Review on the intricate structure, composition and function of lipid body organelles as modulators of immune responses in human eosinophils.
Keywords: lipid droplets, inflammation, eicosanoids, immune responses, cell activation, electron microscopy
Abstract
Lipid-rich organelles are common in many cell types. In cells, such as adipocytes, these organelles are termed LDs, whereas in other cells, such as leukocytes, they are called LBs. The study of leukocyte LBs has attracted attention as a result of their association with human diseases. In leukocytes, such as eosinophils, LB accumulation has been documented extensively during inflammatory conditions. In these cells, LBs are linked to the regulation of immune responses by compartmentalization of several proteins and lipids involved in the control and biosynthesis of inflammatory mediators (eicosanoids). However, it has been unclear how diverse proteins, including membrane-associated enzymes involved in eicosanoid formation, incorporate into LBs, especially if the internal content of LBs is assumed to consist solely of stores of neutral lipids, as present within adipocyte LDs. Studies of the formation, function, and ultrastructure of LBs in eosinophils have been providing insights pertinent to LBs in other leukocytes. Here, we review current knowledge of the composition and function of leukocyte LBs as provided by studies of human eosinophil LBs, including recognitions of the internal architecture of eosinophil LBs based on 3D electron tomographic analyses.
Introduction
In recent decades, there has been a renaissance of interest and recognition in the structure and function of intracellular lipid inclusions. Such lipid-rich organelles in cells, such as adipocytes and steroidogenic cells, are termed LDs. The dynamic nature of LDs has led to their recognition as highly active organelles within most cell types involved in different biological functions and containing not only lipids but also many proteins (reviewed in refs. [1–3]). In leukocytes, there has been a historically delayed recognition of the presence of intracellular lipid organelles that have been mainly termed LBs. Although classic LDs from adipocytes and LBs from leukocytes share some structural features, there are striking differences between them, and they likely represent different types of lipid-rich organelles. Classic LDs, as in adipocytes, have principal roles related to neutral lipid storage and metabolism. In contrast, LBs, as present in leukocytes and other cells, have functions, compositions, and structural aspects distinct from classic LDs. To highlight distinct features of these organelles, in this review, we will use the nomenclature LB and LD to refer to lipid-rich organelles from leukocytes and adipocytes, respectively.
LBs in leukocytes are remarkably linked to inflammatory responses and considered structural markers of inflammation (reviewed in refs. [4, 5]). The association of LBs with inflammation in leukocytes, such as eosinophils, was demonstrated by the following: (1) accentuated and rapid formation of these organelles in response to inflammatory stimuli or allergic/inflammatory human diseases, including infections with parasites and bacteria; (2) presence of arachidonyl phospholipids, which serve as precursors for synthesis of inflammatory mediators (eicosanoids), within LBs; (3) correlative associations of LB formation (or inhibition) with levels of eicosanoids secretion; (4) localizations of eicosanoid-generating enzymes within LBs; and (5) in situ synthesis of eicosanoids (PGs and LTs) in activated cells (reviewed in refs. [4, 6]). Enzymes involved in the biogenesis of these inflammatory lipid mediators, as well as eicosanoids themselves, were fully and directly localized within leukocyte LBs [4]. Another key finding in LBs related to inflammation was the demonstration of the proinflammatory cytokine TNF-α within LBs from human eosinophils [7], confirming that LBs in leukocytes contain multiple molecular mechanisms for regulating inflammatory events. Leukocyte LBs are therefore likely, intimately linked to the functional capabilities of these leukocytes.
Although a phospholipid hemimembrane and proteins decorate the surface of leukocyte LBs, as found with LDs from adipocytes and other cells, the internal lipid-rich “cores” of leukocyte LBs are much more complex than recognized in LDs. The spatial organization of LB organelles is now beginning to be uncovered. Here, we integrate our current knowledge on the composition, structure, and function of LBs in human eosinophils, cells involved in a diversity of allergic and inflammatory diseases (reviewed in refs. [8–10]), including recent insights into the internal architecture of eosinophil LBs based on 3D electron tomographic analyses [11], which altogether, have provided new insights in the structure and function of LBs in leukocytes.
LBs: TYPICAL ORGANELLES OF HUMAN EOSINOPHILS
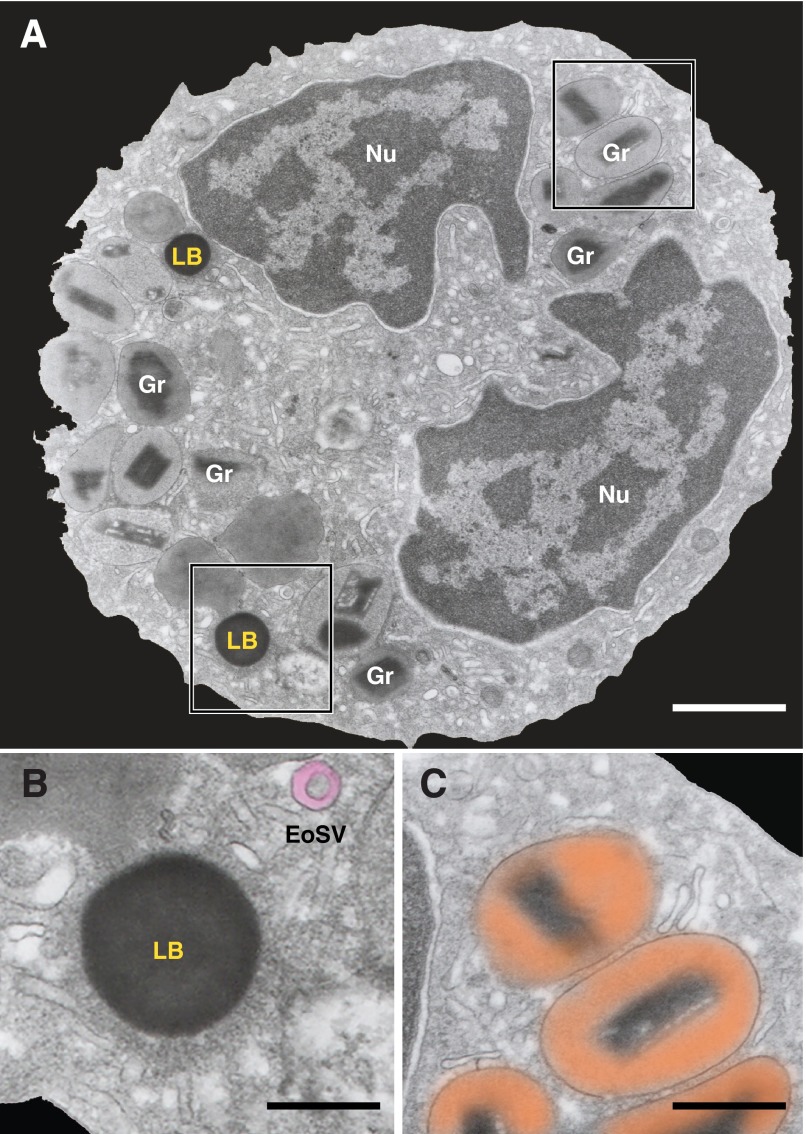
Mature human eosinophils are distinguished by a major population of secretory granules, at times, termed secondary, crystalline, or specific granules. Eosinophils are also characterized by a typically bilobed nucleus with condensed, marginated nuclear chromatin, a prominent vesicular system, including large vesiculotubular carriers, termed EoSVs, and LBs (Fig. 1; reviewed in refs. [10, 12–14]).
Figure 1. TEM of a human eosinophil.
(A) The eosinophil cytoplasm contains a major population of specific granules (Gr), LBs, transport vesicles, and an usually bilobed nucleus (Nu) with condensed, marginated chromatin. (B and C) The boxed areas are shown in higher magnification. (B) An electron-dense LB and EoSV (highlighted in pink), with their typical morphology, are observed. (C) The unique ultrastructure of specific granules with an internal, often electron-dense crystalline core surrounded by an electron-lucent matrix (highlighted in orange). Original scale bars, 1.0 μm (A); 300 nm (B); 800 nm (C).
Although LBs are distinctive organelles of human eosinophils, the existence of LBs in the cytoplasm of these cells was unappreciated in the past because of technical issues. Identification of LBs by light microscopy has methodological limitations, as LBs are neither resistant to drying nor to fixation and recognition with commonly used alcohol-based hematological stains (that solubilize LBs). For instance, cold methanol fixation extracts the majority of cellular phospholipids and promotes fusion of LBs [15], whereas May-Grünwald-Giemsa staining causes dissolution of LBs [16].
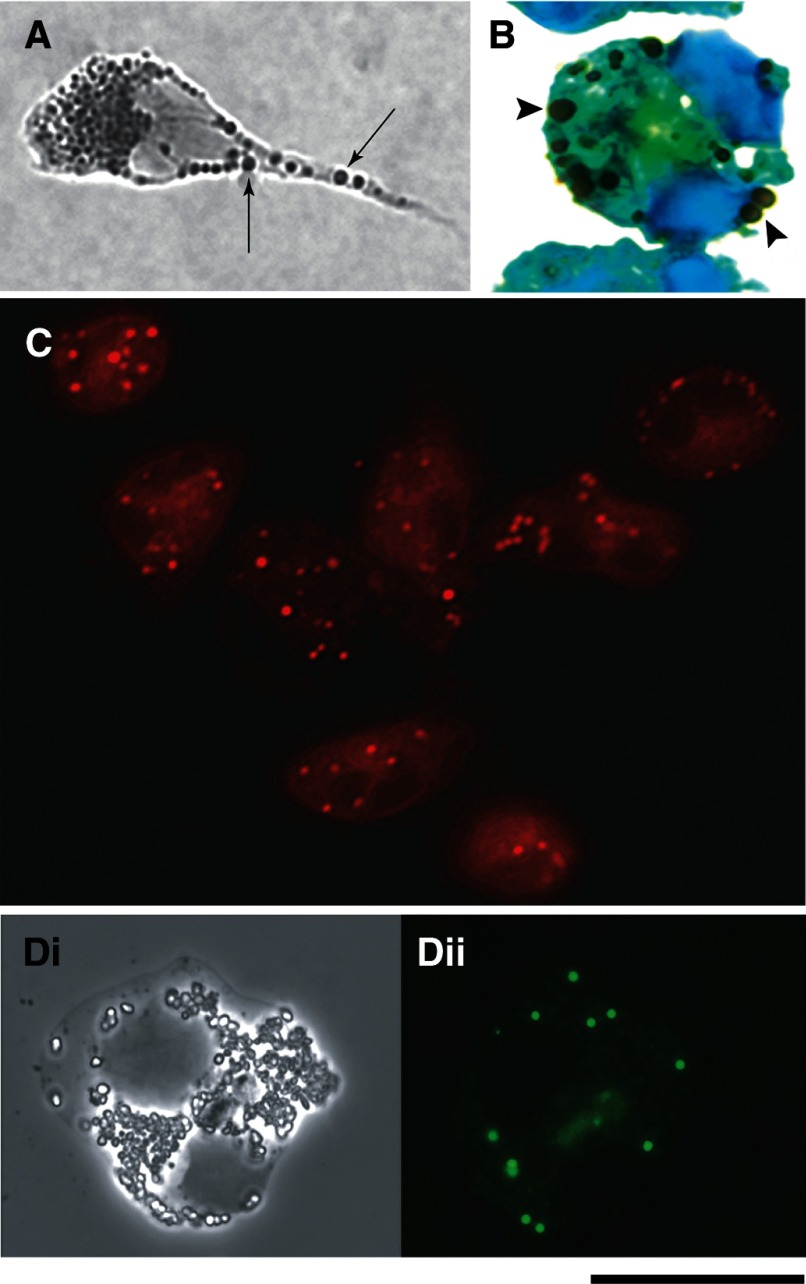
During the 1970s, the applications of osmium to fix and preserve lipids led to identification of LB as a “regular inclusion of normal eosinophil and neutrophil leukocytes,” but LBs were misconsidered as candidate granules [17, 18]. Later, the use of other methodologies, including the use of fluorescent lipophilic dyes (see Fig. 2), definitively identified LBs as regular organelles within eosinophils and other leukocytes. The main techniques currently used to visualize LBs in leukocytes by light microscopy are summarized in recent reviews [4, 19].
Figure 2. LB imaging within human blood eosinophils by light microscopy.
Cells were stained with osmium (A and B), Nile red (C), or green fluorescent boron-dipyrromethene phosphatidic acid (Bodipy) (Dii). LBs appear as round and dark (A, arrows; B, arrowheads) or fluorescent (C and Dii) organelles, distributed throughout the cytoplasm. Di, by phase contrast microscopy, shows the same cell in Dii. (B) Cells were counterstained with Hema 3. (A and B) Agarose cell and cytospin preparations, respectively. Eosinophils were obtained from the blood of normal human donors and stimulated with CCL11 (eotaxin 1; A and B), calcium ionophore (C), or medium alone (D), as before [21]. Reprinted from ref. [20], with permission.
By TEM, eosinophil LBs are imaged as round, highly osmiophilic organelles and therefore, appear as very electron-dense organelles in TEM images (Fig. 1, and see Fig. 3A). The high electron density of LBs, in conjunction with the fact that LBs are not delimited by a true bilayer membrane, enables their unambiguous, ultrastructural identification within eosinophils (see Fig. 3A). LBs can also be easily identified within eosinophils by wet-scanning EM as highly contrasted organelles [20].
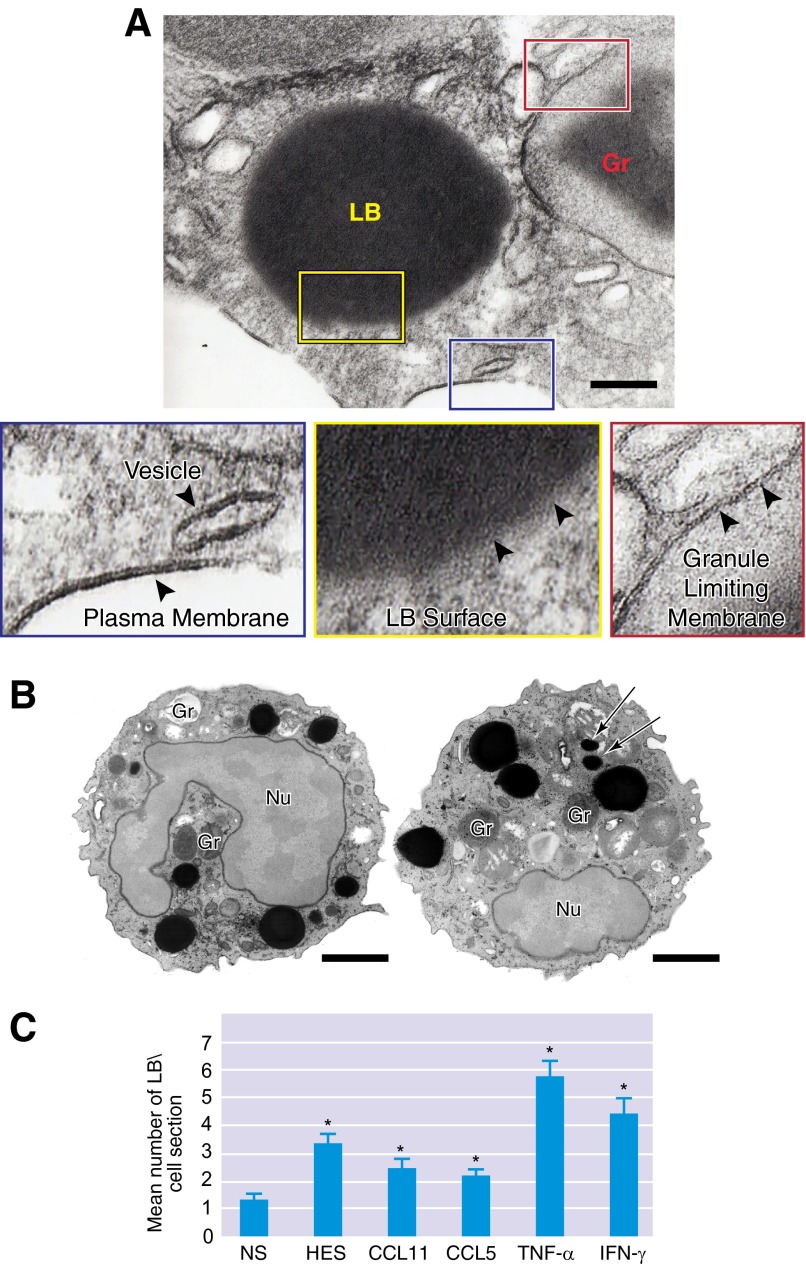
Figure 3. LBs, cytoplasmic organelles not delimited by a bilayer membrane, are formed rapidly in response to leukocyte activation.
(A) LBs are delimited by a monolayer of phospholipids (yellow box, arrowheads), differing from the classical, structural organization (phospholipid bilayer membrane) of cytoplasmic granules (red box, arrowheads), vesicles, and plasma membranes (blue box, arrowheads). (B) In TNF-α-activated eosinophils, a large number of LBs with different sizes are seen in the cytoplasm as dark, osmium-stained organelles. Arrows indicate small LBs. (C) Stimulation of eosinophils with inflammatory chemokines and cytokines induces significant formations (*P≤0.05) of LBs compared with NS eosinophils. Naturally activated eosinophils from HES patients also showed increased numbers of LBs. Cells were isolated from the peripheral blood of healthy donors or an HES donor, stimulated or not with CCL11/eotaxin-1, CCL5/RANTES, TNF-α, or IFN-γ, and processed for TEM using conventional (A) or reduced (B) osmium [11]. Original scale bars, 500 nm (A) and 900 nm (B). (B and C) Reprinted from ref. [11], with permission.
Another morphological feature of eosinophil LBs is their considerable variation in size. By TEM, under favorable conditions, which may include postfixation with reduced osmium to increase contrast, LBs, as small as 30 nm, can be identified in conjunction with other LBs of greater size (see Fig. 3B). Cell activation induces notable increases in LB numbers and sizes, as recognized even by light microscopy (Fig. 2A and B) and demonstrated extensively during inflammatory responses (Fig. 3B and C, and see Table 1).
Table 1. Stimulus-induced LB Formation in Human Eosinophils.
| Stimulus | LB formation | Cell origin | Refs. |
|---|---|---|---|
| Cytokines/chemokines | |||
| CCL5 | + | Peripheral blood | [11, 39] |
| CCL11 | + | Peripheral blood | [11, 20, 39, 43] |
| GM-CSF | + | Peripheral blood | [33] |
| IL-5 | + | Peripheral blood | [64] |
| IL-3 + IL-5 + GM-CSF | + | Peripheral blood | [33] |
| IL-16 | + | Peripheral blood | [65] |
| IFN-γ | + | Peripheral blood | [11] |
| TNF-α | + | Peripheral blood | [11] |
| MIF | + | Peripheral blood | [43] |
| Lipids | |||
| AA | + | Peripheral blood | [43, 66] |
| OAG | + | Peripheral blood | [66] |
| OA | + | Peripheral blood | [66] |
| PAF | + | Peripheral blood | [31, 43, 66] |
| Other molecules | |||
| IgG | + | Peripheral blood | [64] |
| Antimicrobial peptide LL-37 | + | Peripheral blood | [34] |
| Diseases | |||
| Crohn's disease | + | Colonic tissue | [7] |
| Eosinophilic pneumonia | + | Bronchoalveolar lavage fluid | [67] |
| HES | + | Peripheral blood | [29, 33] |
| Inflammatory lesions | + | Small bowel tissue | [29] |
| + | Pelvic pouch tissue (bacterial pouchitis) | [68] |
+, Induced; OAG, 1-oleyl-2-acetyl-glycerol.
LB CONSTITUENTS IN HUMAN EOSINOPHILS
As a feature common to LDs in diverse cell types [22, 23], eosinophil LBs contain an internum rich in neutral lipids, surrounded by a monolayer of phospholipids with associated proteins. LD/LB-specific structural proteins, the PAT family of proteins (renamed to PLIN family proteins) [24]—PLIN/PLIN1, adipose differentiation-related protein (ADRP/adipophilin/PLIN2) [25], and tail-interacting protein of 47 kDa (PLIN3) [26]—are constitutively associated with the circumferential rim of these organelles [27].
AA
AA, a 20-carbon fatty acid, when enzymatically released from arachidonyl-phospholipids, is the substrate for enzymatic conversion into diverse eicosanoid lipid mediators (e.g., PGs, LTs) [28]. A role for LBs in the cellular metabolism of AA was first determined with eosinophils isolated from the blood of eosinophilic patients with HES [29]. Isolated LBs of human eosinophils contained stores of AA, mainly esterified in phospholipids [29, 30]. This means that arachidonyl-phospholipid pools, in addition to their classical localizations in cell membranes (e.g., nuclear envelope), reside in LBs, and therefore, these organelles are potentially able to initiate cascades that culminate in the formation of eicosanoid lipid mediators. In fact, even in anucleate eosinophil cytoplasts, LBs were inducible, were correlated with enhanced eicosanoid formation, and were discrete sites of eicosanoid-forming enzyme localization [31].
Enzymes involved in the conversion of AA into eicosanoids localized to LBs
The major enzymes involved in the enzymatic conversion of AA into eicosanoids are localized specifically within LBs of activated eosinophils. These membrane-associated or membrane-inserted enzymes include COX [31, 32], 5- and 15-LO [31, 33, 34], and LTC4 synthase [31, 34]. Notably, an early study using immunogold EM localized 5-LO, not at the surrounding peripheral membrane of eosinophil LBs but rather, localized fully within eosinophil LBs [31].
Studies in other leukocytes also localized AA-mobilizing phospholipase A2 to LBs [35, 36]. Thus, the varied, often membrane-associated, enzymes involved in the regulated release of AA from phospholipids and their subsequent enzymatic conversions into varied eicosanoids were localized to and often within LBs of eosinophils and other leukocytes.
Eicosanoid formation at LBs
As noted, LBs from human eosinophils compartmentalize and colocalize substrate AA esterified in phospholipids and the entire enzymatic machinery for eicosanoid synthesis. Moreover, stimuli that enhanced eosinophil LB formation dose-dependently, coordinately enhanced eicosanoid synthesis in activated eosinophils. Inhibitors of agonist-elicited LB formation in eosinophils and other leukocytes coordinately inhibited eicosanoid release in activated leukocytes. Significant correlations between LB formation and enhanced generation of eicosanoids by eosinophils and other leukocytes have been observed (reviewed in ref. [37]).
As eicosanoids are not stored as preformed mediators, the intracellular sites of newly formed eicosanoid lipids, following cell stimulation, had lacked specific documentation. If eosinophil and other leukocyte LBs were specific sites of synthesis of eicosanoids, a “smoking-gun” demonstration was needed. With the adaptation to a method to carbodiimide immobilize eicosanoid carboxyl groups to proximate proteins to document local, new formation of eicosanoids [38], the first proof that LBs were sites of formation of an eicosanoid was reported for eosinophil LBs as sites of LTC4 formation [39]. Notably, ionophore activation led to localized eicosanoid formation at the perinuclear membrane, whereas more “physiologic” stimuli led to eicosanoid formation at LBs [40].
With the use of this technique (EicosaCell) [41], LTC4 formation was demonstrated specifically at LBs from human eosinophils stimulated with the chemokines CCL11 and CCL5 [39, 41] and in eosinophil LBs from murine models of allergic inflammation [42]. PGD2 was also demonstrated within LBs from human eosinophils stimulated with AA or CCL11, whereas virtually no PGD2 immunolabeling was observed within NS eosinophils [43]. Likewise, in other leukocytes, LBs are documented focal sites of eicosanoid synthesis (see LB Formation and Function in Other Leukocytes below).
Cytokines
An as-yet, under-investigated role of LBs relates to cytokines localized within LBs from eosinophils and other leukocytes. For instance, TNF-α was clearly detected by immunogold EM within cytoplasmic LBs of eosinophils present in vivo in colonic Crohn's disease biopsies [7]. Evidence for localization of TNF-α was also provided by immunofluorescence microscopy after in vivo LPS stimulation of peripheral blood neutrophils and monocytes from septic patients [16]. However, to date, the association of cytokines with signaling functions within LBs is a question that remains to be addressed.
THE INTERNAL ULTRASTRUCTURE OF LBs: INSIGHTS FROM 3D STUDIES IN HUMAN EOSINOPHILS
Some of the difficulties in the study of LBs originate from their unique architecture. In contrast to vesicles and membranous organelles that have an aqueous content surrounded by a phospholipid bilayer membrane, LBs, as mentioned, are encircled just by a monolayer of phospholipids [4] (Fig. 3). LBs, therefore, lack a true delimiting unit membrane structure. Because of this distinct organization, it is not understood how LBs interact with other membranous organelles and vesicles and how molecules are transported to and from these organelles. Although vesicles are seen around or in contact with LBs, a vesicular transport route to/from these organelles has not yet been documented.
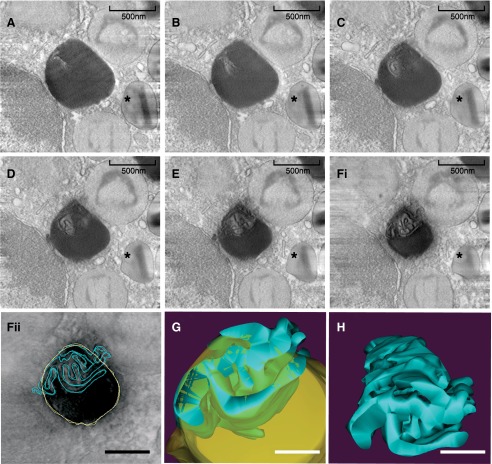
With the recognition that leukocyte LBs are sites for the regulated biosynthesis of eicosanoid lipid mediators [31, 42, 44, 45], with membrane-inserting proteins present within their cores, an intriguing question has been raised. How are diverse membrane-associated proteins, including enzymes, involved in eicosanoid formation, such as COX, 5-LO, and LTC4, spatially organized within leukocyte LBs? Recent work using human eosinophils has shed light on how proteins with transmembrane domains stay associated with the LB core, not only in leukocytes but also in other cell types [11]. Automated electron tomography, a technique that offers 3D information at very high resolution [46, 47], as well as conventional TEM, provided evidence for the presence of membranes within LBs (Fig. 4). After 3D reconstruction and modeling, analyses revealed that indeed, eosinophil LBs enclose an intricate system of membranes within their cores [11]. Computational reconstruction showed that these membranes are organized as a network of tubules that resemble the ER (Fig. 4). The recognition of ER-derived membranes within LBs may be crucial to understanding the functional activities of LBs. In addition to explaining the association of polar proteins within LB cores, this finding may be important to understanding LB biogenesis [11].
Figure 4. Electron tomography reveals membranes within the internal core of eosinophil LBs.
(A–F) A gallery of 4 nm digital, serial slices of a tomogram taken from a representative human eosinophil stimulated with eotaxin-1. An eosinophil-specific granule is indicated (*). LB boundaries and internal membranes in consecutive tomogram slices were manually contoured in yellow and blue, respectively (as in Fii), and a computer-based 3D model is shown (G and H). LB membranes are seen as interconnected tubules (H). Virtual slices were extracted from 3D reconstructions of a 400-nm eosinophil plastic section, analyzed by fully automated electron tomography at 200 kV. Original scale bars, 500 nm (A–Fi); 400 nm (Fii); 300 nm (G and H). Reprinted from ref. [11], with permission.
LBs AS KEY MARKERS OF EOSINOPHIL ACTIVATION AND MODULATORS OF IMMUNE RESPONSES
In response to multiple and varied inflammatory diseases and stimuli, the number of LBs increases within eosinophils (see Table 1). For example, higher LB formation was documented within human eosinophils after stimulation with the chemokines CCL11 or CCL5 [11, 20, 39] and with the proinflammatory cytokines TNF-α and IFN-γ (Fig. 3B and C) [11]. Eosinophils from patients with HES also exhibited increased number of LBs [11, 33]. Interestingly, HES eosinophils are naturally activated, i.e., show a number of functional and biochemical measures indicative of activation [48], including increased numbers of EoSVs [49] in the cytoplasm, and have enhanced LTC4 production [50, 51]. Other situations and diseases that trigger significant formation of LBs in human eosinophils are listed (see Table 1).
As noted, eosinophil LBs compartmentalize several proteins and lipids involved in the control and biosynthesis of inflammatory mediators. Therefore, LBs, formed in response to cell activation, act as competent organelles for modulation of immune responses in leukocytes. Moreover, as biogenesis of LBs is now a well-documented event within inflammatory cells, LBs are considered key structural markers of cell activation and inflammation (reviewed in ref. [4]).
Is the blocking of allergen-induced LB formation beneficial in controlling aspects of allergic inflammation? With the consideration that eicosanoids, such as LTC4, have central roles in the pathogenesis of allergic diseases, such as asthma, inhibition of the biogenesis of LBs, in which consistent synthesis of this LT occurs under eosinophil activation, may have therapeutic effects (reviewed in ref. [37]). It is well-documented that specific blockage of CCL11-, MIF-, or PGD2-driven effects is able to impair LB formation and LT synthesis by recruited eosinophils [37]. Interestingly, the effect of natural, anti-inflammatory products on the formation of LB within eosinophils has also been studied. For example, by investigating potential antiallergic properties of the Brazilian bromeliaceae Nidularium procerum in an experimental model of allergic pleurisy, it was observed that pretreatment with N. procerum not only reduced pleural eosinophil influx triggered by allergen challenge but also decreased LB numbers in infiltrating eosinophils [52]. Moreover, pretreatment with N. procerum blocked pleural eosinophil influx triggered by PAF or CCL11, key mediators of the development of allergic pleural eosinophilia [52]. Thus, LBs within eosinophils and other leukocytes are emerging as key organelles involved in inflammation signaling and as such, are an attractive target candidate for therapeutic intervention.
ARE EOSINOPHIL LBs SITES FOR COMPARTMENTALIZED PROTEIN SYNTHESIS?
As a growing list of proteins has been identified within LBs, as revealed by many proteomic studies in different cell types, it is believed that these organelles act as transient sites for proteins that will be released, delivered, or catabolized [53], but are LBs stations for protein synthesis?
A characteristic of LBs in eosinophils and other cells is their association with the ER, which appears frequently around or even apparently intermingled in the periphery of LBs in conventional, thin TEM sections [11, 54]. Interestingly, ultrastructural analyses identified the presence of ribosomes attached to the circumferential surfaces of eosinophil LBs and even spread within their electron-dense core content [54]. The ribosomal localization at and within LBs in eosinophils may be linked to compartmentalized protein synthesis at LBs. This is fully consonant with prior ultrastructural localization studies of LBs in human mast cells: (1) 3H-uridine was shown to accumulate in LBs; (2) RNA was localized within LBs by hybridization with an RNase-gold probe and by anti-RNA antibody immunogold labeling; (3) poly(A) mRNA was detected within LBs by in situ hybridization with a poly(U) probe; and (4) several human autoimmune sera-to-ribosomal component proteins immunolabeled LBs [55, 56]. Ribosomal component proteins and proteins involved in regulation of ribosomal protein translation, as well as ER-associated, glycosylation-mediating proteins, were also documented within LBs from human monocyte U937 line cells by our group in a proteomic study [54].
Although there is no direct evidence for protein synthesis within eosinophil LBs until now, our group has identified by immunogold EM the presence of the ER protein PDI, which is required for constitutive events of protein formation, in LBs from human eosinophils [57]. This finding supports the view that events of protein synthesis may be indeed occurring in these organelles, as PDI is involved in the proper folding and in the formation and reshuffling of the disulfide bridges in new, synthesized proteins (reviewed in refs. [58, 59]). Moreover, PDI-positive vesicular compartments were found in interactions with LBs in the eosinophil cytoplasm, which may be reflecting a pathway for proteins that are being processed within these organelles and/or released from them [57]. Future studies are needed to determine if indeed protein synthesis events take place within eosinophil LBs.
BIOGENESIS OF LIPID-RICH ORGANELLES: INSIGHTS FROM STUDIES USING HUMAN EOSINOPHILS
Although it is extensively recognized that lipid-rich organelles originate from the ER, it is still a matter of debate on how these organelles are formed. There are different models to explain lipid-rich organelles biogenesis. The prevailing model assumes that these organelles are formed by accumulating neutral lipids between the cytoplasmic and luminal leaflets of ER bilayer membranes, followed by the budding off of these organelles surrounded by a phospholipid monolayer derived from the cytoplasmic leaflets of ER membranes (reviewed in ref. [60]). This model is in agreement with the recognized phospholipid monolayer that surrounds lipid-rich organelles [23] but fails to elucidate a noncircumferential topology of membrane-associated proteins within them. By this model, proteins could insert only in the circumferential monolayer membrane of LDs/LBs, but as noted, several proteins, including the PLIN family proteins, which were described initially only at the LD/LB periphery, are recognized as proteins incorporated within their cores [61]. In fact, little evidence supports the LD/LB-budding model, and alternative models have been proposed (reviewed in ref. [60]).
Studies using human eosinophils and other leukocytes are helping to elucidate biogenesis of lipid-rich organelles. Conventional TEM demonstrated portions of ER at the LB periphery and even intermingled in the lipid content [11, 54]. More recently, electron tomography revealed ER-like, membranous structures within LBs from human eosinophils [11]. These findings support evidence for a different model of leukocyte LB formation by incorporating bilayer membranes of the ER within their cores [11, 54]. By this model, LBs would be formed by incorporating multiple loops of ER membranes (both cytoplasmic and luminal leaflets of membranes) within developing LBs. Accumulations of neutral lipids would develop among the internal, ER-originated LB membranes, i.e., triacylglycerol, and other neutral lipids would be locally synthesized. Indeed, several enzymes involved in the synthesis of triacylglycerol have been demonstrated in isolated LDs/LBs and associated with local synthesis of these lipids [62, 63].
The synthesis of lipids within LBs would elucidate the rapid and extraordinary enlargement of LBs in the cytoplasm of leukocytes during different diseases. Our group has been demonstrating LBs occupying large portions of the cytoplasm of human eosinophils and other leukocytes in response to clinical and experimental inflammatory conditions (Fig. 3B and C and Table 1; reviewed in ref. [4]).
LB FORMATION AND FUNCTION IN OTHER LEUKOCYTES
Since the pioneer work in eosinophilic patients, demonstrating not only LB formation in eosinophils but also a role for this organelle in AA metabolism [29], further studies in leukocytes have greatly contributed to our understanding of LBs as inflammation-associated intracellular sites (reviewed in refs. [4, 37]). Over the past years, substantial progress has been made, demonstrating that LBs in leukocytes, as well as in other cells, are actively formed in response to different stimuli and serve as discrete sites for the synthesis of PGs and LTs.
Neutrophils
In human neutrophils from the peripheral blood, LBs can be elicited rapidly in vitro after stimulation with PAF, protein kinase C activators, or cis-unsaturated fatty acids, such as OA and AA [69–71]. COX was directly demonstrated within LBs from these cells [72]. More recently, formation of LBs was observed in cultured HL-60-derived neutrophils, after stimulation with Porphyromonas gingivalis LPS, a major pathogen in adult periodontitis [73]. Interestingly, PLIN-3 was linked with LD biogenesis and PGE2 production in these cells [73].
Basophils
The chemokines CCL5 and CCL11 activate basophils for enhanced LTC4 generation by distinct signaling and compartmentalization mechanisms involving the induced formation of new cytoplasmic LBs [39].
Mast cells
Since the demonstration that LBs from human mast cells contain large amounts of AA [74], these organelles have been implicated in eicosanoid biology (reviewed in refs. [75, 76]).
Macrophages
Research over the last decade has identified LBs as critical organelles of inflammatory macrophages, as discussed in different reviews [4, 5, 37, 77]. Numerous stimuli and inflammatory conditions, including experimental and clinical infections with different pathogens, such as bacteria, parasites, and viruses, induce LB formation within macrophages, and it was shown that stimulated macrophages respond with synthesis of both eicosanoids and their forming enzymes within LBs (reviewed in refs. [4, 5, 37, 77]). In addition to LB accumulation, interaction of these organelles with pathogen-containing phagosomes within macrophages has increasingly been recognized [78, 79]. Recent observations have indicated that this intriguing and intimate association is a pathogen-driven process, evolved to survive within the host cells by sequestering mainly host lipids or as part of an anti-immunity strategy [78]. However, future studies will be necessary to address whether LBs have a major role in the intracellular survival or destruction of pathogens and/or if these organelles are able to interfere with phagocytosis pathways.
Other cells
There is increasing evidence that LBs may act as sites for inflammatory mediators in other cell types. For example, enzymes involved in PG synthesis, such as phospholipase A2, were associated with LB formation in cells from human fetal membranes [80] and in an AA- or OA-stimulated epithelial cell line [81]. Moreover, PGE2 production was directly detected within LBs from epithelial cells, indicating a potential role for these organelles in epithelial cell-driven inflammatory functions [81]. Another example from a recent study is the biogenesis of LBs in muscle cells cultured with the intracellular protozoan parasite Toxoplasma gondii. PGE2 quantified in the supernatant from noninfected and infected muscle cells showed a significant time-dependent increase in PGE2 generation that positively correlated with LB formation in T. gondii-infected muscle cells but not in uninfected cells [82].
CONCLUDING REMARKS AND PERSPECTIVES
LBs are common organelles of human eosinophils and other leukocytes with functions distinct from classic LDs from adipocytes. Leukocyte LBs reside in the cytoplasm as highly dynamic stations able to change their composition in response to inflammatory events. Under cell activation, enzymes involved in the synthesis of lipid inflammatory mediators are activated in leukocyte LBs and convert esterified AA into eicosanoids, mediating intracrine and paracrine activities. Moreover, LBs of activated human eosinophils represent additional subcellular storage compartments for the inflammatory cytokine TNF-α. Indeed, leukocyte LBs are not only lipid-rich organelles but also organelles in which protein compartmentalization and management occur during cellular mechanisms of diseases. Therefore, in addition to their distinct molecular machinery, leukocyte LBs are likely structurally complex organelles, but we are just beginning to understand fundamental aspects of LB architecture in leukocytes and other cells. Recent studies using advanced EM techniques applied to human eosinophils have revealed the internal organization of LBs in 3D. LBs from these cells enclose an intricate system of membranous structures within their cores, organized as a network of tubules that resemble the ER organization. This is important to understand how LBs are formed from the ER and how membrane-bound proteins, including enzymes responsible for the formation of inflammatory mediators, are linked to LBs. The recognition of ER-derived membranes within LBs may be crucial to understand the functional activities of LBs and potentially, to distinguish LBs from LDs. However, several aspects of LB life in leukocytes remain to be defined. It is not understood how inflammatory lipid mediators, as well as proteins localized within leukocyte LBs, are mobilized from within these organelles. Is there a vesicular transport-mediated secretion from or to LBs? If yes, would it be possible to block this pathway? These and other aspects, including the development of appropriate LB inhibitors, need to be investigated so that the functional significance of LBs can be fully appreciated as critical regulators of different inflammatory diseases in leukocytes.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (Grants USA- R37AI020241 and R01AI022571) and by grants (305983/2011-3 and 477475/2013-2) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Brazil) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; Brazil).
We gratefully acknowledge the helpful discussions with Dr. Ann Dvorak and the skillful assistance of Ellen Morgan, Rita Monahan-Earley, and Tracey Sciutto (Electron Microscopy Unit, Dept. of Pathology, Beth Israel Deaconess Medical Center, Harvard Medical School).
Footnotes
- 3D
- three-dimensional
- AA
- arachidonic acid
- COX
- cyclooxygenase
- EM
- electron microscopy
- EoSV
- eosinophil sombero vesicle
- ER
- endoplasmic reticulum
- HES
- hypereosinophilic syndrome
- LB
- lipid body
- LD
- lipid droplet
- LO
- lipoxygenase
- LTC4S
- leukotriene C4 synthase
- MIF
- macrophage migration inhibitory factor
- NS
- nonstimulated
- OA
- oleic acid
- PAF
- platelet-activating factor
- PDI
- protein disulfide isomerase
- PGD2
- prostaglandin D2
- PLIN
- perilipin
- TEM
- transmission electron microscopy
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Murphy D. J. (2012) The dynamic roles of intracellular lipid droplets: from archaea to mammals. Protoplasma 249, 541–585. [DOI] [PubMed] [Google Scholar]
- 2. Beller M., Thiel K., Thul P. J., Jackle H. (2010) Lipid droplets: a dynamic organelle moves into focus. FEBS Lett. 584, 2176–2182. [DOI] [PubMed] [Google Scholar]
- 3. Fujimoto T., Ohsaki Y., Cheng J., Suzuki M., Shinohara Y. (2008) Lipid droplets: a classic organelle with new outfits. Histochem. Cell Biol. 130, 263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melo R. C. N., D'Avila H., Wan H. C., Bozza P. T., Dvorak A. M., Weller P. F. (2011) Lipid bodies in inflammatory cells: structure, function, and current imaging techniques. J. Histochem. Cytochem. 59, 540–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saka H. A., Valdivia R. (2012) Emerging roles for lipid droplets in immunity and host-pathogen interactions. Annu. Rev. Cell Dev. Biol. 28, 411–437. [DOI] [PubMed] [Google Scholar]
- 6. Bozza P. T., Melo R. C. N., Bandeira-Melo C. (2007) Leukocyte lipid bodies regulation and function: contribution to allergy and host defense. Pharmacol. Ther. 113, 30–49. [DOI] [PubMed] [Google Scholar]
- 7. Beil W. J., Weller P. F., Peppercorn M. A., Galli S. J., Dvorak A. M. (1995) Ultrastructural immunogold localization of subcellular sites of TNF-α in colonic Crohn's disease. J. Leukoc. Biol. 58, 284–298. [DOI] [PubMed] [Google Scholar]
- 8. Hogan S. P., Rosenberg H. F., Moqbel R., Phipps S., Foster P. S., Lacy P., Kay A. B., Rothenberg M. E. (2008) Eosinophils: biological properties and role in health and disease. Clin. Exp. Allergy 38, 709–750. [DOI] [PubMed] [Google Scholar]
- 9. Shamri R., Xenakis J. J., Spencer L. A. (2011) Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 343, 57–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenberg H. F., Dyer K. D., Foster P. S. (2013) Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 13, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Melo R. C. N., Paganoti G. F., Dvorak A. M., Weller P. F. (2013) The internal architecture of leukocyte lipid body organelles captured by three-dimensional electron microscopy tomography. PLoS One 8, e59578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melo R. C. N., Weller P. F. (2010) Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol. Histopathol. 25, 1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Melo R. C. N., Dvorak A. M., Weller P. F. (2012) Eosinophil ultrastructure. In Eosinophils in Health and Disease (Lee J., Rosenberg H. eds.), Vol. 1, Elsevier, New York, 20–27. [Google Scholar]
- 14. Melo R. C. N., Dvorak A. M., Weller P. F. (2010) Contributions of electron microscopy to understand secretion of immune mediators by human eosinophils. Microsc. Microanal. 16, 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DiDonato D., Brasaemle D. L. (2003) Fixation methods for the study of lipid droplets by immunofluorescence microscopy. J. Histochem. Cytochem. 51, 773–780. [DOI] [PubMed] [Google Scholar]
- 16. Pacheco P., Bozza F. A., Gomes R. N., Bozza M., Weller P. F., Castro-Faria-Neto H. C., Bozza P. T. (2002) Lipopolysaccharide-induced leukocyte lipid body formation in vivo: innate immunity elicited intracellular Loci involved in eicosanoid metabolism. J. Immunol. 169, 6498–6506. [DOI] [PubMed] [Google Scholar]
- 17. Coimbra A., Lopes-Vaz A. (1971) The presence of lipid droplets and the absence of stable sudanophilia in osmium-fixed human leukocytes. J. Histochem. Cytochem. 19, 551–557. [DOI] [PubMed] [Google Scholar]
- 18. Solley G. O., Maldonando J. E., Gleich G. J., Giulani E. R., Hoagland H. C., Pierre R. V., Brown A. L., Jr., (1976) Endomyocardiopathy with eosinophilia. Mayo Clin. Proc. 51, 697–708. [PubMed] [Google Scholar]
- 19. Melo R. C. N., D'Avila H., Bozza P. T., Weller P. F. (2011) Imaging lipid bodies within leukocytes with different light microscopy techniques. Methods Mol. Biol. 689, 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Melo R. C. N., Sabban A., Weller P. F. (2006) Leukocyte lipid bodies: inflammation-related organelles are rapidly detected by wet scanning electron microscopy. J. Lipid Res. 47, 2589–2594. [DOI] [PubMed] [Google Scholar]
- 21. Bandeira-Melo C., Perez S. A., Melo R. C. N., Ghiran I., Weller P. F. (2003) EliCell assay for the detection of released cytokines from eosinophils. J. Immunol. Methods 276, 227–237. [DOI] [PubMed] [Google Scholar]
- 22. Brasaemle D. L. (2007) Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48, 2547–2559. [DOI] [PubMed] [Google Scholar]
- 23. Tauchi-Sato K., Ozeki S., Houjou T., Taguchi R., Fujimoto T. (2002) The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 277, 44507–44512. [DOI] [PubMed] [Google Scholar]
- 24. Kimmel A. R., Brasaemle D. L., McAndrews-Hill M., Sztalryd C., Londos C. (2010) Adoption of perilipin as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J. Lipid Res. 51, 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brasaemle D. L., Dolios G., Shapiro L., Wang R. (2004) Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279, 46835–46842. [DOI] [PubMed] [Google Scholar]
- 26. Wolins N. E., Rubin B., Brasaemle D. L. (2001) TIP47 associates with lipid droplets. J. Biol. Chem. 276, 5101–5108. [DOI] [PubMed] [Google Scholar]
- 27. Bickel P. E., Tansey J. T., Welte M. A. (2009) PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim. Biophys. Acta 1791, 419–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yaqoob P. (2003) Fatty acids as gatekeepers of immune cell regulation. Trends Immunol. 24, 639–645. [DOI] [PubMed] [Google Scholar]
- 29. Weller P. F., Dvorak A. M. (1985) Arachidonic acid incorporation by cytoplasmic lipid bodies of human eosinophils. Blood 65, 1269–1274. [PubMed] [Google Scholar]
- 30. Weller P. F., Monahan-Earley R. A., Dvorak H. F., Dvorak A. M. (1991) Cytoplasmic lipid bodies of human eosinophils. Subcellular isolation and analysis of arachidonate incorporation. Am. J. Pathol. 138, 141–148. [PMC free article] [PubMed] [Google Scholar]
- 31. Bozza P. T., Yu W., Penrose J. F., Morgan E. S., Dvorak A. M., Weller P. F. (1997) Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J. Exp. Med. 186, 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dvorak A. M., Morgan E. S., Tzizik D. M., Weller P. F. (1994) Prostaglandin endoperoxide synthase (cyclooxygenase): ultrastructural localization to nonmembrane-bound cytoplasmic lipid bodies in human eosinophils and 3T3 fibroblasts. Int. Arch. Allergy Immunol. 105, 245–250. [DOI] [PubMed] [Google Scholar]
- 33. Bozza P. T., Yu W., Cassara J., Weller P. F. (1998) Pathways for eosinophil lipid body induction: differing signal transduction in cells from normal and hypereosinophilic subjects. J. Leukoc. Biol. 64, 563–569. [DOI] [PubMed] [Google Scholar]
- 34. Sun J., Dahlen B., Agerberth B., Haeggstrom J. Z. (2013) The antimicrobial peptide LL-37 induces synthesis and release of cysteinyl leukotrienes from human eosinophils—implications for asthma. Allergy 68, 304–311. [DOI] [PubMed] [Google Scholar]
- 35. Yu W., Bozza P. T., Tzizik D. M., Gray J. P., Cassara J., Dvorak A. M., Weller P. F. (1998) Co-compartmentalization of MAP kinases and cytosolic phospholipase A2 at cytoplasmic arachidonate-rich lipid bodies. Am. J. Pathol. 152, 759–769. [PMC free article] [PubMed] [Google Scholar]
- 36. Wooten R. E., Willingham M. C., Daniel L. W., Leslie C. C., Rogers L. C., Sergeant S., O'Flaherty J. T. (2008) Novel translocation responses of cytosolic phospholipase A2alpha fluorescent proteins. Biochim. Biophys. Acta 1783, 1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bozza P. T., Bakker-Abreu I., Navarro-Xavier R. A., Bandeira-Melo C. (2011) Lipid body function in eicosanoid synthesis: an update. Prostaglandins Leukot. Essent. Fatty Acids 85, 205–213. [DOI] [PubMed] [Google Scholar]
- 38. Liu L. X., Buhlmann J. E., Weller P. F. (1992) Release of prostaglandin E2 by microfilariae of Wuchereria bancrofti and Brugia malayi. Am. J. Trop. Med. Hyg. 46, 520–523. [DOI] [PubMed] [Google Scholar]
- 39. Bandeira-Melo C., Phoofolo M., Weller P. F. (2001) Extranuclear lipid bodies, elicited by CCR3-mediated signaling pathways, are the sites of chemokine-enhanced leukotriene C4 production in eosinophils and basophils. J. Biol. Chem. 276, 22779–22787. [DOI] [PubMed] [Google Scholar]
- 40. Tedla N., Bandeira-Melo C., Tassinari P., Sloane D. E., Samplaski M., Cosman D., Borges L., Weller P. F., Arm J. P. (2003) Activation of human eosinophils through leukocyte immunoglobulin-like receptor 7. Proc. Natl. Acad. Sci. USA 100, 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bandeira-Melo C., Weller P. F., Bozza P. T. (2011) EicosaCell—an immunofluorescent-based assay to localize newly synthesized eicosanoid lipid mediators at intracellular sites. Methods Mol. Biol. 689, 163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vieira-de-Abreu A., Assis E. F., Gomes G. S., Castro-Faria-Neto H. C., Weller P. F., Bandeira-Melo C., Bozza P. T. (2005) Allergic challenge-elicited lipid bodies compartmentalize in vivo leukotriene C4 synthesis within eosinophils. Am. J. Respir. Cell Mol. Biol. 33, 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luna-Gomes T., Magalhaes K. G., Mesquita-Santos F. P., Bakker-Abreu I., Samico R. F., Molinaro R., Calheiros A. S., Diaz B. L., Bozza P. T., Weller P. F., Bandeira-Melo C. (2011) Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. J. Immunol. 187, 6518–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. D'Avila H., Melo R. C. N., Parreira G. G., Werneck-Barroso E., Castro-Faria-Neto H. C., Bozza P. T. (2006) Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J. Immunol. 176, 3087–3097. [DOI] [PubMed] [Google Scholar]
- 45. D'Avila H., Freire-de-Lima C. G., Roque N. R., Teixeira L., Barja-Fidalgo C., Silva A. R., Melo R. C. N., Dosreis G. A., Castro-Faria-Neto H. C., Bozza P. T. (2011) Host cell lipid bodies triggered by Trypanosoma cruzi infection and enhanced by the uptake of apoptotic cells are associated with prostaglandin E generation and increased parasite growth. J. Infect. Dis. 204, 951–961. [DOI] [PubMed] [Google Scholar]
- 46. Melo R. C. N., Perez S. A., Spencer L. A., Dvorak A. M., Weller P. F. (2005) Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic 6, 866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McIntosh R., Nicastro D., Mastronarde D. (2005) New views of cells in 3D: an introduction to electron tomography. Trends Cell Biol. 15, 43–51. [DOI] [PubMed] [Google Scholar]
- 48. Ackerman S. J., Bochner B. S. (2007) Mechanisms of eosinophilia in the pathogenesis of hypereosinophilic disorders. Immunol. Allergy Clin. North Am. 27, 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Melo R. C. N., Spencer L. A., Perez S. A., Neves J. S., Bafford S. P., Morgan E. S., Dvorak A. M., Weller P. F. (2009) Vesicle-mediated secretion of human eosinophil granule-derived major basic protein. Lab. Invest. 89, 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weller P. F., Lee C. W., Foster D. W., Corey E. J., Austen K. F., Lewis R. A. (1983) Generation and metabolism of 5-lipoxygenase pathway leukotrienes by human eosinophils: predominant production of leukotriene C4. Proc. Natl. Acad. Sci. USA 80, 7626–7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Henderson W. R., Harley J. B., Fauci A. S. (1984) Arachidonic acid metabolism in normal and hypereosinophilic syndrome human eosinophils: generation of leukotrienes B4, C4, D4 and 15-lipoxygenase products. Immunology 51, 679–686. [PMC free article] [PubMed] [Google Scholar]
- 52. Vieira-de-Abreu A., Amendoeira F. C., Gomes G. S., Zanon C., Chedier L. M., Figueiredo M. R., Kaplan M. A., Frutuoso V. S., Castro-Faria-Neto H. C., Weller P. F., Bandeira-Melo C., Bozza P. T. (2005) Anti-allergic properties of the bromeliaceae Nidularium procerum: inhibition of eosinophil activation and influx. Int. Immunopharmacol. 5, 1966–1974. [DOI] [PubMed] [Google Scholar]
- 53. Welte M. A. (2007) Proteins under new management: lipid droplets deliver. Trends Cell Biol. 17, 363–369. [DOI] [PubMed] [Google Scholar]
- 54. Wan H. C., Melo R. C., Jin Z., Dvorak A. M., Weller P. F. (2007) Roles and origins of leukocyte lipid bodies: proteomic and ultrastructural studies. FASEB J. 21, 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dvorak A. M., Morgan E. S., Weller P. F. (2003) RNA is closely associated with human mast cell lipid bodies. Histol. Histopathol. 18, 943–968. [DOI] [PubMed] [Google Scholar]
- 56. Dvorak A. M. (2005) Mast cell secretory granules and lipid bodies contain the necessary machinery important for the in situ synthesis of proteins. Chem. Immunol. Allergy 85, 252–315. [DOI] [PubMed] [Google Scholar]
- 57. Dias F. F., Amaral K., Carmo L., Shamri R., Dvorak A., Weller P., Melo R. C. N. (2014) Human eosinophil leukocytes express protein disulfide isomerase in secretory granules and vesicles: ultrastructural studies. J. Histochem. Cytochem. 62, 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Turano C., Coppari S., Altieri F., Ferraro A. (2002) Proteins of the PDI family: unpredicted non-ER locations and functions. J. Cell. Physiol. 193, 154–163. [DOI] [PubMed] [Google Scholar]
- 59. Laurindo F. R., Pescatore L. A., Fernandes Dde C. (2012) Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic. Biol. Med. 52, 1954–1969. [DOI] [PubMed] [Google Scholar]
- 60. Walther T. C., Farese R. V., Jr., (2009) The life of lipid droplets. Biochim. Biophys. Acta 1791, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Robenek H., Buers I., Hofnagel O., Robenek M. J., Troyer D., Severs N. J. (2009) Compartmentalization of proteins in lipid droplet biogenesis. Biochim. Biophys. Acta 1791, 408–418. [DOI] [PubMed] [Google Scholar]
- 62. Fujimoto Y., Itabe H., Kinoshita T., Homma K. J., Onoduka J., Mori M., Yamaguchi S., Makita M., Higashi Y., Yamashita A., Takano T. (2007) Involvement of ACSL in local synthesis of neutral lipids in cytoplasmic lipid droplets in human hepatocyte HuH7. J. Lipid Res. 48, 1280–1292. [DOI] [PubMed] [Google Scholar]
- 63. Kuerschner L., Moessinger C., Thiele C. (2008) Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic 9, 338–352. [DOI] [PubMed] [Google Scholar]
- 64. Bartemes K. R., McKinney S., Gleich G. J., Kita H. (1999) Endogenous platelet-activating factor is critically involved in effector functions of eosinophils stimulated with IL-5 or IgG. J. Immunol. 162, 2982–2989. [PubMed] [Google Scholar]
- 65. Bandeira-Melo C., Sugiyama K., Woods L. J., Phoofolo M., Center D. M., Cruikshank W. W., Weller P. F. (2002) IL-16 promotes leukotriene C(4) and IL-4 release from human eosinophils via CD4- and autocrine CCR3-chemokine-mediated signaling. J. Immunol. 168, 4756–4763. [DOI] [PubMed] [Google Scholar]
- 66. Weller P. F., Bozza P. T., Yu W., Dvorak A. M. (1999) Cytoplasmic lipid bodies in eosinophils: central roles in eicosanoid generation. Int. Arch. Allergy Immunol. 118, 450–452. [DOI] [PubMed] [Google Scholar]
- 67. Ishida T., Matsumura Y., Miyake A., Amitani R. (1992) [Ultrastructural observation of eosinophils in bronchoalveolar lavage fluid in eosinophilic pneumonia]. Nihon Kyobu Shikkan Gakkai Zasshi 30, 1951–1956. [PubMed] [Google Scholar]
- 68. Dvorak A., Monahan-Earley R. (1992) Gastrointestinal system. Pouchitis of continental ileal pelvic pouch (familial multiple polyposis). In Diagnostic Ultrastructural Pathology I (Dvorak A. M., Monahan-Earley R., eds.), Vol. 1, CRC, Boca Raton, FL, USA, 66–81. [Google Scholar]
- 69. Weller P. F., Ryeom S. W., Picard S. T., Ackerman S. J., Dvorak A. M. (1991) Cytoplasmic lipid bodies of neutrophils: formation induced by cis-unsaturated fatty acids and mediated by protein kinase C. J. Cell Biol. 113, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Weller P. F., Ackerman S. J., Nicholson-Weller A., Dvorak A. M. (1989) Cytoplasmic lipid bodies of human neutrophilic leukocytes. Am. J. Pathol. 135, 947–959. [PMC free article] [PubMed] [Google Scholar]
- 71. Bozza P. T., Payne J. L., Goulet J. L., Weller P. F. (1996) Mechanisms of platelet-activating factor-induced lipid body formation: requisite roles for 5-lipoxygenase and de novo protein synthesis in the compartmentalization of neutrophil lipids. J. Exp. Med. 183, 1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dvorak A. M., Morgan E., Schleimer R. P., Ryeom S. W., Lichtenstein L. M., Weller P. F. (1992) Ultrastructural immunogold localization of prostaglandin endoperoxide synthase (cyclooxygenase) to non-membrane-bound cytoplasmic lipid bodies in human lung mast cells, alveolar macrophages, type II pneumocytes, and neutrophils. J. Histochem. Cytochem. 40, 759–769. [DOI] [PubMed] [Google Scholar]
- 73. Nose F., Yamaguchi T., Kato R., Aiuchi T., Obama T., Hara S., Yamamoto M., Itabe H. (2013) Crucial role of perilipin-3 (TIP47) in formation of lipid droplets and PGE2 production in HL-60-derived neutrophils. PLoS One 8, e71542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dvorak A. M., Dvorak H. F., Peters S. P., Shulman E. S., MacGlashan D. W., Jr., Pyne K., Harvey V. S., Galli S. J., Lichtenstein L. M. (1983) Lipid bodies: cytoplasmic organelles important to arachidonate metabolism in macrophages and mast cells. J. Immunol. 131, 2965–2976. [PubMed] [Google Scholar]
- 75. Dvorak A. M. (2002) Ultrastructure of human mast cells. Int. Arch. Allergy Immunol. 127, 100–105. [DOI] [PubMed] [Google Scholar]
- 76. Dichlberger A., Kovanen P. T., Schneider W. J. (2013) Mast cells: from lipid droplets to lipid mediators. Clin. Sci. (Lond). 125, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dias F. F., Zarantonello V. C., Parreira G. G., Chiarini-Garcia H., Melo R. C. N. (2014) The intriguing ultrastructure of lipid body organelles within activated macrophages. Microsc. Microanal. 20, 869–878. [DOI] [PubMed] [Google Scholar]
- 78. Melo R. C. N., Dvorak A. M. (2012) Lipid body-phagosome interaction in macrophages during infectious diseases: host defense or pathogen survival strategy? PLoS Pathog. 8, e1002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mattos K. A., Oliveira V. C., Berredo-Pinho M., Amaral J. J., Antunes L. C., Melo R. C. N., Acosta C. C., Moura D. F., Olmo R., Han J., Rosa P. S., Almeida P. E., Finlay B. B., Borchers C. H., Sarno E. N., Bozza P. T., Atella G. C., Pessolani M. C. (2014) Mycobacterium leprae intracellular survival relies on cholesterol accumulation in infected macrophages: a potential target for new drugs for leprosy treatment. Cell. Microbiol. 16, 797–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Meadows J. W., Pitzer B., Brockman D. E., Myatt L. (2005) Expression and localization of adipophilin and perilipin in human fetal membranes: association with lipid bodies and enzymes involved in prostaglandin synthesis. J. Clin. Endocrinol. Metab. 90, 2344–2350. [DOI] [PubMed] [Google Scholar]
- 81. Moreira L. S., Piva B., Gentile L. B., Mesquita-Santos F. P., D'Avila H., Maya-Monteiro C. M., Bozza P. T., Bandeira-Melo C., Diaz B. L. (2009) Cytosolic phospholipase A2-driven PGE2 synthesis within unsaturated fatty acids-induced lipid bodies of epithelial cells. Biochim. Biophys. Acta 1791, 156–165. [DOI] [PubMed] [Google Scholar]
- 82. Gomes A. F., Magalhaes K. G., Rodrigues R. M., de Carvalho L., Molinaro R., Bozza P. T., Barbosa H. S. (2014) Toxoplasma gondii-skeletal muscle cells interaction increases lipid droplet biogenesis and positively modulates the production of IL-12, IFN-g and PGE2. Parasit. Vectors 7, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]