Abstract
Purpose.
To characterize age-related differences in the magnitude of spectral-domain optical coherence tomography (SD-OCT) structural change in early experimental glaucoma (EG).
Methods.
Both eyes from four young (1.4–2.6 years) and four old (18.6–21.9 years) rhesus monkeys were imaged at least three times at baseline, and then every 2 weeks after laser-induced, chronic, unilateral IOP elevation until the onset of EG (confocal scanning laser tomographic surface change confirmed twice). Two to 20 weeks after EG onset, animals were euthanized and optic nerve axon counts for all eyes were performed. Masked operators delineated retinal and ONH landmarks in 40 radial B-scans from each eye and imaging session to quantify change from baseline in five SD-OCT neural and connective tissue parameters. The effects of EG, age, and EG × age interactions on the magnitude, rate (magnitude per postlaser time), and IOP responsiveness (magnitude per cumulative IOP insult) of postlaser parameter change were individually assessed using general estimating equation models.
Results.
Presac SD-OCT RNFLT and minimum rim width change and postmortem axon loss was not significantly different in old compared with young EG eyes. The rate of change and IOP responsiveness of the parameters anterior lamina cribrosa surface depth relative to Bruch's membrane opening (BMO) and BMO depth relative to peripheral Bruch's membrane were significantly lower (P < 0.05) in the old compared with the young EG eyes.
Conclusions.
At similar postlaser times, levels of cumulative IOP insult and axonal damage, SD-OCT–detected ONH connective tissue structural change is greater in young compared with old monkey EG eyes.
Keywords: glaucoma, optic nerve head, lamina cribrosa, BMO minimum rim width, optical coherence tomography
SD-OCT ONH structural change at the onset of experimental glaucoma is greater in young compared with old monkey eyes. Age-related differences in ONH connective tissue structural stiffness and/or remodeling may contribute to age-related differences in the appearance of glaucomatous cupping.
Introduction
“Cupping” is a clinical term that is used to describe enlargement of the optic nerve head (ONH) cup in all forms of optic neuropathy.1–3 However, “cupping” also is used as a synonym for the pathophysiology of glaucomatous damage to the ONH neural and connective tissues.4 Because of this link to pathophysiology, it is often suggested that “cupping” is pathognomonic of glaucoma, yet there is much literature that discusses the presence, importance, and meaning of “cupping” in a variety of optic neuropathies, including those of glaucoma, compressive orbital masses, ischemia, inflammation, hereditary disorders and, most recently, primary cerebrospinal fluid pressure lowering.5–9 This is because the optic cup should enlarge if the ONH neuroretinal rim thins due to degeneration and loss of axons in all forms of optic neuropathy regardless of etiology.
What distinguishes glaucoma as an optic neuropathy is not cupping, per se, but rather the type of cupping, which is classically deep; more pronounced within the superior and inferior temporal quadrants; and is associated with excavation, nerve fiber layer (NFL) hemorrhages, and a pattern of retinal ganglion cell (RGC) axon injury and peripapillary retinal NFL (RNFL) loss that leads to characteristic patterns of visual field loss.2,4 This is in contrast to “nonglaucomatous cupping,” which manifests greater pallor and less deformation for a given amount of visual field loss.2,6
However, the clinical appearance of glaucomatous cupping is variable.10 We have previously proposed that in all optic neuropathies, regardless of the location and etiology of the primary insult to the visual system, the clinical phenomenon of cup enlargement (herein referred to as clinical cupping) has two principal pathophysiologic components: “prelaminar thinning” and “ONH connective tissue deformation.”11,12 We define “prelaminar thinning” to be that portion of cup enlargement that results from thinning of the prelaminar tissues, which consist of axonal, astroglial, and vascular components. We define “ONH connective tissue deformation” to be that portion of cup enlargement that results from permanent posterior lamina cribrosa deformation and/or remodeling that may be associated with enlargement of the scleral canal and posterior deformation of the scleral flange and peripapillary scleral connective tissues.11–13
We have further proposed that regardless of its contribution to the mechanisms of RGC axonal insult, it is the presence of ONH connective tissue deformation and/or remodeling that underlies and defines a “glaucomatous” form of cupping, and its character and magnitude that determine the cupping “type” (i.e., its glaucoma phenotype)11–14 in a given eye (Fig. 1). With these concepts in mind, it is important to note that with the development of spectral-domain optical coherence tomography (SD-OCT) imaging, the prelaminar and ONH connective tissue components of cupping are beginning to be separately detected and quantified.15,16
Figure 1.
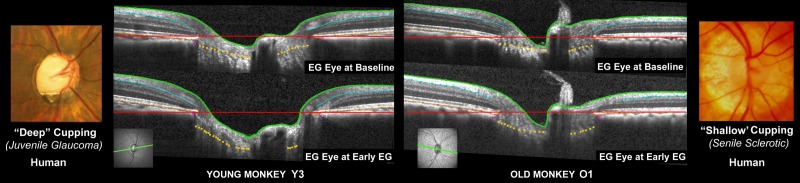
Differences in ONH connective tissue structural stiffness and/or remodeling may underlie “shallow” and “deep” forms of glaucomatous cupping in monkeys and humans. Deep (far left) and shallow (far right) forms of human glaucomatous cupping occur at all ages and IOP levels, but are classically seen in youthful and elderly eyes, respectively. We have proposed that the ONH connective tissues “harden” with age and that, on average, aged eyes should demonstrate a shallower form of cupping (i.e., a shallower “phenotype”) as a result.13 Spectral-domain OCT ONH B-scans (green, lower left) from the EG eye of a young (left) and old (right) monkey, when the eye was normal (upper) and at the second confirmation of CSLT detection of ONH surface change in the young eye (lower left) and at the (later) pre-euthanasia data set in the old eye (lower right). All images were obtained after 30 minutes of manometer-controlled IOP (10 mm Hg). In both eyes, although prelaminar neural tissue thickness alterations are present, laminar deformation is also apparent as an increase in the magnitude of space between the BMO reference plane (red line) and the anterior lamina cribrosa surface (gold dots). Laminar deformation in the old eye is far less than in the young eye and this profound difference in laminar deformation occurred in the setting of a cumulative IOP insult that was approximately five times greater in the old eye (Table 3).
From a biomechanical standpoint, the clinical manifestation of glaucomatous damage to a given ONH should depend on a host of neural and connective tissue factors, including eye-specific differences in ONH and peripapillary scleral connective tissue structural stiffness and remodeling.12,17–19 The structural stiffness of a tissue is determined by its geometry (i.e., its thickness and curvature) and its material properties (i.e., the arrangement, amount, and character of its constituents). A growing body of direct and indirect evidence suggests that the structural stiffness of the monkey and human lamina cribrosa and peripapillary scleral connective tissues increases with age.20–27 Practically speaking, this should translate to the aged eye (or stiff eye regardless of age) deforming less for a given level of loading than a young eye (or compliant eye regardless of age).
Two recent cross-sectional studies used SD-OCT lamina cribrosa imaging to report that in human patients with glaucoma or ocular hypertension, the eyes of the younger patients demonstrated a deeper laminar position relative to Bruch's membrane opening (BMO) when controlled for the level of visual field loss and RNFL loss.28,29 These observations are clinically important because they support the notion that age exerts an important influence on structure/structure relationships (e.g., lamina cribrosa deformation versus RNFLT change) and structure/function relationships (e.g., lamina cribrosa deformation versus visual field change).30,31 They also suggest that age-related differences in ONH connective tissue structural stiffness and/or remodeling may be two mechanisms by which this occurs.
Eye-specific differences in structural stiffness and/or remodeling, regardless of age, should therefore contribute to the glaucomatous phenotype expressed by an individual eye (Fig. 1).13 We recently reported that SD-OCT ONH change detection commonly preceded or coincided with ONH surface change detection by confocal scanning laser tomography (CSLT) in four young and four old monkeys with chronic unilateral experimental glaucoma (EG).15 In this study, SD-OCT ONH change also preceded detection of RNFL thickness (RNFLT) change by SD-OCT, RNFL retardance change by scanning laser polarimetry, and retinal functional change by multifocal electroretinogram.15 In the present report, we analyze the same longitudinal SD-OCT data sets to test the hypothesis that SD-OCT–detected ONH structural change was greater in the four young as compared with the four old monkey EG eyes at similar postlaser time intervals, similar levels of postlaser cumulative IOP insult, and at the onset of CSLT ONH surface change.
Methods
Acronyms and Abbreviations
Please see Table 1 for definitions and descriptions of all acronyms and abbreviations.
Table 1.
Full Parameter Names, Acronyms, and Their Descriptions
|
Parameters/Abbreviations |
Full Name/Description |
| BM | Bruch's membrane |
| BMO | Bruch's membrane opening |
| BMO depth | BMO depth relative to a BM reference plane 1500 μm from BMOcentroid |
| CSLT | Confocal scanning laser tomography |
| Cumulative IOP difference | Cumulative IOP difference between experimental glaucoma eye and control eye |
| EG | Experimental glaucoma |
| GEE | Generalized estimating equation |
| ILM | Internal limiting membrane |
| IOP | Intraocular pressure |
| Laminar surface depth | Anterior lamina cribrosa surface depth relative to BMO |
| MPD | Mean of all CSLT topographic values within the operator-assigned disc margin |
| MRW | Minimum rim width measured from BMO |
| ONH | Optic nerve head |
| Prelaminar tissue thickness | Prelaminar tissue thickness measured from anterior laminar surface to ILM surface |
| RPE | Retinal pigment epithelium |
| RNFLT | Retinal nerve fiber layer thickness measured 12° from the disc center |
| SD-OCT | Spectral-domain optical coherence tomography |
| TCA | CSLT topographic change analysis |
Animals
All experiments were performed in accordance with an institutionally approved animal use protocol at Legacy Health and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Four young (<3 years old) and four old (>18 years old) rhesus monkeys (Macaca mulatta) were used in the current study.
Overall Experimental Design
Both eyes of each animal underwent CSLT and SD-OCT imaging on at least three separate occasions during baseline (i.e., pre-laser, when both eyes were normal). One eye of each monkey was then randomly chosen to receive 360° of argon laser photocoagulation to the trabecular meshwork in two sessions of 180°, 2 weeks apart.32,33 Postlaser imaging sessions in both the lasered (hereafter referred to as the EG) eye and contralateral control eye continued in 1- to 3-week intervals. Laser photocoagulation was repeated in additional 90° increments as necessary to achieve evidence of structural change or to maintain a target of 20 to 30 mm Hg in its absence. Where additional laser was indicated, we chose to perform more rather than less laser in the old eyes so as to maintain the old EG eyes at higher IOPs than the young EG eyes throughout the study. Although our goal was equal IOP exposure in both groups, we sought to minimize risk of undermining the hypothesis that older eyes would deform less for a given IOP exposure by maintaining a bias toward slightly higher IOPs in the old group (i.e., if the older group exhibited less deformation but also lower average IOP levels, the hypothesis test would be confounded). All animals were followed to the onset of CSLT-detected ONH surface change confirmed on two subsequent occasions in the EG eye.
Confocal Scanning Laser Tomography and SD-OCT Imaging Protocol
Animals were initially anesthetized with 15 mg/kg intramuscular ketamine and 0.2 mg/kg midazolam followed by a 0.05-mg/kg subcutaneous bolus of atropine sulfate. The monkeys were then intubated and maintained on mixed oxygen (100%) and isoflurane gas (1%–2%). Vital signs were monitored and maintained at normal ranges throughout all the experiments. Intravenous lactated Ringer's solution or 6% hydroxyethyl starch was administered to maintain blood pressure whenever mean arterial pressure fell below 65 mm Hg. One drop of topical 0.5% proparacaine was applied to each eye before a lid speculum was placed. Intraocular pressure was measured three times by applanation tonometry (Tonopen XL; Reichert, Inc., Depew, NY, USA). Pupils were then dilated with 0.5% tropicamide and 2.5% phenylephrine before a plano rigid contact lens was placed on each eye with topical lubricant (0.5% carboxymethylcellulose sodium; Allergan, Inc., Irvine, CA, USA) to keep the cornea moistened during the experiments.
A 27-gauge cannula was connected to a manometer and inserted through the temporal cornea into the anterior chamber. By adjusting the manometer, IOP was set at 10 mm Hg and stabilized for 30 minutes before CSLT and SD-OCT imaging commenced. The goal of this IOP 10 mm Hg period was to reduce or remove reversible components of ONH deformation present in the EG eyes (due to the ambient IOP elevation at the time of imaging), so as to capture permanent glaucomatous ONH alterations.34,35 Optical nerve head surface topography was measured by CSLT using a Heidelberg Retina Tomograph II (Heidelberg Engineering GmbH, Heidelberg, Germany).32,36 A minimum of three individual scans were acquired at each CSLT imaging session and averaged to create a mean topography for each eye.
Eighty standard, nonenhanced depth imaging, 870-nm SD-OCT (Spectralis; Heidelberg Engineering, GmbH, Heidelberg, Germany) radial B-scans were acquired over a 15° area centered on the operator's estimate of the clinical disc margin (768 A-scans per B-scan, n = 9 repetitions), followed by a peripapillary circular scan with a 12° diameter (1536 A-scans per B-scan, n = 9–16 repetitions) for measurement of RNFLT. All repetitive scans (i.e., at the time of individual B-scan acquisition) were acquired using eye tracking (Spectralis Manual) and averaged to reduce speckle noise. For each monkey eye, the center of the ONH was estimated and registered during the first imaging session and eye-tracking was used during each subsequent imaging session to acquire each B-scan of the radial B-scan data set at the same retinal location as the first imaging session.
Confocal Scanning Laser Tomography–Detected EG Onset
A trained technician outlined the optic disc margin within the baseline image of each eye using a disc photograph for reference where necessary. This contour line was automatically transferred to all subsequent images in the longitudinal series. For the current study, the parameter mean position of the disc (MPD) was calculated for each CSLT session as described previously.32,37 Mean position of the disc was defined as the height of the surface of the ONH (i.e., average height of all pixels located within the disc margin contour line) relative to the height of a standard reference plane (a plane that runs parallel to the peripapillary retinal surface, and is set 50 μm below the retinal surface height at the papillomacular bundle, which is located in the −10° to −4° section temporal of the contour line (HRT II Operating Instructions; software version 1.6). Mean position of the disc onset was defined as the first postlaser session in which the MPD value was outside of the eye-specific 95% confidence interval (95% CI) determined by the baseline testing sessions, which was then confirmed in the two subsequent imaging sessions. Therefore once three consecutive imaging sessions resulted in MPD outside the confidence interval, the first of those three sessions was defined as MPD onset.32,38
Tomographic change analysis (TCA) is a native change-detection algorithm embedded within the HRT II Explorer software package (Heidelberg Engineering GmbH).39 Tomographic change analysis onset was independently evaluated in each eye by two experienced investigators (CFB and BF) masked to eye condition (EG versus control) reading the TCA maps retrospectively. Change onset was defined as the first image showing ONH surface change exceeding baseline variability that was confirmed in the two subsequent sessions exceeding the magnitude and position of red TCA super-pixels within the baseline images. Tomographic change analysis onset at a given postlaser imaging session required a clinician to see similar TCA change in the two subsequent sessions. Where necessary, interobserver differences in TCA were adjudicated by the two readers through discussion. For this study, EG onset was CSLT onset determined by either MPD or TCA, whichever occurred earlier (four TCA, two MPD, and two simultaneous).
Animal Euthanization and Axon Count
Seven of the eight animals were euthanized within 2 to 8 weeks post-EG onset (i.e., the time it took to confirm CSLT onset twice). One monkey (O1) was euthanized 20 weeks after retrospective assignment of a CSLT end point that was not appreciated at the time it occurred. Post mortem, optic nerve axon counts for both eyes of each animal were performed using a previously described automated segmentation algorithm that samples 100% of the optic nerve cross-section and counts 100% of the detected axons.40
Spectral-Domain OCT Data Set Delineation
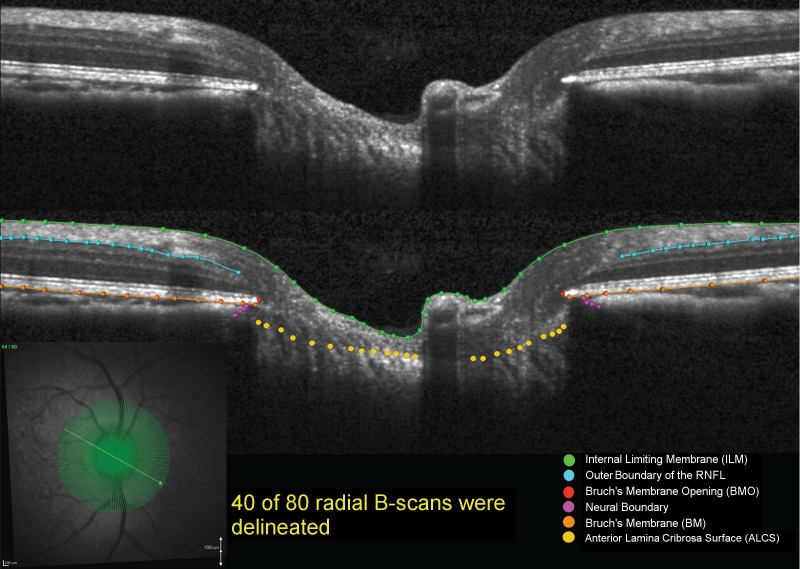
Our methodology for delineation of SD-OCT ONH data sets has been described in detail in prior studies.15,41–43 Customized “Multiview” software (built on the Visualization Toolkit [VTK; Kitware, Inc., Clifton Park, NY, USA]) was used to delineate the following anatomic features within 40 of the 80 radial B-scans (every other scan) of each SD-OCT data set by two trained technicians who were masked to the status of each eye (EG versus control) and time point (baseline or postlaser): internal limiting membrane (ILM), outer boundary of RNFL, Bruch's membrane/retinal pigment epithelium complex (BM/RPE), BMO (innermost termination of the BM/RPE complex), neural boundary (inner border of the neural canal), and anterior lamina cribrosa surface (Fig. 2).
Figure 2.
Original and delineated SD-OCT ONH data sets in a normal monkey eye. Green lines/points: ILM; blue lines/points: outer boundary of the RNFL; orange lines/points: BM/RPE; red points: BMO; purple points: neural boundary; yellow points: anterior lamina cribrosa surface. One of two B-scans was delineated and thus yielded 40 delineated data sets for each monkey eye at a time.
For the ILM, RNFL, BM/RPE, and neural boundary categories, each layer was delineated using discrete points interconnected by a linear Bézier curve. The position of each point in each category was finely adjusted so that the fitted Bézier curve matched the feature of interest as closely as possible. Bruch's membrane opening was delineated using two discrete points at either side of the neural canal. The anterior lamina cribrosa surface was delineated based on previous direct comparisons between SD-OCT B-scans and matched histologic sections,41 recent comparisons to three-dimensional (3D) histomorphometric reconstructions (Zangalli C, et al. IOVS 2014;55:ARVO E-Abstract 4747), as well as our previous publications on SD-OCT laminar visualization42 and longitudinal change detection.15,43 For each SD-OCT B-scan data set, a point cloud including all the above landmarks was generated (Figs. 3, 4). Because the Spectralis x,y transverse dimensions are calibrated for human eyes, a scaling factor of 0.857 was used to correct the difference between the ocular magnification of the average monkey eye and the human eye assumed by the instrument, as previously described.43,44
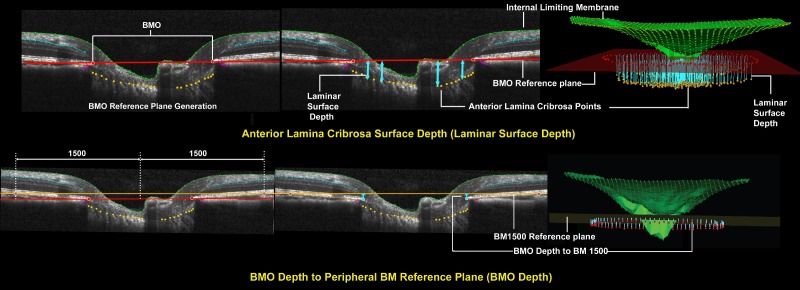
Figure 3.
Representative ONH connective tissue parameters within each B-scan and in 3D space (set 1). Top left: Bruch's membrane opening reference plane is defined using a best fit ellipse that fit through all 80 delineated BMO points (two in each radial). Top middle: Anterior lamina cribrosa surface depth (light blue arrows) is measured at each delineated anterior lamina cribrosa point as the perpendicular distance from the BMO reference plane (laminar surface depth) seen in cross-section as a red line. It is positive when anterior lamina cribrosa is above or anterior to the reference plane and negative when anterior lamina cribrosa is below or negative to the reference plane. Top right: Anterior lamina cribrosa depth in 3D space for a full 3D volume. Bottom left: Bruch's membrane reference plane definition. The BM reference plane is constructed in two steps. First, to identify constituent BM points, a circle within the BMO reference plane 1500 μm from the BMO centroid is identified and perpendiculars are dropped to the RPE/BM complex, in 7.5° intervals. Second, a best-fitting ellipse is fit through the identified RPE/BM complex points. Bruch's membrane opening depth is positive when BMO is above or anterior to the BM reference plane and negative when BMO is below or negative to the BM reference plane. Bottom middle: Bruch's membrane opening depth is measured at each delineated BMO point as the perpendicular distance from the BM reference plane (orange line) (bottom right).
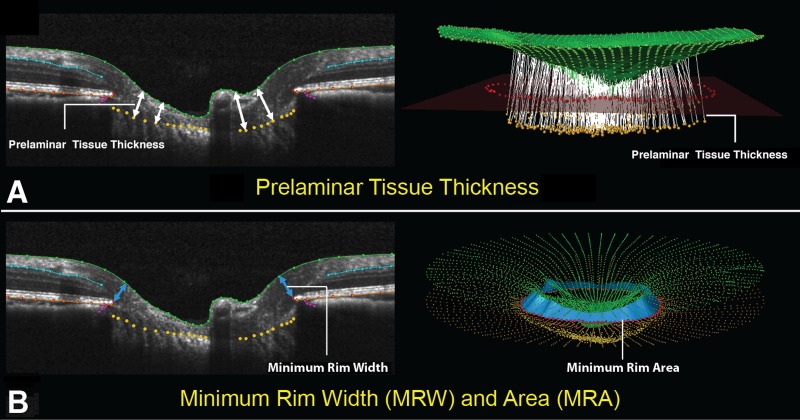
Figure 4.
Optic nerve head prelaminar and RNFL parameter definition within each B-scan and in 3D space (set 2). (A) Left: Prelaminar tissue thickness is measured as the normal distance from the tangent to the anterior lamina cribrosa surface to the ILM (green line). Right: Prelaminar tissue thickness measurement normals for a full 3D volume. (B) Left: Minimum rim width (blue arrows) is measured at each delineated BMO point (red) as the minimum distance to the ILM. Right: A 3D representation of interpolated rim width (rim area) is shown in a fully delineated volume (light blue band).
Spectral-Domain OCT ONH Connective Tissue, Prelaminar Tissue and RNFLT Parameterization
To parameterize each SD-OCT ONH data set, two reference planes were used (Figs. 3, 4). A BMO reference plane was based on the best-fitting ellipse through the 80 delineated BMO points in a 3D space (2 points/B-scan × 40 B-scans). A BM reference plane was constructed in two steps as follows. First, to identify constituent BM points, a circle within the BMO reference plane 1500 μm from the BMO centroid was identified and perpendiculars were dropped from the BMO reference plane to the RPE/BM complex, in 7.5° intervals. Second, a best-fitting ellipse was fit through the identified RPE/BM complex landmark points. Bruch's membrane opening depth was defined to be positive when BMO was above or anterior to the BM reference plane and negative when BMO was below or negative to the BM reference plane (Fig. 3). The ONH connective tissue parameters, anterior lamina cribrosa surface depth relative to the BMO reference plane (laminar surface depth), and BMO depth relative to the BM reference plane (BMO depth) are also described in Figure 3. The ONH prelaminar parameters, prelaminar tissue thickness, and BMO minimum rim width (MRW) are described in Figure 4. A standard RNFLT circle scan at 12° angle was also performed (not shown).
Data Analysis
To estimate the magnitude of IOP insult for each EG eye, we calculated the parameter cumulative IOP difference as the IOP difference between the EG and its control eye on each measurement day multiplied by the number of days from the last measurement and summed over the period of postlaser follow-up (cumIOP, in units of mm Hg × days).
The effect of age and EG on the postlaser data of the eight EG and eight control eyes were analyzed for each parameter in turn using generalized estimating equation (GEE) models. In this analysis, the effects of EG, age and EG × age interactions on (1) the magnitude, (2) rate (magnitude per postlaser time), and (3) IOP responsiveness (magnitude per cumulative IOP insult) of postlaser parameter change (relative to baseline) were individually assessed.
Rates of change (relative to postlaser day) for laminar surface depth and BMO depth, prelaminar tissue thickness, MRW, and RNFLT were derived from the slope coefficients from the GEE model in original scale (days). Intraocular pressure responsiveness (change/cumulative IOP insult) for laminar surface depth, BMO depth, prelaminar tissue thickness, MRW, and RNFLT were also derived from the slope coefficients from the GEE model in original scale (mm Hg × days).
Statistical analyses were performed in R (The R Foundation for Statistical Computing, Vienna, Austria) or Excel (Microsoft Corporation, Redmond, WA, USA).
Results
Animal demographics are listed in Table 2. The median age of the young animals was 1.9 years (range, 1.4–2.6) at study initiation and the median length of postlaser follow-up was 33 weeks (range, 26–34 weeks). The median age of the old animals was 20.95 years (range, 18.6–21.9) at study initiation and the median length of postlaser follow-up was 23 weeks (range, 21–39 weeks) (P = 0.34 for postlaser follow-up times, Wilcoxon rank sum test).
Table 2.
Demographics, IOP Characteristics, and Measures of EG Eye MRW, RFNLT, and Optic Nerve Axon Loss for Each Study Animal
|
Animal No. |
Animal ID |
Sex |
Age, y |
Weight, kg |
Baseline Mean IOP C/EG, mm Hg |
Postlaser Mean IOP C/EG, mm Hg |
Postlaser Peak IOP C/ EG, mm Hg |
EG Eye Cumulative IOP Insult: CSLT Onset/Pre-Euthanasia, mm Hg × day* |
RNFLT Change in EG at Pre- Euthanasia, μm† |
MRW Change in EG at Pre- Euthanasia, μm‡ |
Optic Nerve Axon Count in C Eye |
EG Eye Optic Nerve Axon Loss, %§ |
| Y1 | 25357 | M | 2.6 | 5.5 | 10/9 | 9/11 | 13/29 | 309/556 | −5 (−5.1%) | −15 (−8.3%) | 1,086,264 | −29% |
| Y2 | 25564 | F | 2.3 | 3.7 | 8/8 | 9/13 | 13/28 | 183/578 | −6 (−6.3%) | −49 (−21.5%) | 1,178,562 | −19% |
| Y3 | 26072 | F | 1.5 | 4.1 | 5/6 | 9/10 | 15/32 | 61/231 | 9 (9.7%) | −64 (−30.9%) | 1,160,765 | −15% |
| Y4 | 26161 | F | 1.4 | 3.3 | 6/6 | 8/11 | 12/37 | 17/524 | −17 (−16.8%) | −126 (−33.6%) | 1,039,990 | −4% |
| O1 | AM76 | F | 21.9 | 8.6 | 9/9 | 11/16 | 19/38 | 80/1185 | −8 (−8.3%) | −48 (−17.3%) | 1,392,210 | −12% |
| O2 | AM89 | M | 21.9 | 8.9 | 11/11 | 14/16 | 20/42 | 94/330 | 0 (0%) | −10 (−3.1%) | 904,345 | 2% |
| O3 | AO23 | F | 20 | 7.2 | 9/9 | 10/18 | 15/38 | 649/1164 | 1 (1.2%) | −66 (−23.9%) | 1,251,884 | −13% |
| O4 | AP02 | F | 18.6 | 5.6 | 7/7 | 9/11 | 13/41 | 193/548 | −2 (−2.2%) | −46 (−17.3%) | 1,153,703 | −23% |
C, control eyes; EG, experimental glaucoma eyes; F, female; M, male; O, old monkeys; Y, young monkeys.
Cumulative IOP difference between the EG eye and control eye from the onset of trabecular meshwork lasering to CSLT onset and at pre-euthanasia session.
The SD-OCT conventional circle scan RNFLT change magnitude and change in percentage at the pre-euthanasia session from the average of RNFLT in the baseline sessions.
Bruch's membrane opening MRW change magnitude and change in percentage at the pre-euthanasia session from the average of MRW in the baseline sessions.
Optic nerve axon loss in the EG eye expressed as percentage of its contralateral control eye.
Age-Related RNFLT and Orbital Optic Nerve Axon Loss Differences at Euthanization
The percent change of RNFLT and MRW relative to the baseline average for each individual EG eye is shown in Table 2. Median RNFLT change when animals were euthanized was −5.7% (range, −16.8% to 9.7%) in the four young EG eyes and −1.1% (range, −8.3% to 1.2%) in the four old EG eyes (P = 0.69, Wilcoxon rank sum test). Median MRW change when animals were euthanized was −26.2% (range, −33.6% to −8.3%) in the four young EG eyes and −17.3% (range, −23.9% to −3.1%) in the four old EG eyes (P = 0.20, Wilcoxon rank sum test). Within postmortem orbital optic nerve axon counts, median EG eye axon loss relative to contralateral control was −17% (range, −29% to −4%) in the young EG eyes and −12.5% (range, −23% to 2%) in the old EG eyes (P = 0.49, Wilcoxon rank sum test).
Age-Related IOP Differences in Control and EG Eye Postlaser IOP
Median control eye postlaser IOP was slightly higher in the old (10.5 mm Hg, range, 9–14 mm Hg) compared with the young (9 mm Hg, range, 8–9 mm Hg) eyes (P = 0.07, Wilcoxon rank sum test) (Table 3). Experimental glaucoma eye peak postlaser IOP was greater in the old (median 39.5 mm Hg, range, 38–42 mm Hg) compared with the young eyes (median 30.5 mm Hg, range, 28–37 mm Hg) (P = 0.03, Wilcoxon rank sum test). Although mean and median EG eye cumulative IOP insult at the CSLT onset, and pre-euthanization sessions were greater (nearly doubled) in the old compared with young EG eyes, the difference did not achieve significance.
Table 3.
Age-Related Differences in Control and EG Eyes Pre- and Postlaser IOP
|
Mean Prelaser IOP in Control Eyes, mm Hg |
Mean Prelaser IOP in EG Eyes, mm Hg |
Mean Postlaser IOP in Control Eyes, mm Hg |
Mean Postlaser IOP in EG Eyes, mm Hg |
Peak IOP in Control Eyes, mm Hg |
Peak IOP in EG Eyes, mm Hg |
EG Eye Cumulative IOP Insult: CSLT Onset, mm Hg × day |
EG Eye Cumulative IOP Insult: Pre-Euthanasia, mm Hg × day |
|
| Mean ± SD in young eyes | 8.1 ± 2.2 | 8.2 ± 1.7 | 8.8 ± 0.5 | 11.3 ± 1.3 | 13.3 ± 1.3 | 31.5 ± 4.0 | 143 ± 131 | 472 ± 162 |
| Median (range) in young eyes | 8.2 (5.3–10.6) | 8.3 (6.1–10.3) | 9 (8–9) | 11 (10–13) | 13 (12–15) | 30.5 (28–37) | 122 (17–309) | 540 (231–578) |
| Mean ± SD in old eyes | 10.0 ± 1.3 | 10.3 ± 1.3 | 11.0 ± 2.2 | 15.3 ± 3 | 16.8 ± 3.3 | 39.8 ± 2.1 | 254 ± 268 | 814 ± 428 |
| Median (range) in old eyes | 9.6 (8.9–11.7) | 9.7 (9.4–12.2) | 10.5 (9–14) | 16 (11–18) | 17 (13–20) | 39.5 (38–42) | 144 (80–649) | 871 (330–1185) |
| P value | 0.2000 | 0.2000 | 0.0650 | 0.1016 | 0.1367 | 0.0294 | 0.4857 | 0.2454 |
P values are based on Wilcoxon rank sum test. Bold text indicates P < 0.05, which is statistically significant.
Age-Related Differences in the Magnitude of Parameter Change at CSLT Onset
By GEE analysis, at CSLT onset in the EG eye, the effect of EG was significant for SD-OCT laminar surface depth, prelaminar tissue thickness, and MRW in the young animals but achieved significance only for SD-OCT laminar surface depth and MRW in the old animals (Table 4). Among all parameters, an EG × age interaction effect was significant for laminar surface depth only.
Table 4.
Age-Related Differences in the Magnitude of Parameter Change at CSLT-Detected EG Onset
|
SD-OCT Parameters |
|||||
|
Laminar Surface Depth, μm |
BMO Depth, μm |
Prelaminar Tissue Thickness, μm |
MRW, μm |
RNFLT, μm |
|
| Mean ± SD change* in young EG eyes (EG − Control) | −95 ± 36 | −9 ± 5 | 26 ± 34 | −39 ± 30 | 3.6 ± 7.0 |
| Mean ± SD change* in old eyes (EG − Control) | −22 ± 10 | −7 ± 6 | −17 ± 12 | −24 ± 10 | −0.2 ± 4.0 |
| P value EG: young effect† | 0.0001 | 0.4472 | 0.0262 | 0.0007 | 0.8758 |
| P value EG: old effect† | <0.0001 | 0.1074 | 0.2824 | 0.0065 | 0.2424 |
| P value EG × age interactions‡ | 0.0008 | 0.8302 | 0.0896 | 0.3252 | 0.3281 |
| Positive change | Anterior to BMO | Anterior to PeriBM | Thickening | Thickening | Thickening |
| Negative change | Posterior to BMO | Posterior to PeriBM | Thinning | Thinning | Thinning |
Mean ± SD change: difference between the changes from the mean baseline value to CSLT onset of the EG eye and control eye for the four young and four old animals. Bold text indicates P < 0.05, which is statistically significant.
GEE test if mean change above is significantly different between EG and control eyes at CLST onset in young or old monkeys, respectively.
GEE test if there is interaction between eye condition (EG or control) and age (young and old animals) on changes in parameters at CSLT onset.
Age-Related Differences in the Rate of Postlaser Parameter Change
Although the mean postlaser IOP, peak postlaser IOP, and cumulative IOP insult were considerably lower in the young EG eyes (Table 5), the rate of posterior (outward) laminar surface deformation was 4.5 times faster in young (−0.460 μm/d) compared with old (−0.102 μm/d) EG eyes (P < 0.0001, GEE). Deformation of BMO relative to a peripheral BM reference plane (BMO depth) also demonstrated a significantly faster rate of postlaser change in the young (−0.109 μm/d) compared with the old EG eyes (−0.003 μm/d, P < 0.0001, GEE). In fact, the rate in old EG eyes was not significantly different from old control eyes for this parameter. Postlaser prelaminar tissue thickness significantly increased in young eyes (0.088 μm/d) and decreased in old eyes (−0.040 μm/d, P < 0.0001, GEE).
Table 5.
Age-Related Differences in the Rate of Parameter Change in EG Eyes
|
SD-OCT |
|||||
|
Anterior Laminar Depth,* μm/d |
BMO Depth, μm/d |
Prelaminar Tissue Thickness, μm/d |
MRW, μm/d |
RNFLT, μm/d |
|
| Change/day† in young EG eyes | −0.460 | −0.109 | 0.088 | −0.202 | −0.004 |
| Change/day† in old EG eyes | −0.102 | −0.003 | −0.040 | −0.099 | −0.010 |
| Change rates young vs. old EG eyes, P value‡ | <0.0001 | <0.0001 | <0.0001 | 0.1433 | 0.4901 |
| Change rates in EG vs. control eyes: young animals, P value§ | <0.0001 | 0.0002 | 0.2013 | 0.0029 | 0.7957 |
| Change rates in EG vs. control eyes: old animals, P value‖ | 0.0001 | 0.6175 | 0.0186 | 0.0221 | 0.3559 |
| Positive change | Anterior to BMO | Anterior to PeriBM | Thickening | Thickening | Thickening |
| Negative change | Posterior to BMO | Posterior to PeriBM | Thinning | Thinning | Thinning |
Rates of change were derived from the slope coefficients from the GEE model for each SD-OCT parameter. Bold text indicates P < 0.05, which is statistically significant.
The longitudinal change rate of each parameter represents how much the parameter changed for each unit of time (units in day) relative to mean baseline. The data reported here are the mean value for the n = 4 old and n = 4 young EG eyes.
P value for whether the rate of parameter change is significant between young and old EG eyes.
P value for whether the rate of parameter change in the EG eye is significantly different from in control eyes in young animals.
‖ P value for whether the rate of parameter change in the EG eye is significantly different from in control eyes in old animals.
Age-Related Differences in the IOP Responsiveness of Parameter Change
Because the rate of postlaser parameter change versus time (in days, Table 5) does not take into account the magnitude of IOP exposure, the magnitude of parameter change was also evaluated per unit of cumulative IOP insult (mm Hg × day), as reported in Table 6. For a given increase in cumulative IOP insult, the anterior laminar surface deformed posteriorly at magnitudes that were 3.64 times faster (P < 0.0001, GEE) in the young (−0.237 μm/mm Hg × day) compared with the old EG eyes (−0.065 μm/mm Hg × day). Bruch's membrane opening depth (3.39 times more posterior deformation, P = 0.0106) also demonstrated age-related differences in IOP responsiveness.
Table 6.
Age-Related Differences in Parameter IOP Responsiveness in EG Eyes (Magnitude of Parameter Change per Cumulative IOP Insult)
|
SD-OCT Parameters, μm/(mm Hg × day) |
|||||
|
Laminar Surface Depth* |
BMO Depth |
Prelaminar Tissue Thickness |
MRW |
RNFLT |
|
| Change/CumIOP† in young EG eyes | −0.237 | −0.035 | 0.0156 | −0.152 | −0.022 |
| Change/CumIOP† in old EG eyes | −0.065 | −0.010 | −0.0143 | −0.048 | −0.004 |
| P value for change/CumIOP in young EG eyes‡ | <0.0001 | 0.0001 | 0.5794 | 0.0216 | 0.0554 |
| P value for change/CumIOP in old EG eyes‡ | <0.0001 | 0.0015 | 0.0001 | <0.0001 | 0.0722 |
| P value for difference in rates of change§ | <0.0001 | 0.0106 | 0.2911 | 0.1177 | 0.1302 |
| Positive change | Anterior to BMO | Anterior to PeriBM | Thickening | Thickening | Thickening |
| Negative change | Posterior to BMO | Posterior to PeriBM | Thinning | Thinning | Thinning |
Rates of change were derived from the slope coefficients from the GEE model for each SD-OCT parameter. Bold text indicates P < 0.05, which is statistically significant.
The longitudinal change rate of each parameter represents how much the parameter changed for each unit of cumulative IOP insult relative to mean baseline. The data reported here are the mean value for the n = 4 old and n = 4 young EG eyes.
P value for whether the rate of parameter change is significant in the young or old EG eyes.
P value for the comparison of the rate of parameter change within the young versus the old EG eyes.
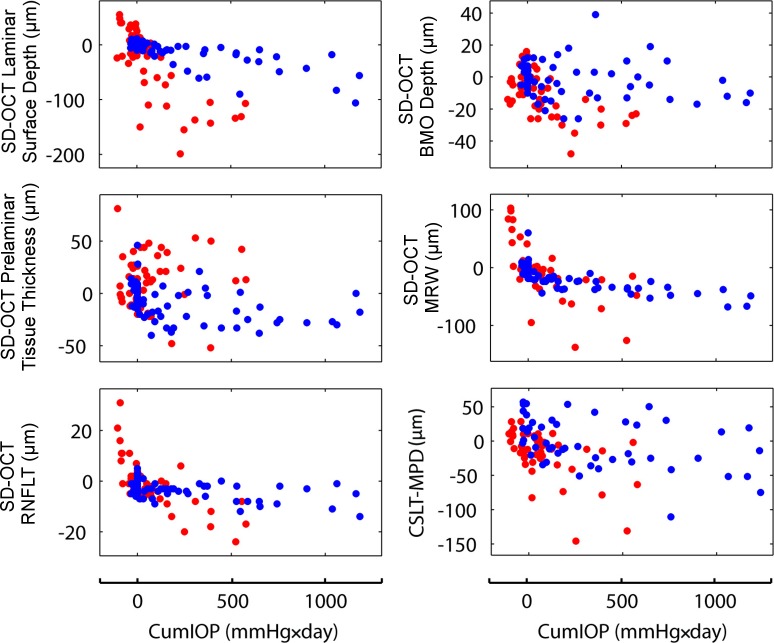
Change from baseline data at each postlaser imaging session is plotted for a subset of parameters relative to the imaging session cumulative IOP insult in Figure 5. In general, it is evident that the young eyes were followed to lower levels of cumulative IOP insult than the old eyes, in that the young eye data points (red dots) end at less than 600 mm Hg × day, whereas the old eye data points (blue dots) extend to more than 1200 mm Hg × day). These data again indicate that CSLT-detected ONH surface change occurred (and was confirmed on two subsequent occasions) at lower levels of cumulative IOP insult in the young EG eyes.
Figure 5.
Change from baseline for selected testing modalities and parameters at each postlaser testing session in the young (red) and old (blue) EG eyes plotted relative to cumulative IOP insult. Testing sessions are ordered by EG eye cumulative IOP insult (bottom of each column). Change from baseline for each parameter at each postlaser testing session is plotted for all four young (red dots) and all four old (blue dots) EG eyes. Note the following. First, in general, the young eyes were followed to lower levels of cumulative IOP insult than the old eyes (red dots end at less than 600 mm Hg × day and blue dots extend to more than 1200 mm Hg × day), reflecting the fact that ONH surface change as detected by CSLT occurred at lower levels of cumulative IOP insult in young eyes. Second, age-related differences in the overall rates of change are apparent qualitatively for most of the parameters and were confirmed as statistically significant for a subset, as reported in Tables 5 and 6.
Discussion
This is the first study to longitudinally characterize age-related differences in the character of glaucomatous “cupping” at its onset, that is, at the conversion from (experimental) ocular hypertension to ONH structural change detected by SD-OCT. In these four young and four old animals, the magnitude of SD-OCT ONH parameter change was greater in the young compared with the old eyes both at the point of CSLT-detected ONH surface change and also when measured as continuous variables relative to postlaser time (in days) and postlaser cumulative IOP insult (in mm Hg × days). The data also suggest that structural change was greater for deep connective tissues in the young eyes.
Regarding the ONH deep connective tissues, when compared by age group versus time or versus cumulative IOP insult, lamina cribrosa deformation relative to BMO (laminar surface depth) and BMO deformation relative to peripheral BM (BMO depth) were both greater in young compared with old monkey eyes. We believe that two mechanisms likely underlie the age-related differences in EG eye ONH connective tissue deformation we report: age-related differences in ONH connective tissue structural stiffness13 and age-related differences in ONH connective tissue remodeling.12 Although the two can be thought of as separate mechanisms, they are also linked.
The findings of our current study offer indirect rather than direct evidence that increased structural stiffness is a contributing mechanism to age-related differences in ONH structural change in early EG. We did not perform acute IOP 10/30 compliance testing45 on the young and old animals of this report at baseline (in our laboratory, acute IOP compliance testing refers to obtaining SD-OCT ONH imaging 30 minutes after IOP is manometrically lowering to 10 mm Hg and again 30 minutes after increasing IOP to 30 mm Hg). Therefore, we do not have direct evidence that the old eyes of this report were structurally stiffer than the young eyes before the onset of unilateral chronic IOP elevation. However, we recently reported that SD-OCT–detected ONH structural change is greater in a separate group of young versus old normal monkey eyes following acute IOP elevation (Qin L, et al. IOVS 2013;54:ARVO E-Abstract 53).
We have previously reported that ONH connective tissue remodeling in early monkey EG includes outward migration of the lamina cribrosa insertions17 and retrolaminar septal recruitment of orbital optic nerve septa into more transversely oriented laminar beams.18 Age-related differences in ONH remodeling,12,13,19 if present, may be multifactorial. Inherent differences in the ONH of young versus old eyes, such as astroglial and peripapillary scleral fibroblast responsiveness to stretch and/or less connective tissue deformation and/or basement membrane strain, in stiffer, aged eyes at similar levels of mechanical load may also contribute.
The ONH rim tissue parameter MRW showed a tendency to decrease more in young compared with old EG eyes versus time or versus cumulative IOP insult (although neither comparison achieved significance), which suggests the ONH rim tissue underwent greater compression and/or thinning in young eyes. Interestingly, even though the lamina bowed more posteriorly and the rim thinned more in young eyes, the prelaminar tissue thickness actually increased in young eyes (but thinned in old eyes) over time and cumulative IOP insult (with only the comparison versus time achieving significance). These data support our previous report of prelaminar tissue thickening within 3D histomorphometric reconstructions of the EG versus control eyes of three young-adult animals with early EG.11 Age-related differences in this finding and its clinical importance need to be studied in larger groups of animals. Optic nerve head rim tissue and prelaminar tissue change (whether detected by MRW measurements made relative to BMO or thickness measurements made relative to the anterior lamina cribrosa surface) may represent RGC axonal loss or degenerative changes that could include both increased and decreased axon caliber, astroglial loss, hypertrophy and/or proliferation, and vascular constriction or engorgement. Although all of these changes may represent varying degrees of pathophysiologic response, it is also possible that all (except RGC axonal loss) are potentially reversible responses to conformational change that follow anterior or posterior lamina cribrosa deformation and/or expansion or contraction of the scleral canal and BMO. Because axon loss was not greater in the young eyes, we believe that both the greater MRW thinning and prelaminar tissue thickening they demonstrate reflect either conformational changes or early degenerative changes that precede frank axonal loss. The nature and clinical significance of conformational change requires further clarification.
Not all structural change results in RGC injury lead to a change in visual function.46,47 Optic nerve head conformational change that is not due to RGC axon loss may not result in visual field progression. However, it is important to note that six of the eight EG eyes in this report (three young and three old), had 12% to 29% optic nerve axon loss by postmortem histology. Thus in those six EG eyes, although there was no detectible RNFLT (−8.3% to 9.7% change), RGC axon loss was modest to moderate in magnitude. It is also important to note that one old (O2) and one young (Y4) EG eye demonstrated similar SD-OCT ONH changes to those observed in the other EG eyes while demonstrating minimal orbital optic nerve axon loss (+2% and −4%, respectively) by postmortem histology. Additional studies in the eight EG eyes of this report are under way to correlate SD-OCT ONH sectoral change to adjacent SD-OCT RNFL thinning and colocalized optic nerve axon loss so as to identify those forms of SD-OCT ONH and RNFL parameter change that most consistently and closely correlate to RGC axon injury.
The fact that the old animals experienced similar or higher IOPs at the point of CSLT-detected ONH surface change is important because it suggests that if our study is biased by IOP exposure, it was biased to detect more, rather than less, deformation in the old eyes. The mean postlaser IOP, the mean postlaser peak IOP, and the cumulative IOP insult at CSLT onset were either significantly higher in the old compared with the young EG eyes, or not significantly different. Where possible, we chose to maintain the old EG eyes at higher IOPs than the young EG eyes, so as to achieve a bias toward more rather than less deformation in the old EG eyes. Our IOP data suggest we achieved this bias, although measurements were made only weekly, whereas continuous telemetric IOP characterization48 would have been necessary to most accurately quantify IOP insult.
The fact that the final measurement of SD-OCT peripapillary RNFLT demonstrated minimal change when using standard peripapillary RNFLT circle scan data in both young and old EG eyes and that postmortem, optic nerve axon loss was not detectibly greater in these four old compared with these four young EG eyes is important because it is not consistent with the concept that old eyes are more susceptible than young eyes to RGC axon loss at all levels of IOP. We have previously outlined the logic and literature that suggests aged eyes should be more susceptible to glaucomatous RGC axon damage than young eyes.12,13 This finding is unexpected and needs to be confirmed in a larger study.
Interpretation of our results should be limited by the issues we have mentioned as well as the following additional considerations. All parameters in this report were calculated on a global basis, which may diminish their ability to detect focal and regional change. In particular, because the major ONH blood vessels most commonly shadow the superior and inferior quadrants, the superior and inferior sectors of the lamina cribrosa (where early change in monkey and human glaucoma is expected) are less robustly represented in our SD-OCT ONH connective tissue parameters.42 This effectively gave more weight to the nasal and temporal quadrants when lamina cribrosa–associated parameters were averaged for a global calculation. Regionalization of each structural parameter relative to an anatomically consistent, foveal-BMO nasal temporal axis49 will be the focus of the next report in this series.
In summary, our findings suggest that at similar levels of cumulative IOP insult and at the onset of CSLT ONH surface change, SD-OCT detected ONH prelaminar and connective tissue structural change is greater in young compared with old monkey eyes. These data support the concept that age-related differences in ONH connective tissue structural stiffness and/or remodeling may contribute to age-related differences in the appearance of early glaucomatous cupping in a given eye. Our findings also suggest that although SD-OCT–detectible ONH change that precedes detectible RNFL change was associated with minimal optic nerve axon loss in two eyes, it was associated with modest to moderate (12% to 29%) axon loss in the majority of monkey eyes.
Acknowledgments
The authors thank the following individuals for their assistance with this study: Jonathan Grimm for his assistance with software and hardware, Erica Dyrud for her assistance with imaging, and Joanne Couchman for her assistance with manuscript preparation and submission.
Aspects of this paper were presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO) in Ft. Lauderdale, Florida, United States, in 2011.
Supported by National Institutes of Health Grants R01-EY011610 (CFB), R01-EY022128 (CFB), and R01-E019327 (BF), and unrestricted research support from Legacy Good Samaritan Foundation, Heidelberg Engineering, Alcon Research Institute, and Sears Medical Trust. NGS acknowledges a proportion of his financial support from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital National Health Service Foundation Trust and University College London Institute of Ophthalmology for a Biomedical Research Centre for Ophthalmology.
The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
Disclosure: H. Yang, None; L. He, None; S.K. Gardiner, None; J. Reynaud, None; G. Williams, None; C. Hardin, None; N.G. Strouthidis, None; J.C. Downs, None; B. Fortune, Heidelberg Engineering (F), Carl Zeiss Meditec (F); C.F. Burgoyne, Heidelberg Engineering (F, R), Reichert (F)
References
- 1. Bianchi-Marzoli S, Rizzo JF III, Brancato R, Lessell S. Quantitative analysis of optic disc cupping in compressive optic neuropathy. Ophthalmology. 1995; 102: 436–440 [DOI] [PubMed] [Google Scholar]
- 2. Greenfield DS, Siatkowski RM, Glaser JS, Schatz NJ, Parrish RK II. The cupped disc. Who needs neuroimaging? Ophthalmology. 1998; 105: 1866–1874 [DOI] [PubMed] [Google Scholar]
- 3. Klein BE, Klein R, Lee KE, Hoyer CJ. Does the intraocular pressure effect on optic disc cupping differ by age? Trans Am Ophthalmol Soc. 2006; 104: 143–148 [PMC free article] [PubMed] [Google Scholar]
- 4. Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979; 86: 1803–1830 [DOI] [PubMed] [Google Scholar]
- 5. Quigley H, Anderson DR. Cupping of the optic disc in ischemic optic neuropathy. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977; 83: 755–762 [PubMed] [Google Scholar]
- 6. Trobe JD, Glaser JS, Cassady J, Herschler J, Anderson DR. Nonglaucomatous excavation of the optic disc. Arch Ophthalmol. 1980; 98: 1046–1050 [DOI] [PubMed] [Google Scholar]
- 7. Ambati BK, Rizzo JF III. Nonglaucomatous cupping of the optic disc. Int Ophthalmol Clin. 2001; 41: 139–149 [DOI] [PubMed] [Google Scholar]
- 8. Greenfield DS. Glaucomatous versus nonglaucomatous optic disc cupping: clinical differentiation. Semin Ophthalmol. 1999; 14: 95–108 [DOI] [PubMed] [Google Scholar]
- 9. Yang D, Fu J, Hou R, et al. Optic neuropathy induced by experimentally reduced cerebrospinal fluid pressure in monkeys. Invest Ophthalmol Vis Sci. 2014; 55: 3067–3073 [DOI] [PubMed] [Google Scholar]
- 10. Broadway DC, Nicolela MT, Drance SM. Optic disk appearances in primary open-angle glaucoma. Surv Ophthalmol. 1999; 43: S223–S243 [DOI] [PubMed] [Google Scholar]
- 11. Yang H, Downs JC, Bellezza A, Thompson H, Burgoyne CF. 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: prelaminar neural tissues and cupping. Invest Ophthalmol Vis Sci. 2007; 48: 5068–5084 [DOI] [PubMed] [Google Scholar]
- 12. Burgoyne CF. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 2011; 93: 120–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burgoyne CF, Downs JC. Premise and prediction—how optic nerve head biomechanics underlies the susceptibility and clinical behavior of the aged optic nerve head. J Glaucoma. 2008; 17: 318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005; 24: 39–73 [DOI] [PubMed] [Google Scholar]
- 15. He L, Yang H, Gardiner SK, et al. Longitudinal detection of optic nerve head changes by spectral domain optical coherence tomography in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2014; 55: 574–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshikawa M, Akagi T, Hangai M, et al. Alterations in the neural and connective tissue components of glaucomatous cupping after glaucoma surgery using swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2014; 55: 477–484 [DOI] [PubMed] [Google Scholar]
- 17. Yang H, Williams G, Downs JC, et al. Posterior (outward) migration of the lamina cribrosa and early cupping in monkey experimental glaucoma. Invest Ophthalmol Vis Sci. 2011; 52: 7109–7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roberts MD, Grau V, Grimm J, et al. Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2009; 50: 681–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Downs JC, Roberts MD, Sigal IA. Glaucomatous cupping of the lamina cribrosa: a review of the evidence for active progressive remodeling as a mechanism. Exp Eye Res. 2011; 93: 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Albon J, Purslow PP, Karwatowski WS, Easty DL. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000; 84: 318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albon J, Karwatowski WS, Avery N, Easty DL, Duance VC. Changes in the collagenous matrix of the aging human lamina cribrosa. Br J Ophthalmol. 1995; 79: 368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Albon J, Karwatowski WS, Easty DL, Sims TJ, Duance VC. Age related changes in the non-collagenous components of the extracellular matrix of the human lamina cribrosa. Br J Ophthalmol. 2000; 84: 311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998; 106: 1–56 [DOI] [PubMed] [Google Scholar]
- 24. Kotecha A, Izadi S, Jeffrey G. Age related changes in the thickness of the human lamina cribrosa. Br J Ophthalmol. 2006; 90: 1531–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fazio MA, Grytz R, Morris JS, et al. Age-related changes in human peripapillary scleral strain. Biomech Model Mechanobiol. 2014; 13: 551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Scleral biomechanics in the aging monkey eye. Invest Ophthalmol Vis Sci. 2009; 50: 5226–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci. 2012; 53: 1714–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rho CR, Park HY, Lee NY, Park CK. Clock-hour laminar displacement and age in primary open-angle glaucoma and normal tension glaucoma. Clin Experiment Ophthalmol. 2012; 40: e183–e189 [DOI] [PubMed] [Google Scholar]
- 29. Ren R, Yang H, Gardiner SK, et al. Anterior lamina cribrosa surface depth, age, and visual field sensitivity in the Portland Progression Project. Invest Ophthalmol Vis Sci. 2014; 55: 1531–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harwerth RS, Wheat JL, Rangaswamy NV. Age-related losses of retinal ganglion cells and axons. Invest Ophthalmol Vis Sci. 2008; 49: 4437–4443 [DOI] [PubMed] [Google Scholar]
- 31. Harwerth RS, Wheat JL, Fredette MJ, Anderson DR. Linking structure and function in glaucoma. Prog Retin Eye Res. 2010; 29: 249–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burgoyne CF, Mercante DE, Thompson HW. Change detection in regional and volumetric disc parameters using longitudinal confocal scanning laser tomography. Ophthalmology. 2002; 109: 455–466 [DOI] [PubMed] [Google Scholar]
- 33. Gardiner SK, Fortune B, Wang L, Downs JC, Burgoyne CF. Intraocular pressure magnitude and variability as predictors of rates of structural change in non-human primate experimental glaucoma. Exp Eye Res. 2012; 103: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burgoyne CF, Quigley HA, Thompson HW, Vitale S, Varma R. Early changes in optic disc compliance and surface position in experimental glaucoma. Ophthalmology. 1995; 102: 1800–1809 [DOI] [PubMed] [Google Scholar]
- 35. Burgoyne CF, Quigley HA, Thompson HW, Vitale S, Varma R. Measurement of optic disc compliance by digitized image analysis in the normal monkey eye. Ophthalmology. 1995; 102: 1790–1799 [DOI] [PubMed] [Google Scholar]
- 36. Chauhan BC, McCormick TA, Nicolela MT, LeBlanc RP. Optic disc and visual field changes in a prospective longitudinal study of patients with glaucoma: comparison of scanning laser tomography with conventional perimetry and optic disc photography. Arch Ophthalmol. 2001; 119: 1492–1499 [DOI] [PubMed] [Google Scholar]
- 37. Heickell AG, Bellezza AJ, Thompson HW, Burgoyne CF. Optic disc surface compliance testing using confocal scanning laser tomography in the normal monkey eye. J Glaucoma. 2001; 10: 369–382 [DOI] [PubMed] [Google Scholar]
- 38. Fortune B, Reynaud J, Wang L, Burgoyne CF. Does optic nerve head surface topography change prior to loss of retinal nerve fiber layer thickness: a test of the site of injury hypothesis in experimental glaucoma. PLoS One. 2013; 8: e77831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chauhan BC, Blanchard JW, Hamilton DC, LeBlanc RP. Technique for detecting serial topographic changes in the optic disc and peripapillary retina using scanning laser tomography. Invest Ophthalmol Vis Sci. 2000; 41: 775–782 [PubMed] [Google Scholar]
- 40. Reynaud J, Cull G, Wang L, et al. Automated quantification of optic nerve axons in primate glaucomatous and normal eyes—method and comparison to semi-automated manual quantification. Invest Ophthalmol Vis Sci. 2012; 53: 2951–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strouthidis NG, Grimm J, Williams GA, Cull GA, Wilson DJ, Burgoyne CF. A comparison of optic nerve head morphology viewed by spectral domain optical coherence tomography and by serial histology. Invest Ophthalmol Vis Sci. 2010; 51: 1464–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang H, Qi J, Hardin C, et al. Spectral-domain optical coherence tomography enhanced depth imaging of the normal and glaucomatous nonhuman primate optic nerve head. Invest Ophthalmol Vis Sci. 2012; 53: 394–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strouthidis NG, Fortune B, Yang H, Sigal IA, Burgoyne CF. Longitudinal change detected by spectral domain optical coherence tomography in the optic nerve head and peripapillary retina in experimental glaucoma. Invest Ophthalmol Vis Sci. 2011; 52: 1206–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL III. A comparison of refractive development between two subspecies of infant rhesus monkeys (Macaca mulatta). Vision Res. 2007; 47: 1668–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strouthidis NG, Fortune B, Yang H, Sigal IA, Burgoyne CF. Effect of acute intraocular pressure elevation on the monkey optic nerve head as detected by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 9431–9437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Medeiros FA, Lisboa R, Zangwill LM, et al. Evaluation of progressive neuroretinal rim loss as a surrogate end point for development of visual field loss in glaucoma. Ophthalmology. 2014; 121: 100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chauhan BC, Nicolela MT, Artes PH. Incidence and rates of visual field progression after longitudinally measured optic disc change in glaucoma. Ophthalmology. 2009; 116: 2110–2118 [DOI] [PubMed] [Google Scholar]
- 48. Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011; 52: 7365–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chauhan BC, Burgoyne CF. From clinical examination of the optic disc to clinical assessment of the optic nerve head: a paradigm change. Am J Ophthalmol. 2013; 156: 218–227.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]