Post-injury differentiation of dysfunctional CD1a+ monocytederived dendritic cells can result from elevated thrombospondin-1, triggering CD47, and increasing inhibitory SHP-1 activation and immunodepression.
Keywords: monocyte-derived DC, SHP-1 phosphatase, trauma patient
Abstract
A subset of Pts develops dysfunctional MO to inflammatory DC differentiation and immunosuppression. MDDC, a newly described DC subset, is pivotal in initiating antibacterial responses. Endogenous proteins are known to alter MO to MDDC differentiation. In particular, trauma-elevated TSP-1, a protein that is known to affect MO functions, could trigger MDDC differentiation defects. We hypothesized that TSP-1-deranged differentiation of inflammatory CD1a+MDDC would negatively alter activation of immune functions, thereby increasing the risk of postinjury infections. Post-trauma increased TSP-1 levels in patients' plasma and MO correlated with two distinct MDDC differentiation dysfunctions: the previously described decreased CD1a+DC yields but also, development of an immunoincompetent CD1a+MDDC. The Pts' development of Dysf DC correlated to increased infectious complications. TSP-1 triggered its inhibitory receptor, CD47, activating an inhibitory phosphatase, SHP-1. Increased pSHP-1, decreased antigen processing, and depressed T cell stimulation characterized Pt Dysf DC. TSP-1 mimics added during Cnt MDDC differentiation depressed CD1a+DC yields but more importantly, also induced defective CD1a+MDDC, reproducing Pts' MDDC differentiation dysfunctions. CD47 triggering during Cnt MDDC differentiation increased SHP-1 activation, inhibiting IL-4-induced STAT-6 activation (critical for CD1a+MDDC differentiation). SHP-1 inhibition during MDDC differentiation in the presence of TSP-1 mimics restored pSTAT-6 levels and CD1a+MDDC immunogenicity. Thus, postinjury-elevated TSP-1 can decrease CD1a+DC yields but more critically, also induces SHP-1 hyperactivity, deviating MDDC differentiation to defective CD1a+ inflammatory MDDCs by inhibiting STAT-6.
Introduction
The ability of DCs to process and present antigen, to stimulate or suppress specific T cell response, to serve as a link between innate and adaptive components of immune systems, and to maintain immune homeostasis makes them pivotal in induction and modulation of immune responses [1–3]. These specialized APCs are relatively short-lived and are continuously replenished from blood and tissue DC precursors or from circulating MO under inflammatory or infectious conditions [1, 4, 5]. The MDDCs are differentiated from circulating human peripheral blood MO in response to specific inflammatory cytokine milieu and/or pathogenic challenges [3, 6, 7]. The in vitro-generated CD1a+MDDC is now considered the closest parallel of the in vivo inflammatory CD1a+MDDC [3, 6]. These inflammatory CD1a+MDDCs are described as a crucial source of APCs that can be readily mobilized to initiate a pathogen-specific immune response, thus playing a critical role during acute inflammation and infection [1, 3, 6–10]. Consequently, MDDC differentiation defects can result in severe immunosuppression and increased infection risk. Postinjury-altered MO activation, decreased differentiated DC numbers, and altered DC function in Pts and septic patients have been reported [11–15]. We previously showed diminished MO to CD1a+MDDC differentiation numbers in a cohort of Pts, resulting in a total APC population with depressed T cell-stimulatory capacity as a result of decreased MDDC numbers [11]. Here, we investigated further whether the few patient CD1a+MDDCs induced may themselves be dysfunctional. As the CD1a-expressing DC was demonstrated as the T cell-stimulatory and IL-12-producing immunogenic MDDC subset in the in vitro differentiation cultures, Dysf DC differentiation would be an even more serious threat to bacterial resistance [3, 6, 16]. Multiple mechanisms can be operative to depress postinjury MDDC differentiation and function. One likely mechanism is the increased release of those endogenous proteins from damaged tissue with direct effects on MO [12, 13, 17]. In particular, increased levels of an endogenous mediator, TSP-1, have been reported postinjury [18–20]. Postinjury-elevated TSP-1 levels have been linked to delayed wound healing and prolonged inflammation, as well as to increased sepsis-related mortality in murine models [21–23]. TSP-1 is a large glycoprotein that can bind and trigger multiple membrane receptors, including MO-expressed CD36 and CD47, in a species-specific manner [24, 25]. Recent reports demonstrate that TSP-1 binds to CD47 with higher affinity than to CD36 [24–26]. CD47 triggering by TSP-1 and subsequent activation of G-coupled proteins can negatively regulate APC function by inhibiting IL-12 production, decreasing costimulatory receptor expression, and diminishing phagocytic capability [25, 27–29]. The signaling mechanisms behind this inhibitory TSP-1-triggered CD47 action have not been totally explored, particularly in humans [25]. One known inhibitory effect of triggering MO CD47 by interaction with some of its ligands/bidirectional receptors, such as SIRP-1α, is activation of an inhibitory phosphatase, [29–31]. SHP-1 negatively modulates a variety of lymphokine-induced and antigen-binding initiated signaling pathways by dephosphorylating necessary signaling components, such as STATs; transcription factors, such as NF-κB and AP-1; as well as components of the MAPK pathway [32–35]. The capacity of activated SHP-1 to target numerous crucial signaling components in a variety of cell-signaling pathways makes it a pivotal regulator of immune signaling. SHP-1 has been demonstrated as a critical regulator of murine DC function but has not been assessed for any role in human MDDC differentiation [35]. In addition, although CD47-triggered inhibition by TSP-1 may proceed through a similar signaling pathway, as does triggering SIRP-1α, this is not established [25]. In this report, we assessed whether (1) post-trauma Dysf DC differentiation significantly increased infectious complications, (2) postinjury-increased TSP-1 levels positively correlate with depressed MDDC differentiation, (3) the few patients' CD1a+MDDCs generated were capable of raising an immunogenic response, and (4) elevated TSP-1 triggering of increased SHP-1 activity in patients' MO contributes to this MDDC dysfunction.
Finally, to evaluate the mechanism of TSP-1-induced MDDC differentiation defects, we differentiated in vitro the MO of healthy donors to MDDCs in the absence or presence of the αCD47 agonist antibody or the peptide CD47-binding domain of TSP-1 to mimic TSP-1 triggering of CD47 [25]. We then assessed whether SHP-1 activation of these differentiating cells decreased the STAT-6 activation and nuclear translocation considered crucial for IL-4-driven CD1a+MDDC differentiation and function [2, 36]. Additionally, CD47-induced, differentiating MDDCs were treated with a pharmacological SHP-1 inhibitor, SSG, to determine whether SHP-1 inhibition would reverse MDDC differentiation defects caused by TSP-1 triggering of CD47 [37].
MATERIALS AND METHODS
Study population
A total of 98 adult burn and mechanical Pts [74 men and 24 women; average age, 41.8 ± 17.3 (mean±sd) with APACHE scores ≥21], admitted to the Trauma Intensive Care Unit of University of Rochester Medical Center, were enrolled in this study. In addition to an APACHE score ≥21, enrolled thermal Pts had a total burned area ≥30% (or 15% after adjustment for age >55). Patients that had severe cerebral damage, were pregnant, had an HIV-positive diagnosis, or had a history of immunosuppressive medications were excluded from the study. After a 24-h IRB-mandated delay postadmission, the included patients' samples were collected approximately every 4 days postinjury, twice/week, until Intensive Care Unit release/demise or onset of infectious complications. All patient samples were assayed in parallel with age-, sex-, and ethnicity-matched Cnt samples. Representative healthy volunteers from our institution served as Cnt. The IRB of University of Rochester approved the study. Informed consents were obtained from all subjects enrolled in the study. Only deidentified patients' information has been used in data analysis.
During data analysis, patients were divided into two groups: (1) patients whose isolated MO to MDDC differentiation was dysfunctional (i.e., MO to MDDC differentiation <40% of parallel processed Cnt MO) and (2) patients with MDDC differentiation-competent MO (>60% of parallel-processed Cnt MDDC differentiation). Eighteen out of 98 patients studied were designated Pt Dysf DC for at least two time-points. Fifty-four patients were designated differentiation Dysf DC and had differentiation-Pt Comp DC at all assay points. Detailed demographic and clinical information (age, sex, injury type, occurrences of infection, initial ISS, maximum MODS experienced) for Pt Comp DC and Pt Dysf is listed in Supplemental Table 1. We found that dividing patients into this “>60% vs. <40%” group allowed a comparison between MO of patients with a definite MDDC differentiation defect and patients' MO that were differentiation Comp DC. During this study period, 26 patients experienced MO to CD1a+MDDC differentiation in the >40% to <60% of Cnt range, at least, at one postinjury time-point. These patients' transient 40–60% CD1a+MDDC differentiation depression could include normal individual variances, as well as indicating a differentiation defect. Patients' CD1a+MDDCs from those cultures with a 40–60% differentiation range could not be homogenously fit into either competent or dysfunctional (or even a separate intermediate) group. Consequently, these patients' data were excluded to allow a definitive comparison between patients with significant Dysf DC and Pt with Comp DC differentiation. However, in correlation studies between two parameters (see Figs. 1C and D and 2C and D), all patient assays were included to avoid introducing false-significance values arising from comparing two already separated groups. Reduced DC numbers in the Pt Dysf DC group only allowed a limited number of the assays to be performed at a time. Patients were tested for APC function, SHP-1 levels, and other parameters, as CD1a+MDDC numbers permitted. Experimental n numbers represent the numbers of patients tested in that type of assay.
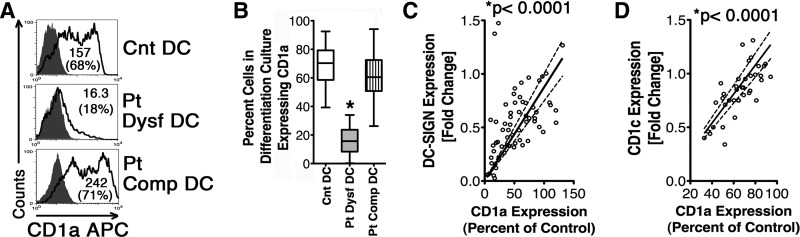
Figure 1. Some severely injured Pts experience MO to MDDC differentiation defects characterized by diminished CD1a, CD1c, and DC-SIGN expression.
Pt and matched Cnt MO were differentiated in vitro to MDDC. (A) Histograms show CD1a expression on harvested cells from MDDC differentiation cultures assessed by flow cytometry. Numbers in parentheses denote percentage of CD1a+ cells. Open numbers indicate net MFI. Patients' MDDC differentiation was considered: (1) dysfunctional (Pt Dysf DC) if yield of CD1a+MDDCs were ≤40% of parallel-processed Cnt MDDC differentiation and (2) competent (Pt Comp DC) when CD1a+MDDC differentiation was ≥60% of Cnt. (B) Box and whisker plot showing percentage of Pts' CD1a+DC in differentiation cultures (boxes representing interquartile range and whiskers showing the maximum and minimum values; horizontal lines inside of the boxes showing the median). Patients were grouped as above. *P < 0.0001 by Mann-Whitney U test, n =18 Pt Dysf DC, 54 Pt Comp DC (collected ∼1-week postinjury to match Pt Dysf DC), and 68 parallel-processed Cnt. (C) Six-day-differentiated patients' MDDCs were simultaneously checked for CD1a and DC-SIGN expression. The graph illustrates a positive correlation between CD1a and DC-SIGN expression. Patients' DC CD1a expression is percent of Cnt MFI. Fold change in DC-SIGN expression was determined by MFI in patients' cells harvested from differentiation cultures/MFI in parallel-processed, similarly differentiated Cnt cells). *P < 0.0001 by Spearman's r test, n = 62. (D) Graph of positive correlation between CD1a and CD1c expression by patients' MDDCs. CD1a expression is percent of Cnt MFI, and CD1c fold change was determined as above. *P < 0.0001 by Spearman's r test, n = 40.
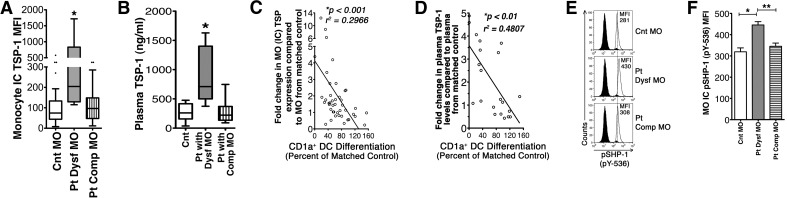
Figure 2. Postinjury MO to MDDC differentiation dysfunction is positively correlated with increased MO TSP production and elevated plasma TSP-1 levels.
(A) Intracellular (IC) TSP levels were assessed in seven patients' dysfunctional MO (Pt Dysf MO) 25 patients' differentiation-competent MO (Pt Comp MO), and 32 matched Cnt MO by flow cytometry (boxes representing interquartile range, whiskers showing the maximum and minimum values, dots showing outliers, and horizontal lines inside of the boxes showing the median). *P < 0.01 by Mann-Whitney U test. (B) Patients and matched Cnt plasma samples obtained on the same assay date as MO were assessed for TSP-1 by ELISA. *P < 0.01 by Mann-Whitney U test; Comp MO n = plasma samples from 12 patients who never had Dysf MO, nine patients experiencing MO dysfunction, and 21 matched Cnt samples. (C) Linear regression analysis correlating patients' increased MO intracellular TSP levels and the capacity of MO to differentiate in vitro into CD1a+MDDCs (percentage of CD1a+ cells) in differentiation culture (expressed as percent of Cnt differentiation). *P < 0.001 by Spearman's r test, n = 43. (D) Linear regression analysis correlating patients' increased plasma TSP-1 levels and MO with CD1a+MDDC differentiation capacity, percent CD1a+MDDCs in differentiation culture as percent of Cnt differentiation. *P < 0.01 by Spearman's r test, n = 21. (E) Histograms showing intracellular pSHP-1 levels in patients' and matched Cnt' isolated MO. (F) Graphs showing elevated intracellular pSHP-1 levels in Pt Dysf MO. Data are represented as mean ± sem. *P < 0.05 and **P < 0.01 by two-tailed t test, n = 6 Cnt MO, 3 Pt Dysf MO, and 4 Pt Comp MO.
Reagents
Leukocyte culture media was RPMI (Invitrogen-Gibco, Grand Island, NY, USA)-supplemented with 10% v/v FBS (Hyclone, Logan, UT, USA), HEPES (10 mM; CellGro, Manassas, VA, USA), penicillin G (50 IU/ml; CellGro), gentamycin (50 μg/ml; CellGro), streptomycin (50 μg/ml; CellGro), fungizone (2.5 μg/ml; CellGro), L-glutamine (4 mM; Invitrogen-Gibco), MEM nonessential amino acids (1% v/v; CellGro), and 0.05 mM β-mercaptoethanol (Sigma, St. Louis, MO, USA). Polymyxin B (100 IU/ml; Sigma) was added to all experiments. The 4N1K and 4N1GG peptides were from American Peptide (Sunnyvale, CA, USA). The αCD47 (no azide low endotoxin; clone B6H12) and its isotype-matched Cnt antibody were from BD Biosciences (San Jose, CA, USA).
Isolation of MO and T cells from whole blood
PBMCs were isolated by density gradient centrifugation over Ficoll-Hypaque. T cells were isolated further by sheep red blood cell rosetting, as described previously [18]. Cnt T cells were kept frozen to be used later in MLR with allogenic Cnt or patient DCs. MO were isolated from the nonrosette fraction of the PBMC by magnetic bead negative selection using αCD3-, αCD19-, αCD56-, and αCD66b-coated beads (Dynabeads; Invitrogen-Gibco). Isolated MO populations had >95% CD14+ cells, as assessed by flow cytometry.
In vitro differentiation of DCs
Freshly isolated CD14+, CD36+ MO were differentiated to MDDCs by culturing with 1200 IU/ml rhIL-4 (Humanzyme, Chicago, IL, USA) and 1500 IU/ml rhGM-CSF (PeproTech, Rocky Hill, NJ, USA) for 6 days in six-well tissue-culture plates. On Day 3 of differentiation, 1.5 ml culture median was removed from each well and replenished with fresh medium containing IL-4 and GM-CSF.
Assay of antigen processing using DQ-OVA
DC antigen-processing capacity was assessed by measuring green fluorescent-cleaved DQ-OVA fragments in the DC intracellular antigen-processing organelle by flow cytometry, as described previously [38].
MLR
T cells (1.5×105) in 200 μl media were cultured for 6 days in 96-well plates with DCs at a ratio of 20:1 (T cell:DC), pulsed with [3H] thymidine (1 μCi/well; PerkinElmer, Wellesley, MA, USA), and then harvested after 18 h. T cell proliferation was expressed as CPM in triplicate cultures. Culture supernatants were collected before tritiated thymidine addition for cytokine assays.
Flow cytometry
Cells were stained for membrane or intracellular receptors/proteins, as described previously [18]. Cells were analyzed in multicolor flow cytometry using a Cyan ADP flow cytometer (Beckman Coulter, Brea, CA, USA). CD1a positivity and CD14 negativity are used as MDDC markers. With the use of a viability detector nuclear dye, Aqua (Molecular Probes, Invitrogen-Gibco), dead cells were excluded from analysis. Intracellular TSP levels in freshly isolated MO were detected with flow cytometry using an αTSP-PE antibody. CD36 and CD14 were used as MO markers. To detect intracellular pSHP-1, cells were fixed with 1× Cytofix buffer (BD Biosciences) for 10 min at 37°C, washed, and then permeabilized with chilled Perm Buffer III (BD Phosflow; BD Biosciences) for 30 min over ice. After washing twice, cells were stained with rabbit α-pSHP-1 (pY536) antibody. Rabbit polyclonal Ig was used to detect nonspecific binding. The α-rabbit Ig Pacific Blue secondary antibody was used to detect α-pSHP-1 antibody binding.
Assessments of TSP-1 levels in patients' plasma
The plasma TSP-1 levels were measured using Quantikine human TSP-1 ELISA kits (R&D Systems, Minneapolis, MN, USA), following the manufacturer's instructions.
CD47 triggering using TSP-1 mimics during Cnt MO to DC differentiation
To assess the effect of TSP-1 binding to CD47R during DC differentiation, MO, from healthy subjects, were differentiated to DC using IL-4 + GM-CSF in the presence of 50 μg/ml 4N1K peptide (the CD47-binding domain of TSP-1; sequence KRFYVVMWKK) or 10 μg/ml αCD47 antibody. Use of whole TSP-1 protein to treat MO was avoided (1) because of the difficulty in keeping this large trimeric glycoprotein in its native structure throughout the 6-day culture period and (2) to avert TSP-1 binding to its multiple other receptors on MO with consequent positive and negative signaling (e.g., CD36). DCs differentiated in parallel in the presence of a Cnt, nonspecific peptide, 4NGG (KRFYGGMWKK, 50 μg/ml), or isotype-matched Cnt antibody (10 μg/ml) served as Cnt. Cells were differentiated for 6 days following the same MDDC differentiation protocol stated above. As the agonist antibody has a longer shelf-life, is readily available from commercial sources, and gives the same results as the peptide, we used the agonist antibody in the majority of our experiments. In some experiments, a pharmacological SHP-1 inhibitor, SSG (7.5 μg/ml; Calbiochem, San Diego, CA, USA), was used to inhibit CD47-triggered, increased pSHP-1.
Assessment of STAT-6 activation levels
DC pSTAT-6 (green fluorescence) and subcellular localization [against nuclear stain DRAQ5 (red fluorescence)] in CD1a+ cells were measured by ImageStream X image cytometry (Amnis, Seattle, WA, USA). Cells were stained and processed following the same procedure, as described previously [18]. STAT-6 nuclear localization was determined by fluorescence overlay with DRAQ5. In some experiments, pSTAT-6, using the same antibody, has been measured in a CyAn flow cytometer (Beckman Coulter).
Statistical analysis
The statistical analyses were done using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). Mann-Whitney U test, Spearman's r test, t test, or Wilcoxon matched-pairs test (as appropriate; indicated in the figure legend) was used to determine significant variance between each of the parameters. Where nonparametric statistical analyses were used, data are presented as box and whisker plots to show the median (horizontal bar inside of the box), the interquartile 95% confidence range (box), the minimum and maximum (whiskers), and the outliers (dots). Data analyzed using parametric statistics are shown as mean ± sem. Data were flagged significant when P < 0.05.
RESULTS
Pts with dysfunctional MO to CD1a+MDDC differentiation have increased infectious complications
To delineate which patient had MDDC differentiation dysfunction, patients' isolated MO were differentiated in vitro to MDDCs, parallel to similarly isolated and treated MO from age-, sex-, and ethnicity-matched, healthy donors. CD1a positivity has been chosen as the primary marker for MDDC differentiation, as these CD1a+ cells are the closest parallel to in vivo-induced inflammatory CD1a+MDDCs [3]. Patients' isolated MO showed a varied degree of differentiation potential. Patients' MO to MDDC differentiation was considered dysfunctional (Pt Dysf DC) if yields of CD1a+MDDCs were <40% of parallel-processed Cnt MDDC differentiation. Patients' MDDC differentiation was considered competent when CD1a+MDDC differentiation was ≥60% of Cnt (Pt Comp DC; Fig. 1A and B). During this study period, 18 patients experienced this defined MDDC differentiation dysfunction, whereas 54 patients had Comp MO differentiation at all assays. We first examined whether decreased yields of CD1a+ cells in Pt dysfunctional differentiation culture truly represent decreased MDDC differentiation and not selective down-regulation of CD1a expression. We assessed two other MDDC membrane proteins considered as human in vitro-differentiated myeloid MDDC markers: DC-SIGN (CD209) and CD1c (BDCA1) [36]. Although MO are known to express the C-type lectin receptor, DC-SIGN, after prolonged IL-4 exposure, MDDCs express this membrane-adhesion molecule in much higher copy numbers, and decreased DC-SIGN expression has been linked with depressed DC differentiation [38, 39]. CD1c (BDCA1) has been demonstrated as a membrane receptor expressed by in vitro- and in vivo-differentiated MDDCs and is considered another marker for T cell-stimulating inflammatory MDDCs [3, 6, 40, 41]. Pt Dysf DC also had decreased CD1c and DC-SIGN compared with Pt Comp DC (in Pt Dysf DC, the MFI for CD1a, CD1c, and DC-SIGN was 21.92%, 45%, and 33.2% of parallel-processed Cnt MDDCs, respectively, whereas in Pt Comp DC differentiation, the same respective values were 88.15%, 107%, and 91.8%). As expected, altered expression of DC-SIGN and CD1c positively correlated with CD1a expression in patients' differentiating MDDCs (Fig. 1C and D). To examine whether MDDC differentiation dysfunction is linked to poor prognosis, we compared occurrences of infectious complication and maximum MODS suffered by these two patient groups (Table 1). We found that the patients with a Dysf DC group had significantly increased the rate of infection occurrence and later experienced higher degrees of organ failure rates. No significant difference in age or injury severity at admission was detected between the two groups (Supplemental Table 1).
Table 1. Patients Who Experienced Development of Dysfunctional MO Had Higher Occurrences of Infectious Complications.
| Group | Number of patients who acquired infection | Number of patients who did not acquire infection |
|---|---|---|
| Patients detected with MO to DC differentiation defect: total, 18; female, 3; male, 15; average age: 40.8 ± 8; average ISS at admission: 25.9 ± 0.8; maximum MODS during study period: 9.6 ± 0.7a | Total: 11;b pneumonia (positive sputum culture):, 7 pneumonia leading to sepsis: 4; bacteremia (positive blood culture): 3; bacteremia leading to sepsis, 1; both positive sputum culture and positive blood culture leading to sepsis, 1; percentage of infected patients who developed sepsis: 54.6% | 7 |
| Patients never detected with MO to DC differentiation defect: total, 54; female, 15; male, 39; average age: 38.6 ± 2.3; average ISS at admission: 26.2 ± 0.7; maximum MODS during study period: 7.5 ± 0.3 | Total: 15; pneumonia (positive sputum culture): 13; pneumonia leading to sepsis: 4; bacteremia (positive blood culture): 2; bacteremia leading to sepsis: 1; both positive sputum culture and positive blood culture leading to sepsis: 0; percentage of infected patients who developed sepsis: 33.3% | 39 |
P = 0.0041 by t test compared with average maximum MODS suffered by patients with differentiation-competent MDDCs.
P = 0.0215 by two-tailed Fishers exact test and 0.0108 by χ2 test compared with infection-occurrence rate of patients with differentiation-competent MO. Averages of age, ISS at admission, and maximum MODS suffered during the study period are presented as mean ± sem.
Concomitantly increased intracellular MO and plasma TSP-1 levels paralleled post-trauma Dysf DC differentiation and elevated MO SHP-1 levels
Exposure to altered endogenous mediator levels can affect MO differentiation to DCs or to macrophage [12, 13, 17]. Increased TSP-1 levels at early time-points postinjury have been described by us [18] and other researchers [25]. We found that patients' differentiation dysfunctional MO had significantly increased intracellular TSP-1 levels compared with Cnt and with Pts' differentiation-competent MO (Fig. 2A). These same patients also had significantly elevated plasma TSP-1 concentrations (Fig. 2B). MO and plasma TSP-1 elevation negatively correlated with the ability of patients' isolated MO to differentiate CD1a+MDDCs in vitro (Fig. 2C and D). In addition, increased TSP-1 levels correlated to increased infection risk. Patients who developed infectious complications experienced plasma TSP-1 levels increased to 2.97 ± 0.46-fold (mean±sem) over Cnt levels, whereas patients with no infectious complications had a mean of 1.01 ± 0.20-fold increase over Cnt (P=0.02).
The inhibitory receptor for TSP-1, CD47, is constitutively expressed on MO, and the triggering of CD47 by some ligands can lead to activation of the inhibitory phosphatase, SHP-1 [29]. SHP-1 is a known regulator of DC function [35]. We therefore assessed whether isolated MO from patients experiencing elevated intracellular TSP-1 and plasma TSP-1 levels had concomitantly increased SHP-1 activation. Isolated MO from patients with elevated TSP-1 levels had increased SHP-1 activation, as determined by assessing intracellular pSHP-1 (pY-536) levels (Fig. 2E and F).
The small number of CD1a+MDDCs generated from differentiation of patients' dysfunctional MO has a decreased immunogenic potential
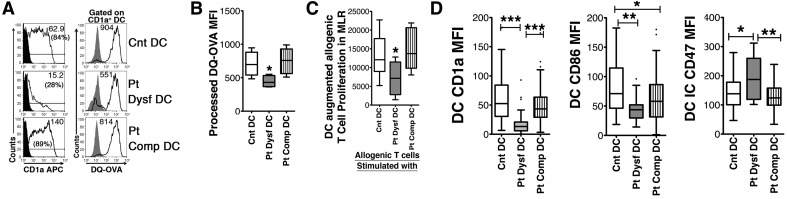
We [11] and others [15] previously had shown diminished CD1a+MDDC differentiation in patients. However, as even a few immunocompetent CD1a+MDDCs are capable of triggering T cells, the diminished CD1a+ cell numbers could still be enough to trigger T cell responses [3, 6, 7]. Consequently, we questioned whether the immunosuppression seen in the patients with reduced CD1a+MDDC numbers resulted more from differentiation of a defective CD1a+MDDC rather than just from reduced CD1a+MDDC numbers. In this set of experiments, we assessed the few CD1a+MDDCs generated from patients' differential dysfunctional MO for their ability to process antigen and stimulate T cells. We gated only on CD1a+MDDCs and assessed their ability to process antigen. Those CD1a+MDDCs differentiated from patients' dysfunctional MO (Pt Dysf DC) showed decreased antigen-processing capacity, as determined by their inability to process nonfluorescent DQ-OVA into cleaved green fluorescent peptide (Fig. 3A and B). The MDDC produced from patients' differential dysfunctional MO cultures also showed significantly decreased, allogenic T cell-stimulatory capacity in mixed leukocyte cultures compared with parallel-processed MDDC differentiated from Cnt MO obtained from healthy subjects (Cnt DC) or MDDCs induced from Pt Comp DC differentiation (Fig. 3C). The CD1a+MDDCs differentiated from patients' dysfunctional MO cultures also had decreased CD86 costimulator expression (Fig. 3D). In addition, the few CD1a+MDDCs from dysfunctional differentiation cultures had increased expression of the inhibitory receptor for TSP-1 (CD47), augmenting TSP-1-triggering potential (Fig. 3D). Consequently, those patients with Dysf DC would have a critically reduced potential as pivotal initiators of immune responses during pathogenic challenges.
Figure 3. The few CD1a+MDDCs differentiated from patients' differentiation dysfunctional MO had decreased antigen processing, depressed T cell-stimulatory capability, decreased costimulatory CD86R, and increased CD47-inhibitory receptor expression.
(A) Harvested patients' and matched Cnt CD1a+MDDCs were assessed for antigen-processing capacity by measuring processing of DQ-OVA gating on CD1a+ cells. Numbers in parentheses denote percentage of CD1a+ cells, and numbers without parentheses indicate net MFI. (B) Graphical representation of DQ-OVA processing assays. *P < 0.05 by Mann-Whitney U test, n = 6 Pt Dysf DC, 7 Pt Comp DC, and 11 Cnt DC. (C) Isolated Cnt MDDCs, Pt Dysf MDDCs, and Pt Comp MDDCs were cocultured with allogenic T cells to assay MDDC-augmented T cell proliferation. *P < 0.05 by t test, n = 5 Pt Dysf DC, 6 Pt Comp DC, and 8 Cnt DC. (D) MDDC expression of CD1a and costimulatory CD86R and inhibitory CD47R expression in a CD1a+-gated population were assessed by flow cytometry. Comp DC differentiation and Dysf DC differentiation were grouped as in Fig. 1. All receptor expressions are presented as median of net MFI, as determined by subtracting isotype Cnt MFI from specific MFI. ***P < 0.0001, **P < 0.01, and *P < 0.05 by Mann-Whitney U test, n = 18 Pt Dysf DC, 54 Pt Comp DC (collected ∼1 week postinjury to match Pt Dysf DC), and 68 parallel-processed Cnt. All of the graphs are presented as box and whisker plots (boxes representing interquartile range, whiskers showing the maximum and minimum values, dots showing outliers, and horizontal lines inside of the boxes showing the median).
Does CD47 triggering in differentiating Cnt MO diminish their MDDC differentiation potential and alter MDDC function similarly to the differentiation dysfunctions of patients' MO?
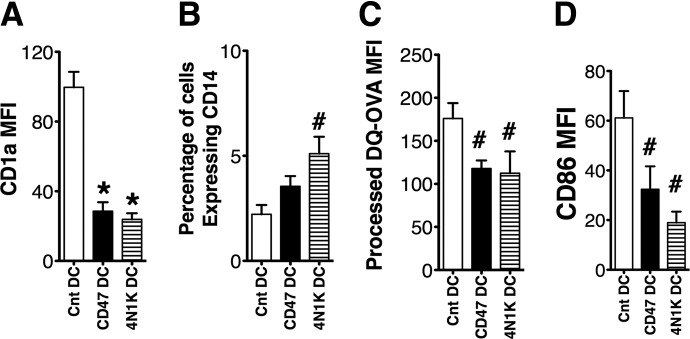
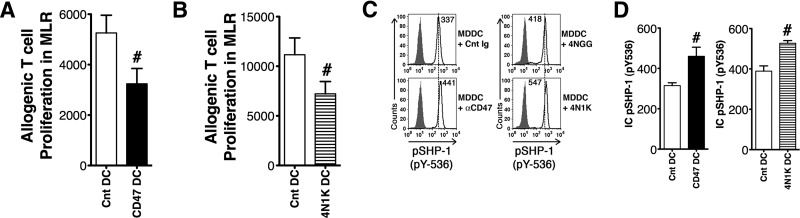
To determine whether the patients' increased TSP-1 levels could be contributing to their depressed MDDC differentiation, we assessed the effect of CD47 triggering during MDDC differentiation of Cnt MO isolated from healthy donors. Cnt CD47Rs of MO were triggered by an agonist αCD47 antibody, targeted to a TSP-1-binding domain or the 4N1K peptide (sequence KRFYVVMWKK) that simulates the CD47-binding domain of TSP-1 [25]. The 4N1K peptide and the αCD47 agonist antibody (clone B6H12) have been used to mimic TSP-1-inhibitory triggering of CD47 and are considered complementary validation for TSP-1 activation of CD47 [24, 25, 28]. The CD1a+MDDC yields were decreased significantly when MO were differentiated in the presence of 4N1K peptide (4N1K DC) or αCD47 antibody (CD47DC) compared with differentiation cultures where either a nonspecific peptide 4NGG or an isotype-matched Cnt Ig was added (Cnt DC; Fig. 4A). This decreased differentiation of CD1a+ cells is similar to that of Pt Dysf DC differentiation. The 2% increase in CD14+ macrophage in the total differentiated population was insufficient to explain the 70% diminished MDDC differentiation and ∼50% reduced T cell-stimulatory function of this TSP-1 mimic-pretreated population (Fig. 4B). Other prominent defects following TSP-1 mimic treatment were decreased antigen processing, depressed costimulatory receptor expression, and decreased T cell-stimulatory capacity, similar to the defects we see in Pt Dysf DC. The small numbers of differentiated CD1a+ CD47 DC and 4N1K DC had decreased antigen-processing capacity, as assessed by DQ-OVA processing (Fig. 4C). The actual CD1a+DC produced during TSP-1 mimic treatment of differentiating MO also expressed significantly depressed CD86 (Fig. 4D). Similar to previous reports, CD47 triggering during MDDC differentiation by αCD47 antibody or 4N1K peptide depressed T cell-stimulatory capacity in allogenic MLR cultures (Fig. 5A). Again, similarly to patients' defective CD1a+MDDC differentiation, TSP-1 mimic treatment increased pSHP-1 in the CD1a+MDDCs (Fig. 5B and C). The pSHP-1 levels remained elevated even after 6 days of differentiation culture. Treatment with the 4NIK peptide or αCD47 yielded similar results, validating TSP-1 triggering of CD47 as the mediator of this inhibition [25].
Figure 4. CD47 triggering in differentiating Cnt MO results in decreased CD1a+MDDC differentiation and development of a CD1a+DC population with down-regulated costimulatory receptors and decreased antigen-processing capability.
MO from healthy volunteers were differentiated to MDDC in the presence of CD47 triggering by αCD47 antibody (CD47 DC) or 4N1K peptide (4N1K DC). MDDCs similarly differentiated in the presence of an isotype Cnt antibody, or a nonspecific peptide served as Cnt (Cnt DC). Six day-differentiated MDDCs were assessed for CD1a (A) and CD14 (B) expression by flow cytometry. (C) CD1a+MDDC antigen processing capacity was assessed by gating on CD1a+MDDCs and measuring DQ-OVA processing with flow cytometry. (D) CD1a+MDDC expression of CD86 was assessed by flow cytometry. For all graphs, #P < 0.05 and *P < 0.01 by Wilcoxon matched pairs test, n = 7 Cnt DC, 4 CD47 DC, and 3 4N1K DC. Data are represented as mean ± sem.
Figure 5. CD47 triggering of differentiating MDDCs reduces T cell-stimulatory capacity and increased CD1a+MDDC-activated pSHP-1 levels.
Cnt MO were differentiated to MDDCs in the presence of αCD47 antibody (CD47 DC) or 4N1K peptide (4N1K DC). Parallel-differentiated MDDCs, in the presence of an isotype-matched, nonspecific antibody or a scramble peptide, served as Cnt (Cnt DC). (A and B) Allogenic T cell-stimulatory capacity of CD47 DC (A) and 4N1K DC (B) in MLRs was determined by titrated thymidine incorporation assay. #P < 0.05 by Wilcoxon matched-pairs test, n = 4 CD47 DC and matched Cnt, 3 4N1K DC with paired Cnt. Data are represented as mean ± sem. (C) Intracellular pSHP-1 (pY-536) levels of 6-day-differentiated MDDCs were determined by flow cytometry in the CD1a+-gated population. Numbers in the histogram indicate net MFI, as determined by subtracting isotype Cnt MFI from MFI of α-pSHP-1-specific fluorescence. CD47 triggering by αCD47 (left) and 4N1K peptide (right) increased pSHP-1. (D) Graphs representing mean ± sem showing increased intracellular pSHP-1 after in CD47 DC and 4N1K DC compared with their matched Cnt. #P < 0.05 by Wilcoxon matched-pairs test, n = 3 CD47 DC, 3 4N1K DC, and their paired Cnt.
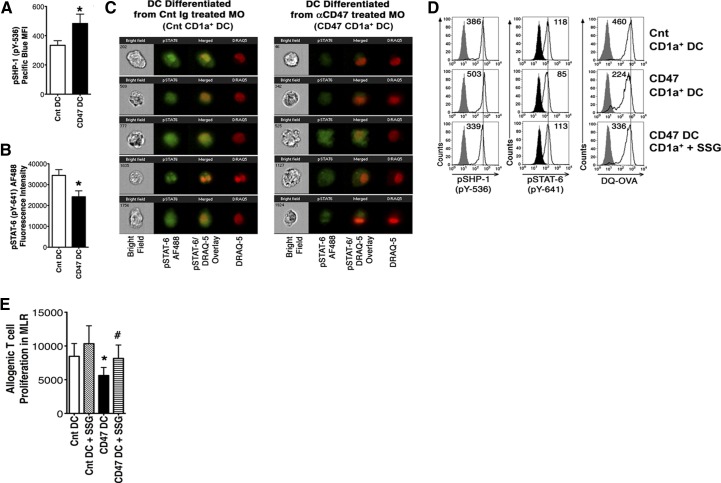
Elevated SHP-1 activation inhibits IL-4-induced pSTAT-6 and nuclear localization in differentiating DCs
To explore further a possible mechanism for TSP-1 mimic-triggered, SHP-1-mediated inhibition of MDDC differentiation, we assessed its effects on one of the crucial MO to MDDC differentiation signals. IL-4 is the critical cytokine for in vitro differentiation of MDDC through its induction of STAT-6 activation, whereas human MO differentiated in GM-CSF alone become macrophages rather than MDDCs [42–45]. STAT-6 is considered to be the primary transcription initiator of genes activated by IL-4 [40, 45, 46]. SHP-1 transfection has been shown to reduce IL-4-triggered STAT-6 activation and STAT-6-initiated transcription in NIH 3T3 cell lines but has not been investigated during MO to CD1a+MDDC differentiation [36]. We investigated whether CD47 mimic-triggered SHP-1 activation during MO to MDDC differentiation interferes with STAT-6 activation and nuclear localization. Cnt MO were differentiated in vitro to MDDCs with IL-4 + GM-CSF in the absence (Cnt DC) or presence of αCD47 antibody (CD47 DC). When assessed for pSTAT-6 and nuclear translocation, CD47 DC (with increased pSHP-1 levels) had significantly decreased pSTAT-6 levels (Fig. 6A and B). The major portions of pSTAT-6-specific fluorescence in untreated Cnt CD1a+MDDC overlapped the nuclear dye, DRAQ5, indicating pSTAT-6 nuclear translocation. In contrast, pSTAT-6-specific fluorescence and its nuclear translocation were diminished in CD47 DC (Fig. 6C). To validate further the inhibitory effect of CD47-triggered SHP-1 hyperactivation on MDDC differentiation and function, we included a small pharmacological SHP-1 inhibitor, SSG, in MO differentiation cultures, along with αCD47 antibody. Treatment with 7.5 μg/ml SSG prevented a CD47-triggered increase in pSHP-1 levels (CD47-treated pSHP-1 levels=mean 190±47.7% of Cnt vs. CD47+ SSG levels of 108±14.0% of Cnt). SSG treatment also reversed most of the pSTAT-6 depression in the CD1a+MDDCs, returning their activated pSTAT-6 levels to 88.5% ± 5.1 of Cnt (Fig. 6D). Importantly, CD47 DCs, differentiated in the simultaneous presence of SSG, also restored the MDDC antigen-processing capacity, as assessed by DQ-OVA processing to 96.1 ± 6.9% of Cnt versus 65.3 ± 6.7% after CD47 alone (Fig. 6D). However, we did not see a significant increase in CD1a+MDDC numbers in the SSG-treated CD47 DC group compared with the untreated group. Nevertheless, SSG treatment did increase significantly the T cell-stimulatory capacities of the few differentiated MDDCs (Fig. 6E). These data clearly demonstrate that TSP-1 triggering of MO CD47 can contribute to development of a defective CD1a+MDDC through SHP-1-mediated deactivation of STAT-6 but reduces the number of CD1a+MDDCs generated though a different mechanism. The restoration of MDDC antigen processing and reduction of SHP-1 levels still increased the T cell-stimulatory capacity of the entire population, even in the presence of decreased CD1a+MDDC numbers. Inhibiting elevated MO pSHP-1 levels reverses IL-4-induced STAT-6 inhibition and ameliorates dysfunction of TSP-1-induced differentiated CD1a+MDDCs.
Figure 6. Increased SHP-1 activation and subsequently, decreased STAT-6 recruitment parallel CD47-induced MO to DC differentiation dysfunction.
Cnt MO were differentiated to MDDCs in the presence of αCD47 (CD47 DC) antibody or a nonspecific isotype-matched antibody (Cnt DC). (A) After 6 days, the pSHP-1 (pY-536) levels of CD1a+MDDCs were assessed, *P < 0.05 by Wilcoxon matched-pairs test, n = 6, data shown as mean ± sem. (B) pSTAT-6 (pY-641) in CD1a-gated Cnt and CD47 DCs was assessed by flow cytometry. *P < 0.05 by Wilcoxon test, n = 6, graph shows mean ± sem. (C) DC intracellular pSTAT-6 levels (green fluorescence) and subcellular localization [against nuclear stain DRAQ5 (red fluorescence)] were also assessed by Amnis Image cytometry. STAT-6 nuclear localization was determined by fluorescence overlay (yellow); data are representative of six experiments. To choose images from each group, CD1a-gated single cells were first assessed for pSTAT-6 intensity. With the use of Amnis IDEAS software, CD1a+MDDCs showing pSTAT-6 intensity at the median level were selected per the software directions. Then, five randomly chosen images from each group from a single experiment were presented, as is the standard Amnis data-presentation procedure. (D) CD47 DC were differentiated in the absence or presence of a small pharmacological SHP-1 inhibitor, SSG. Intracellular pSHP-1 and pSTAT-6 levels and DQ-OVA processing were determined in gated CD1a+MDDCs by flow cytometry. MDDCs differentiated in the absence of CD47 triggering (Cnt DC) served as Cnt (top). Histograms in the middle and bottom panels represent CD47 DC and SSG-treated CD47 DCs, respectively. Numbers in the histograms are presented as net MFI. Data are representative of five experiments with similar results. (E) SHP-1 inhibition during MDDC differentiation increased the CD47-triggered ability of MDDC to stimulate allogenic T cell proliferation in MLR. Cnt DC and CD47 DC were differentiated in the presence of SHP-1 inhibitor SSG (7.5 μg/ml). Harvested cells were cultured with allogenic Cnt T cells to assess total MDDC capacity to induce MLR. *P < 0.05 between Cnt DC and CD47 DC; #P < 0.05 between CD47 DC and CD47 DC + SSG group; by t test, n = 5, values shown as mean ± sem.
DISCUSSION
Previously, we demonstrated that a subset of Pts have a low MLR-inducing MO population that is associated with decreased MDDC differentiation and low MDDC IL-12 production [11]. We [12] and others [15] had suggested that a loss of CD1a+MDDC numbers and an increase of a more macrophage-like CD1a− population might be the main cause of immunosuppression. However, diminished MDDC numbers alone seemed insufficient to explain the degree of APC-associated immunodepression seen in those patients. In this report, we reiterated that the immunosuppressed patients' MO differentiated a reduced number of CD1a+MDDCs. More importantly, we demonstrated novelly that the few CD1a+MDDCs generated were themselves dysfunctional with reduced antigen processing and depressed costimulatory (CD86) receptor expression. The inflammatory human CD1a+CD1c+ (BDCA1+) MDDC subset has been shown recently to be pivotally important in induction of T cell IL-17 production and thus, in initiating antibacterial host-defense mechanisms [3, 6, 7, 10]. We hypothesized that a major elevation in the early trauma-induced mediators in this patient population could be contributing to their MDDC differentiation defects. Severe trauma causes release of multiple endogenous mediators that can influence MO activation and alter MO differentiation capability [12, 18, 19]. We explored a known trauma-induced inhibitory endogenous mediator of DC function, TSP-1, for its effects on MDDC differentiation [20, 23, 25, 27, 28]. High levels of TSP-1 are linked to poor prognosis in several injury models, and increased mortality in septic mice is linked to a TSP-1 effect on macrophage phagocytosis [21–23]. Only a subset of Pts enrolled in our study experienced Dysf DC differentiation, and this dysfunction appeared in the early days' postinjury period. TSP-1 has been shown as elevated as early as 3 days postinjury [47]. TSP-1-mediated triggering of its inhibitory CD47R can induce overall APC defects by inhibiting phagocytic capacity, cytokine production, and T cell-stimulatory function [21–23, 25, 26]. Thus, TSP-1 seemed a strong candidate for mediating patients' MO differentiation defect. We found a significant correlation among patients' elevated plasma TSP-1, intracellular MO TSP-1 level increases, MDDC differentiation failure, and infectious complications. TSP-1 triggering of its inhibitory CD47R increases SHP-1 activation (phosphorylation) in some cell types [18, 31]. To date, an increase of SHP-1 in macrophages has only been demonstrated for SIRP-1α triggering of CD47 [29]. However, some of the signaling targets of TSP-1 binding of CD47 are shared by SIRP-1α CD47 signaling [25]. Consequently, our demonstration that CD47 triggering by a αCD47 or TSP-1 peptide activates SHP-1 is new but not totally unexpected. However, TSP-1 binds CD47 at a well-separated domain from SIRP-1α, and the CD47 antibody clone we used does not trigger SIRP-1α [25, 48]. The TSP-1–CD47 interaction triggers alterations in MAPKs and down-regulates cGMP in a pertussis toxin-sensitive manner, suggesting a G protein signaling mechanism. However, the TSP-1 triggering of CD47 inhibition in T cells is proposed also to require a modification of CD47R [48]. How this TSP-1–CD47 signaling pathway relates to activation of SHP-1 is unclear. Increased pSHP-1 occurred in our patients' differentiation dysfunctional MO populations. SHP-1 can play an important role in immune response regulation by inhibitory dephosphorylation of a variety of signaling molecules associated with immune cell-stimulatory activation [18, 32–35, 49]. A recent study described SHP-1 as an intrinsic regulator of murine DCs [35]. Preliminary experiments on patients' differentiation dysfunctional MO adding a SHP-1 inhibitor (SSG) reduced intracellular SHP-1 levels in the Pt Dysf DC and restored the total MDDC populations' T cell stimulation from 60% to 84% of SSG-treated Cnt MDDC and 88% of untreated Cnt MDDC. These preliminary data suggest that the CD1a+MDDC SHP-1 increase is a more pivotal cause of Pt MDDC immunoincompetence rather than the decrease in CD1a+MDDC numbers. The interpretation that CD47–TSP-1 triggering of SHP-1 is a major contributor to CD1a+MDDC defects is supported by our Cnt MDDC experiments.
CD47 triggering by the 4N1K peptide or αCD47 during Cnt MO → MDDC differentiation significantly reduced the ability of MO to differentiate into CD1a+ immunogenic MDDC. TSP-1 mimic triggering of Cnt MO induced elevation of intracellular-activated SHP-1 phosphatase and depression of critical STAT-6 activation [35, 36]. Thus, TSP-1 mimic triggering of MO through CD47 reproduced Pts' depressed CD1a+MDDC numbers and their generation of a defective CD1a+MDDC. We again specifically assessed the small number of CD1a+MDDCs derived from Cnt CD47-triggered MO, showing that these CD1a+DCs had decreased antigen-processing capacity and depressed costimulatory receptor expression. Inhibition of the TSP-1 mimic-triggered increases in SHP-1 activation reversed the generation of defective CD1a+MDDCs, improving the CD1a+MDDC antigen-processing capacity and CD86 expression. In contrast, CD47-triggered depression of CD1a+MDDC differentiation numbers was not reversed by the SHP-1 inhibitor. However, the T cell-stimulatory capacity of the total generated MDDC population was improved. These data imply that improving the CD1a+MDDC function was capable of restoring immunostimulatory function, even in the face of decreased CD1a+MDDC numbers. This interpretation is contrary to what we [11, 12] and others [15] suggested previously. The data also suggest that the mechanism by which TSP-1 triggering of MO CD47 diminishes the numbers of differentiating DCs is not a result of increases in pSHP-1 or of induced increases in classic CD14+ macrophage numbers. Instead, depressed CD1a+MDDC numbers may represent generation of an altered CD14low MO subset differentiation through TSP-1 triggering of other MO mediators, such as activated TGF-β [21, 25].
The generation of a Dysf DC seems the more deleterious effect of post- trauma-elevated TSP-1 triggering of MO CD47. Decreasing MO-derived CD1a+MDDC numbers may reflect the competing need for increased MO differentiation subsets involved in wound-healing or other necessary postinjury functions and therefore, may be less deleterious [22, 50, 51]. The presence of two separate MO-differentiation defects, decreasing CD1a+MDDC numbers versus generation of a Dysf DC, may account for our findings that not all patients who develop decreased MO to CD1a+MDDC differentiation numbers developed infectious complication. The development of infectious complications in patients who were never detected with DC differentiation defects also highlights the role of other immune cell alterations in post-trauma immunosuppression [18, 52]. However, the TSP-1-mediated generation of CD1a+MDDC with defective T cell stimulation capacity can lead to T cell anergy and apoptosis, which are serious immune defects [53].
Our demonstration that patients' intracellular MO expression of TSP-1 corresponds to development of defective DC differentiation and infectious complications indicates a role for TSP-1 in post-trauma pathological MO dysfunctions beyond the MDDC effects. Our own [18] and others' data [45, 47, 54] show that CD47 triggering can induce T cell apoptosis and immunosuppression indirectly and directly. These TSP-1–CD47 T cell effects also involve activation of SHP-1 but in T cells. Consequently, the immune-inhibitory effects of elevated TSP-1 levels in Pts extend well beyond their effect in inducing dysfunction of MO to CD1a+MDDC differentiation. Nevertheless, our demonstration that deviation of patients' MO differentiation to defective CD1a+MDDCs correlates to immunosuppression, and infectious complications imply that this TSP-1-mediated CD1a+MDDC defect has pathological consequences.
In summary, our data suggest that at least one of the mechanisms by which elevated postinjury TSP-1 levels cause MO to CD1a+MDDC differentiation defects is to activate SHP-1. This TSP-1–CD47-activated SHP-1 deviates MO to CD1a+MDDC differentiation, producing a Dysf DC, thereby contributing to the development of postinjury immunosuppression. The data also suggest that postinjury inhibition of SHP-1 might be a viable therapy.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by U.S. National Institutes of Health grants R01-GM065237 and R01-GM036214.
We thank Leanne Staples for excellent technical support, Joyce Krieger and Charles Graziano for helping with the manuscript preparation, and Sabrina Fisher for her role as clinical coordinator.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- α
- anti- (antibody)
- APACHE
- Acute Physiology and Chronic Health Evaluation
- BDCA1
- blood dendritic cell antigen 1
- Cnt
- control
- Comp DC
- competent MDDCs
- DC
- dendritic cell
- DC-SIGN
- dendritic cell-specific ICAM-3-grabbing nonintegrin
- DQ-OVA
- DQ ovalbumin
- Dysf DC
- dysfunctional MDDC
- IRB
- Institutional Review Board
- ISS
- injury severity score
- MDDC
- monocyte-derived CD1a+ dendritic cell
- MFI
- median fluorescence intensity
- MLR
- mixed leukocyte reaction
- MO
- monocyte
- MODS
- Marshall's multiple organ dysfunction score
- p
- phosphorylated
- Pt
- trauma patient
- pY
- phosphorylated tyrosine
- rh
- recombinant human
- SHP-1
- Src homology domain 2-containing phosphatase-1
- SIRP-1α
- signal regulatory protein 1α
- SSG
- sodium stibogluconate
- TSP-1
- thrombospondin-1
AUTHORSHIP
G.B. and S.B. performed and designed experiments and analyzed data. G.B. participated in writing the manuscript and figure design. C.L.M.-G. designed experiments, analyzed data, and participated in writing the manuscript and in data presentation. P.E.B. supervised patient sample collection and provided clinical data and patient infection designations.
DISCLOSURES
The authors declare no commercial or other conflicts of interest.
REFERENCES
- 1. Belz G. T., Nutt S. L. (2012) Transcriptional programming of the dendritic cell network. Nat. Rev. Immunol. 12, 101–113. [DOI] [PubMed] [Google Scholar]
- 2. Arima K., Watanabe N., Hanabuchi S., Chang M., Sun S. C., Liu Y. J. (2010) Distinct signal codes generate dendritic cell functional plasticity. Sci. Signal. 3, ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Segura E., Touzot M., Bohineust A., Cappuccio A., Chiocchia G., Hosmalin A., Dalod M., Soumelis V., Amigorena S. (2013) Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 38, 336–348. [DOI] [PubMed] [Google Scholar]
- 4. Van de Laar L., Coffer P. J., Woltman A. M. (2012) Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood 119, 3383–3393. [DOI] [PubMed] [Google Scholar]
- 5. Li H. S., Watowich S. S. (2012) A STATus report on DC development. J. Leukoc. Biol. 92, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Segura E., Amigorena S. (2013) Inflammatory dendritic cells in mice and humans. Trends Immunol. 34, 440–445. [DOI] [PubMed] [Google Scholar]
- 7. Walsh K. P., Mills K. H. (2013) Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 34, 521–530. [DOI] [PubMed] [Google Scholar]
- 8. Remoli M. E., Giacomini E., Petruccioli E., Gafa V., Severa M., Gagliardi M. C., Iona E., Pine R., Nisini R., Coccia E. M. (2011) Bystander inhibition of dendritic cell differentiation by Mycobacterium tuberculosis-induced IL-10. Immunol. Cell Biol. 89, 437–446. [DOI] [PubMed] [Google Scholar]
- 9. Ivanov S., Fontaine J., Paget C., Macho Fernandez E., Van Maele L., Renneson J., Maillet I., Wolf N. M., Rial A., Leger H., Ryffel B., Frisch B., Chabalgoity J. A., Sirard J. C., Benecke A., Faveeuw C., Trottein F. (2012) Key role for respiratory CD103(+) dendritic cells, IFN-γ, and IL-17 in protection against Streptococcus pneumoniae infection in response to α-galactosylceramide. J. Infect. Dis. 206, 723–734. [DOI] [PubMed] [Google Scholar]
- 10. Goldszmid R. S., Caspar P., Rivollier A., White S., Dzutsev A., Hieny S., Kelsall B., Trinchieri G., Sher A. (2012) NK cell-derived interferon-γ orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 36, 1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De A. K., Laudanski K., Miller-Graziano C. L. (2003) Failure of monocytes of trauma patients to convert to immature dendritic cells is related to preferential macrophage-colony-stimulating factor-driven macrophage differentiation. J. Immunol. 170, 6355–6362. [DOI] [PubMed] [Google Scholar]
- 12. Laudanski K., De A., Brouxhon S., Kyrkanides S., Miller-Graziano C. (2004) Abnormal PGE(2) regulation of monocyte TNF-α levels in trauma patients parallels development of a more macrophage-like phenotype. Shock 22, 204–212. [DOI] [PubMed] [Google Scholar]
- 13. Nasi A., Fekete T., Krishnamurthy A., Snowden S., Rajnavolgyi E., Catrina A. I., Wheelock C. E., Vivar N., Rethi B. (2013) Dendritic cell reprogramming by endogenously produced lactic acid. J. Immunol. 191, 3090–3099. [DOI] [PubMed] [Google Scholar]
- 14. Van den Berg L. M., de Jong M. A., Witte L., Ulrich M. M., Geijtenbeek T. B. (2011) Burn injury suppresses human dermal dendritic cell and Langerhans cell function. Cell. Immunol. 268, 29–36. [DOI] [PubMed] [Google Scholar]
- 15. Faivre V., Lukaszewicz A. C., Alves A., Charron D., Payen D., Haziot A. (2012) Human monocytes differentiate into dendritic cells subsets that induce anergic and regulatory T cells in sepsis. PLoS One 7, e47209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cernadas M., Lu J., Watts G., Brenner M. B. (2009) CD1a expression defines an interleukin-12 producing population of human dendritic cells. Clin. Exp. Immunol. 155, 523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takahara M., Kang K., Liu L., Yoshida Y., McCormick T. S., Cooper K. D. (2003) iC3b arrests monocytic cell differentiation into CD1c-expressing dendritic cell precursors: a mechanism for transiently decreased dendritic cells in vivo after human skin injury by ultraviolet B. J. Invest. Dermatol. 120, 802–809. [DOI] [PubMed] [Google Scholar]
- 18. Bandyopadhyay G., Bankey P. E., Miller-Graziano C. L. (2012) Trauma patients' elevated tumor necrosis related apoptosis inducing ligand (TRAIL) contributes to increased T cell apoptosis. Clin. Immunol. 145, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cornell T. T., Wynn J., Shanley T. P., Wheeler D. S., Wong H. R. (2010) Mechanisms and regulation of the gene-expression response to sepsis. Pediatrics 125, 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rogers N. M., Yao M., Novelli E. M., Thomson A. W., Roberts D. D., Isenberg J. S. (2012) Activated CD47 regulates multiple vascular and stress responses: implications for acute kidney injury and its management. Am. J. Physiol. Renal Physiol. 303, F1117–F1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kyriakides T. R., Maclauchlan S. (2009) The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J. Cell. Commun. Signal. 3, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agah A., Kyriakides T. R., Lawler J., Bornstein P. (2002) The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am. J. Pathol. 161, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McMaken S., Exline M. C., Mehta P., Piper M., Wang Y., Fischer S. N., Newland C. A., Schrader C. A., Balser S. R., Sarkar A., Baran C. P., Marsh C. B., Cook C. H., Phillips G. S., Ali N. A. (2011) Thrombospondin-1 contributes to mortality in murine sepsis through effects on innate immunity. PLoS One 6, e19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamauchi Y., Kuroki M., Imakiire T., Uno K., Abe H., Beppu R., Yamashita Y., Kuroki M., Shirakusa T. (2002) Opposite effects of thrombospondin-1 via CD36 and CD47 on homotypic aggregation of monocytic cells. Matrix Biol. 21, 441–448. [DOI] [PubMed] [Google Scholar]
- 25. Frazier W. A., Isenberg J. S., Kaur S., Roberts D. D. (2010, February 16) CD47. Basis sequence: mouse. UCSD Signaling Gateway Molecule Pages Database, University of California, San Diego, USA, from http://www.signaling-gateway.org/molecule/query?afcsid=A002870# [Google Scholar]
- 26. Isenberg J. S., Ridnour L. A., Dimitry J., Frazier W. A., Wink D. A., Roberts D. D. (2006) CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J. Biol. Chem. 281, 26069–26080. [DOI] [PubMed] [Google Scholar]
- 27. Johansson U., Londei M. (2004) Ligation of CD47 during monocyte differentiation into dendritic cells results in reduced capacity for interleukin-12 production. Scand. J. Immunol. 59, 50–57. [DOI] [PubMed] [Google Scholar]
- 28. Demeure C. E., Tanaka H., Mateo V., Rubio M., Delespesse G., Sarfati M. (2000) CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J. Immunol. 164, 2193–2199. [DOI] [PubMed] [Google Scholar]
- 29. Okazawa H., Motegi S., Ohyama N., Ohnishi H., Tomizawa T., Kaneko Y., Oldenborg P. A., Ishikawa O., Matozaki T. (2005) Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J. Immunol. 174, 2004–2011. [DOI] [PubMed] [Google Scholar]
- 30. Bandyopadhyay G., De A., Laudanski K., Li F., Lentz C., Bankey P., Miller-Graziano C. (2007) Negative signaling contributes to T-cell anergy in trauma patients. Crit. Care Med. 35, 794–801. [DOI] [PubMed] [Google Scholar]
- 31. Burger P., Hilarius-Stokman P., de Korte D., van den Berg T. K., van Bruggen R. (2012) CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 119, 5512–5521. [DOI] [PubMed] [Google Scholar]
- 32. Zhang J., Somani A. K., Siminovitch K. A. (2000) Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin. Immunol. 12, 361–378. [DOI] [PubMed] [Google Scholar]
- 33. Paling N. R., Welham M. J. (2002) Role of the protein tyrosine phosphatase SHP-1 (Src homology phosphatase-1) in the regulation of interleukin-3-induced survival, proliferation and signalling. Biochem. J. 368, 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pandey M. K., Sung B., Ahn K. S., Aggarwal B. B. (2009) Butein suppresses constitutive and inducible signal transducer and activator of transcription (STAT) 3 activation and STAT3-regulated gene products through the induction of a protein tyrosine phosphatase SHP-1. Mol. Pharmacol. 75, 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramachandran I. R., Song W., Lapteva N., Seethammagari M., Slawin K. M., Spencer D. M., Levitt J. M. (2011) The phosphatase SRC homology region 2 domain-containing phosphatase-1 is an intrinsic central regulator of dendritic cell function. J. Immunol. 186, 3934–3945. [DOI] [PubMed] [Google Scholar]
- 36. Haque S. J., Harbor P., Tabrizi M., Yi T., Williams B. R. (1998) Protein-tyrosine phosphatase Shp-1 is a negative regulator of IL-4- and IL-13-dependent signal transduction. J. Biol. Chem. 273, 33893–33896. [DOI] [PubMed] [Google Scholar]
- 37. Pathak M. K., Yi T. (2001) Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J. Immunol. 167, 3391–3397. [DOI] [PubMed] [Google Scholar]
- 38. Daro E., Pulendran B., Brasel K., Teepe M., Pettit D., Lynch D. H., Vremec D., Robb L., Shortman K., McKenna H. J., Maliszewski C. R., Maraskovsky E. (2000) Polyethylene glycol-modified GM-CSF expands CD11b(high)CD11c(high) but not CD11b(low)CD11c(high) murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J. Immunol. 165, 49–58. [DOI] [PubMed] [Google Scholar]
- 39. Relloso M., Puig-Kroger A., Pello O. M., Rodriguez-Fernandez J. L., de la Rosa G., Longo N., Navarro J., Munoz-Fernandez M. A., Sanchez-Mateos P., Corbi A. L. (2002) DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-β, and anti-inflammatory agents. J. Immunol. 168, 2634–2643. [DOI] [PubMed] [Google Scholar]
- 40. den Dekker E., Grefte S., Huijs T., ten Dam G. B., Versteeg E. M., van den Berk L. C., Bladergroen B. A., van Kuppevelt T. H., Figdor C. G., Torensma R. (2008) Monocyte cell surface glycosaminoglycans positively modulate IL-4-induced differentiation toward dendritic cells. J. Immunol. 180, 3680–3688. [DOI] [PubMed] [Google Scholar]
- 41. Leslie D. S., Dascher C. C., Cembrola K., Townes M. A., Hava D. L., Hugendubler L. C., Mueller E., Fox L., Roura-Mir C., Moody D. B., Vincent M. S., Gumperz J. E., Illarionov P. A., Besra G. S., Reynolds C. G., Brenner M. B. (2008) Serum lipids regulate dendritic cell CD1 expression and function. Immunology 125, 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chaitidis P., O'Donnell V., Kuban R. J., Bermudez-Fajardo A., Ungethuem U., Kuhn H. (2005) Gene expression alterations of human peripheral blood monocytes induced by medium-term treatment with the TH2-cytokines interleukin-4 and -13. Cytokine 30, 366–377. [DOI] [PubMed] [Google Scholar]
- 43. Solary E. (2012) When monocyte life hangs by a thread. Blood 119, 2699–2700. [DOI] [PubMed] [Google Scholar]
- 44. Ahn J. S., Agrawal B. (2005) IL-4 is more effective than IL-13 for in vitro differentiation of dendritic cells from peripheral blood mononuclear cells. Int. Immunol. 17, 1337–1346. [DOI] [PubMed] [Google Scholar]
- 45. Wurster A. L., Tanaka T., Grusby M. J. (2000) The biology of Stat4 and Stat6. Oncogene 19, 2577–2584. [DOI] [PubMed] [Google Scholar]
- 46. Johnson D. J., Pao L. I., Dhanji S., Murakami K., Ohashi P. S., Neel B. G. (2013) Shp 1 regulates T cell homeostatis by limiting IL-4 signals. J. Exp. Med. 210, 1419–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Isenberg J. S., Annis D. S., Pendrak M. L., Ptaszynska M., Frazier W. A., Mosher D. F., Roberts D. D. (2009) Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J. Biol. Chem. 284, 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaur S., Kuznetsova S. A., Pendrak M. L., Sipes J. M., Romeo M. J., Li Z., Zhang L., Roberts D. D. (2011) Heparan sulfate modification of the transmembrane receptor CD47 is necessary for inhibition of T cell receptor signaling by thrombospondin-1. J. Biol. Chem. 286, 14991–15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chong Z. Z., Maiese K. (2007) The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol. Histopathol. 22, 1251–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mantovani A., Biswas S. K., Galdiero M. R., Sica A., Locati M. (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185. [DOI] [PubMed] [Google Scholar]
- 51. West S. D., Goldberg D., Ziegler A., Krencicki M., Du Clos T. W., Mold C. (2012) Transforming growth factor-β, macrophage colony-stimulating factor and C-reactive protein levels correlate with CD14(high)CD16+ monocyte induction and activation in trauma patients. PLoS One 7, e52406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hotchkiss R. S., Chang K. C., Swanson P. E., Tinsley K. W., Hui J. J., Klender P., Xanthoudakis S., Roy S., Black C., Grimm E., Aspiotis R., Han Y., Nicholson D. W., Karl I. E. (2000) Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat. Immunol. 1, 496–501. [DOI] [PubMed] [Google Scholar]
- 53. Flohe S. B., Agrawal H., Schmitz D., Gertz M., Flohe S., Schade F. U. (2006) Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J. Leukoc. Biol. 79, 473–481. [DOI] [PubMed] [Google Scholar]
- 54. Manna P. P., Frazier W. A. (2003) The mechanism of CD47-dependent killing of T cells: heterotrimeric Gi-dependent inhibition of protein kinase A. J. Immunol. 170, 3544–3553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.