Abstract
A complex regulatory network of morphogens and transcription factors is essential for normal cardiac development. Nkx2-5 is among the earliest known markers of cardiac mesoderm that is central to the regulatory pathways mediating second heart field (SHF) development. Here, we have examined the specific requirements for Nkx2-5 in the SHF progenitors. We show that Nkx2-5 potentiates Wnt signaling by regulating the expression of the R-spondin3 (Rspo3) gene during cardiogenesis. R-spondins are secreted factors and potent Wnt agonists that in part regulate stem cell proliferation. Our data show that Rspo3 is markedly downregulated in Nkx2-5 mutants and that Rspo3 expression is regulated by Nkx2-5. Conditional inactivation of Rspo3 in the Isl1 lineage resulted in embryonic lethality secondary to impaired development of SHF. More importantly, we find that Wnt signaling is significantly attenuated in Nkx2-5 mutants and that enhancing Wnt/β-catenin signaling by pharmacological treatment or by transgenic expression of Rspo3 rescues the SHF defects in the conditional Nkx2-5+/− mutants. We have identified a previously unrecognized genetic link between Nkx2-5 and Wnt signaling that supports continued cardiac growth and proliferation during development. Identification of Rspo3 in cardiac development provides a new paradigm in temporal regulation of Wnt signaling by cardiac-specific transcription factors.
Keywords: Wnt signaling, R-spondins, Cardiac development, Transcriptional regulation, Angiogenesis, Nkx2-5, Rspo3
INTRODUCTION
The spatiotemporal aspects of cardiac development are mediated by interactions of transcription factors and morphogen-mediated signaling pathways that operate in negative- and positive-feedback loops. The mechanism by which signaling pathways, such as Wnt, FGF or Bmp, and transcription factors interact and modulate each others’ function is of crucial importance to our understanding of cardiac cell specification and proliferation. Nkx2-5 is one of the earliest known transcription factors required for cardiac cell specification and proliferation. Mice null for Nkx2-5 die in utero at E10 due to circulatory failure (Lyons et al., 1995; Tanaka et al., 1999). Close analysis of the mutant embryos shows arrest of cardiac development at looping stage and selective loss of second heart field (SHF) progenitor cells with absence of right ventricle (RV) and poorly developed outflow tract (OFT). It is generally accepted that Nkx2-5, among other genes, such as Islet1, Mef2c, Hand2, Pitx2, Foxh1, Fgf8, Fgf1 and Shh (Buckingham et al., 2005), is required for proliferation and/or patterning of SHF. An equally important observation is that Nkx2-5 is required for distinct phases of cardiac development including specification, differentiation and maintenance of function (Lyons et al., 1995; Tanaka et al., 1999; Habets et al., 2002; Pashmforoush et al., 2004; Jay et al., 2004; Zaffran et al., 2006; Prall et al., 2007). These observations reiterate the significance of a conserved spatiotemporal pattern of Nkx2-5 gene expression in the context of early cardiac mesoderm specification, proliferation and overall heart tube morphogenesis (Biben and Harvey, 1997; Prall et al., 2007). How Nkx2-5 expression and function are integrated into morphogen-mediated signaling pathways is currently not clearly understood.
A crucial interaction of Bmp signaling and Nkx2-5 was demonstrated by the Nkx2-5/Bmp2/Smad1 negative-feedback loop that controls heart progenitor specification and proliferation (Prall et al., 2007). In the cardiac fields of Nkx2-5 mutants, genes controlling cardiac specification and maintenance of the progenitor state, such as Bmp2, were upregulated, leading initially to progenitor overspecification, but subsequently failed SHF proliferation and OFT truncation. In the Nkx2-5 hypomorphic mutant mice, OFT abnormalities were partially rescued by Smad1 deletion (Prall et al., 2007).
Wnt/β-catenin signaling has emerged as a key regulator of cardiac progenitor cell specification, and several in vivo and in vitro studies have examined its function in cardiogenesis (Wu et al., 1995; Schneider and Mercola, 2001; Kwon et al., 2007; Qyang et al., 2007; Ai et al., 2007). Targeted inactivation of β-catenin in the precardiac mesoderm in the SHF using Isl1Cre resulted in complete loss of RV and a foreshortened OFT. However, the induction of precardiac mesoderm was not affected. Accordingly, constitutive activation of β-catenin resulted in the expansion of SHF and an enlarged, hypercellular RV (Kwon et al., 2007). These studies provide compelling evidence that this Wnt/β-catenin pathway plays a positive regulatory role in precardiac and cardiac mesoderm, and furthermore promotes committed cardiac cell proliferation and differentiation.
In vitro studies using embryonic stem cells (ESCs) have provided further evidence for a biphasic role of canonical Wnt signaling in promoting cardiogenic fate (Ueno et al., 2007; Kwon et al., 2007; Wang et al., 2007). Whereas early (prior to mesoderm formation) ectopic Wnt signaling can have inhibitory affects on cardiogenesis, later Wnt signals are essential for cardiogenesis. In this regard, inhibition of canonical Wnt signaling after formation of mesoderm progenitor cells completely abolished beating embryoid bodies (EBs), despite the early presence of Nkx2-5 (Kwon et al., 2007, 2008). These findings point to the crucial role of Wnt/β-catenin signaling even after initial commitment to the cardiac fate. However, the molecular mechanism by which Wnt signaling promotes expansion of cardiac mesoderm and proliferation of cardiomyocytes is not understood.
Here, we show that the conditional loss of Nkx2-5 in the SHF results in small RV and persistent truncus arteriosus (PTA), and that Rspo3, a key mediator of the canonical Wnt signaling, is markedly downregulated in the Nkx2-5 mutants. Accordingly, we see a reduction of Wnt/β-catenin signal in the SHF of Nkx2-5 mutants. Our studies demonstrate that Rspo3 is a target of Nkx2-5 and that conditional loss of Rspo3 resulted in early embryonic lethality secondary to SHF defects. We were able to rescue OFT septation defects in the conditional Nkx2-5 mutants by in vivo pharmacological treatment with LiCl. Similarly, transgenic overexpression of Rspo3 was able to rescue SHF defects in the Nkx2-5 mutants. Our studies point to a complex regulatory mechanism by which Nkx2-5 regulates multiple aspects of cardiac development. We can further unravel a novel pathway by which Nkx2-5 upregulates Wnt signaling and promotes cardiac cell growth by regulating the expression of Rspo3.
RESULTS
Nkx2-5 is required for the proper specification and expansion of SHF progenitors
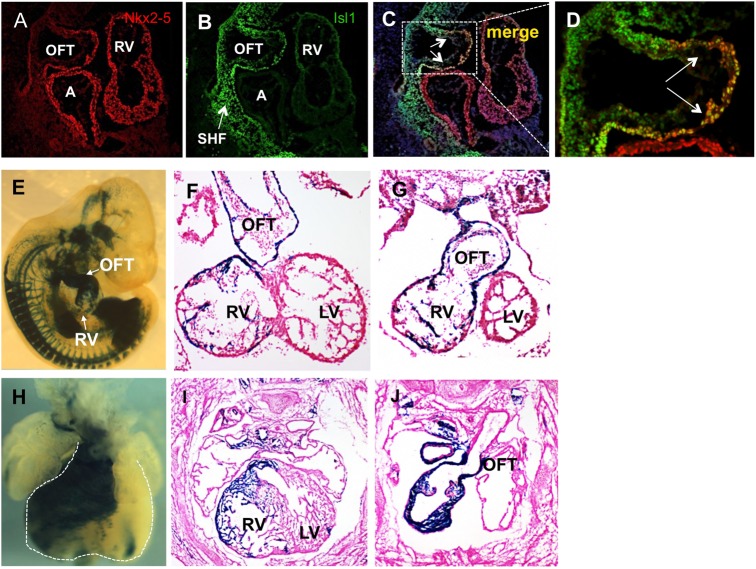
Nkx2-5+ progenitors give rise to the majority of cardiac mesoderm, but the selective role of Nkx2-5 in the SHF progenitors has not been examined. We inactivated Nkx2-5 in the pre-cardiac mesoderm using Isl1-Ires-Cre (Srinivas et al., 2001; Cai et al., 2003), Isl1-Cre (Yang et al., 2006) and Mef2c-Cre lines (Verzi et al., 2005). Conditional inactivation of Nkx2-5 by Isl1-Cre or Mef2c-Cre resulted in early embryonic lethality at E10-E10.5, which led to a loss of RV and foreshortened OFT (supplementary material Fig. S1). By contrast, inactivation of Nkx2-5 by Isl1-Ires-Cre consistently resulted in perinatal (P1) lethality due to OFT septation defects. Both Isl1-Cre and Isl1-Ires-Cre lines mediate early recombination very early in the SHF progenitors (Cai et al., 2003, Yang et al., 2006; Sun et al., 2007); however, we found that Isl1-Cre-mediated recombination is far more efficient in the SHF than Isl1-Ires-Cre (Milgrom-Hoffman et al., 2011). Consistent with Isl1-Cre cell lineage studies (supplementary material Fig. S1), we observe that Islet 1 immunoreactive protein is widely present in the SHF progenitors. Furthermore, coincident expression of Isl1 and Nkx2-5 occurred in this region at E9.5 (Fig. 1A-D) (Sun et al., 2007). By contrast, descendants of SHF progenitors from Isl1-Ires-Cre-mediated recombination predominantly contributed to the OFT, and less efficiently to the RV (Fig. 1E-J), suggesting that Isl1-Ires-Cre effectively labels only a subset of SHF precursors.
Fig. 1.
Fate map of Isl1-Ires-Cre in R26R-lacZ reporter mice. (A-D) Immunofluorescence analysis on sagittal sections of E9.5 embryos shows the presence of Isl1 in the SHF and the colocalization of Nkx2-5 and Isl1 in the OFT precursors (arrows). (E-J) X-gal staining of whole-mount (E,H) and sections (F,G,I,J) of embryos from Isl1-Ires-Cre crossed with R26R-lacZ reporter line showing efficient recombination in a subset of SHF precursors that contribute to OFT myocardium at E10.5 (E-G) and E12.5 (H-J). By contrast, RV myocytes are not labeled efficiently (F,I). A, atrium; LV, left ventricle; OFT, outflow tract; RV, right ventricle; SHF, second heart field.
All Nkx2-5 conditional mutants generated by Isl1-Ires-Cre showed a small RV with a foreshortened OFT when compared with the heterozygous littermates (Fig. 2A-F). There was a complete penetrance of OFT defects in all mutants examined, which resulted in cyanosis and death within a few hour of birth (Fig. 2F,J). A substantial number (92%) of the mutants analyzed (n>140) demonstrated a single OFT arising from the RV (Fig. 2J), and only a small number of embryos showed double outlet RV (DORV). The compound heterozygotes of Nkx2-5 and Isl1 were all viable and recovered at the expected Mendelian ratio. Gross examination and histology showed no evidence of OFT or RV abnormalities in the heterozygotes. Examination of Nkx2-5 mutants on the R26R-lacZ background revealed that lacZ+ cells contributing to the RV and OFT are substantially reduced (Fig. 2G-J).
Fig. 2.
Cardiac defects in the conditional Nkx2-5 mutants. Compared with the heterozygous littermates (A,C,E), conditional mutants of Nkx2-5 by Isl1-Ires-Cre display a single OFT and a considerably smaller RV (B,D,F) at E12.5. Examination of Nkx2-5 mutants and littermate controls at E10.5 (G,H, n=4) and P1 (I,J, n=5) on R26R-lacZ background shows reduced number of lacZ+ cells in the RV and OFT of mutant hearts. However, compared with RV, a large number of lacZ+ cells are still present in the mutant OFT. Ao, aorta; LA, left atrium; LV, left ventricle; OFT, outflow tract; PA, pulmonary artery; PTA, persistent truncus arteriosus; RA, right atrium; RV, right ventricle.
Defective trabeculae and endocardial cushions in the Nkx2-5 mutants
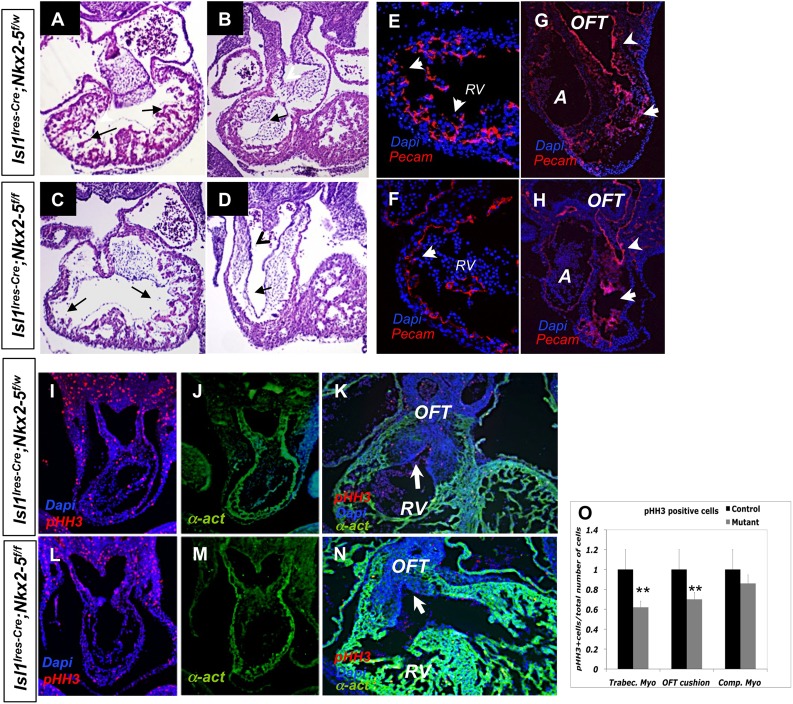
In the conventional Nkx2-5 mutants the early cardiac tube was formed but endocardial cushions and trabeculae were distinctly absent. These findings suggest that Nkx2-5 is required for certain phases of myocardial growth and proliferation, as well as interaction of myocytes with the adjacent non-myocardial tissue (Lyons et al., 1995; Tanaka et al., 1999). Histological examination of the Isl1-Ires-Cre; Nkx2-5f/f mutants (from here on referred to as conditional Nkx2-5 mutants) similarly showed a marked reduction of trabecular networks in the RV, whereas the left ventricle (LV) was spared (Fig. 3A-D, arrows). Furthermore, we noted smaller developing endocardial cushions in the proximal OFT (Fig. 3B,D). In this regard, Nkx2-5 immunoreactive protein was predominantly absent in the RV and OFT, but consistently present in the LV of the mutant embryos at E12.5, suggesting at least a cell-autonomous role for Nkx2-5 in this region (supplementary material Fig. S2).
Fig. 3.
Conditional Nkx2-5 mutants show selective loss of RV trabeculae and endocardial cushions. (A-D) Hematoxylin and Eosin (H&E) staining of paraffin sections show poorly developed trabeculae and endocardial networks in the RV of the mutant hearts at E11.5, whereas the LV is relatively preserved. (E-H) Similarly, immunofluorescence staining for Pecam1 shows significant loss of trabeculae in the mutant RV (E,F, arrows) and diminished endocardial networks in the conotruncus (G,H, arrowheads) at E9.5. (I,J,L,M) Immunofluorescent analysis for pHH3 (I,L) and for α-actinin (J,M). Mutant hearts show diminished relative cell proliferation in the trabeculae and endocardial cushions as assessed by pHH3 immunostaining. (K,N) At E12.5, the mutant hearts show hypoplastic cushions in the OFT (arrows; n=5). (O) Statistical analyses show a significant reduction of the relative cell proliferation in the trabecular myocardium and OFT cushions at E10.5 (n=7; **P=0.014). α-act, α-actinin; A, atrium; OFT, outflow tract; RV, right ventricle.
To further confirm these findings, we next examined the formation of trabecular networks and OFT cushions in mutant hearts by immunofluorescence staining for CD31 antigen (Pecam1). We similarly observed a marked reduction of trabeculae and endocardial networks in the conditional mutant RV (Fig. 3E,F; n=7). Examination of the proximal OFT (arrows) in mutants showed defective endocardial networks, whereas the neural crest cell-derived distal OFT endocardial cushions appeared relatively unperturbed (Fig. 3G,H, arrowheads; n=7). Examination of the mutant hearts by apoptotic markers (TUNEL assay) failed to show a difference compared with the heterozygous littermates (data not shown). By contrast, examination of the trabecular myocardium and OFT cushions, but not the compact myocardium, by phospho-histone-H3 immunostaining (and 5-bromo-2-deoxyuridine, BrdU; data not shown) showed a statistically significant reduction in the relative proliferative rate in the mutant embryos (Fig. 3I-O) (Prall et al., 2007). The decrease in the relative proliferative rate of SHF was evident as early as E10, which ultimately resulted in hypoplastic RV and OFT in the mutants at E12.5 (Fig. 3K,N). Consistent with prior studies, these observations suggest that Nkx2-5 is a crucial factor in the formation of the trabecular myocardium and OFT cushions (Waldo et al., 2001; Jiang et al., 2000; Prall et al., 2007).
In our studies, we did not find a significant change in either Bmp2 or Bmp4 expressions in the conditional Nkx2-5 mutants. Therefore, ectopic expression of Bmp2 in the conventional Nkx2-5 mutants might be dependent on spatial and/or temporal factors that are not present in our conditional model (Prall et al., 2007). In support of this notion, examination of the compound conditional mutants of Bmp2 and Nkx2-5 did not show a change in the phenotype of the conditional Nkx2-5 mutants (supplementary material Figs S3 and S4).
Gene expression profile in the RV/OFT of the Nkx2-5 mutants
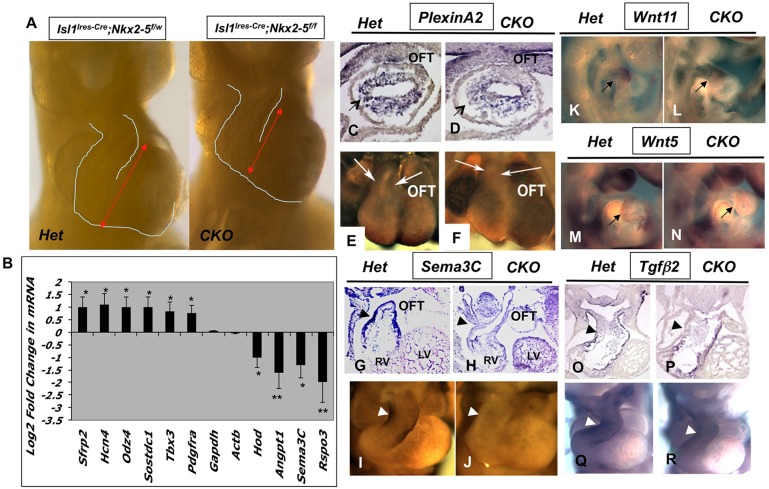
To gain further insight into the molecular basis of Nkx2-5 functions in the SHF, we examined the mutant and heterozygous littermates by gene expression microarray on two independent sample pairs and subsequently performed quantitative RT-PCR on ten independent sample pairs (Fig. 4A,B). In agreement with previously reported findings (Pashmforoush et al., 2004; Prall et al., 2007), we noted that several genes, including Hcn4, Odz4, Tbx3 and Pdgfrα, were upregulated in the RNA isolated from RV/OFT of conditional Nkx2-5 mutants. Strikingly, we observed that some of the key regulators of Wnt signaling pathway were altered in the Nkx2-5 mutants (Fig. 4B). Among these, Rspo3, a secreted factor and a reported potent agonist of Wnt signaling, was significantly diminished in the conditional Nkx2-5 mutants. Rspo3 is the predominant member of the R-spondin gene family (1-4) that is initially expressed in the embryonic heart at around E8.5, whereas other R-spondin members showed very low expression levels (Nam et al., 2007; Aoki et al., 2007; Kazanskaya et al., 2008). Further expression analysis of several other secreted factors, including Wnt11, Wnt5, Wnt8, Fgf8, Fgf10, Bmp2 and Bmp4, failed to show a significant change in mRNA levels by qRT-PCR in several independent mutants examined (supplementary material Fig. S5).
Fig. 4.
Altered gene expressions in the Nkx2-5 mutant hearts. (A) RV and OFT were dissected from the conditional mutants (n=12) and control littermates (n=11) at E10.5. (B) qRT-PCR analysis showing selected genes with statistically significant variations (*P<0.05; **P<0.01) in expression between mutants and control littermates. (C-J) Whole-mount and section in situ hybridization for Plxna2 (C-F) and for Sema3C (G-J) showing reduced expression levels (arrows and arrowheads) in the mutants compared with controls. In situ hybridizations show similar levels of expression for Wnt11 (K,L, arrows), Wnt5 (M,N, arrows) and Tgfβ2 (O-R, arrowheads) in the mutant and control embryos at E10.5. CKO, Nkx2-5 mutants; Het, heterozygous control; LV, left ventricle; OFT, outflow tract; RV, right ventricle.
Several studies have pointed to the inductive interaction of cardiac neural crest cells and SHF progenitors in the septation of the OFT (Kirby et al., 1985; Kirby and Waldo, 1995; Zaffran et al., 2004). We therefore examined the expression of several key factors implicated in the development and septation of OFT by in situ hybridization. semaphorin 3C (Sema3C) is expressed in the OFT/subpulmonary myocardium (Feiner et al., 2001), whereas plexin A2 (Plxna2), a receptor for semaphorin signaling, is expressed in the cardiac neural crest cells (CNCC; Brown et al., 2001). Plxna2 transcripts were present at a reduced level in the OFT by section and whole-mount in situ hybridization (Fig. 4C-F). We also noted that expression of Sema3C was markedly diminished in the mutant hearts (Fig. 4G-J). We did not observe an appreciable difference in Wnt11, Wtn5 or Tgfb2 expression in the mutant hearts at E10.5 (Fig. 4K-R). Our data suggest that loss of Nkx2-5 negatively impacts myocardial:CNCC interaction, thus contributing to OFT septation defects seen in the Nkx2-5 mutants.
Rspo3 is markedly downregulated in the Nkx2-5 mutants
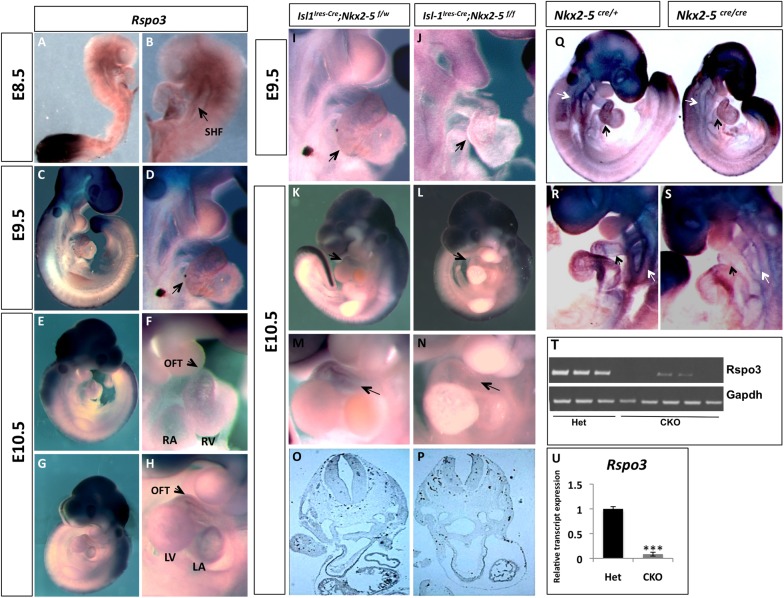
R-spondin3 is a member of the R-spondin gene family (Rspo1-4), a group of secreted Wnt signaling agonists that regulate embryonic patterning and stem cell proliferation (Kazanskaya et al., 2004, 2008; de Lau et al., 2011; Glinka et al., 2011). Gene expression analyses indicated that Rspo3 is markedly downregulated in the Nkx2-5 mutants. To validate these observations, we examined the expression of Rspo3 by in situ hybridization. Rspo3 is expressed at low levels in the developing heart at E8.5. However, at E9.5 to E10.5, Rspo3 is predominantly seen in the AV canal and the OFT, whereas lower levels are detectable in the entire heart including atria and ventricles (Fig. 5A-H) (Nam et al., 2006, 2007; Aoki et al., 2007; Kazanskaya et al., 2008). In late gestation, expression of Rspo3 becomes restricted to the sites of active Wnt signaling, such as cardiac valves and the myocardium just adjacent to the valvular apparatus (supplementary material Fig. S6). By in situ hybridization we noted marked reduction of Rspo3 transcripts in the RV and OFT of the conditional Nkx2-5 mutants (Fig. 5I-P, arrows; n=8). Examination of the conventional Nkx2-5 mutants similarly showed reduced Rspo3 expression in the mutant SHF (white arrows) and OFT (black arrows) at E9 (Fig. 5Q-S; n=4). To further confirm these findings, we micro-dissected RV and OFT from conditional KO (n=5) and from their littermate controls (n=3) at E10.5 and subjected them to semi-quantitative (Fig. 5T) and quantitative (Fig. 5U) RT-PCR analysis. The conditional Nkx2-5 mutants have a marked reduction in the Rspo3 transcripts compared with heterozygous controls (Fig. 5T-U). These observations provide support for a genetic link between Nkx2-5 and Rspo3 in the developing heart.
Fig. 5.
Rspo3 expression is significantly reduced in Nkx2-5 mutants. (A-H) Whole-mount in situ hybridization in E8.5-E10.5 embryos show that Rspo3 is highly expressed in the tail bud and neural tissue. Lower levels of Rspo3 transcripts are detected in the SHF (arrow in B) and the rest of the developing heart (arrow in D). At E10.5, Rspo3 is highly expressed the AV canal and the OFT (arrow in F). (I-P) Whole-mount and section in situ hybridization showing markedly diminished expression of Rspo3 (arrows) in the Nkx2-5 mutant OFT at E9.5 and E10.5. (Q-S) Whole-mount in situ hybridizations showing markedly diminished Rspo3 expression in the RV/OFT (black arrows) and SHF/pharyngeal arches (white arrows) of the Nkx2-5 conventional mutants. (T) Semi-quantitative and (U) quantitative RT-PCR showing markedly diminished Rspo3 transcripts in the conditional mutant hearts (P=0.0008). LA, left atrium; LV, left ventricle; OFT, outflow tract; RA, right atrium; RV, right ventricle.
Rspo3 is directly regulated by Nkx2-5
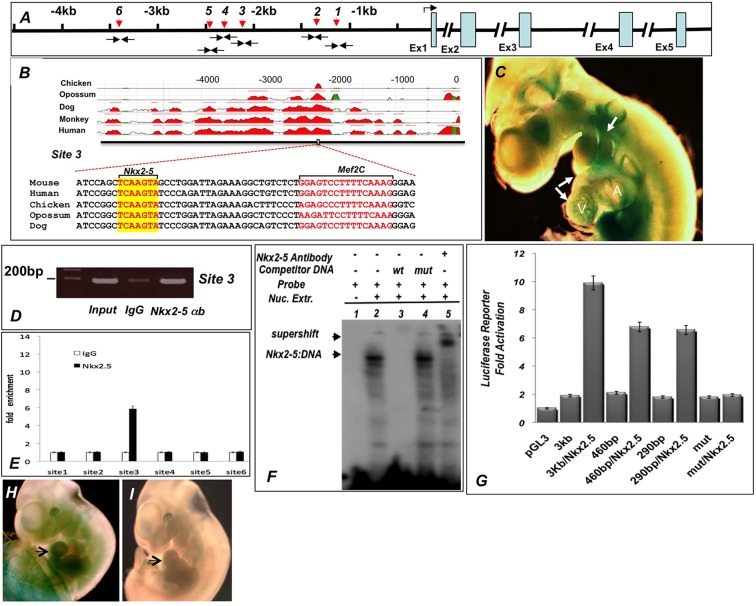
To determine whether Nkx2-5 can directly regulate the expression of Rspo3 in the developing heart, we performed an in silico analysis of Rspo3 and identified multiple potential Nkx2-5 binding sites in the immediate 5′-region of the gene by using BLAST and VISTA analyses (Fig. 6A,B) (Altschul et al., 1990; Mayor et al., 2000). Among the putative Nkx2-5 binding sites, we identified a site in a highly conserved region of the 5′-regulatory sequence conserved from chick to human (Fig. 6B). To test the functionality of the Rspo3 enhancer in vivo, we first generated transient transgenic mice harboring the immediate 5 kb upstream of Rspo3 (from ATG codon) that were cloned upstream of an Hsp68-lacZ reporter plasmid (Kothary et al., 1989). Such analysis revealed that three out of the five embryos carrying the transgene demonstrated β-galactosidase activity in the developing heart at E10.5 (Fig. 6C). The 5 kb enhancer sequences also drove the expression of lacZ in the pharyngeal arches, otic vesicles and the neural tube, sites consistent with in situ hybridizations for Rspo3 (Aoki et al., 2007; this study).
Fig. 6.
Nkx2-5 directly regulates Rspo3 expression. (A) Schematic representation of the Rspo3 locus showing the relative positions of the six putative Nkx2-5 binding sites (red arrows) and ChIP PCR primers (black arrows). (B) The position of site 3 in the Rspo3 promoter is highlighted and shows marked conservation across species. The putative Nkx2-5 and Mef2c binding sites in close proximity are shown. (C) The cloned 5 kb promoter construct drives the expression of Hsp68-lacZ reporter transgene in the embryonic heart and pharyngeal arches at E10.5 (white arrows). Three out of five transient transgenic embryos showed β-galactosidase staining that closely resembles the in situ hybridization. (D,E) ChIP showing that site 3 is highly enriched compared with the other sites. ChIP assays were repeated three times in triplicate assays (P<0.01). (F) EMSA demonstrating specific binding of Nkx2-5 to oligos containing Nkx2-5 binding sequences in site 3. (G) Luciferase assays in transient transfections of 293T cells showing that a 3 kb fragment upstream of ATG codon is significantly activated by Nkx2-5. The 460 bp (position −2505 to −2965) and 290 bp (−2505 to −2795) fragments containing site 3 are also activated by Nkx2-5 co-transfection. The expression vector containing a mutated Nkx2-5 binding site in the Rspo3 promoter is not activated by co-transfection with Nkx2-5 expression vector. (H,I) Four out of seven transient transgenic embryos harboring the 290 bp fragment showed expression of the Hsp68-lacZ reporter in the heart (arrow in H), whereas only one out of six transgenic embryo carrying the mutated Nkx2-5 binding site did not show β-galactosidase expression (arrow in I).
To determine whether Nkx2-5 can directly bind the Rspo3 regulatory sequences, we examined the conserved 5′-region of the Rspo3 gene by chromatin immunoprecipitation (ChIP) using Nkx2-5 antibody. Six potential Nkx2-5 binding sites were identified and subsequently examined by qPCR analysis of the immunoprecipitated chromatin. Among these, site 3 (−2704 bp from ATG codon), which is located in the highly conserved region, showed statistically significant enrichment by ChIP assay (Fig. 6D,E). To confirm further whether site 3 is a potential Nkx2-5 binding site, we examined the ability of Nkx2-5 expressed protein to bind these sequences by electrophoretic mobility shift assays (EMSAs). Using radiolabeled oligos containing the putative Nkx2-5 binding sequences at site 3, we consistently observed that the electrophoretic mobility shift of in vitro translated Nkx2-5 in these assays (Fig. 6F).
We next tested the ability of Nkx2-5 to activate the Rspo3 promoter in 293T cell transfection luciferase assays. Accordingly, we found that plasmids harboring DNA fragments, which contained site 3, demonstrated robust activation by Nkx2-5 (Fig. 6G). Mutation of the putative Nkx2-5 consensus sequence in site 3 abolished activation by Nkx2-5 in luciferase assays. We next generated transient transgenic mice harboring the 290 bp conserved region (including site 3) upstream of the Hsp68-lacZ gene. Transient transgenic assays showed β-galactosidase expression in the heart of four out of seven embryos harboring the transgene (Fig. 6H,I). These results provide further evidence that Nkx2-5 can directly regulate the expression of Rspo3 in vitro. Collectively, our data support direct regulation of Rspo3 by Nkx2-5. In agreement with our data, a recent study showed loss of expression of Rspo3 in Nkx2-5-deficient mouse ESCs. By contrast, overexpression of Nkx2-5 in the ESCs led to a marked increase in expression of Rspo3 (Nakashima et al., 2009).
Wnt/β-catenin signaling is diminished in the Nkx2-5 mutants
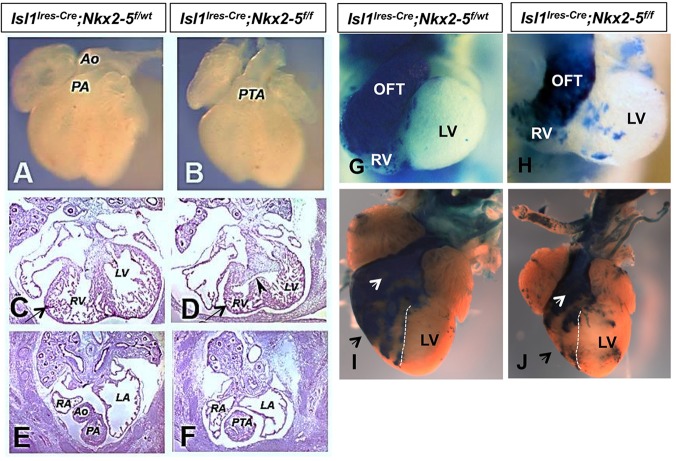
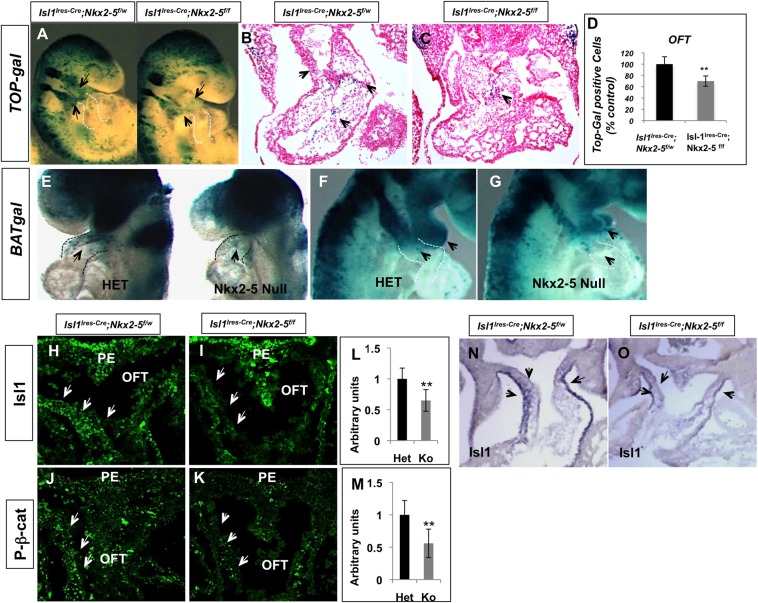
Several studies have demonstrated that canonical Wnt signaling is essential for normal cardiac development and that the conditional loss of β-catenin in the SHF leads to profound cardiac defects (Ai et al., 2007; Klaus et al., 2007; Lin et al., 2007; Kwon et al., 2007). We hypothesized that loss of Rspo3 and dysregulation of Wnt signaling is one of the potential mechanisms that can lead to SHF defects in the Nkx2-5 mutants. We assessed the extent of Wnt signaling by crossing TOPGAL and BATGAL reporter lines into the conditional and conventional Nkx2-5 mutants, respectively. Examination of the conditional (TOPGAL; Fig. 7A-C) and conventional (BATGAL; Fig. 7E-G) Nkx2-5 mutants on the Wnt reporter backgrounds showed a reduced number of lacZ+ cells in the OFT and pharyngeal arches (sites of expression of Nkx2-5 and Isl1) in the conditional and conventional Nkx2-5 mutants. We quantified the number of lacZ+ cells in tissue sections of the conditional Nkx2-5 mutant embryos (n=6). These analyses revealed a 32% reduction in the number of lacZ+ cells in mutant OFT compared with heterozygous littermates.
Fig. 7.
Decreased Wnt-β-catenin signaling in the SHF of the conditional Nkx2-5 mutants. (A-C) Whole-mount and section X-gal staining of the conditional Nkx2-5 mutants on the TOPGAL background showing statistically significant diminished number of lacZ+ cells in the pharyngeal arches and OFT (black arrows) of the E9.5 mutant embryos (n=9 embryos). (D) Quantification of lacZ+ cells in mutants and control OFT (n=6; **P<0.01). (E-G) X-gal staining of the conventional Nkx2-5 mutants (Nkx2-5cre/cre, Nkx2-5 Null) and heterozygous (Nkx2-5cre/+, HET) embryos on the BATGAL reporter background, showing significant reduction of lacZ+ in the developing OFT of Nkx2-5 null embryos (black arrows; n=10). (H-K) Immunofluorescence staining showing reduced number of Isl1+ (H,I, arrows) and activated β-catenin+ (J,K, arrows) cells in the SHF of controls (Isl1-Ires-Cre xNkx2-5f/w; n=4) and mutants (Isl1Cre×Nkx2-5f/f; n=6) at E10.5. Quantitation of Isl1+ (L) and activated β-catenin+ (M) cells shows statistically significant reduced number of cells in the mutant sections (10 μm). (N,O) In situ hybridization showing diminished expression of Isl1 in SHF of the Nkx2-5 mutants (Isl1Cre×Nkx2-5f/f) at E10.5. OFT, outflow tract; PE, pharyngeal endoderm.
To further validate these observations, we examined the expressions of known targets of Wnt signaling, including Isl1 and activated β-catenin, in the SHF of the Nkx2-5 mutants (Lin et al., 2007; Cohen et al., 2007). We used a combination of immunofluorescence staining and in situ hybridization to identify Isl1+ and activated β-catenin+ cells in the conditional Nkx2-5 mutants (Fig. 7H-O) (Rhee et al., 2007). At E10.5, during proliferation and deployment of the SHF progenitors in the OFT, we observed a significant reduction in the number of Isl1+ and activated β-catenin+ nuclei in the conditional Nkx2-5 mutants (Fig. 7L,M; n=5 mutants analyzed). Further examination of the conditional Nkx2-5 mutants by section in situ hybridization also confirmed reduced Isl1 expression in the OFT in the mutants (Fig. 7N,O). These data support the contention that canonical Wnt signaling is reduced in the SHF of Nkx2-5 mutants.
R-spondin3 is required for the proper development of SHF
Conditional inactivation of β-catenin by Isl1-Cre was previously shown to result in embryonic lethality at E12.5 secondary to cardiac and pharyngeal arch artery defects (Lin et al., 2007; Kwon et al., 2007). We hypothesized that the phenotype in the Nkx2-5 mutants could be in part due to the loss of Rspo3 and attenuation of Wnt signaling in the SHF. Prior studies have shown that Rspo3 null mutants are growth retarded and die at E10 due to placental and global vascular defects (Aoki et al., 2007; Kazanskaya et al., 2008). The early embryonic lethality precluded examination of later cardiac development in Rspo3 null mice. To circumvent this problem, we crossed mice carrying the conditional allele of Rspo3 (Kazanskaya et al., 2008) with Isl1-Ires-Cre mice and Isl1Cre lines. Conditional inactivation of Rspo3 by Isl1-Ires-Cre resulted in late gestational lethality (E14.5-16.5) in most mutants secondary to cardiovascular failure. All mutant embryos analyzed had evidence of small RVs and/or DORV (supplementary material Fig. S7).
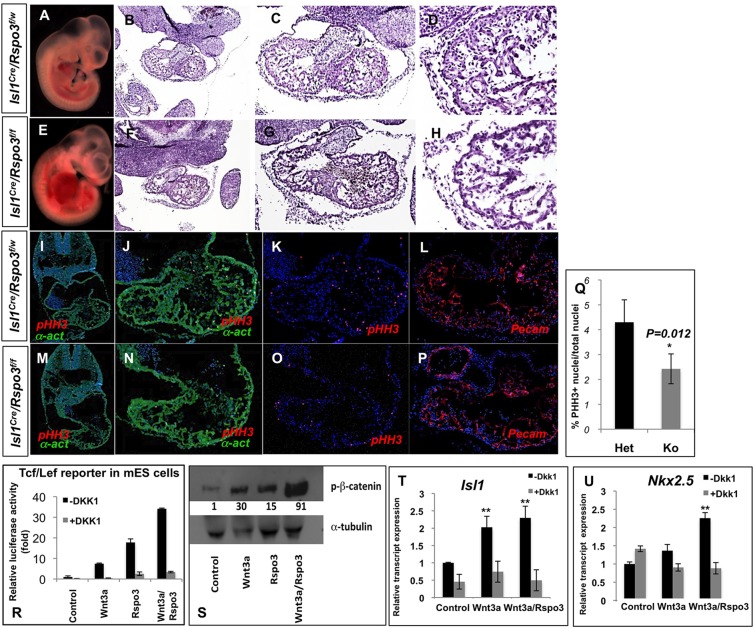
By contrast, inactivation of Rspo3 by the more efficient Isl1-Cre line resulted in uniform embryonic lethality at E11.5 (>100 mutants analyzed; from here on referred to as Rspo3 mutants). All Rspo3 mutants demonstrated pericardial edema and marked vascular congestion, evidence of early circulatory failure (Fig. 8A,E). Histological analysis of Rspo3 mutant hearts consistently revealed defects of RV and OFT, consisting of hypocelluar cushions and a thin-walled myocardium (Fig. 8B-H). The heterozygous littermates were recovered in the expected Mendelian ratio without any noticeable abnormality. The cardiac defects in Rspo3 mutants were evident as early as E10, when myocardial wall thickness was diminished in the mutant embryos (data not shown). Immunofluorescence analysis of the mutant hearts by anti-CD31 and anti-α-actinin antibodies suggested proper specification of myocardial and endocardial lineages in the Rspo3 mutants (Fig. 8I-P). We next examined the proliferation rate in the mutant embryos by immunostaining for phospho-histone H3 (pHH3) (Fig. 8K,O) and BrdU (Fig. 8Q). These independent studies showed reduced relative proliferation rates in the Rspo3 mutant hearts. These results are thus consistent with the suggested role of Wnt/β-catenin signaling in cardiac progenitor cell proliferation (Kwon et al., 2007, 2008).
Fig. 8.
Rspo3 is essential for cardiac development. (A-H) Conditional inactivation of Rspo3 by Isl1Cre resulted in embryonic lethality at E11.5. The mutant embryos show evidence of vascular congestion and pericardial edema (A,E). H&E sections of the Rspo3 mutants (Isl1Cre; Rspo3f/f) and heterozygotes (Isl1Cre; Rspo3f/w) show poorly developed RV and OFT in the mutant embryos. (I-P) Examination of Rspo3 mutants by immunofluorescence for α-actinin, phospho-histone-H3 and Pecam1 (CD31) shows a poorly developed heart. Endocardium and myocardium appear to be specified properly. (Q) Quantitation of cell proliferation, as assessed by BrdU, shows a statistically significant reduction of the relative proliferation rate in cardiomyocytes of the mutant hearts (Ko) compared with wild type (Het). (R) Rspo3 potentiates canonical Wnt signaling in mouse ESCs transfected with Tcf/Lef reporter plasmid. Rspo3 activation of Tcf/Lef reporter plasmid is inhibited by Dkk1. (S) Western blot showing a significant increase in activated β-catenin in EBs (day 5) derived from ESCs treated with Wnt3a and/or Rspo3. (T,U) Mouse ESCs were treated (days 3, 4) with Wnt3a- or Wnt3a/Rspo3-conditioned media in the presence or absence of Dkk1. RNA was isolated from EBs at day 6 and was subjected to qRT-PCR for Isl1 and Nkx2-5 transcripts. Rspo3 potentiates Wnt3a by significantly increasing Isl1 and Nkx2-5 transcripts in EBs.
To further explore the role of Rspo3 as a positive modulator of Wnt signaling, we tested the ability of Rspo3 to drive the expression of the Tcf/Lef luciferase reporter system in mouse ESCs (Biechele et al., 2009). ESCs were transfected with Tcf/Lef luciferase reporter plasmids and after 24 h were treated with Wnt3a and/or Rspo3 recombinant proteins. These experiments demonstrated the potent ability of Rspo3 in activating canonical Wnt signaling and further showed that Rspo3 synergizes with Wnt3a in activating the β-catenin protein (Fig. 8R,S). The activation of the Tcf/Lef reporter was promptly abolished by addition of Dkk1, thus showing the specificity of Rspo3 in activating the canonical Wnt pathway (Fig. 8R).
Several studies have indicated a positive role for the canonical Wnts in promoting cardiogenesis in ESC-derived embryoid bodies (EBs; Ueno et al., 2007; Kwon et al., 2007). To this end, we used previously described methods (Kwon et al., 2007) to test whether Rspo3 can positively influence cardiogenesis in EBs derived from mouse ESCs. We treated mouse ESCs (R1) with Rspo3 recombinant protein on days 3-5 of differentiation, and subsequently collected EBs at days 5 and 6. Quantitative RT-PCR analysis of RNA derived from Rspo3-treated ESCs consistently showed that Rspo3 potentiates the expression of the cardiogenic fate markers Islet1 and Nkx2-5 (Fig. 8T,U). Therefore, our studies point to Rspo3 as a positive modulator of cardiogenic fate in mouse ESCs.
Activation of canonical Wnt signaling rescues SHF defects in the Nkx2-5 mutants
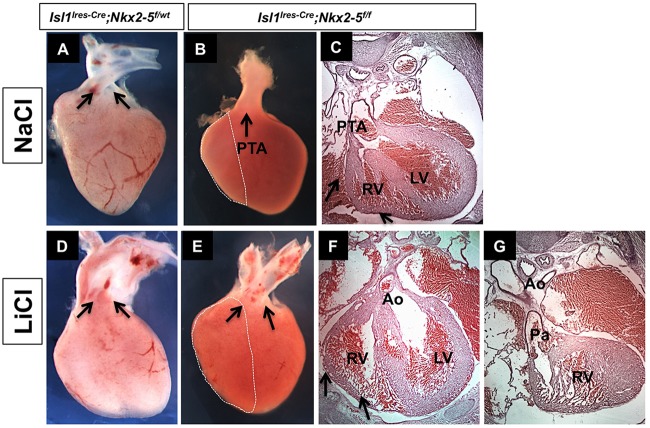
Our data suggested that loss of Nkx2-5 negatively impacts the extent of Wnt signaling in the SHF by downregulating the expression of Rspo3. Furthermore, Rspo3 is a crucial modulator of Wnt signaling required for cardiac development. To determine whether temporal activation of Wnt/β-catenin signaling can rescue the phenotype in the Nkx2-5 mutants, we chose a pharmacological approach using LiCl, an inhibitor of Gsk3β and potent activator of Wnt/β-catenin pathway (Klein and Melton, 1996; Nakamura et al., 2003; Tian et al., 2010). We administered LiCl or NaCl (control) once a day intraperitoneally to pregnant females (Isl1Cre; Nkx2-5f/wt×Nkx2-5f/f) from E8 to E10 at the time of expansion and proliferation of SHF. Whereas all mutant neonates born to females treated with NaCl had a small RV and a single OFT (n=12), the majority of the mutants (10/15, 67%) born to females treated with LiCl showed a significantly larger RV and drastic septation of the OFT (Fig. 9). However, treatment with LiCl failed to correct the alignment defects of the OFT in the Nkx2-5 mutant embryos (Fig. 9E). These data show that loss of Nkx2-5 leads to attenuation of the Wnt/β-catenin pathway and that pharmacological activation of Wnt signaling can significantly ameliorate the phenotype in the conditional Nkx2-5 mutants.
Fig. 9.
Pharmacological rescue of SHF defects in conditional Nkx2-5 mutants. Pregnant females were treated with intraperitoneal injections of LiCl or NaCl from E8-E10 of gestation. (A-C) All Nkx2-5 conditional mutants treated with NaCl had a single OFT (PTA) at E17.5 (n=12). (D-G) 67% (n=10/15; P=0.01) of the conditional Rspo3 mutants (E-G) treated with LiCl had a significantly larger RV (arrows) and showed OFT septation. Treatment with LiCl did not correct the OFT alignment defects in the mutants (E). In A,D,E, aorta and pulmonary artery are indicated by arrows. Ao, aorta; LV, left ventricle; OFT, outflow tract; Pa, pulmonary artery; PTA, persistent truncus arteriosus; RV, right ventricle.
Conditional expression of Rspo3 rescues Nkx2-5 mutants
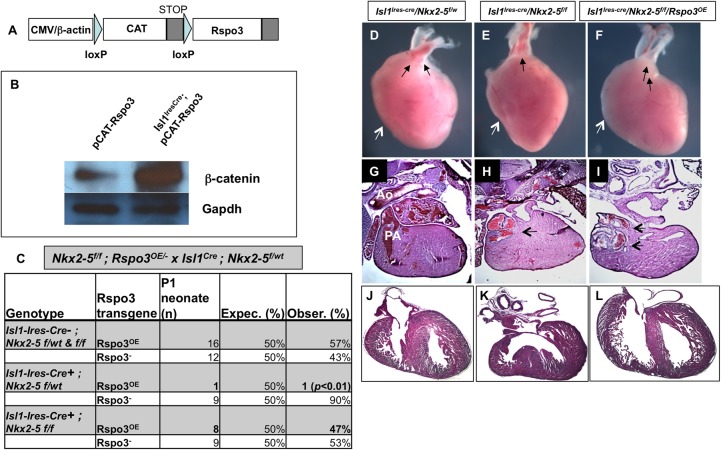
We next sought to determine whether gain-of-function of Rspo3 could rescue the SHF phenotype in the Nkx2-5 mutants. To this end, we generated transgenic mice that conditionally express Rspo3 after they are crossed with a Cre-driver line (Fig. 10A,B). Conditional overexpression of Rspo3 transgene (Rspo3OE) using an Isl1-Ires-Cre line resulted in embryonic lethality in most transgenic embryos at mid-gestation (9/10 at E11.5), and we were unable to recover any newborns (Fig. 10C). These results suggest that excess Wnt signaling mediated by Rspo3 results in embryonic lethality (Fig. 10B). These data are consistent with prior studies that also demonstrated that constitutive activation of Wnt/β-catenin signaling in cardiac mesoderm results in early embryonic lethality (Kwon et al., 2007; Grigoryan et al., 2008). Surprisingly, when the Rspo3OE line was crossed into the conditional Nkx2-5 line (Isl1Ires-Cre; Nkx2-5f/w×Rspo3OE; Nkx2-5f/f), we were able to recover the majority of Rspo3OE neonates on the Nkx2-5 mutant background (Fig. 10C,F). Therefore, it appears that loss of Nkx2-5 in the precardiac mesoderm attenuates excess Wnt signaling in Rspo3-overexpressing embryos. We next examined the conditional Nkx2-5 mutants that carried the Rspo3OE transgene. Examination of Rspo3OE neonates on the Nkx2-5 mutant background in several litters consistently showed that selective activation of Rspo3 in the SHF by Isl1-Ires-Cre rescues the SHF defects of the conditional Nkx2-5 mutants and results in a larger RV and septation of the OFT (Fig. 10D-L). Our data provide further evidence for the genetic interaction between Nkx2-5 and Wnt signaling in the SHF.
Fig. 10.
In vivo expression of Rspo3 rescues the SHF defects in conditional Nkx2-5 mutants. (A) Transgene construct for the conditional Rspo3 expression, in which a chloramphenicol acetyl transferase loxP (CAT)-STOP loxP-Rspo3 gene cassette is driven by CMV/chicken β-actin promoter. (B) Western blot showing a significant increase in active β-catenin in embryonic hearts (E10.5) isolated from Rspo3OE transgenic mice crossed with Isl1-Ires-Cre line. (C) The majority of embryos carrying the Cre-activated Rspo3OE transgene die in utero. By contrast, most of Rspo3-overexpressing mice on the Nkx2-5 mutant background are recovered at birth. (D-L) Whole-mount and H&E stain of sections from control and rescued Nkx2-5 conditional mutants. Conditional activation of Rspo3 on the Nkx2-5 mutant background results in the rescue of RV/OFT defects (F,I,L) compared with the simple mutants (E,H,K), and is comparable to the control neonates (D,G,J). The ventricular septal defect is absent in the rescued heart and RV size appears larger (white arrows). Black arrows represent aorta and pulmonary artery in D,F,I and PTA in E,H. PA, pulmonary artery.
DISCUSSION
Recent data have identified that Wnt/β-catenin signals control distinct sets of transcription factors in cardiac progenitor cells (Klaus et al., 2012). Here, we find a genetic link between Nkx2-5 and Wnt signaling that is mediated by Rspo3. We show that Nkx2-5 is required for the expansion of SHF progenitors and that loss of Nkx2-5 in the SHF is associated with loss of RV and defects of OFT septation. In our study, conditional mutants of Nkx2-5 generated by Mef2cCre and Isl1-Cre lines display early embryonic lethality with a virtually absent RV and a foreshortened OFT, consistent with defects of SHF development (supplementary material Fig. S1). By contrast, mutants generated by Isl1-Ires-Cre survived to term with a smaller RV being present. It has previously been shown that Isl1-Ires-Cre is active very early in precardiac mesoderm and that it acts upstream of Mef2c (Cai et al., 2003; Dodou et al., 2004; Verzi et al., 2005). The data presented here suggest that it is the efficiency of the Cre lines in the SHF that results in the observed phenotypes, and not necessarily a temporal difference. To this end, we observed that Isl1-Ires-Cre-mediated recombination is very efficient in the SHF progenitors that ultimately contribute to the myocardium of the OFT, whereas recombination in progenitors that contribute to RV is modest (Figs 1 and 2). We find that lacZ+, Nkx2-5-deficient cells are predominantly present in the myocardium of OFT compared with RV (supplementary material Fig. S1J,M and Fig. S2I,J), suggesting that Nkx2-5 might not be as crucially required in this population of cardiomyocytes. The predominant presence of Nkx2-5− cells in the OFT, but not RV, raises the interesting possibility that requirements for Nkx2-5 are not uniform in the SHF progenitors.
Nkx2-5 regulates Rspo3 expression and modulates Wnt signaling
Perturbation of both canonical and non-canonical Wnt signaling affects the development of SHF-derived structures resulting in RV and OFT defects. Conditional mutants of β-catenin and Lrp6 in the canonical pathway, as well as Wnt5a and Wnt11 in the non-canonical pathway, result in OFT defects in mice (Kwon et al., 2007; Lin et al., 2007; Schleiffarth et al., 2007; Zhou et al., 2007; Song et al., 2010). In the present study, we provide evidence that Nkx2-5 is required for growth and proliferation of SHF progenitor cells, in part by regulating the expression of Rspo3 and by modulation of Wnt signaling. Identification of Rspo3, a potent Wnt agonist, as a target of Nkx2-5 in the developing heart, has significant implication for the growth and development of the embryonic heart. Whereas R-spondins are shown to synergize with Wnt3a to activate canonical Wnt signaling (our data; Nam et al., 2006; Kazanskaya et al., 2008; Baljinnyam et al., 2012), recent studies also provide strong evidence for the activation of the non-canonical Wnt pathway by Rspo3 (Ohkawara et al., 2011; Glinka et al., 2011). Multiple independent studies have now shown that stem cell markers and orphan receptors, Lgr5 and Lgr4, bind R-spondins and activate both canonical and non-canonical Wnt signaling pathways (Carmon et al., 2011; de Lau et al., 2011; Glinka et al., 2011). Given the well-studied role of Wnt/β-catenin signaling in growth and development of SHF, it is plausible that Rspo3 potentiates this process and thus promotes progenitor cell proliferation.
Supporting the notion that Wnt signaling is a positive regulator of cardiomyocyte proliferation is the finding that conditional loss of β-catenin in cardiac progenitors resulted in diminished myocardial growth and hypoplasia of both cardiac fields (Kwon et al., 2008). Furthermore, heterozygous loss of β-catenin was sufficient to suppress the cardiomyocyte overgrowth in the Hippo pathway mutants, suggesting a crucial link between canonical Wnt pathway and myocardial proliferation (Heallen et al., 2011). Therefore, it is plausible that potentiation of Wnt signaling by Rspo3 is crucial for cardiac growth and development. Whether Rspo3 functions in the cardiomyocytes, endocardial cells, or both, is currently being investigated. Isl1Cre operates in the majority of cardiac myocytes and in only a subset of endocardial cells (Yang et al., 2006; Milgrom-Hoffman et al., 2011). In this regard, we consistently observe a diminished number of endocardial cells in Rspo3 mutants. Therefore, it would be important to test whether Rspo3 from myocardium can attenuate cell growth or survival in endocardium.
In line with our hypothesis, we were able to rescue the majority of SHF defects in the Nkx2-5 mutants by activation of Wnt signaling, suggesting that Nkx2-5 serves cell-autonomous and non-cell-autonomous functions in the SHF. The molecular link between Nkx2-5 and Wnt signaling was suggested by a recent study (Klaus et al., 2012), thus providing further evidence for transcriptional attenuation of specific signaling pathways. Pharmacological rescue of OFT septation in the Nkx2-5 mutants provides strong evidence that Nkx2-5 and canonical Wnt signaling overlap significantly in genetic pathways leading to cardiogenesis. The attenuation of Wnt signaling by Nkx2-5 may have implication in the temporal aspect of cell division and differentiation of SHF progenitors.
A recent study suggested that Wnt and Bmp signals control distinct sets of transcription factors in cardiac progenitors of SHF (Klaus et al., 2012). Our data are in line with these observations, as Rspo3 was able to rescue the SHF defects in the Nkx2-5 mutants, thus providing strong evidence for the genetic interaction of Nkx2-5 and the Wnt pathway. Prior studies have shown that excess activation of the Wnt pathway by a constitutively active β-catenin results in early embryonic lethality (Kwon et al., 2007; Grigoryan et al., 2008). We similarly observed that conditional overexpression of Rspo3 in the precardiac/cardiac mesoderm results in excess activation of β-catenin and embryonic lethality. Surprisingly, we find that the only surviving Rspo3OE mutants are on the Nkx2-5 mutant background, thus providing an independent line of evidence that loss of Nkx2-5 can attenuate excess Wnt signal by Rspo3. Therefore, our studies point to the previously unrecognized genetic interaction of Nkx2-5 and Wnt signaling in the development of SHF.
MATERIALS AND METHODS
Mice
All mouse lines have been described previously: Rspo3-floxed allele (Kazanskaya et al., 2008); Nkx2-5-floxed allele (Pashmforoush et al., 2004), Mef2cCre (Dodou et al., 2004), Islet1Cre (Cai et al., 2003), Bmp2-floxed allele (Rivera-Feliciano and Tabin, 2006), Foxa2Cremcm (Park et al., 2008), TOPGAL (DasGupta and Fuchs, 1999) and BATGAL (Maretto et al., 2003). All mouse lines were of mixed C57BL6/129SVEJ background. The embryos were collected at E8.5 to neonatal stage. Transgenic mice carrying the Rspo3 overexpression transgene were generated at the transgenic facility of the University of California, Irvine. Ten founder mice were initially determined by PCR analysis of tail DNA. Subsequently, the lines expressing high levels of chloramphenicol acetyl transferase (CAT) were identified by a colorimetric assay of CAT enzyme isolated from tail tissue. The transgenic mice were crossed into the Nkx2-5-floxed allele for at least six generations. For in vivo pharmacological rescues, pregnant mice were injected interperitoneally with 200 mg/kg LiCl once a day at the indicated times. Tamoxifen-induced gene knockout is described in the Methods in the supplementary material. All animal procedures were performed in accordance with the Institute for Animal Care and Use Committee at the University of Southern California.
Constructs
Rspo3 enhancer constructs for transient transgenic experiments and luciferase assays were created by PCR amplification using high-fidelity Taq polymerase using primers that span the Rspo3 promoter region and site 3. The PCR fragments were cloned into pGH-lacZ, which contains the HSP68 core promoter upstream of lacZ coding sequences as previously described (Creemers et al., 2006). For luciferase assays the PCR fragments were cloned into pGL3 (Promega). Constructs were verified by sequencing.
Histology and immunofluorescence
Embryos were isolated and fixed in 4% PFA/PBS overnight and then dehydrated and embedded in OCT (Sakura Finetek). Sections of 10 μm thickness were collected on slides and subjected to immunostaining. The primary antibodies were PY489-β-catenin (DHSB, Balsamo; 1/100), Nkx2-5 (Santa Cruz Biotechnology, Sc-8697; 1/100), Pecam1 (BD Pharmingen, 550274; 1/100), Isl1 (DSHB, 40.3A4; 1/100), α-actinin (Sigma, A7811; 1/1000); and Rspo3 (R&D Systems, MAB 41201; 1/50).
In situ hybridization
Whole-mount and section in situ hybridization were performed according to previously described protocols (Moormann et al., 2001). Briefly, the fixed embryos were digested with proteinase K to facilitate probe penetration. After washes, the embryos were incubated with digoxigenin-labeled antisense RNA (Roche) at 70°C overnight. After several high-stringency washes post-hybridization, embryos were blocked for 2 hours at room temperature, followed by incubation with alkaline phosphatase-conjugated anti-digoxigenin antibody (Roche). After extensive washes embryos were incubated with BM Purple (Roche) for colorimetric detection of labeled transcript.
β-galactosidase staining
Embryos from timed pregnancies were isolated and fixed for 30 min in 4% PFA/PBS at 4°C. β-galactosidase staining was performed as described (Yamagishi et al., 2003). Sections were counterstained by nuclear Fast Red.
Electrophoretic mobility shift assay (EMSA)
Experiments were performed using annealed double-stranded oligonucleotides derived from the indicated region (site 3) of the Rspo3 promoter and recombinant Nkx2-5 protein. Briefly, wild-type double-stranded oligonucleotides for the Nkx2-5 binding sequence from the Rspo3 promoter region, Nkx2-5-2214WT: 5′-CCAGCTCAAGTAGCCTGGAT-TAGA-3′ and Nkx2-5-2214Mut: CCGCTCCCTCAGCCTGGATTAGA, were synthesized, and the two complementary oligonucleotides were annealed and labeled with [α-32P]-dATP and [α-32P]-dCTP using the Klenow enzyme. The labeled probes were incubated with 10 µl of in vitro-translated protein product and binding buffer containing 0.25 mg/ml poly dI-dC for 30 min at room temperature. For the super gel shift, 1 µl of Nkx-2.5 (N-19) was added and kept at room temperature for 30 min longer. For the competition reaction, 200-fold excess of unlabeled wild-type probe was added. The protein/DNA mixture was resolved on a 6% polyacrylamide gel.
ESC culture and EBs
Murine ESCs were propagated undifferentiated on mytomycin-C-treated MEFs in maintenance medium of Glasgow MEM (Sigma; G5154) supplemented with 10% FBS (HyClone; SH30071.03), 1 mM 2-mercaptoethanol (Sigma; M7522), 2 mM l-glutamine (Gibco-BRL; 25030-081), 1 mM sodium pyruvate (GIBCO-BRL; 11360-070), 0.1 mM nonessential amino acids and 1000 units/ml leukemia inhibitory factor (LIF) (Chemicon International; ESG1107). All ESC manipulations and formation of EBs were carried out according to Kwon et al. (2007). EBs were formed by the hanging drop method in differentiation medium (DM) that contained the same components as the maintenance medium but with 20% FBS added and without LIF. For treatment, the medium was replaced with DM containing Wnt3a (150 ng/ml, R&D Systems) or Rspo3 (150-200 ng/ml, R&D Systems) at the start of day 3. On day 5, the medium was replaced with fresh DM. Protein and RNA were isolated from EBs on days 5 and 6 of differentiation, respectively (Kwon et al., 2007).
In vivo ChIP assays
Whole heart was extracted from wild-type E10.5 embryos and processed as detailed in the Methods in the supplementary material. Chromatin was immunoprecipitated with either the Nkx-2.5 (N-19; Santa Cruz Biotechnology) or a normal goat IgG control antibody. Amplified fragments were analyzed on a 1.5% agarose gel or qPCR was performed using a DNA Engine Oticon2 thermal cycler (MJ Research) with SYBR Green Supermix (Bio-Rad) and quantitative differences were determined. Each ChIP assay was performed at least three times, and qPCR results represent the mean±s.e.m. of three assays. ChIP assay primers are detailed in the Methods in the supplementary material.
Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated from E9.5 or 10.5 hearts with Trizol reagent (Invitrogen) and 1 µg was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad). Real-time PCR reactions were performed using the iQTM SYBR Green Supermix (Bio-Rad). The qPCR data were normalized to the expression of β-actin or Gapdh (primers are listed in supplementary material Table S1). Statistical analyses were performed using Student's t-test. The results are the average of three or more independent experiments in triplicate.
Promoter cloning and luciferase reporter assays
The 3 kb genomic DNA fragment containing the putative Nkx2-5 binding site upstream of the Rspo3 (ENSMUSG00000019880) start codon, and 290 bp and 460 bp fragments were amplified as described in the Methods in the supplementary material. The luciferase transfection assay was performed in 293T cells as described in the Methods in the supplementary material.
Supplementary Material
Acknowledgements
We thank Mr and Mrs Paul Simon for their generosity. We are grateful for the support of the California Institute for Regenerative Medicine (CIRM).
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
L.C. and M. Pashmforoush developed the concepts and approach, analyzed the data for these studies and wrote the manuscript. L.C. performed most of the experiments with help from M. Plate. H.M.S. participated in developing the study concept and editing the manuscript.
Funding
This work was supported by CIRM grant RN1-00562. The authors were also supported by generous gifts from the Simon-Strauss Foundation. H.M.S. was funded by the National Institutes of Health [HL070123]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.103416/-/DC1
References
- Ai D., Fu X., Wang J., Lu M.-F., Chen L., Baldini A., Klein W. H., Martin J. F. (2007). Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc. Natl. Acad. Sci. USA 104, 9319-9324 10.1073/pnas.0701212104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403-410 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- Aoki M., Mieda M., Ikeda T., Hamada Y., Nakamura H., Okamoto H. (2007). R-spondin3 is required for mouse placental development. Dev. Biol. 301, 218-226 10.1016/j.ydbio.2006.08.018 [DOI] [PubMed] [Google Scholar]
- Baljinnyam B., Klauzinska M., Saffo S., Callahan R., Rubin J. S. (2012). Recombinant R-spondin2 and Wnt3a up- and down-regulate novel target genes in C57MG mouse mammary epithelial cells. PLoS ONE 7, e29455 10.1371/journal.pone.0029455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biben C., Harvey R. P. (1997). Homeodomain factor Nkx2-5 controls left/right asymmetric expression of bHLH gene eHand during murine heart development. Genes Dev. 11, 1357-1369 10.1101/gad.11.11.1357 [DOI] [PubMed] [Google Scholar]
- Biechele T. L., Adams A. M., Moon R. T. (2009). Transcription-based reporters of Wnt/beta-catenin signaling. Cold Spring Harb. Protoc. 2009, pdb.prot5223 10.1101/pdb.prot5223 [DOI] [PubMed] [Google Scholar]
- Brown C. B., Feiner L., Lu M. M., Li J., Ma X., Webber A. L., Jia L., Raper J. A., Epstein J. A. (2001). PlexinA2 and semaphorin signaling during cardiac neural crest development. Development 128, 3071-3080. [DOI] [PubMed] [Google Scholar]
- Buckingham M., Meilhac S., Zaffran S. (2005). Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 6, 826-837 10.1038/nrg1710 [DOI] [PubMed] [Google Scholar]
- Cai C.-L., Liang X., Shi Y., Chu P.-H., Pfaff S. L., Chen J., Evans S. (2003). Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 5, 877-889 10.1016/S1534-5807(03)00363-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon K. S., Gong X., Lin Q., Thomas A., Liu Q. (2011). R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 108, 11452-11457 10.1073/pnas.1106083108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers E. E., Sutherland L. B., McAnally J., Richardson J. A., Olson E. N. (2006). Myocardin is a direct transcriptional target of Mef2, Tead and Foxo proteins during cardiovascular development. Development 133, 4245-4256 10.1242/dev.02610 [DOI] [PubMed] [Google Scholar]
- Cohen E. D., Wang Z., Lepore J. J., Lu M. M., Taketo M. M., Epstein D. J., Morrisey E. E. (2007). Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J. Clin. Invest. 117, 1794-1804 10.1172/JCI31731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R., Fuchs E. (1999). Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557-4568. [DOI] [PubMed] [Google Scholar]
- de Lau W., Barker N., Low T. Y., Koo B.-K., Li V. S. W., Teunissen H., Kujala P., Haegebarth A., Peters P. J., van de Wetering M., et al. (2011). Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293-297 10.1038/nature10337 [DOI] [PubMed] [Google Scholar]
- Dodou E., Verzi M. P., Anderson J. P., Xu S.-M., Black B. L. (2004). Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development 131, 3931-3942 10.1242/dev.01256 [DOI] [PubMed] [Google Scholar]
- Feiner L., Webber A. L., Brown C. B., Lu M. M., Jia L., Feinstein P., Mombaerts P., Epstein J. A., Raper J. A. (2001). Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 128, 3061-3070. [DOI] [PubMed] [Google Scholar]
- Glinka A., Dolde C., Kirsch N., Huang Y.-L., Kazanskaya O., Ingelfinger D., Boutros M., Cruciat C.-M., Niehrs C. (2011). LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 12, 1055-1061 10.1038/embor.2011.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan T., Wend P., Klaus A., Birchmeier W. (2008). Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 22, 2308-2341 10.1101/gad.1686208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets P. E. M. H., Moorman A. F. M., Clout D. E. W., van Roon M. A., Lingbeek M., van Lohuizen M., Campione M., Christoffels V. M. (2002). Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation. Genes Dev. 16, 1234-1246 10.1101/gad.222902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T., Zhang M., Wang J., Bonilla-Claudio M., Klysik E., Johnson R. L., Martin J. F. (2011). Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458-461 10.1126/science.1199010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay P. Y., Harris B. S., Maguire C. T., Buerger A., Wakimoto H., Tanaka M., Kupershmidt S., Roden D. M., Schultheiss T. M., O'Brien T. X., et al. (2004). Nkx2-5 mutation causes anatomic hypoplasia of the cardiac conduction system. J. Clin. Invest. 113, 1130-1137 10.1172/JCI19846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Rowitch D. H., Soriano P., McMahon A. P., Sucov H. M., et al. (2000). Fate of the mammalian cardiac neural crest. Development 127, 1607-1616. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O., Glinka A., del Barco Barrantes I., Stannek P., Niehrs C., Wu W. (2004). R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev. Cell 7, 525-534 10.1016/j.devcel.2004.07.019 [DOI] [PubMed] [Google Scholar]
- Kazanskaya O., Ohkawara B., Heroult M., Wu W., Maltry N., Augustin H. G., Niehrs C. (2008). The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development 135, 3655-3664 10.1242/dev.027284 [DOI] [PubMed] [Google Scholar]
- Kirby M. L., Waldo K. L. (1995). Neural crest and cardiovascular patterning. Circ. Res. 77, 211-215 10.1161/01.RES.77.2.211 [DOI] [PubMed] [Google Scholar]
- Kirby M. L., Turnage K. L., III, Hays B. M. (1985). Characterization of conotruncal malformations following ablation of “cardiac” neural crest. Anat. Rec. 213, 87-93 10.1002/ar.1092130112 [DOI] [PubMed] [Google Scholar]
- Klaus A., Saga Y., Taketo M. M., Tzahor E., Birchmeier W. (2007). Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc. Natl. Acad. Sci. USA 104, 18531-18536 10.1073/pnas.0703113104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A., Müller M., Schulz H., Saga Y., Martin J. F., Birchmeier W. (2012). Wnt/β-catenin and Bmp signals control distinct sets of transcription factors in cardiac progenitor cells. Proc. Natl. Acad. Sci. USA 109, 10921-10926 10.1073/pnas.1121236109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. S., Melton D. A. (1996). A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 93, 8455-8459 10.1073/pnas.93.16.8455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary R., Clapoff S., Darling S., Perry M. D., Moran L. A., Rossant J. (1989). Inducible expression of an hsp68-lacZ hybrid gene in transgenic mice. Development 105, 707-714. [DOI] [PubMed] [Google Scholar]
- Kwon C., Arnold J., Hsiao E. C., Taketo M. M., Conklin B. R., Srivastava D. (2007). Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc. Natl. Acad. Sci. USA 104, 10894-10899 10.1073/pnas.0704044104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C., Cordes K. R., Srivastava D. (2008). Wnt/β-catenin signaling acts at multiple developmental stages to promote mammalian cardiogenesis. Cell Cycle 7, 3815-3818 10.4161/cc.7.24.7189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Cui L., Zhou W., Dufort D., Zhang X., Cai C.-L., Bu L., Yang L., Martin J., Kemler R., et al. (2007). Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc. Natl. Acad. Sci. USA 104, 9313-9318 10.1073/pnas.0700923104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons I., Parsons L. M., Hartley L., Li R., Andrews J. E., Robb L., Harvey R. P. (1995). Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 9, 1654-1666 10.1101/gad.9.13.1654 [DOI] [PubMed] [Google Scholar]
- Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A. B., Volpin D., Bressan G. M., Piccolo S. (2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299-3304 10.1073/pnas.0434590100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor C., Brudno M., Schwartz J. R., Poliakov A., Rubin E. M., Frazer K. A., Pachter L. S., Dubchak I. (2000). VISTA: visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 16, 1046-1047 10.1093/bioinformatics/16.11.1046 [DOI] [PubMed] [Google Scholar]
- Milgrom-Hoffman M., Harrelson Z., Ferrara N., Zelzer E., Evans S. M., Tzahor E. (2011). The heart endocardium is derived from vascular endothelial progenitors. Development 138, 4777-4787 10.1242/dev.061192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman A. F., Houweling A. C., de Boer P. A. J., Christoffels V. M. (2001). Sensitive nonradioactive detection of mRNA in tissue sections: novel application of the whole-mount in situ hybridization protocol. J. Histochem. Cytochem. 49, 1-8 10.1177/002215540104900101 [DOI] [PubMed] [Google Scholar]
- Nakamura T., Sano M., Songyang Z., Schneider M. D. (2003). A Wnt- and beta-catenin-dependent pathway for mammalian cardiac myogenesis. Proc. Natl. Acad. Sci. USA 100, 5834-5839 10.1073/pnas.0935626100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y., Ono K., Yoshida Y., Kojima Y., Kita T., Tanaka M., Kimura T. (2009). The search for Nkx2-5-regulated genes using purified embryonic stem cell-derived cardiomyocytes with Nkx2-5 gene targeting. Biochem. Biophys. Res. Commun. 390, 821-826 10.1016/j.bbrc.2009.10.056 [DOI] [PubMed] [Google Scholar]
- Nam J.-S., Turcotte T. J., Smith P. F., Choi S., Yoon J. K. (2006). Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J. Biol. Chem. 281, 13247-13257 10.1074/jbc.M508324200 [DOI] [PubMed] [Google Scholar]
- Nam J.-S., Turcotte T. J., Yoon J. K. (2007). Dynamic expression of R-spondin family genes in mouse development. Gene Expr. Patterns 7, 306-312 10.1016/j.modgep.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Ohkawara B., Glinka A., Niehrs C. (2011). Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev. Cell 20, 303-314 10.1016/j.devcel.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Park E. J., Sun X., Nichol P., Saijoh Y., Martin J. F., Moon A. M. (2008). System for tamoxifen-inducible expression of cre-recombinase from the Foxa2 locus in mice. Dev. Dyn. 237, 447-453 10.1002/dvdy.21415 [DOI] [PubMed] [Google Scholar]
- Pashmforoush M., Lu J. T., Chen H., Amand T. S., Kondo R., Pradervand S., Evans S. M., Clark B., Feramisco J. R., Giles W., et al. (2004). Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell 117, 373-386 10.1016/S0092-8674(04)00405-2 [DOI] [PubMed] [Google Scholar]
- Prall O. W. J., Menon M. K., Solloway M. J., Watanabe Y., Zaffran S., Bajolle F., Biben C., McBride J. J., Robertson B. R., Chaulet H., et al. (2007). An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell 128, 947-959 10.1016/j.cell.2007.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qyang Y., Martin-Puig S., Chiravuri M., Chen S., Xu H., Bu L., Jiang X., Lin L., Granger A., Moretti A., et al. (2007). The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell 1, 165-179 10.1016/j.stem.2007.05.018 [DOI] [PubMed] [Google Scholar]
- Rhee J., Buchan T., Zukerberg L., Lilien J., Balsamo J. (2007). Cables links Robo-bound Abl kinase to N-cadherin-bound beta-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat. Cell Biol. 9, 883-892 10.1038/ncb1614 [DOI] [PubMed] [Google Scholar]
- Rivera-Feliciano J., Tabin C. J. (2006). Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev. Biol. 295, 580-588 10.1016/j.ydbio.2006.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiffarth J. R., Person A. D., Martinsen B. J., Sukovich D. J., Neumann A., Baker C. V., Lohr J. L., Cornfield D. N., Ekker S. C., Petryk A. (2007). Wnt5a is required for cardiac outflow tract septation in mice. Pediatr. Res. 61, 386-391 10.1203/pdr.0b013e3180323810 [DOI] [PubMed] [Google Scholar]
- Schneider V. A., Mercola M. (2001). Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 15, 304-315 10.1101/gad.855601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Li Y., Wang K., Zhou C.J. (2010). Cardiac neural crest and outflow tract defects in Lrp6 mutant mice. Dev. Dyn. 239, 200-210. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.-S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP in the ROSA26 locus. BMC Dev. Biol. 1, 4 10.1186/1471-213X-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Liang X., Najafi N., Cass M., Lin L., Cai C.-L., Chen J., Evans S. M. (2007). Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev. Biol. 304, 286-296 10.1016/j.ydbio.2006.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Chen Z., Bartunkova S., Yamasaki N., Izumo S. (1999). The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 126, 1269-1280. [DOI] [PubMed] [Google Scholar]
- Tian Y., Yuan L., Goss A. M., Wang T., Yang J., Lepore J. J., Zhou D., Schwartz R. J., Patel V., Cohen E. D., et al. (2010). Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev. Cell 18, 275-287 10.1016/j.devcel.2010.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S., Weidinger G., Osugi T., Kohn A. D., Golob J. L., Pabon L., Reinecke H., Moon R. T., Murry C. E. (2007). Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA 104, 9685-9690 10.1073/pnas.0702859104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzi M. P., McCulley D. J., De Val S., Dodou E., Black B. L. (2005). The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev. Biol. 287, 134-145 10.1016/j.ydbio.2005.08.041 [DOI] [PubMed] [Google Scholar]
- Waldo K. L., Kumiski D. H., Wallis K. T., Stadt H. A., Hutson M. R., Platt D. H., Kirby M. L. (2001). Conotruncal myocardium arises from a secondary heart field. Development 128, 3179-3188. [DOI] [PubMed] [Google Scholar]
- Wang J., Li S., Chen Y., Ding X. (2007). Wnt/beta-catenin signaling controls Mespo expression to regulate segmentation during Xenopus somitogenesis. Dev. Biol. 304, 836-847 10.1016/j.ydbio.2006.12.034 [DOI] [PubMed] [Google Scholar]
- Wu X., Golden K., Bodmer R. (1995). Heart development in Drosophila requires the segment polarity gene wingless. Dev. Biol. 169, 619-628 10.1006/dbio.1995.1174 [DOI] [PubMed] [Google Scholar]
- Yamagishi H., Maeda J., Hu T., McAnally J., Conway S. J., Kume T., Meyers E. N., Yamagishi C., Srivastava D. (2003). Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer. Genes Dev. 17, 269-281 10.1101/gad.1048903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Cai C.-L., Lin L., Qyang Y., Chung C., Monteiro R. M., Mummery C. L., Fishman G. I., Cogen A., Evans S. (2006). Isl1Cre reveals a common Bmp pathway in heart and limb development. Development 133, 1575-1585 10.1242/dev.02322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffran S., Kelly R. G., Meilhac S. M., Buckingham M. E., Brown N. A. (2004). Right ventricular myocardium derives from the anterior heart field. Circ. Res. 95, 261-268 10.1161/01.RES.0000136815.73623.BE [DOI] [PubMed] [Google Scholar]
- Zaffran S., Reim I., Qian L., Lo P. C., Bodmer R., Frasch M. (2006). Cardioblast-intrinsic Tinman activity controls proper diversification and differentiation of myocardial cells in Drosophila. Development 133, 4073-4083 10.1242/dev.02586 [DOI] [PubMed] [Google Scholar]
- Zhou W., Lin L., Majumdar A., Li X., Zhang X., Liu W., Etheridge L., Shi Y., Martin J., Van de Ven W., et al. (2007). Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat. Genet. 39, 1225-1234 10.1038/ng2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.