Abstract
Although many regulatory networks involved in defining definitive endoderm have been identified, the mechanisms through which these networks interact to pattern the endoderm are less well understood. To explore the mechanisms involved in midgut patterning, we dissected the transcriptional regulatory elements of nephrocan (Nepn), the earliest known midgut specific gene in mice. We observed that Nepn expression is dramatically reduced in Sox17−/− and Raldh2−/− embryos compared with wild-type embryos. We further show that Nepn is directly regulated by Sox17 and the retinoic acid (RA) receptor via two enhancer elements located upstream of the gene. Moreover, Nepn expression is modulated by Activin signaling, with high levels inhibiting and low levels enhancing RA-dependent expression. In Foxh1−/− embryos in which Nodal signaling is reduced, the Nepn expression domain is expanded into the anterior gut region, confirming that Nodal signaling can modulate its expression in vivo. Together, Sox17 is required for Nepn expression in the definitive endoderm, while RA signaling restricts expression to the midgut region. A balance of Nodal/Activin signaling regulates the anterior boundary of the midgut expression domain.
Keywords: Midgut definitive endoderm, Nephrocan (Nepn), Retinoic acid, Sox17, Nodal/Activin A, Mouse
INTRODUCTION
The definitive endoderm (DE), one of the three primary germ layers formed during gastrulation, gives rise to a vast array of highly specialized epithelial cell types that line the respiratory and digestive systems, and which contribute to associated organs, such as thyroid, thymus, lungs, liver, pancreas, stomach and intestines. Before the onset of gastrulation, the mouse embryo is a cup-shaped bilayer, composed of an inner epiblast layer of columnar epithelial cells and an outer visceral endoderm (VE) layer of epithelial cells. Gastrulation is initiated at approximately embryonic day (E) 6.5, when epiblast cells undergo an epithelial-to-mesenchymal transition (EMT) and migrate through the primitive streak to form either mesoderm or DE (reviewed by Nowotschin and Hadjantonakis, 2010). Cells that will contribute to the pluripotent endodermal layer of the embryo undergo a rapid mesenchymal-to-epithelial transition (MET) and intercalate with the VE. Following gastrulation, morphogenetic movements transform the naïve DE, with the integrated VE, into a primitive gut tube. Morphogenesis involves invagination of the foregut and hindgut pockets, expansion of the pockets towards the posterior and anterior, respectively, and closure of the intervening midgut region upon embryonic turning at E9.0 (Lewis and Tam, 2006).
Nodal, a member of the TGFβ family of ligands that includes TGFβs, Activins and BMPs, is essential for gastrulation and formation of mesoderm and DE, with higher levels of Nodal promoting DE formation rather than mesoderm (Vincent et al., 2003). Anterior-to-posterior patterning of the DE is initiated by both the time and position of cell ingression along the primitive streak (Lawson, 1999), with the earliest cells to emerge being directed to the foregut. Gradients of Nodal signaling can also regulate patterning within the DE, with high levels specifying foregut endoderm (Norris et al., 2002). Embryos deficient in Foxh1, a transcription factor that mediates a Nodal auto-regulatory circuit resulting in high levels of Nodal, cannot form foregut endoderm, although midgut and hindgut endoderm are present (McKnight et al., 2010). However, the fate of the endoderm is not fully determined at this stage, as transplantation of posterior endoderm to anterior regions can acquire anterior characteristics and vice versa (Wells and Melton, 2000; Kimura et al., 2007). Further endoderm patterning is controlled by a series of reciprocal interactions with nearby mesoderm tissues and the various regions becoming determined at different times around somitogenesis. The broad gene expression patterns within the foregut, midgut and hindgut become progressively refined into precise domains in which specific organs will form (Zorn and Wells, 2009; Kraus and Grapin-Botton, 2012).
In addition to Nodal, DE formation is controlled by a core group of transcription factors, including Eomesodermin [Eomes, eomesodermin homolog (Xenopus laevis) – Mouse Genome Informatics], Foxa2 and Sox17. Eomesodermin is required for specification of DE and limits the expression of mesoderm genes (Arnold et al., 2008; Teo et al., 2011). Embryos deficient in Foxa2, a forkhead transcription factor, fail to form foregut endoderm, although midgut and hindgut endoderm are present (Dufort et al., 1998; McKnight et al., 2010), a phenotype similar to Foxh1−/− embryos (Hoodless et al., 2001; Yamamoto et al., 2001). By contrast, embryos lacking Sox17, an HMG-box transcription factor, exhibit deficiencies in gut endoderm in which mid- and hindgut tissues fail to expand (Kanai-Azuma et al., 2002). Endoderm patterning is further refined with several transcription factors defining specific domains for organogenesis, such as Cdx2 for intestine and colon, Hex1 (Hhex – Mouse Genome Informatics) for liver and thyroid, Pdx1 for pancreas and duodenum and Nkx2-1 for lung (reviewed by Grapin-Botton, 2008). However, the patterning mechanisms after endoderm formation but before organ-specific development are poorly understood.
Endoderm and mesoderm exchange instructive signals that induce specific anteroposterior identities as well as permissive signals required for organogenesis from previously patterned fields. Many growth factor pathways, including FGF, BMP, Wnt and retinoic acid (RA), play multiple stage-specific roles (reviewed by Duester, 2008; Zorn and Wells, 2009; Kraus and Grapin-Botton, 2012). In mouse and chick embryos, FGF4 promotes Cdx expression in the hindgut and represses expression of Hex and Foxa2 in the foregut (Wells and Melton, 2000; Dessimoz et al., 2006). Wnt initiates posteriorization, acting directly on endoderm to induce Cdx2 (Gregorieff et al., 2004; McLin et al., 2007; Goessling et al., 2008; Li et al., 2008; Sherwood et al., 2011). Recently, Engert et al. reported that Wnt/β-catenin signaling also regulates endoderm formation through Sox17 (Engert et al., 2013). In zebrafish, Xenopus and chick, BMP posteriorizes the endoderm (Tiso et al., 2002; Wills et al., 2008). In zebrafish and Xenopus, RA is important to establish the foregut-midgut boundary (Stafford and Prince, 2002; Chen et al., 2004), and in mouse RA generated in the mesoderm is required for dorsal pancreas formation (Molotkov et al., 2005). RA is also required for posterior endoderm fate establishment (Bayha et al., 2009). Despite studies implicating many signaling pathways in DE patterning, less is known about the mechanisms that link these pathways and how the effects of the different signaling pathways are integrated during endoderm patterning.
We previously identified a novel domain-specific marker, nephrocan (Nepn) (Hou et al., 2007), which is first expressed at E7.25 in a limited population of posterior endodermal cells. At E8.0-8.5, expression becomes restricted to the definitive endoderm in the open region of the gut tube between the anterior and posterior intestinal portals. Here, we refer to this region as midgut, and Nepn is the earliest known marker specific to this region. By E9.5, Nepn is expressed in the dorsal pancreas and duodenum. In adult mice, Nepn is expressed in the kidney. Nepn is a member of the small leucine-rich repeat protein (SLRP) family, which includes Biglycan and Decorin. Nepn can act as a secreted inhibitor of TGFβ signaling (Mochida et al., 2006), suggesting a potential functional importance in endoderm development in mice.
Nepn provides a unique opportunity to explore the mechanisms guiding formation and patterning of the midgut DE. We previously showed that two crucial endoderm regulators, Foxa2 and Foxh1, are not required for Nepn expression and midgut DE formation in the mouse embryo (McKnight et al., 2010). Here, we describe in vivo and in vitro experiments examining the mechanisms regulating Nepn expression. Our results demonstrate that Nepn expression is directly upregulated by RA and Sox17 in a cooperative fashion. Moreover, Nepn expression exhibits a concentration-dependent response to Activin signaling, with low levels inducing expression and high levels inhibiting expression. Together, regulation of the midgut-specific gene Nepn demonstrates that a network composed of TGFβ family ligands, RA and Sox17, cooperatively patterns the midgut DE.
RESULTS
Sox17 directly induces Nepn expression in midgut definitive endoderm
To explore the molecular mechanisms that pattern the midgut endoderm, we analyzed the regulation of Nepn gene expression. As the transcription factor Sox17 is essential for mid- and hindgut expansion, we first examined Nepn expression in Sox17−/− mice. At E8.25, Nepn expression was completely lost in Sox17−/− embryos, demonstrating that Sox17 is necessary for induction of Nepn expression, thus supporting previous data showing that Sox17 is required in midgut formation (Fig. 1Ai-iv). Of interest, expression of Nepn was observed at E9.25 in Sox17−/− embryos, but was dramatically reduced compared with a heterozygous control (Fig. 1Av,vi), suggesting that factors other than Sox17 can induce Nepn expression at later stages.
Fig. 1.
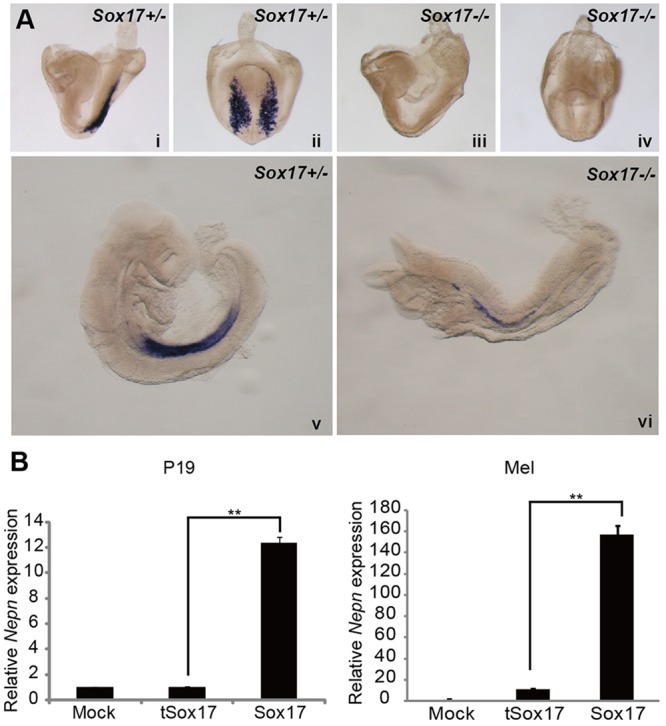
Sox17 regulates Nepn expression. (A) WISH showing Nepn expression in Sox17−/− and Sox17+/− embryos at E8.25 (side views i and iii, posterior views ii and iv) and E9.25 (side views v and vi). Nepn is substantially downregulated in Sox17−/− embryos. (B) Quantitative RT-PCR of Nepn mRNA in P19 and MEL cells transfected with vectors ectopically expressing Sox17, a truncated isoform of Sox17 (tSox17) or mock transfected. Samples were normalized to β-actin expression. Nepn expression is upregulated in the presence of Sox17.
As Nepn expression is downstream of Sox17 during endoderm development, we next explored the mechanisms through which Sox17 regulates Nepn. First, we screened several cell lines for endogenous Nepn expression (supplementary material Fig. S1A) and selected the murine erythroleukemia cell line (MEL), in which Nepn is expressed, and the embryonic carcinoma cell line (P19), with undetectable Nepn expression, for further study. To determine whether Sox17 can regulate Nepn expression in vitro, we ectopically expressed Sox17 in the cell lines. Supporting the above in vivo data, enforced expression of Sox17 was able to upregulate endogenous Nepn expression both in MEL and P19 cells (Fig. 1B). A truncated isoform of Sox17 (tSox17), that lacks part of the HMG domain and thus lacks DNA-binding ability (Kanai et al., 1996), did not induce Nepn expression, demonstrating that induction of Nepn is dependent on Sox17 transcriptional activity.
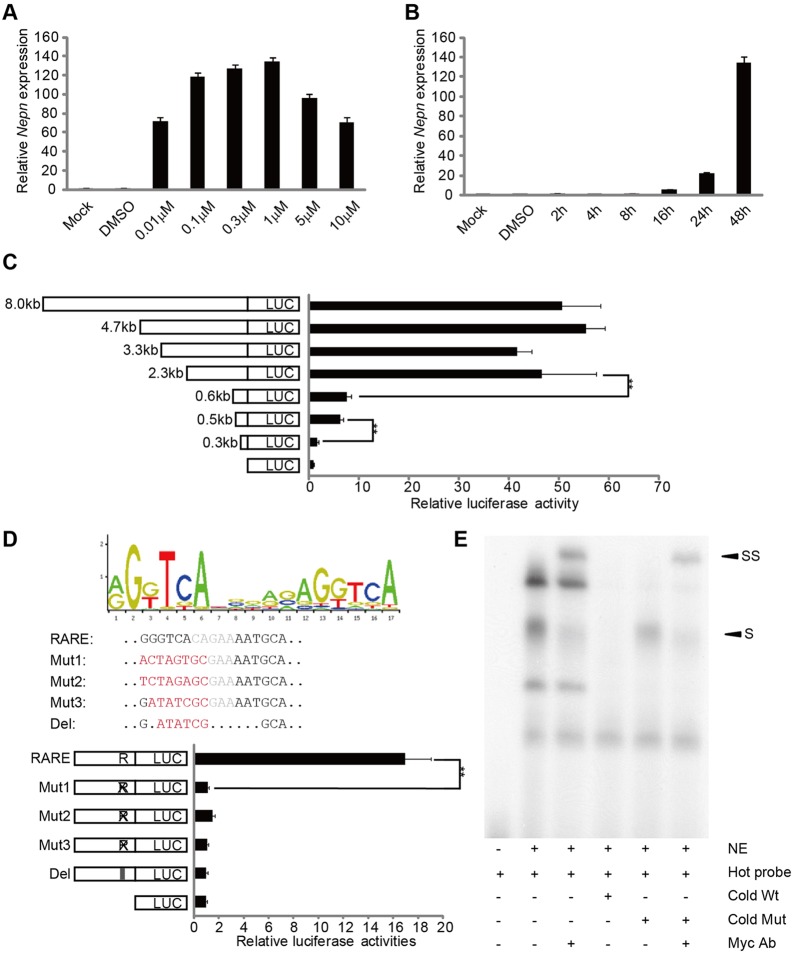
To determine if Nepn can directly respond to Sox17 expression, we generated a series of luciferase reporter constructs containing the Nepn promoter, including the transcriptional start site (TSS) and various sizes of upstream genomic regions up to −4.7 kb. The luciferase activity of these constructs was evaluated in P19 cells in the presence of ectopically expressed Sox17 (Fig. 2A). Constructs containing upstream regions with at least −842 bp and as much as −4.7 kb were induced 6- to 12-fold by Sox17 expression. A significant drop in Sox17-inducible activity occurred when the sequence between −842 bp and −640 bp upstream of the Nepn TSS was deleted, suggesting that an enhancer region that is dependent on Sox17 lies in this region. To explore whether Sox17 regulates Nepn expression through direct binding on the Nepn promoter, we screened the Nepn upstream promoter region up to −842 bp for Sox17 DNA-binding motifs using the JASPAR database (Bryne et al., 2008). One potential binding site, site A, was identified at −678 bp and scored over 9 in JASPAR. We also observed a second high-scoring site, site B, at −513 bp. Conservation of these two sites is shown in supplementary material Fig. S1B. To evaluate the functionality of these sites, we generated mutations in the two putative binding sequences in the −842 bp promoter luciferase construct. Mutagenesis of site B did not affect the ability of Sox17 to induce the Nepn promoter, whereas either mutation or deletion of site A significantly decreased Nepn promoter activity in the presence of ectopic Sox17 expression (Fig. 2B). In addition, mutation of site B had little effect on the promoter activity when site A was deleted (Fig. 2B), suggesting that Sox17 induces Nepn expression through direct binding to site A. The ability of Sox17 to directly bind to site A was further confirmed by an electrophoretic mobility shift assay (EMSA) (Fig. 2C). Nuclear extracts from HEK293T cells ectopically expressing Myc-tagged Sox17 were incubated with radioactively labeled oligonucleotides containing site A. Several protein-DNA complexes were observed that were competitively reduced by both wild-type and mutated unlabeled binding sites, indicating non-Sox17-specific binding. One complex (S) was enhanced in the presence of unlabeled oligonucleotides containing a mutated Sox17-binding site and could be super-shifted with an anti-Myc antibody (SS), indicating that the complex contained Sox17 and the binding was specific (Fig. 2C). Together, our in vivo and in vitro data demonstrate that Sox17 positively controls Nepn transcription within the midgut definitive endoderm through direct binding to the Nepn promoter region.
Fig. 2.
Sox17 regulates the Nepn promoter through a direct binding site. (A) Luciferase constructs containing the Nepn transcriptional start site and upstream promoter regions were assayed in P19 cells co-transfected with either pBOS-Sox17 or pBOS-Venus. The length of the upstream region for each construct is indicated in the graphic. Relative luciferase activity represents the fold increase observed in the presence of ectopic Sox17 expression relative to vector control. (B) Schematic view of Nepn promoter indicating the two Sox17-binding sites and the consensus Sox17-binding motif from the JASPAR database. Mutations or deletions of either site A or site B in the 0.8 kb (842 bp) Nepn promoter luciferase vectors were generated, with mutations as indicated in red, and the vectors were tested in P19 cells. Relatively luciferase activity was determined as described above. (C) EMSA assay of nuclear protein binding to site A in the Nepn promoter. Myc-tagged Sox17 was ectopically expressed in HEK293T cells and nuclear extracts were incubated with 32P-labeled Site A. Competition with a 100-fold excess of unlabeled wild-type or mutated Site A is indicated. Anti-Myc antibodies were included in the binding assay as indicated. S indicates the Sox17-Site A complex, SS indicates the super-shifted complex in the presence of anti-Myc antibody. **P<0.01. Mut, mutated; NE, nuclear extracts; wt, wild type.
RA signaling enhances Nepn expression
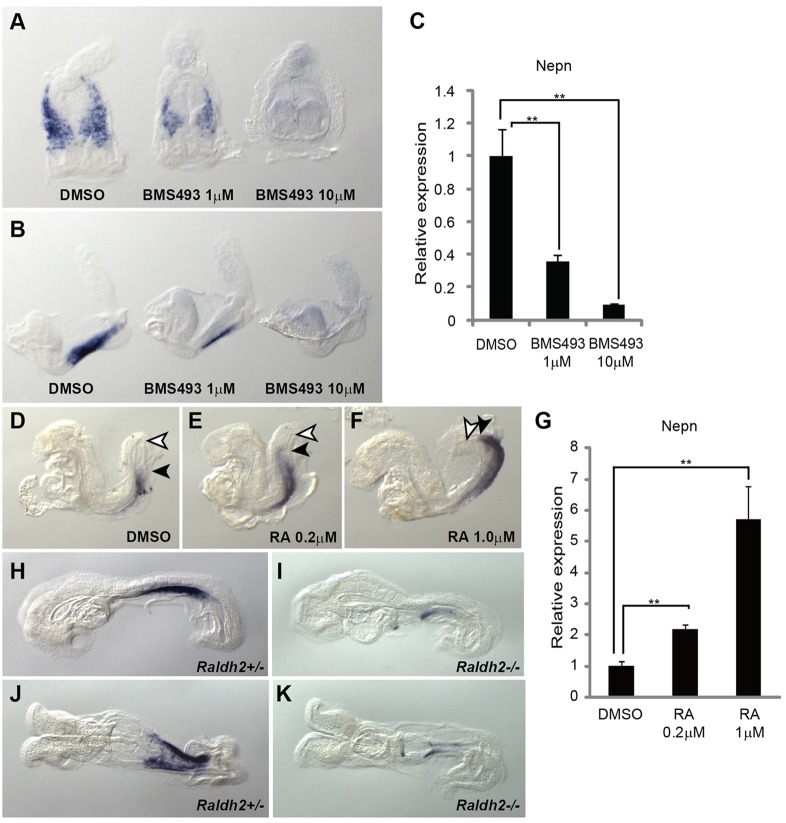
Nepn is highly expressed in the midgut region of the embryo, but expression is absent in the foregut and hindgut pockets. As Sox17 is expressed throughout the entire DE, additional factors must function to restrict the Nepn expression domain to the midgut. In the early mouse embryo, the RA-responsive domain, as shown using an RA response element (RARE)-lacZ reporter, is restricted to all three germ layers in the trunk region of the embryo (Rossant et al., 1991; Sakai et al., 2001; Mic et al., 2002), overlapping with expression of Nepn. Thus, we investigated whether RA signaling regulates Nepn expression by employing an ex vivo whole-embryo culture system, in which embryos were incubated in the presence of RA or the RA inhibitor BMS493. After culture for 24 h, embryos in the presence of BMS493 showed reduced expression of Nepn in a dosage-dependent manner (Fig. 3A-C). By contrast, Nepn expression was significantly upregulated in a dose-dependent manner in embryos cultured in the presence of exogenous RA for 24 h (Fig. 3D-G). Of note, the Nepn expression domain appears to extend further caudally in the presence of RA (arrow in Fig. 3E,F). To further confirm the role of RA in Nepn regulation, we analyzed Nepn expression in Raldh2−/− embryos (Mic et al., 2002). Raldh2 (Aldh1a2) encodes retinaldehyde dehydrogenase 2 (Raldh2), which mediates an essential step in the synthesis of RA and is the primary homolog active in the gastrulating embryo (Duester, 2008). At E8.5, Nepn expression was drastically reduced in Raldh2−/− embryos, indicating that RA signaling is required for Nepn expression in vivo (Fig. 3H-K). In summary, Nepn expression is substantially decreased when RA signaling is reduced by deletion of Raldh2 or inclusion of RA inhibitors, but upregulated with excess RA signaling.
Fig. 3.
RA signaling controls Nepn expression. (A,B) Posterior (A) and lateral (B) views of Nepn expression by WISH in embryos cultured for 24 h in either DMSO or the indicated concentration of BMS493, an RA inhibitor. (C) Quantitation of Nepn expression by RT-PCR of DMSO- and BMS493-treated embryos. Data obtained from three embryos were plotted. Nepn expression was reduced in the presence of BMS493. (D-F) Lateral views of Nepn expression by WISH in embryos cultured for 24 h in either DMSO or the indicated concentration of exogenous RA. Black arrowheads indicate the posterior boundary of Nepn domain; white arrowheads indicate the posterior end of the gut. For better comparison, these embryos were understained. (G) Quantitation of Nepn expression by RT-PCR of DMSO- and RA-treated embryos. Data obtained from three embryos were plotted. Nepn expression was increased in embryos cultured in the presence of RA. (H-K) Lateral (H,I) and ventral (J,K) views of Nepn expression in Raldh2+/− and Raldh2−/− embryos by WISH. Nepn was downregulated in Raldh2−/− embryos.
As P19 is a characterized RA-responsive cell line, we tested whether RA is able to regulate Nepn expression in P19 cells. We found that endogenous Nepn expression was induced in P19 cells in a dosage-dependent manner (Fig. 4A). After 48 h of 0.1 μM RA treatment, Nepn expression was increased over 100-fold (Fig. 4B). Moreover, luciferase constructs containing the Nepn promoter region from the TSS to −8 kb upstream showed a strong activation in P19 cells treated with 0.1 μM RA (Fig. 4C). By using truncations of the −8 kb promoter to further localize the RA-responsive regions, we observed two dramatic drops in promoter activity, one between −2.3 kb and −0.6 kb upstream and the second between −0.3 kb and −0.5 kb upstream from the TSS (Fig. 4C). To investigate whether RA directly regulates Nepn expression, we analyzed the Nepn genomic region for RARE from the TSS to −8 kb with the JASPAR database. Four sites matching the RAR:RXR_DR5 consensus motif were identified. One of these sites, located at −381 bp upstream of the TSS, was highly conserved between several species by analysis using MULAN (Ovcharenko et al., 2005) (supplementary material Fig. S2A,B). This putative RARE consists of a canonical half site (GGGTCA) and a more diverse half site (AATGCA) separated by five nucleotides. Interestingly, mutation of this site within the −2.3 kb construct abolished the promoter's ability to respond to RA signaling (Fig. 4D), suggesting that this site is crucial for Nepn expression. We further confirmed direct binding of the RA receptor, RARα, with the DNA sequence of this region through EMSA, in which we expressed Myc-tagged RARα in P19 cells. Together, these results indicate that RA signaling directly regulates Nepn expression through a RARE located at −381 bp.
Fig. 4.
RA signal directly regulates Nepn expression through an RARE. (A) P19 cells were treated with DMSO or RA for 48 h as indicated. Endogenous Nepn expression was measured by quantitative RT-PCR. (B) P19 cells were treated with DMSO or 0.1 μM RA as indicated. Endogenous Nepn expression was measured by quantitative RT-PCR. (C) Luciferase constructs containing the Nepn TSS and upstream promoter regions were assayed in P19 cells treated with DMSO or RA (0.1 μM). The length of the upstream region for each construct is indicated in the graphic. Relative luciferase activity represents the fold increase observed in the presence of RA relative to DMSO. (D) Consensus RARE (DR5) motif from the JASPAR database is shown with the sequence of the RARE from the Nepn promoter indicated below. Mutations or deletions of the Nepn RARE in the 2.3 kb Nepn promoter luciferase vectors were generated, with mutations as indicated in red, and the vectors were tested in P19 cells in the presence of DMSO or RA (0.1 μM). Relative luciferase activity was determined as described above. (E) EMSA assay of nuclear protein binding to the proposed RARE from the Nepn promoter. Myc-tagged RARα was ectopically expressed in P19 cells treated with RA (0.1 μM) and nuclear extracts were incubated with 32P-labelled RARE from the Nepn promoter. Competition with a 100-fold excess of unlabeled wild-type or mutated RARE is indicated. Anti-Myc antibodies were included in the binding assay as indicated. S indicates the RARα-DNA complex; SS indicates the super shifted complex in the presence of anti-Myc antibody. **P<0.01. Mut, mutated; NE, nuclear extracts; Wt, wild type.
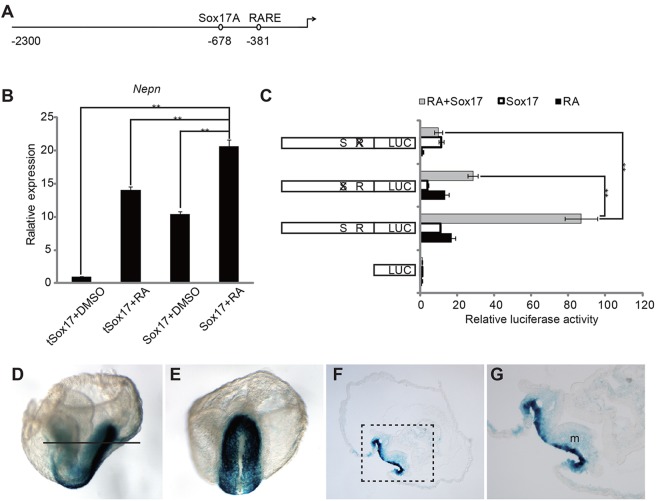
Given that both Sox17 and RA can directly regulate Nepn expression, we next explored whether Sox17 and RA regulate Nepn expression in a cooperative fashion. Sox17 or tSox17 (Kanai et al., 1996) were ectopically expressed in P19 cells in the presence of RA or DMSO. Indeed, combination of ectopic Sox17 expression and RA increased endogenous Nepn expression approximately twofold over either factor alone (Fig. 5B). Moreover, luciferase assays indicated that promoter activity of the construct (2.3 kb) that contains both Sox17-binding motif and RARE was dramatically enhanced with ectopic expression of Sox17 in the presence of RA. Mutation of either the Sox17 DNA-binding site or the RARE significantly reduced this synergistic effect (Fig. 5C). Together, Sox17 and RA cooperate to promote Nepn expression.
Fig. 5.
RA signaling and Sox17 cooperate to enhance Nepn expression. (A) Schematic view of Nepn promoter indicating the Sox17-binding site and RARE. (B) P19 cells were transfected with pBOS-Sox17 or pBOS-tSox17 and cultured in the presence of DMSO (0.1%) or RA (0.1 μM). Endogenous Nepn expression was measured by quantitative RT-PCR. (C) P19 cells were co-transfected with the 2.3 kb Nepn promoter luciferase vector that was either unmodified or mutated in the Sox17 Site A or the Nepn RARE, as indicated, and with pBOS-Sox17 in the presence of DMSO, pBOS-Sox17 in the presence of RA or pBOS-Venus in the presence of RA or DMSO. The gray bars indicate fold change in relative luciferase activity of Nepn promoter within cells transfected with pBOS-Sox17 and treated with RA relative to cells transfected with pBOS-Venus and treated with DMSO; the white bars indicate the fold change of cells transfected with pBOS-Sox17 to cells with pBOS-venus; the black bars indicate the fold change of cells treated with RA to cells treated with DMSO. Sox17 expression and RA synergize to activate the Nepn promoter. **P<0.01. (D,E) Lateral view (D) and posterior view (E) of whole-mount lacZ staining of somite 5 embryo. A cross section (indicated by the line) of the embryo in D is shown in F and G. The DE shows strong lacZ staining, whereas mesoderm has weak staining. m, mesoderm.
To evaluate whether the Nepn promoter containing the Sox17-binding site and RARE is sufficient to drive midgut DE expression in vivo, we generated transient transgenic embryos using a lacZ reporter gene driven by the 2.3 kb Nepn promoter. Strong lacZ staining was observed in the definitive endoderm in E7.5-8.5 transgenic embryos (four out of four) (Fig. 5D-G), overlapping with the Nepn expression domain (Hou et al., 2007). Of note, although the expression did not extend into the foregut region, expression in the hindgut was observed in two of the embryos that showed strong lacZ staining (Fig. 5D,E), suggesting that both Sox17 and RA signaling can regulate gene expression in the hindgut in vivo. Thus, additional elements are likely required to refine the posterior boundary. Low levels of lacZ expression were also detected in mesoderm (three out of four embryos) (Fig. 5F), suggesting that the 2.3 kb promoter region is able to weakly respond to RA signaling in mesoderm. Together, our results support a model in which Sox17 and RA are required for expression of Nepn in midgut definitive endoderm.
The anterior Nepn expression boundary is dependent on Nodal signaling
Nodal signaling is essential for DE formation. Removal of maternal RA by embryonic Cyp26 (Cyp26a1b1/c1 – Mouse Genome Informatics)-mediated RA degradation has been shown to be necessary to correctly regulate Nodal expression in the embryo (Uehara et al., 2009). To explore whether Nodal signaling regulates Nepn expression, we examined RA-dependent induction of endogenous Nepn in P19 cells in the presence and absence of exogenous ActivinA. In these studies, we used ActivinA as a ligand, as it activates the same receptor, ALK4 (also known as Acvr1b), and intracellular signaling pathways as Nodal without a requirement for a co-receptor (e.g. Cripto). In P19 cells, ActivinA alone did not induce Nepn expression (supplementary material Fig. S3A). However, Nepn expression demonstrated a concentration-dependent response to addition of ActivinA in the presence of RA. ActivinA at low concentrations (5-10 ng/ml) strongly synergized with RA and induced Nepn expression nearly 300-fold. By contrast, ActivinA at a high concentration (100 ng/ml), which is commonly used for endoderm differentiation of stem cells, inhibited RA-dependent induction of Nepn (Fig. 6A). As RA is able to induce Sox17 in F9, an embryonic carcinoma cell line (Futaki et al., 2003), we examined whether induction of Sox17 was responsible for the dose-dependent response of Nepn to RA and ActivinA in P19 cells. Of note, Sox17 was induced by RA in P19 cells, but its expression was not varied by addition of low amounts of ActivinA (1-20 ng/ml) (Fig. 6A), indicating that the mechanism of synergistic activity of low Activin with RA is not through increased expression of Sox17.
Fig. 6.
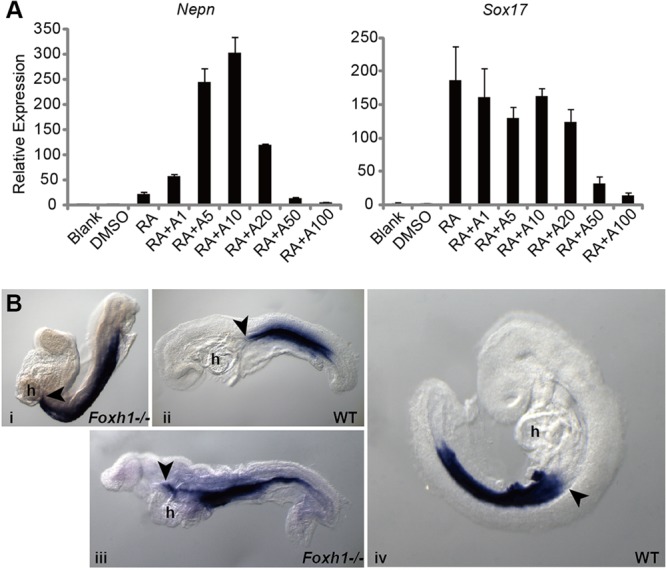
The Nepn expression domain is expanded anteriorly in Foxh1−/− embryos. (A) P19 cells were cultured in reduced serum (1% FBS) in the presence of RA (0.1 μM) in combination with increasing doses of ActivinA as indicated (in ng/ml) for 48 h. Endogenous Nepn (left) and Sox17 (right) expressions were quantified by RT-PCR. Total RNA was extracted 48 h after each treatment. (B) WISH for Nepn expression in Foxh1−/− embryos at E8.5 (i,ii) and E9.5 (iii,iv) (lateral views shown). A representative image of mutants is shown at each stage. Nepn expression was upregulated in Foxh1−/− embryos, which exhibited an anterior extension of Nepn domain. Black arrowheads indicate the anterior boundary of Nepn. h, heart; WT, wild type.
We next examined if the Nodal/Activin signaling pathway alters the Nepn expression domain in vivo by examining Foxh1−/− embryos. Foxh1 is a transcription factor that mediates high levels of Nodal signaling during gastrulation through an auto-regulatory loop (Saijoh et al., 2000; Norris et al., 2002). Loss of Foxh1 results in a failure to form foregut endoderm, whereas midgut endoderm is evident (McKnight et al., 2010). This phenotype is similar to mice carrying a deletion in the Foxh1-dependent auto-regulatory enhancer in Nodal (Norris et al., 2002). To determine whether reduced Nodal signaling affected Nepn expression, we examined E8.5-9.5 Foxh1−/− embryos (Fig. 6B). In embryos in which morphogenesis is less severely disrupted (Hoodless et al., 2001; Yamamoto et al., 2001), we observed that the Nepn expression domain was expanded into the anterior gut region. In wild-type embryos, Nepn expression is always posterior to the heart and anterior intestinal portal (AIP), whereas in Foxh1−/− embryos, Nepn expression extends anteriorly, dorsal to the heart (arrow in Fig. 6B), suggesting that high levels of Nodal signaling define the anterior boundary by inhibiting midgut marker Nepn expression. Together, our data suggest that Nodal/Activin signaling can modulate RA-dependent Nepn expression.
DISCUSSION
The mechanisms that define developmental domains and in particular the boundaries that limit those domains is crucial to understanding organogenesis in the embryo. Multiple signaling pathways have been implicated in endoderm patterning, including Nodal, RA, Wnt and FGF. Much of our understanding has been developed from in vitro studies of human and mouse stem cell differentiation. How these pathways interact in vivo is less well understood. Moreover, given the lack of DE-domain-specific markers, most of the work on DE patterning has been done using organ-specific markers that turned on at later stages of organogenesis (Wendling et al., 2000; Chen et al., 2007; Bayha et al., 2009). We previously identified Nepn as a unique, specific marker of midgut DE (Hou et al., 2007). Its expression initiates at E7.25, before overt formation of midgut endoderm, suggesting that midgut patterning begins early in development. Thus, Nepn provides a valuable tool to study early midgut patterning mechanisms, before organogenesis. Here, using a combination of in vivo, ex vivo and in vitro approaches, we identified two regulatory elements in the upstream promoter regions of Nepn: one at −381 bp is an RARE and a second at −678 bp is responsive to direct binding of Sox17. We observed that Sox17 and RA can cooperate to significantly increase Nepn promoter activity in vitro and that this promoter region can direct strong lacZ expression to DE in vivo. Furthermore, Nepn expression is tightly regulated by Nodal/Activin A signaling, with high levels inhibiting its expression and low levels enhancing its expression. Our data further support the idea that the level of Activin/Nodal signaling is crucial to define the anterior boundary of Nepn expression in vivo. Together, these signaling pathways and transcription factors form a network that regulates Nepn expression and defines the midgut domain.
Nepn expression is directly regulated by Sox17 and RA signaling
The transcription factor Sox17 functions as an endoderm determinant (Kanai-Azuma et al., 2002). Embryos lacking Sox17 exhibit reduced DE in the mid- and hindgut and Sox17-null embryonic stem cells are completely excluded from the mid- and hindgut in chimera assays, demonstrating the essential role of Sox17 in mid- and hindgut patterning (Kanai-Azuma et al., 2002). Consistent with the lack of midgut and hindgut, Nepn is absent in Sox17−/− embryos at E8.5. Moreover, our data indicate that Nepn is also a direct target of Sox17. Of note, by E9.0, weak expression of Nepn is observed in Sox17−/− embryos (Fig. 1Avi), suggesting that Sox17 is not essential at this stage. As Sox9 is able to induce Nepn in MEL cells (supplementary material Fig. S4), it may be compensating for the loss of Sox17. Interestingly, in β-catenin (Ctnnb1 – Mouse Genome Informatics) conditional knockout embryos in which Sox17 is almost absent, Nepn expression was also substantially decreased (Engert et al., 2013), suggesting that Wnt/β-catenin indirectly regulates Nepn expression through Sox17.
As Sox17 is more broadly expressed than Nepn, including in the visceral endoderm and throughout the foregut, midgut and hindgut (Kanai-Azuma et al., 2002), factors in addition to Sox17 are required to control Nepn expression in the midgut. We found that RA signaling is a potent inducer of Nepn expression and it is dramatically reduced in Raldh2−/− embryos, confirming its dependence on RA. Moreover, we observed that RA signaling can cooperate with Sox17 in P19 cells. Nepn is directly regulated by RA through a responsive element that is able to bind RARα. Of note, in P19 cells, Nepn expression is dramatically increased with relatively low concentrations of RA (0.1 μM), suggesting that Nepn promoter is highly sensitive to RA signaling. However, Nepn expression is not significantly upregulated until after 16 h of RA treatment in P19 cells, implying that induction of additional co-regulators, such as Sox17, are required. Notably, the 2.3 kb promoter region of Nepn, which contains the Sox17-binding site and the RARE, is sufficient to drive gene expression in the DE of transgenic embryos.
Nepn is first expressed at E7.25 in the mouse embryo and by E8.0, Nepn is expressed in the lateral DE. By E10.5, Nepn is expressed caudally from the stomach throughout dorsal pancreas and intestine (Hou et al., 2007), encompassing the Pdx1 expression domain (supplementary material Fig. S5). By E11.5, the expression of Nepn and Pdx1 substantially overlap, although the expression of Nepn in the ventral pancreatic bud is substantially weaker compared with its expression in the dorsal pancreatic bud (supplementary material Fig. S5). By E14.5, Nepn is restricted to the exocrine pancreas (Anderson et al., 2009). Most recently, Nepn was found to label pancreatic progenitors in embryonic stem cell differentiation (De Angelis et al., 2014). Previous studies have shown that Sox17 function is essential for Pdx1 expression in the dorsal and ventral pancreatic primordial (Kanai-Azuma et al., 2002). In those studies, Hex expression was normal in Sox17−/− embryos, indicating that liver and thyroid primordia are formed. Moreover, development of the dorsal pancreatic bud was absent in Raldh2−/− embryos (Martin et al., 2005; Molotkov et al., 2005). In these mutants, Pdx1 was expressed in the ventral pancreatic bud but was absent in the dorsal pancreatic region. Thus, Nepn and Pdx1 expression share several common regulatory mechanisms in vivo, although Nepn is expressed substantially earlier than Pdx1. Together, the regulation of Nepn by Sox17 and RA signaling supports a crucial role for these factors in the patterning of the midgut DE.
RA and Activin/Nodal signaling define the boundaries of Nepn expression
Based on a RARE-lacZ reporter transgenic mouse line, the region responsive to RA signaling is localized to the trunk region of the embryo, posterior to the preotic sulcus, with lower levels of RA responsiveness extending into the hindgut (Sirbu and Duester, 2006). The limits of the domain are regulated by a balance between the synthesis and metabolism of RA. Raldh2, the primary enzyme that synthesizes RA in the embryo, is not present in the DE but is found in the adjacent mesoderm. Raldh2 expression in the trunk mesoderm extends from the anterior region of the tailbud to the level of the posterior foregut (Molotkov et al., 2005). Cyp26a1 encodes an RA-metabolizing enzyme and is expressed in the head and posterior region of the tailbud, thus restricting high RA activity to the trunk (Abu-Abed et al., 2001; Sakai et al., 2001; Sirbu et al., 2005). In vivo, Nepn is exclusively expressed in the DE, and the anterior and posterior boundaries of Nepn are similar to those of the high RA-responsive domain in the trunk of the embryo, hinting that RA signaling may modulate the anterior and posterior boundaries of Nepn expression. A recent study in chick embryos showed that organogenesis in the entire endoderm is patterned along anteroposterior axis through RA signaling, in particular by regulating CdxA (Bayha et al., 2009). In our transgenic embryos, although the anterior boundary is maintained, the 2.3 kb promoter region of Nepn that contains the Sox17-binding site and the RARE is sufficient to drive lacZ expression throughout the hindgut region, suggesting that the posterior boundary is not maintained. It is possible that the Nepn promoter is highly sensitive to RA and is responding to the lower levels of RA in the hindgut region. Together, our results support a model in which Sox17 restricts Nepn expression to the DE while RA signaling promotes expression in the midgut and hindgut. Additional factors, possibly Cdx related, are probably necessary to refine the posterior boundary of Nepn expression (Fig. 7).
Fig. 7.
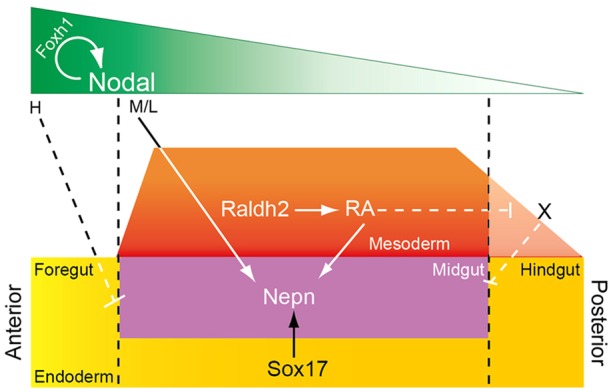
Pathway interactions regulating midgut patterning. During gastrulation, Nodal establishes a morphogen gradient to specify nascent endoderm from primitive streak. The Foxh1-mediated high Nodal signal (H) is crucial for foregut patterning and the establishment of foregut-midgut boundary; medium/low (M/L) amount of Nodal enhances Nepn expression. The expression of Sox17 restricts Nepn expression domain to the DE. RA is secreted from the adjacent mesoderm and diffuses to the endoderm to further restrict Nepn expression in the midgut region. Higher RA signaling facilitates the expansion of Nepn expression domain posteriorly.
As high levels of Nodal are required for foregut formation, we examined Foxh1−/− embryos in which Nodal signaling is reduced and foregut formation is disrupted (McKnight et al., 2010). In Foxh1−/− embryos, Nepn expression extends into the anterior pocket region, indicating that Nepn expression can be expressed anterior to the anterior intestinal portal and suggesting that the foregut region in these embryos has a midgut identity. We further observed that Nepn expression can be controlled in P19 cells by Activin in a dose-dependent manner, in conjunction with RA. At high Activin concentrations normally used for endoderm induction in stem cells (100 ng/ml), RA-dependent induction of Nepn is inhibited; at lower doses of Activin (10 ng/ml), Nepn expression is significantly enhanced. We were not able to identify an Activin response element in the Nepn promoter, although one could be outside of the domain studied here (supplementary material Fig. S3B). Alternatively, Activin may indirectly enhance Nepn RA-dependent expression, as Activin may promote differentiation of the P19 cells. Although the levels of Sox17 were not affected by inclusion of Activin, Nepn expression in response to Activin and RA correlates with the expression of Cdx2 in P19 cells (supplementary material Fig. S6A), suggesting that at the lower concentrations of Activin, P19 cells have a posterior identity. Of note, Nepn expression is absent in Cdx1−/−;Cdx2−/− embryos (supplementary material Fig. S6B), indicating that midgut endoderm is not formed and supporting studies showing that in the absence of Cdx2, the endoderm is converted to foregut/esophageal-like cells (Gao et al., 2009). Of interest, we were not able to identify an Activin responsive element in luciferase assays using the 8 kb promoter region upstream of Nepn, suggesting that the Activin regulatory region lies outside of the region analyzed. Alternatively, Nepn induction by Activin may be indirect by promoting differentiation of the cells to foregut endoderm and establishing the anterior boundary through regulation of RA signaling.
Nepn is a member of the small leucine-rich repeat (SLRP) family of proteins. It is a secreted, N-glycosylated inhibitor of TGFβ signaling (Mochida et al., 2006) and a potent inhibitor of Activin signaling (supplementary material Fig. S7). Due to the similarities between Nodal and Activin signaling, it is possible that Nepn can also inhibit Nodal signaling in the embryo. Moreover, Nodal has an RA-responsive element and can be ectopically induced in E6.5 embryos lacking all three Cyp26 genes that fail to degrade RA derived from the maternal bloodstream (Uehara et al., 2009); embryonic RA synthesis does not begin until E7.5 when Raldh2 expression is first observed (Sirbu et al., 2005). Thus, RA does not normally interfere with Foxh1 function to properly induce high levels of Nodal to promote foregut formation. As development proceeds, Nepn may be induced by RA to inhibit Nodal signaling and prevent posterior expansion of the foregut domain in the DE. As high levels of Nodal signaling are required for foregut, but not midgut or hindgut formation, the balance between foregut and midgut could be altered by RA signaling. Thus, our data suggest that Nodal, RA and Nepn may form a feedback loop to regulate the foregut-midgut boundary in the embryo (Fig. 7). Further studies to explore the relationship of these factors will be required.
In summary, our data support a model in which a core regulatory network comprised of Nodal/Activin signaling, RA signaling and Sox17 regulate Nepn expression and pattern the midgut DE. In this core network, Sox17 is required for Nepn expression in DE. RA synthesized from adjacent mesoderm cells, diffuses to regulate Nepn expression within the midgut domain and, along with an unknown factor, contribute to the posterior boundary. The Nepn anterior boundary is refined by a feedback loop formed by Nepn, RA and Nodal/Activin signaling (Fig. 7). Our work supports and extends previous models (Zorn and Wells, 2009; Kraus and Grapin-Botton, 2012) with in vivo molecular data in the mouse. Moreover, other signaling pathways, such as Wnt and FGF, are involved in both endoderm formation and patterning and are likely to contribute to Nepn regulation.
MATERIALS AND METHODS
Whole-mount in situ hybridization (WISH)
All mouse studies conformed to the regulatory standards and protocols adopted by the appropriate animal oversight committees at the University of British Columbia, University of Utah, Sanford–Burnham Medical Research Institute, University of Ottawa or McGill University Animal Research Committee at the Sanford–Burnham Medical Research Institute. Embryos were obtained from crosses of Foxh1 (Hoodless et al., 2001), Sox17 (Saund et al., 2012) or Raldh2 (Mic et al., 2002) heterozygous mice. Embryos were staged based on morphology and the number of somites, as previously described (Downs and Davies, 1993). Embryos were dissected from the uterus in DPBS (Invitrogen), fixed with 4% paraformaldehyde overnight at 4°C, dehydrated through a graded methanol series and stored at −20°C. WISH was carried out as previously described (Hou et al., 2007). Staged-matched heterozygous littermates were used as controls. For each experiment, at least five embryos were examined, and representative embryos are shown in the figures. The Nepn in situ probe was previously described (Hou et al., 2007).
Cell culture, vector construction and luciferase reporter assay
P19 and MEL cells were cultured in DMEM (STEMCELL Technologies) supplemented with 10% FBS (Hyclone). RA was reconstituted in DMSO to a working stock of 10−3 M. Corresponding amounts of DMSO were supplemented as a control in all experiments. Nepn upstream regions were generated by PCR from bacterial artificial clone (BAC) DNA, RP24-251C12, and cloned into pGL3 basic luciferase reporter vector (Promega). Reporter vectors were co-transfected with the Renilla luciferase construct, TK-RL, and, if indicated, with pBOS-Sox17 or pBOS-Venus. P19 cells were seeded at 5×104/well in a 24-well plate and transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For each well, 0.8 μg of the reporter luciferase vector, 0.02 μg TK-RL and 0.04 μg Sox17 or control vector were typically used. The medium was refreshed with indicated treatments 6 h after transfection. Cell lysates were collected 48 h after transfection, and Firefly and Renilla luciferase activities were measured using the Dual Luciferase Assay (Promega) according to the manufacturer's instructions. Firefly luciferase was normalized to Renilla luciferase, and normalized to the respective negative controls (DMSO or pBOS-Venus).
Transient transgenic reporter assay
To construct the lacZ reporter vector, lacZ was subcloned into pGL3 to replace firefly luciferase. The microinjection and lacZ staining were performed as previously described (Uehara et al., 2009; Yamanaka et al., 2010).
RNA quantification
Total RNA was extracted with Trizol (Invitrogen) using MaXtracthigh density phase-lock gels (Qiagen). RNA was reverse-transcribed and quantified using SYBR Green-based real-time PCR (qRT-PCR) according to manufacturer's instructions (Roche) with ABI PRISM 7300 Sequence Detection System. Gapdh or actin served as controls for normalization.
Whole-embryo culture
E7.5 Institute of Cancer Research (ICR) mouse embryos were dissected in warm DMEM supplemented with 10% FBS. Embryos with similar developmental stage were transferred to 50 ml falcon tubes containing 50% rat serum in DMEM in the presence of either RA, BMS493 or DMSO, and rotated for 24 h under 5% CO2 at 37°C as described (Yamamoto et al., 2003). For each condition, at least five embryos were examined, and representative embryos are shown.
Electrophoretic mobility supershift assay
Myc-Sox17 and Myc-RARα were generated from pBOS-Sox17, which was cloned from mouse cDNA (Kanai et al., 1996) and pSG5-RARα (Underhill et al., 1994), respectively, by subcloning into the vector N′ Myc-V517 (Turkson et al., 2004; Colwill et al., 2006). For Sox17 binding assay, cell nuclear extracts were prepared from HEK293T cells with V518-Myc-Sox17. For RARα binding assay, cell nuclear extracts were prepared from RA-treated P19 cells transfected with V518-Myc-RARα. Wild-type or mutant oligos containing transcription factor-binding sites were annealed with their antisense. Then, double-stranded wild-type oligonucleotides were radiolabeled with (γ-32P) ATP using T4 polynucleotide kinase according to the manufacturer's instructions (Invitrogen). Gel shift assays were performed as previously described using Novex EMSA gel system (Invitrogen) (Xiang et al., 2010). Anti-Myc antibody was purchased from Covance.
Statistics
All data presented are representative of at least three independent experiments unless indicated otherwise. Data are presented as mean±s.d. Statistical analysis was performed using Student's t-test. Statistical significance was inferred at *P<0.05 and **P<0.01.
Supplementary Material
Acknowledgements
We thank Dr Masami and Yoshi Kanai for Sox17 and tSox17 expression vector, Dr Michael Underhill for kindly sharing pSG5-RARα, and Dr Se-Wing Grace Cheng and Dr Gregg B. Morin for N′ V517-Myc.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
J.H., W.W., Yu.Y., D.Y.D.L., O.A., N.A.J.K., R.M., R.C. and N.H. performed experimental procedures under the guidance of P.A.H. J.H. performed the embryo culture, luciferase assay and analyzed the Foxh1 mutant. W.W. performed the cell culture experiments and molecular cloning. D.Y.D.L., N.A.J.K., R.M. and R.C. performed quantitative RT-PCR assay. P.X., O.A. and R.K.H. performed EMSA assay. R.S.S. and Y.S. analyzed the Sox17 mutant; T.J.C. and G.D. analyzed the Raldh2 mutant; J.G.A.S. and D.L. analyzed the double mutant of Cdx1 and Cdx2; N.H. and Yo.Y. performed transient transgenic assay. J.H., W.W. and P.A.H. wrote the manuscript.
Funding
This work was supported by funding from the Canadian Institute of Health Research [FRN 89806 to P.A.H.]; the Natural Sciences and Engineering Research Council of Canada [RGPIN 418298-12 to P.A.H., RGPIN 418720-12 to Yo.Y.]; and National Institutes of Health grants [R01HD0066121 to Y.S., GM062848 to G.D.]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.108274/-/DC1
References
- Abu-Abed, S., Dollé, P., Metzger, D., Beckett, B., Chambon, P. and Petkovich, M. (2001). The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 15, 226-240 10.1101/gad.855001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, K. R., White, P., Kaestner, K. H. and Sussel, L. (2009). Identification of known and novel pancreas genes expressed downstream of Nkx2.2 during development. BMC Dev. Biol. 9, 65 10.1186/1471-213X-9-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, S. J., Hofmann, U. K., Bikoff, E. K. and Robertson, E. J. (2008). Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Development 135, 501-511 10.1242/dev.014357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayha, E., Jørgensen, M. C., Serup, P. and Grapin-Botton, A. (2009). Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS ONE 4, e5845 10.1371/journal.pone.0005845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryne, J. C., Valen, E., Tang, M.-H. E., Marstrand, T., Winther, O., da Piedade, I., Krogh, A., Lenhard, B. and Sandelin, A. (2008). JASPAR, the open access database of transcription factor-binding profiles: new content and tools in the 2008 update. Nucleic Acids Res. 36, D102-D106 10.1093/nar/gkm955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Pan, F. C., Brandes, N., Afelik, S., Sölter, M. and Pieler, T. (2004). Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev. Biol. 271, 144-160 10.1016/j.ydbio.2004.03.030 [DOI] [PubMed] [Google Scholar]
- Chen, F., Desai, T. J., Qian, J., Niederreither, K., Lu, J. and Cardoso, W. V. (2007). Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 134, 2969-2979 10.1242/dev.006221 [DOI] [PubMed] [Google Scholar]
- Colwill, K., Wells, C. D., Elder, K., Goudreault, M., Hersi, K., Kulkarni, S., Hardy, W. R., Pawson, T. and Morin, G. B. (2006). Modification of the Creator recombination system for proteomics applications--improved expression by addition of splice sites. BMC Biotechnol. 6, 13 10.1186/1472-6750-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis, M. T., Russo, F., D'Angelo, F., Federico, A., Gemei, M., Del Vecchio, L., Ceccarelli, M., De Felice, M. and Falco, G. (2014). Novel Pancreas Organogenesis Markers Refine the Pancreatic Differentiation Roadmap of Embryonic Stem cells. Stem Cell Rev. 10, 269-279 10.1007/s12015-013-9489-5 [DOI] [PubMed] [Google Scholar]
- Dessimoz, J., Opoka, R., Kordich, J. J., Grapin-Botton, A. and Wells, J. M. (2006). FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech. Dev. 123, 42-55 10.1016/j.mod.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Downs, K. M. and Davies, T. (1993). Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development 118, 1255-1266. [DOI] [PubMed] [Google Scholar]
- Duester, G. (2008). Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921-931 10.1016/j.cell.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufort, D., Schwartz, L., Harpal, K. and Rossant, J. (1998). The transcription factor HNF3beta is required in visceral endoderm for normal primitive streak morphogenesis. Development 125, 3015-3025. [DOI] [PubMed] [Google Scholar]
- Engert, S., Burtscher, I., Liao, W. P., Dulev, S., Schotta, G. and Lickert, H. (2013). Wnt/beta-catenin signalling regulates Sox17 expression and is essential for organizer and endoderm formation in the mouse. Development 140, 3128-3138 10.1242/dev.088765 [DOI] [PubMed] [Google Scholar]
- Futaki, S., Hayashi, Y., Yamashita, M., Yagi, K., Bono, H., Hayashizaki, Y., Okazaki, Y. and Sekiguchi, K. (2003). Molecular basis of constitutive production of basement membrane components: gene expression profiles of Engelbreth-Holm-Swarm tumor and F9 embryonal carcinoma cells. J. Biol. Chem. 278, 50691-50701 10.1074/jbc.M304985200 [DOI] [PubMed] [Google Scholar]
- Gao, N., White, P. and Kaestner, K. H. (2009). Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev. Cell 16, 588-599 10.1016/j.devcel.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling, W., North, T. E., Lord, A. M., Ceol, C., Lee, S., Weidinger, G., Bourque, C., Strijbosch, R., Haramis, A.-P., Puder, M.et al. (2008). APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev. Biol. 320, 161-174 10.1016/j.ydbio.2008.05.526 [DOI] [PubMed] [Google Scholar]
- Grapin-Botton, A. (2008). Endoderm Specification StemBook. Cambridge, MA: Harvard Stem Cell Institute. [PubMed] [Google Scholar]
- Gregorieff, A., Grosschedl, R. and Clevers, H. (2004). Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(−/−)/Tcf1(−/−) embryos. EMBO J. 23, 1825-1833 10.1038/sj.emboj.7600191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoodless, P. A., Pye, M., Chazaud, C., Labbé, E., Attisano, L., Rossant, J. and Wrana, J. L. (2001). FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev. 15, 1257-1271 10.1101/gad.881501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, J., Charters, A. M., Lee, S. C., Zhao, Y., Wu, M. K., Jones, S. J. M., Marra, M. A. and Hoodless, P. A. (2007). A systematic screen for genes expressed in definitive endoderm by Serial Analysis of Gene Expression (SAGE). BMC Dev. Biol. 7, 92 10.1186/1471-213X-7-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai, Y., Kanai-Azuma, M., Noce, T., Saido, T. C., Shiroishi, T., Hayashi, Y. and Yazaki, K. (1996). Identification of two Sox17 messenger RNA isoforms, with and without the high mobility group box region, and their differential expression in mouse spermatogenesis. J. Cell Biol. 133, 667-681 10.1083/jcb.133.3.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai-Azuma, M., Kanai, Y., Gad, J. M., Tajima, Y., Taya, C., Kurohmaru, M., Sanai, Y., Yonekawa, H., Yazaki, K., Tam, P. P.et al. (2002). Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129, 2367-2379. [DOI] [PubMed] [Google Scholar]
- Kimura, W., Yasugi, S. and Fukuda, K. (2007). Regional specification of the endoderm in the early chick embryo. Dev. Growth Differ. 49, 365-372 10.1111/j.1440-169X.2007.00933.x [DOI] [PubMed] [Google Scholar]
- Kraus, M. R. C. and Grapin-Botton, A. (2012). Patterning and shaping the endoderm in vivo and in culture. Curr. Opin. Genet. Dev. 22, 347-353 10.1016/j.gde.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Lawson, K. A. (1999). Fate mapping the mouse embryo. Int. J. Dev. Biol. 43, 773-775. [PubMed] [Google Scholar]
- Lewis, S. L. and Tam, P. P. L. (2006). Definitive endoderm of the mouse embryo: formation, cell fates, and morphogenetic function. Dev. Dyn. 235, 2315-2329 10.1002/dvdy.20846 [DOI] [PubMed] [Google Scholar]
- Li, Y., Rankin, S. A., Sinner, D., Kenny, A. P., Krieg, P. A. and Zorn, A. M. (2008). Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 22, 3050-3063 10.1101/gad.1687308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín, M., Gallego-Llamas, J., Ribes, V., Kedinger, M., Niederreither, K., Chambon, P., Dollé, P. and Gradwohl, G. (2005). Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev. Biol. 284, 399-411 10.1016/j.ydbio.2005.05.035 [DOI] [PubMed] [Google Scholar]
- McKnight, K. D., Hou, J. and Hoodless, P. A. (2010). Foxh1 and Foxa2 are not required for formation of the midgut and hindgut definitive endoderm. Dev. Biol. 337, 471-481 10.1016/j.ydbio.2009.10.040 [DOI] [PubMed] [Google Scholar]
- McLin, V. A., Rankin, S. A. and Zorn, A. M. (2007). Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development 134, 2207-2217 10.1242/dev.001230 [DOI] [PubMed] [Google Scholar]
- Mic, F. A., Haselbeck, R. J., Cuenca, A. E. and Duester, G. (2002). Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development 129, 2271-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida, Y., Parisuthiman, D., Kaku, M., Hanai, J.-I., Sukhatme, V. P. and Yamauchi, M. (2006). Nephrocan, a novel member of the small leucine-rich repeat protein family, is an inhibitor of transforming growth factor-beta signaling. J. Biol. Chem. 281, 36044-36051 10.1074/jbc.M604787200 [DOI] [PubMed] [Google Scholar]
- Molotkov, A., Molotkova, N. and Duester, G. (2005). Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev. Dyn. 232, 950-957 10.1002/dvdy.20256 [DOI] [PubMed] [Google Scholar]
- Norris, D. P., Brennan, J., Bikoff, E. K. and Robertson, E. J. (2002). The Foxh1-dependent autoregulatory enhancer controls the level of Nodal signals in the mouse embryo. Development 129, 3455-3468. [DOI] [PubMed] [Google Scholar]
- Nowotschin, S. and Hadjantonakis, A.-K. (2010). Cellular dynamics in the early mouse embryo: from axis formation to gastrulation. Curr. Opin. Genet. Dev. 20, 420-427 10.1016/j.gde.2010.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovcharenko, I., Loots, G. G., Nobrega, M. A., Hardison, R. C., Miller, W. and Stubbs, L. (2005). Evolution and functional classification of vertebrate gene deserts. Genome Res. 15, 137-145 10.1101/gr.3015505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant, J., Zirngibl, R., Cado, D., Shago, M. and Giguere, V. (1991). Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 5, 1333-1344 10.1101/gad.5.8.1333 [DOI] [PubMed] [Google Scholar]
- Saijoh, Y., Adachi, H., Sakuma, R., Yeo, C.-Y., Yashiro, K., Watanabe, M., Hashiguchi, H., Mochida, K., Ohishi, S., Kawabata, M.et al. (2000). Left-right asymmetric expression of lefty2 and nodal is induced by a signaling pathway that includes the transcription factor FAST2. Mol. Cell 5, 35-47 10.1016/S1097-2765(00)80401-3 [DOI] [PubMed] [Google Scholar]
- Sakai, Y., Meno, C., Fujii, H., Nishino, J., Shiratori, H., Saijoh, Y., Rossant, J. and Hamada, H. (2001). The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 15, 213-225 10.1101/gad.851501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saund, R. S., Kanai-Azuma, M., Kanai, Y., Kim, I., Lucero, M. T. and Saijoh, Y. (2012). Gut endoderm is involved in the transfer of left-right asymmetry from the node to the lateral plate mesoderm in the mouse embryo. Development 139, 2426-2435 10.1242/dev.079921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood, R. I., Maehr, R., Mazzoni, E. O. and Melton, D. A. (2011). Wnt signaling specifies and patterns intestinal endoderm. Mech. Dev. 128, 387-400 10.1016/j.mod.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu, I. O. and Duester, G. (2006). Retinoic-acid signalling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nat. Cell Biol. 8, 271-277 10.1038/ncb1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu, I. O., Gresh, L., Barra, J. and Duester, G. (2005). Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development 132, 2611-2622 10.1242/dev.01845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford, D. and Prince, V. E. (2002). Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr. Biol. 12, 1215-1220 10.1016/S0960-9822(02)00929-6 [DOI] [PubMed] [Google Scholar]
- Teo, A. K. K., Arnold, S. J., Trotter, M. W. B., Brown, S., Ang, L. T., Chng, Z., Robertson, E. J., Dunn, N. R. and Vallier, L. (2011). Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev. 25, 238-250 10.1101/gad.607311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiso, N., Filippi, A., Pauls, S., Bortolussi, M. and Argenton, F. (2002). BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech. Dev. 118, 29-37 10.1016/S0925-4773(02)00252-6 [DOI] [PubMed] [Google Scholar]
- Turkson, J., Kim, J. S., Zhang, S., Yuan, J., Huang, M., Glenn, M., Haura, E., Sebti, S., Hamilton, A. D. and Jove, R. (2004). Novel peptidomimetic inhibitors of signal transducer and activator of transcription 3 dimerization and biological activity. Mol. Cancer Ther. 3, 261-269. [PubMed] [Google Scholar]
- Uehara, M., Yashiro, K., Takaoka, K., Yamamoto, M. and Hamada, H. (2009). Removal of maternal retinoic acid by embryonic CYP26 is required for correct Nodal expression during early embryonic patterning. Genes Dev. 23, 1689-1698 10.1101/gad.1776209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill, T. M., Cash, D. E. and Linney, E. (1994). Constitutively active retinoid receptors exhibit interfamily and intrafamily promoter specificity. Mol. Endocrinol. 8, 274-285. [DOI] [PubMed] [Google Scholar]
- Vincent, S. D., Dunn, N. R., Hayashi, S., Norris, D. P. and Robertson, E. J. (2003). Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 17, 1646-1662 10.1101/gad.1100503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, J. M. and Melton, D. A. (2000). Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development 127, 1563-1572. [DOI] [PubMed] [Google Scholar]
- Wendling, O., Dennefeld, C., Chambon, P. and Mark, M. (2000). Retinoid signaling is essential for patterning the endoderm of the third and fourth pharyngeal arches. Development 127, 1553-1562. [DOI] [PubMed] [Google Scholar]
- Wills, A., Dickinson, K., Khokha, M. and Baker, J. C. (2008). Bmp signaling is necessary and sufficient for ventrolateral endoderm specification in Xenopus. Dev. Dyn. 237, 2177-2186 10.1002/dvdy.21631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, P., Lo, C., Argiropoulos, B., Lai, C. B., Rouhi, A., Imren, S., Jiang, X., Mager, D. and Humphries, R. K. (2010). Identification of E74-like factor 1 (ELF1) as a transcriptional regulator of the Hox cofactor MEIS1. Exp. Hematol. 38, 798-808e2 10.1016/j.exphem.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, M., Meno, C., Sakai, Y., Shiratori, H., Mochida, K., Ikawa, Y., Saijoh, Y. and Hamada, H. (2001). The transcription factor FoxH1 (FAST) mediates Nodal signaling during anterior-posterior patterning and node formation in the mouse. Genes Dev. 15, 1242-1256 10.1101/gad.883901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, M., Mine, N., Mochida, K., Sakai, Y., Saijoh, Y., Meno, C. and Hamada, H. (2003). Nodal signaling induces the midline barrier by activating Nodal expression in the lateral plate. Development 130, 1795-1804 10.1242/dev.00408 [DOI] [PubMed] [Google Scholar]
- Yamanaka, Y., Lanner, F. and Rossant, J. (2010). FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 137, 715-724 10.1242/dev.043471 [DOI] [PubMed] [Google Scholar]
- Zorn, A. M. and Wells, J. M. (2009). Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221-251 10.1146/annurev.cellbio.042308.113344 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.