Abstract
Discussion of myeloid-derived suppressor cells expansion in patients with gram-positive sepsis.
Keywords: Sepsis, myeloid-derived suppressor cells, neutrophils, monocytes, gram negative bacteria, gram positive bacteria
Sepsis is a major cause of death in the Western world with high mortality rates in ICUs. The disease is characterized by an excessive and dysregulated immune response to microbial infections, coagulation abnormalities leading to capillary leakage, lung damage, and finally, multiple organ failure [1]. It is known that most septic patients in ICUs, in addition to hyperinflammatory response, suffer from a hypoinflammatory state, which often leads to sepsis-induced multiorgan dysfunction and death. This suggests that sepsis-induced immunosuppression is a significant factor contributing to these deaths. MDSCs may be a critical element in the development of such a hypoinflammatory state and thus, in the outcome of the disease.
Although MDSCs were described originally in cancer [2], it has now become increasingly clear that MDSCs play an important role in the regulation of immune responses in many pathological conditions not directly associated with cancer. MDSCs are pathologically activated immature myeloid cells comprised of immature neutrophils, monocytes, and myeloid precursors. It is important to point out that the immature state and myeloid origin are necessary, but not sufficient, characteristics to define MDSCs. These cells have rather unique genetic and biochemical features and most importantly, the ability to inhibit immune responses by using a large array of different mechanisms. MDSCs consist of two main subsets: PMN-MDSC and M-MDSC. The phenotype of these populations is now well defined in mice and humans. PMN-MDSCs consist of relatively immature and pathologically activated neutrophils and are defined in mice as CD11b+Ly6ClowLy6G+ cells, whereas M-MDSCs, pathologically activated inflammatory monocytes, are defined as CD11b+Ly6ChighLy6G− cells. Historically, human MDSCs were defined as lineage marker (CD3, CD14, CD19, CD56)-negative, HLA-DR−, and common myeloid marker CD33-positive cells, copurified with mononuclear cells on a ficoll gradient. More recently, the existence of two subsets of cells (similar to murine models) has been reported, and PMN-MDSCs are commonly characterized as CD11b+CD14− cells expressing a granulocytic marker: CD15 or CD66b. M-MDSCs are most commonly defined by two combinations of markers: CD11b+CD14−CD15− (or CD66b−) or CD11b+CD14+HLA-DR−/lo. It appears that at least in cancer, M-MDSCs may play a central role in the development of immune-suppressive myeloid cells. In tumor sites, they differentiate to tumor-associated macrophages with potent immune-suppressive activity and in the periphery, may give rise to PMN-MDSCs.
Ample evidence demonstrated that MDSCs are expanded and play an important functional role in chronic inflammation of different origins, bacterial and viral infections, autoimmune diseases, etc. [3]. Delano et al. [4] have demonstrated that polymicrobial sepsis in mice was associated with accumulation of MDSCs. These cells effectively suppressed antigen-specific CD8+ T cells but only modestly suppressed CD4+ T cells. MyD88 was implicated in MDSC expansion in sepsis.
Mouse models of inflammatory diseases poorly mimic human diseases [5]. Therefore, human studies are necessary to identify the role of MDSC in sepsis. The current study by Janols et al. [6] investigated MDSC in patients with septic shock and sepsis. Samples of peripheral blood from 56 patients with septic shock or sepsis caused by G− or G+ bacteria were studied. The authors have demonstrated that patients with sepsis had increased proportions of M-MDSC (defined as CD14+HLA-DRlow cells). However, only patients with G+ sepsis had a significant increase in immune-suppressive CD14−/lowCD15+/lowCD11b+ CD33+ PMN-MDSC. This immune suppressive activity was linked to the reactive oxygen species level in these cells [6]. PMN-MDSCs from patients with G+ sepsis were heterogeneous with a large numbers of blasts (monoblasts, promonocytes, or immature neutrophils). In contrast, the majority of PMN-MDSCs from the G− sepsis patients was morphologically similar to mature PMN. Based on their findings, the authors raised the fascinating possibility that different types of bacteria can give rise to, or activate, distinct MDSC populations. Based on the observation that PMN-MDSCs in G+ sepsis patients show an immature, blast-like morphology while suppressing T cell functions more efficiently than PMN-MDSCs from G− sepsis patients, authors speculate that the immature cells released from the bone marrow in G+ sepsis have a distinct function. They interpret their results such as that G+, but not G− sepsis, induces the export of immature myeloid cells that are functionally and phenotypically related to PMN-MDSCs.
This interesting study describes a dichotomy in the nature of myeloid cells present in septic patients caused by different types of bacteria. It suggests the intriguing possibility that different types of bacteria can determine the type and function of myeloid cells produced by the host. This study provides impetus for a more detailed investigation of the mechanisms regulating MDSC expansion and activation in bacterial infections and sepsis.
However, in the absence of direct experimental clarification, the authors' interpretation of their data may be challenged. In recent years, it has become increasingly clear that the accumulation of MDSC is the result of the convergence of several factors that stimulate myelopoiesis, induce pathological activation of immature myeloid cells, and cause emigration of these cells from bone marrow. These processes are governed by partially overlapping but largely different factors and mechanisms. Each of these factors separately is not sufficient to generate MDSC. For instance, activation of neutrophils with LPS is not enough to convert these cells to MDSC. Mobilization of neutrophils from bone marrow during acute bacterial infection also does not result in generation of typical MDSC.
It appears that there are fundamental differences in host responses to G+ and G− bacterial infections. It is known that TLR2 and TLR4 differentially recognize G+ and G− bacteria. TLR4 recognizes LPS on G− bacteria, whereas TLR2 plays a major role in detecting G+ bacteria by recognizing lipoproteins and lipoteichoic acid [7]. Some studies showed that G− bacteria cause a much more potent proinflammatory response than G+ bacteria. C-Reactive protein and IL-6 blood level were found to be significantly higher in G− than in G+ bacteremia [8]. Moreover, the triggering of costimulatory 4-1BB (CD137) molecules enhanced responses against G+ bacteria, whereas it decreased these responses against G− bacteria. Stimulation of CD137 enhanced activities of neutrophils against G+ Staphylococcus aureus but decreased these activities against G− Escherichia coli [9]. Although this issue is far from being settled, and opposing views exist, these data may suggest that in G− infections, neutrophils are more activated than neutrophils in G+ infections. Previously, Cuenca et al. [10] suggested a paradoxical role of cells with the MDSC phenotype in a mouse model of polymicrobial sepsis. Cells with the MDSC phenotype during early phases of sepsis had potent antibacterial activity and very little immune suppression, whereas cells with the same phenotype at later stages had potent immune-suppressive activity. A similar paradigm has been described in viral infections. MDSCs were accumulated only in mice infected with strains of the lymphocytic choriomeningitis virus causing chronic infection but not acute infection [11].
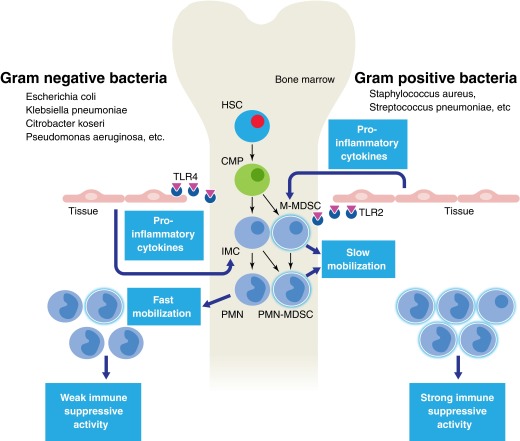
Janols et al. [6] evaluated most of the samples of patients within 2 days of admission. Given all of these facts, their data could be explained within a paradigm on biological differences between G+ and G− sepsis. G− sepsis causes a more potent proinflammatory response, resulting in hyperactivation and quick mobilization of neutrophils from bone marrow. This explains why the authors observed an increase in cells with a rather homogenous neutrophil phenotype lacking immune-suppressive activity. These cells are likely not MDSCs but rather typical neutrophils. In contrast, G+ sepsis is associated with a less potent and therefore, more prolonged exposure of bone marrow to proinflammatory factors. This exposes the myeloid compartment to various factors associated with bacterial infection. The proportion of cells with the PMN-MDSC phenotype in peripheral blood was similar to that in G− sepsis. However, these cells were much more heterogeneous, reflecting the natural history of MDSC expansion. These cells include some M-MDSC and myeloid precursors. More importantly, these cells acquired the ability to suppress immune responses (Fig. 1).
Figure 1. Different effects of G+ and G− bacteria on MDSC in sepsis.
PMNs are generated in bone marrow via sequential steps of differentiation from hematopoietic stem cells (HSC), common myeloid progenitors (CMP), and immature myeloid cells (IMC), represented by different myeloid precursors. G− sepsis causes a potent proinflammatory response, resulting in hyperactivation and quick mobilization of PMNs from bone marrow. These cells lack immune-suppressive activity. G+ sepsis is associated with less potent and therefore, more prolonged exposure of bone marrow to proinflammatory factors. This exposes the myeloid compartment to various factors associated with bacterial infection and supports the generation of MDSCs. The population of cells emigrated from bone marrow is more heterogeneous than in G− sepsis and includes M-MDSCs, as well as more immature precursors, and these cells are immune suppressive.
The answer to the question of the mechanisms regulating MDSC expansion in different types of sepsis may come from future model experiments in vitro and in vivo. The main challenge is how to distinguish neutrophils phenotypically from PMN-MDSCs. In mice, several molecules can be used for this goal. However, in humans, this issue is not yet resolved. Identification of these molecules will help to clarify this interesting concept presented in current studies.
ACKNOWLEDGMENTS
This work was supported, in part, by U.S. National Institutes of Health Grant CA 100062.
SEE CORRESPONDING ARTICLE ON PAGE 685
- G−
- gram-negative
- G+
- gram-positive
- ICU
- intensive care unit
- M-MDSC
- monocytic-myeloid-derived suppressor cell
- MDSC
- myeloid-derived suppressor cell
- PMN
- polymorphonuclear neutrophil
- PMN-MDSC
- polymorphonuclear neutrophil-myeloid-derived suppressor cell
DISCLOSURES
The author declares no conflict of interest.
REFERENCES
- 1. Riedemann N. C., Guo R. F., Ward P. A. (2003) The enigma of sepsis. J. Clin. Invest. 112, 460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Talmadge J. E., Gabrilovich D. I. (2013) History of myeloid derived suppressor cells (MDSCs) in the macro- and micro-environment of tumour-bearing hosts. Nat. Rev. Cancer 10, 739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagaraj S., Youn J. I., Gabrilovich D. I. (2013) Reciprocal relationship between myeloid-derived suppressor cells and T cells. J. Immunol. 191, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delano M. J., Scumpia P. O., Weinstein J. S., Coco D., Nagaraj S., Kelly-Scumpia K. M., O'Malley K. A., Wynn J. L., Antonenko S., Al-Quran S. Z., Swan R., Chung C. S., Atkinson M. A., Ramphal R., Gabrilovich D. I., Reeves W. H., Ayala A., Phillips J., Laface D., Heyworth P. G., Clare-Salzler M., Moldawer L. L. (2007) MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 204, 1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seok J., Warren H. S., Cuenca A. G., Mindrinos M. N., Baker H. V., Xu W., Richards D. R., McDonald-Smith G. P., Gao H., Hennessy L., Finnerty C. C., Lopez C. M., Honari S., Moore E. E., Minei J. P., Cuschieri J., Bankey P. E., Johnson J. L., Sperry J., Nathens A. B., Billiar T. R., West M. A., Jeschke M. G., Klein M. B., Gamelli R. L., Gibran N. S., Brownstein B. H., Miller-Graziano C., Calvano S. E., Mason P. H., Cobb J. P., Rahme L. G., Lowry S. F., Maier R. V., Moldawer L. L., Herndon D. N., Davis R. W., Xiao W., Tompkins R. G., Inflammation and Host Response to Injury, Large Scale Collaborative Research Program (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 110, 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Janols H., Bergenfelz C., Allaoui R., Larsson A., Rydén L., Björnsson S., Janciauskiene S., Wullt M., Bredberg A., Leandersson K. (2014) A high frequency of myeloid-derived suppressor cells in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J. Leukoc. Biol. 96, 685–693. [DOI] [PubMed] [Google Scholar]
- 7. Kawai T., Akira S. (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650. [DOI] [PubMed] [Google Scholar]
- 8. Abe R., Oda S., Sadahiro T., Nakamura M., Hirayama Y., Tateishi Y., Shinozaki K., Hirasawa H. (2010) Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit. Care 14, R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen Q. T., Nguyen T. H., Ju S. A., Lee Y. S., Han S. H., Lee S. C., Kwon B. S., Yu R., Kim G. Y., Lee B. J., Kim B. S. (2013) CD137 expressed on neutrophils plays dual roles in antibacterial responses against Gram-positive and Gram-negative bacterial infections. Infect. Immun. 81, 2168–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cuenca A. G., Delano M. J., Kelly-Scumpia K. M., Moreno C., Scumpia P. O., Laface D. M., Heyworth P. G., Efron P. A., Moldawer L. L. (2012) A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol. Med. 17, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Norris B. A., Uebelhoer L. S., Nakaya H. I., Price A. A., Grakoui A., Pulendran B. (2013) Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity. Immunity 38, 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]