Abstract
In mammals, a set of anatomically diverse polarity processes – including axon growth and guidance, hair follicle orientation, and stereociliary bundle orientation in inner ear sensory hair cells – appear to be mechanistically related, as judged by their dependence on vertebrate homologues of core tissue polarity/planar cell polarity (PCP) genes in Drosophila. To explore more deeply the mechanistic similarities between different polarity processes, we have determined the extent to which frizzled 3 (Fz3) can rescue the hair follicle and Merkel cell polarity defects in frizzled 6-null (Fz6−/−) mice, and, reciprocally, the extent to which Fz6 can rescue the axon growth and guidance defects in Fz3−/− mice. These experiments reveal full rescue of the Fz6−/− phenotype by Fz3 and partial rescue of the Fz3−/− phenotype by Fz6, implying that these two proteins are likely to act in a conserved manner in these two contexts. Stimulated by these observations, we searched for additional anatomical structures that exhibit macroscopic polarity and that might plausibly use Fz3 and/or Fz6 signaling. This search has revealed a hitherto unappreciated pattern of papillae on the dorsal surface of the tongue that depends, at least in part, on redundant signaling by Fz3 and Fz6. Taken together, these experiments provide compelling evidence for a close mechanistic relationship between multiple anatomically diverse polarity processes.
Keywords: Planar cell polarity, Skin, Brain, Hair follicle, Tongue, Mouse
INTRODUCTION
Complex metazoan animals are replete with structures that exhibit a high degree of spatial order. One type of order is apparent in the orientation of polar structures relative to local anatomic landmarks and/or the body axes. The genetic dissection of this type of spatial order – referred to as tissue polarity or, more restrictively, planar cell polarity (PCP) – began 30 years ago with the discovery and characterization of a core set of genes in Drosophila that regulate the orientations of wing hairs and cuticular bristles (Adler, 2002; Goodrich and Strutt, 2011; Gubb and Garcia-Bellido, 1982). Subsequent work showed that these genes also control ommatidial chirality, implying a more general role in influencing vectorial processes during development (Jenny, 2010).
Homologues of Drosophila PCP genes are found in all vertebrates, with the added complexity that there are typically several homologues for each Drosophila gene. Targeted disruption of these genes in mice – including the genes coding for frizzled (Fz; ten family members), dishevelled (Dsh; three family members), Van Gogh-like (Vangl; two family members) and Celsr (three family members) proteins – has revealed multiple anatomic structures that appear to require polarity signaling to attain their correct orientations (Tissir and Goffinet, 2013; Wang and Nathans, 2007; Wynshaw-Boris, 2012). These include: (1) hair follicles and their associated structures in the skin; (2) stereociliary bundles on the apical faces of inner ear sensory hair cells; and (3) motile cilia in the trachea and on the walls of the cerebral ventricles that direct the vectorial movement of mucus and cerebrospinal fluid, respectively. Two processes that involve oriented cell movements – neural tube closure in mammals and the related process of convergent extension in amphibia and fish – also require core PCP gene function (Munoz-Soriano et al., 2012; Tada and Heisenberg, 2012).
In epithelia, where PCP has been most extensively studied, current evidence suggests that PCP signaling involves the assembly of asymmetric cell-surface complexes that organize the underlying cytoskeleton (Peng and Axelrod, 2012). In these complexes, Fz proteins are localized in the plasma membrane of one cell and face Vang/Vangl proteins in the plasma membrane of the neighboring cell. Importantly, PCP protein assemblies exhibit a macroscopic asymmetry: Fz proteins assemble exclusively on one side of each cell and Vang/Vangl proteins assemble exclusively on the opposite side. The multiple cadherin-domain protein Fmi/Stan/Celsr is present on both sides of the cell and forms homophilic interactions between adjacent cells that stabilize the complex. In current models of PCP signaling, a self-assembly process in which a Fz- or Vang/Vangl-containing hemi-complex on one cell promotes the assembly of the opposite type of hemi-complex on the neighboring cell is hypothesized to be the mechanism by which polarity information is created in and propagates across the epithelial sheet (Peng and Axelrod, 2012; Simons and Mlodzik, 2008).
The present work focuses on Fz3 and Fz6, two mammalian Fz family members that are implicated in tissue polarity signaling. As judged by their amino acid sequences and intron-exon structures, Fz3 and Fz6 form a distinct branch within the mammalian Fz family tree (Fig. 1A,B). Fz6 is expressed in the skin and hair follicles, and Fz6−/− mice exhibit a nearly complete randomization of hair follicle orientations at early times in skin development, a phenotype that resembles the phenotypes of PCP mutants in the Drosophila cuticle (Wang et al., 2006a, 2010). By contrast, Fz3 is expressed in the developing central nervous system (CNS), and Fz3−/− mice exhibit multiple defects in axon growth and guidance, including: (1) the mis-routing of thalamocortical axons to an intra-thalamic trajectory; (2) the failure of corticothalamic axons to enter the internal capsule and reach the thalamus; (3) the absence of the corticospinal tract; (4) the randomization of spinal cord sensory axon trajectories after midline crossing; (5) the failure of some cranial motor axons to reach their muscle targets; and (6) the irreversible stalling of most hindlimb and some forelimb dorsal motor axons in the nerve plexus at the base of the limbs (Hua et al., 2013; Lyuksyutova et al., 2003; Wang et al., 2002, 2006c). Many of these defects are also seen in Celsr3−/− mice (Tissir et al., 2005; Zhou et al., 2008). Some of the axon guidance phenotypes observed in Fz3−/− mice – such as the failure of spinal cord sensory axons to turn rostrally – suggest a polarity signaling defect, whereas other phenotypes – such as the stalling of dorsal limb motor axons – do not. Evidence that Fz3 can engage the polarity signaling machinery in other contexts comes from the redundancy of Fz3 and Fz6 in closing the neural tube and eyelids, and in orienting inner ear sensory hair cells (Wang et al., 2006b).
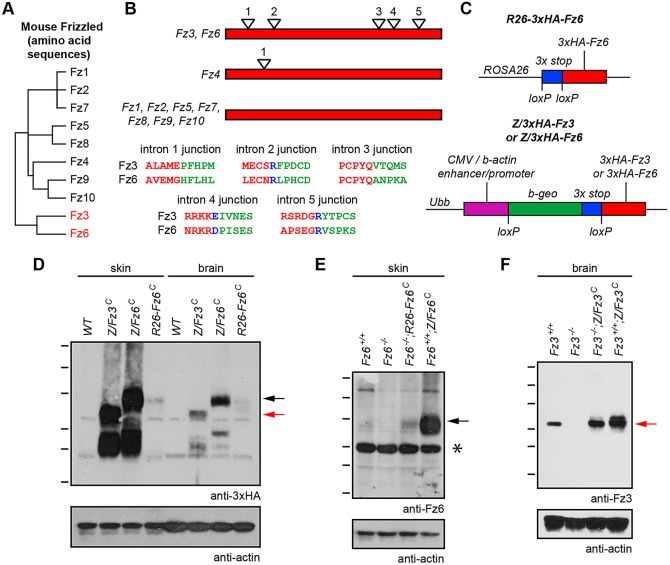
Fig. 1.
Knock-in alleles for constitutive production of Fz3 and Fz6. (A) Dendrogram showing amino acid sequence identities among the 10 mouse Fz proteins. Fz3 and Fz6 show 48% amino acid identity. (B) Schematic of coding region intron-exon structures of mouse Fz family members. Fz3 and Fz6 each have five introns, and the intron positions are precisely conserved, as seen by the alignment of encoded amino acids near each exon-exon junction. Red lettering: amino acids encoded by the 5′ exon. Green lettering: amino acids encoded by the 3′ exon. Blue lettering: the intron is located within that codon. Fz4 has one coding region intron; all other genes in the Fz family lack coding region introns. (C) Schematic of ROSA26-3xHA-Fz6 (top), and Z/3xHA-Fz3 and Z/3xHA-Fz6 (bottom). At the Z locus, Cre-mediated deletion of the loxP-beta-geo-stop-loxP cassette leads to constitutive expression of 3xHA-Fz3 or 3xHA-Fz6 driven by the CAG promoter. At the ROSA26 (R26) locus, Cre-mediated deletion of the loxP-stop-loxP cassette leads to constitutive expression of 3xHA-Fz6 driven by the relatively weak ROSA26 promoter. The constitutively active derivatives of these alleles are referred to as Z/Fz3C, Z/Fz6C and R26-Fz6C, respectively. (D-F) Anti-3xHA, anti-Fz6 and anti-Fz3 immunoblots of P1 brain and skin extracts from wild-type, Z/Fz3C, Z/Fz6C and R26-Fz6C mice in the presence or absence of endogenous Fz3 or Fz6 alleles, as indicated. The Fz3−/− brain was harvested at E18.5. The ubiquitously expressed (D) and endogenous (E) Fz6 proteins migrate at higher apparent molecular weights than the ubiquitously expressed (D) and endogenous (F) Fz3 proteins. Molecular weight (MW) heterogeneity may reflect heterogenous glycosylation. Black arrows, Fz6 protein; red arrows, Fz3 protein. Asterisk indicates an irrelevant cross-reacting band. MW standards are 180, 115, 82, 64, 49 and 37 kDa.
The present study is aimed at determining the degree to which Fz3 and Fz6 are interchangeable, and, by inference, the degree to which polarity signaling in the skin and nervous system are mechanistically related. Our approach is to test whether it is possible to rescue Fz3−/− mice with ubiquitously expressed Fz6 or to rescue Fz6−/− mice with ubiquitously expressed Fz3. The results show a complete phenotypic rescue of Fz6−/− hair patterning by Fz3 and a partial rescue of Fz3−/− axon growth and guidance defects by Fz6. We have also searched for additional anatomic structures that exhibit macroscopic polarity to examine the contributions of Fz3 and Fz6 to that polarity. That search has revealed a hitherto unappreciated epithelial pattern that covers the dorsal surface of the mouse tongue and is partially disrupted by the combined loss of Fz3 and Fz6.
RESULTS
Alleles for ubiquitous production of Fz3 and Fz6
To test the potential of Fz6 to genetically rescue Fz3−/− mice and of Fz3 to genetically rescue Fz6−/− mice, we reasoned that the simplest experimental design would be one in which the rescuing constructs were ubiquitously expressed. To this end, triple-hemaglutinin epitope (HA)-tagged Fz3- and Fz6-coding regions were inserted into the ubiquitously expressed ubiquitin B (Ubb) locus. The Ubb locus is referred to hereafter as the ‘Z’ locus because it is the site into which a Cre reporter transgene, Z/AP, was found to have randomly integrated (Lobe et al., 1999; Rotolo et al., 2008). As shown in Fig. 1C, the Z/3xHA-Fz3 and Z/3xHA-Fz6 knock-in alleles carry a CMV enhancer/beta-actin (CAG) promoter, followed by a β-galactosidase/neo (‘beta-geo’)-triple transcription stop cassette flanked by loxP sites (loxP-beta-geo-stop-loxP). In all of the experiments described here, the loxP-beta-geo-stop-loxP cassette was first removed by Cre-mediated recombination in the germline, generating derivatives in which the Fz3- or Fz6-coding regions were constitutively expressed, referred to as Z/Fz3C and Z/Fz6C, respectively. Additionally, the 3×HA-Fz6-coding region with a triple transcription stop cassette flanked by loxP sites was inserted at the ROSA26 (R26) locus to generate R26-3xHA-Fz6. Constitutive activation of this allele by germline Cre-mediated recombination generated the constitutively expressed R26-Fz6C allele. Expression of a single copy of Z/Fz3C, Z/Fz6C or R26-Fz6C in a wild-type background produced no visible effect on viability, growth, fertility or overall health, implying that ubiquitous production of Fz3 or Fz6 is relatively innocuous.
Comparisons of the levels of protein production from the three ubiquitously expressed knock-in loci and from the endogenous Fz3 and Fz6 loci by immunoblotting show that: (1) the CAG promoter at the Z locus produces many fold higher levels of Fz3 and Fz6 than the ROSA26 locus in both brain and skin (Fig. 1D); (2) the levels of Fz3 and Fz6 produced from the Z locus are higher in skin than in brain, but the levels of Fz6 produced from the ROSA26 locus in skin and brain are more nearly equivalent (Fig. 1D); (3) the level of endogenous Fz6 in skin is barely detectable (compare Fz6+/+ and Fz6−/− lanes in Fig. 1E), is several fold below the level of Fz6 produced from the ROSA26 locus, and is many fold below the level of Fz6 produced from the Z locus (Fig. 1E); and (4) the level of endogenous Fz3 in brain is readily detectable and is close to the level of Fz3 produced from the Z locus (Fig. 1F). We note that in both brain and skin, the comparison between endogenous and ubiquitously expressed proteins does not correct for the more limited anatomic distribution of the endogenous protein, an effect that acts to minimize endogenous versus ubiquitous protein concentration differences in the relevant cell types compared with the ratios observed in the whole tissue immunoblots. For example, in the skin, endogenous Fz6 is expressed only in the epidermis and hair follicles, whereas the Z and ROSA26 loci are active in the epidermis, hair follicles, dermis and vasculature, all of which were included in the tissue homogenate.
Ubiquitous production of Fz3 rescues the Fz6−/− hair polarity phenotype
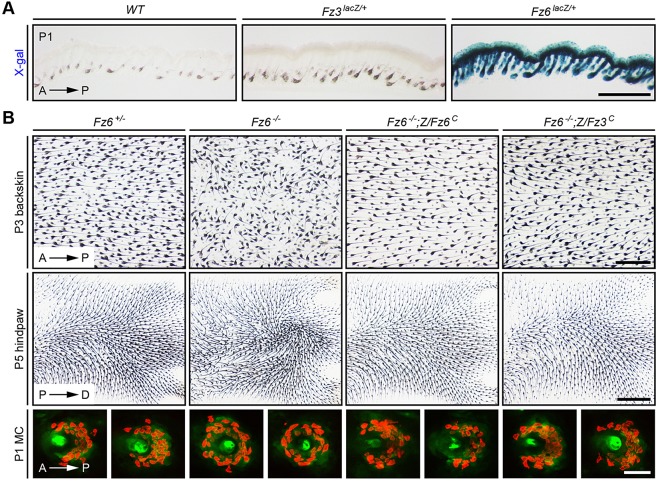
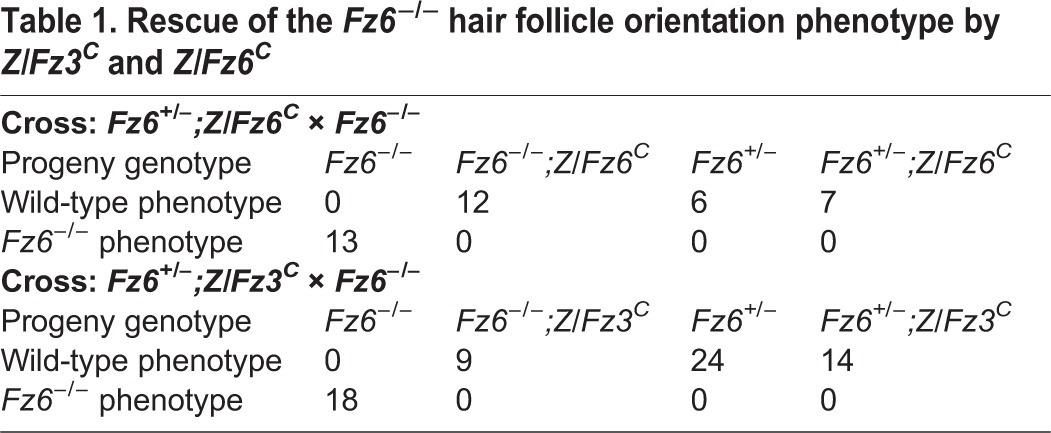
In the skin, Fz6 expression – as measured with a Fz6lacZ knock-in allele (Guo et al., 2004) – is readily detected in the epidermis and in hair follicles, whereas Fz3 expression – as measured with a Fz3lacZ knock-in allele (Wang et al., 2002) – is undetectable (Fig. 2A). Crossing Z/Fz6C or R26-Fz6C into the Fz6−/− background showed, as expected, a complete rescue of the Fz6−/− hair orientation phenotype. More interestingly, crossing Z/Fz3C into the Fz6−/− background also showed a complete rescue of the Fz6−/− hair orientation phenotype, as assessed on both the back and the paws (Table 1, Fig. 2B). At postnatal day (P) 3, hair follicles on the back are well oriented in an anterior-to-posterior direction in Fz6+/− mice but are severely disordered in Fz6−/− mice. In Fz6−/−;Z/Fz3C and Fz6−/−;Z/Fz6C mice, the normal anterior-to-posterior follicle orientation in back skin is restored. Similarly, at P5, hair follicles on the dorsal surface of the paws are aligned in a proximal-to-distal direction in Fz6+/− mice, whereas they form a macroscopic whorl in the center of the paws in Fz6−/− mice. In Fz6−/−;Z/Fz3C and Fz6−/−;Z/Fz6C mice, the normal proximal-to-distal follicle orientation on the paws is restored.
Fig. 2.
Complete rescue of the Fz6−/− hair follicle and Merkel cell phenotypes by Z/Fz3C. (A) X-gal staining of transverse sections of P1 back skin from Fz6lacZ/+, Fz3lacZ/+ and wild-type control mice. Fz6 is expressed in epidermis and hair follicles, but Fz3 expression is undetectable. Melanin pigmentation (brown) is visible in the follicles in each image. Similar data have been presented by Chang and Nathans (2013). Scale bar: 500 μm. (B) Upper panels show rescue of Fz6−/− hair follicle orientation phenotype by Z/Fz6C and by Z/Fz3C on the back [upper panels; anterior (A) is to the left and posterior (P) is to the right] and hindpaw [lower panels; proximal (P) is to the left and distal (D) is to the right]. Follicles in skin flat mounts are visualized with melanin pigment. Lower panels show rescue of Fz6−/− Merkel cell cluster phenotype in the back skin by Z/Fz3C and Z/Fz6C (A, anterior; P, posterior). Merkel cells are visualized with anti-cytokeratin 8 immunostaining. Scale bars: 500 μm for back skin; 1 mm for paws; 50 μm for Merkel cell clusters.
Table 1.
Rescue of the Fz6−/− hair follicle orientation phenotype by Z/Fz3C and Z/Fz6C
In wild-type mice, a semicircle of ∼30 Merkel cells partially surrounds each guard hair on the back skin, with the opening of the semicircle facing anteriorly. In Fz6−/− mice, the anterior-posterior polarity of the Merkel cell cluster is lost and the Merkel cells are arranged in a complete circle (Chang and Nathans, 2013; Fig. 2B). In Fz6−/−;Z/Fz3C and Fz6−/−;Z/Fz6C mice, Merkel cell polarity is restored (Fig. 2B).
To assess the specificity of Z/Fz3C function, we asked whether it could rescue the palate closure defect that occurs in Fz1−/−;Fz2−/− embryos (Yu et al., 2010). In 9/9 Fz1−/−;Fz2−/−;Z/Fz3C embryos examined, there was a failure of palate closure indistinguishable from the palate closure defect seen in Fz1−/−;Fz2−/− embryos (supplementary material Fig. S1). Thus, Z/Fz3C is capable of rescuing some Fz mutations but not others.
Ubiquitous production of Fz6 partially rescues Fz3−/− axon growth and guidance phenotypes
In the embryonic brain, Fz3 is widely expressed (Tissir and Goffinet, 2006; Wang et al., 2002) but Fz6 expression is largely confined to the vasculature (Z.L.H., H.C., Y.W., P.M.S. and J.N., unpublished; Daneman et al., 2009; Stenman et al., 2008). Crossing Z/Fz3C into the Fz3−/− background showed that the neonatal lethality exhibited by Fz3−/− mice is completely rescued by ubiquitous production of Fz3 (Table 2) and, as described more fully below, the Fz3−/− axon growth and guidance phenotypes are also completely rescued (Table 3). Indeed, adult Fz3−/−;Z/Fz3C mice are healthy, fertile and indistinguishable from wild-type controls. By contrast, early postnatal lethality is not rescued in Fz3−/−;Z/Fz6C and Fz3−/−;R26-Fz6C mice (Table 2).
Table 2.
Fz3−/− neonatal lethality: quantification of Z/Fz3C, Z/Fz6C and R26-Fz6C rescue experiments
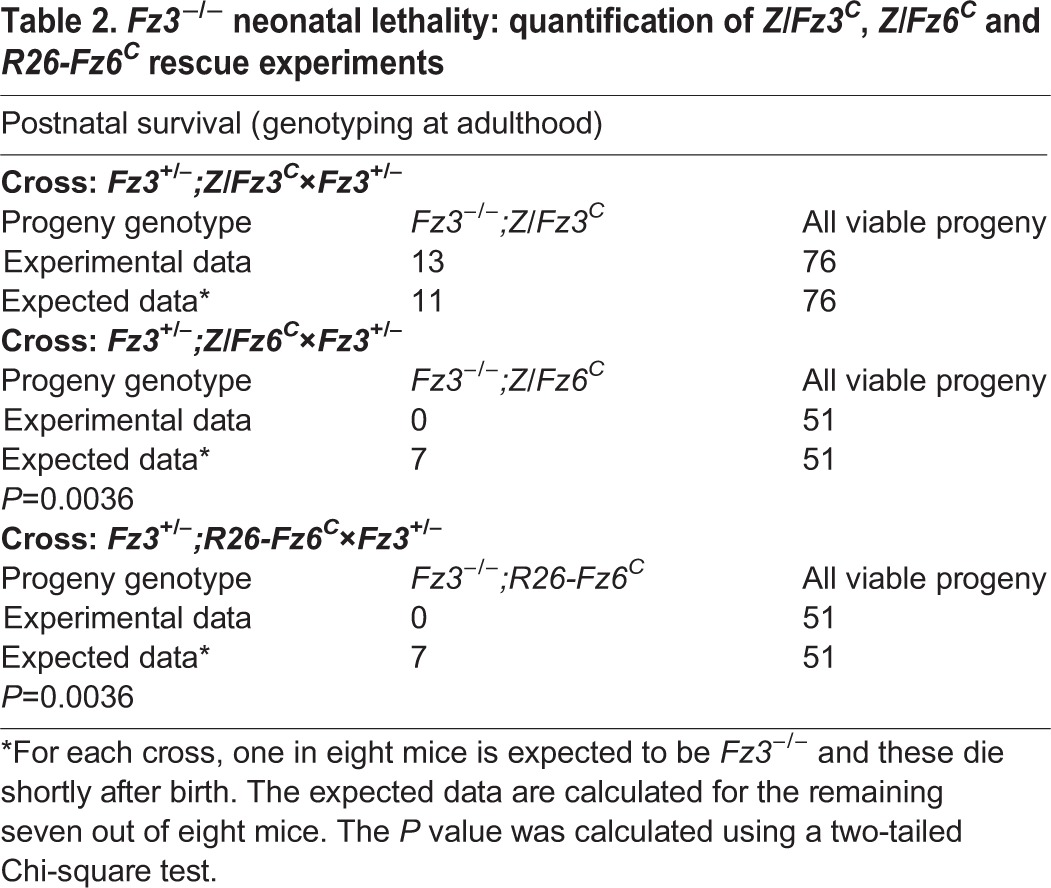
Table 3.
Fz3−/− axon growth and guidance phenotypes: quantification of Z/Fz3C and Z/Fz6C rescue experiments
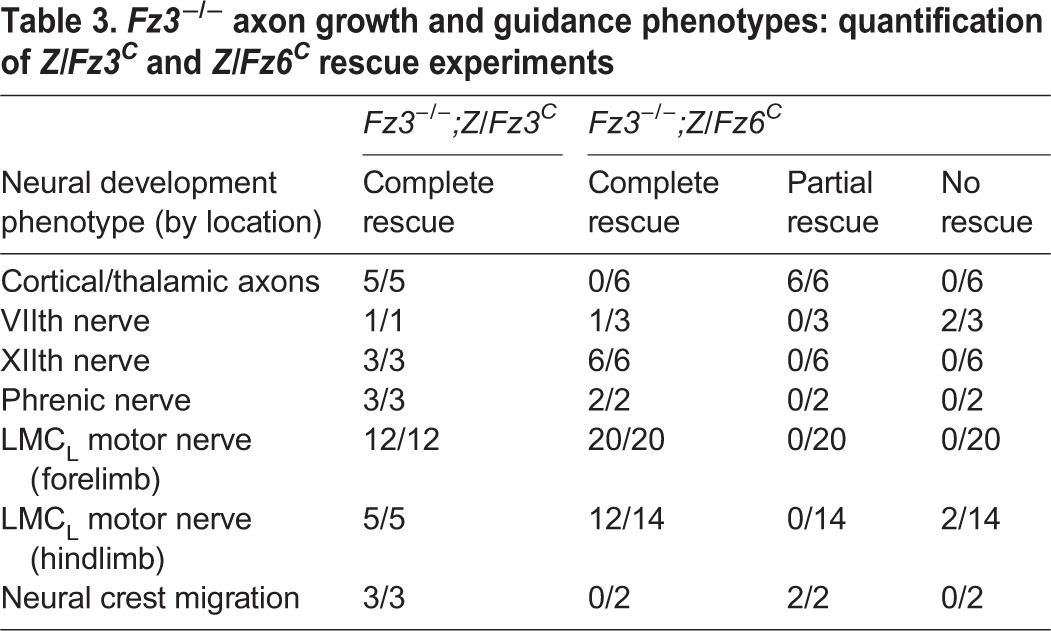
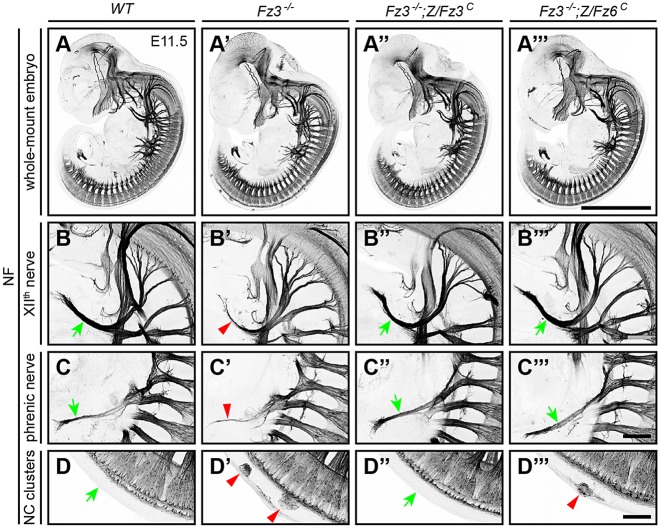
The failure of the Z/Fz6C allele to rescue the Fz3−/− lethal phenotype does not necessarily imply that the Z/Fz6C allele is without effect. To determine whether the Z/Fz6C allele can correct some of the Fz3−/− axon growth and guidance defects, axon tracts were visualized with neurofilament (NF) immunostaining, with wild-type and Fz3−/−;Z/Fz3C embryos serving as controls (Table 3). At embryonic day (E) 11.5, the thinning of the XIIth cranial and phrenic nerves that characterizes Fz3−/− embryos (Hua et al., 2013) was rescued in both Fz3−/−;Z/Fz3C and Fz3−/−;Z/Fz6C embryos (Fig. 3A-C‴). Fz3−/−;Z/Fz6C embryos also exhibited a nearly complete rescue of the neural crest migration defect that leads to the retention of clusters of neural crest cells along the dorsal spinal cord (Fig. 3D-D‴). At E11.5, a mean of 9.5 cell clusters were seen per Fz3−/− embryo (range 7-13; n=4) compared with one cluster in each of two Fz3−/−;Z/Fz6C embryos and no clusters in each of three Fz3−/−;Z/Fz3C embryos.
Fig. 3.
Complete rescue of axon growth defect in the XIIth and phrenic nerves and partial rescue of neural crest cell migration defect in Fz3−/− embryos by Z/Fz6C. (A-A‴) NF immunostaining of whole-mount E11.5 embryos. (B-B‴) Fz3−/− XIIth nerve is completely rescued by Z/Fz3C and Z/Fz6C. In Fz3−/− embryos, the XIIth nerve is markedly thinned after making a rostral turn towards the tongue (B′). (C-C‴) Fz3−/− phrenic nerve is completely rescued by Z/Fz3C and Z/Fz6C. In Fz3−/− embryos, the phrenic nerve is thinned (C′). (D-D‴) Neural crest cell migration defects in Fz3−/− embryos are completely rescued by Z/Fz3C and partially rescued by Z/Fz6C. In Fz3−/− embryos, neural crest cells that failed to migrate from the dorsal neural tube form NF-rich clusters along the caudal half of the spinal cord (A′,D′). In three Fz3−/−;Z/Fz3C embryos examined, the clusters were absent (D″). Each of two Fz3−/−;Z/Fz6C embryos examined contains one cluster (D‴). In B-D‴, green arrows show normal structures and red arrowheads show abnormal structures. Scale bars: 1 mm in A; 100 μm in B-D.
The severe defect in thalamocortical and corticothalamic tract formation in Fz3−/− embryos was completely rescued in Fz3−/−;Z/Fz3C embryos (n=5), but only partially rescued in Fz3−/−;Z/Fz6C embryos (n=6; Fig. 4A-D′). In Fz3−/− embryos, thalamocortical and corticothalamic axons do not enter the internal capsule. In Fz3−/−;Z/Fz6C embryos, many of these axons enter the internal capsule, but the resulting tracts appear narrower (arrow in Fig. 4D) and some of the axons exhibit aberrant trajectories around the globus pallidus (arrowhead in Fig. 4D′).
Fig. 4.
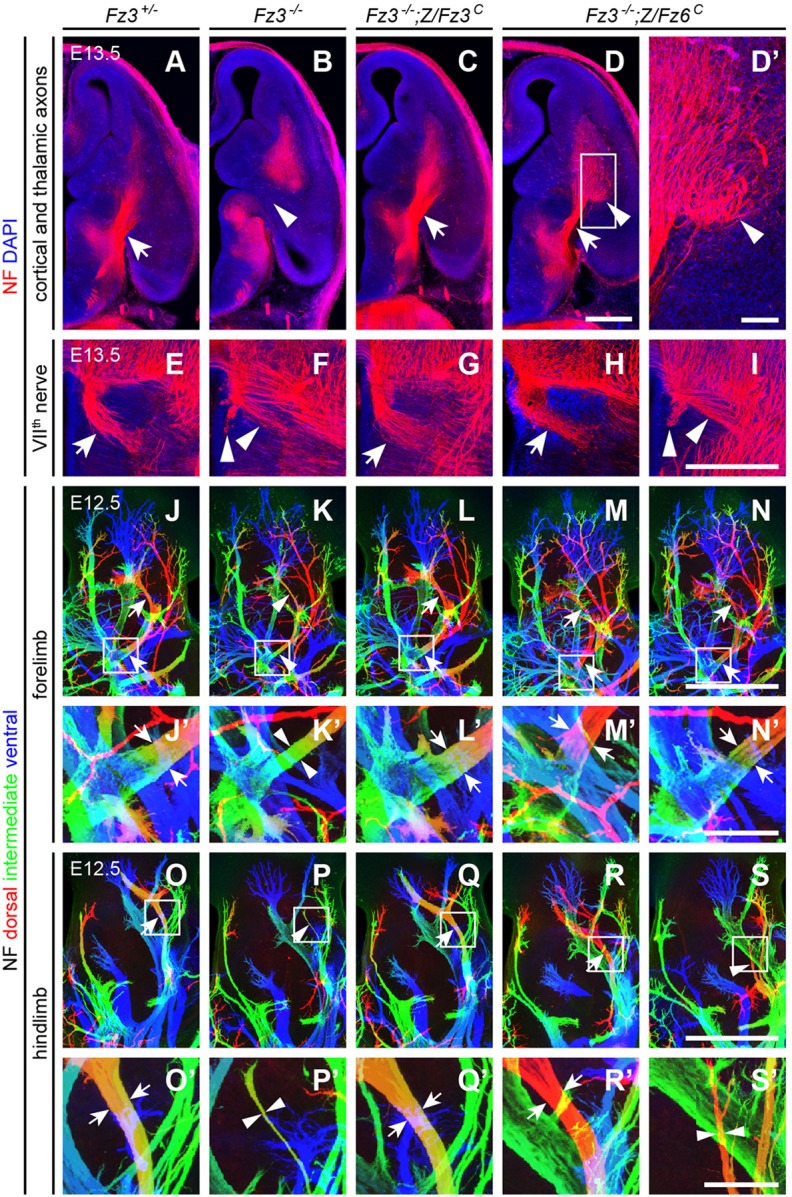
Partial rescue of Fz3−/− axon growth and guidance defects in the forebrain, VIIth cranial nerve and dorsal limb motor axons by Z/Fz6C. (A-D′) NF immunostaining of horizontal sections from E13.5 brains showing cortical and thalamic axons. The boxed region in D is enlarged in D′. Scale bars: 500 μm in D; 100 μm in D′. (E-I) NF immunostaining of horizontal sections from E13.5 brainstems showing VIIth nerve axons. Scale bar: 500 μm. (J-N′) NF immunostaining of whole-mount E13.5 forelimbs. (J′-N′) Magnified view of boxed regions in J-N. Depth within the z-stack along the dorsal-to-ventral axis is color-coded from red to blue. Scale bars: 500 μm in N; 100 μm in N′. (O-S′) NF immunostaining of whole-mount E13.5 hindlimbs. (O′-S′) Magnified view of boxed regions in O-S. Depth within the z-stack along the dorsal to ventral axis is color-coded from red to blue. Scale bars: 500 μm in S; 100 μm in S′. In A-S′, arrows show normal axon arrangements and arrowheads show abnormal arrangements.
Fz3−/−;Z/Fz6C embryos showed variable rescue of the migratory defects of VIIth cranial nerve cell bodies. In the Fz3−/− brainstem, these cells fail to migrate tangentially and caudally from rhombomere 4 to rhombomere 6, and, as a consequence, their axons fail to loop around the VIth cranial nerve nucleus (compare Fig. 4E with Fig. 4F). In Fz3−/−;Z/Fz3C embryos this defect was fully rescued (Fig. 4G). Among three Fz3−/−;Z/Fz6C embryos examined, one was completely rescued (Fig. 4H) and two showed no rescue (Fig. 4I).
In earlier work, we characterized the stalling of dorsal motor axons at the base of both fore- and hindlimbs in midgestation Fz3−/− embryos by determining the width of the dorsal nerve at different locations along its trajectory (Hua et al., 2013). Stalling is more complete in the hindlimb and leads to rapid death of the corresponding motor neurons in the spinal cord. By whole-limb NF immunostaining, Fz3−/−;Z/Fz3C fore- and hindlimbs showed a complete rescue of the axon stalling defect (Table 3; Fig. 4J-L′,O-Q′; n=12 forelimbs and n=5 hindlimbs). In Fz3−/−;Z/Fz6C embryos, the forelimb phenotype was completely rescued (n=20 forelimbs), but a complete rescue was observed in only 12/14 hindlimbs, with the remaining 2/14 showing little or no rescue (Fig. 4M-N′,R-S′).
The complete rescue of the dorsal motor axon defect in the majority of Fz3−/− limbs by Z/Fz6C raises the issue of whether Fz6 normally plays any role in the development of this axon tract. To address this, we examined whole-mount limbs from E13.5 embryos in which homozygous deletion of Fz6 in motor neurons was paired with: (1) deletion of both copies of Fz3 (Olig2Cre/+;Fz3CKO/−;Fz6−/−); or (2) deletion of one copy of Fz3 (Olig2Cre/+;Fz3CKO/+;Fz6−/−). [The use of Olig2Cre to drive Cre-mediated recombination of Fz3CKO avoids the potentially confounding effect of the neural tube closure defect that is part of the Fz3−/−;Fz6−/− phenotype (Wang et al., 2006b).] With both Fz3 and Fz6 deleted (supplementary material Fig. S2C-D′,H-I′), dorsal axon thinning was indistinguishable from the Fz3−/− phenotype (supplementary material Fig. S2B,B′,G, G′; see also Fig. 4); and with one copy of Fz3 present, loss of either one or both copies of Fz6 did not produce any alteration in the appearance of the dorsal axon (supplementary material Fig. S2B,B′,E,E′,G,G′,J,J′). We conclude that Fz6 has neither an independent effect on dorsal axon development nor an effect that is redundant with Fz3.
The complete rescue of all aspects of the Fz3−/− phenotype by Z/Fz3C, together with the nearly identical levels of 3×HA-tagged Fz3 and Fz6 proteins produced in the brain from the Z/Fz3C and Z/Fz6C alleles (Fig. 1D), implies that the incomplete rescue effected by Z/Fz6C cannot be explained by a failure of the Z/Fz6C allele to express sufficient protein in the appropriate spatiotemporal pattern. Moreover, the rescue of the Fz6−/− skin phenotype by Z/Fz6C and R26-Fz6C implies that both of these knock-in alleles produce functional Fz6. The data suggest either that Fz6 is quantitatively less active in promoting axon growth and guidance than Fz3 or that Fz6 differs in some qualitative manner so that it cannot fully recapitulate Fz3 function.
Polarization of lingual papillae involves Fz3 and Fz6
The observation that Fz3 can replace Fz6 in skin PCP signaling prompted us to examine whether Fz3 might normally play a role in patterning epithelial structures. In keeping with the absence of detectable Fz3 expression in the skin over most of the body (Fig. 2A), loss of Fz3 did not perturb hair follicle orientation at E18.5 (supplementary material Fig. S3A,B), and the combined loss of Fz3 and Fz6 in the epidermis (Fz3CKO/−;Fz6−/−;K14-Cre;K17-GFP) produced a phenotype of hair follicle mis-orientation at P0 that was indistinguishable from the phenotype produced by global loss of Fz6 (supplementary material Fig. S3C). These data imply that Fz3 plays little or no role in hair follicle orientation. [In these experiments and the ones described below, we have bypassed the CNS defects that lead to neonatal lethality in Fz3−/− mice by using a Keratin14-Cre (K14-Cre) transgene to selectively delete Fz3 in the epidermis starting at ∼E12.5-E13.5 (Beronja et al., 2010). In unpublished work we observed that Fz6CKO/CKO;K14-Cre and Fz6−/− mice exhibit identical hair patterning phenotypes, implying that K14-Cre acts sufficiently early to eliminate PCP in the developing epidermis.]
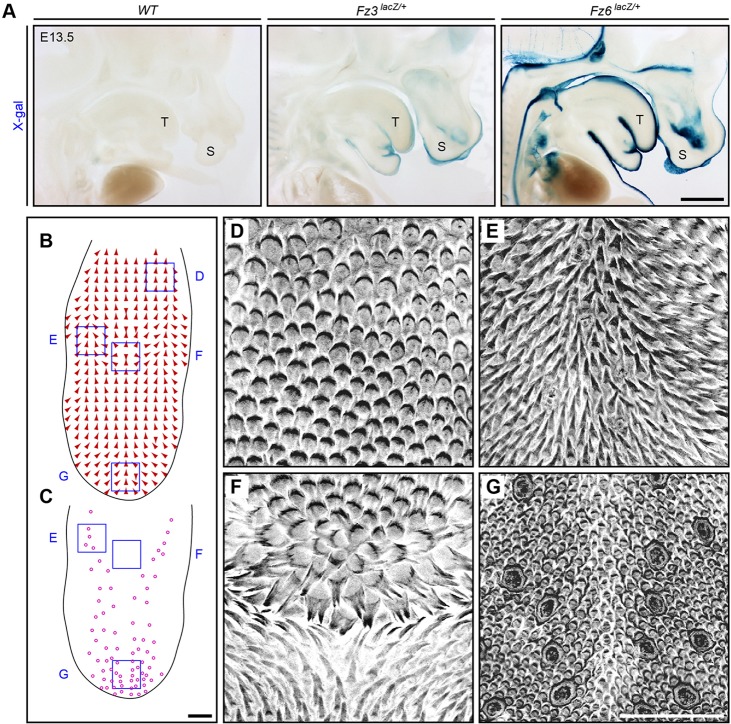
In a search for other epithelial structures that exhibit large-scale polarity and that might reveal effects of Fz3 and/or cooperative effects of Fz3 and Fz6, we investigated the dorsal surface of the tongue, which in most mammals, including rodents, is covered with asymmetric epithelial protrusions (papillae). The development and micro-anatomy of lingual (i.e. tongue) papillae have been extensively studied, but their large-scale spatial organization has received little attention (Hume and Potten, 1976; Iwasaki et al., 1996). Both Fz3 and Fz6 are expressed in the tongue epithelium at E13.5, as judged by expression of the corresponding lacZ knock-in alleles (Fig. 5A). (We note that the X-gal staining intensities of these two alleles are not directly comparable: the lacZ knock-in at the Fz3 locus includes an intron in the 3′ UTR and lacks a nuclear localization signal, and it is therefore likely to be a weaker reporter than the nuclear localized lacZ knock-in at the Fz6 locus.)
Fig. 5.
Large-scale patterning of lingual papillae and expression of Fz3 and Fz6 in the dorsal epithelium of the wild-type mouse tongue. (A) X-gal staining of sagittal sections of E13.5 Fz6lacZ/+, Fz3lacZ/+ and wild-type embryos. Fz6 is strongly expressed throughout the epidermis and in the surface epithelium of the tongue. Fz3 is expressed in the epidermis of the snout and the surface epithelium of the tongue. Anterior is towards the right. T, tongue; S, snout. Scale bar: 1 mm. (B,C) Epithelial patterns on the dorsal surface of a wild-type tongue from a 6-month-old mouse injected with AM4-65. The global orientation of papillae (arrows) are shown in B; the locations of taste buds (circles) are shown in C. The tongue is outlined in black. Anterior is downwards. Scale bar: 1 mm. (D-G) Grayscale images of epithelial AM4-65 fluorescence correspond to the locations of the blue squares in B and C. The rosette pattern is seen in F. Anterior is downwards. Scale bar: 500 μm.
To obtain a global assessment of the polarity of lingual papillae, we viewed the tongue as a flat-mount, using a combination of GFP expression from a Keratin17-GFP (K17-GFP) transgene and in vivo labeling with AM4-65 to assist in visualizing papillae. The wild-type mouse tongue shows a highly stereotyped pattern of papillae orientations (Fig. 5B-G). Along the front and sides, papillae make an angle of ∼45° to the midline, with their raised edges pointing toward the center and posterior of the tongue. Moving more medially from each side, the papillae progressively rotate until they point posteriorly at the midline. The most distinctive feature of the papillae pattern is the presence of a single circularly symmetric rosette located at the midline ∼70% of the distance from the anterior tip to the base of the tongue. The orientations of papillae at progressively greater distances from the rosette change smoothly to accommodate the vector fields at the sides and front of the tongue (Fig. 5B,F; supplementary material Fig. S4). The result is that the tongue surface exhibits two ‘singularities’, i.e. locations where there are large differences in the orientations of neighboring papillae. One singularity is at the center of the rosette and the second is ∼1 mm anterior to the rosette, where posteriorly directed papillae from the anterior half of the tongue encounter anteriorly directed papillae from the rosette (supplementary material Fig. S4). As the entire papillae pattern is almost perfectly left-right symmetric, both singularities are located at the midline.
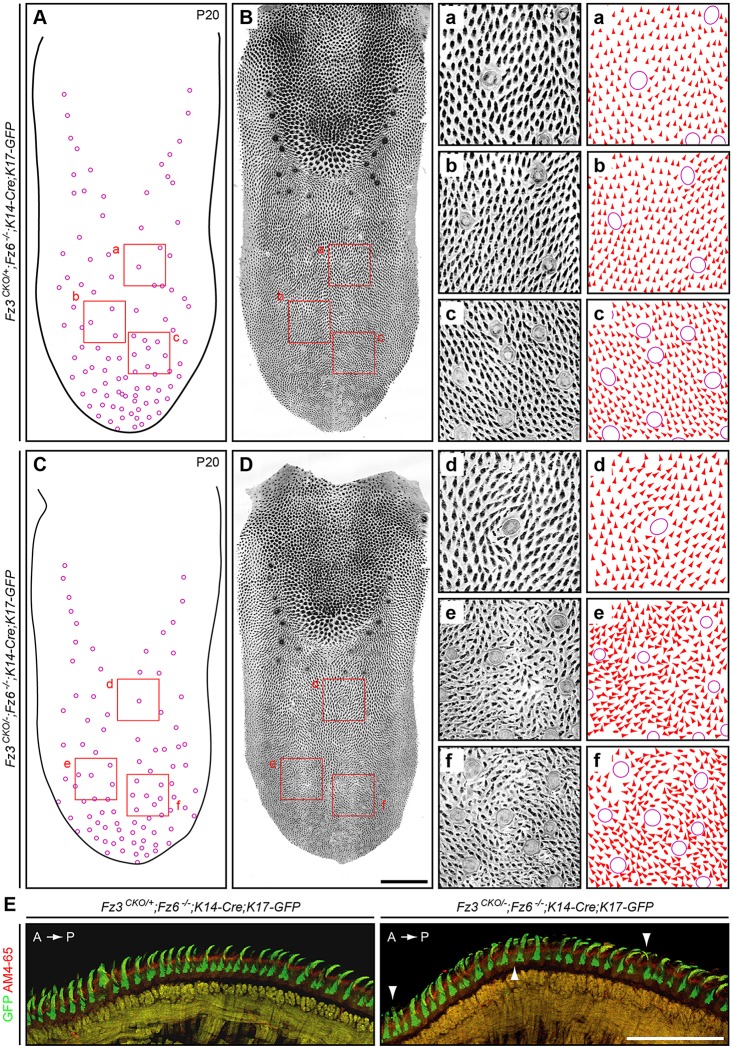
To determine whether Fz3 and/or Fz6 controls the patterning of lingual papillae, we examined tongues from mice missing either or both genes in the tongue epithelium at ages ranging from P3 to 9 months. As noted above in the context of supplementary material Fig. S3C, the Fz3CKO allele was recombined with K14-Cre, which is expressed uniformly in the dorsal epithelium of the mature tongue, as assessed with a Hprt-LSL-tdTomato reporter (Wu et al., 2014; data not shown). The genotype/phenotype relationships are summarized in Table 4. In the absence of both Fz3 and Fz6 (Fz3CKO/−;Fz6−/−;K14-Cre;K17-GFP), the dorsal tongue surface at P20 shows no changes in the number and locations of taste buds or in the density of lingual papillae. However, papillae in the anterior half of the tongue show a general disorganization in their orientations within the plane of the tongue epithelium that is strongly reminiscent of the bristle and wing hair patterning defects observed in PCP mutants in Drosophila (Fig. 6C,D; Adler, 2002). In cross-sections near the midline, Fz3CKO/−;Fz6−/−;K14-Cre;K17-GFP tongues reveal numerous papillae that lack the regular anterior-to-posterior orientation observed in control tongues (Fig. 6E). By contrast, the presence of single Fz3 allele in the absence of Fz6 (Fz3CKO/+;Fz6−/−;K14-Cre;K17-GFP) or a single Fz6 allele in the absence of Fz3 (Fz3CKO/−;Fz6+/−;K14-Cre;K17-GFP) produces a papillae pattern that is indistinguishable or nearly indistinguishable from the wild type (Fig. 6A,B; supplementary material Figs S4, S5; Table 4). These data imply that Fz3 and Fz6 function redundantly in tongue epithelial patterning.
Table 4.
Tongue patterning phenotypes with loss of Fz3 and/or Fz6
Fig. 6.
Patterning of lingual papillae is partially disrupted in the anterior of the Fz3CKO/−;Fz6−/−;K14-Cre tongue. (A-D) Dorsal views of a Fz3CKO/+;Fz6−/−;K14-Cre;K17-GFP tongue (A,B) and a Fz3CKO/−;Fz6−/−;K14-Cre;K17-GFP tongue (C,D) at P20 showing the locations of taste buds (A,C) and epithelial morphology with AM4-65 and GFP fluorescence (B,D). The pattern in B matches that of wild-type tongues. Anterior is downwards. The rosette pattern is present along the midline at ∼70% of the distance from the tip to the base of the tongue. The grayscale images on the right (panels a-f) correspond to the locations of the red squares in the low-magnification images on the left. The orientation of each papilla in a-f was scored with an arrowhead or, in cases where the polarity of the papillae is ambiguous, with an oval. Scale bar: 1 mm. (E) Cross-sections of the anterior dorsal surface of a control Fz3CKO/+;Fz6−/−;K14-Cre;K17-GFP tongue (left; wild-type pattern) and a Fz3CKO/−;Fz6−/−;K14-Cre;K17-GFP tongue (right) at 3 months of age. Arrowheads indicate some of the mis-oriented papillae in the right panel. The mice were injected intraperitoneally with AM4-65 1 day prior to sacrifice. A, anterior; P, posterior. Scale bar: 500 μm.
The localization of the patterning defect to the anterior of the tongue in Fz3CKO/−;Fz6−/−;K14-Cre;K17-GFP mice is puzzling. As lingual papillae are part of a larger integrated pattern, one might predict that any PCP gene mutations that affect lingual patterning would affect the entire pattern. Although we do not have a resolution to this apparent paradox, we speculate that if the patterning of lingual papillae roughly coincides with the time when expression of the K14-Cre transgene begins, and if the anterior tongue pattern develops 1-2 days later than the posterior tongue pattern or if Cre-mediated recombination occurs 1-2 days earlier in the anterior tongue, then the patterning defect in Fz3CKO/−;Fz6−/−;K14-Cre;K17-GFP mice could be limited to the anterior tongue.
Sequence divergence and evolutionary history of Fz3 and Fz6
Which domains of Fz3 and Fz6 might account for the subtly different activities of these two proteins? Alignment of mouse Fz3 and Fz6 sequences reveals a pattern of amino acid conservation that differs between domains, with 50% identity in the extracellular N-terminal cysteine-rich domain and 68% amino acid identity in the transmembrane domain, but only 23-29% identity in the cytoplasmic C-terminal tail (the range reflecting the different lengths of Fz3 and Fz6 C-terminal tails; supplementary material Fig. S6). Similar results are obtained in comparisons across other vertebrate species. Additionally, the C-terminal tails of Fz3 (169 amino acids) and Fz6 (212 amino acids) are substantially longer than the C-terminal tails of other Fz family members. The high degree of divergence between Fz3 and Fz6 in the C-terminal tail suggests that part of the activity difference between these two proteins in the context of CNS development may reside in this region, an idea that could be tested by swapping this domain.
Alignments of Fz amino acid sequences deduced from genomic and cDNA sequences of diverse vertebrates and invertebrates show that the Fz3/Fz6 branch within the Fz family arose in the vertebrate lineage (Fig. 7). Moreover, the division of that branch into Fz3 and Fz6 sub-branches appears to have occurred early in the vertebrate lineage, as the two sub-branches are found among all major vertebrate divisions. By contrast, the genome of C. intestinalis, a primitive chordate, codes for only one Fz3/Fz6-like sequence. [The identification of a Fz3 homologue but not a Fz6 homologue among P. marinus (lamprey) sequences should be interpreted cautiously, as genomic sequences from this species are substantially incomplete.] In sum, current evidence indicates that all jawed vertebrates (Gnathostomata) use distinct Fz3 and Fz6 proteins, suggesting that the division of function between these two family members occurred at least 400 million years ago.
Fig. 7.
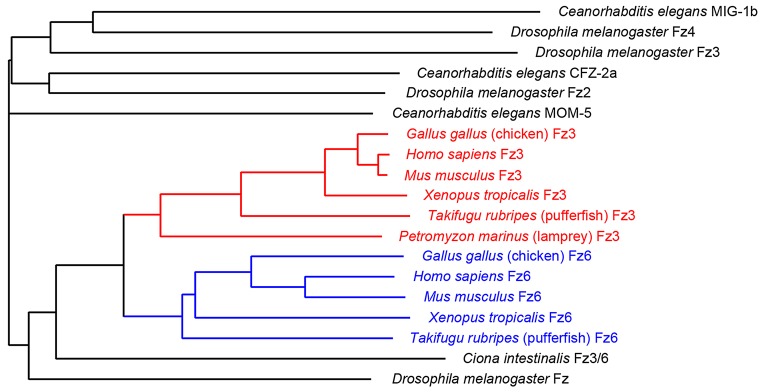
Evolutionary divergence of Fz3 and Fz6 sequences. Dendrogram showing amino acid divergence of Fz3 (red) and Fz6 (blue) from select vertebrates, the closest Frizzled homologue from Ciona intestinalis, and all of the Frizzled family members from C. elegans and D. melanogaster. The ID numbers of the sequences used are listed in supplementary material Table S1.
DISCUSSION
The experiments reported here provide strong evidence that Fz3 and Fz6 have broadly similar functional properties. They also imply that the molecular mechanisms of transmembrane signaling in Fz3-mediated axon growth and guidance and in Fz6-mediated hair follicle polarity involve at least some homologous protein-protein interactions. As Fz3 and Fz6 homologues exist in amphibia, birds, fish and mammals, the signaling mechanisms that use their shared structural and functional motifs likely pre-date the ancestral duplication event that created separate Fz3 and Fz6 genes at the beginning of the vertebrate radiation. This general view is further supported by the observation that loss-of-function mutation of the core PCP gene Celsr1 produces a hair follicle orientation phenotype closely resembling the Fz6−/− phenotype (Ravni et al., 2009), and, as noted in the Introduction, loss-of-function mutation of the homologous Celsr3 gene produces an axon guidance phenotype closely resembling the Fz3−/− phenotype (Tissir et al., 2005). The emerging picture is one in which Celsr and Fz represent the core components of an ancient and versatile polarity signaling complex. It is not clear whether other core PCP genes, which were initially defined in the context of epithelial polarity, also play a role in axon growth and guidance.
Partial redundancy and partial interchangeability of Fz3 and Fz6
The Fz3 and Fz6 rescue experiments described here can be conceptualized as a genetically engineered extension of the ‘experiment of nature’ in which Fz3 and Fz6 function redundantly in the context of neural tube and eyelid closure, inner ear sensory hair cell orientation and (as shown here) patterning of lingual papillae. Because functional redundancy is predicated on spatiotemporal overlap in expression, a failure to observe redundancy in any particular context could simply reflect the absence of expression of one of the genes, as appears to be the case for Fz3 in skin and hair follicles. In the experiments described here, we engineered ubiquitous expression of the rescuing constructs to maximize the chances of observing functional rescue, essentially creating a synthetic form of redundancy. Conveniently, it appears that ubiquitous production of Fz3 or Fz6 is not deleterious, although we cannot rule out the possibility of subtle effects. It also appears that ubiquitous production of Fz3 or Fz6 does not elicit the induction of aberrantly oriented epithelial structures of the type observed in the Drosophila wing and abdomen at the boundaries between clones that differ in the level of Fz gene expression (Adler, 2002; Struhl et al., 2012).
The contrast between the ability of ubiquitous Fz3 to fully rescue the Fz6−/− hair follicle orientation/epithelial polarity phenotype and the failure of ubiquitous Fz6 to fully rescue the Fz3−/− axon growth and guidance phenotype suggests that epithelial polarity may be the more fundamental of the two processes, whereas axon growth/guidance may represent a process for which Fz3 has evolved subtle alterations relative to Fz6. The conserved nature of epithelial PCP signaling can be seen in the morphological and molecular similarities between PCP in mammalian epithelia and in the Drosophila cuticle and wing, both of which require Frizzled, Stan/Fmi/Celsr and Vang/Vangl genes, and both of which feature asymmetric PCP protein complexes (Devenport and Fuchs, 2008; Goodrich and Strutt, 2011; Wang et al., 2006b). In Drosophila and C. elegans, Stan/Fmi/Celsr and Frizzled genes have been implicated in axon guidance, branching and target selection, and Drosophila Stan/Fmi has been implicated in self-avoidance in sensory dendrite tiling (Huarcaya Najarro and Ackley, 2013; Lee et al., 2003; Matsubara et al., 2011; Ng, 2012; Senti et al., 2003; Steinel and Whitington, 2009). In view of the subtly different biological activities of Fz3 and Fz6 observed here, it would be interesting to investigate whether specific classes of mutations in Drosophila Stan/Fmi might differentially affect epidermal polarity versus axonal/dendritic pathfinding/target selection/tiling.
Evolution of PCP genes
A general issue in the study of genome evolution relates to the biochemical and evolutionary forces that determine the sizes of gene/protein families in different species and the extent of sequence divergence among family members. In some instances, the functional properties of a gene/protein family are sufficiently well understood to provide some insights into this issue; this is especially true when the relevant biochemical properties of the proteins can be defined in vitro. For example, the diversification of the mammalian globin family was likely driven by the advantages associated with the different oxygen affinities of embryonic, fetal and adult hemoglobins, which promote oxygen transfer from the mother to the embryo or fetus; the diversification of the vertebrate immunoglobin family was likely driven by the advantages associated with distinct binding specificities imparted by different variable regions, which increase the diversity of the immune repertoire; and the diversification of the visual pigment family was likely driven by the advantages associated with the different absorbance spectra of visual pigment receptors, which determine the wavelengths of light that can be detected, and by the advantages associated with increasing numbers of receptors, which permits a higher dimensionality in the resulting color vision. However, for many gene families, defining the relationships between sequence, function and evolutionary pressure is more challenging, especially if the encoded proteins function in multiple biological processes. Proteins in this category include, for example, myosin (over 40 family members in mammals), small GTPases (over 100 family members in mammals) and matrix metalloproteinases (over 25 family members in mammals).
For the gene families encoding PCP signaling proteins, the evolutionary pressures and relationships are rendered more complex by the additional involvement of some members of these protein families in canonical Wnt signaling and/or Wnt/calcium signaling. For example, Drosophila Fz, one of four Drosophila Frizzled proteins, functions in both canonical and PCP signaling (Strutt et al., 2012), as do Drosophila Dsh and mammalian Dvl proteins (Gao and Chen, 2010; Wynshaw-Boris, 2012). In mammals, the extent to which each of the ten Fz proteins participates in more than one signaling pathway is not clear, although current in vivo data are consistent with a division of labor in which some Fz proteins, such as Fz4, signal predominantly or exclusively via the canonical Wnt pathway, and others, such as Fz3 and Fz6, signal predominantly or exclusively via the PCP pathway (Wang and Nathans, 2007; Xu et al., 2004). One evolutionary force for PCP gene diversification is likely to act at the level of gene expression, as the requirements for spatially and temporally distinctive and cell type-specific patterns of expression may exert selective pressures that can only be satisfied by evolving multiple family members with distinct promoter and enhancer sequences. The success of our genetic rescue experiments based on ubiquitous production of Fz3 and Fz6 serves as a reminder that we are still largely ignorant of the role that spatiotemporal control of gene expression plays in polarity signaling.
MATERIALS AND METHODS
Mouse lines
The Z/3xHA-Fz3, Z/3xHA-Fz6 and R26-3xHA-Fz6 alleles were generated by homologous recombination in mouse embryonic stem (ES) cells using standard techniques. Targeting constructs (Fig. 1C) were electroporated into R1 mouse ES cells. Colonies were grown in medium containing G418 and ganclovir, and were screened by karyotyping and Southern blot hybridization. Positive clones were injected into C57BL/6 blastocysts to generate chimeric founders, and germline transmission was confirmed by Southern blot hybridization and PCR.
The following mouse alleles were also used: Fz1− and Fz2− (Yu et al., 2010), Fz3− (Wang et al., 2002), Fz3CKO (Hua et al., 2013), Fz6− (Guo et al., 2004), Hprt-LSL-tdTomato (Wu et al., 2014), K14-Cre (Dassule et al., 2000), K17-GFP (Bianchi et al., 2005), Olig2Cre (Dessaud et al., 2007) and Sox2-Cre (Hayashi et al., 2002). Mice were handled and housed according to the approved Institutional Animal Care and Use Committee (IACUC) protocol MO13M469 of the Johns Hopkins Medical Institutions.
Reagents and immunohistochemistry
The following primary antibodies were used for immunohistochemistry or western blotting: mouse monoclonal anti-neurofilament (165 kDa; 2H3, Developmental Studies Hybridoma Bank; 1:1000), rabbit anti-K17 (a gift from Dr Pierre Coulombe, Johns Hopkins Univeristy, Baltimore, MD, USA; 1:1000), rat mAb anti-cytokeratin8 (CK8; TROMA-1; Developmental Studies Hybridoma Bank; 1:500), rabbit anti-Fz3 and anti-Fz6 antibodies (Wang et al., 2006b; 1:1000-1:5000), and rabbit anti-3×HA (T. Rotolo and J.N., unpublished; 1:10,000). Secondary antibodies were Alexa Fluor 488 or 594 conjugated (Invitrogen; 1:500). AM4-65 was from Biotium (#70039; 20 µg per mouse).
Immunostaining was performed on: (1) vibratome sections from embryos that were immersion fixed in 4% (w/v) paraformaldehyde (PFA) dissolved in phosphate-buffered saline (PBS) (pH 7.4) at 4°C overnight, washed three times in ice-cold PBS, embedded in 3% (w/v, dissolved in PBS) low melting point agarose and sectioned at 120 μm on a vibratome; and (2) whole-mount embryos and limbs that were immersion fixed in 4% PFA at 4°C for 2 h and washed three times in ice-cold PBS.
For immunostaining of 120 μm vibratome sections, sections were blocked in PBST (PBS with 0.3% Triton X-100) containing 5% normal goat serum (NGS) at room temperature for 1 h, and incubated with primary antibody in PBST containing 5% NGS at 4°C overnight. Sections were then washed five times in PBST and incubated with secondary antibody in PBST containing 5% NGS at 4°C overnight. Finally, sections were washed five times in PBST and mounted on slides with Fluoromount-G (Southern Biotech).
For immunostaining of whole-mount embryos and limbs, samples were first incubated in Dent's Bleach [10% H2O2, 13.3% dimethyl sulfoxide (DMSO), 53.3% methanol] at 4°C for 24 h, washed in methanol five times, and fixed in Dent's Fix (20% DMSO, 80% methanol) at 4°C overnight. Samples were washed in PBS three times, incubated with primary antibody in blocking solution (20% DMSO, 75% PBST, 5% NGS, 0.025% sodium azide) at room temperature for 5 days to 1 week with gentle end-over-end rotation, and then washed five times in PBST. Samples were incubated with secondary antibody in blocking solution at room temperature for 2 days with gentle end-over-end rotation and then washed five times in PBST. Finally samples were dehydrated in 50% methanol/PBS and methanol, and cleared in benzyl benzoate: benzyl alcohol (BBBA) as described previously (Hua et al., 2013).
Skin whole mounts
The procedures for preparation and processing of skin whole mounts for Merkel cells immunostaining and imaging of hair follicles based on melanin content are described by Chang and Nathans (2013) and Chang et al. (2014).
Tongue dorsal surface whole mounts
To visualize the geometry of the lingual papillae, mice were injected intraperitoneally with ∼10 μg AM4-65 dye 12-24 h prior to sacrifice, and the tissues were fixed either by perfusion (older mice) or immersion (embryos and early postnatal mice) in PFA/PBS. For mice younger than P20, epithelial GFP fluorescence from the K17-GFP transgene also helps to visualize the papillae. Tongues from mice between E18 and 9 months of age were processed for flat-mount imaging by cutting off the ventral half of the tongue and then trimming away most of the remaining connective tissue and muscle from the inner surface of the dorsal tongue. The final flat-mount preparation was 0.5-1 mm thick. As the tongue surface is gently curved, the pattern of papillae was most clearly visualized with a projection of the z-stack image.
Immunoblots
Skins and brains [E18.5 and postnatal day (P)1] were homogenized with a Polytron in 1.5 ml of ice-cold lysis buffer [50 mM Tris-HCl (pH 7.4), 50 mM NaCl and 2% Triton X-100] supplemented with protease inhibitors (Roche, complete mini cocktail tablets). The tissue homogenates were further incubated at 4°C for 1 h, followed by centrifugation at 10,000 g for 5 min at 4°C. The samples were resolved by SDS-PAGE on a 10% gel. Immunoblots were incubated at 4°C overnight in the following primary antibodies: affinity-purified rabbit anti-Fz3 and anti-Fz6 (Wang et al., 2006b); rabbit anti-3×HA (T. Rotolo and J.N., unpublished); or mouse anti-actin (Chemicon, MAB1501). The blots were incubated for 2 h at room temperature in HRP-conjugated secondary antibodies (Bio-Rad), and the immunoreactive bands were visualized with the SuperSignal West Pico Substrate (Pierce).
Microscopy and image analysis
Immunostained samples were imaged using a Zeiss LSM700 confocal microscope with Zen software. Images of whole-mount samples were acquired with a 10× air objective at 10 μm intervals in the z dimension, and the entire z stack was either collapsed using a maximum intensity projection or color-coded based on depth. BBBA-cleared embryos were positioned in custom-built metal embryo holders consisting of a shallow triangular trough (sides, 2 cm×2 cm×1 cm; depths, 1, 2, 3, or 4 mm). The trough was filled with BBBA and coverslipped during imaging.
Sequence alignments
Amino acid sequences were aligned with ClustalW (Vector NTI software). The dendrogram branch lengths are proportional to percent amino acid non-identity.
Supplementary Material
Acknowledgements
This work was supported by the Howard Hughes Medical Institute. The authors thank John Williams for assistance with genotyping, Terry Stromsky for assistance with artwork, and Alisa Mo and Amir Rattner for helpful comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
Z.L.H., H.C., Y.W. and J.N. designed experiments; P.M.S. constructed knock-in mice; Z.L.H., H.C. and Y.W. conducted experiments; Z.L.H., H.C., Y.W. and J.N. analyzed data and wrote the paper.
Funding
This work was supported by the Howard Hughes Medical Institute. Deposited in PMC for release after 6 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.110189/-/DC1
References
- Adler, P. N. (2002). Planar signaling and morphogenesis in Drosophila. Dev. Cell 2, 525-535 10.1016/S1534-5807(02)00176-4 [DOI] [PubMed] [Google Scholar]
- Beronja, S., Livshits, G., Williams, S. and Fuchs, E. (2010). Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nat. Med. 16, 821-827 10.1038/nm.2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi, N., Depianto, D., McGowan, K., Gu, C. and Coulombe, P. A. (2005). Exploiting the keratin 17 gene promoter to visualize live cells in epithelial appendages of mice. Mol. Cell. Biol. 25, 7249-7259 10.1128/MCB.25.16.7249-7259.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H. and Nathans, J. (2013). Responses of hair follicle-associated structures to loss of planar cell polarity signaling. Proc. Natl. Acad. Sci. USA 110, E908-E917 10.1073/pnas.1301430110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H., Wang, Y., Wu, H. and Nathans, J. (2014). Flat mount imaging of mouse skin and its application to the analysis of hair follicle patterning and sensory axon morphology. J. Vis. Exp. e51749 10.3791/51749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman, R., Agalliu, D., Zhou, L., Kuhnert, F., Kuo, C. J. and Barres, B. A. (2009). Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 106, 641-646 10.1073/pnas.0805165106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud, E., Yang, L. L., Hill, K., Cox, B., Ulloa, F., Ribeiro, A., Mynett, A., Novitch, B. G. and Briscoe, J. (2007). Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450, 717-720 10.1038/nature06347 [DOI] [PubMed] [Google Scholar]
- Dassule, H. R., Lewis, P., Bei, M., Maas, R. and McMahon, A. P. (2000). Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127, 4775-4785 [DOI] [PubMed] [Google Scholar]
- Devenport, D. and Fuchs, E. (2008). Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat. Cell Biol. 10, 1257-1268 10.1038/ncb1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, C. and Chen, Y.-G. (2010). Dishevelled: the hub of Wnt signaling. Cell. Signal. 22, 717-727 10.1016/j.cellsig.2009.11.021 [DOI] [PubMed] [Google Scholar]
- Goodrich, L. V. and Strutt, D. (2011). Principles of planar polarity in animal development. Development 138, 1877-1892 10.1242/dev.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb, D. and Garcia-Bellido, A. (1982). A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 68, 37-57. [PubMed] [Google Scholar]
- Guo, N., Hawkins, C. and Nathans, J. (2004). Frizzled6 controls hair patterning in mice. Proc. Natl. Acad. Sci. USA 101, 9277-9281 10.1073/pnas.0402802101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, S., Lewis, P., Pevny, L. and McMahon, A. P. (2002). Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr. Patterns 2, 93-97 10.1016/S0925-4773(02)00292-7 [DOI] [PubMed] [Google Scholar]
- Hua, Z. L., Smallwood, P. M. and Nathans, J. (2013). Frizzled3 controls axonal development in distinct populations of cranial and spinal motor neurons. Elife 2, e01482 10.7554/eLife.01482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarcaya Najarro, E. and Ackley, B. D. (2013). C. elegans fmi-1/flamingo and Wnt pathway components interact genetically to control the anteroposterior neurite growth of the VD GABAergic neurons. Dev. Biol. 377, 224-235 10.1016/j.ydbio.2013.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume, W. J. and Potten, C. S. (1976). The ordered columnar structure of mouse filiform papillae. J. Cell Sci. 22, 149-160. [DOI] [PubMed] [Google Scholar]
- Iwasaki, S., Yoshizawa, H. and Kawahara, I. (1996). Study by scanning electron microscopy of the morphogenesis of three types of lingual papilla in the mouse. Acta Anat. (Basel) 157, 41-52 10.1159/000147865 [DOI] [PubMed] [Google Scholar]
- Jenny, A. (2010). Planar cell polarity signaling in the Drosophila eye. Curr. Top. Dev. Biol. 93, 189-227 10.1016/B978-0-12-385044-7.00007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, R. C., Clandinin, T. R., Lee, C.-H., Chen, P.-L., Meinertzhagen, I. A. and Zipursky, S. L. (2003). The protocadherin Flamingo is required for axon target selection in the Drosophila visual system. Nat. Neurosci. 6, 557-563 10.1038/nn1063 [DOI] [PubMed] [Google Scholar]
- Lobe, C. G., Koop, K. E., Kreppner, W., Lomeli, H., Gertsenstein, M. and Nagy, A. (1999). Z/AP, a double reporter for cre-mediated recombination. Dev. Biol. 208, 281-292 10.1006/dbio.1999.9209 [DOI] [PubMed] [Google Scholar]
- Lyuksyutova, A. I., Lu, C.-C., Milanesio, N., King, L. A., Guo, N., Wang, Y., Nathans, J., Tessier-Lavigne, M. and Zou, Y. (2003). Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302, 1984-1988 10.1126/science.1089610 [DOI] [PubMed] [Google Scholar]
- Matsubara, D., Horiuchi, S.-Y., Shimono, K., Usui, T. and Uemura, T. (2011). The seven-pass transmembrane cadherin Flamingo controls dendritic self-avoidance via its binding to a LIM domain protein, Espinas, in Drosophila sensory neurons. Genes Dev. 25, 1982-1996 10.1101/gad.16531611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Soriano, V., Belacortu, Y. and Paricio, N. (2012). Planar cell polarity signaling in collective cell movements during morphogenesis and disease. Curr. Genomics 13, 609-622 10.2174/138920212803759721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, J. (2012). Wnt/PCP proteins regulate stereotyped axon branch extension in Drosophila. Development 139, 165-177 10.1242/dev.068668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y. and Axelrod, J. D. (2012). Asymmetric protein localization in planar cell polarity: mechanisms, puzzles, and challenges. Curr. Top. Dev. Biol. 101, 33-53 10.1016/B978-0-12-394592-1.00002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravni, A., Qu, Y., Goffinet, A. M. and Tissir, F. (2009). Planar cell polarity cadherin Celsr1 regulates skin hair patterning in the mouse. J. Invest. Dermatol. 129, 2507-2509 10.1038/jid.2009.84 [DOI] [PubMed] [Google Scholar]
- Rotolo, T., Smallwood, P. M., Williams, J. and Nathans, J. (2008). Genetically-directed, cell type-specific sparse labeling for the analysis of neuronal morphology. PLoS ONE 3, e4099 10.1371/journal.pone.0004099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senti, K.-A., Usui, T., Boucke, K., Greber, U., Uemura, T. and Dickson, B. J. (2003). Flamingo regulates R8 axon-axon and axon-target interactions in the Drosophila visual system. Curr. Biol. 13, 828-832 10.1016/S0960-9822(03)00291-4 [DOI] [PubMed] [Google Scholar]
- Simons, M. and Mlodzik, M. (2008). Planar cell polarity signaling: from fly development to human disease. Annu. Rev. Genet. 42, 517-540 10.1146/annurev.genet.42.110807.091432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinel, M. C. and Whitington, P. M. (2009). The atypical cadherin Flamingo is required for sensory axon advance beyond intermediate target cells. Dev. Biol. 327, 447-457 10.1016/j.ydbio.2008.12.026 [DOI] [PubMed] [Google Scholar]
- Stenman, J. M., Rajagopal, J., Carroll, T. J., Ishibashi, M., McMahon, J. and McMahon, A. P. (2008). Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247-1250 10.1126/science.1164594 [DOI] [PubMed] [Google Scholar]
- Struhl, G., Casal, J. and Lawrence, P. A. (2012). Dissecting the molecular bridges that mediate the function of Frizzled in planar cell polarity. Development 139, 3665-3674 10.1242/dev.083550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt, D., Madder, D., Chaudhary, V. and Artymiuk, P. J. (2012). Structure-function dissection of the frizzled receptor in Drosophila melanogaster suggests different mechanisms of action in planar polarity and canonical Wnt signaling. Genetics 192, 1295-1313 10.1534/genetics.112.144592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada, M. and Heisenberg, C.-P. (2012). Convergent extension: using collective cell migration and cell intercalation to shape embryos. Development 139, 3897-3904 10.1242/dev.073007 [DOI] [PubMed] [Google Scholar]
- Tissir, F. and Goffinet, A. M. (2006). Expression of planar cell polarity genes during development of the mouse CNS. Eur. J. Neurosci. 23, 597-607 10.1111/j.1460-9568.2006.04596.x [DOI] [PubMed] [Google Scholar]
- Tissir, F. and Goffinet, A. M. (2013). Atypical cadherins Celsr1-3 and planar cell polarity in vertebrates. Prog. Mol. Biol. Transl. Sci. 116, 193-214 10.1016/B978-0-12-394311-8.00009-1 [DOI] [PubMed] [Google Scholar]
- Tissir, F., Bar, I., Jossin, Y., De Backer, O. and Goffinet, A. M. (2005). Protocadherin Celsr3 is crucial in axonal tract development. Nat. Neurosci. 8, 451-457. [DOI] [PubMed] [Google Scholar]
- Wang, Y. and Nathans, J. (2007). Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 134, 647-658 10.1242/dev.02772 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Thekdi, N., Smallwood, P. M., Macke, J. P. and Nathans, J. (2002). Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J. Neurosci. 22, 8563-8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Badea, T. and Nathans, J. (2006a). Order from disorder: self-organization in mammalian hair patterning. Proc. Natl. Acad. Sci. USA 103, 19800-19805 10.1073/pnas.0609712104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Guo, N. and Nathans, J. (2006b). The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 26, 2147-2156 10.1523/JNEUROSCI.4698-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Zhang, J., Mori, S. and Nathans, J. (2006c). Axonal growth and guidance defects in Frizzled3 knock-out mice: a comparison of diffusion tensor magnetic resonance imaging, neurofilament staining, and genetically directed cell labeling. J. Neurosci. 26, 355-364 10.1523/JNEUROSCI.3221-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Chang, H. and Nathans, J. (2010). When whorls collide: the development of hair patterns in frizzled 6 mutant mice. Development 137, 4091-4099 10.1242/dev.057455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H., Luo, J., Yu, H., Rattner, A., Mo, A., Wang, Y., Smallwood, P. M., Erlanger, B., Wheelan, S. J. and Nathans, J. (2014). Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease. Neuron 81, 103-119 10.1016/j.neuron.2013.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynshaw-Boris, A. (2012). Dishevelled: in vivo roles of a multifunctional gene family during development. Curr. Top. Dev. Biol. 101, 213-235 10.1016/B978-0-12-394592-1.00007-7 [DOI] [PubMed] [Google Scholar]
- Xu, Q., Wang, Y., Dabdoub, A., Smallwood, P. M., Williams, J., Woods, C., Kelley, M. W., Jiang, L., Tasman, W., Zhang, K.et al. (2004). Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883-895 10.1016/S0092-8674(04)00216-8 [DOI] [PubMed] [Google Scholar]
- Yu, H., Smallwood, P. M., Wang, Y., Vidaltamayo, R., Reed, R. and Nathans, J. (2010). Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development 137, 3707-3717 10.1242/dev.052001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L., Bar, I., Achouri, Y., Campbell, K., De Backer, O., Hebert, J. M., Jones, K., Kessaris, N., de Rouvroit, C. L., O'Leary, D.et al. (2008). Early forebrain wiring: genetic dissection using conditional Celsr3 mutant mice. Science 320, 946-949 10.1126/science.1155244 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.