrp1+ Treg and Helios−Nrp1− Tregs differentially express IFN-γ in the spleen.
(A) Tregs (CD4+Foxp3+) were separated into Helios+Nrp1+ and Helios−Nrp1− Tregs, and analyzed for IFN-γ and IL-17 expression from the spleen of TLR4−/−, IL-10−/−, TLR4−/−IL-10−/−, and WT mice. (B) Helios+Nrp1+ Tregs [double-positive (DP) tTreg] and Helios−Nrp1− Tregs [double-negative (DN) iTreg] from the spleen of TLR4−/− IL-10−/− and WT mice were analyzed for IFN-γ and IL-17 production. Bar charts are calculated based on total double-positive tTreg or double-negative iTreg and reflect mean ± sem. *P < 0.05, and **P < 0.01. Data are representative of three or more experiments of two or more mice/group. FACS plots are gated upon live CD4+Foxp3+ cells.
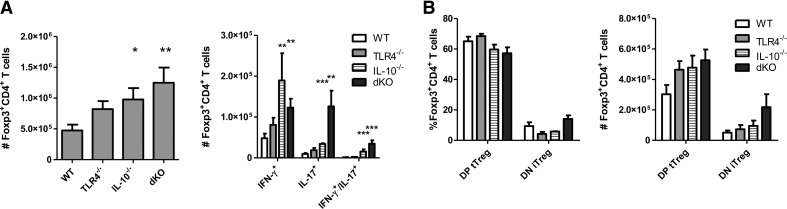
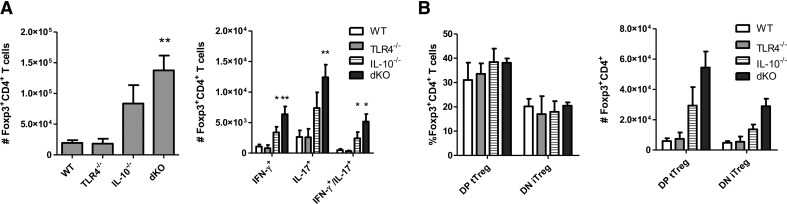
Figure 4. Tregs are increased systemically in the absence of TLR4 and IL-10.
(A) Spleen CD4 cells from TLR4−/−, IL-10−/−, TLR4−/−IL-10−/−, and WT mice were counted for Foxp3 expression and IFN-γ and IL-17 in Tregs. (B) Tregs (CD4+Foxp3+) were analyzed for Helios and Nrp expression. DP tTreg, Helios+Nrp1+ Tregs; DN iTreg, Helios−Nrp1− Tregs. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with WT B6 mice. Data are representative of three or more experiments of two or more mice/group.
Figure 5. Intestinal Helios+ Nrp1+ Tregs and Helios−Nrp1− Tregs differentially produce IFN-γ and IL-17.
(A) Treg (CD4+Foxp3+) cells were separated into Helios+Nrp1+ and Helios−Nrp1− Tregs and analyzed for IFN-γ and IL-17 expression from the intestines of TLR4−/−, IL-10−/−, TLR4−/−IL-10−/−, and WT mice. (B) Helios+Nrp1+ Tregs (DP tTreg) and Helios−Nrp1− Tregs (DN iTreg) from the intestines of TLR4−/−IL-10−/− and WT mice were analyzed for IFN-γ and IL-17 production. Bar charts are calculated based on total double-positive tTreg or double-negative iTreg and reflect mean ± sem. *P < 0.05, **P < 0.01, and ***P < 0.001. Data are representative of three or more experiments of two or more mice/group. FACS plots are gated upon live CD4+Foxp3+ cells.
Figure 6. Intestinal Tregs are increased in the absence of TLR4 and IL-10.
(A) Intestinal CD4 cells from TLR4−/−, IL-10−/−, TLR4−/−IL-10−/− (dKO), and WT mice were counted for Foxp3 expression and IFNγ and IL-17 in Tregs. (B) Tregs (CD4+Foxp3+) were analyzed for Helios and Nrp expression.*P < 0.05, and **P < 0.01 compared with WT B6 mice. Data are representative of three or more experiments of two or more mice/group.
Foxp3+ Tregs expand in the intestine during intestinal inflammation
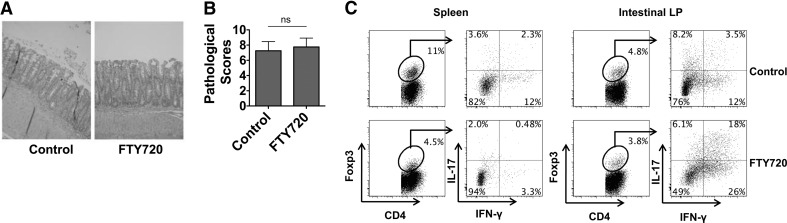
We then investigated whether Treg expansion occurred systemically or within the tissue at the interface of microbial exposure and interaction. We used the CBir1 T cell transfer model to examine the location of Treg expansion. The transfer of CD4+ T cells from CBir1 Tg mice, which are specific for an immunodominant commensal bacterial antigen CBir1 flagellin into immunodeficient RAG−/− mice, induces colitis in the recipients [5, 30]. CBir1 CD4 T cells were i.v. transferred into recipient RAG−/− mice and treated orally with FTY720 on the same day of cell transfer and every other day thereafter for 4 weeks, which inhibits lymphocyte egress from the tissues [31]. In FTY720-treated mice, CD4+ T cells activated in the intestines are expected to remain within the intestinal compartment. Consistent with our previous observations [5], both FTY720-treated and control CBir1 T cell-reconstituted RAG−/− mice developed colitis (Fig. 7A and B). Analysis of the mice receiving CBir1 CD4+ T cells revealed substantial numbers of Th1 cells, Th17 cells (data not shown), as well as Foxp3+ Tregs in the spleen and LP (Fig. 7C). The intestine contained the highest percentage of Foxp3+ Tregs producing IFN-γ, IL-17, or both IFN-γ and IL-17. Treatment with FTY720 greatly decreased the Foxp3+ Tregs in spleen but not in the mesenteric lymph node and intestine. Notably, the proportion of IFN-γ+Foxp3+ Tregs was increased greatly in the intestine after FTY720 treatment. IL-17+Foxp3+ T cells also increased to a lesser extent. Collectively, these data demonstrate that the expansion and subsequent production of proinflammatory cytokines by Foxp3+ Tregs occur primarily in the inflamed intestine with eventual systemic circulation.
Figure 7. Expansion and acquisition of proinflammatory cytokine production by Tregs occur in the intestine.
CBir1 CD4 T cells (0.25×106) were i.v. transferred into RAG2−/− mice. Recipient mice were gavaged with PBS or FTY720 every 2 days. (A) Four weeks post-T cell transfer, the histopathology of intestines was determined. Images are representative of two experiments of four mice/group with similar results. (B) Pathological scores are shown. (C) Tregs were isolated from the spleens and intestinal LP of CBir1 CD4 T cell recipient mice and analyzed for Foxp3, IFN-γ, and IL-17 production by flow cytometry. FACS plots are gated on total live CD4+ T cells. Data are representative of two experiments of four mice/group with similar results.
TLR4 signaling down-regulates development of Foxp3+ Tregs and Treg expression of IFN-γ in a MyD88-dependent and TRIF-independent manner
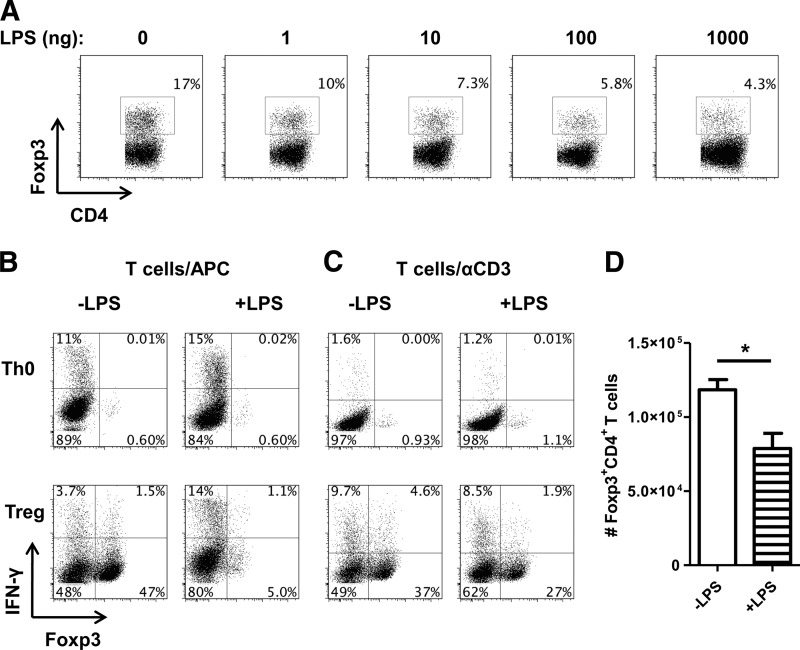
We next investigated how TLR4 signaling regulates Foxp3+ Tregs. As TLR4−/− mice had a higher frequency of Tregs in the intestine, where CD4+ T cells would have the most contact with bacteria and TLR ligands, we questioned whether TLR4 signaling inhibited induction of Foxp3+ Tregs and whether TLR4 signaling on CD4+ T cells or APCs was responsible for down-regulation of Foxp3+ Treg induction. We first cultured naive CD62L+CD4+ T cells from CBir1 Tg mice with splenic APCs under Treg conditions with TGF-β in the presence or absence of a series of doses of LPS. As shown in Fig. 8A, LPS inhibited development of Foxp3+ Tregs in a dose-dependent manner under Treg-polarizing conditions and had no discernible effect on Foxp3 expression on unpolarized T cells in the absence of TGF-β (Fig. 8B). Treatment with LPS led to an increase in IFN-γ expression in Foxp3− effector T cells, both in the presence and absence of TGF-β, but inhibited Foxp3+IFN-γ− and Foxp3+IFN-γ+ Tregs.
Figure 8. LPS inhibits Foxp3 Tregs.
(A) CD62L+CD4+ CBir1 CD4 T cells were cocultured with irradiated splenocytes with CBir1 peptide and 5 ng/ml TGF-β to induce Foxp3 induction. Cells were also treated with or without varying concentrations of LPS and analyzed by flow cytometry after 5 days. (B) CD62L+CD4+ CBir1 CD4 T cells were cocultured with irradiated splenocytes (APC) with CBir1 peptide in the absence of polarization (Th0) or with 5 ng/ml TGF-β (Treg). Cells were also treated with or without LPS (1000 ng) and analyzed by flow cytometry after 5 days. (C) CD62L+CD4+ T cells were cultured with plate-bound αCD3 (1 μg) and αCD28 (2 μg/ml) in the absence of polarization (Th0) or with 5 ng/ml TGF-β (Treg). Cells were also treated with or without LPS (1000 ng) and analyzed by flow cytometry after 5 days. (D) Bar graph reflects total numbers of Foxp3+ cells generated in culture of C and represents mean ± sem. *P < 0.05, n = 4. FACS plots are gated upon live CD4+ cells.
As CD4+ T cells express TLR4 and respond to its ligand LPS [22], we cultured naive CD62L+CD4+ T cells with plate-bound anti-CD3 and anti-CD28 with TGF-β in the presence or absence of LPS to determine the role of LPS-TLR4 signaling in T cells on Treg differentiation. As shown in Fig. 8C, LPS-TLR4 signaling in T cells inhibited Treg development. Although LPS can promote the overall proliferation of CD4+ T cells [24], we observed that the total number of Foxp3+ Tregs was decreased after LPS treatment (Fig. 8D), thus excluding the possibility that hyperproliferation of non-Treg Foxp3− cells was diluting the frequency of Foxp3+ Tregs. It has been reported previously that TLR4 signaling on Th1 cells can decrease IFN-γ production [22]. Whereas we observed little difference in IFN-γ expression in Foxp3− cells, we found that direct treatment with LPS strongly decreased IFN-γ production in Tregs (Fig. 8C).
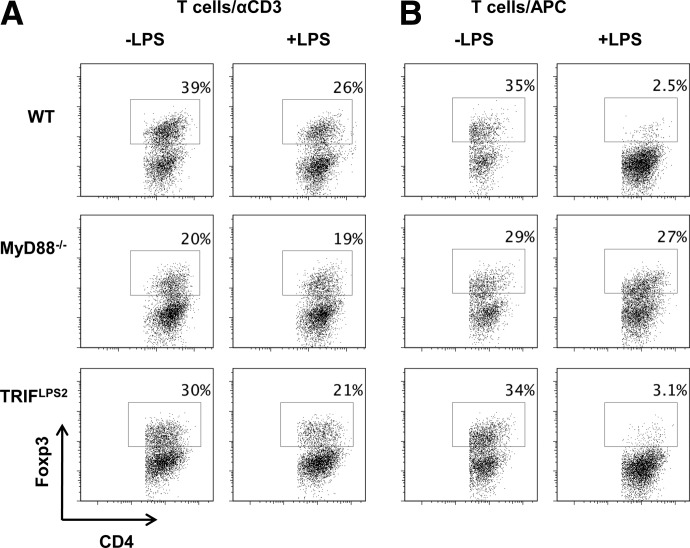
TLR4 signals through two separate pathways: the MyD88 pathway and the TRIF pathway [32]. To determine the mechanism behind TLR4 inhibition of Foxp3+ Tregs, we used MyD88−/− or TRIF−/− CD4+ T cells to identify which pathway was responsible for the effects directly in T cells. After treatment with LPS and TGF-β, MyD88−/− naive CD4+ T cells exhibited no discernible change in induction of Foxp3+ Tregs, whereas LPS down-regulated Foxp3+ Treg induction in TRIF−/− naive CD4+ T cells (Fig. 9A), revealing that CD4+ T cell TLR4 inhibition of Foxp3+ Tregs depends on MyD88 but not TRIF.
Figure 9. LPS partially inhibits Treg generation through MyD88 signaling on CD4 cells and APCs.
(A) MyD88−/−, TRIF−/−, or WT CD4 cells were cultured with plate-bound αCD3 (1 μg) and αCD28 (2 μg/ml) with 5 ng/ml TGF-β (Treg) in the presence or absence of LPS (1 μg) and analyzed by flow cytometry after 5 days. (B) MyD88−/−, TRIF−/−, or WT irradiated splenocytes (APC) were cultured with WT CD4 cells with plate-bound αCD3 (1 μg) and 5 ng/ml TGF-β in the presence or absence of LPS (1000 ng) and analyzed by flow cytometry after 5 days. FACS plots are gated upon live CD4+ cells.
As LPS signaling on APCs drastically inhibited Foxp3+ Tregs at a much greater level compared with TLR4 signaling solely on CD4+ T cells (Fig. 8), we then examined the role of the MyD88 and TRIF pathways in APCs by coculturing WT CD4+ T cells with MyD88−/− or TRIF−/− APCs under Treg conditions in the presence or absence of LPS. As addition of LPS in the culture of naive CD4+ T cells with WT APCs under Treg conditions almost completely inhibited induction of Foxp3+ Tregs, addition of LPS did not result in a decrease of induction of Foxp3+ Tregs in the absence of APC MyD88 signaling (Fig. 9B). In contrast, when WT naive CD4+ T cells were cultured with TRIF−/− APCs, LPS inhibited induction of Foxp3+ Tregs at a similar level to that with WT APCs. Collectively, these data demonstrated that LPS modulates Foxp3+ Tregs in a MyD88-dependent, TRIF-independent manner. This effect is mediated directly on CD4+ T cells, as well as indirectly through APCs.
SOCS3 mediates LPS-TLR4 inhibition of Foxp3+ Treg development
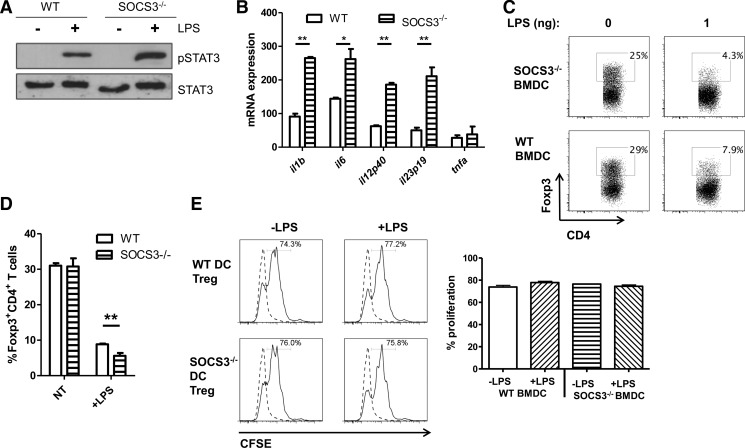
LPS-TLR4 signaling stimulates SOCS3 expression and STAT3 activation, and SOCS3 serves as a key negative regulator of the LPS-TLR4 pathway through inhibition of STAT3 [33]. Therefore, SOCS3 has a crucial role in modulating STAT3 activation and inflammatory gene expression. We next investigated whether SOCS3 regulates LPS inhibition of Foxp3+ Tregs. We first generated BMDCs from WT and LysMCre-SOCS3fl/fl mice and stimulated them with LPS. SOCS3−/− BMDCs expressed higher levels of activation of STAT3 (Fig. 10A) and produced higher levels of IL-1β, IL-6, IL-12, IL-23, and TNF-α compared with levels in WT BMDCs (Fig. 10B). To determine whether SOCS3 regulates LPS-TLR4 inhibition of Foxp3+ Tregs, we cultured WT naive CD4+ T cells with WT and SOCS3−/− BMDCs with anti-CD3 under Treg conditions with TGF-β. To delineate the effect of SOCS3, we selected a low dose of 1 ng/ml LPS that only partially inhibited induction of Foxp3+ Tregs. SOCS3−/− in APCs enhanced LPS down-regulation of Foxp3+ Tregs (Fig. 10C and D). Collectively, these data indicated that SOCS3 negatively regulates LPS inhibition of Foxp3+ Treg development.
Figure 10. SOCS3 negatively regulates LPS inhibition of Foxp3+ Tregs.
BMDCs from SOCS3−/− or WT mice were derived with GM-CSF after 8 days. (A) BMDCs were treated with medium or LPS (10 ng/ml) for 4 h. Cell lysates were subjected to immunoblotting with antiphospho-STAT3 (pSTAT3), anti-STAT3, and GAPDH antibodies. (B) Primary BMDCs from WT and SOCS3−/− mice were treated with medium or LPS (10 ng/ml) for 4 h. mRNA was analyzed for IL-1β, IL-6, IL-12p40, IL-23p19, and TNF-α by quantitative RT-PCR. Representative of three independent experiments. *P > 0.01, and **P > 0.001. (C) BMDCs were cocultured with CBir1 CD4 cells with TGF-β (5 ng/ml) in the presence or absence of LPS (1 ng) and analyzed by flow cytometry after 5 days. (D) Bar graph reflects percentage of Foxp3+ cells generated in culture of C and represents mean ± sem. **P < 0.01, n = 6. FACS plots are gated upon live CD4+ cells. NT, non-treated. (E) Tregs were sorted from Foxp3-GFP CBir1 CD45.2 CD4 T cells after culture with 5 ng/ml TGF-β in the presence or absence of LPS (1 ng/ml) and WT or SOCS3−/− BMDCs. FACS-sorted GFP+ Tregs were cultured with CFSE-labeled CBir1 CD45.1 CD4 T cells (1:4) and irradiated APCs with 1 μg/ml CBir1 peptide. Proliferation of CD45.1 responder CD4 T cells was measured after 4 days. FACS plots are gated upon live CD45.1+ CD4+ cells (dashed lines, naive T cell alone; solid lines, with Tregs).
To assess whether the functionality of Tregs is affected after generation under the influence of LPS and SOCS3, we cultured naive CD45.2+ Foxp3-GFP CBir1 CD4 T cells with WT and SOCS3−/− BMDCs under Treg conditions with TGF-β in the presence or absence of LPS (1 ng/ml). After sorting GFP+ Tregs, we assessed their ability to suppress effector T cell proliferation by coculturing with CFSE-labeled splenic CD45.1 CBir1 CD4 T cells and measured effector T cell proliferation via CFSE dilution after 4 days. As illustrated in Fig. 10E, LPS treatment during Treg induction had no discernible effect on Treg function. Whereas the frequency of Treg generation is strongly inhibited by LPS, the Tregs that arise continue to function normally. SOCS3−/− in APCs during Treg generation also does not have significant bearing on the function of Tregs after induction of Foxp3.
DISCUSSION
IBD is dependent on the microbiota for providing signals to modulate the immune system and control inflammation. An inability to regulate the intestinal environment properly coupled with constant exposure to microbial ligands induces chronic inflammation. In both patients with IBD and experimental colitis, high levels of Foxp3+ Tregs are found to produce proinflammatory cytokines within the inflamed intestine [5, 6, 8], potentially intensifying inflammation or stifling regulation. Recently, it has been shown that microbial ligands modulate CD4+ T cell activation. Although CD4+ T cells respond in an antigen-specific manner, the presence of TLR ligands can potentiate CD4+ T cell responses [34, 35]. TLR4−/− on CD4+ T cells aggravates colitis in IL-10−/− mice by increasing Treg and T effector cell production of IFN-γ and IL-17 [22, 23]. As Tregs can arise from the thymus or in the periphery, there is a question of whether there are differences in immunoregulation and cytokine production between Treg subsets. It has been reported that Helios and Nrp1 are expressed predominantly by tTregs, although not absolutely. Helios+Nrp1+Foxp3+ is considered to be the best indicator of tTregs, whereas Helios−Nrp1− Foxp3+ is the best indicator of iTregs [27, 28, 36, 37]. We report here that Helios+Nrp1+ and Helios−Nrp1− Foxp3+ Tregs can be induced to express IFN-γ and/or IL-17 under homeostatic conditions and inflammatory conditions, a process that is negatively regulated by TLR4 in a MyD88-dependent but TRIF-independent manner.
It has been observed consistently that Foxp3+ Tregs accumulate in inflamed tissue of animal models of colitis and IBD patients [6, 38]. Whereas it would appear that the influx of Tregs is a response at controlling extensive inflammation, a substantial number of them produce proinflammatory cytokines. This highlights the challenge of using expanded Treg populations to treat autoimmune diseases, which might result in the uncontrolled production of proinflammatory cytokines by Tregs [39–41]. Although recent reports demonstrated that encounter with microbiota resulted in the peripheral generation of Tregs, and microbiota antigens play an important role in shaping the colonic Treg population [42, 43], it is still highly debatable as to whether only iTregs can convert into proinflammatory cytokine-producing T cells or whether tTregs can also do so under certain conditions in the intestine. In contrast to some previous reports [44] indicating that tTregs were stable and resistant to conversion into Th1 or Th17 cells, our current findings revealed that Helios+Nrp1+Foxp3+ Tregs produced IFN-γ and IL-17 at a higher frequency and even in greater numbers than Helios−Nrp1− Foxp3+ Tregs. These data corroborate previous findings that tTregs are apt to produce IL-17 after treatment with IL-6 [45], while continuing to express Foxp3. Although severe inflammation was not a prerequisite for Treg conversion, as it can be seen in the intestine of healthy WT mice, the degree of inflammation did contribute to the acquisition of proinflammatory cytokines in Tregs. Given our previous observations that the acquisition of proinflammatory cytokines can occur in tandem with the loss of Foxp3 expression from “ex-Tregs”; at this point, it remains unclear how the chronic inflammation in TLR4−/−IL-10−/− mice affects stability of Foxp3 expression in tTregs or iTregs. It has been shown that Treg expression of a number of factors contributes to Treg function and stability through improving Treg-mediated immunoregulation, including OX40 [12, 13], which enhances Treg responsiveness to IL-2, and TNFRII [14, 15], which enhances Treg function in the presence of TNF-α. However, it is currently not known completely how TLR4 and its signaling components regulate such conversion. Furthermore, it remains unclear whether microbial antigens and pathogen-associated molecular patterns are able to impair Foxp3 and Treg stability and promote conversion to other subsets, perhaps as a result of consistent or repeated TLR stimulation.
Interestingly, although we observed an increase in intestinal Foxp3+ Tregs in TLR4−/− mice housed in the animal facilities at The University of Chicago and at UTMB, there was no observable difference in Foxp3+ Treg numbers between the colons of WT and TLR4−/− mice housed in the animal facilities at Massachusetts General Hospital in our previous studies [23], indicating that different commensal bacteria differentially stimulate gut TLR4 signaling and host immune responses. The acquisition or loss of particular bacterial populations may have altered the propensity for induction of Foxp3 in the context of TLR4 signaling, as it has been reported that identical strains of mice often have considerable phenotypic variation in their intestinal microbiota when relocated to different facilities.
During colitis, antigen-specific Tregs induced by microbial signals produce proinflammatory cytokines that accumulate not only in the inflamed intestine but also in the spleen (Fig. 7). That raises important questions: where do Treg expansion and conversion into proinflammatory cytokine-producing T cells occur? Are they induced systemically or locally in the mucosa where they are activated after encountering their cognate microbial antigens in the intestinal lumen? Treatment with FTY720, which inhibits lymphocyte egress from lymph nodes [31], decreased total Foxp3+ Tregs as well as proinflammatory, cytokine-expressing Foxp3+ Tregs in the spleens of colitic RAG−/− mice that received CBir1 Tg T cells. However, FTY720 treatment did not affect Foxp3+ Tregs in the LP, indicating that Foxp3+ Treg expansion and production of proinflammatory cytokines occurred in the intestine, where they were activated by their cognate microbiota antigens, and migrated into systemic lymphoid organs.
Activation of CD4+ T cells requires the presentation and coreceptor binding by APCs. Thus, the effects of LPS cannot be isolated solely on CD4+ T cells or on the APCs. When APCs are taken into account, LPS strongly abrogates Foxp3+ Treg development under Treg conditions. With the decrease in Foxp3+ Tregs, increases in IFN-γ and IL-17 are also observed in Foxp3− cells, thereby indicating that polarizing cytokines are being produced by APCs upon LPS exposure, which, in turn, inhibits induction of Foxp3+ Tregs and skews CD4+ T cell differentiation. The effect of LPS directly upon Th1 cells has been detailed previously [22], with LPS able to suppress IFN-γ. We found that the presence of LPS during Treg differentiation via αCD3/αCD28 was able to suppress Foxp3 induction. Additionally, LPS was able to suppress IFN-γ selectively in Tregs (Fig. 8). It is likely that the absence of TLR4 signaling on T cells partially contributes to the increases in cytokine production from Tregs, as seen in TLR4−/− and TLR4−/−/IL-10−/− mice (Figs. 2B and 4A).
The LPS-TLR4 pathway has been shown to stimulate SOCS3 expression and STAT3 activation, with SOCS3 serving as a crucial negative regulator of LPS-TLR4 responses by inhibiting activation of STAT3 and NF-κB, thus limiting inflammatory cytokine production [33]. By using BMDCs from myeloid-specific SOCS3−/− mice, we demonstrated that SOCS3 negatively regulated LPS-TLR4 inhibition of Treg Foxp3 expression, in that SOCS3−/− further enhanced LPS inhibition of Foxp3+ Tregs. This is most likely mediated by higher levels of inflammatory cytokine production in SOCS3−/− DCs in response to LPS, such as IL-1, IL-6, IL-12, and IL-23, which have been shown to be able to inhibit Foxp3+ Tregs [16, 25, 46].
In summary, our data demonstrated that Helios+Nrp1+ tTregs and Helios−Nrp1− iTregs are able to produce IFN-γ and/or IL-17 in the intestine and that intestinal inflammation promotes Treg accumulation and proinflammatory cytokine production. TLR4 negatively regulates Treg generation through a MyD88-dependent, TRIF-independent manner. TLR4 regulation of Foxp3+ Tregs acts indirectly through APCs, as well as directly through CD4+ T cells. These data thus provide insights into Treg regulation under TLR4-mediated conditions.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health grants DK079918, DK098370, DK071176, and DK089999 and the National Natural Science Foundation of China (81061120521, 81270470). A.T.C. is a recipient of the James W. McLaughlin Postdoctoral Fellowship, University of Texas Medical Branch.
We thank Kabir Matharu for critical review of the manuscript.
Footnotes
- −/−
- deficient
- B6
- C57BL/6
- BM
- bone marrow
- BMDC
- bone marrow-derived dendritic cell
- CBir1 Tg
- C57BL/6.CBir1 flagellin-specific TCR transgenic
- CD62L
- CD62 ligand
- DC
- dendritic cell
- Foxp3
- forkhead box p3
- IBD
- inflammatory bowel disease
- iTreg
- inducible regulatory T cell
- LP
- lamina propria
- LysMCre-SOCS3fl/fl
- C57BL/6.SOCS3 conditional knockout
- Nrp
- neuropilin
- SOCS
- suppressor of cytokine signaling
- Treg
- regulatory T cell
- TRIF
- Toll-IL-1R domain-containing adapter-inducing IFN-β
- tTreg
- thymic regulatory T cell
- UTMB
- The University of Texas Medical Branch
- WT
- wild-type
AUTHORSHIP
A.T.C. performed experiments and wrote the manuscript. S.Y., H.L., and H.L.E-M. performed intestinal cell isolations. A.T.S. contributed reagents and technical advice. H.Q. performed SOCS3 experiments. Z.L., C.O.E., and C.R.N. contributed reagents and to the manuscript review. Y.C. conceived of experiments, supervised the study, and wrote the manuscript.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1. Fontenot J. D., Gavin M. A., Rudensky A. Y. (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336. [DOI] [PubMed] [Google Scholar]
- 2. Khattri R., Cox T., Yasayko S. A., Ramsdell F. (2003) An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342. [DOI] [PubMed] [Google Scholar]
- 3. Bluestone J. A., Abbas A. K. (2003) Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3, 253–257. [DOI] [PubMed] [Google Scholar]
- 4. Uhlig H. H., Coombes J., Mottet C., Izcue A., Thompson C., Fanger A., Tannapfel A., Fontenot J. D., Ramsdell F., Powrie F. (2006) Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 177, 5852–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feng T., Cao A. T., Weaver C. T., Elson C. O., Cong Y. (2011) Interleukin-12 converts Foxp3+ regulatory T cells to interferon-γ-producing Foxp3+ T cells that inhibit colitis. Gastroenterology 140, 2031–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holmen N., Lundgren A., Lundin S., Bergin A. M., Rudin A., Sjovall H., Ohman L. (2006) Functional CD4+CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm. Bowel Dis. 12, 447–456. [DOI] [PubMed] [Google Scholar]
- 7. Xu L., Kitani A., Fuss I., Strober W. (2007) Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-β. J. Immunol. 178, 6725–6729. [DOI] [PubMed] [Google Scholar]
- 8. Hovhannisyan Z., Treatman J., Littman D. R., Mayer L. (2011) Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology 140, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blatner N. R., Mulcahy M. F., Dennis K. L., Scholtens D., Bentrem D. J., Phillips J. D., Ham S., Sandall B. P., Khan M. W., Mahvi D. M., Halverson A. L., Stryker S. J., Boller A. M., Singal A., Sneed R. K., Sarraj B., Ansari M. J., Oft M., Iwakura Y., Zhou L., Bonertz A., Beckhove P., Gounari F., Khazaie K. (2012) Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci. Transl. Med. 4, 164ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Z., Arijs I., De Hertogh G., Vermeire S., Noman M., Bullens D., Coorevits L., Sagaert X., Schuit F., Rutgeerts P., Ceuppens J. L., Van Assche G. (2010) Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab. Inflamm. Bowel Dis. 16, 1299–1310. [DOI] [PubMed] [Google Scholar]
- 11. Chen X., Oppenheim J. J. (2011) Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett. 585, 3611–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Griseri T., Asquith M., Thompson C., Powrie F. (2010) OX40 is required for regulatory T cell-mediated control of colitis. J. Exp. Med. 207, 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piconese S., Pittoni P., Burocchi A., Gorzanelli A., Care A., Tripodo C., Colombo M. P. (2010) A non-redundant role for OX40 in the competitive fitness of Treg in response to IL-2. Eur. J. Immunol. 40, 2902–2913. [DOI] [PubMed] [Google Scholar]
- 14. Chen X., Wu X., Zhou Q., Howard O. M., Netea M. G., Oppenheim J. J. (2013) TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J. Immunol. 190, 1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Housley W. J., Adams C. O., Nichols F. C., Puddington L., Lingenheld E. G., Zhu L., Rajan T. V., Clark R. B. (2011) Natural but not inducible regulatory T cells require TNF-α signaling for in vivo function. J. Immunol. 186, 6779–6787. [DOI] [PubMed] [Google Scholar]
- 16. Josefowicz S. Z., Rudensky A. (2009) Control of regulatory T cell lineage commitment and maintenance. Immunity 30, 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang X. O., Nurieva R., Martinez G. J., Kang H. S., Chung Y., Pappu B. P., Shah B., Chang S. H., Schluns K. S., Watowich S. S., Feng X. H., Jetten A. M., Dong C. (2008) Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29, 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koch M. A., Tucker-Heard G., Perdue N. R., Killebrew J. R., Urdahl K. B., Campbell D. J. (2009) The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsuji M., Komatsu N., Kawamoto S., Suzuki K., Kanagawa O., Honjo T., Hori S., Fagarasan S. (2009) Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 323, 1488–1492. [DOI] [PubMed] [Google Scholar]
- 20. Sutmuller R. P., Morgan M. E., Netea M. G., Grauer O., Adema G. J. (2006) Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 27, 387–393. [DOI] [PubMed] [Google Scholar]
- 21. Uematsu S., Fujimoto K., Jang M. H., Yang B. G., Jung Y. J., Nishiyama M., Sato S., Tsujimura T., Yamamoto M., Yokota Y., Kiyono H., Miyasaka M., Ishii K. J., Akira S. (2008) Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 9, 769–776. [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez-Navajas J. M., Fine S., Law J., Datta S. K., Nguyen K. P., Yu M., Corr M., Katakura K., Eckman L., Lee J., Raz E. (2010) TLR4 signaling in effector CD4+ T cells regulates TCR activation and experimental colitis in mice. J. Clin. Invest. 120, 570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matharu K. S., Mizoguchi E., Cotoner C. A., Nguyen D. D., Mingle B., Iweala O. I., McBee M. E., Stefka A. T., Prioult G., Haigis K. M., Bhan A. K., Snapper S. B., Murakami H., Schauer D. B., Reinecker H. C., Mizoguchi A., Nagler C. R. (2009) Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology 137, 1380–1390.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukata M., Breglio K., Chen A., Vamadevan A. S., Goo T., Hsu D., Conduah D., Xu R., Abreu M. T. (2008) The myeloid differentiation factor 88 (MyD88) is required for CD4+ T cell effector function in a murine model of inflammatory bowel disease. J. Immunol. 180, 1886–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qin H., Holdbrooks A. T., Liu Y., Reynolds S. L., Yanagisawa L. L., Benveniste E. N. (2012) SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 189, 3439–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubtsov Y. P., Niec R. E., Josefowicz S., Li L., Darce J., Mathis D., Benoist C., Rudensky A. Y. (2010) Stability of the regulatory T cell lineage in vivo. Science 329, 1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weiss J. M., Bilate A. M., Gobert M., Ding Y., Curotto de Lafaille M. A., Parkhurst C. N., Xiong H., Dolpady J., Frey A. B., Ruocco M. G., Yang Y., Floess S., Huehn J., Oh S., Li M. O., Niec R. E., Rudensky A. Y., Dustin M. L., Littman D. R., Lafaille J. J. (2012) Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 209, 1723–1742, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thornton A. M., Korty P. E., Tran D. Q., Wohlfert E. A., Murray P. E., Belkaid Y., Shevach E. M. (2010) Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 184, 3433–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., Deroos P., Liu H., Cross J. R., Pfeffer K., Coffer P. J., Rudensky A. Y. (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 19, 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cong Y., Feng T., Fujihashi K., Schoeb T. R., Elson C. O. (2009) A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. USA 106, 19256–19261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Radi Z. A., Heuvelman D. M., Masferrer J. L., Benson E. L. (2011) Pharmacologic evaluation of sulfasalazine, FTY720, and anti-IL-12/23p40 in a TNBS-induced Crohn's disease model. Dig. Dis. Sci. 56, 2283–2291. [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K., Akira S. (2003) Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301, 640–643. [DOI] [PubMed] [Google Scholar]
- 33. Stoiber D., Kovarik P., Cohney S., Johnston J. A., Steinlein P., Decker T. (1999) Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-γ. J. Immunol. 163, 2640–2647. [PubMed] [Google Scholar]
- 34. Caramalho I., Lopes-Carvalho T., Ostler D., Zelenay S., Haury M., Demengeot J. (2003) Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 197, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Maren W. W., Jacobs J. F., de Vries I. J., Nierkens S., Adema G. J. (2008) Toll-like receptor signalling on Tregs: to suppress or not to suppress? Immunology 124, 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dhamne C., Chung Y., Alousi A. M., Cooper L. J., Tran D. Q. (2013) Peripheral and thymic foxp3(+) regulatory T cells in search of origin, distinction, and function. Front. Immunol. 4, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yadav M., Stephan S., Bluestone J. A. (2013) Peripherally induced Tregs—role in immune homeostasis and autoimmunity. Front. Immunol. 4, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maul J., Loddenkemper C., Mundt P., Berg E., Giese T., Stallmach A., Zeitz M., Duchmann R. (2005) Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology 128, 1868–1878. [DOI] [PubMed] [Google Scholar]
- 39. McMurchy A. N., Bushell A., Levings M. K., Wood K. J. (2011) Moving to tolerance: clinical application of T regulatory cells. Semin. Immunol. 23, 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. June C. H., Blazar B. R. (2006) Clinical application of expanded CD4+25+ cells. Semin. Immunol. 18, 78–88. [DOI] [PubMed] [Google Scholar]
- 41. Riley J. L., June C. H., Blazar B. R. (2009) Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity 30, 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lathrop S. K., Bloom S. M., Rao S. M., Nutsch K., Lio C. W., Santacruz N., Peterson D. A., Stappenbeck T. S., Hsieh C. S. (2011) Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. (2004) Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241. [DOI] [PubMed] [Google Scholar]
- 44. Reynolds J. M., Martinez G. J., Chung Y., Dong C. (2012) Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc. Natl. Acad. Sci. USA 109, 13064–13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zheng S. G., Wang J., Horwitz D. A. (2008) Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-β are resistant to Th17 conversion by IL-6. J. Immunol. 180, 7112–7116. [DOI] [PubMed] [Google Scholar]
- 46. Pasare C., Medzhitov R. (2003) Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299, 1033–1036. [DOI] [PubMed] [Google Scholar]