Mouse alveolar macrophage PDE2A significantly inhibits the increased iNOS expression resulting from LPS exposure.
Keywords: cyclic nucleotide, pneumonia, acute lung injury, nitric oxide, atrial natriuretic peptide
Abstract
PDE2A is a dual-function PDE that is stimulated by cGMP to hydrolyze cAMP preferentially. In a two-hit model of ALI, we found previously that PDE2A decreased lung cAMP, up-regulated lung iNOS, and exacerbated ALI. Recent data suggest that macrophage iNOS expression contributes to ALI but later, promotes lung-injury resolution. However, macrophage iNOS is increased by cAMP, suggesting that PDE2A could negatively regulate macrophage iNOS expression. To test this, we examined the effects of manipulating PDE2A expression and function on LPS-induced iNOS expression in a mouse AM cell line (MH-S) and primary mouse AMs. In MH-S cells, LPS (100 ng/ml) increased PDE2A expression by 15% at 15 min and 50% at 6 h before decreasing at 24 h and 48 h. iNOS expression appeared at 6 h and remained increased 48 h post-LPS. Compared with control Ad, Ad.PDE2A-shRNA enhanced LPS-induced iNOS expression further by fourfold, an effect mimicked by the PDE2A inhibitor BAY 60–7550. Adenoviral PDE2A overexpression or treatment with ANP decreased LPS-induced iNOS expression. ANP-induced inhibition of iNOS was lost by knocking down PDE2A and was not mimicked by 8-pCPT-cGMP, a cGMP analog that does not stimulate PDE2A activity. Finally, we found that in primary AMs from LPS-treated mice, PDE2A knockdown also increased iNOS expression, consistent with the MH-S cell data. We conclude that increased AM PDE2A is an important negative regulator of macrophage iNOS expression.
Introduction
In the normal lung, NO is generated predominantly by the constitutive endothelial and neuronal isoforms of NOS. In the presence of the bacterial endotoxin LPS, however, large amounts of NO are generated from an up-regulation of iNOS, which is expressed in lung epithelium [1, 2] and macrophages [3]. Once expressed, iNOS is constitutively active so that activity correlates directly with expression when substrates are not rate limiting [4, 5]. NO from iNOS is known to contribute to the pathogenesis of murine acute lung-injury models in a complex fashion with pro- and anti-inflammatory effects, depending on the specific insult [6, 7] and the temporal stage of injury [8]. For example, IT LPS-induced lung injury was attenuated in iNOS−/−mice at 1 day following IT LPS administration with decreased BAL neutrophil counts and concentrations of protein, MIP-2, and TNF-α [6, 9]. D'Alessio et al. [8] found a similar, early protection in lung injury in iNOS−/−mice after IT LPS, but the lung inflammation failed to resolve in the iNOS−/−mice, supporting a late anti-inflammatory role for iNOS. Importantly, the early proinflammatory effects [10] and the later injury resolution effects [8] of iNOS expression following IT LPS were mediated by macrophage iNOS expression.
Murine macrophage iNOS expression is potently induced by LPS and cytokines, such as IFN-γ, but the signaling pathways regulating iNOS expression have not been fully elucidated. Increasing evidence suggests that the second messenger cAMP plays a key role in up-regulating macrophage iNOS expression following LPS exposure [5, 11–15], primarily through increased transcription [5]. Conversely, increasing cGMP by particulate GC activation with ANP resulted in dose-dependent inhibition of LPS-stimulated macrophage iNOS expression [16, 17]. LPS was shown to cause ANP release from macrophages, suggesting an autocrine cGMP-mediated feedback control of iNOS expression [17].
Cyclic nucleotide concentrations are tightly controlled by PDEs [18]. Several PDE family members have been identified in macrophages, but PDE2A consistently appears as a key PDE in activated human and rat primary macrophages [19, 20]. PDE2A hydrolyzes cGMP and cAMP but preferentially targets cAMP in the presence of high cGMP levels, thus allowing a cGMP-mediated decrease in cAMP signaling [21]. This occurs when cGMP stimulates catalytic activity by binding to a regulatory GAF domain. We recently demonstrated the critical importance of lung PDE2A as a proinflammatory mediator in the ALI caused by the combination of IT LPS and VILI in mice [22]. We found that lung PDE2A and iNOS expression were synergistically up-regulated in this injury. Moreover, knocking down PDE2A by IT infection with an Ad that expressed Ad.PDE2A-shRNA decreased mortality, lung injury, and lung iNOS expression in association with an increased lung cAMP concentration [22].
The inverse correlation between cAMP and iNOS expression that we found in the lung was consistent with observations indicating that increased cAMP attenuates epithelial iNOS expression [23]. However, cAMP has been shown to increase macrophage iNOS expression [5, 11–15]. We therefore hypothesized that PDE2A expression might serve as a major negative regulator of iNOS expression in lung macrophages after LPS exposure. To test this hypothesis, we examined the effects of manipulating PDE2A expression and function on LPS-induced iNOS expression in a mouse AM cell line (MH-S) and primary mouse AMs. We found that PDE2A and iNOS expression is increased in LPS-treated MH-S cells and that down-regulation of PDE2A expression with Ad.PDE2A-shRNA infection or pharmacologic PDE2A inhibition increased LPS-induced iNOS expression. Conversely, PDE2A overexpression with Ad.PDE2A infection attenuated LPS-induced iNOS expression, as did treatment with ANP. The inhibitory effect of ANP was lost by knocking down PDE2A and was not mimicked by administration of 8-pCPT-cGMP, a membrane-permeable cGMP analog that is unable to interact with the regulatory GAF site. Finally, we found that PDE2A knockdown in primary AMs from LPS-treated mice also showed increased iNOS expression consistent with the MH-S cell data. We conclude that increased AM PDE2A is an important negative regulator of macrophage iNOS expression.
MATERIALS AND METHODS
Experimental animals
All procedures described here had prior approval from the Johns Hopkins Animal Care and Use Committee. Male C57BL/6 mice (8–10 weeks) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and housed in our facilities at the Johns Hopkins University Asthma and Allergy Center under standard conditions in a pathogen-free environment and had free access to commercial chow and water.
Adenoviral vectors
The construction of a mouse PDE2A2 gene-expressing plasmid, pCHA-PDE2A, was reported previously [22]. Briefly, we generated a GFP tag in-frame using a 2.8-kb EcoRI fragment harboring HA-PDE2A of pcHA-PDE2A subcloned into the EcoRI site of pEGFP-C1 (Clontech, Mountain View, CA, USA). Positive transformant pGFP-PDE2A was then introduced into the shuttle vector pAdCMV.DEST-V5 (Invitrogen, Carlsbad, CA, USA) to produce pAd.PDE2A. Replication-defective virus amplification was prepared by transfecting pAd.PDE2A into 293A cells (Life Technologies, Carlsbad, CA, USA). Cytopathic effect and GFP expression were monitored to confirm the amplification of recombinant viruses. Ad.PDE2A2 purification was carried out using the Ad purification kit Adenopure (Puresyn, Malvern, PA, USA), according to the manufacturer's instructions.
To achieve suppression of macrophage PDE2A expression, we used a previously described [22] recombinant replication-defective Ad that expresses GFP and an artificial shRNAmiR directed against PDE2A expression (Ad.PDE2A-shRNA). Briefly, the shRNA sequences in the murine miRNA (miR-155) [24] were replaced by shRNA sequences targeting PDE2A. This was incorporated into a commercially available vector pcDNA6.2-DEST (Gateway recombination cloning system; Invitrogen), downstream of a cDNA encoding a modified GFP. To control for the effects of viral infection, we used Ad.Neg-shRNA designed to express GFP and a control shRNAmiR that was designed to avoid targeting all known vertebrate genes [22, 25]. Amplification and purification of Ad.PDE2A-shRNA and Ad.Neg-shRNA were performed by Puresyn.
Adenoviral gene silencing or overexpression of PDE2A in MH-S macrophages
Transformed mouse AMs (MH-S; CRL-2019; American Type Culture Collection, Manassas, VA, USA) were subcultured at 50% confluence in complete media in 12-well dishes and incubated in a humidified 5% CO2 incubator at 37°C. At 24 h, the MH-S cells were infected with Ad after achieving 80–90% confluence, corresponding to ∼5 × 106 cells/well. For Ad.Neg-shRNA or Ad.PDE2A-shRNA infection, the media were replaced with complete media, containing 20, 50, or 100 MOI. To achieve a comparable level of infection with the noncomercially purified Ad.PDE2A, we used 200, 500, and 1000 MOI. The Ads were incubated for 48 h, followed by 8–16 h of exposure to 100 ng/ml LPS. Fluorescent images of infected cells were acquired with an Olympus IX51 inverted fluorescent microscope after fixation in 4% paraformaldehyde, as described previously [26]. In experiments examining the effect of ANP (Biomedicals, Solon, OH, USA) on iNOS expression, MH-S macrophages were infected with 100 MOI Ad for 48 h before ANP and LPS exposure.
Drug treatment protocol in MH-S cells
MH-S macrophages were cultured in six- or 12-well plates until 70–80% confluence in a complete media. The cells were serum deprived for 1 h (1% FBS-DMEM, with no antibiotics) and then treated with ANP, 8-pCPT-cGMP, or 8-CPT-cAMP (BioLog, Bremen, Germany) or the selective PDE2A inhibitor BAY 60–7550 (Cayman Chemical, Ann Arbor, MI, USA) [21] for 30 min before the addition of 100 ng/ml LPS. After various incubation times, the cells were harvested for Western protein analysis.
In vivo mouse AM adenoviral infection
On Day 0, mice were lightly anesthetized with chloroform before inducing pharyngeal aspiration of 50 μl/mouse Escherichia coli LPS (2.5 mg/g mouse weight O55:B5; Sigma-Aldrich, St. Louis, MO, USA) was performed. On Day 3, mice were reanesthetized i.p. with ketamine and acetylpromazine, and 35 μl water containing 3 × 109 pfu/ml Ad.Neg-shRNA, Ad.PDE2A-shRNA, Ad.PDE2A, or water alone was instilled IT via a 20-g catheter, as described previously [22]. On Day 6, mice were reanesthetized and killed by exsanguination from the inferior vena cava. BAL cells, including AMs, were then harvested by lavaging both lungs with 8 ml PBS in 1-ml increments. Cells were pooled, centrifuged, and plated for 2 h in DMEM high-glucose media, supplemented with 10% heat-inactivated FBS and 1% penicillin-streptomycin, allowing AM to adhere. Nonadherent cells were removed by rinsing with PBS. AMs were added in vitro to 100 ng/ml LPS in a complete media (DMEM high glucose, supplemented with 10% heat-inactivated FBS, 1% penicillin-streptomycin, 1% L-glutamine, and 2.4% 1 M Hepes) for 12 h. Cells were harvested by gentle scraping in a protein-extraction buffer for Western analysis.
Western blot analysis
Immunoblots were performed as described previously [22]. Briefly, cells were washed twice with ice-cold PBS; lysed in a cold, 1× cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA); and enriched with 100× protease inhibitor cocktail (Sigma-Aldrich), 50× PhosSTOP (Roche Applied Science, Mannheim, Germany), and 1 mM PMSF for 30 min on the ice. Lysates were centrifuged, and the supernatants were assayed for protein concentration using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). Equal amounts of protein/sample (25–30 μg) were resolved on 8% SDS-PAGE gels; transferred to polyvinylidene difluoride membrane (iBlot; Invitrogen, Kiryat Shmona, Israel); incubated with antibodies to PDE2A (FabGennix International, Frisco, TX, USA), iNOS (Transduction Laboratories, Rockville, MD, USA), β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and GAPDH-peroxidase (Sigma-Aldrich); and detected using ECL Plus (Amersham, Piscataway, NJ, USA). Relative band intensities were quantified by densitometry using UN-SCAN-IT imaging software (Bio-Medicine, Orem, UT, USA).
cGMP and cAMP enzyme immunoassay
Changes in intracellular cGMP and cAMP in MH-S cells, following LPS in the absence or presence of BAY 60–7550, were measured by using a commercially available kit (Assay Design, Ann Arbor, MI, USA), as described previously [22].
Statistics
Multiple treatment groups were compared by one- or two-factor ANOVA with least significant difference post hoc testing. Non-normally distributed data were converted to logarithms before analysis. Values presented in the text are means ± se. Differences were considered significant when P ≤ 0.05.
RESULTS
PDE2A and iNOS expression is increased in LPS-treated MH-S cells
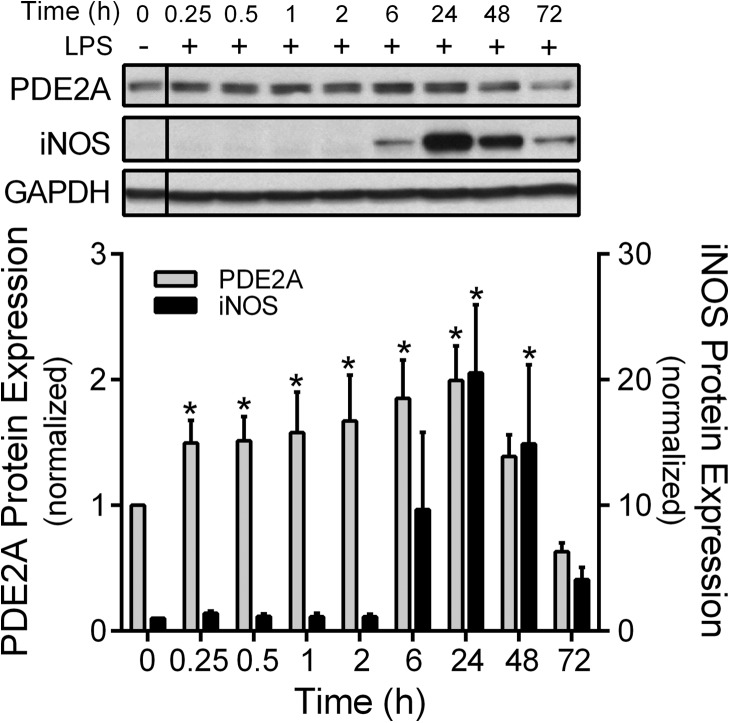
We first examined the time course effect of 100 ng/ml LPS on MH-S PDE2A and iNOS protein expression from 15 min to 72 h after LPS exposure (Fig. 1). PDE2A protein expression was present at baseline in MH-S macrophages, whereas protein expression of iNOS was not detectable. LPS treatment significantly (P<0.001) increased PDE2A protein expression by 50% at 15 min and by twofold at 6 and 24 h before decreasing to basal levels. iNOS expression increased 20-fold (P<0.01) at 24 h and remained markedly elevated at 48 h before declining at 72 h.
Figure 1. Time course of PDE2A and iNOS protein expression by Western immunoblot in MH-S macrophages following LPS (100 ng/ml).
The PDE2A and iNOS protein levels are normalized to GAPDH loading control and then to control MH-S cells not exposed to LPS (n=3 independent experiments). The vertical line separating the control LPS cells indicates that this lane came from a different part of the same gel. Values are means ± se. *P < 0.05 versus the Time 0 control cells.
Down-regulation of PDE2A expression increases LPS-induced iNOS expression in MH-S cells
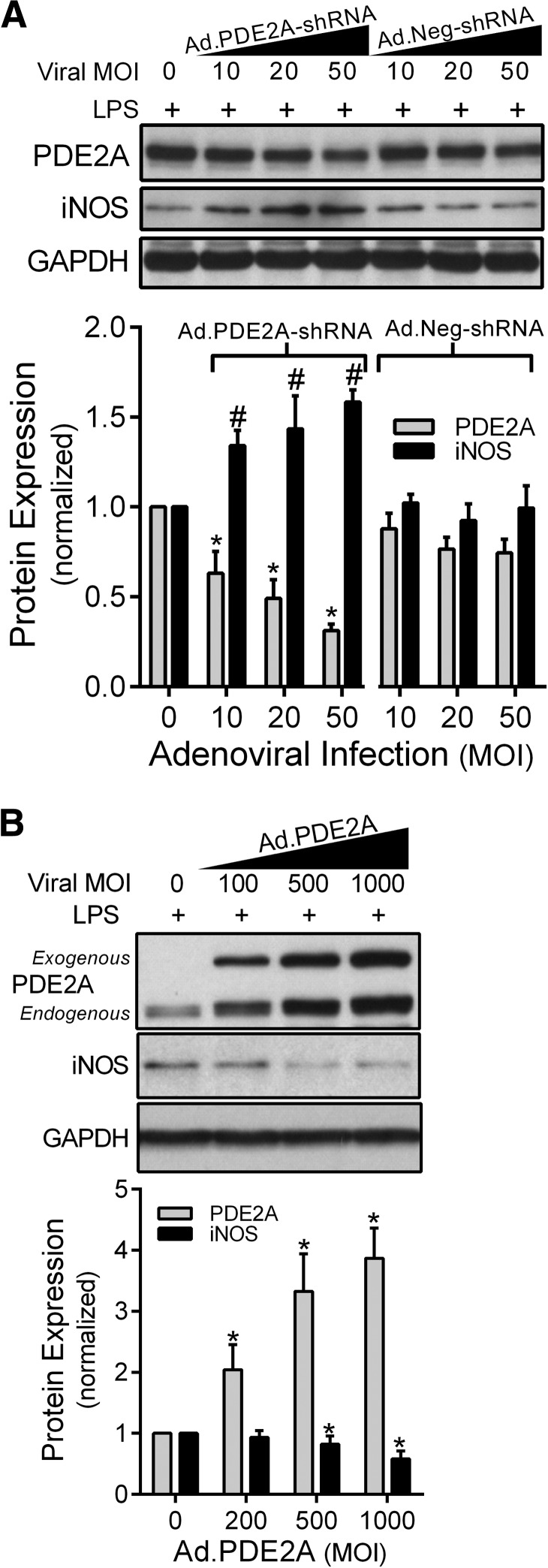
Next, we examined the effect of knocking down the LPS-induced increase in PDE2A expression on iNOS expression. MH-S macrophages were infected with Ad.PDE2A-shRNA for 48 h before adding LPS and incubating for an additional 8 h. Compared with Ad.Neg-shRNA control virus, Ad.PDE2A-shRNA decreased the PDE2A protein level by 70% (P=0.05) and caused a near twofold increase (P<0.03) in iNOS protein expression (Fig. 2A), suggesting that in the presence of LPS, macrophage PDE2A down-regulates iNOS expression. Note that the presence of viral infection resulted in the appearance of iNOS 8 h after LPS, which was not observed this early after LPS alone. Next, MH-S cells were infected with Ad.PDE2A to overexpress PDE2A for 48 h before 16 h of LPS treatment. As shown in Fig. 2B, overexpression of PDE2A by Ad attenuated iNOS protein expression by 50%, consistent with the inverse relationship between PDE2A and iNOS expression suggested in Fig. 2A. Interestingly, exogenous PDE2A expression by Ad.PDE2A was associated with an increased endogenous PDE2A expression in a dose-dependent fashion, whereas Ad.Neg-shRNA had no effect, suggesting that the increase in endogenous PDE2A was not a nonspecific effect of viral infection.
Figure 2. PDE2A and iNOS protein expression by Western immunoblot in MH-S macrophages that were first infected with increasing Ad MOI to manipulate PDE2A expression, followed 48 h later by LPS (100 ng/ml) exposure for an additional 8 h.
(A) The effect of Ad.PDE2A-shRNA versus the control Ad.Neg-shRNA. (B) The effect of PDE2A overexpression with Ad.PDE2A. PDE2A has two bands, as the exogenous PDE2A is coupled with GFP. The PDE2A and iNOS protein levels are normalized to GAPDH loading control and then to control MH-S cells exposed to LPS but not Ad (n=3 independent experiments). Values are means ± se. #P = 0.05 versus Ad.Neg-shRNA by ANOVA interaction; *P < 0.03 versus Ad.Neg-shRNA by ANOVA interaction.
Pharmacologic inhibition of PDE2A increases LPS-induced iNOS expression and cyclic nucleotide concentrations in MH-S macrophages
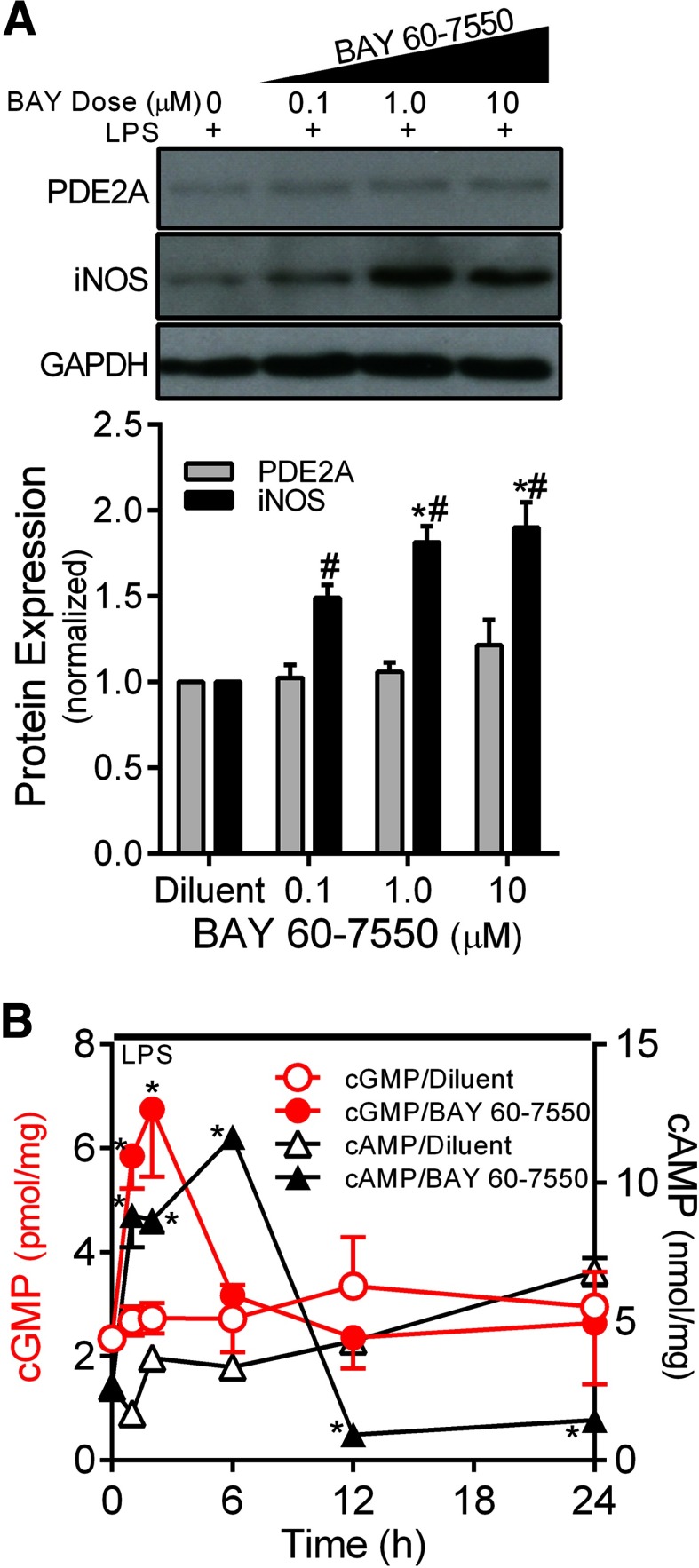
To determine whether PDE2A enzymatic activity was responsible for the inverse relationship between PDE2A and iNOS protein expression, we exposed MH-S macrophages to LPS in the presence or absence of increasing doses of BAY 60–7550 for 24 h. As shown in Fig. 3A, LPS-induced iNOS expression was increased (P<0.001) in BAY 60–7550-treated cells in a dose-response manner, mimicking the effect of Ad.PDE2A-shRNA infection shown in Fig. 2. Figure 3B shows the cyclic nucleotide signaling resulting from LPS in the presence of PDE2A inhibition with 1 μM BAY 65–7550. PDE2A inhibition uncovered a rapid LPS-induced, significant increase (P<0.002) in intracellular cGMP to ∼7 pmol/mg protein, which was followed by a more prolonged increase in cAMP to 12 nmol/mg protein (P<0.0001), likely explaining the resulting increase in LPS-induced iNOS expression. The fact that cGMP and cAMP increased in the presence of PDE2A inhibition is consistent with the dual-function activity of PDE2A. In separate experiments, we measured cGMP in MH-S cells after exposure to increasing ANP concentrations or the direct soluble GC agonist [3-(4-amino-5-cyclopropylpyrimidine-2-yl)-1-(2-fluorobenzyl)-1 H-pyrazolo[3,4-b]pyridine [27], in the presence of 3-isobutyl-1-methylxanthine but without LPS, and found increased cGMP only from ANP (data not shown), suggesting a lack of soluble GC in these cells, consistent with observations in other macrophage types [17, 20].
Figure 3. (A) Effect of increasing concentrations of the PDE2A inhibitor BAY 65–7550 on MH-S macrophage PDE2A and iNOS expression by Western blot with densitometric analysis measured after 24 h of exposure to LPS (100 ng/ml).
The PDE2A and iNOS protein levels are normalized to GAPDH loading control and then to control cells that were exposed to LPS with BAY 65–7550 diluent (n=3 independent experiments). Values are means ± se. #P < 0.001 versus diluent; *P < 0.001 versus 0.1 μM BAY 60–7550. (B) Effect of BAY 65–7550 (1 μM) versus the DMSO diluent on the time course of intracellular cGMP (red) and cAMP (black) concentrations in MH-S macrophages exposed to LPS (100 ng/ml; n=3–6 independent experiments). Values are means ± se. *P < 0.002 versus diluent by ANOVA interaction.
An increase in cGMP from ANP regulates LPS-induced iNOS in MH-S through PDE2A
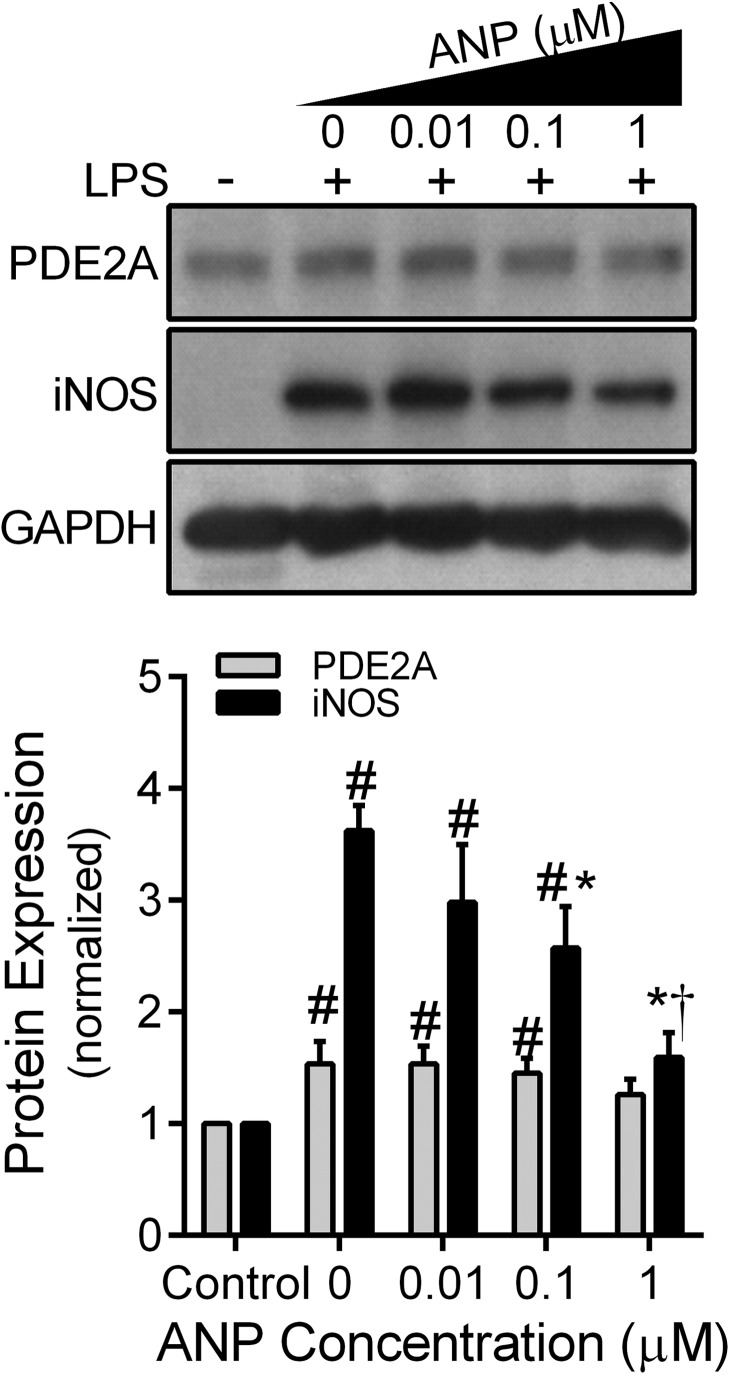
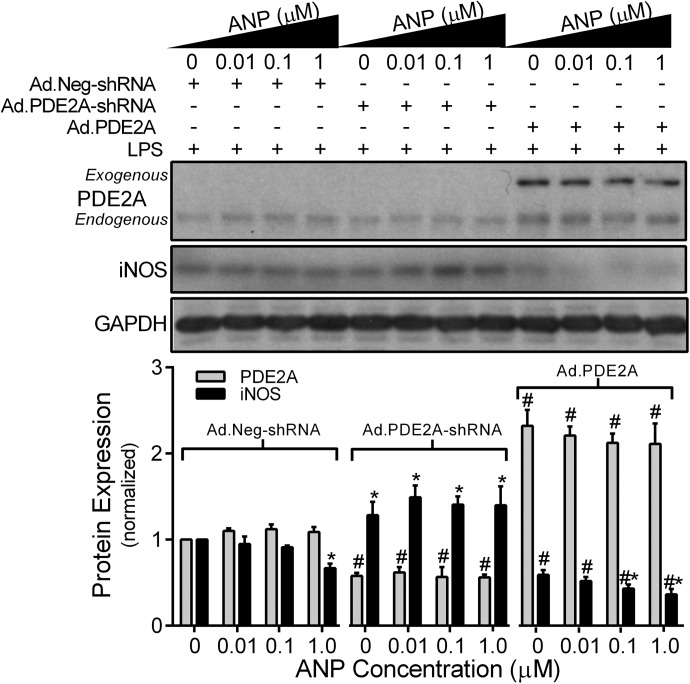
As PDE2A- mediated hydrolysis of cAMP can be enhanced by cGMP [21, 28], and cAMP has been shown to stimulate AM iNOS expression [29], we explored the effect of ANP on LPS-induced iNOS expression in MH-S cells. MH-S macrophages were treated with LPS (100 ng/ml) for 12 h in the presence of increasing ANP (0, 0.01, 0.1, 1 μM) concentration to increase cGMP from particulate GC. In the absence of ANP, LPS significantly increased (P<0.03) both PDE2A and iNOS expression, consistent with Fig. 1. Increasing ANP concentrations decreased LPS-induced iNOS expression in a dose-response fashion (P<0.001) without a significant effect on PDE2A (Fig. 4). We then examined the influence of down- or up-regulating PDE2A expression on the ANP effect by treating with the Ads for 48 h before the 12-h ANP-LPS protocol. Similar to uninfected cells, MH-S macrophages infected with the control Ad.Neg.shRNA demonstrated ANP-induced inhibition (P<0.05) of iNOS expression (Fig. 5). In contrast, Ad.PDE2A-shRNA increased iNOS expression and eliminated the ANP-induced inhibitory effect. Overexpression of PDE2A by Ad.PDE2A significantly attenuated LPS-induced iNOS expression, but a dose-dependent, further inhibition of iNOS by ANP could still be observed (P<0.05). These data suggest that ANP/pGC-derived cGMP decreased LPS-induced iNOS expression through stimulation of PDE2A.
Figure 4. MH-S macrophage PDE2A and iNOS expression by Western blot with densitometric analysis measured after 12 h of exposure to an increasing concentration of ANP administered with LPS (100 ng/ml).
The PDE2A and iNOS protein levels are normalized to GAPDH loading control and then to control MH-S cells not exposed to LPS or ANP (n=6 independent experiments). Values are means ± se. #P < 0.03 versus control cells; *P < 0.001 versus iNOS expression following LPS with no ANP; †P < 0.001 versus iNOS expression in all other LPS-treated groups.
Figure 5. Effect of down- or up-regulating PDE2A expression on MH-S macrophage PDE2A and iNOS expression by Western blot with densitometric analysis measured after 12 h of exposure to an increasing concentration of ANP administered with LPS (100 ng/ml).
Ad.Neg-shRNA, Ad.PDE2A-shRNA, or Ad.PDE2A were administered 48 h before ANP/LPS. The PDE2A and iNOS protein levels are normalized to GAPDH loading control and then to control Ad treatment in the absence of ANP (n=8 independent experiments). Values are means ± se. *P < 0.05 versus 0 ANP value within each adenoviral treatment group; #P < 0.001 versus Ad.Neg-shRNA treatment groups.
8-pCPT-cGMP fails to decrease LPS-induced iNOS expression in MH-S macrophages
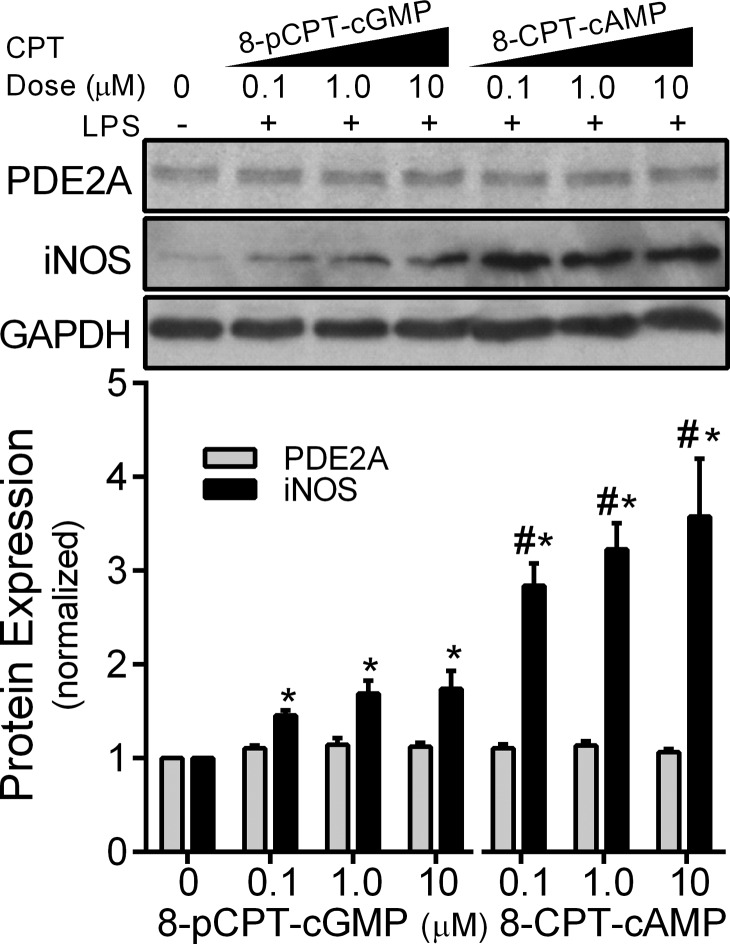
To implicate further cGMP-mediated regulation of PDE2A activity, we treated MH-S macrophages with the membrane-permeable analog 8-pCPT-cGMP. This agent is a potent activator of cGMP-dependent protein kinase I or cGMP-gated ion channels but is unable to stimulate or inhibit cyclic nucleotide-sensitive PDEs [21, 30, 31]. MH-S macrophages were treated with LPS for 8 h in the presence of an increasing dose of 8-pCPT-cGMP (0.1, 1, or 10 μM). We found that 8-pCPT-cGMP failed to decrease LPS-induced iNOS in MH-S cells (Fig. 6), supporting the notion that the ANP effect observed in Figs. 2 and 3 was mediated through PDE2A rather than a non-PDE cGMP effect. In comparison, the membrane-permeable cAMP analog 8-CPT-cAMP potently increased iNOS expression.
Figure 6. Effect of 8-pCPT-cGMP and 8-CPT-cAMP on MH-S macrophage PDE2A and iNOS expression by Western blot with densitometric analysis measured after 8 h of exposure to LPS (100 ng/ml).
The PDE2A and iNOS protein levels are normalized to GAPDH loading control and then to control cells not exposed to LPS or cyclic nucleotide analog (n=3 independent experiments). Values are means ± se. *P < 0.005 versus 0 cyclic nucleotide value within each treatment group; #P < 0.002 versus 8-pCPT-cGMP by ANOVA interaction.
PDE2A controls iNOS in mouse AMs isolated from LPS-treated mice
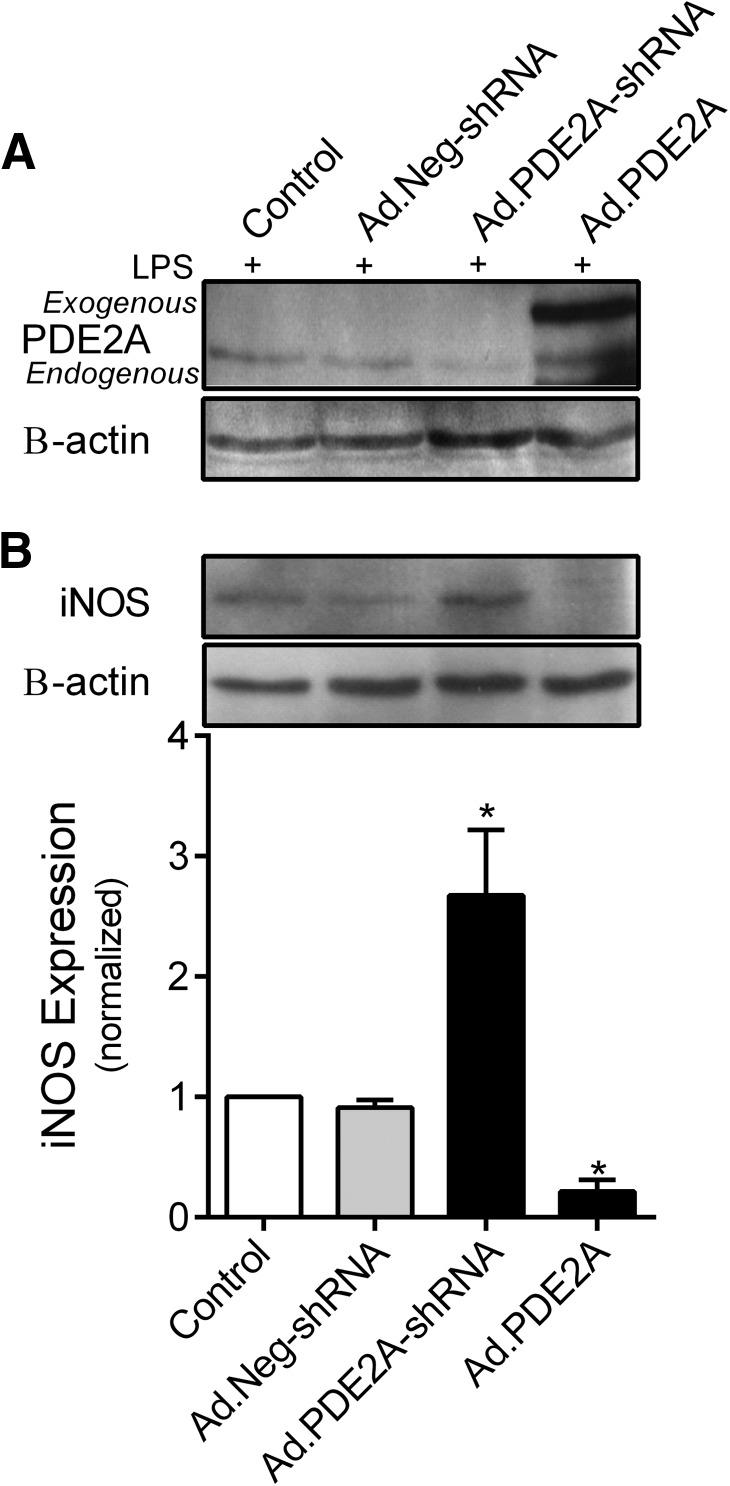
To determine whether PDE2A is a key regulator of iNOS expression in primary mouse AMs, C57BL6 mice underwent pharyngeal-LPS on Day 0 to initiate AM influx into the lungs. On Day 3, IT Ads were delivered to down- or up-regulate PDE2A expression. On Day 6, BAL cells were harvested, and AMs were isolated and exposed to 100 ng/ml LPS for an additional 18 h in vitro. The PDE2A protein bands from individual experiments were too faint to quantify, so the cell homogenates from four independent experiments were combined to generate Fig. 7A. These data show that PDE2A protein expression was detected in mouse AMs and was successfully manipulated downward and upward by Ad.PDE2A-shRNA and Ad.PDE2A, respectively, similar to the MH-S cells. In contrast, iNOS was easily detected in the individual experiments, so Fig. 7B shows a representative Western blot of iNOS expression above the average densitometry from the same four experiments represented in Fig. 7A. Similar to the results found in MH-S-transformed AMs, down-regulation of PDE2A with Ad.PDE2A-shRNA caused a near threefold increase in LPS-induced iNOS expression, whereas overexpression of PDE2A decreased iNOS by 80% compared with Ad.Neg-shRNA infection (P<0.0001).
Figure 7. Effect of down- or up-regulating PDE2A expression on mouse AM PDE2A and iNOS expression by Western blot with densitometric analysis of iNOS expression.
AMs were obtained by lavage 6 days after pharyngeal-LPS (2.5 mg/g body weight) and 3 days after IT installation of Ad.PDE2A-shRNA, Ad.PDE2A, or water diluent. The isolated AMs were exposed to an additional 18 h of LPS (100 ng/ml) in vitro before processing. (A) Mouse AM homogenates from four mice were combined to generate the PDE2A and B-actin blots. (B) iNOS and B-actin blots are representative blots from a single mouse with densitometric analysis (n=4 mice). Values are means ± se. *P < 0.0001 versus all other groups.
DISCUSSION
The major new finding presented in this study is that PDE2A is a major negative regulator of LPS-induced iNOS expression in murine AMs. Moreover, our data suggest that PDE2A contributes to the previously observed ANP-mediated inhibition of LPS-induced iNOS induction in lung macrophages [16, 17, 32]. These results, taken together with our recent findings demonstrating the protective effects of knocking down PDE2A in a two-hit mouse model of LPS/VILI [22], suggest that PDE2A is a critical upstream determinate of cyclic nucleotide signaling and iNOS expression in parenchymal cells and AMs in ALI.
Of the 11 known families of mammalian PDEs, the most significant forms in pulmonary endothelium and epithelium are PDE2A, -3, -4, and -5 [21, 33–37]. The spectrum of macrophage PDEs differs by tissue origin and species but includes PDE1, -2A, -3, -4, -5, -10, and -11 [19, 20, 38, 39], with PDE2A and PDE3 as the most active PDEs in mouse exudative peritoneal macrophages [20]. PDE2A and -3 are dual-function PDEs capable of mediating cross-talk between cGMP and cAMP signaling [40]. PDE2A contains a regulatory GAF domain that increases cAMP catalytic activity when bound by cGMP, whereas increased cGMP decreases cAMP hydrolysis by PDE3 through catalytic site competition [18, 21]. Thus, only PDE2A is capable of causing cGMP-mediated decreases in cAMP concentration [21].
Increasing evidence points to the importance of cAMP in macrophage signaling [41]. For example, cAMP suppresses cytokine release, oxidant generation [42], and phagocytosis [43]. PDEs, particularly PDE2A, were hypothesized to play a key role in controlling cAMP signaling in activated macrophages [20, 28], but we could find no published studies examining the effects of specific pharmacologic inhibition or molecular knockdown of PDE2A on macrophage signaling. If PDE2A is a critical negative regulator of cAMP in macrophages, one would expect that perturbations known to increase cGMP in activated macrophages, such as LPS or ANP [17], would counter cAMP-induced effects because of cGMP-dependent cAMP hydrolysis. Consistent with this pattern, macrophage iNOS expression was previously shown to be inhibited by cGMP [17] and stimulated by cAMP [5, 11–15], although some investigators have reported cAMP-mediated inhibition of macrophage iNOS expression [44, 45].
We chose to examine the role of PDE2A in regulating LPS-induced iNOS expression in MH-S cells, a well-characterized murine AM cell line [46–48]. Similar to rat peritoneal macrophages [20], LPS caused a rapid, significant increase in PDE2A protein expression in MH-S macrophages that was followed by an increase in iNOS expression peaking at 24 h (Fig. 1). When we knocked down PDE2A with Ad.PDE2A-shRNA (Fig. 2A) or used the specific PDE2A inhibitor BAY 60–7550 (Fig. 3A), there was a dose-dependent, further increase in LPS-mediated iNOS induction. Consistent with these data, overexpression of PDE2A with Ad.PDE2A markedly attenuated iNOS expression (Fig. 2B). These data confirmed a significant role for PDE2A in the regulation of macrophage iNOS expression. Examination of the time course of intracellular cyclic nucleotide concentrations in the presence of PDE2A inhibition (Fig. 3B) uncovered an early LPS-induced increase in cGMP, followed by an increase in cAMP, consistent with the dual hydrolytic effects of PDE2A [28] and previous work by others showing that LPS increased macrophage cGMP by generating ANP [17] and cAMP by stimulating PGE2 and ATP release [15, 49–52].
In murine bone marrow-derived macrophages [17] and two macrophage cell lines (RAW 264.7 and J774), Kiemer and Vollmar [16] showed a dose-dependent inhibition of iNOS expression and activity by ANP that was blocked by a natriuretic peptide receptor A antagonist and partially mimicked by high concentrations of dibutyryl-cGMP. They concluded that the effect of ANP was cGMP mediated but did not consider a cGMP-mediated decrease in cAMP. We also found that the addition of exogenous ANP inhibited LPS-induced iNOS expression in a dose-response manner in MH-S macrophages (Fig. 4). We confirmed a significant role for PDE2A in this response, as the ANP inhibitory effect was lost when PDE2A was knocked down and accentuated by PDE2A overexpression (Fig. 5). Moreover, the inhibitory effect of ANP was not mimicked by 8-pCPT-cGMP (Fig. 6), a cGMP analog that does not interact with the PDE2A GAF site, suggesting that the ANP effect was not mediated by protein kinase G activation. Unlike 8-pCPT-cGMP, millimolar concentrations of dibutyryl-cGMP are capable of a PDE2A GAF site interaction [31] so the results of Kiemer and Vollmar [16] are not inconsistent with a PDE2A-mediated effect.
Based on the effects we observed by manipulating PDE2 expression and activity, we were not surprised that a cAMP analog that is not susceptible to PDE hydrolysis caused increased iNOS expression in MH-S macrophages (Fig. 6), similar to the results in other macrophage types [5, 11–15]. We also confirmed that altering PDE2A expression in mouse primary AMs exposed to IT, and then in vitro LPS resulted in the same effects observed in the MH-S macrophage cell line (Fig. 7). Kiemer et al. [49] showed that ANP caused a dose-dependent decrease in LPS-induced cAMP in bone marrow-derived macrophages that was attributed to activation of the natriuretic protein clearance receptors, causing a direct inhibition of adenylyl cyclase. Whether this PDE2A-independent mechanism also contributes to ANP-mediated iNOS expression inhibition in MH-S cells or primary AMs remains to be determined. It is also important to note that although iNOS has been identified in human AMs by immunohistochemistry in diseased lungs [3], iNOS induction in human macrophages in vitro has been difficult to demonstrate [53]. Thus, the control of human macrophage iNOS expression and its role in modulating human ALI remain unclear.
PDE2A is an important emerging contributor to the pathogenesis of ALI. Pharmacologic PDE2A inhibition proved protective in a severe murine model of pneumococcal pneumonia [54]. We previously found an important injurious role for PDE2A in an isolated, perfused mouse lung model of ventilator-induced lung endothelial barrier dysfunction [55]. More recently, we examined the role of PDE2A in a two-hit in vivo mouse model of IT LPS combined with high tidal-volume ventilation [22]. IT LPS rapidly increased PDE2A expression in lung homogenate by Western blot and in endothelium, epithelium, and macrophages by immunohistochemistry, followed by alveolar inflammation and protein leak, which peaked at Days 3 and 4. The addition of high tidal-volume ventilation for 4 h synergistically increased lung PDE2A, iNOS, and BAL inflammation on Day 1. Ad.PDE2A-shRNA, given IT before the LPS/high tidal-volume ventilation, decreased lung PDE2A expression, BAL inflammation, iNOS expression, and mortality compared with a control virus-infected group, confirming a significant pathologic PDEA contribution [22, 56].
Based on the current study, it is likely that the marked lung-injury protection caused by IT Ad.PDE2A-shRNA in our previous study [22] was simultaneously associated with decreased epithelial iNOS and increased macrophage iNOS expression, both secondary to increased cAMP. At present, we cannot separate the effects of these iNOS expression changes from the other generally anti-inflammatory effects of increasing cAMP in both cell types. Although epithelial iNOS was not shown to play a significant, injurious role in blood-born infection models of ALI [57–59], the early, direct injury to the epithelium in our IT LPS model, associated with a marked alveolitis and protein leak, could be more dependent on increased epithelial iNOS. Taken together with the results of D'Alessio et al. [8], we propose that PDE2A inhibition may be an attractive treatment for pneumonia-induced ALI by attenuating early epithelial activation and leak through increased epithelial cAMP and decreased iNOS, while promoting the increased macrophage iNOS that is necessary to trigger injury resolution.
ACKNOWLEDGMENTS
The work was supported by the National Heart, Lung, and Blood Institute, U.S. National Institutes of Health Grants HL067189 (to D.B.P.) and HL103793 (to F.R.D.).
The authors thank Dr. Michael Crow for assistance in the construction of the shRNA-expressing Ads.
Footnotes
- 8-pCPT
- 8-(4-chlorophenylthio)
- Ad
- adenovirus
- Ad.Neg-shRNA
- adenovirus expressing a short hairpin RNA designed to avoid targeting all known vertebrate genes
- Ad.PDE2A-shRNA
- phosphodiesterase 2A knockdown by an adenovirus expressing a short hairpin RNA
- ALI
- acute lung injury
- AM
- alveolar macrophage
- ANP
- atrial natriuretic peptide
- BAL
- bronchoalveolar lavage
- BAY 60–7550
- 2-(3,4-dimethoxybenzyl)-7-[(1R)-1-[(1R)-1-hydroxyethyl]-4-phenylbutyl]-5-methylimidazo[5,1-f] [1,2,4]triazin-4(3H)-1
- cGMP
- cyclic GMP
- GAF
- cyclic GMP-activated phosphodiesterases, Anabaena adenylyl cyclase, and Escherichia coli Fh1A
- GC
- guanylyl cyclase
- iNOS−/−
- iNOS-deficient
- IT
- intratracheal
- miR
- micro RNA
- miRNA
- micro RNA
- MOI
- multiplicity of infection
- PDE
- phosphodiesterase
- shRNA
- short hairpin RNA
- VILI
- ventilator-induced lung injury
AUTHORSHIP
O.R. performed the experiments. O.R., F.R.D., and D.B.P. designed the study, analyzed the data, and wrote the paper.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1. Lane C., Knight D., Burgess S., Franklin P., Horak F., Legg J., Moeller A., Stick S. (2004) Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax 59, 757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Warner R. L., Paine R., III, Christensen P. J., Marletta M. A., Richards M. K., Wilcoxen S. E., Ward P. A. (1995) Lung sources and cytokine requirements for in vivo expression of inducible nitric oxide synthase. Am. J. Respir. Cell Mol. Biol. 12, 649–661. [DOI] [PubMed] [Google Scholar]
- 3. Kobzik L., Bredt D. S., Lowenstein C. J., Drazen J., Gaston B., Sugarbaker D., Stamler J. S. (1993) Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am. J. Respir. Cell Mol. Biol. 9, 371–377. [DOI] [PubMed] [Google Scholar]
- 4. Kleinert H., Schwarz P. M., Forstermann U. (2003) Regulation of the expression of inducible nitric oxide synthase. Biol. Chem. 384, 1343–1364. [DOI] [PubMed] [Google Scholar]
- 5. Galea E., Feinstein D. L. (1999) Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP. FASEB J. 13, 2125–2137. [DOI] [PubMed] [Google Scholar]
- 6. Okamoto T., Gohil K., Finkelstein E. I., Bove P., Akaike T., van der Vliet A. (2004) Multiple contributing roles for NOS2 in LPS-induced acute airway inflammation in mice. Am. J. Physiol. Lung Cell.Mol. Physiol. 286, L198–L209. [DOI] [PubMed] [Google Scholar]
- 7. Kobayashi H., Hataishi R., Mitsufuji H., Tanaka M., Jacobson M., Tomita T., Zapol W. M., Jones R. C. (2001) Antiinflammatory properties of inducible nitric oxide synthase in acute hyperoxic lung injury. Am. J. Respir. Cell Mol. Biol. 24, 390–397. [DOI] [PubMed] [Google Scholar]
- 8. D'Alessio F. R., Tsushima K., Aggarwal N. R., Mock J. R., Eto Y., Garibaldi B. T., Files D. C., Avalos C. R., Rodriguez J. V., Waickman A. T., Reddy S. P., Pearse D. B., Sidhaye V. K., Hassoun P. M., Crow M. T., King L. S. (2012) Resolution of experimental lung injury by monocyte-derived inducible nitric oxide synthase. J. Immunol. 189, 2234–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shanley T. P., Zhao B., Macariola D. R., Denenberg A., Salzman A. L., Ward P. A. (2002) Role of nitric oxide in acute lung inflammation: lessons learned from the inducible nitric oxide synthase knockout mouse. Crit. Care Med. 30, 1960–1968. [DOI] [PubMed] [Google Scholar]
- 10. Koay M. A., Gao X., Washington M. K., Parman K. S., Sadikot R. T., Blackwell T. S., Christman J. W. (2002) Macrophages are necessary for maximal nuclear factor-kappa B activation in response to endotoxin. Am. J. Respir. Cell Mol. Biol. 26, 572–578. [DOI] [PubMed] [Google Scholar]
- 11. Hwang T. L., Tang M. C., Kuo L. M., Chang W. D., Chung P. J., Chang Y. W., Fang Y. C. (2012) YC-1 potentiates cAMP-induced CREB activation and nitric oxide production in alveolar macrophages. Toxicol. Appl. Pharmacol. 260, 193–200. [DOI] [PubMed] [Google Scholar]
- 12. Okado-Matsumoto A., Matsumoto A., Fujii J., Taniguchi N. (2000) Effect of cAMP on inducible nitric oxide synthase gene expression: its dual and cell-specific functions. Antioxid. Redox Signal 2, 631–642. [DOI] [PubMed] [Google Scholar]
- 13. Greenberg S. S., Zhao X., Wang J. F., Hua L., Ouyang J. (1997) cAMP and purinergic P2y receptors upregulate and enhance inducible NO synthase mRNA and protein in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 273, L967–L979. [DOI] [PubMed] [Google Scholar]
- 14. Won J. S., Im Y. B., Singh A. K., Singh I. (2004) Dual role of cAMP in iNOS expression in glial cells and macrophages is mediated by differential regulation of p38-MAPK/ATF-2 activation and iNOS stability. Free Radic. Biol. Med. 37, 1834–1844. [DOI] [PubMed] [Google Scholar]
- 15. Chen C. C., Chiu K. T., Sun Y. T., Chen W. C. (1999) Role of the cyclic AMP-protein kinase A pathway in lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages. Involvement of cyclooxygenase-2. J. Biol. Chem. 274, 31559–31564. [DOI] [PubMed] [Google Scholar]
- 16. Kiemer A. K., Vollmar A. M. (1997) Effects of different natriuretic peptides on nitric oxide synthesis in macrophages. Endocrinology 138, 4282–4290. [DOI] [PubMed] [Google Scholar]
- 17. Kiemer A. K., Vollmar A. M. (1998) Autocrine regulation of inducible nitric-oxide synthase in macrophages by atrial natriuretic peptide. J. Biol. Chem. 273, 13444–13451. [DOI] [PubMed] [Google Scholar]
- 18. Conti M., Beavo J. (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 76, 481–511. [DOI] [PubMed] [Google Scholar]
- 19. Bender A. T., Ostenson C. L., Giordano D., Beavo J. A. (2004) Differentiation of human monocytes in vitro with granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor produces distinct changes in cGMP phosphodiesterase expression. Cell. Signal. 16, 365–374. [DOI] [PubMed] [Google Scholar]
- 20. Witwicka H., Kobialka M., Siednienko J., Mitkiewicz M., Gorczyca W. A. (2007) Expression and activity of cGMP-dependent phosphodiesterases is up-regulated by lipopolysaccharide (LPS) in rat peritoneal macrophages. Biochim. Biophys. Acta 1773, 209–218. [DOI] [PubMed] [Google Scholar]
- 21. Surapisitchat J., Jeon K. I., Yan C., Beavo J. A. (2007) Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circ. Res. 101, 811–818. [DOI] [PubMed] [Google Scholar]
- 22. Rentsendorj O., Damarla M., Aggarwal N. R., Choi J. Y., Johnston L., D'Alessio F. R., Crow M. T., Pearse D. B. (2011) Knockdown of lung phosphodiesterase 2A attenuates alveolar inflammation and protein leak in a two-hit mouse model of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L161–L170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo F. H., De Raeve H. R., Rice T. W., Stuehr D. J., Thunnissen F. B., Erzurum S. C. (1995) Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc. Natl. Acad. Sci. USA 92, 7809–7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Angelini D. J., Su Q., Yamaji-Kegan K., Fan C., Skinner J. T., Champion H. C., Crow M. T., Johns R. A. (2009) Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMα) induces the vascular and hemodynamic changes of pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L582–L593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang M., Koitabashi N., Nagayama T., Rambaran R., Feng N., Takimoto E., Koenke T., O'Rourke B., Champion H. C., Crow M. T., Kass D. A. (2008) Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell. Signal. 20, 2231–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moldobaeva A., Welsh-Servinsky L. E., Shimoda L. A., Stephens R. S., Verin A. D., Tuder R. M., Pearse D. B. (2006) Role of protein kinase G in barrier-protective effects of cGMP in human pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L919–L930. [DOI] [PubMed] [Google Scholar]
- 27. Evgenov O. V., Pacher P., Schmidt P. M., Hasko G., Schmidt H. H., Stasch J. P. (2006) NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat. Rev. Drug Discov. 5, 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bender A. T., Beavo J. A. (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol. Rev. 58, 488–520. [DOI] [PubMed] [Google Scholar]
- 29. Peters-Golden M. (2009) Putting on the brakes: cyclic AMP as a multipronged controller of macrophage function. Sci. Signal. 2, e37. [DOI] [PubMed] [Google Scholar]
- 30. Thomas M. K., Francis S. H., Beebe S. J., Gettys T. W., Corbin J. D. (1992) Partial mapping of cyclic nucleotide sites and studies of regulatory mechanisms of phosphodiesterases using cyclic nucleotide analogues. Adv. Second Messenger Phosphoprotein Res. 25, 45–53. [PubMed] [Google Scholar]
- 31. Jäger R., Schwede F., Genieser H. G., Koesling D., Russwurm M. (2010) Activation of PDE2 and PDE5 by specific GAF ligands: delayed activation of PDE5. Br. J. Pharmacol. 161, 1645–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kiemer A. K., Vollmar A. M. (2001) Elevation of intracellular calcium levels contributes to the inhibition of nitric oxide production by atrial natriuretic peptide. Immunol. Cell Biol. 79, 11–17. [DOI] [PubMed] [Google Scholar]
- 33. Thompson W. J., Ashikaga T., Kelly J. J., Liu L., Zhu B., Vemavarapu L., Strada S. J. (2002) Regulation of cyclic AMP in rat pulmonary microvascular endothelial cells by rolipram-sensitive cyclic AMP phosphodiesterase (PDE4). Biochem. Pharmacol. 63, 797–807. [DOI] [PubMed] [Google Scholar]
- 34. Moore T. M., Chetham P. M., Kelly J. J., Stevens T. (1998) Signal transduction and regulation of lung endothelial cell permeability. Interaction between calcium and cAMP. Am. J. Physiol. 275, L203–L222. [DOI] [PubMed] [Google Scholar]
- 35. Zhu B., Strada S., Stevens T. (2005) Cyclic GMP-specific phosphodiesterase 5 regulates growth and apoptosis in pulmonary endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L196–L206. [DOI] [PubMed] [Google Scholar]
- 36. Zhu B., Kelly J., Vemavarapu L., Thompson W. J., Strada S. J. (2004) Activation and induction of cyclic AMP phosphodiesterase (PDE4) in rat pulmonary microvascular endothelial cells. Biochem. Pharmacol. 68, 479–491. [DOI] [PubMed] [Google Scholar]
- 37. Rousseau E., Gagnon J., Lugnier C. (1994) Biochemical and pharmacological characterization of cyclic nucleotide phosphodiesterase in airway epithelium. Mol. Cell. Biochem. 140, 171–175. [DOI] [PubMed] [Google Scholar]
- 38. Tenor H., Hatzelmann A., Kupferschmidt R., Stanciu L., Djukanovic R., Schudt C., Wendel A., Church M. K., Shute J. K. (1995) Cyclic nucleotide phosphodiesterase isoenzyme activities in human alveolar macrophages. Clin. Exp. Allergy 25, 625–633. [DOI] [PubMed] [Google Scholar]
- 39. Bender A. T., Beavo J. A. (2004) Specific localized expression of cGMP PDEs in Purkinje neurons and macrophages. Neurochem. Int. 45, 853–857. [DOI] [PubMed] [Google Scholar]
- 40. Omori K., Kotera J. (2007) Overview of PDEs and their regulation. Circ. Res. 100, 309–327. [DOI] [PubMed] [Google Scholar]
- 41. Serezani C. H., Ballinger M. N., Aronoff D. M., Peters-Golden M. (2008) Cyclic AMP: master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 39, 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aronoff D. M., Canetti C., Serezani C. H., Luo M., Peters-Golden M. (2005) Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J. Immunol. 174, 595–599. [DOI] [PubMed] [Google Scholar]
- 43. Aronoff D. M., Canetti C., Peters-Golden M. (2004) Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J. Immunol. 173, 559–565. [DOI] [PubMed] [Google Scholar]
- 44. Raddassi K., Petit J. F., Lemaire G. (1993) LPS-induced activation of primed murine peritoneal macrophages is modulated by prostaglandins and cyclic nucleotides. Cell. Immunol. 149, 50–64. [DOI] [PubMed] [Google Scholar]
- 45. Marotta P., Sautebin L., Di R. M. (1992) Modulation of the induction of nitric oxide synthase by eicosanoids in the murine macrophage cell line J774. Br. J. Pharmacol. 107, 640–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mbawuike I. N., Herscowitz H. B. (1989) MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. J. Leukoc. Biol. 46, 119–127. [DOI] [PubMed] [Google Scholar]
- 47. Matsunaga K., Klein T. W., Friedman H., Yamamoto Y. (2001) Alveolar macrophage cell line MH-S is valuable as an in vitro model for Legionella pneumophila infection. Am. J. Respir. Cell Mol. Biol. 24, 326–331. [DOI] [PubMed] [Google Scholar]
- 48. Sankaran K., Herscowitz H. B. (1995) Phenotypic and functional heterogeneity of the murine alveolar macrophage-derived cell line MH-S. J. Leukoc. Biol. 57, 562–568. [DOI] [PubMed] [Google Scholar]
- 49. Kiemer A. K., Lehner M. D., Hartung T., Vollmar A. M. (2002) Inhibition of cyclooxygenase-2 by natriuretic peptides. Endocrinology 143, 846–852. [DOI] [PubMed] [Google Scholar]
- 50. Denlinger L. C., Fisette P. L., Garis K. A., Kwon G., Vazquez-Torres A., Simon A. D., Nguyen B., Proctor R. A., Bertics P. J., Corbett J. A. (1996) Regulation of inducible nitric oxide synthase expression by macrophage purinoreceptors and calcium. J. Biol. Chem. 271, 337–342. [DOI] [PubMed] [Google Scholar]
- 51. Mauel J., Ransijn A., Corradin S. B., Buchmuller-Rouiller Y. (1995) Effect of PGE2 and of agents that raise cAMP levels on macrophage activation induced by IFN-γ and TNF-α. J. Leukoc. Biol. 58, 217–224. [DOI] [PubMed] [Google Scholar]
- 52. Tonetti M., Sturla L., Bistolfi T., Benatti U., De F. A. (1994) Extracellular ATP potentiates nitric oxide synthase expression induced by lipopolysaccharide in RAW 264.7 murine macrophages. Biochem. Biophys. Res. Commun. 203, 430–435. [DOI] [PubMed] [Google Scholar]
- 53. Kobzik L. (2009) Translating NO biology into clinical advances: still searching for the right dictionary? Am. J. Respir. Cell Mol. Biol. 41, 9–13. [DOI] [PubMed] [Google Scholar]
- 54. Witzenrath M., Gutbier B., Schmeck B., Tenor H., Seybold J., Kuelzer R., Grentzmann G., Hatzelmann A., van L., V, Tschernig T., Mitchell T. J., Schudt C., Rosseau S., Suttorp N., Schutte H. (2009) Phosphodiesterase 2 inhibition diminished acute lung injury in murine pneumococcal pneumonia. Crit. Care Med. 37, 584–590. [DOI] [PubMed] [Google Scholar]
- 55. Schmidt E. P., Damarla M., Rentsendorj O., Servinsky L. E., Zhu B., Moldobaeva A., Gonzalez A., Hassoun P. M., Pearse D. B. (2008) Soluble guanylyl cyclase contributes to ventilator-induced lung injury in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L1056–L1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuebler W. M. (2011) The Janus-faced regulation of endothelial permeability by cyclic GMP. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L157–L160. [DOI] [PubMed] [Google Scholar]
- 57. Wang l. F., Patel M., Razavi H. M., Weicker S., Joseph M. G., McCormack D. G., Mehta S. (2002) Role of inducible nitric oxide synthase in pulmonary microvascular protein leak in murine sepsis. Am. J. Respir. Crit. Care Med. 165, 1634–1639. [DOI] [PubMed] [Google Scholar]
- 58. Razavi H. M., Wang L. F., Weicker S., Rohan M., Law C., McCormack D. G., Mehta S. (2004) Pulmonary neutrophil infiltration in murine sepsis: role of inducible nitric oxide synthase. Am. J. Respir. Crit. Care Med. 170, 227–233. [DOI] [PubMed] [Google Scholar]
- 59. Farley K. S., Wang L. F., Razavi H. M., Law C., Rohan M., McCormack D. G., Mehta S. (2006) Effects of macrophage inducible nitric oxide synthase in murine septic lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L1164–L1172. [DOI] [PubMed] [Google Scholar]