Review of clinical and scientific evidence of different aspects of CD4 T cells affected by sepsis-induced immunosuppression.
Keywords: apoptosis, lymphopenia, homeostatic proliferation, immune suppression
Abstract
Sepsis remains the primary cause of death from infection in hospital patients, despite improvements in antibiotics and intensive-care practices. Patients who survive severe sepsis can display suppressed immune function, often manifested as an increased susceptibility to (and mortality from) nosocomial infections. Not only is there a significant reduction in the number of various immune cell populations during sepsis, but there is also decreased function in the remaining lymphocytes. Within the immune system, CD4 T cells are important players in the proper development of numerous cellular and humoral immune responses. Despite sufficient clinical evidence of CD4 T cell loss in septic patients of all ages, the impact of sepsis on CD4 T cell responses is not well understood. Recent findings suggest that CD4 T cell impairment is a multipronged problem that results from initial sepsis-induced cell loss. However, the subsequent lymphopenia-induced numerical recovery of the CD4 T cell compartment leads to intrinsic alterations in phenotype and effector function, reduced repertoire diversity, changes in the composition of naive antigen-specific CD4 T cell pools, and changes in the representation of different CD4 T cell subpopulations (e.g., increases in Treg frequency). This review focuses on sepsis-induced alterations within the CD4 T cell compartment that influence the ability of the immune system to control secondary heterologous infections. The understanding of how sepsis affects CD4 T cells through their numerical loss and recovery, as well as function, is important in the development of future treatments designed to restore CD4 T cells to their presepsis state.
Introduction
Historical accounts of sepsis help to explain why this syndrome—currently defined as a SIRS in the presence of a disseminated infection—remains a serious challenge to modern medicine [1]. The term “sepsis” (σηψιζ) is first found in relation to disease in the writings of the Greek physician Hippocrates (c. 460–370 BC) as the reason behind the “odiferous biological decay of the body” and a bad prognosis for the wound-healing process [2]. Galen (Roman gladiatorial surgeon; 130–200 AD) would misinterpret this notion 500 years later [3], claiming that sepsis was essentially a good omen in infections (e.g., pus bonum et laudabile, or part of aöhealthy” and “welcomed” suppuration) [4]. Galen's humoristic views about the nature of sepsis became medical dogma for more than 15 centuries, until the germ theory of infection gained acceptance and shed light on the nature and propagation of disseminated infections [5]. To this day, sepsis remains a poorly understood disease process [6]. In spite of the technological leaps in critical care, overall case mortality from septic events is still high, ranging between 30% and 50% [7]. Septic causes are responsible for ∼200,000 deaths/year in the United States [8], making it a leading cause of death in hospitals of the 21st century. The elderly are a patient population with a high incidence (accounting for nearly 60% of all septic cases) that is vulnerable to the consequences of sepsis [9], showing 100-fold higher mortality rates than the general population [10]. Collectively, the burden of morbidity, mortality, reduced quality of life, and excessive cost of sepsis on the healthcare system ($14–16 billion/year) [11] are clear indicators of how much of an unmet medical challenge this condition truly represents [12].
Within the last 40 years, our collective knowledge regarding the pathophysiology of sepsis has grown exponentially. Specifically, it has become clear that sepsis is not just the symptoms of a complicated infection; instead, we now know that sepsis is more like a “bad” immune response to a complicated infection [6]. In other words, sepsis represents the dysregulation of immune responses as a result of an invading pathogen and the ensuing system-wide collateral damage. The crux of the sepsis mystery resides in knowing the parts of the immune system that remain defective after sepsis and are ultimately detrimental to patients. In this review, we will dissect how sepsis affects the recovery and maintenance of a diverse, functional T cell repertoire, as well as to investigate potential therapies that improve survival and enhance function of T cells early and late after a septic event. The understanding of these areas is crucial for the development and translation of potential therapies to restore immune system function in recovering sepsis patients.
SEPSIS-INDUCED IMMUNOPATHOLOGY
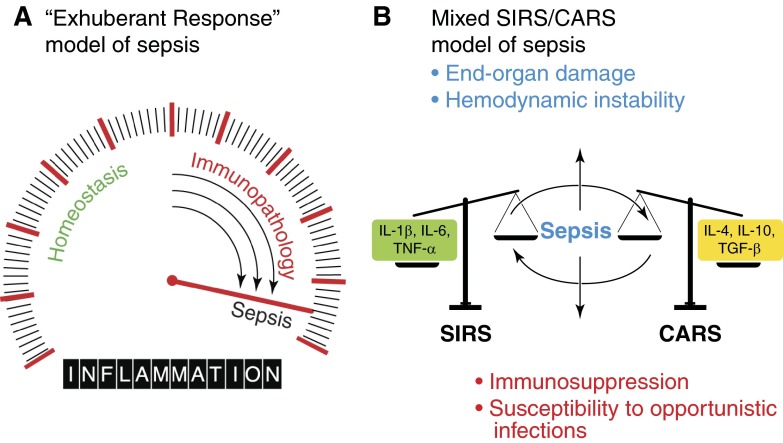
The birth of molecular immunology paved the way for the earliest interpretations of what happens to the immune system during/after a septic event. At first, the reproducible observation of elevated inflammatory markers in the serum of patients, coupled with the high mortality rates, led to the idea that the systemic invasion of pathogens was forcing our own bodies to use massive retaliation to regain homeostasis (Fig. 1A) [13], a phenomenon referred to as SIRS.
Figure 1. Evolving concepts in the etiological basis for sepsis.
The conceptual understanding of the pathophysiology of sepsis has evolved over the past 40 years from a simple, linear model of “exuberant” inflammation to a complicated interplay between opposing factions within the immune response. (A) The classic theory (and current consensus definition) of sepsis was popularized in the 1970s and views sepsis as a linear consequence of uncontrolled inflammation caused by the innate immune system in response to an invading pathogen. The inflammatory response is here depicted as a dial or gradient that encompasses immunological states ranging from homeostasis to sepsis. (B) Currently, one of the more widely accepted theories about sepsis is that it stems from the interplay between two opposite immunological poles or forces (depicted here as scales). Several clues point to this as a reasonable alternative to the classic model. First, clinical studies have found that undesirable concentrations of pro- and anti-inflammatory cytokines can be detected in the serum of septic patients. In addition, lymphocytes can be detected as undergoing apoptosis and proliferating simultaneously. Balance between immunological extremes varies from patient to patients and influences the outcome of the septic episode: some patients may experience cardiovascular collapse and organ ischemia, whereas others might recover from hemodynamic instability but end up immunosuppressed and vulnerable to secondary opportunistic infections.
This theory of hyperinflammation has dictated the direction of basic and translational sepsis research for the last 30 years [14]. This is not surprising, given that SIRS is a key component of septic pathophysiology. SIRS represents the spillover of elevated inflammatory mediators into the circulation [15] that are released during the course of an immune response [16]. These mediators locally promote cell death and leukocyte recruitment, as well as coagulation events that limit the systemic spread of infection and create an uninviting environment for the offending pathogen [17]. When amplified systemically, the same mediators cause localized edema and promote neutrophil infiltration that can lead to cardiovascular dysfunction [18], and factors causing local thrombosis can initiate disseminated intravascular coagulation [11]. In accordance with this “exhuberant immune response” model of sepsis, the vast majority of therapeutics tested in the 1980s and 1990s was aimed at blocking proinflammatory responses [19–22]. Unfortunately, strategies that dampen inflammation have been overwhelmingly ineffective in reducing mortality when tested in clinical settings [5, 23, 24].
Perhaps the most important contributor to re-evaluating the immunopathophysiological mechanisms of sepsis was Roger Bone [25], who in light of numerous therapeutic failures, noted several phenomena that were not consistent with the traditional “exuberant inflammation” model of sepsis. Bone noticed that most patients would survive the SIRS phase with adequate supportive care, and the “cytokine storm” would eventually subside, but mortality remained elevated long after a septic episode resolved. There was also clear evidence of anti-inflammatory cytokines circulating during sepsis, including IL-4, IL-10, TGF-β, and CSFs [26]. In addition, he observed that apoptotic cell death seemed to be present in sepsis in a variety of cell types, including lymphocytes. These largely ignored pieces of the sepsis puzzle led to Bone's postulate that a large population of patients surviving the early events of sepsis would enter into an immunological state characterized by hypoinflammation and immunosuppression, which he termed a CARS [25]. More recent evidence suggests that SIRS and CARS are interdependent and concurrent during the course of sepsis [27] (Fig. 1B). Indeed, we now know that a sizeable number of patients surviving the early events of sepsis enter into an immunological state characterized by T cell exhaustion, unresolved infection, and defective antigen presentation [28]. It is also becoming clear that apoptosis of lymphoid and nonlymphoid tissue [29, 30] and suppression of lymphocyte responses after the acute phase events [31] are of paramount importance to the protracted course and infectious complications often seen in septic patients [32].
APOPTOSIS AND LYMPHOCYTE IMMUNOSUPPRESSION IN SEPSIS
The focus of basic and clinical research regarding lymphocyte apoptosis in sepsis has grown considerably over the past 20 years. The studies published have added credibility to the idea that apoptosis and immune suppression are not only important players in the pathophysiology of sepsis but are also intricately intertwined. Work performed in the 1990s dramatically advanced the understanding of apoptotic cell death through the identification of numerous cell death-inducing molecules and their cognate receptors, as well as the molecular components of the cell death machinery. Incorporation of the wide range of cell death reagents and genetically modified mice into the sepsis arena helped to define some of the proteins important in sepsis-induced lymphocyte apoptosis and the importance of sepsis-induced apoptosis on the development of the subsequent immune suppression. General inhibition of apoptosis, via overexpression of Bcl-2, increased survival after sepsis induction [33]. In addition, a variety of caspases is activated during sepsis-induced apoptosis, and the administration of caspase inhibitors also improves survival [34, 35]. However, the molecular mechanism by which lymphocyte apoptosis occurs after sepsis has remained difficult to define, as no single extrinsic or intrinsic pathway appears to be dominant [36]. Interestingly, there are data to suggest that the TRAIL pathway is important in the establishment of sepsis-induced immune suppression [37, 38].
In support of the numerous animal-based studies examining the relationship between sepsis-induced lymphocyte apoptosis and immune suppression, Hotchkiss and colleagues [39] showed that postmortem tissue samples from septic patients had considerable amounts of apoptotic cell death (specifically, 56.3% of spleens, 47.1% of colons, and 27.7% of ileums sampled). Furthermore, tissue immunohistochemistry revealed increased caspase-3 activity in septic versus nonseptic patients, with 25–50% of cells positive in the splenic white pulp of six septic but none of the nonseptic patients, providing evidence that lymphocyte apoptosis was increased significantly in septic patients [40]. Several other studies have added credibility to the theory that lymphocyte apoptosis plays a role in the immune suppression characteristic of the late events in sepsis. Le Tulzo et al. [41] examined freshly isolated lymphocytes of critically ill septic patients and showed a higher degree of apoptosis in the earlier stages of septic shock, as well as delayed T cell reconstitution, compared with nonseptic individuals. Evidence for the expression of intracellular proapoptotic molecules has also been reported in human lymphocytes from septic patients. Weber and colleagues [42] analyzed mRNA expression of several Bcl-2 family molecules in circulating lymphocytes, comparing patients with severe sepsis with nonseptic critically ill patients. One interesting finding in this study was the marked up-regulation of Bim, a proapoptotic molecule whose deletion is associated with complete protection from apoptosis in animal sepsis in sepsis-derived lymphocytes. This is a potentially insightful finding into the specific pathways of apoptotic death that are dominant in sepsis, given that Bim is the only component of the apoptosis cascade whose deletion induces complete protection from apoptotic cell death in septic mice [36].
IMPACT OF SEPSIS ON CD4 T CELL RESPONSES
CD4 Th cells are among the most important peripheral lymphocyte subsets when it comes to the orchestration of successful immune responses, influencing innate and adaptive immune cells through cytokine production and cell-to-cell interaction [43]. CD4 T cells are essential for effective primary CD8 T cell responses [44, 45] and the formation of functional CD8 T cell memory [46–49], as well as efficient isotype switching in primary and memory B cell responses [50, 51]. A defining feature of antigen-specific CD4 T cells is that upon recognition of antigen—and depending on the cytokines and costimulatory molecules present—subsets of effector CD4 T cells take on a specific phenotype best suited to drive a response against the perceived threat. These differentiation pathways enable the activated and differentiated CD4 T cell to exert specific effector functions, such as produce cytokines, activate other cells, and change immune cell migration patterns necessary to clear the pathogen recognized.
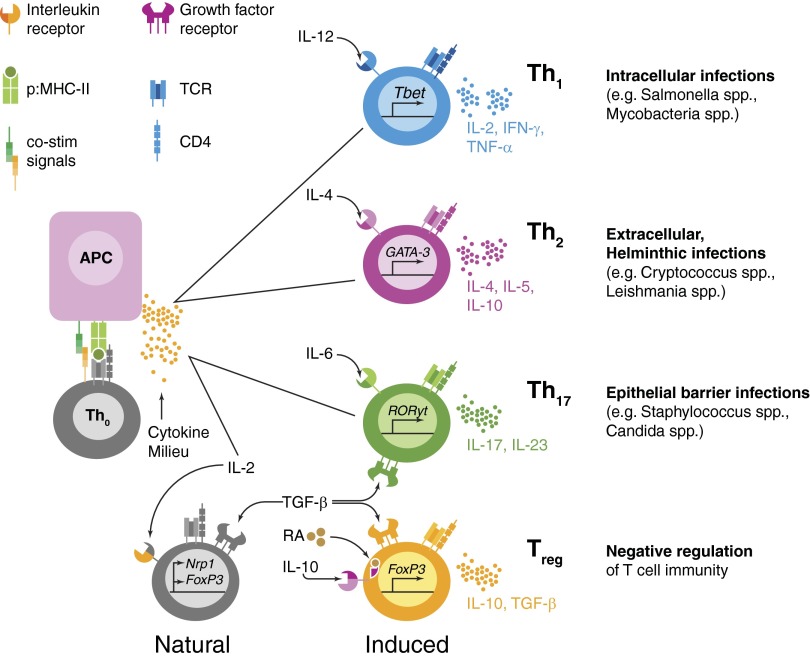
Several “polarities” or effector differentiation pathways are well-described within CD4 T cells (Fig. 2). For example, Th1 cells are induced in response to viral, bacterial, and protozoan intracellular infections [52]. Classically, CD4 T cells from this subset are induced by available IL-12 and IFN-γ in the inflammatory milieu and produce cytokines, such as IL-2 and IFN-γ, which go on to activate intracellular killing mechanisms in macrophages [53, 54]. In addition, Th1 cells provide necessary signals for isotype switching in B cell responses (e.g., IgG2a in mice) [55]. In contrast, Th2 cells (activated in the presence of IL-4) produce predominantly IL-4, IL-5, and IL-13 and are important for the clearance of helminthic infections [56]. Th2 cells also enhance B cell isotype switching (to IgG1 and IgE) via IL-4 secretion, as well as the alternative activation of macrophages to promote tissue repair [57]. Lastly, Th17 cells, which are effector CD4 T cells that can produce IL-17, IL-22, and TNF-α, are important in immunity to extracellular fungal and bacterial pathogens (especially at mucosal surfaces) [58] through the recruitment and activation of neutrophils [59]. Thus, the loss or improper function of CD4 T cell responses is detrimental for immunity to a wide range of pathogens.
Figure 2. Plasticity of CD4 T cell phenotype is essential for generation of optimal responses to a wide variety of pathogens.
A naive CD4 T cell has the ability to execute one of several effector programs. During an infection, APCs present antigenic epitopes from invading pathogens to CD4 T cells via MHC II. Along with TCR stimulation, APCs also provide CD4 T cells with costimulatory (co-stim) ligands and cytokine signals that are optimized for the antigen in question. The ensuing cytokine milieu, created for a specific infection at a specific infection site, will polarize CD4 T cells into an effector phenotype most suitable for helping the innate and adaptive components of the immune response. As our discussion has focused on the activity of Th1, Th2, and Th17 CD4 T cells, we have only included these subtypes in the figure. Thus, optimal immunity is dependent on the “correct” polarization of CD4 T cells, which is driven by the context, as well as the type of antigen encountered (e.g., type of pathogen in question, innate adjuvant effects, and route of infection). Polarization can also induce naive CD4 T cells to become Treg, which work alongside thymus-derived, “natural” Treg to suppress excessive inflammation and modulate the damage inflicted upon the host by the immune response generated. p:MHC-II, peptide:MHC II; RA, retinoic acid; Nrp1, neuropilin-1.
Whether CD4 T cells are involved directly in the early stages of septic injury is debated. Several animal studies have shown CD4 T cells to mediate directly the host response to sepsis [60, 61], whereas others have concluded that CD4 T cells have no impact in the inflammatory response [62]. Regardless of their direct effect on the acute response to septic injury, several observations point to CD4 T cells as a subset of leukocytes that might be important to consider when discussing sepsis-induced immunosuppression. These observations can be grouped into three general categories: (1) altered effector CD4 T cell phenotype and/or function, (2) altered peripheral CD4 T cell diversity, and (3) altered Treg frequency and/or function.
Altered effector CD4 T cell phenotype and/or function
During initial TCR engagement, costimulatory signals lower the threshold for T cell activation [63]. A T cell receiving only antigen-specific TCR stimulation in the absence (or inhibition) of costimulation is rendered unresponsive or anergic to subsequent challenges [64]. The inhibition of costimulation by the immune system helps to attenuate T cell responses by way of “clonal anergy” [65] and reduces responses by way of “clonal deletion” [57, 66]. Similarly, T cell exhaustion, as seen in situations of chronic viral infection [67] and cancer [68], as a result of prolonged antigen exposure in the presence of low-grade inflammation, makes use of the same mechanisms to attenuate and reduce T cell responses. Modulation of the overall “strength of signal” transmitted to a T cell by inflammation can potentially give rise to an exhuberant immune response [69], and in such cases, the immune system attempts to regain homeostasis through the same mechanisms that we have described [70]. Thus, it is now accepted that an important aspect of postseptic T cell dysfunction is a phenomenon similar to anergy or T cell exhaustion [71]. This state includes decreases in cytokine production, epigenetic changes to T cell transcription factors, and the up-regulation of inhibitory cell surface proteins, such as TRAIL [37, 38], PD-1 [72–74], and BTLA [75, 76].
The available empirical data supporting the idea of altered effector CD4 T cell function in critically ill sepsis patients date back to studies in the 1970s and 1980s, showing impaired DTH skin reactions [77]. Early studies using peripheral blood showed that cytokines produced under Th1 or Th2 conditions were altered in sepsis [78–82]. More recently, Boomer and colleagues [83] used freshly isolated, postmortem spleen and lung tissue samples from 40 patients who died in intensive care units as a result of severe sepsis compared with similar samples from nonseptic, control patients. The authors found almost no production of IFN-γ, TNF-α, IL-6, and IL-10 after 5 h stimulation with α-CD3/α-CD28, which strongly suggests of a state of impaired T cell function. Whereas some investigators believed initially that these findings pointed toward a phenotypic switch in CD4 T cells from Th1 to Th2 [84], changes in cytokine secretion are more likely a result of a global state of anergy [78]. This fact has been reinforced by the finding that in human septic lymphocytes, there is decreased expression of T-bet, GATA3, and ROR-γt—transcription factors that regulate the Th1, Th2, and Th17 effector CD4 T cell phenotypes, respectively [85]. Animal studies have also shown that histone methylation and chromatin remodeling can occur within the T-bet and GATA3 promoter regions of lymphocytes after sepsis, thereby contributing to the anergic state of CD4 T cells [86].
Other studies have also pointed to indirect evidence of defective CD4 T cell function. For example, effective CD4 T cell immunity is essential for the decrease in frequency and severity of recrudescence in human herpesviral infections [87], such as CMV [88] or HSV [89, 90], and recent studies have shown a significantly higher rate of CMV/HSV reactivation in septic patients [89, 91]. CD4 T cell function is also integral to adequate B cell function, including antibody isotype switching and maintenance of an effective humoral memory. B cells are diminished severely by sepsis-induced apoptosis [28], and there exists some evidence of perturbations within peripheral B cell subsets early on after septic injury [92]. Moreover, certain B cells are thought to play a role in the success of the innate immune response during sepsis [93, 94]. Recent animal studies have shown that administration of Ig fractions modified by mild oxidation with ferrous ions improves survival after sepsis [95, 96]. Interestingly, several investigators have also observed alterations in humoral responses after sepsis, specifically in terms of antigen-specific immunity (e.g. T cell-dependent antibody responses) [97–99].
Altered peripheral CD4 T cell diversity
Another important observation in sepsis patients is the considerable reduction in circulating CD4 T cells (along with other lymphocyte populations), which is documented in patients of all ages [41, 100–107] and occurring at the time of high pathogen burden [108–110]. However, very little is known about how CD4 T cells recover following septic injury, particularly the extent to which thymic function and homeostatic proliferation are involved. Naive CD4 T cells are normally maintained in the periphery after thymic egress by frequent low-level signals from self-peptide:MHC II and cytokine signals (most notably, IL-7 signaling for naive CD4 T cells [111], with IL-15 signaling more important for naive CD8 T cells [112]). In situations where T cell numbers drop acutely (such as during cytotoxic drug regimens, irradiation, and certain viral infections), the increased availability of these resources turns survival signals into mitogenic stimuli in a process known as homeostatic proliferation, which promotes a proliferative expansion to restore T cell numbers [113]. During expansion (and despite the antigen-independent nature of this proliferative mechanism), naive T cells acquire the phenotypic features of antigen-experienced, memory T cells [114]. One study, examining recovery of T cells after sepsis-induced lymphopenia, argued that (OT-I) CD8 T cells were able to proliferate when adoptively transferred into a septic host, but (OT-II) CD4 T cells could only proliferate if cognate antigen or IL-7 was administered [115]. The authors accordingly concluded that homeostatic proliferation was not the main mechanism for CD4 T cell recovery. This conclusion leads to an interesting dilemma: what “endogenous source” is reconstituting CD4 T cells after sepsis? It is evident that thymic output cannot explain T cell reconstitution in elderly human patients, given that the export rate of naive, thymus-derived cells is not modulated by alterations to the peripheral T cell pool (neither by lymphocytosis nor lymphopenia) [116]. Indeed, circulating levels of IL-7 in athymic (but not necessarily lymphopenic) and elderly individuals are increased significantly [117], which adds more evidence to the fact that peripheral mechanisms play a bigger role than the thymus in the maintenance of circulating T cell numbers after puberty. Furthermore, animal studies demonstrate that thymic function is impaired severely by sepsis via massive apoptosis and thymic involution [115, 118]. It is more plausible that CD4 T cells rely in peripheral (rather than central) maintenance mechanisms to recover full numerical strength after sepsis.
The almost “laissez faire” maintenance of naive T cell numbers in the periphery—autoregulation through the availability of IL-7 and tonic TCR signaling in the context of available space within the T cell compartment—has one important compromise. To anticipate an ever-changing world of pathogens, the immune system has evolved to give T cells impressive diversity [119]. However, homeostatic proliferation does not create diversity, so much as it can maintain some of the diversity. This diversity begins at the antigen-specific population level, where each CD4 T cell binds a specific complex of peptide antigen and MHC II via their TCR [120]. That is, one seemingly homogenous group of CD4 T cells, recognizing the same antigen, is formed by a diverse set of clones with divergent capabilities to form TCR/peptide:MHC II complexes [121]. Thus, an optimal diversity of the CD4 T cell repertoire (both in terms of breadth of antigen recognition, as well as heterogeneity of clonotypes within each antigen-specific population) is crucial for effective immune responses against invading pathogens.
As we age, the competition within one population of antigen-specific T cells might give rise to a “culled” repertoire. In animal studies of lymphopenia, proliferative expansion of naive T cells also becomes more dependent on TCR/self-peptide:MHC II “tonic” signaling. The resultant environment enforces competition between clones and minimizes diversity within antigen-specific populations. This is akin to a “democratization” process of the antigen-specific repertoire, whereby the clonal “elite” is culled in favor of the “mediocre” majority [122]. In agreement with this, both mouse and human studies show a dramatic, age-related decline in the diversity of antigen-specific T cells, preferentially losing reactivity over time to epitopes recognized by T cells with low precursor frequencies [123]. This effort to maintain some recognition of a pathogen can sacrifice clonal and antigenic diversity, plausibly generating “gaps” in the immunological repertoire. Extrapolating from these observations, we can reasonably argue that clonal diversity in antigen-specific cells might be reduced to a minimum in an effort to maintain naive homeostasis and that in the aging individual, this eventually results in the selection of clones with poor affinity. In the context of sepsis, a recent study showed drastic reductions in clonotype diversity of septic patients [124], making it tempting to speculate that sepsis could effectively “age” the adaptive immune system by accelerating the selection of clones with poor affinity within the resultant peripheral repertoire.
Recent findings from our group have also shed light on the impact of sepsis on the recovery of antigen-specific diversity within the peripheral T cell pool. Specifically, we studied the effect of sepsis on a range of antigen-specific CD8 T cell populations specific for LCMV and found significant changes to the antigen-specific precursor populations after sepsis that correlate with impaired priming for some epitope-specific responses [125]. The data in this study ultimately hinted at changes to the immunodominance hierarchy of LCMV-specific responses in septic animals. In agreement with these results examining antigen-specific CD8 T cell populations, we have seen that a similar phenomenon occurs in antigen-specific CD4 T cell populations (unpublished data). As the survival of naive and memory antigen-specific cells correlates inversely with clonal abundance [126], it is plausible that the massive apoptosis of peripheral T cells in septic patients can drive a recovery of antigen-specific populations with diminished repertoire diversity, whereby surviving clones do not adequately represent the immunodominance hierarchy against a specific antigen [127]. In the context of a pathogen-specific response, this idea implies that the recovery of a less-diverse repertoire within antigen-specific populations could lead to aberrant responses as a result of changes in the affinity for dominant antigen peptides. This effect would account (at least in part) for the susceptibility to opportunistic infections and diminished lymphocyte function seen in sepsis.
Increases in Treg frequency and/or function
An increased frequency of Treg has been found in the periphery of septic patients, particularly in the early stages after diagnosis [102, 128, 129]. These results were later clarified by a study showing that the increased frequency of Treg was a result of decreases in the effector populations of CD4 T cells [81]. Thus, one conclusion drawn about Treg in sepsis is that they are more resistant to apoptosis than conventional CD4 T cells [28]. Despite these findings, the role of Treg in septic injury is still debated. Excessive Treg formation decreases survival in animal models of sepsis [130], as well as improving outcomes and immunity [131–133]. These contrasting results, particularly in human studies, may be related to the sensitivity of analyzing FoxP3 expression via flow cytometry, the timing of analysis, and the ability to discern the methylation status of the FoxP3 promoter in circulating septic lymphocytes [134]. In animal models, the removal of Treg by anti-CD25 mAb has not led to any improvements in survival [135], but this may be a result of the expression of CD25 in activated CD4 T cells (and thus, depletion is not limited to CD4 Treg). More recently, some investigators have used GITR agonistic antibodies to block Treg function, and results from these studies show improved immune function and microbial killing in septic animals [99].
Complement depletion, which often occurs as a result of disseminated intravascular coagulation, can also have an effect on the balance between CD4 Treg and effector T cells. Recently, several reports showed how signaling via C3aR/C5aR diminishes the function of Treg [136–138]. Along these lines, studies in human septic patients have shown strong correlations between complement C3 depletion and an increase in the frequency of Treg, as well as significantly higher postoperative complications and hospital stay [139, 140]. Moreover, the administration of exogenous C3 inhibited Treg expansion and improved survival in animal models of sepsis [141, 142]. Whereas it is true that C3 might also be improving outcomes by preventing organ dysfunction, it is just as plausible that changes in the T cell compartments and the paralysis of immune responses can be, in part, a result of systemic cell death.
STRATEGIES TO ENHANCE T CELL RECOVERY AND FUNCTION AFTER SEPSIS
Administration of immune-modulatory therapy is a promising treatment approach for treating sepsis survivors. Of particular interest are two therapeutic approaches: the use of γc receptor-dependent cytokines, such as IL-2, IL-7, and IL-15 [143–145], and the blockade of certain inhibitory molecules (particularly, PD-1 [146, 147], CTLA-4 [148], and TRAIL [38]). However, the potency and safety of some these therapies have to be enhanced and toxicity minimized before their efficacy can be tested in clinical settings. Here, we will review the extent to which these therapies can improve pathogen clearance, increase CD4 T cell responsiveness, and promote survival in sepsis.
Cytokines of the common γc receptor family
Cytokine-based, immune-modulatory strategies have proven effective in boosting anticancer responses, rejuvenating T cell-mediated immunity in settings of chronic viral infections, as well as accelerating immune reconstitution after bone marrow transplantation. It is now apparent that although their rejuvenating effects apply differentially to T cell subsets, several members in the common γc family of cytokines promote homeostasis and survival in CD4 and CD8 T cells. For example, IL-2 becomes a master regulator of homeostasis during T cell-mediated responses, as well as during T cell recovery in lymphopenic hosts (promoting survival, expansion of Treg, or activation-induced death in a context-dependent manner). IL-7 and IL-15, conversely, are much more specifically required for the survival and expansion of CD4 and CD8 memory and T cells during homeostatic expansion. IL-7 is one of the most promising cytokine-based therapies to date, with 13 currently ongoing or recently closed clinical trials examining the effect of IL-7 adjuvant therapy in anti-tumor responses or as a booster of immunity in chronic viral infections. In the context of sepsis, studies published within the last 3 years have shown IL-7 to improve survival of murine T cells after sepsis induced by CLP, to increase pathogen clearance and DTH responses in a mouse model of sepsis with candidiasis, and to promote proliferation in hyporesponsive PBMC from septic patients [144, 145, 149].
The biggest potential drawbacks of a cytokine-based therapy are potential off-target effects. For example, although IL-7 can enhance immune reconstitution after bone marrow transplantation in humans, patients with acute graft-versus-host disease display significantly higher circulating concentrations of IL-7 [150]. Similarly, IL-15 can exacerbate autoimmunity (such as in celiac disease), and side-effects of high-dose IL-2 immunotherapy stem from its ability to cause vascular leak syndrome, an accumulation of intravascular fluid in organs, causing prominent pulmonary edema and liver cell damage [151].
Immune checkpoint modulation
As mentioned previously, sepsis-induced immune dysfunction shares many similarities with cancers refractory to standard therapies. One of the newest strategies for therapeutic intervention in cancer—immune checkpoint modulation—might lead to benefits in sepsis as well. Most of these new strategies are based on blockade of pathways that negatively regulate T cell survival and/or activation following TCR engagement. Thus, whereas cytokine therapies might prevent or lessen irreparable losses in the T cell repertoire, immune checkpoint modulation therapies would serve to reverse sepsis-induced immunosuppression.
Perhaps the most popular target for checkpoint modulation in current sepsis research is PD-1. A growing number of biologicals targeting the PD-1 pathway have been evaluated as immunotherapy for several types of solid tumors and have shown impressive efficacy in clinical trials [152, 153], as well as in the effective reversal of T cell exhaustion in mycobacterial and chronic CMV infections [147, 154]. Interestingly, PD-1 expression has also correlated with mortality and nosocomial infections in sepsis patients [73]. Correspondingly, the pharmacologically relevant ligand for PD-1, PD-L1, is up-regulated on circulating monocytes in human sepsis patients and mouse macrophages during experimental sepsis [73, 74]. Indeed, blockade of the PD-1:PD-L1 signaling pathway improves survival in animal models of sepsis [155, 156] and reverses T cell exhaustion in patients with sepsis [146].
Another potential target for blockade or inhibition is TRAIL, which has been under investigation in clinical trials in the cancer and bone marrow transplantation fields [157]. TRAIL is up-regulated in situations of immune privilege and T cell exhaustion to induce apoptotic cell death [48, 158]. As mentioned, one interesting phenomenological defect in sepsis patients is a loss of CD4 T cell-dependent DTH responses. In other tolerance models where there is administration of antigen-coupled apoptotic cells or generation of a large number of apoptotic cells in vivo, one mechanism to explain the lack of CD4 T cell immunity is immune regulation by a TRAIL-expressing CD8 T cell population [66, 159, 160]. In line with these experimental models of tolerance, a population of TRAIL-expressing CD8 T cells potently inhibits CD4 T cell function in sepsis, and the blockade of TRAIL by mAb treatment increases T cell function and decreased heterologous pathogen burden during a secondary infection in CLP-induced sepsis [37, 38]. Interestingly, low concentrations of soluble TRAIL in the plasma correlated with reduced immune function and a higher risk of mortality in patients with septic shock [161]. Clearly, additional research is needed to determine the relationship between the mouse and human data on the potential function of TRAIL in sepsis.
CONCLUDING REMARKS
Sepsis is a complex medical condition that exerts a variety of consequences on the immune system. We have examined the impact of sepsis specifically on CD4 T cell immunity, as these cells factor into the development of numerous types of responses by the immune system. Moreover, we have focused our discussion on the impact of sepsis on the quantity and quality of CD4 T cells, given that these characteristics dictate the magnitude and success of any prospective CD4 T cell response to pathogenic challenge. The apoptotic attrition within the CD4 T cell compartment during a septic event stochastically affects all antigen-specific effector CD4 T cell populations (Fig. 3). Acutely, this sudden loss of CD4 T cells dramatically affects a variety of adaptive-immune functions. As CD4 T cells recover numerically, multiple mechanisms are potentially involved in sepsis-induced immune suppression of CD4 T cell responses. Changes in TCR repertoire use, antigen-binding affinity, proliferative capacity, and cytokine production can act collectively to alter qualitative and compositional aspects of postseptic CD4 T cell responses. Furthermore, sepsis-induced alterations in the composition of the antigen-specific CD4 T cell repertoire persist and may ultimately account for the increased risk of mortality [162, 163] and the more poorly perceived general health that is documented in survivors for years after resolution of sepsis [164].
Figure 3. Sepsis-associated lymphocyte apoptosis is followed by a quantitatively and qualitatively impaired recovery of CD4 T cell pathogen-specific responses.
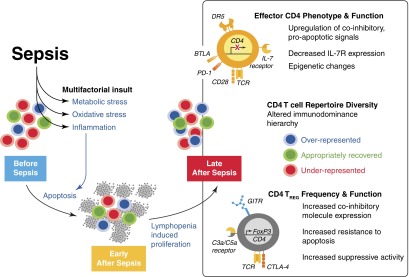
The colored cells represent three different antigen-specific populations within an immunodominance hierarchy. Sepsis causes a stochastic loss of CD4 T cells by apoptosis, but the causative agent(s) responsible for this decline are not clear. It is thought that the drop in circulating lymphocytes stems from a multifactorial insult that includes excessive proinflammatory cytokine levels, metabolic stress, increased levels of toxic metabolites, reactive oxygen species. and hypoxia/ischemia. Nevertheless, the end result for a significant group of patients is a state of lymphopenia that is most pronounced for certain cell populations (one such population includes CD4 T cells) with clear reductions in diversity, as well as the eventual numerical recovery of T cells. However, several changes occur to CD4 T cells in the process of recovery. These include cell-intrinsic changes (anergic and proapoptotic phenotypes, as well as hypermethylation of promoter regions for important Th cell transcription factors) and regulatory changes (increased fraction of Treg and/or perhaps increases in the functional capacity of Treg). Finally, changes to CD4 T cell repertoire diversity are depicted here by showing how antigen-specific populations may be altered after lymphopenia and recovery, thereby altering the immunodominance hierarchy of a response: one population has an impaired recovery, whereas another is over-represented after recovery, and a third population recovers numerically to its level at homeostasis. This change can be demonstrated at the level of single antigen-specific populations but is not evident otherwise given the numerical recovery of total CD4 T cells. DR5, death receptor 5.
We realize that the sepsis-induced changes within the CD4 T cell compartment that we have highlighted are just a few of the many ways in which sepsis affects the immune system. As medical advancements have increased the ability of clinicians to reduce morbidity to acute sepsis, the continued testing of therapeutics that prevent CD4 T cell loss, accelerate numerical recovery, boost cellular function, and/or block immunosuppressive pathways is needed to decrease mortality rates associated with the increased susceptibility to secondary infection. As each of these aspects of CD4 T cell function act at different points, it is likely that targeting multiple points will be needed to restore the CD4 T cell response to (near) normal states.
ACKNOWLEDGMENTS
This study was supported by the University of Minnesota Center for Immunology Training Grant T32 AI007313 and Medical Scientist Training Program T32 GM008244 (to J.C-P.), an American Heart Association Postdoctoral Fellowship (to S.A.C.), U.S. National Institutes of Health Grant AI83286 (to V.P.B.), and a U.S. Department of Veterans Affairs Merit Review Award (to T.S.G.).
Footnotes
- γc
- γ chain
- BTLA
- B- and T-lymphocyte attenuator
- CARS
- compensatory anti-inflammatory response syndrome
- CLP
- cecal ligation and puncture
- DTH
- delayed-type hypersensitivity
- FoxP3
- forkhead box P3
- GITR
- glucocorticoid-induced TNFR-related protein
- HSV
- herpes simplex virus
- LCMV
- lymphocytic choriomeningitis virus
- PD-1
- programmed cell death protein 1
- PD-L1
- programmed cell death protein 1 ligand
- ROR-γt
- retinoic acid receptor-related orphan receptor γ
- SIRS
- systemic inflammatory response syndrome
- Treg
- CD4+CD25+forkhead box P3+ regulatory T cell
AUTHORSHIP
J.C-P. designed and wrote the paper. S.A.C., V.P.B., and T.S.G. wrote and reviewed the paper.
DISCLOSURES
The authors disclose no financial conflicts of interest.
REFERENCES
- 1. Geroulanos S., Douka E. T. (2006) Historical perspective of the word “sepsis”. Intensive Care Med. 32, 2077. [DOI] [PubMed] [Google Scholar]
- 2. Hippocrates (1886) On regimen on acute disease. In The Genuine Works of Hippocrates (Adams F., translator), Gryphon Editions, New York, 59–97. [Google Scholar]
- 3. Galeni C. (1549) De symptomatum causis (On the causes of symptoms). In Pergameni Medicorum Facile Principis (Lateranensem I. ed.), Apud Iacobum Dupuys, Paris, 56–169. [Google Scholar]
- 4. Thurston A. J. (2000) Of blood, inflammation and gunshot wounds: the history of the control of sepsis. Aust. N. Z. J. Surg. 70, 855–861. [DOI] [PubMed] [Google Scholar]
- 5. Baue A. E. (2001) Sepsis research: what did we do wrong? What would Semmelweis do today? Shock 16, 1–8. [DOI] [PubMed] [Google Scholar]
- 6. Vincent J. L., Opal S. M., Marshall J. C., Tracey K. J. (2013) Sepsis definitions: time for change. Lancet 381, 774–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwashyna T. J., Netzer G., Langa K. M., Cigolle C. (2012) Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am. J. Respir. Crit. Care Med. 185, 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang H. E., Shapiro N. I., Angus D. C., Yealy D. M. (2007) National estimates of severe sepsis in United States emergency departments. Crit. Care Med. 35, 1928–1936. [DOI] [PubMed] [Google Scholar]
- 9. Turnbull I. R., Clark A. T., Stromberg P. E., Dixon D. J., Woolsey C. A., Davis C. G., Hotchkiss R. S., Buchman T. G., Coopersmith C. M. (2009) Effects of aging on the immunopathologic response to sepsis. Crit. Care Med. 37, 1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Angus D. C., Wax R. S. (2001) Epidemiology of sepsis: an update. Crit. Care Med. 29, S109–S116. [DOI] [PubMed] [Google Scholar]
- 11. Hall M. J., Williams S. N., DeFrances C. J., Golosinskiy A. (June 2011) Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief, U.S. Department of Health and Human Services, Hyattsville, MD, USA, No. 62, 1–8. [PubMed] [Google Scholar]
- 12. Heyland D. K., Hopman W., Coo H., Tranmer J., McColl M. A. (2000) Long-term health-related quality of life in survivors of sepsis. Short form 36: a valid and reliable measure of health-related quality of life. Crit. Care Med. 28, 3599–3605. [DOI] [PubMed] [Google Scholar]
- 13. Stoecklein V. M., Osuka A., Lederer J. A. (2012) Trauma equals danger—damage control by the immune system. J. Leukoc. Biol. 92, 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hotchkiss R. S., Karl I. E. (2001) Cytokine blockade in sepsis—are two better than one? Crit. Care Med. 29, 671–672. [DOI] [PubMed] [Google Scholar]
- 15. Pinsky M. R., Vincent J. L., Deviere J., Alegre M., Kahn R. J., Dupont E. (1993) Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest 103, 565–575. [DOI] [PubMed] [Google Scholar]
- 16. Bone R. C., Balk R. A., Cerra F. B., Dellinger R. P., Fein A. M., Knaus W. A., Schein R. M., Sibbald W. J. (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101, 1644–1655. [DOI] [PubMed] [Google Scholar]
- 17. Hotchkiss R. S., Karl I. E. (2003) The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348, 138–150. [DOI] [PubMed] [Google Scholar]
- 18. Landry D. W., Oliver J. A. (2001) The pathogenesis of vasodilatory shock. N. Engl. J. Med. 345, 588–595. [DOI] [PubMed] [Google Scholar]
- 19. Abraham E., Laterre P. F., Garbino J., Pingleton S., Butler T., Dugernier T., Margolis B., Kudsk K., Zimmerli W., Anderson P., Reynaert M., Lew D., Lesslauer W., Passe S., Cooper P., Burdeska A., Modi M., Leighton A., Salgo M., Van der Auwera P. (2001) Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit. Care Med. 29, 503–510. [DOI] [PubMed] [Google Scholar]
- 20. Dhainaut J. F., Tenaillon A., Le Tulzo Y., Schlemmer B., Solet J. P., Wolff M., Holzapfel L., Zeni F., Dreyfuss D., Mira J. P., et al. (1994) Platelet-activating factor receptor antagonist BN 52021 in the treatment of severe sepsis: a randomized, double-blind, placebo-controlled, multicenter clinical trial. BN 52021 Sepsis Study Group. Crit. Care Med. 22, 1720–1728. [PubMed] [Google Scholar]
- 21. Fisher C. J., Jr., Agosti J. M., Opal S. M., Lowry S. F., Balk R. A., Sadoff J. C., Abraham E., Schein R. M., Benjamin E. (1996) Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N. Engl. J. Med. 334, 1697–1702. [DOI] [PubMed] [Google Scholar]
- 22. Fisher C. J., Jr., Slotman G. J., Opal S. M., Pribble J. P., Bone R. C., Emmanuel G., Ng D., Bloedow D. C., Catalano M. A. (1994) Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit. Care Med. 22, 12–21. [DOI] [PubMed] [Google Scholar]
- 23. Carlet J. (2003) Drotrecogin α (activated) administration: too many subgroups. Crit. Care Med. 31, 2564; author reply 2564–2565. [DOI] [PubMed] [Google Scholar]
- 24. Marti-Carvajal A. J., Sola I., Lathyris D., Cardona A. F. (2012) Human recombinant activated protein C for severe sepsis. Cochrane Database Syst. Rev. 3, CD004388. [DOI] [PubMed] [Google Scholar]
- 25. Bone R. C. (1996) Sir Isaac Newton, sepsis, SIRS, and CARS. Crit. Care Med. 24, 1125–1128. [DOI] [PubMed] [Google Scholar]
- 26. Freeman B. D., Yatsiv I., Natanson C., Solomon M. A., Quezado Z. M., Danner R. L., Banks S. M., Hoffman W. D. (1995) Continuous arteriovenous hemofiltration does not improve survival in a canine model of septic shock. J. Am. Coll. Surg. 180, 286–292. [PubMed] [Google Scholar]
- 27. Gomez H. G., Gonzalez S. M., Londono J. M., Hoyos N. A., Nino C. D., Leon A. L., Velilla P. A., Rugeles M. T., Jaimes F. A. (2014) Immunological characterization of compensatory anti-inflammatory response syndrome in patients with severe sepsis: a longitudinal study. Crit. Care Med. 42, 771–780. [DOI] [PubMed] [Google Scholar]
- 28. Hotchkiss R. S., Monneret G., Payen D. (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 13, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hotchkiss R. S., Karl I. E. (2004) Endothelial cell apoptosis in sepsis: a case of habeas corpus? Crit. Care Med. 32, 901–902. [DOI] [PubMed] [Google Scholar]
- 30. Moldawer L. L. (1999) LOrgan apoptosis in the septic patient: a potential therapeutic target? Crit. Care Med. 27, 1381–1382. [DOI] [PubMed] [Google Scholar]
- 31. Castelino D. J., McNair P., Kay T. W. (1997) Lymphocytopenia in a hospital population–what does it signify? Aust. N. Z. J. Med. 27, 170–174. [DOI] [PubMed] [Google Scholar]
- 32. Abraham E. (1991) Physiologic stress and cellular ischemia: relationship to immunosuppression and susceptibility to sepsis. Crit. Care Med. 19, 613–618. [DOI] [PubMed] [Google Scholar]
- 33. Hotchkiss R. S., Swanson P. E., Knudson C. M., Chang K. C., Cobb J. P., Osborne D. F., Zollner K. M., Buchman T. G., Korsmeyer S. J., Karl I. E. (1999) Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J. Immunol. 162, 4148–4156. [PubMed] [Google Scholar]
- 34. Tinsley K. W., Cheng S. L., Buchman T. G., Chang K. C., Hui J. J., Swanson P. E., Karl I. E., Hotchkiss R. S. (2000) Caspases-2, -3, -6, and -9, but not caspase-1, are activated in sepsis-induced thymocyte apoptosis. Shock 13, 1–7. [DOI] [PubMed] [Google Scholar]
- 35. Hotchkiss R. S., Chang K. C., Swanson P. E., Tinsley K. W., Hui J. J., Klender P., Xanthoudakis S., Roy S., Black C., Grimm E., Aspiotis R., Han Y., Nicholson D. W., Karl I. E. (2000) Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat. Immunol. 1, 496–501. [DOI] [PubMed] [Google Scholar]
- 36. Chang K. C., Unsinger J., Davis C. G., Schwulst S. J., Muenzer J. T., Strasser A., Hotchkiss R. S. (2007) Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 21, 708–719. [DOI] [PubMed] [Google Scholar]
- 37. Unsinger J., Kazama H., McDonough J. S., Griffith T. S., Hotchkiss R. S., Ferguson T. A. (2010) Sepsis-induced apoptosis leads to active suppression of delayed-type hypersensitivity by CD8+ regulatory T cells through a TRAIL-dependent mechanism. J. Immunol. 184, 6766–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gurung P., Rai D., Condotta S. A., Babcock J. C., Badovinac V. P., Griffith T. S. (2011) Immune unresponsiveness to secondary heterologous bacterial infection after sepsis induction is TRAIL dependent. J. Immunol. 187, 2148–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hotchkiss R. S., Tinsley K. W., Karl I. E. (2003) Role of apoptotic cell death in sepsis. Scand. J. Infect. Dis. 35, 585–592. [DOI] [PubMed] [Google Scholar]
- 40. Hotchkiss R. S., Swanson P. E., Freeman B. D., Tinsley K. W., Cobb J. P., Matuschak G. M., Buchman T. G., Karl I. E. (1999) Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 27, 1230–1251. [DOI] [PubMed] [Google Scholar]
- 41. Le Tulzo Y., Pangault C., Gacouin A., Guilloux V., Tribut O., Amiot L., Tattevin P., Thomas R., Fauchet R., Drenou B. (2002) Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock 18, 487–494. [DOI] [PubMed] [Google Scholar]
- 42. Weber S. U., Schewe J. C., Lehmann L. E., Muller S., Book M., Klaschik S., Hoeft A., Stuber F. (2008) Induction of Bim and Bid gene expression during accelerated apoptosis in severe sepsis. Crit. Care 12, R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pepper M., Jenkins M. K. (2011) Origins of CD4(+) effector and central memory T cells. Nat. Immunol. 12, 467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Green A. M., Difazio R., Flynn J. L. (2013) IFN-γ from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J. Immunol. 190, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Phares T. W., Stohlman S. A., Hinton D. R., Bergmann C. C. (2012) Enhanced CD8 T-cell anti-viral function and clinical disease in B7-H1-deficient mice requires CD4 T cells during encephalomyelitis. J. Neuroinflammation 9, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Badovinac V. P., Messingham K. A., Griffith T. S., Harty J. T. (2006) TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J. Immunol. 177, 999–1006. [DOI] [PubMed] [Google Scholar]
- 47. Church S. E., Jensen S. M., Antony P. A., Restifo N. P., Fox B. A. (2014) Tumor-specific CD4(+) T cells maintain effector and memory tumor-specific CD8(+) T cells. Eur. J. Immunol. 44, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Janssen E. M., Droin N. M., Lemmens E. E., Pinkoski M. J., Bensinger S. J., Ehst B. D., Griffith T. S., Green D. R., Schoenberger S. P. (2005) CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434, 88–93. [DOI] [PubMed] [Google Scholar]
- 49. Sacks J. A., Bevan M. J. (2008) TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J. Immunol. 180, 4570–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weinstein J. S., Hernandez S. G., Craft J. (2012) T cells that promote B-cell maturation in systemic autoimmunity. Immunol. Rev. 247, 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yates J. L., Racine R., McBride K. M., Winslow G. M. (2013) T cell-dependent IgM memory B cells generated during bacterial infection are required for IgG responses to antigen challenge. J. Immunol. 191, 1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. (2005) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. J. Immunol. 175, 5–14. [PubMed] [Google Scholar]
- 53. Munk M. E., Emoto M. (1995) Functions of T-cell subsets and cytokines in mycobacterial infections. Eur. Respir. J. Suppl. 20, 668s–675s. [PubMed] [Google Scholar]
- 54. Wargnier A., Lagrange P. H. (1993) [Bactericidal activity of cells of the immune system]. Pathol. Biol. (Paris) 41, 887–896. [PubMed] [Google Scholar]
- 55. Mahon B. P., Katrak K., Nomoto A., Macadam A. J., Minor P. D., Mills K. H. (1995) Poliovirus-specific CD4+ Th1 clones with both cytotoxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. J. Exp. Med. 181, 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Allen J. E., Maizels R. M. (2011) Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 11, 375–388. [DOI] [PubMed] [Google Scholar]
- 57. Chen F., Liu Z., Wu W., Rozo C., Bowdridge S., Millman A., Van Rooijen N., Urban J. F., Jr., Wynn T. A., Gause W. C. (2012) An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med. 18, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cohen J. M., Khandavilli S., Camberlein E., Hyams C., Baxendale H. E., Brown J. S. (2011) Protective contributions against invasive Streptococcus pneumoniae pneumonia of antibody and Th17-cell responses to nasopharyngeal colonisation. PLoS One 6, e25558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Basu R., Hatton R. D., Weaver C. T. (2013) The Th17 family: flexibility follows function. Immunol. Rev. 252, 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kasten K. R., Tschop J., Goetzman H. S., England L. G., Dattilo J. R., Cave C. M., Seitz A. P., Hildeman D. A., Caldwell C. C. (2010) T-cell activation differentially mediates the host response to sepsis. Shock 34, 377–383. [DOI] [PubMed] [Google Scholar]
- 61. Martignoni A., Tschop J., Goetzman H. S., Choi L. G., Reid M. D., Johannigman J. A., Lentsch A. B., Caldwell C. C. (2008) CD4-expressing cells are early mediators of the innate immune system during sepsis. Shock 29, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Enoh V. T., Lin S. H., Etogo A., Lin C. Y., Sherwood E. R. (2008) CD4+ T-cell depletion is not associated with alterations in survival, bacterial clearance, and inflammation after cecal ligation and puncture. Shock 29, 56–64. [DOI] [PubMed] [Google Scholar]
- 63. Schwartz R. H., Mueller D. L., Jenkins M. K., Quill H. (1989) T-cell clonal anergy. Cold Spring Harbor Symp. Quant. Biol. 54, 605–610. [DOI] [PubMed] [Google Scholar]
- 64. Jenkins M. K., Schwartz R. H. (1987) Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 165, 302–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sharpe A. H. (2009) Mechanisms of costimulation. Immunol. Rev. 229, 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Griffith T. S., Brincks E. L., Gurung P., Kucaba T. A., Ferguson T. A. (2011) Systemic immunological tolerance to ocular antigens is mediated by TRAIL-expressing CD8+ T cells. J. Immunol. 186, 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zajac A. J., Blattman J. N., Murali-Krishna K., Sourdive D. J., Suresh M., Altman J. D., Ahmed R. (1998) Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pardoll D. M. (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Richer M. J., Nolz J. C., Harty J. T. (2013) Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling. Immunity 38, 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ayres J. S., Schneider D. S. (2012) Tolerance of infections. Ann. Rev. Immunol. 30, 271–294. [DOI] [PubMed] [Google Scholar]
- 71. Wherry E. J. (2011) T cell exhaustion. Nat. Immunol. 12, 492–499. [DOI] [PubMed] [Google Scholar]
- 72. Guignant C., Lepape A., Huang X., Kherouf H., Denis L., Poitevin F., Malcus C., Cheron A., Allaouchiche B., Gueyffier F., Ayala A., Monneret G., Venet F. (2011) Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit. Care 15, R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Huang X., Venet F., Wang Y. L., Lepape A., Yuan Z., Chen Y., Swan R., Kherouf H., Monneret G., Chung C. S., Ayala A. (2009) PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl. Acad. Sci. USA 106, 6303–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Y., Li J., Lou J., Zhou Y., Bo L., Zhu J., Zhu K., Wan X., Cai Z., Deng X. (2011) Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit. Care 15, R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shubin N. J., Chung C. S., Heffernan D. S., Irwin L. R., Monaghan S. F., Ayala A. (2012) BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J. Leukoc. Biol. 92, 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shubin N. J., Monaghan S. F., Heffernan D. S., Chung C. S., Ayala A. (2013) B and T lymphocyte attenuator expression on CD4+ T-cells associates with sepsis and subsequent infections in ICU patients. Crit. Care 17, R276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Meakins J. L., Pietsch J. B., Bubenick O., Kelly R., Rode H., Gordon J., MacLean L. D. (1977) Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann. Surg. 186, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. De A. K., Kodys K. M., Pellegrini J., Yeh B., Furse R. K., Bankey P., Miller-Graziano C. L. (2000) Induction of global anergy rather than inhibitory Th2 lymphokines mediates posttrauma T cell immunodepression. Clin. Immunol. 96, 52–66. [DOI] [PubMed] [Google Scholar]
- 79. Heidecke C. D., Hensler T., Weighardt H., Zantl N., Wagner H., Siewert J. R., Holzmann B. (1999) Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am. J. Surg. 178, 288–292. [DOI] [PubMed] [Google Scholar]
- 80. Roth G., Moser B., Krenn C., Brunner M., Haisjackl M., Almer G., Gerlitz S., Wolner E., Boltz-Nitulescu G., Ankersmit H. J. (2003) Susceptibility to programmed cell death in T-lymphocytes from septic patients: a mechanism for lymphopenia and Th2 predominance. Biochem. Biophys. Res. Commun. 308, 840–846. [DOI] [PubMed] [Google Scholar]
- 81. Venet F., Pachot A., Debard A. L., Bohe J., Bienvenu J., Lepape A., Monneret G. (2004) Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25- lymphocytes. Crit. Care Med. 32, 2329–2331. [DOI] [PubMed] [Google Scholar]
- 82. Wick M., Kollig E., Muhr G., Koller M. (2000) The potential pattern of circulating lymphocytes TH1/TH2 is not altered after multiple injuries. Arch. Surg. 135, 1309–1314. [DOI] [PubMed] [Google Scholar]
- 83. Boomer J. S., To K., Chang K. C., Takasu O., Osborne D. F., Walton A. H., Bricker T. L., Jarman S. D., II, Kreisel D., Krupnick A. S., Srivastava A., Swanson P. E., Green J. M., Hotchkiss R. S. (2011) Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. O'Sullivan S. T., Lederer J. A., Horgan A. F., Chin D. H., Mannick J. A., Rodrick M. L. (1995) Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann. Surg. 222, 482–490; discussion 490–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pachot A., Monneret G., Voirin N., Leissner P., Venet F., Bohe J., Payen D., Bienvenu J., Mougin B., Lepape A. (2005) Longitudinal study of cytokine and immune transcription factor mRNA expression in septic shock. Clin. Immunol. 114, 61–69. [DOI] [PubMed] [Google Scholar]
- 86. Carson W. F. t., Cavassani K. A., Ito T., Schaller M., Ishii M., Dou Y., Kunkel S. L. (2010) Impaired CD4+ T-cell proliferation and effector function correlates with repressive histone methylation events in a mouse model of severe sepsis. Eur. J. Immunol. 40, 998–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ouwendijk W. J., Laing K. J., Verjans G. M., Koelle D. M. (2013) T-cell immunity to human αherpesviruses. Curr. Opin. Virol. 3, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Limaye A. P., Kirby K. A., Rubenfeld G. D., Leisenring W. M., Bulger E. M., Neff M. J., Gibran N. S., Huang M. L., Santo Hayes T. K., Corey L., Boeckh M. (2008) Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 300, 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Laing K. J., Dong L., Sidney J., Sette A., Koelle D. M. (2012) Immunology in the Clinic Review Series; focus on host responses: T cell responses to herpes simplex viruses. Clin. Exp. Immunol. 167, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rinaldo C. R., Jr., Torpey D. J., III (1993) Cell-mediated immunity and immunosuppression in herpes simplex virus infection. Immunodeficiency 5, 33–90. [PubMed] [Google Scholar]
- 91. Luyt C. E., Combes A., Deback C., Aubriot-Lorton M. H., Nieszkowska A., Trouillet J. L., Capron F., Agut H., Gibert C., Chastre J. (2007) Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am. J. Respir. Crit. Care Med. 175, 935–942. [DOI] [PubMed] [Google Scholar]
- 92. Monserrat J., de Pablo R., Diaz-Martin D., Rodriguez-Zapata M., de la Hera A., Prieto A., Alvarez-Mon M. (2013) Early alterations of B cells in patients with septic shock. Crit. Care 17, R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kelly-Scumpia K. M., Scumpia P. O., Weinstein J. S., Delano M. J., Cuenca A. G., Nacionales D. C., Wynn J. L., Lee P. Y., Kumagai Y., Efron P. A., Akira S., Wasserfall C., Atkinson M. A., Moldawer L. L. (2011) B cells enhance early innate immune responses during bacterial sepsis. J. Exp. Med. 208, 1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rauch P. J., Chudnovskiy A., Robbins C. S., Weber G. F., Etzrodt M., Hilgendorf I., Tiglao E., Figueiredo J. L., Iwamoto Y., Theurl I., Gorbatov R., Waring M. T., Chicoine A. T., Mouded M., Pittet M. J., Nahrendorf M., Weissleder R., Swirski F. K. (2012) Innate response activator B cells protect against microbial sepsis. Science 335, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Djoumerska-Alexieva I., Pashova S., Vassilev T., Pashov A. (2013) The protective effect of modified intravenous immunoglobulin in LPS sepsis model is associated with an increased IRA B cells response. Autoimmunity Rev. 12, 653–656. [DOI] [PubMed] [Google Scholar]
- 96. Nakamura K., Doi K., Okamoto K., Arai S., Ueha S., Matsushima K., Nakajima S., Yahagi N., Noiri E. (2013) Specific antibody in IV immunoglobulin for postsplenectomy sepsis. Crit. Care Med. 41, e163–e170. [DOI] [PubMed] [Google Scholar]
- 97. Mohr A., Polz J., Martin E. M., Griessl S., Kammler A., Potschke C., Lechner A., Broker B. M., Mostbock S., Mannel D. N. (2012) Sepsis leads to a reduced antigen-specific primary antibody response. Eur. J. Immunol. 42, 341–352. [DOI] [PubMed] [Google Scholar]
- 98. Potschke C., Kessler W., Maier S., Heidecke C. D., Broker B. M. (2013) Experimental sepsis impairs humoral memory in mice. PLoS One 8, e81752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Scumpia P. O., Delano M. J., Kelly-Scumpia K. M., Weinstein J. S., Wynn J. L., Winfield R. D., Xia C., Chung C. S., Ayala A., Atkinson M. A., Reeves W. H., Clare-Salzler M. J., Moldawer L. L. (2007) Treatment with GITR agonistic antibody corrects adaptive immune dysfunction in sepsis. Blood 110, 3673–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cheadle W. G., Pemberton R. M., Robinson D., Livingston D. H., Rodriguez J. L., Polk H. C., Jr., (1993) Lymphocyte subset responses to trauma and sepsis. J. Trauma 35, 844–849. [DOI] [PubMed] [Google Scholar]
- 101. Chen X., Ye J. (2011) Analysis of peripheral blood lymphocyte subsets and prognosis in patients with septic shock. Microbiol. Immunol. 55, 736–742. [DOI] [PubMed] [Google Scholar]
- 102. Gouel-Cheron A., Venet F., Allaouchiche B., Monneret G. (2012) CD4+ T-lymphocyte alterations in trauma patients. Crit. Care 16, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Heffernan D. S., Monaghan S. F., Thakkar R. K., Machan J. T., Cioffi W. G., Ayala A. (2012) Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Crit. Care 16, R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hoser G. A., Skirecki T., Zlotorowicz M., Zielinska-Borkowska U., Kawiak J. (2012) Absolute counts of peripheral blood leukocyte subpopulations in intraabdominal sepsis and pneumonia-derived sepsis: a pilot study. Folia Histochem. Cytobiol. 50, 420–426. [DOI] [PubMed] [Google Scholar]
- 105. Hotchkiss R. S., Tinsley K. W., Swanson P. E., Schmieg R. E., Jr., Hui J. J., Chang K. C., Osborne D. F., Freeman B. D., Cobb J. P., Buchman T. G., Karl I. E. (2001) Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 166, 6952–6963. [DOI] [PubMed] [Google Scholar]
- 106. Inoue S., Suzuki-Utsunomiya K., Okada Y., Taira T., Iida Y., Miura N., Tsuji T., Yamagiwa T., Morita S., Chiba T., Sato T., Inokuchi S. (2013) Reduction of immunocompetent T cells followed by prolonged lymphopenia in severe sepsis in the elderly. Crit. Care Med. 41, 810–819. [DOI] [PubMed] [Google Scholar]
- 107. Roger P. M., Hyvernat H., Ticchioni M., Kumar G., Dellamonica J., Bernardin G. (2012) The early phase of human sepsis is characterized by a combination of apoptosis and proliferation of T cells. J. Crit. Care 27, 384–393. [DOI] [PubMed] [Google Scholar]
- 108. Baker C. C., Miller C. L., Trunkey D. D., Lim R. C., Jr., (1979) Identity of mononuclear cells which compromise the resistance of trauma patients. J. Surg. Res. 26, 478–487. [DOI] [PubMed] [Google Scholar]
- 109. Hansbrough J. F., Bender E. M., Zapata-Sirvent R., Anderson J. (1984) Altered helper and suppressor lymphocyte populations in surgical patients. A measure of postoperative immunosuppression. Am. J. Surg. 148, 303–307. [DOI] [PubMed] [Google Scholar]
- 110. Munster A. M. (1976) Post-traumatic immunosuppression is due to activation of suppressor T cells. Lancet 1, 1329–1330. [DOI] [PubMed] [Google Scholar]
- 111. Sportes C., Hakim F. T., Memon S. A., Zhang H., Chua K. S., Brown M. R., Fleisher T. A., Krumlauf M. C., Babb R. R., Chow C. K., Fry T. J., Engels J., Buffet R., Morre M., Amato R. J., Venzon D. J., Korngold R., Pecora A., Gress R. E., Mackall C. L. (2008) Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 205, 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Surh C. D., Sprent J. (2008) Homeostasis of naive and memory T cells. Immunity 29, 848–862. [DOI] [PubMed] [Google Scholar]
- 113. Boyman O., Letourneau S., Krieg C., Sprent J. (2009) Homeostatic proliferation and survival of naive and memory T cells. Eur. J. Immunol. 39, 2088–2094. [DOI] [PubMed] [Google Scholar]
- 114. Sprent J., Surh C. D. (2011) Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat. Immunol. 12, 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Unsinger J., Kazama H., McDonough J. S., Hotchkiss R. S., Ferguson T. A. (2009) Differential lymphopenia-induced homeostatic proliferation for CD4+ and CD8+ T cells following septic injury. J. Leukoc. Biol. 85, 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Houston E. G., Jr., Boursalian T. E., Fink P. J. (2012) Homeostatic signals do not drive post-thymic T cell maturation. Cell. Immunol. 274, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sauce D., Larsen M., Fastenackels S., Roux A., Gorochov G., Katlama C., Sidi D., Sibony-Prat J., Appay V. (2012) Lymphopenia-driven homeostatic regulation of naive T cells in elderly and thymectomized young adults. J. Immunol. 189, 5541–5548. [DOI] [PubMed] [Google Scholar]
- 118. Hiramatsu M., Hotchkiss R. S., Karl I. E., Buchman T. G. (1997) Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock 7, 247–253. [DOI] [PubMed] [Google Scholar]
- 119. Tubo N. J., Pagan A. J., Taylor J. J., Nelson R. W., Linehan J. L., Ertelt J. M., Huseby E. S., Way S. S., Jenkins M. K. (2013) Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell 153, 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Surh C. D., Lee D. S., Fung-Leung W. P., Karlsson L., Sprent J. (1997) Thymic selection by a single MHC/peptide ligand produces a semidiverse repertoire of CD4+ T cells. Immunity 7, 209–219. [DOI] [PubMed] [Google Scholar]
- 121. Malherbe L., Hausl C., Teyton L., McHeyzer-Williams M. G. (2004) Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity 21, 669–679. [DOI] [PubMed] [Google Scholar]
- 122. Moses C. T., Thorstenson K. M., Jameson S. C., Khoruts A. (2003) Competition for self ligands restrains homeostatic proliferation of naive CD4 T cells. Proc. Natl. Acad. Sci. USA 100, 1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nagata M. P., Gentry C. A., Hampton E. M. (1996) Is there a therapeutic or pharmacokinetic rationale for amphotericin B dosing in systemic Candida infections? Ann. Pharmacother. 30, 811–818. [DOI] [PubMed] [Google Scholar]
- 124. Venet F., Filipe-Santos O., Lepape A., Malcus C., Poitevin-Later F., Grives A., Plantier N., Pasqual N., Monneret G. (2013) Decreased T-cell repertoire diversity in sepsis: a preliminary study. Crit. Care Med. 41, 111–119. [DOI] [PubMed] [Google Scholar]
- 125. Condotta S. A., Rai D., James B. R., Griffith T. S., Badovinac V. P. (2013) Sustained and incomplete recovery of naive CD8+ T cell precursors after sepsis contributes to impaired CD8+ T cell responses to infection. J. Immunol. 190, 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hataye J., Moon J. J., Khoruts A., Reilly C., Jenkins M. K. (2006) Naive and memory CD4+ T cell survival controlled by clonal abundance. Science 312, 114–116. [DOI] [PubMed] [Google Scholar]
- 127. Condotta S. A., Cabrera-Perez J., Badovinac V. P., Griffith T. S. (2013) T-cell-mediated immunity and the role of TRAIL in sepsis-induced immunosuppression. Crit. Rev. Immunol. 33, 23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Leng F. Y., Liu J. L., Liu Z. J., Yin J. Y., Qu H. P. (2013) Increased proportion of CD4(+)CD25(+)Foxp3(+) regulatory T cells during early-stage sepsis in ICU patients. J. Microbiol. Immunol. Infect. 46, 338–344. [DOI] [PubMed] [Google Scholar]
- 129. Monneret G., Debard A. L., Venet F., Bohe J., Hequet O., Bienvenu J., Lepape A. (2003) Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit. Care Med. 31, 2068–2071. [DOI] [PubMed] [Google Scholar]
- 130. Ono S., Kimura A., Hiraki S., Takahata R., Tsujimoto H., Kinoshita M., Miyazaki H., Yamamoto J., Hase K., Saitoh D. (2013) Removal of increased circulating CD4+CD25+Foxp3+ regulatory T cells in patients with septic shock using hemoperfusion with polymyxin B-immobilized fibers. Surgery 153, 262–271. [DOI] [PubMed] [Google Scholar]
- 131. Okeke E. B., Okwor I., Mou Z., Jia P., Uzonna J. E. (2013) CD4+CD25+ regulatory T cells attenuate lipopolysaccharide-induced systemic inflammatory responses and promotes survival in murine Escherichia coli infection. Shock 40, 65–73. [DOI] [PubMed] [Google Scholar]
- 132. Zheng Y. S., Wu Z. S., Ni H. B., Ke L., Tong Z. H., Li W. Q., Li N., Li J. S. (2014) Codonopsis pilosula polysaccharide attenuates CLP sepsis via circuiting Tregs in mice. Shock 41, 250–255. [DOI] [PubMed] [Google Scholar]
- 133. Kuhlhorn F., Rath M., Schmoeckel K., Cziupka K., Nguyen H. H., Hildebrandt P., Hunig T., Sparwasser T., Huehn J., Potschke C., Broker B. M. (2013) Foxp3+ regulatory T cells are required for recovery from severe sepsis. PLoS One 8, e65109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tatura R., Zeschnigk M., Adamzik M., Probst-Kepper M., Buer J., Kehrmann J. (2012) Quantification of regulatory T cells in septic patients by real-time PCR-based methylation assay and flow cytometry. PLoS One 7, e49962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Carrigan S. O., Yang Y. J., Issekutz T., Forward N., Hoskin D., Johnston B., Lin T. J. (2009) Depletion of natural CD4+CD25+ T regulatory cells with anti-CD25 antibody does not change the course of Pseudomonas aeruginosa-induced acute lung infection in mice. Immunobiology 214, 211–222. [DOI] [PubMed] [Google Scholar]
- 136. Kwan W. H., van der Touw W., Paz-Artal E., Li M. O., Heeger P. S. (2013) Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J. Exp. Med. 210, 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Strainic M. G., Shevach E. M., An F., Lin F., Medof M. E. (2013) Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3(+) regulatory T cells. Nat. Immunol. 14, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Van der Touw W., Cravedi P., Kwan W. H., Paz-Artal E., Merad M., Heeger P. S. (2013) Cutting edge: receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells. J. Immunol. 190, 5921–5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ren J., Zhao Y., Yuan Y., Han G., Li W., Huang Q., Tong Z., Li J. (2012) Complement depletion deteriorates clinical outcomes of severe abdominal sepsis: a conspirator of infection and coagulopathy in crime? PLoS One 7, e47095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yuan Y., Yan D., Han G., Gu G., Ren J. (2013) Complement C3 depletion links to the expansion of regulatory T cells and compromises T-cell immunity in human abdominal sepsis: a prospective pilot study. J. Crit. Care 28, 1032–1038. [DOI] [PubMed] [Google Scholar]
- 141. Yuan Y., Ren J., Cao S., Zhang W., Li J. (2012) Exogenous C3 protein enhances the adaptive immune response to polymicrobial sepsis through down-regulation of regulatory T cells. Int. Immunopharmacol. 12, 271–277. [DOI] [PubMed] [Google Scholar]
- 142. Yuan Y., Ren J., Wu X., Cao S., Li J. (2011) Exogenous C3 postpones complement exhaustion and confers organ protection in murine sepsis. J. Surg. Res. 168, e87–e94. [DOI] [PubMed] [Google Scholar]
- 143. Inoue S., Unsinger J., Davis C. G., Muenzer J. T., Ferguson T. A., Chang K., Osborne D. F., Clark A. T., Coopersmith C. M., McDunn J. E., Hotchkiss R. S. (2010) IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J. Immunol. 184, 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Unsinger J., Burnham C. A., McDonough J., Morre M., Prakash P. S., Caldwell C. C., Dunne W. M., Jr., Hotchkiss R. S. (2012) Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J. Infect. Dis. 206, 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Unsinger J., McGlynn M., Kasten K. R., Hoekzema A. S., Watanabe E., Muenzer J. T., McDonough J. S., Tschoep J., Ferguson T. A., McDunn J. E., Morre M., Hildeman D. A., Caldwell C. C., Hotchkiss R. S. (2010) IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J. Immunol. 184, 3768–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chang K., Svabek C., Vazquez-Guillamet C., Sato B., Rasche D., Wilson S., Robbins P., Ulbrandt N., Suzich J., Green J., Patera A. C., Blair W., Krishnan S., Hotchkiss R. (2014) Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit. Care 18, R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Dirks J., Egli A., Sester U., Sester M., Hirsch H. H. (2013) Blockade of programmed death receptor-1 signaling restores expression of mostly proinflammatory cytokines in anergic cytomegalovirus-specific T cells. Transplant. Infect. Dis. 15, 79–89. [DOI] [PubMed] [Google Scholar]
- 148. Inoue S., Bo L., Bian J., Unsinger J., Chang K., Hotchkiss R. S. (2011) Dose-dependent effect of anti-CTLA-4 on survival in sepsis. Shock 36, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Venet F., Foray A. P., Villars-Mechin A., Malcus C., Poitevin-Later F., Lepape A., Monneret G. (2012) IL-7 restores lymphocyte functions in septic patients. J. Immunol. 189, 5073–5081. [DOI] [PubMed] [Google Scholar]
- 150. Dean R. M., Fry T., Mackall C., Steinberg S. M., Hakim F., Fowler D., Odom J., Foley J., Gress R., Bishop M. R. (2008) Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J. Clin. Oncol. 26, 5735–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Goodman L., Gilman A. (2011) Goodman and Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill, New York. [Google Scholar]
- 152. Brahmer J. R., Tykodi S. S., Chow L. Q., Hwu W. J., Topalian S. L., Hwu P., Drake C. G., Camacho L. H., Kauh J., Odunsi K., Pitot H. C., Hamid O., Bhatia S., Martins R., Eaton K., Chen S., Salay T. M., Alaparthy S., Grosso J. F., Korman A. J., Parker S. M., Agrawal S., Goldberg S. M., Pardoll D. M., Gupta A., Wigginton J. M. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Topalian S. L., Hodi F. S., Brahmer J. R., Gettinger S. N., Smith D. C., McDermott D. F., Powderly J. D., Carvajal R. D., Sosman J. A., Atkins M. B., Leming P. D., Spigel D. R., Antonia S. J., Horn L., Drake C. G., Pardoll D. M., Chen L., Sharfman W. H., Anders R. A., Taube J. M., McMiller T. L., Xu H., Korman A. J., Jure-Kunkel M., Agrawal S., McDonald D., Kollia G. D., Gupta A., Wigginton J. M., Sznol M. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J,. Med. 366, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Singh A., Mohan A., Dey A. B., Mitra D. K. (2013) Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon γ-producing T cells from apoptosis in patients with pulmonary tuberculosis. J. Infect. Dis. 208, 603–615. [DOI] [PubMed] [Google Scholar]
- 155. Brahmamdam P., Inoue S., Unsinger J., Chang K. C., McDunn J. E., Hotchkiss R. S. (2010) Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J. Leukoc. Biol. 88, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zhang Y., Zhou Y., Lou J., Li J., Bo L., Zhu K., Wan X., Deng X., Cai Z. (2010) PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit. Care 14, R220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Den Hollander M. W., Gietema J. A., de Jong S., Walenkamp A. M., Reyners A. K., Oldenhuis C. N., de Vries E. G. (2013) Translating TRAIL-receptor targeting agents to the clinic. Cancer Lett. 332, 194–201. [DOI] [PubMed] [Google Scholar]
- 158. Lee H. O., Herndon J. M., Barreiro R., Griffith T. S., Ferguson T. A. (2002) TRAIL: a mechanism of tumor surveillance in an immune privileged site. J. Immunol. 169, 4739–4744. [DOI] [PubMed] [Google Scholar]
- 159. Griffith T. S., Kazama H., VanOosten R. L., Earle J. K., Jr., Herndon J. M., Green D. R., Ferguson T. A. (2007) Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J. Immunol. 178, 2679–2687. [DOI] [PubMed] [Google Scholar]
- 160. Gurung P., Kucaba T. A., Schoenberger S. P., Ferguson T. A., Griffith T. S. (2010) TRAIL-expressing CD8+ T cells mediate tolerance following soluble peptide-induced peripheral T cell deletion. J. Leukoc. Biol. 88, 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Tian Y., Tao T., Zhu J., Zou Y., Wang J., Li J., Bo L., Deng X. (2013) Soluble tumor necrosis factor related apoptosis inducing ligand level as a predictor of severity of sepsis and the risk of mortality in septic patients. PLoS One 8, e82204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Quartin A. A., Schein R. M., Kett D. H., Peduzzi P. N. (1997) Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA 277, 1058–1063. [PubMed] [Google Scholar]
- 163. Wang H. E., Szychowski J. M., Griffin R., Safford M. M., Shapiro N. I., Howard G. (2014) Long-term mortality after community-acquired sepsis: a longitudinal population-based cohort study. BMJ Open 4, e004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Perl T. M., Dvorak L., Hwang T., Wenzel R. P. (1995) Long-term survival and function after suspected gram-negative sepsis. JAMA 274, 338–345. [PubMed] [Google Scholar]