Abstract
Glia constitute the majority of cells in the mammalian central nervous system and are crucial for neurological function. However, there is an incomplete understanding of the molecular control of glial cell development. We find that the transcription factor Ascl1 (Mash1), which is best known for its role in neurogenesis, also functions in both astrocyte and oligodendrocyte lineages arising in the mouse spinal cord at late embryonic stages. Clonal fate mapping in vivo reveals heterogeneity in Ascl1-expressing glial progenitors and shows that Ascl1 defines cells that are restricted to either gray matter (GM) or white matter (WM) as astrocytes or oligodendrocytes. Conditional deletion of Ascl1 post-neurogenesis shows that Ascl1 is required during oligodendrogenesis for generating the correct numbers of WM but not GM oligodendrocyte precursor cells, whereas during astrocytogenesis Ascl1 functions in balancing the number of dorsal GM protoplasmic astrocytes with dorsal WM fibrous astrocytes. Thus, in addition to its function in neurogenesis, Ascl1 marks glial progenitors and controls the number and distribution of astrocytes and oligodendrocytes in the GM and WM of the spinal cord.
Keywords: Astrocyte heterogeneity, Glial specification, Oligodendrogenesis, Spinal cord gliogenesis
INTRODUCTION
Glia, which include astrocytes and oligodendrocytes, are the most abundant cell types in the mammalian central nervous system (CNS) and are crucial for regulating and maintaining neuronal architecture and function. During development, glial progenitor cells undergo an extended precursor cell stage, referred to as intermediate astrocyte precursors (IAPs) or oligodendrocyte precursor cells (OPCs), which can migrate and continue to proliferate in the surrounding gray matter (GM) and white matter (WM) prior to terminal differentiation into mature astrocytes or oligodendrocytes, respectively (Nishiyama et al., 2009; Ge et al., 2012; Tien et al., 2012). Astrocytes in the GM, known as protoplasmic astrocytes, are morphologically distinct from those in the WM, described as fibrous astrocytes (Rowitch, 2004; Oberheim et al., 2012). Similarly, oligodendrocytes in the GM can also be distinguished from those in the WM on the basis of their level of myelin marker expression (Rowitch and Kriegstein, 2010). These differences suggest that astrocytes and oligodendrocytes in the GM are developmentally and functionally distinct from their respective counterparts in the WM. However, how this heterogeneity develops is not known.
The developing vertebrate spinal cord is segmentally organized along the dorsal-ventral (DV) axis into distinct progenitor domains (Jessell, 2000). The pattern of transcription factor expression in the progenitor domains and the unique spatial organization of the spinal cord into GM and WM make it an ideal setting to elucidate the mechanisms of glial heterogeneity (Rowitch and Kriegstein, 2010). The ventral spinal cord is the site of the earliest waves of IAPs and OPCs, where, following the completion of neurogenesis, oligodendrocytes are generated from the pMN domain (Zhou et al., 2000, 2001; Lu et al., 2002; Zhou and Anderson, 2002), whereas the surrounding ventral p1-p3 domains give rise to three distinct ventral WM astrocyte populations (Pringle et al., 2003; Muroyama et al., 2005; Hochstim et al., 2008). A second wave of gliogenesis arises from neural progenitors in the intermediate and dorsal domains that generates oligodendrocytes and astrocytes that reside at similar DV positions in both the GM and WM (Fogarty et al., 2005; Zhu et al., 2011; Tsai et al., 2012). Currently, it is unclear which molecular mechanisms are utilized for specifying IAPs and OPCs in the dorsal spinal cord, or if IAPs and OPCs from this region are derived from the same or separate populations of progenitor cells.
One transcription factor that is highly expressed in neural progenitor cells in the developing dorsal spinal cord is the basic helix-loop-helix (bHLH) factor Ascl1. Ascl1 mutant mice have been shown to exhibit significant alterations in differentiation and specification of both neurons and OPCs (Helms et al., 2005; Mizuguchi et al., 2006; Wildner et al., 2006; Sugimori et al., 2007, 2008). However, because Ascl1 functions in neurogenesis prior to gliogenesis, it is unclear whether the disruption in oligodendrocyte specification and differentiation is a direct effect or secondary to the fundamental changes to the overall progenitor and/or neuronal pools. Furthermore, Ascl1 mutant mice die neonatally, thereby precluding any analysis of the postnatal spinal cord. Thus, the extent of Ascl1 function in glial cell development remains largely undefined, particularly in dorsal spinal cord domains where Ascl1 is expressed in progenitor populations that have been shown to also give rise to astrocytes (Zhu et al., 2011; Tsai et al., 2012).
In this study, we identify the fate of Ascl1-expressing glial progenitors in the mouse spinal cord and determine if Ascl1 is required for the development of glial lineages. We confirm that Ascl1-expressing glial progenitors directly give rise to oligodendrocytes, and also to dorsally restricted spinal cord astrocytes. Clonal analysis in vivo reveals that a subset of Ascl1-expressing progenitors in the ventricular zone (VZ) is already lineage restricted toward only the GM or WM, but not to both, either as astrocytes or oligodendrocytes. We further show that, in the absence of Ascl1, Ascl1 lineage cells give rise to an increased number of GM protoplasmic astrocytes and a decreased number of WM OPCs/oligodendrocytes and WM fibrous astrocytes. Thus, Ascl1 marks a heterogeneous population of glial progenitor cells and is crucial for generating a normal distribution of both astrocytes and oligodendrocytes in the GM and WM of the spinal cord.
RESULTS
Ascl1 is expressed in progenitor and precursor cells during gliogenesis in the spinal cord
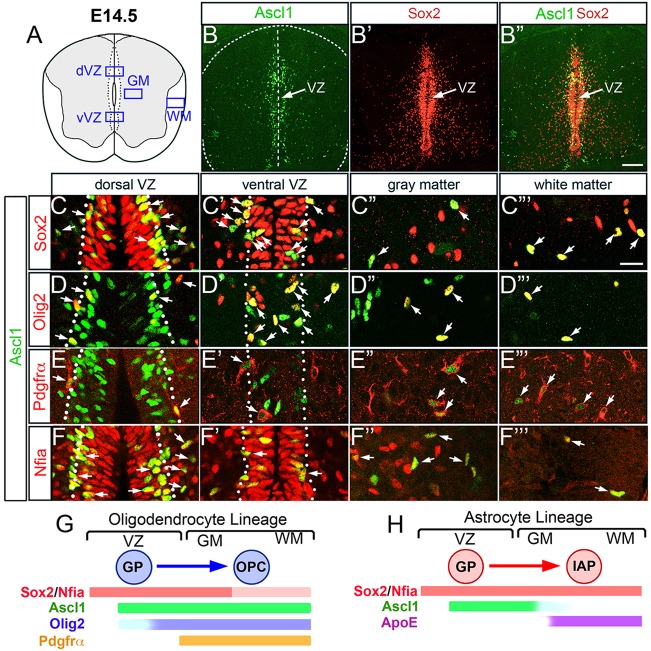
It was previously reported that Ascl1 is expressed during oligodendrogenesis in the spinal cord of rat and mouse (Battiste et al., 2007; Sugimori et al., 2007, 2008). To assess Ascl1 expression during gliogenesis in the spinal cord in more detail, double immunofluorescence for Ascl1 along with glial lineage markers was performed from embryonic day (E) 14.5 to E18.5. At E14.5, Ascl1 is broadly expressed throughout the VZ as well as in some scattered cells in the GM and WM (Fig. 1B). As development proceeds, the number of Ascl1-expressing cells increases in the GM and WM but is diminished in the VZ by E17.5 (supplementary material Fig. S1A,F,K). All Ascl1+ cells in the VZ, GM and WM are positive for Sox2 (Fig. 1B-C‴; supplementary material Fig. S1A-D,F-I,K-N), which at these stages is expressed in glial progenitor and precursor cells (Hoffmann et al., 2014). A few of the Ascl1+ cells in or near the VZ are positive for Olig2 or the OPC-specific marker Pdgfrα, especially in the dorsal spinal cord (Fig. 1D,D′,E,E′), whereas all Ascl1+ cells in the GM and WM are Olig2+, and most of these are also Pdgfrα+ (Fig. 1D″,D‴,E″,E‴; supplementary material Fig. S1E,J). By contrast, all of the Ascl1+ cells in the VZ and most Ascl1+ cells in the GM and WM from E14.5 to E18.5 are positive for Nfia (Fig. 1F′-F‴), a transcription factor that is required for the specification and differentiation of IAPs (Deneen et al., 2006; Kang et al., 2012). However, none of the Nfia+ Ascl1+ cells in the GM and WM expressed the astrocyte lineage marker ApoE (supplementary material Fig. S1O) (Bachoo et al., 2004), indicating that Ascl1 is downregulated in the astrocyte lineage once glial progenitors transition out of the VZ into IAPs (Fig. 1G,H).
Fig. 1.
. Ascl1 is transiently expressed in glial progenitor and precursor cells in the developing mouse spinal cord. Immunofluorescence for Ascl1 (green) and glial lineage markers (red) on thoracic spinal cord sections at E14.5. (A) Schematic of imaged areas. (B-F‴) Ascl1 (green) colocalizes (arrows) with Sox2 (B-C‴), Olig2 (D-D‴), Pdgfrα (E-E‴) and Nfia (F-F‴). Dotted lines indicate the lateral boundary of the ventricular zone, as determined by the density of Sox2 expression. (G,H) Schematic of Ascl1 expression in oligodendrocyte and astrocyte lineages in the post-E14.5 spinal cord relative to other markers used in this study. GP, glial progenitor; OPC, oligodendrocyte precursor cell; IAP, intermediate astrocyte precursor; VZ, ventricular zone (d, dorsal; v, ventral); GM, gray matter; WM, white matter. Scale bars: 100 μm for B-B″; 25 μm for C-F‴.
Although at these stages Olig2 and Nfia are dynamically expressed and are not completely restricted to the oligodendrocyte or astrocyte lineages, respectively (Cai et al., 2007; Deneen et al., 2006), these findings indicate that Ascl1 is expressed in glial progenitor and precursor cells to both the oligodendrocyte and astrocytes lineages in the spinal cord.
Ascl1 lineage cells include astrocytes and oligodendrocytes that are found in both the GM and WM of the spinal cord
Previous lineage analysis using bacterial artificial chromosome (BAC) transgenic mice showed that Ascl1-expressing cells are restricted to neuronal and oligodendroglial lineages in the telencephalon and spinal cord, and do not become astrocytes (Battiste et al., 2007; Parras et al., 2007). By contrast, recent fate mapping using Ascl1CreERT2 knock-in (KI) mice showed that both astrocytes and oligodendrocytes are labeled during gliogenesis in the cerebellum (Sudarov et al., 2011), indicating that the Ascl1CreERT2 KI allele may be able to label more progenitor types or an earlier, multipotent progenitor population that was not detected in the Ascl1 BAC transgenic lines.
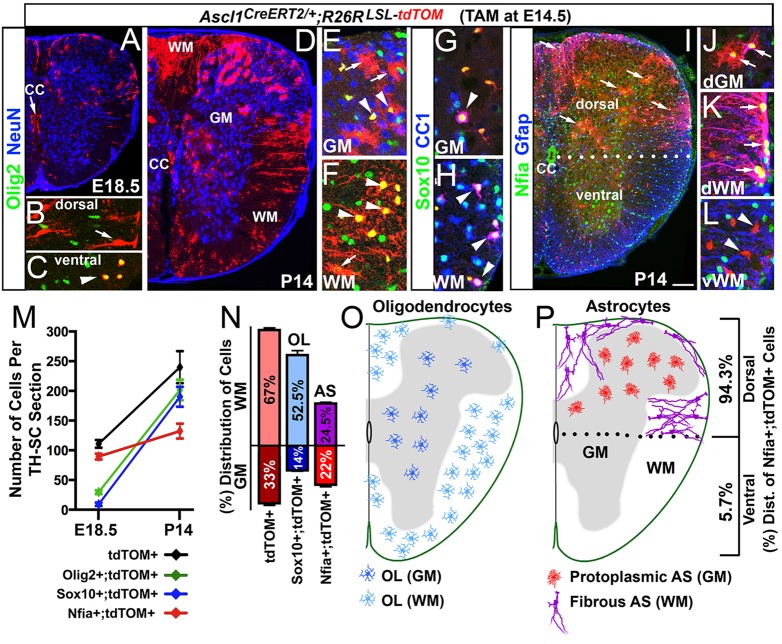
To determine whether Ascl1-expressing glial progenitors in the spinal cord are capable of giving rise to both oligodendrocytes and astrocytes, Ascl1CreERT2/+ KI mice were crossed with the Cre recombinase-dependent reporter R26RLSL-tdTOM. Cre recombinase activity was induced at E14.5 by administering tamoxifen (2.5 mg/40 g body weight) to gravid females to permanently label Ascl1-expressing cells. This stage was specifically chosen because neurogenesis is largely complete and Ascl1+ glial progenitors are present in high numbers, especially in the dorsal spinal cord VZ (Fig. 1). Analysis at E18.5 and postnatal day (P) 14 showed that tdTOM+ cells were widely distributed in both GM and WM and, as expected, very few if any neurons (NeuN+; Rbfox3 – Mouse Genome Informatics) were labeled (Fig. 2A,D,E). Indeed, unlike Ascl1-CreERTM BAC transgenic mice (data not shown; Battiste et al., 2007), the tdTOM+ cells co-express either oligodendrocyte [Olig2, Sox10, CC1 (Apc)] (Fig. 2C,E-H) or astrocyte (Nfia, Gfap) markers (Fig. 2I-L). Quantification at the level of the thoracic region showed that 112±3.5 tdTOM+ cells were labeled per spinal cord section at E18.5, and this increased ∼2-fold to 269±17.4 tdTOM+ cells per section by P14 (black line, Fig. 2M). Notably, Ascl1 lineage OPCs (Olig2+;tdTOM+ and Sox10+;tdTOM+), despite constituting only ∼20% of the labeled tdTOM+ cells at E18.5, increased to make up the majority (66.5%) of the labeled cells by P14 and were found mostly in the WM (Fig. 2M-O). By contrast, the Ascl1 lineage astrocytes (Nfia+;tdTOM+) increased to a lesser extent between E18.5 and P14 (red line, Fig. 2M), were restricted predominantly to the dorsal half of the spinal cord and were evenly distributed into the GM (22%) and WM (24.5%) (Fig. 2I-N,P). This dorsal restriction of tdTOM+ astrocytes suggests that they are derived from the dorsal Ascl1+ glial progenitors, since astrocytes in the spinal cord are not known to undergo extensive tangential migration (Zhu et al., 2011; Tsai et al., 2012).
Fig. 2.
Ascl1-expressing glial cells give rise to both astrocytes and oligodendrocytes in the GM and WM of the spinal cord. Immunofluorescence on E18.5 (A-C) or P14 (D-L) thoracic spinal cord (TH-SC) sections of Ascl1CreERT2/+;R26LSL-tdTOM mice treated with tamoxifen at E14.5. Red is tdTOM fluorescence marking Ascl1 lineage cells. (A-H) tdTOM does not colocalize with neuronal marker NeuN (A,D), which was used here to delineate the GM, but colocalizes with the oligodendrocyte (OL) markers Olig2, Sox10 and CC1 (B,C,E-H, arrowheads) in the GM and WM. Note that Olig2 and Sox10 are not expressed in tdTOM+ astrocytes (B,E,F, arrows). (I-L) tdTOM also colocalizes with the astrocyte (AS) markers Nfia and Gfap in the GM and WM in the dorsal spinal cord (arrows). Ventral tdTOM+ cells do not express Nfia and Gfap (arrowheads, L). (M-P) Quantification and schematic summary of the number (mean±s.d.) and distribution of tdTOM-labeled astrocyte (Nfia+;tdTOM+) and oligodendrocyte (Olig2+;tdTOM+ and Sox10+;tdTOM+) lineage cells per thoracic spinal cord section. Percentage (N) refers to the percentage of total tdTOM+ cells for each cell type in GM or WM at P14. Dotted lines (I,P) delineate the dorsal-ventral boundary at the level of the central canal (CC). Number of spinal cords analyzed: N=4 at E18.5 and N=3 at P14. Scale bar: 100 μm for A,D,I; 25 μm for B,C,E-H,J-L.
There was a less than 15% overlap in the tdTOM+ cells marked by Olig2 or Nfia at the various stages analyzed, which is consistent with the known overlap of these markers early in the glial lineages. However, taken together with the later oligodendrocyte- and astrocyte-specific markers, this long-term fate-mapping analysis into the postnatal spinal cord illustrates that Ascl1 is expressed in glial progenitors that are fated to both the oligodendrocyte and astrocyte lineages.
Individual Ascl1+ glial cells are restricted in distribution to the GM or WM
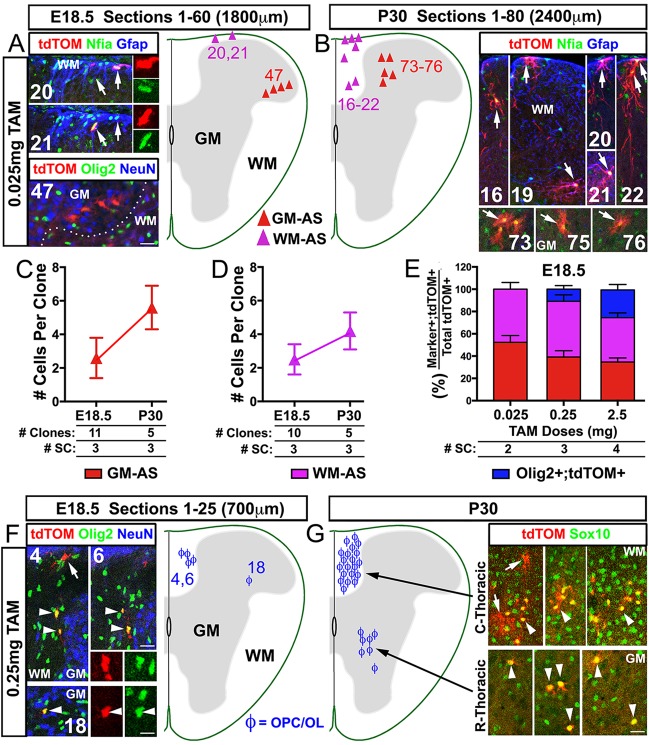
To determine if individual Ascl1+ progenitor cells in the spinal cord could be shown to be bipotential in giving rise to both astrocytes and oligodendrocytes, a low dose of tamoxifen (0.025 mg/40 g body weight) was administered to Ascl1CreERT2/+;R26LSL-tdTOM mice at E14.5 to sparsely label single Ascl1-expressing cells. To verify the sparse labeling of cells, 50 consecutive sections (totaling 1500 μm thickness) through the thoracic (N=2) or lumbar (N=2) regions were examined 12 h post tamoxifen administration at E15.0. tdTOM+ cells were found only in the dorsal spinal cord primarily in or immediately adjacent to the VZ (64%), and in the GM (36%), but not the WM (supplementary material Fig. S2A-G,N). To define the tdTOM+ cells as clones, a radius of 150 μm (∼20 cell diameters) was measured for each tdTOM+ cell along the anterior-posterior (AP) axis, and all labeled cells within this radius were considered a clone. Out of 16 putative clones identified, 11 consisted of a single cell (supplementary material Fig. S2A,C). Marker analysis revealed that these tdTOM+ cells were not positive for Olig2 (supplementary material Fig. S2D-G) or the OPC marker Pdgfrα (not shown), and thus were likely to be astrocyte lineage cells. This was distinctly different from the tdTOM+ cells initially labeled at the higher doses of tamoxifen (supplementary material Fig. S2H-N), which, as demonstrated below, contained both astrocyte and oligodendrocyte lineages.
Screening serial sections through spinal cords at E18.5 showed that, unlike at E15.0, the sparsely labeled tdTOM+ cells were absent from the VZ and were found only in the GM or WM. The tdTOM+ cells still consisted of astrocyte (Nfia+, Gfap+) and not oligodendrocyte (Olig2−) lineage cells (Fig. 3A,E), and were evenly distributed to the GM and WM in the dorsal spinal cord. This distribution pattern was similar to that seen at the higher tamoxifen doses at this stage (GM-AS and WM-AS bars, Fig. 3E), and suggests that within the astrocyte lineage, both low and high tamoxifen doses are labeling a similar set of cells. Because IAPs are known to undergo local proliferation (Ge et al., 2012), the sparsely labeled tdTOM+ IAPs were defined as clonal if they were found in the same or neighboring sections within 150 μm of each other, but at least 300 μm away from other labeled cells along the AP axis. Strikingly, all tdTOM+ IAPs in a defined clone were found in close proximity to each other within the same or adjacent sections, as clusters of 2-4 cells in the GM or WM (Fig. 3A,C,D). This spatial restriction of Ascl1 lineage tdTOM+ astrocytes in the dorsal spinal cord to just the GM or WM was also observed at P30, although the number of cells per clone at this stage had doubled (Fig. 3B-D). These findings suggest that Ascl1+ progenitors in the dorsal VZ, as labeled at the low tamoxifen dose, are not bipotential but instead are restricted in fate to the GM or WM as astrocytes.
Fig. 3.
The development of Ascl1 lineage marked clones is spatially restricted to the GM or WM in the spinal cord. Immunofluorescence on spinal cord sections of Ascl1CreERT2/+;R26LSL-tdTOM mice treated with low (0.025 mg/40 g body weight, A,B) or intermediate (0.25 mg/40 g body weight, F,G) doses of tamoxifen at E14.5. (A) Sixty consecutive sections of E18.5 spinal cord contained only astrocyte labeled clones, separated by 780 μm in this example, and were restricted to WM (sections 20, 21) or GM (section 47). (B) Eighty consecutive sections of P30 spinal cord contained the progeny of two astrocyte labeled clones, separated by 1530 μm in this example, and were restricted to WM (sections 16-22) or GM (sections 73-76). (C,D) Number (mean±s.d.) of cells per GM astrocyte (red triangle) or WM astrocyte (purple triangle) clone at E18.5 and P30 from the lowest tamoxifen doses as in A,B. (E) Tamoxifen dose curve of E18.5 spinal cord showing the percentage (mean±s.e.m.) of tdTOM+ AS (Olig2− or Nfia+) and Olig2+;tdTOM+ cells labeled. (F) Twenty-five consecutive sections of E18.5 spinal cord showing the presence of two Ascl1 lineage OPC clusters, separated by 360 μm in this example, that were restricted to WM (sections 4, 6) or GM (section 18). An Olig2−;tdTOM+ cell (astrocyte) is also present (arrow) but the clonal relationship to the Olig2+;tdTOM+ cells cannot be determined. (G) P30 thoracic spinal cord from caudal or rostral thoracic sections showing spatial restriction of Ascl1 lineage oligodendrocyte clusters to either WM or GM, but not both. Arrows indicate IAPs/astrocytes and arrowheads indicate OPCs/oligodendrocytes. Scale bars: 25 μm for A,B,F,G; 12.5 μm for insets in A,F.
At the very low dose of tamoxifen administered, no OPCs or oligodendrocytes were labeled. To increase the probability of capturing Ascl1+ clones of the oligodendrocyte lineage at E14.5, an intermediate dose of tamoxifen (0.25 mg/40 g body weight) was used. At 24 h post tamoxifen administration, a few tdTOM+ cells were observed per spinal cord section (supplementary material Fig. S2H), all of which were Nfia+ (supplementary material Fig. S2J). Occasionally, some of these tdTOM+ cells were also Olig2+ (supplementary material Fig. S2I). The distribution of the tdTOM+ cells in the VZ, GM and WM at the intermediate dose is similar to that of the high (2.5 mg/40 g body weight) tamoxifen dose (supplementary material Fig. S2K-N). By E18.5, with the intermediate tamoxifen dose clusters of 2-4 tdTOM+ OPCs (Olig2+;tdTOM+) were sparsely labeled in the dorsal WM or GM (Fig. 3F), along with consistent labeling of astrocytes. These Olig2+;tdTOM+ clusters account for ∼11% of the total tdTOM+ cells, compared with 24% at the high tamoxifen dose at this time point (Fig. 3E). Unfortunately, due to the density of cells labeled at the intermediate dose, it cannot be determined whether the labeled OPC clusters (Fig. 3F) and surrounding astrocytes (Fig. 3F) arise from a common or separate Ascl1-expressing cell(s). By P30, the size of tdTOM+ OPCs/oligodendrocyte clusters had dramatically increased from 2-4 to 10-50 cells, as assessed by Sox10 expression (Sox10+;tdTOM+), to occupy large portions (0.5-1 mm) of the spinal cord along the AP axis. Despite this expansion, however, all the Sox10+;tdTOM+ cells within a cluster were found together and restricted only to the dorsal WM or GM (Fig. 3G). This spatial restriction of tdTOM+ oligodendrocyte lineage clusters at both E18.5 and P30 to only the WM or GM implies that the labeled Ascl1+ cells in the oligodendrocyte lineage, similar to what was seen for the astrocyte lineage, are already restricted to localize in the GM or WM. These findings do not however exclude the possible existence of Ascl1-expressing bipotential or multipotential cells that might not be marked with the low doses of tamoxifen required for these experiments.
The number and distribution of glial cells in the dorsal spinal cord are altered in the absence of Ascl1
It was previously shown that Ascl1 mutant mice exhibit defects in OPC specification and differentiation in the spinal cord (Sugimori et al., 2007, 2008). However, it was unclear whether these defects were a direct effect or the result of fundamental changes to the overall progenitor and/or neuronal pools resulting from the loss of earlier Ascl1 roles in neurogenesis (Helms et al., 2005; Mizuguchi et al., 2006; Wildner et al., 2006). Furthermore, because it was not known that Ascl1-expressing glial progenitors also give rise to dorsal astrocytes (Figs 2 and 3), the effects on astrocyte development were not assessed. Finally, Ascl1 null mutants die shortly after birth, so the consequences for glial cell development and distribution in the GM and WM, which occur postnatally, could not be addressed.
To circumvent these limitations, Ascl1 was conditionally knocked out (Ascl1 CKO) only in Ascl1-expressing cells post-neurogenesis at E14.5 in the spinal cord. This was accomplished by crossing Ascl1CreERT2/+ KI mice with R26RLSL-tdTOM Cre reporter mice that also carry an Ascl1FL allele, then Cre recombinase was induced with tamoxifen (2.5 mg/40 mg body weight) at E14.5. Although the initial function of Ascl1 in glial progenitor cells cannot be assessed, a unique advantage with this paradigm is that only those cells that express Ascl1 at the time of tamoxifen administration will be labeled and affected, while others in the surrounding neural landscape will be spared. The efficient deletion of Ascl1 was determined at E15.5, 24 h after tamoxifen administration in control (Ascl1CreERT2/+;R26LSL-tdTOM) or Ascl1 CKO (Ascl1CreERT2/FL;R26LSL-tdTOM) spinal cord. In control, Ascl1 lineage tdTOM+ cells in the VZ and some in the GM continued to express Ascl1 (supplementary material Fig. S3B). However, Ascl1 expression was no longer detected in the majority of tdTOM+ cells in the Ascl1 CKO (supplementary material Fig. S3B′). Extending this analysis to E18.5, Ascl1 was also not expressed in Olig2+;tdTOM+ cells of the Ascl1 CKO (supplementary material Fig. S3F′-H′), whereas it was in controls (supplementary material Fig. S3F-H).
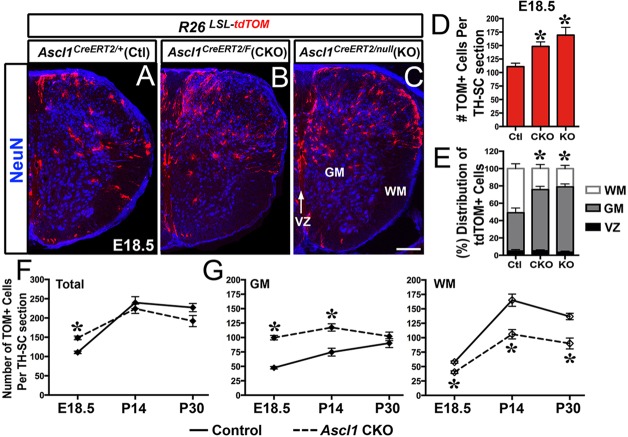
To evaluate whether the post-neurogenesis conditional knockout of Ascl1 had a disruption in spinal cord gliogenesis, the total number and distribution of tdTOM+ cells in the Ascl1 CKO were compared with those in control and Ascl1 KO mice (Ascl1CreERT2/null;R26LSL-tdTOM treated with tamoxifen at E14.5). Analysis at E18.5 showed that over 90% of the tdTom+ cells had migrated out of the VZ in all three conditions; therefore, Ascl1 lineage cells are not maintained as radial glial even in the absence of Ascl1 (Fig. 3E). Additionally, the tdTOM+ cells were largely non-neuronal and were mostly restricted to the dorsal spinal cord. Notably, the Ascl1 CKO and Ascl1 KO had a ∼40% increase in the number of tdTOM+ cells (Fig. 4A-D). This could be due in part to the loss of negative feedback on Ascl1 expression, which is known to occur in this locus (Horton et al., 1999), a confound that is difficult to eliminate. However, this increase in labeled cells could also result from additional rounds of division, an interpretation supported by clonal experiments (see Fig. 8 and related text below). Another notable phenotype was a shift in the distribution of the tdTOM+ cells from WM to GM (Fig. 4E-G), with the absolute number and ratio of Ascl1 lineage oligodendrocytes (Olig2+;tdTOM+ and Sox10+;tdTOM+) and astrocytes (Nfia+;tdTOM+) in the GM and WM permanently altered in the Ascl1 CKO into postnatal stages (supplementary material Fig. S4). These findings implicate a functional role for Ascl1 during gliogenesis in the spinal cord.
Fig. 4.
The number and distribution of Ascl1 lineage glial cells into the GM and WM are altered in Ascl1 CKO and Ascl1 KO spinal cords. Immunofluorescence on thoracic sections of E18.5 Ascl1CreERT2/+;R26LSL-tdTOM control, Ascl1CreERT2/FL;R26LSL-tdTOM (Ascl1 CKO) or Ascl1CreERT2/null;R26LSL-tdTOM (Ascl1 KO) mice treated with tamoxifen at E14.5. (A-E) NeuN delineates the boundaries between VZ, GM and WM. The number and distribution of tdTOM+ cells are increased in the GM of Ascl1 CKO and Ascl1 KO at E18.5. (F,G) Number of tdTOM+ cells is altered in the GM and WM of Ascl1 CKO into postnatal stages. Number of spinal cords analyzed: E18.5, N=4 control, N=3 Ascl1 CKO and Ascl1 KO; P14, N=3 control and Ascl1 CKO; P30, N=2 control and Ascl1 CKO. *P<0.005 indicates statistical significance using linear regression analysis. Values in graphs are mean±s.e.m. Scale bar: 100 μm.
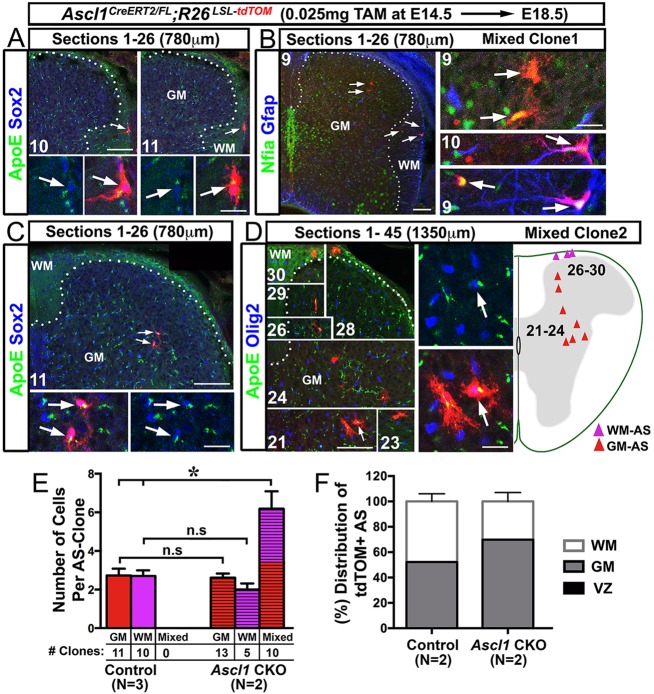
Fig. 8.
The proliferation and distribution of astrocyte clones in the GM and WM are altered in Ascl1 CKO spinal cords. Immunofluorescence on spinal cord sections of Ascl1CreERT2/FL;R26LSL-tdTOM mice treated with a low dose of tamoxifen (0.025 mg/40 g body weight) at E14.5. (A,C) Consecutive sections showing the presence of WM-only (A) or GM-only (C) labeled astrocyte clones found at the center of two separate 780 μm spinal cord portions. (B,D) Consecutive sections showing the presence of two mixed (GM and WM) astrocyte-labeled clones that are found separately in a 780 μm or 1350 μm spinal cord segment. Note that the number of cells in the mixed clones is increased compared with GM-only or WM-only clones. Dotted lines delineate the GM/WM boundary. Arrows indicate labeled AS noted in both low- and high-magnification images. (E) Number (mean±s.e.m.) of cells per AS clone. The number of each type of clone is also shown. (F) Distribution of sparsely labeled tdTOM+ AS cells in the VZ, GM and WM between control and Ascl1 CKO littermates. *P<0.005, Student's t-test; n.s, not significant. Scale bars: 100 μm for low-magnification images; 25 μm for high-magnification insets.
Ascl1 is required to generate the correct number of WM OPCs in the spinal cord
To understand the role of Ascl1 during oligodendrogenesis in the spinal cord, the developmental dynamics of Ascl1 lineage tdTOM+ OPCs in the GM and WM were analyzed in the thoracic region over time in Ascl1CreERT2/+;R26LSL-tdTOM control mice following tamoxifen (2.5 mg/40 mg body weight) at E14.5. At E18.5, the number of tdTOM+ cells co-expressing Olig2 (Olig2+;tdTOM+), which should mark oligodendrocyte lineage cells at this stage, was distributed evenly into the GM (11.3±3.6) and WM (14.5±3.5) (Fig. 5A,I,J,K). Fewer tdTOM+ cells at this stage co-expressed Sox10 (Sox10+;tdTOM+) (Fig. 5O-Q), most likely because Sox10 is a more mature marker, and Olig2 is also expressed in a small subset of immature astrocytes (Fig. 6B,C) (Cai et al., 2007).
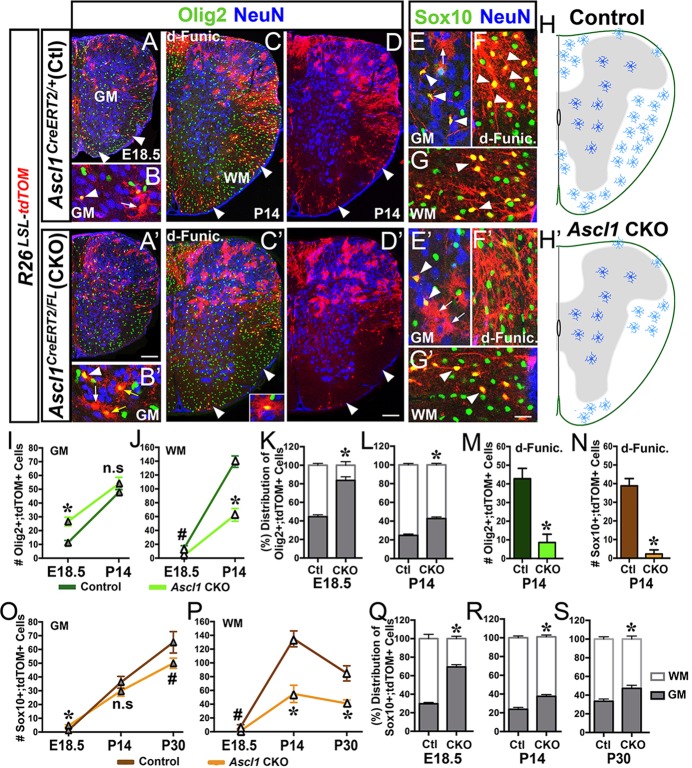
Fig. 5.
Number of OPCs and oligodendrocytes is decreased in WM of Ascl1 CKO spinal cords. Immunofluorescence on thoracic sections of Ascl1CreERT2/+;R26LSL-tdTOM control or Ascl1CreERT2/FL;R26LSL-tdTOM CKO mice treated with tamoxifen at E14.5. (A-G) Control spinal cords showing very few Olig2+;tdTOM+ cells at E18.5 (arrowheads, A,B), but many Olig2+;tdTOM+ (arrowheads, C) and Sox10+;tdTOM+ (arrowheads, F,G) cells in the WM at P14. (A′-G′) Ascl1 CKO spinal cords showing Olig2+;tdTOM+ cells in GM at E18.5 (yellow arrows, A′,B′), and Olig2+;tdTOM+ (arrowheads, C′,D′) and Sox10+;tdTOM+ cells (arrowheads, F′,G′) in the WM. A dramatic decrease in Sox10+;tdTOM+ cells is detected especially in the dorsal funiculus at P14 (compare yellow cells in F′ and F). (H,H′) Schematic summary of the oligodendrocyte phenotype of control and Ascl1 CKO at P14/P30. (I-S) The number and distribution of Olig2+;tdTOM+ (I-M) and Sox10+;tdTOM+ (N-S) cells per thoracic section in the GM, WM and dorsal funiculus of control and Ascl1 CKO. Number of spinal cords analyzed: E18.5, N=4 control, N=3 Ascl1 CKO; P14, N=3 control and Ascl1 CKO; P30, N=2 control and Ascl1 CKO. #P<0.05 and *P<0.005 indicate statistical significance using linear regression analysis. Values in graphs are mean±s.e.m. Scale bars: 100 μm for A,A′,C-D′; 25 μm for B,B′,E-G′.
Fig. 6.
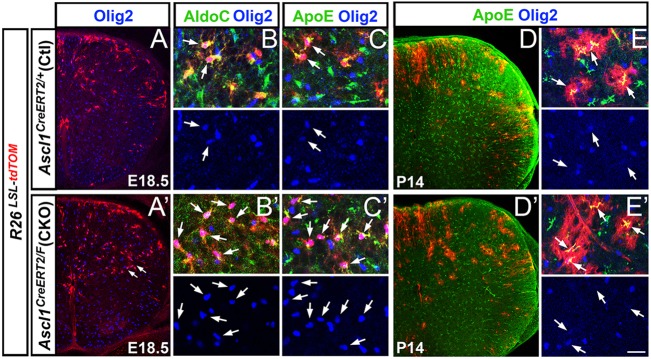
Number of Ascl1 lineage Olig2+ astrocytes is increased in the GM of Ascl1 CKO spinal cords at E18.5. Immunofluorescence on thoracic sections of Ascl1CreERT2/+;R26LSL-tdTOM control or Ascl1CreERT2/FL;R26LSL-tdTOM CKO mice treated with tamoxifen at E14.5. (A-C) Control spinal cord showing the Ascl1 lineage from E14.5 includes a few Olig2+;tdTOM+ cells in the GM that also co-express the astrocyte markers AldoC and ApoE (arrows, B,C) at E18.5. (A′-C′) Number of Olig2+;tdTOM+ astrocytes (AldoC+ ApoE+) is dramatically increased in the GM of Ascl1 CKO spinal cord at E18.5 (arrows, A′-C′). (D-E′) Olig2+;ApoE+;tdTOM+ astrocytes are no longer as apparent by P14 in the Ascl1 CKO compared with controls. Scale bar: 100 μm for A,A′,D,D′; 25 μm for B-C′,E,E′.
By P14, the number of Olig2+;tdTOM+ cells per section was increased ∼4-fold in the GM (47.8±3.8) and 10-fold in the WM (139.4±8.2) (Fig. 5I,J). A similar number of Sox10+;tdTOM+ cells was also observed in the GM (36.2±4.3) and WM (134.9±11.5) (Fig. 5C-G,O,P), consistent with Olig2 and Sox10 both marking similar sets of cells at this stage. Some Olig2+;tdTOM+ or Sox10+;tdTOM+ cells in the GM and most in the WM at this stage are also CC1+ (Fig. 2G,H), illustrating that they have matured into oligodendrocytes. Interestingly, analysis at P30 revealed that the number of Sox10+;tdTOM+ cells had increased by 80% in the GM (65.2±7.8) but decreased by 37% in the WM (84.8±10.9) compared with that at P14 (Fig. 5O,P). The reason for this decrease in the WM at P30 is unclear, but it could reflect a faster rate of WM OPC maturation to oligodendrocyte and/or a turnover of WM oligodendrocytes within this time frame. Overall, these findings indicate that the proliferation and differentiation of Ascl1 lineage OPCs in the GM and WM of control spinal cords are highly dynamic.
The development of the oligodendrocyte lineage in the absence of Ascl1 was then analyzed. At E18.5, the effect on the GM oligodendrocyte lineage is complex. In particular, both Olig2+;tdTOM+ and Sox10+;tdTOM+ cells in the GM were doubled in number in the Ascl1 CKO compared with control (Fig. 5I,O). Unexpectedly, the morphology of most of the Olig2+;tdTOM+ cells in the GM of the Ascl1 CKO resembled that of astrocytes (Fig. 5B′) and were negative for the OPC marker Pdgfrα (supplementary material Fig. S3C′-E′). This increase in Olig2+;tdTOM+ astrocyte-like cells was also noted in the GM of Ascl1 KO spinal cords at E18.5 (supplementary material Fig. S3F″-H″). Marker analysis revealed that, indeed, most of the Olig2+;tdTOM+ cells in the GM of the Ascl1 CKO co-express astrocyte markers such as AldoC and ApoE (Fig. 6A-C,A′-C′) (Walther et al., 1998; Bachoo et al., 2004). However, whether this phenotype is the result of a transfating of Olig2+ OPCs to GM astrocytes, or a delay in the downregulation of Olig2 expression in the astrocyte lineage, could not be determined. But by P14, these Olig2+;tdTOM+ astrocytes (AldoC+ ApoE+) were no longer as apparent in the Ascl1 CKO (Fig. 6D,E versus 6D′,E′). Accordingly, the numbers of Olig2+;tdTOM+ and of Sox10+;tdTOM+ cells in the GM of the Ascl1 CKO were not significantly different to the control (Fig. 5I,O). Interestingly, by P30 the number of Sox10+;tdTOM+ cells in the GM of the Ascl1 CKO had decreased to 80% of controls (Fig. 5O), suggesting that Ascl1 might be required for continued proliferation or survival of the GM oligodendrocyte lineage.
By contrast, in the WM there was a persistent and significant decrease in the number of Olig2+;tdTOM+ and Sox10+;tdTOM+ cells in the Ascl1 CKO from E18.5 to P30 (compare Fig. 5A-G with 5A′-G′,J,P), suggesting that the proliferation or expansion of WM OPCs is disrupted. This decrease was especially severe in the dorsal funiculus of the Ascl1 CKO at P14, in which there was almost a complete absence of Olig2+;tdTOM+ and Sox10+;tdTOM+ cells in contrast to the control (Fig. 5F versus 5F′,M,N). This preferential loss in the WM resulted in an apparent proportional shift in the distribution of Ascl1 lineage OPC/oligodendrocytes from the WM to the GM in the spinal cord (Fig. 5K,L,Q-S).
Collectively, these findings demonstrate a differential requirement for Ascl1 for OPCs in the GM and WM, and, together with the results from the lineage tracing using the intermediate tamoxifen dose (Fig. 3F,G), imply that GM and WM oligodendrocytes arise from distinct Ascl1 lineage cells (see Fig. 9A,B).
Fig. 9.
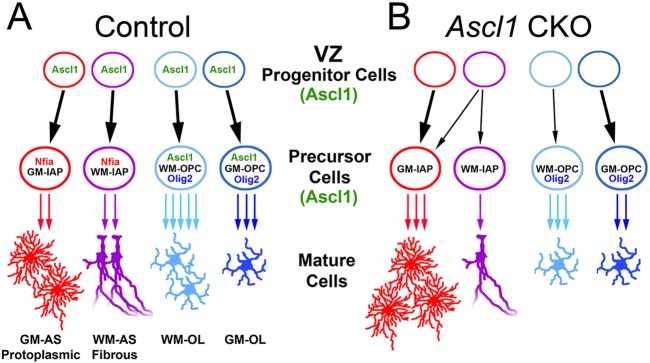
Summary of Ascl1 expression and function during gliogenesis in the spinal cord. (A) Ascl1 is expressed in progenitors to four different glial lineages. The number of arrows at precursor cell stage indicates the degree of proliferation for each lineage, as inferred between E18.5 and P30. (B) Ascl1 CKO showed an increase in the GM-AS lineage and decreases in the WM-AS and WM-OL lineages. In the absence of Ascl1, progenitors that normally give rise to WM-AS also give rise to mixed WM/GM progeny. Ascl1 regulates the development of these lineages in the spinal cord at the progenitor and precursor cell stages.
Ascl1 is required to balance the number of GM and WM astrocytes in the dorsal spinal cord
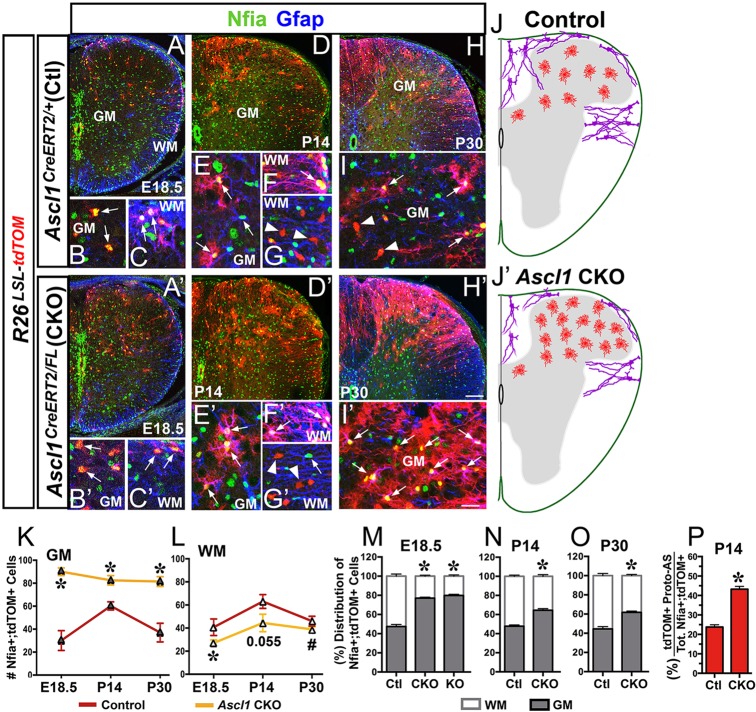
Tracing the lineage of Ascl1-expressing cells at E14.5 in the Ascl1CreER/+;R26LSL-tdTOM control mice revealed that they give rise equally to both protoplasmic and fibrous astrocytes in the dorsal spinal cord (Fig. 2I-K,N and Fig. 7A-I). To determine if Ascl1 plays a role in the development of these two dorsal astrocyte lineages, spinal cords of Ascl1 CKO and Ascl1 KO (2.5 mg tamoxifen/40 mg body weight) were examined in comparison to controls. At E18.5, the number and percentage of Nfia+;tdTOM+ cells were significantly increased in the GM and decreased in the WM of the Ascl1 CKO and Ascl1 KO relative to control (Fig. 7A versus 7A′,K-M), suggesting that Ascl1 normally functions to suppress the generation of GM astrocytes. This phenotype is unlikely to be due to a delay in migration or maturation of these astrocytes because it persists into postnatal stages through P30 of the Ascl1 CKO (Fig. 7D,H versus 7D′,H′,K-O). More importantly, at these postnatal stages the increased GM Nfia+;tdTOM+ cells were able to migrate into the dorsal horn and begin to exhibit a complex morphology resembling that of protoplasmic astrocytes (Fig. 7E,E′,I,I′). Indeed, there were twice as many tdTOM+ cells with a protoplasmic astrocyte morphology in the Ascl1 CKO than in the control (Fig. 7P).
Fig. 7.
The balance of GM and WM astrocytes is altered in the spinal cord of Ascl1 CKO mice. Immunofluorescence on thoracic spinal cord sections of Ascl1CreERT2/+;R26LSL-tdTOM control and Ascl1CreERT2/FL;R26LSL-tdTOM CKO mice treated with tamoxifen at E14.5. (A-I) E18.5 control showing that Nfia+;tdTOM+ cells are Gfap– in GM (arrows, B) and Gfap+ in WM (arrows, C). By P14 and P30, most Nfia+;tdTOM+ cells in the GM exhibited a complex morphology resembling that of protoplasmic astrocytes (arrows, E,I). Note that Nfia and Gfap are not expressed in tdTOM+ OL (arrowheads, G,I). (A′-I′) Ascl1 CKO showing an increase in the number of Nfia+;tdTOM+ cells in the dorsal GM at E18.5 (A′-C′), which exhibited protoplasmic morphology at P14 (D′-G′) and P30 (H′,I′). (J,J′) Schematic summary comparing the astrocyte phenotype of control and Ascl1 CKO spinal cords at P14/P30. (K-O) Number and distribution of Nfia+;tdTOM+ cells per section in the GM and WM of control, Ascl1 CKO and Ascl1 KO spinal cords at E18.5, P14 and P30. (P) Percentage of tdTOM+ cells with protoplasmic morphology among Nfia+;tdTOM+ cells at P14. Number of spinal cords analyzed: E18.5, N=3 control, N=2 Ascl1 CKO and Ascl1 KO; P14, N=3 control and Ascl1 CKO; P30, N=2 control and Ascl1 CKO. #P<0.05 and *P<0.005 indicate statistical significance using linear regression analysis. Values in graphs are mean±s.e.m. Scale bars: 100 μm for A,A′,D,D′,H,H′; 25 μm for B-C′,E-G′,I,I′.
To gain insight into how the imbalance of GM protoplasmic and WM fibrous astrocytes arises in the absence of Ascl1, a low dose of tamoxifen (0.025 mg/40 g body weight) was administered to Ascl1 CKO mice at E14.5. Unlike in the control, in which GM-only and WM-only astrocyte clones were noted at E18.5 (Fig. 3), in the Ascl1 CKO mixed clones, which contained tdTOM+ cells in both the GM and WM, were also observed in addition to GM-only and WM-only clones (Fig. 8A-D). The GM-only and WM-only clones in the Ascl1 CKO were positive for astrocyte markers (Sox2, ApoE, tdTOM) (Fig. 8A,C) and contained a similar number of tdTOM+ cells per clone as in the control (Fig. 8E). By contrast, the mixed clones contained twice as many tdTOM+ cells per clone, suggesting that an additional division occurred between E14.5 and E18.5 in the absence of Ascl1. Thus, additional cell divisions could account for the overall increase in tdTOM+ cells seen in Ascl1 CKO and Ascl1 KO (Fig. 4A-D). The mixed clones can be found on the same or immediately adjacent sections (Fig. 8B, mixed clone 1), or distributed across multiple nearby sections (Fig. 8D, mixed clone 2). Marker analysis showed that these mixed clones express astrocyte markers (Nfia, Gfap), but also contain cells that co-express Olig2 and ApoE (insets, Fig. 8D). Interestingly, extensive analysis through large portions (∼4 mm) of the Ascl1 CKO spinal cords revealed that there was a greater occurrence of GM-only and mixed clones than WM-only clones. This difference in occurrence resulted in an altered distribution of tdTOM+ cells, with more cells settling in the GM (70%) than WM (30%) in the Ascl1 CKO as compared with control littermates (52% GM, 48% WM) (Fig. 8F), and is reminiscent of that seen at the population level with the high tamoxifen dose (73% GM, 27% WM) (Fig. 7O).
In summary, these findings suggest that, at both the population and clonal level, Ascl1 is playing a role in governing the balance of GM protoplasmic and WM fibrous astrocytes generated in the dorsal spinal cord.
DISCUSSION
Ascl1 defines cells that are fated to astrocyte lineages in the dorsal spinal cord
An emerging concept over the past few years is that astrocyte specification and heterogeneity are pre-patterned and segmentally defined by transcription factor codes (for a review see Molofsky et al., 2012). This concept is demonstrated in the ventral spinal cord, where homeodomain (Pax6, Nkx6.1) and bHLH (Scl1) transcription factors mark progenitor cells that give rise to different spatially defined ventral astrocyte populations (Hochstim et al., 2008; Muroyama et al., 2005). Lineage analysis of progenitor cells in the dP6-p0 domains demonstrate that they also give rise to both GM protoplasmic and WM fibrous astrocytes that are found at similar intermediate regions of the spinal cord (Fogarty et al., 2005; Tsai et al., 2012). Not surprisingly, lineage analysis of Ascl1+ glial progenitors, which predominantly comprise those of the dP3-dP5 domains, reveal that they contribute to only dorsal GM protoplasmic and WM fibrous astrocytes, and this spatial restriction is retained into the adult spinal cord. This dorsal restriction of astrocytes deriving from dorsal glial progenitor cells has also been noted for Msx3-Cre and Pax3-Cre lineage cells in the dP1-dP6 domains (Zhu et al., 2011; Tsai et al., 2012). Collectively, findings in this study further support the concept that the expression of homeodomain and bHLH factors spatially defines the progenitor origin of regionally distinct astrocytes along the DV axis of the spinal cord.
Ascl1 balances the number of GM protoplasmic and WM fibrous astrocytes generated
The presence of two classes of morphologically distinct astrocytes, namely protoplasmic and fibrous, was first noted in the GM and WM, respectively, more than 100 years ago (Oberheim et al., 2012). These two classes of astrocytes utilize different mechanisms for calcium wave propagation in the cortex (Haas et al., 2006), and may be functionally distinct in their ability to communicate and regulate neuronal homeostasis and functions. However, how GM protoplasmic and WM fibrous astrocytes are generated from glial progenitor cells during development in the CNS is unknown. Here, Ascl1+ glial progenitors labeled at E14.5 in the dorsal spinal cord contribute equally to both types of astrocytes (Figs 2 and 7). The fact that both GM and WM astrocytes were similarly labeled suggested that Ascl1 could potentially regulate an asymmetric division of astrocyte progenitor cells to generate these two astrocyte cell types. However, by administering low doses of tamoxifen to sparsely label individual Ascl1-expressing cells, the resulting clones were clusters of astrocytes restricted to either the GM or WM but not to both (Fig. 3A,B). This implies that GM protoplasmic and WM fibrous astrocytes in the dorsal spinal cord are not the result of asymmetric division from a common Ascl1+ astrocyte progenitor cell, but are produced from two separate Ascl1+ progenitors (Fig. 9A). A similar heterogeneity in astrocyte progenitors was recently reported from lineage-tracing studies in the cortex, where protoplasmic and fibrous astrocyte clones were distinctly labeled by the combinatorial expression of fluorescent proteins (Garcia-Marques and Lopez-Mascaraque, 2013). However, neither study excludes the possibility of earlier Ascl1-negative or Ascl1low bipotential progenitors that were not labeled in these experiments. Indeed, the presence of mixed clones in the Ascl1 CKO spinal cords supports the existence of a GM/WM bipotential progenitor cell. Nevertheless, examining the Ascl1 astrocyte lineage in the Ascl1 CKO as clones or as a population demonstrated that Ascl1 is necessary for the correct distribution of astrocyte types in the dorsal spinal cord, and, in its absence, there is an increase in the number of GM astrocytes along with a loss of WM astrocytes (Figs 7-9).
Requirement for Ascl1 in the generation of OPCs in the WM of the spinal cord
It has been known for some time that Ascl1 is expressed in progenitors to the oligodendrocyte lineage in the brain (Parras et al., 2004, 2007; Kim et al., 2007) and spinal cord (Battiste et al., 2007; Sugimori et al., 2007, 2008). Furthermore, the number of OPCs is significantly decreased in the spinal cord of germline null Ascl1 mice at the onset of oligodendrogenesis, and this is followed by a lack or delayed expression of more mature oligodendrocyte markers in the ventral WM at E18.5 (Sugimori et al., 2007, 2008). However, because the progenitor and neuronal pools are significantly altered in the Ascl1 mutant prior to gliogenesis as a result of its earlier role in neurogenesis (Torii et al., 1999; Helms et al., 2005; Mizuguchi et al., 2006; Wildner et al., 2006), a direct role for Ascl1 in the specification and differentiation of OPCs in the spinal cord has not been completely addressed. In the neonatal brain, however, conditional deletion of Ascl1 in SVZ stem/progenitor cells showed that Ascl1 is required for OPC specification, and OPC-specific deletion of Ascl1 resulted in an increase in proliferation and a decrease in differentiation of OPCs during remyelination (Nakatani et al., 2013).
We have shown that oligodendrogenesis in the spinal cord is dramatically reduced in the WM of Ascl1 CKO mice (Fig. 5). This loss of OPCs/oligodendrocytes was especially apparent in the dorsal funiculus (Fig. 5F′), a domain where many of the OPCs are likely to be derived from dorsally located Ascl1+ cells (Fig. 3C,D) (Zhu et al., 2011; Tsai et al., 2012). This phenotype could reflect a role for Ascl1 in the specification of glial progenitors into the oligodendrocyte lineage, OPC proliferation, and/or OPC migration from the GM to the WM. In contrast to the consistent decrease in the WM oligodendrocyte lineage, the number of GM oligodendrocyte lineage cells, as assessed by Sox10 expression, was initially increased in the Ascl1 CKO, but eventually decreased back to slightly below normal numbers at postnatal stages (Fig. 5O). This indicates differences in the way the GM and WM OPCs respond to the loss of Ascl1, and supports the concept of heterogeneity between these populations of cells as seen in multiple contexts. For example, the proliferation and differentiation efficiency of adult cortical WM and GM OPCs were shown to be intrinsically distinct when they were transplanted heterotopically to the GM or WM in the cortex (Viganò et al., 2013). Furthermore, WM OPCs can proliferate in response to the growth factor PDGF but GM OPCs cannot (Hill et al., 2013). Given that the function of Ascl1 in the CNS is highly dynamic and context dependent (Helms et al., 2005; Mizuguchi et al., 2006; Wildner et al., 2006; Jacob et al., 2013), and that Ascl1 has been shown to regulate a complex set of downstream targets (Castro et al., 2011; Borromeo et al., 2014), it is possible that Ascl1 in the oligodendrocyte lineages directly contributes to the differences in their intrinsic properties in the GM and WM.
MATERIALS AND METHODS
Mouse strains, tamoxifen administration and tissue preparation
Protocols for the generation and genotyping of mouse strains are as previously described: Ascl1null (Guillemot et al., 1993); Ascl1CreERT2 [Ascl1tm1.1(Cre/ERT2)Jejo/J] (Kim et al., 2011); Ascl1FL (Pacary et al., 2011); and the Cre reporter line R26LSL-tdTom [Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J] (Madisen et al., 2010). All procedures on animals followed NIH guidelines and were approved by the UT Southwestern Institutional Animal Care and Use Committee.
The appearance of a vaginal plug was considered E0.5 and the day of birth was noted as P0. Tamoxifen (Sigma, T55648) administration was accomplished by intraperitoneal injection of gravid females at 2.5 mg tamoxifen/40 g body weight, except for clonal analysis, where doses of 0.025 mg to 0.25 mg tamoxifen/40 g body weight were used. Spinal cords of postnatal animals were fixed via perfusion with 4% paraformaldehyde in PBS, immersion fixed overnight, washed in PBS and submerged in 30% sucrose. All tissues were embedded in O.C.T. (Sakura Finetek) for cryosectioning at 30 μm.
Immunofluorescence
Spinal cord sections were incubated with primary antibody (supplementary material Table S1) in 1% goat (or donkey) serum/0.3% Triton X-100×/PBS overnight, followed by secondary antibody conjugated with Alexa Fluor 488, 568 or 647 (Molecular Probes).
Quantification and statistical analyses
For quantification, cell numbers and distribution in the spinal cord were counted manually using ImageJ (NIH) software on sections from the thoracic region (∼T2-T9). NeuN or Gfap staining was used to demarcate the boundary between GM and WM for each section. TDP43 (Tardbp – Mouse Genome Informatics), a ubiquitously expressed nuclear protein (Sephton et al., 2010), was used as a pan-nuclei marker, and colocalization of TDP43 with tdTomato (tdTOM) was used to determine the total number of tdTOM+ cells per section. For each marker, ∼5-9 sections per spinal cord were counted for marker+;tdTOM+ cells in the VZ, GM and WM. A 10-15% overlap between the oligodendrocyte (Olig2, Sox10) and astrocyte (Nfia) markers with respect to the total number of glial cells labeled by tdTOM was noted, indicating that these markers are mostly specific but not exclusive for their respective lineages.
To assess the differences in cell number and distribution of marker+;tdTOM+ cells between control, Ascl1 CKO and Ascl1 KO mice, we used a linear regression technique to model the observed cell number as a linear combination of fixed effect (genotype) and random effect (animal ID). The animal ID is included as the random effect because multiple spinal cord sections were counted per animal, and multiple animals were counted per genotype. We then performed the likelihood ratio test to determine the statistical significance (chi-square, degree of freedom and P-value) using R lmer function to conduct the linear mixed model fit by maximum likelihood. The output value of this analysis is interpreted as estimates from a traditional least squares linear regression, where the estimate for the control is the reference (or Y intercept), and the estimate for the Ascl1 CKO or Ascl1 KO is the value of the slope of the line from the control. These estimated output values, which are approximately the means, are reported for each genotype. A P<0.05 indicates statistical significance that genotype (or the loss of Ascl1) accounts for the change in cell numbers and percentage cell distributions observed.
Supplementary Material
Acknowledgements
We acknowledge Lauren Tyra and Rachel Yuengert for excellent technical services with mouse genotyping and husbandry. We thank Dr Ben Deneen for Nfia antibodies; Dr Gang Yu for TDP43 antibodies; Dr K. C. Tung for help with statistical analyses; Drs Helen Lai and Richard Lu for critical reading of the manuscript; and all members of the J.E.J. laboratory for many helpful discussions throughout this study.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
E.J.K. initiated the project with J.E.J., performed early experiments and generated the Ascl1CreER KI mice. C.M.P. and F.G. generated the Ascl1FLOX mice. T.Y.V. designed and performed all the experiments and data analysis, and prepared the manuscript with J.E.J. All authors provided scientific insight and edited the manuscript.
Funding
This work was supported by Public Health Service grants from the National Institutes of Health [R01 NS032817 to J.E.J. and F32CA168330 to T.Y.V.] and a Grant-in-Aid from the Medical Research Council [U117570528 to F.G.]. Deposited in PMC for release after 6 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.105270/-/DC1
References
- Bachoo, R. M., Kim, R. S., Ligon, K. L., Maher, E. A., Brennan, C., Billings, N., Chan, S., Li, C., Rowitch, D. H., Wong, W. H.et al. (2004). Molecular diversity of astrocytes with implications for neurological disorders. Proc. Natl. Acad. Sci. USA 101, 8384-8389 10.1073/pnas.0402140101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiste, J., Helms, A. W., Kim, E. J., Savage, T. K., Lagace, D. C., Mandyam, C. D., Eisch, A. J., Miyoshi, G. and Johnson, J. E. (2007). Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development 134, 285-293 10.1242/dev.02727 [DOI] [PubMed] [Google Scholar]
- Borromeo, M. D., Meredith, D. M., Castro, D. S., Chang, J. C., Tung, K.-C., Guillemot, F. and Johnson, J. E. (2014). A transcription factor network specifying inhibitory versus excitatory neurons in the dorsal spinal cord. Development 141, 2803-2812, 3102 10.1242/dev.114728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, J., Chen, Y., Cai, W.-H., Hurlock, E. C., Wu, H., Kernie, S. G., Parada, L. F. and Lu, Q. R. (2007). A crucial role for Olig2 in white matter astrocyte development. Development 134, 1887-1899 10.1242/dev.02847 [DOI] [PubMed] [Google Scholar]
- Castro, D. S., Martynoga, B., Parras, C., Ramesh, V., Pacary, E., Johnston, C., Drechsel, D., Lebel-Potter, M., Garcia, L. G., Hunt, C.et al. (2011). A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev. 25, 930-945 10.1101/gad.627811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneen, B., Ho, R., Lukaszewicz, A., Hochstim, C. J., Gronostajski, R. M. and Anderson, D. J. (2006). The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron 52, 953-968 10.1016/j.neuron.2006.11.019 [DOI] [PubMed] [Google Scholar]
- Fogarty, M., Richardson, W. D. and Kessaris, N. (2005). A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development 132, 1951-1959 10.1242/dev.01777 [DOI] [PubMed] [Google Scholar]
- Garcia-Marques, J. and Lopez-Mascaraque, L. (2013). Clonal identity determines astrocyte cortical heterogeneity. Cereb. Cortex 23, 1463-1472 10.1093/cercor/bhs134 [DOI] [PubMed] [Google Scholar]
- Ge, W.-P., Miyawaki, A., Gage, F. H., Jan, Y. N. and Jan, L. Y. (2012). Local generation of glia is a major astrocyte source in postnatal cortex. Nature 484, 376-380 10.1038/nature10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot, F., Lo, L.-C., Johnson, J. E., Auerbach, A., Anderson, D. J. and Joyner, A. L. (1993). Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75, 463-476 10.1016/0092-8674(93)90381-Y [DOI] [PubMed] [Google Scholar]
- Haas, B., Schipke, C. G., Peters, O., Söhl, G., Willecke, K. and Kettenmann, H. (2006). Activity-dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb. Cortex 16, 237-246 10.1093/cercor/bhi101 [DOI] [PubMed] [Google Scholar]
- Helms, A. W., Battiste, J., Henke, R. M., Nakada, Y., Simplicio, N., Guillemot, F. and Johnson, J. E. (2005). Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development 132, 2709-2719 10.1242/dev.01859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, R. A., Patel, K. D., Medved, J., Reiss, A. M. and Nishiyama, A. (2013). NG2 cells in white matter but not gray matter proliferate in response to PDGF. J. Neurosci. 33, 14558-14566 10.1523/JNEUROSCI.2001-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstim, C., Deneen, B., Lukaszewicz, A., Zhou, Q. and Anderson, D. J. (2008). Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133, 510-522 10.1016/j.cell.2008.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, S. A., Hos, D., Kuspert, M., Lang, R. A., Lovell-Badge, R., Wegner, M. and Reiprich, S. (2014). Stem cell factor Sox2 and its close relative Sox3 have differentiation functions in oligodendrocytes. Development 141, 39-50 10.1242/dev.098418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, S., Meredith, A., Richardson, J. A. and Johnson, J. E. (1999). Correct coordination of neuronal differentiation events in ventral forebrain requires the bHLH factor MASH1. Mol. Cell. Neurosci. 14, 355-369 10.1006/mcne.1999.0791 [DOI] [PubMed] [Google Scholar]
- Jacob, J., Kong, J., Moore, S., Milton, C., Sasai, N., Gonzalez-Quevedo, R., Terriente, J., Imayoshi, I., Kageyama, R., Wilkinson, D. G.et al. (2013). Retinoid acid specifies neuronal identity through graded expression of Ascl1. Curr. Biol. 23, 412-418 10.1016/j.cub.2013.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell, T. M. (2000). Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 1, 20-29 10.1038/35049541 [DOI] [PubMed] [Google Scholar]
- Kang, P., Lee, H. K., Glasgow, S. M., Finley, M., Donti, T., Gaber, Z. B., Graham, B. H., Foster, A. E., Novitch, B. G., Gronostajski, R. M.et al. (2012). Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron 74, 79-94 10.1016/j.neuron.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E. J., Leung, C. T., Reed, R. R. and Johnson, J. E. (2007). In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J. Neurosci. 27, 12764-12774 10.1523/JNEUROSCI.3178-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E. J., Battiste, J., Nakagawa, Y. and Johnson, J. E. (2008). Ascl1 (Mash1) lineage cells contribute to discrete cell populations in CNS architecture. Mol. Cell. Neurosci. 38, 595-606 10.1016/j.mcn.2008.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E. J., Ables, J. L., Dickel, L. K., Eisch, A. J. and Johnson, J. E. (2011). Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS ONE 6, e18472 10.1371/journal.pone.0018472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Q. R., Sun, T., Zhu, Z., Ma, N., Garcia, M., Stiles, C. D. and Rowitch, D. H. (2002). Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75-86 10.1016/S0092-8674(02)00678-5 [DOI] [PubMed] [Google Scholar]
- Madisen, L., Zwingman, T. A., Sunkin, S. M., Oh, S. W., Zariwala, H. A., Gu, H., Ng, L. L., Palmiter, R. D., Hawrylycz, M. J., Jones, A. R.et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133-140 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi, R., Kriks, S., Cordes, R., Gossler, A., Ma, Q. and Goulding, M. (2006). Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat. Neurosci. 9, 770-778 10.1038/nn1706 [DOI] [PubMed] [Google Scholar]
- Molofsky, A. V., Krenick, R., Ullian, E., Tsai, H.-H., Deneen, B., Richardson, W. D., Barres, B. A. and Rowitch, D. H. (2012). Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 26, 891-907 10.1101/gad.188326.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroyama, Y., Fujiwara, Y., Orkin, S. H. and Rowitch, D. H. (2005). Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature 438, 360-363 10.1038/nature04139 [DOI] [PubMed] [Google Scholar]
- Nakatani, H., Martin, E., Hassani, H., Clavairoly, A., Maire, C. L., Viadieu, A., Kerninon, C., Delmasure, A., Frah, M., Weber, M.et al. (2013). Ascl1/Mash1 Promotes Brain Oligodendrogenesis during Myelination and Remyelination. J. Neurosci. 33, 9752-9768 10.1523/JNEUROSCI.0805-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama, A., Komitova, M., Suzuki, R. and Zhu, X. (2009). Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 10, 9-22 10.1038/nrn2495 [DOI] [PubMed] [Google Scholar]
- Oberheim, N. A., Goldman, S. A. and Nedergaard, M. (2012). Heterogeneity of astrocytic form and function. Methods Mol. Biol. 814, 23-45 10.1007/978-1-61779-452-0_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacary, E., Heng, J., Azzarelli, R., Riou, P., Castro, D., Lebel-Potter, M., Parras, C., Bell, D. M., Ridley, A. J., Parsons, M.et al. (2011). Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibition of RhoA signaling. Neuron 69, 1069-1084 10.1016/j.neuron.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras, C. M., Galli, R., Britz, O., Soares, S., Galichet, C., Battiste, J., Johnson, J. E., Nakafuku, M., Vescovi, A. and Guillemot, F. (2004). Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 23, 4495-4505 10.1038/sj.emboj.7600447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras, C. M., Hunt, C., Sugimori, M., Nakafuku, M., Rowitch, D. and Guillemot, F. (2007). The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J. Neurosci. 27, 4233-4242 10.1523/JNEUROSCI.0126-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle, N. P., Yu, W.-P., Howell, M., Colvin, J. S., Ornitz, D. M. and Richardson, W. D. (2003). Fgfr3 expression by astrocytes and their precursors: evidence that astrocytes and oligodendrocytes originate in distinct neuroepithelial domains. Development 130, 93-102 10.1242/dev.00184 [DOI] [PubMed] [Google Scholar]
- Rowitch, D. H. (2004). Glial specification in the vertebrate neural tube. Nat. Rev. Neurosci. 5, 409-419 10.1038/nrn1389 [DOI] [PubMed] [Google Scholar]
- Rowitch, D. H. and Kriegstein, A. R. (2010). Developmental genetics of vertebrate glial-cell specification. Nature 468, 214-222 10.1038/nature09611 [DOI] [PubMed] [Google Scholar]
- Sephton, C. F., Good, S. K., Atkin, S., Dewey, C. M., Mayer, P., Herz, J. and Yu, G. (2010). TDP-43 is a developmentally regulated protein essential for early embryonic development. J. Biol. Chem. 285, 6826-6834 10.1074/jbc.M109.061846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarov, A., Turnbull, R. K., Kim, E. J., Lebel-Potter, M., Guillemot, F. and Joyner, A. L. (2011). Ascl1 genetics reveals insights into cerebellum local circuit assembly. J. Neurosci. 31, 11055-11069 10.1523/JNEUROSCI.0479-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimori, M., Nagao, M., Bertrand, N., Parras, C. M., Guillemot, F. and Nakafuku, M. (2007). Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development 134, 1617-1629 10.1242/dev.001255 [DOI] [PubMed] [Google Scholar]
- Sugimori, M., Nagao, M., Parras, C. M., Nakatani, H., Lebel, M., Guillemot, F. and Nakafuku, M. (2008). Ascl1 is required for oligodendrocyte development in the spinal cord. Development 135, 1271-1281 10.1242/dev.015370 [DOI] [PubMed] [Google Scholar]
- Tien, A.-C., Tsai, H.-H., Molofsky, A. V., McMahon, M., Foo, L. C., Kaul, A., Dougherty, J. D., Heintz, N., Gutmann, D. H., Barres, B. A.et al. (2012). Regulated temporal-spatial astrocyte precursor cell proliferation involves BRAF signalling in mammalian spinal cord. Development 139, 2477-2487 10.1242/dev.077214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii, M., Matsuzaki, F., Osumi, N., Kaibuchi, K., Nakamura, S., Casarosa, S., Guillemot, F. and Nakafuku, M. (1999). Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development 126, 443-456. [DOI] [PubMed] [Google Scholar]
- Tsai, H.-H., Li, H., Fuentealba, L. C., Molofsky, A. V., Taveira-Marques, R., Zhuang, H., Tenney, A., Murnen, A. T., Fancy, S. P. J., Merkle, F.et al. (2012). Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337, 358-362 10.1126/science.1222381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viganò, F., Möbius, W., Götz, M. and Dimou, L. (2013). Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat. Neurosci. 16, 1370-1372 10.1038/nn.3503 [DOI] [PubMed] [Google Scholar]
- Walther, E. U., Dichgans, M., Maricich, S. M., Romito, R. R., Yang, F., Dziennis, S., Zackson, S., Hawkes, R. and Herrup, K. (1998). Genomic sequences of aldolase C (Zebrin II) direct lacZ expression exclusively in non-neuronal cells of transgenic mice. Proc. Natl. Acad. Sci. USA 95, 2615-2620 10.1073/pnas.95.5.2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle, H., Lieberam, I., Porter, J. A. and Jessell, T. M. (2002). Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385-397 10.1016/S0092-8674(02)00835-8 [DOI] [PubMed] [Google Scholar]
- Wildner, H., Müller, T., Cho, S.-H., Bröhl, D., Cepko, C. L., Guillemot, F. and Birchmeier, C. (2006). dILA neurons in the dorsal spinal cord are the product of terminal and non-terminal asymmetric progenitor cell divisions, and require Mash1 for their development. Development 133, 2105-2113 10.1242/dev.02345 [DOI] [PubMed] [Google Scholar]
- Zhou, Q. and Anderson, D. J. (2002). The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109, 61-73 10.1016/S0092-8674(02)00677-3 [DOI] [PubMed] [Google Scholar]
- Zhou, Q., Wang, S. and Anderson, D. J. (2000). Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron 25, 331-343 10.1016/S0896-6273(00)80898-3 [DOI] [PubMed] [Google Scholar]
- Zhou, Q., Choi, G. and Anderson, D. J. (2001). The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron 31, 791-807 10.1016/S0896-6273(01)00414-7 [DOI] [PubMed] [Google Scholar]
- Zhu, Q., Whittemore, S. R., Devries, W. H., Zhao, X., Kuypers, N. J. and Qiu, M. (2011). Dorsally-derived oligodendrocytes in the spinal cord contribute to axonal myelination during development and remyelination following focal demyelination. Glia 59, 1612-1621 10.1002/glia.21203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.