Abstract
Purpose.
We investigated endoglin expression in hypoxic microvascular endothelial cells and retinal endoglin expression in rats that develop experimental oxygen-induced retinopathy (OIR). We also tested neutralizing antibodies (Abs) against endoglin (anti-CD105 Ab) and VEGF (anti-VEGF Ab) either alone or in combination for efficacy against serum-induced retinal microvascular endothelial cell proliferation and retinal neovascularization (NV) in OIR rats. To our knowledge, this marks the first time that a biologic agent has been used to target retinal endoglin and modulate retinal neovascularization.
Methods.
Induction of endoglin by hypoxia was measured by immunohistochemical analysis and ELISA. Proliferation was quantified using a colorimetric 5-bromo-2-deoxyuridine ELISA. Western blots were used to measure endoglin levels in retinas of OIR rats. Immunohistochemical staining was also preformed in OIR rats using anti-CD105 and fluorescein isothiocyanate-conjugated isolectin B4 antibodies.
Results.
Anti-CD105 Ab and Anti-VEGF Ab, administered alone or in combination, reduced serum-induced retinal microvascular endothelial cell proliferation. Additionally, in a rat model of oxygen-induced retinopathy, retinal endoglin was significantly increased at 14(2), 14(3), 14(4) and 14(6) compared with retinal levels in control rats. At 14(2), immunohistochemical analysis demonstrated that endoglin was elevated in newly developed vessels at the peripheral extent of major veins, precisely where NV is expected to develop in OIR rats. Neutralizing anti-CD105 reduced retinal NV in OIR rats.
Conclusions.
Our data support other studies showing that reduction of endoglin expression inhibits retinal NV. Our findings demonstrate that retinal endoglin immunolocalization overlaps with nascent neovascular structures in OIR rats. Therefore, endoglin may serve as a useful predictor of incipient neovascular disease.
Keywords: angiogenesis, early diagnostic biomarker, retinal neovascularization
This manuscript investigated the role of endoglin expression in both cell and animal models of retinal neovascularization. To our knowledge, this marks the first time that a biologic agent has been used to target retinal endoglin and modulate retinal neovascularization.
Introduction
Angiogenesis, the formation of new capillaries from existing blood vessels, occurs in physiologic processes such as reproduction, growth and development, and wound healing.1–6 However, in diseases such as arthritis, tumor growth and retinopathies, a dysregulated and persistent pathologic angiogenesis often referred to as neovascularization (NV) may develop.6–8 A number of blinding conditions, including diabetic retinopathy (DR), retinopathy of prematurity (ROP) and retinal vaso-occlusive disorders all exhibit retinal NV as a critical pathologic feature.9
Evidence strongly suggests that the onset and progression of retinal NV is primarily induced by VEGF.10–12 Accordingly, pharmacologic therapies currently used in the clinic target VEGF.13,14 Widely used bevacizumab (Avastin; Genentech, San Francisco, CA, USA) and ranibizumab (Lucentis; Genentech) are humanized monoclonal antibodies with high affinities for VEGF-A. Recently, aflibercept (Eylea; Regeneron Pharmaceuticals, Tarrytown, NY, USA)—a recombinant fusion protein made from a combination of VEGF receptors 1 and 2—has also been approved for ocular use.15 Although these therapies have proven efficacious, there may be drawbacks related to their chronic use.16,17 For instance, it has been shown that in younger patients, anti-VEGF therapies have the potential to leak into the circulation and potentially damage vascular systems.18 Reports concerning the application of anti-VEGF therapies to the treatment of human ROP have revealed persistent avascular retina, increased preretinal neovascularization, and retinal detachment.19,20 Additionally, Hartnett et al.19 have recently reported that intravitreal injection of a neutralizing antibody against VEGF (anti-VEGF Ab) in a rat model of oxygen-induced retinopathy (OIR, a model of human ROP) led to increased preretinal NV and peripheral retinal avascularity at late time points. There is also evidence showing that VEGF plays an important survival role for retinal neurons.21,22 Lastly, studies using VEGF-targeted therapies for choroidal NV show that as many as 45% of the patients tested showed no improvement in visual outcome.23,24 Considering these findings, continued investigation of other therapies against ocular NV is warranted.
Endoglin (CD105) is a transmembrane glycoprotein that is an accessory to the transforming growth factor β (TGFβ) receptor system.25 Endoglin is likely to be involved in endothelial cell (EC) differentiation, migration, and proliferation.26,27 Endoglin is also a marker of mesenchymal stem cells and it is expressed in progenitor cells involved in vascular remodeling in animal models of myocardial infarction and rheumatic diseases.28,29 Moreover, shRNA-mediated endoglin knockdown inhibits VEGF-induced angiogenic human umbilical vein endothelial cells responses and endoglin haploinsufficiency attenuates retinal NV in a mouse OIR model.30,31 Monoclonal antibodies (mAbs) against endoglin have been tested in vitro and in vivo in angiogenic assays, and the results suggest that endoglin has a proangiogenic function. For example, TEC-11 (mAb) inhibits the proliferation of microvascular and macrovascular endothelial cells and a transformed endothelial cell line. Furthermore, systemic administration of SN6j (mAb), showed significant efficacy against the growth of vessels in human tumors grafted into mice.32,33 Finally, TRC105 (mAb) is currently being tested in combination with VEGF inhibitors to treat several solid tumor types.34
In the present studies, we investigated endoglin induction in hypoxic microvascular endothelial cells and retinal endoglin expression in rats that develop experimental OIR. We also tested neutralizing antibodies against endoglin (anti-CD105 Ab) and VEGF (anti-VEGF Ab) either alone or in combination for efficacy against serum-induced retinal microvascular endothelial cell (RMEC) proliferation and retinal NV in OIR rats. To our knowledge, this marks the first time that a biologic agent has been used to target retinal endoglin and modulate retinal neovascularization.
Materials and Methods
Cell Treatment
Primary cultures of rat or human RMECs (Cell Systems; Kirkland, WA, USA) were seeded into tissue culture flasks coated with attachment factor (Cell Signaling; Danvers, MA, USA). We cultured RMECs in phenol red–free endothelial basal medium (Lonza Group; Walkersville, MD, USA) supplemented with 10% FBS, 1× antibiotic/antimycotic solution, and endothelial cell growth supplements (Lonza Group). When experimental conditions required serum-free (SF) medium, endothelial basal medium with no FBS or growth supplements was used. All cultures were incubated at 37°C, 5% CO2, and 95% relative humidity (20.9% oxygen). Passages six to eight were used for these experiments.
In Vitro Hypoxia Treatment
Rat or human RMECs were grown to 70% confluence in conditions stated previously and were placed in a hypoxia (0.7% O2 level) chamber for 24 hours (using a BBL GasPak system; Becton, Dickinson and Company; Sparks, MD, USA). Some cultures were lysed using a protein prep kit (Qproteome Mammalian Protein Prep; Qiagen; Venlo, The Netherlands) for endoglin ELISAs, (R&D Systems, Inc., Minneapolis, MN, USA) while others were prepared for immunocytochemistry. For immunocytochemistry experiments, cells were grown on chamber slides (Nunc Lab-Tek; Thermo Fisher Scientific, Inc., Nashville, TN, USA) fixed with 4% paraformaldehyde, washed in phosphate buffered saline with Tween 20 (PBST), and blocked overnight in 1.5% bovine serum albumin in PBST. Cells were then probed with anti-CD105 antibody (Millipore Corp., Billerica, MA, USA). These experiments were replicated four times, with n = 4 for each treatment group in each replicate. For ELISA experiments, a human endoglin/CD105 quantikine kit was used following manufacturer's instructions.
Proliferation Assay
Rat RMECs were isolated and seeded at 3 × 103 cells/well in a 96-well plate containing growth medium for 8 hours to allow them to settle and attach. Cells were then serum-starved for 12 hours before being treated with either serum free medium, 10% serum, rat anti-VEGF (100 μg/mL; R&D Systems, Inc.) and/or anti-CD105 (Millipore Corp.) at concentrations ranging from 1 to 100 μg/mL (Sigma Aldrich, St. Louis, MO, USA). After 24 hours of treatment, cells were labeled with 5-bromo-2-deoxyuridine (BrdU) labeling solution for an additional 12 hours, and BrdU incorporation was quantified using a colorimetric BrdU ELISA (Roche Diagnostics Corp., Indianapolis, IN, USA), according to the manufacturer's instructions. The experiment was independently repeated three times.
Western Blot Analysis
For Western blot analysis, the retinas of three eyes were pooled in 300 μL cold lysis buffer (150 mM NaCl, 1.0% Triton X-100, 0.1% SDS, 50 mM Tris-HCl, 100 μg/mL phenylmethylsulfonyl fluoride, 10 mM orthovanadate, 0.3 μg/mL EDTA, 0.5% deoxycholate acid, 50 μM NaF, 0.5 μg/mL leupeptin, 0.7 μg/mL pepstatin A, and 1.0 mg/mL aprotinin) and homogenized by sonication at 4°C. The samples were incubated at 4°C for 30 minutes and then centrifuged at 5000 rpm for 15 minutes at 4°C. Protein concentrations of the supernatants were determined with the BCA kit (Pierce Biotechnology, Rockford, IL, USA). The protein concentration of each sample was adjusted to 2.5 μg/μL with cold lysis buffer containing protease inhibitors. Twenty microliters (50 μg) was mixed with 20 μL of 2× Laemmli buffer (Sigma Aldrich) and heated at 95°C for 10 minutes. The samples were resolved by SDS-PAGE and were transferred to 0.2 μm nitrocellulose membranes (Bio-Rad Laboratories, Inc.; Hercules, CA, USA). Nitrocellulose membranes were blocked with tris-buffered saline and Tween 20, 1% bovine serum albumin (Sigma Aldrich), and were probed with primary antibodies. Either goat anti-mouse IgG horseradish peroxidase (HRP; Chemicon International, Inc., Temecula, CA, USA), goat anti-rabbit IgG-HRP (Chemicon International, Inc.), or donkey anti-goat IgG-HRP (Santa Cruz Biotechnology, Dallas, TX, USA) secondary antibodies were applied to the membranes and were developed with enhanced chemiluminescence (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). The following primary antibodies were used in this study: anti-CD105 (NeoMarkers, Inc.; Fremont, CA, USA) and anti-β-actin (Sigma Aldrich). Each Western blot was repeated at least three times.
Vascular and Immunohistochemical Staining
At 14(2), 14(3), and 14(6), eyes of the rats were enucleated, retinal flat-mounts were prepared, and vasculature was stained with FITC-conjugated isolectin B4, (Sigma Aldrich), and anti-CD105 (Millipore Corp.). The tissue was then preserved with gel mount (Biomedia; Victoria, Australia).
Rat Oxygen Treatment
All animal experiments were approved by the Vanderbilt University School of Medicine Institutional Animal Care and Use Committee, and they were conducted according to the principles expressed in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Within 8 hours after birth, litters of Sprague-Dawley rat pups and their mothers were exposed to alternating periods of 24 hours at 50% and 10% oxygen for 14 days. This variable oxygen treatment protocol predisposes the rat pups to oxygen-induced retinopathy.35 Hereafter, these rats are referred to as OIR rats. Age-matched control rats were maintained in ambient (20.9% oxygen) normoxia. These rats are referred to as room air (RA) rats. After the variable oxygen treatment, OIR rats were removed to room air for up to 6 days, allowing time for retinal NV to develop. We refer to the timing of sacrifice and assessment with two numbers: one representing the time in variable oxygen and one representing the postexposure period. Hence, rats sacrificed immediately upon removal from exposure are termed 14(0), while rats sacrificed at the end of the 6-day postoxygen exposure period are referred to as 14(6).
Intravitreal Injections
Rats were anesthetized by isoflurane (Terrell, Meridian, ID, USA) inhalation, and a single drop of 0.5% proparacaine (Allergan, Hormigueros, PR, USA) was topically applied to the cornea prior to intravitreal injection. For all intravitreal injections, the globe was penetrated posterior to the ora ciliaris retinae using a 30-gauge needle with a 19° bevel and a 10-μL syringe (Hamilton Co., Reno, NV, USA). The needle was advanced to the posterior vitreous while maintaining a steep angle to avoid contact with the lens. The injection bolus was delivered near the trunk of the hyaloid artery, proximal to the posterior pole of the retina. Following injection, a topical antibiotic suspension (neomycin and polymyxin B sulfates and gramicidin; Monarch Pharmaceuticals, Inc.; Bristol, TN, USA) was applied. Noninjected eyes were also treated with topical proparacaine and antibiotic to control for the potential of these agents to influence retinal vessel growth.
Quantification of Retinopathy
Rats with OIR were euthanized by decapitation on 14(6). Rats were enucleated and the neural retinas were dissected and placed in 10% neutral buffered formalin (37% formaldehyde solution; Thermo Fisher Scientific; Fair Lawn, NJ, USA) overnight at 4°C. The retinal vasculature was stained for adenosine diphosphatase (ADPase) activity, according to a previously described method36 adapted for use in this experimental context.37,38 Images of ADPase-stained retinas were digitized, captured, and displayed at ×20 magnification. The total retinal area and the vascularized retinal area were traced and the number of pixels within these areas was converted to mm2. This parameter was used to measure effects of antibody treatment on normal, intraretinal vascular development. To determine the effect of the various treatments on pathological vascular growth, the extent of retinal NV was assessed in flat-mounted retinas stained for ADPase activity. Abnormal, preretinal NV was assessed by digitally measuring NV area in images captured and displayed at ×65 magnification by previously described methods.39,40
Drug Treatment
At 14(0), eyes from OIR and RA rats either remained uninjected, or were injected with 5 μL of vehicle (PBS), rat anti-VEGF (Sigma Aldrich) at 1.0 mg/mL, or anti-CD105 at doses ranging from 0.01 to 1.0 mg/mL. These doses were initially chosen based on published data for the use of anti-VEGF treatments.41,42
Statistical Analysis
Statistically significant differences between treatment and control groups were determined by analysis of variance combined with Dunnett's post hoc procedure. Each in vivo experiment was replicated three times with n = 8 to 12 eyes for each treatment group. In vitro experiments were also performed at least three times.
Results
Hypoxic Induction of Endoglin in Rat and Human RMEC
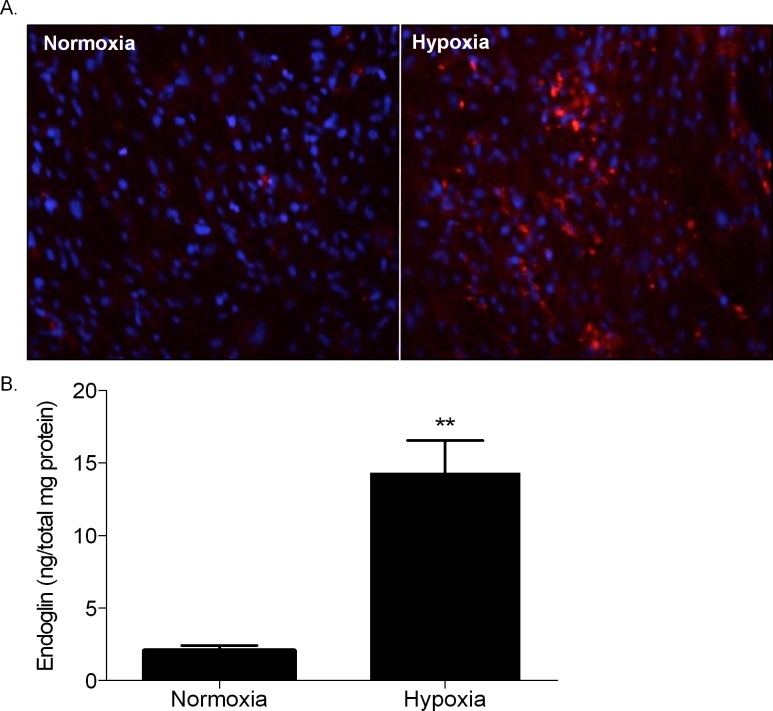
Twenty-four hour exposure of rat RMEC to hypoxia caused a significant increase in endoglin levels. Immunocytochemical staining of hypoxic and normoxic cells confirmed a striking difference in endoglin immunoreactivity within the compared cultures (Fig. 1A). Endoglin protein levels, measured by ELISA, were also elevated in similarly treated human RMEC (Fig. 1B).
Figure 1.
Endoglin induction by hypoxia. (A) Anti-CD105 immunocytochemistry in rat RMECs cultured in normoxia and hypoxia for 24 hours. Endoglin is shown in red, while DAPI-stained cell nuclei are shown in blue. (B) Endoglin (CD105) ELISA on human retinal microvascular endothelial cells exposed to normoxia and hypoxia for 24 hours. Endoglin levels are significantly upregulated in rat and human microvascular endothelial cells cultured in hypoxia versus normoxia. **P < 0.02, relative to normoxia).
Serum-Mediated Proliferation in Rat RMEC
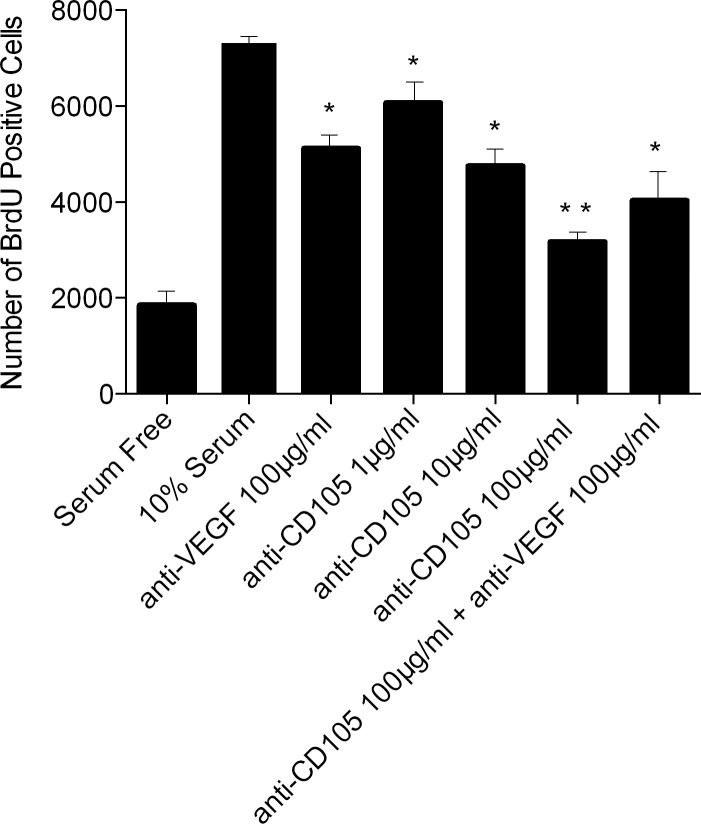
Anti-VEGF (100 μg/mL) treatment resulted in a decrease of serum-stimulated proliferation by 31% (P < 0.05). Anti-CD105 treatment reduced the proliferation in a dose-dependent manner. Doses of 1 μg/mL, 10 μg/mL, and 100 μg/mL reduced proliferation by 17% (P < 0.05); 34% (P < 0.05); and 57% (P < 0.02), respectively (Fig. 2). A combination of anti-VEGF and anti-CD105 (10 μg/mL) significantly reduced proliferation by 44% (P < 0.05). Although the effect of this combination treatment is significantly different from the effect of serum-only treatment, it is not significantly different from the effect of either the anti-VEGF or anti-CD105 (1–100 μg/mL) treatments alone.
Figure 2.
Analysis of anti-CD105 treatment on rat RMEC proliferation. All antibody treatments were performed on a 10% serum background. *P < 0.05. **P < 0.02, relative to 10% serum.
Assessment of Endoglin Levels and Localization in Rat OIR Tissue
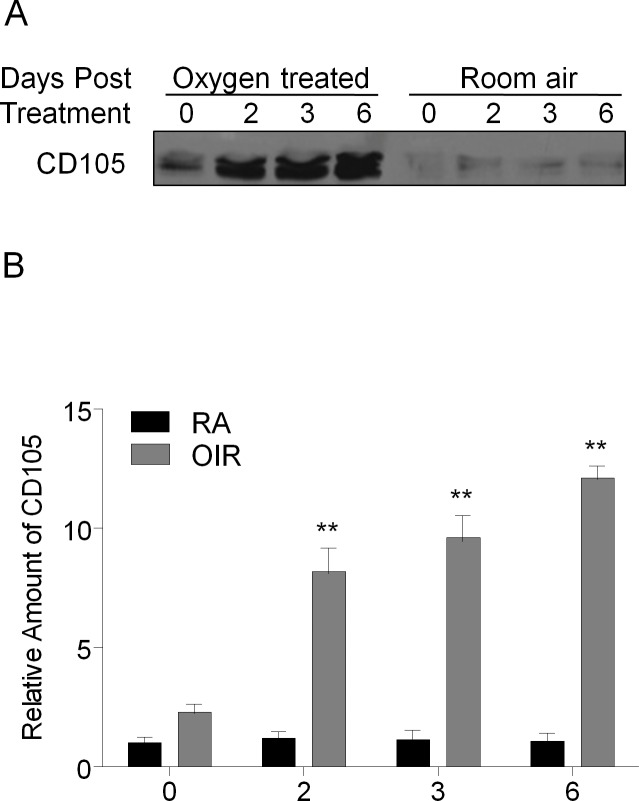
Immunoblotting demonstrated that endoglin levels were elevated during the postoxygen exposure period in OIR retinal tissue when compared with matched RA samples (Fig. 3A). Endoglin protein levels were significantly increased in OIR rat retinas on 14(2), 14(3), and 14(6) (P < 0.02). A representative Western blot and graph depicting the quantified endoglin band densities from four separate Western blot experiments are shown in Figure 3B.
Figure 3.
Oxygen-induced retinopathy increases endoglin protein levels. (A) Western blot showing endoglin levels in OIR and room air–raised rat retinas at several postexposure time points. Samples in every lane were matched for protein concentration and compared after being normalized to beta-actin. (B) Normalized and averaged results of the western blot band densities. Western blots were independently replicated three times. **P < 0.02.
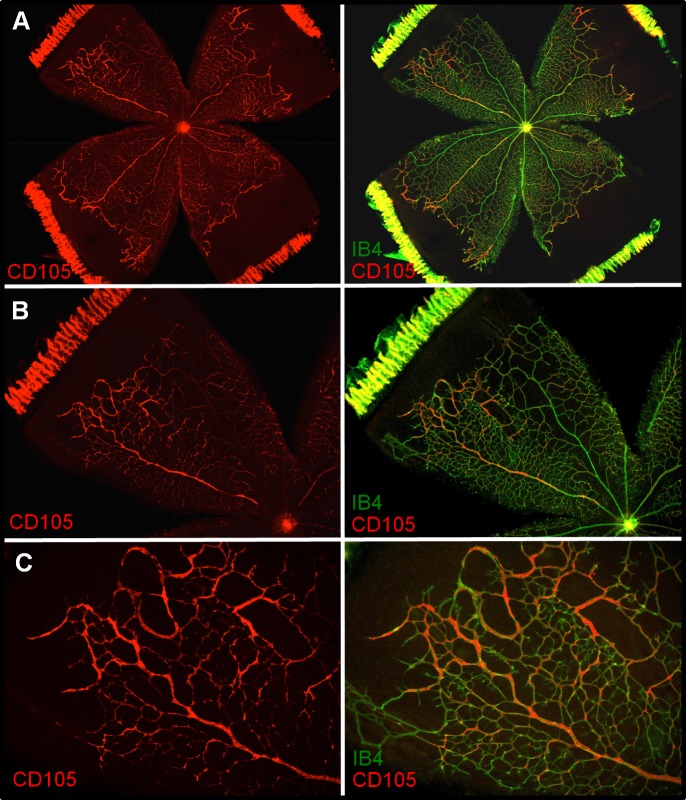
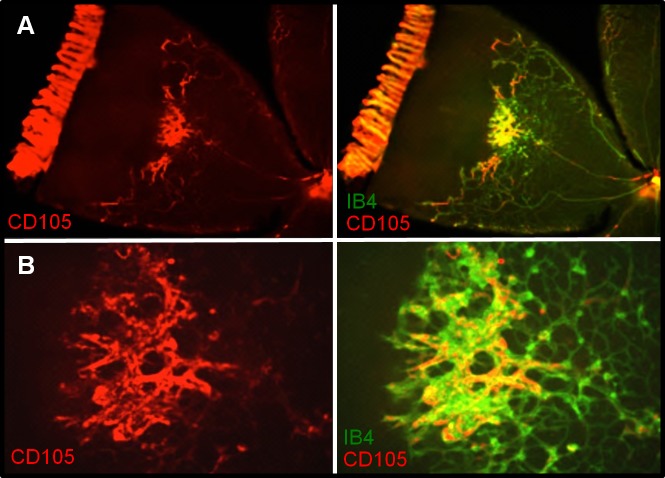
Retinas dissected from RA and OIR animals at time points 14(2), 14(3), and 14(6) were flatmounted; stained with anti-endoglin (anti-CD105); counterstained with isolectin B4 (IB4), a marker of vascular EC; and examined for endoglin localization. Room air samples demonstrated little or no endoglin immunoreactivity (Supplementary Fig. S1). Endoglin immunoreactivity at 14(2) exhibited specific association with vasculature at the leading edge of vessel growth and was primarily localized to the peripheral extent of veins—the future sites of NV (Fig. 4). Staining at 14(3) (Fig. 5) and 14(6) (Fig. 6) revealed precise colocalization with nascent and expanding NV tufts, respectively. Images are representative of four independent experiments.
Figure 4.
Endoglin (CD105) immunohistochemistry of 14(2) retinal flatmounts. Staining with CD105 shows association at the leading edge of vessel growth, but only near veins. Left: CD105 (red). Right: IB4 (green) and CD105/IB4 merged image. (A) ×2. (B) ×4. (C) ×10. Images shown are representative of four independent experiments.
Figure 5.
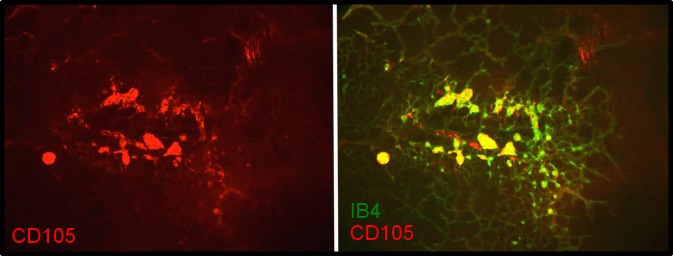
Endoglin (CD105) immunohistochemistry of 14(3) retinal flatmounts. Staining shows close association with the peripheral extent of veins and sites of early NV. Left: CD105. Right: IB4 and CD105/IB4 merged image, ×10. Images are representative of four independent experiments.
Figure 6.
Endoglin (CD105) immunohistochemistry of 14(6) retinal flat-mounts. Staining shows precise colocalization with NV tufts. Left: CD105. Right: IB4 and CD105/IB4 merged image. (A) ×4. (B) ×10. Images are representative of four independent experiments.
Treatment With Anti-Endoglin Inhibits Neovascularization in OIR Rats
Relative to vehicle, anti-VEGF treatment reduced neovascular area by 32% (P < 0.05). Anti-CD105 treatment at 0.01, 0.10, or 1.0 mg/mL reduced the neovascular area in a dose-dependent manner by 18%, 31% (P < 0.05), and 47% (P < 0.05), respectively. Combined treatment with anti-CD105 and anti-VEGF resulted in 62% inhibition of NV (P < 0.02), and this reduction in neovascular area was significantly different from the highest dose of anti-CD105 or anti-VEGF (P < 0.05) alone (Figs. 7A–F). Quantification of the data is showed in Figure 7G.
Figure 7.
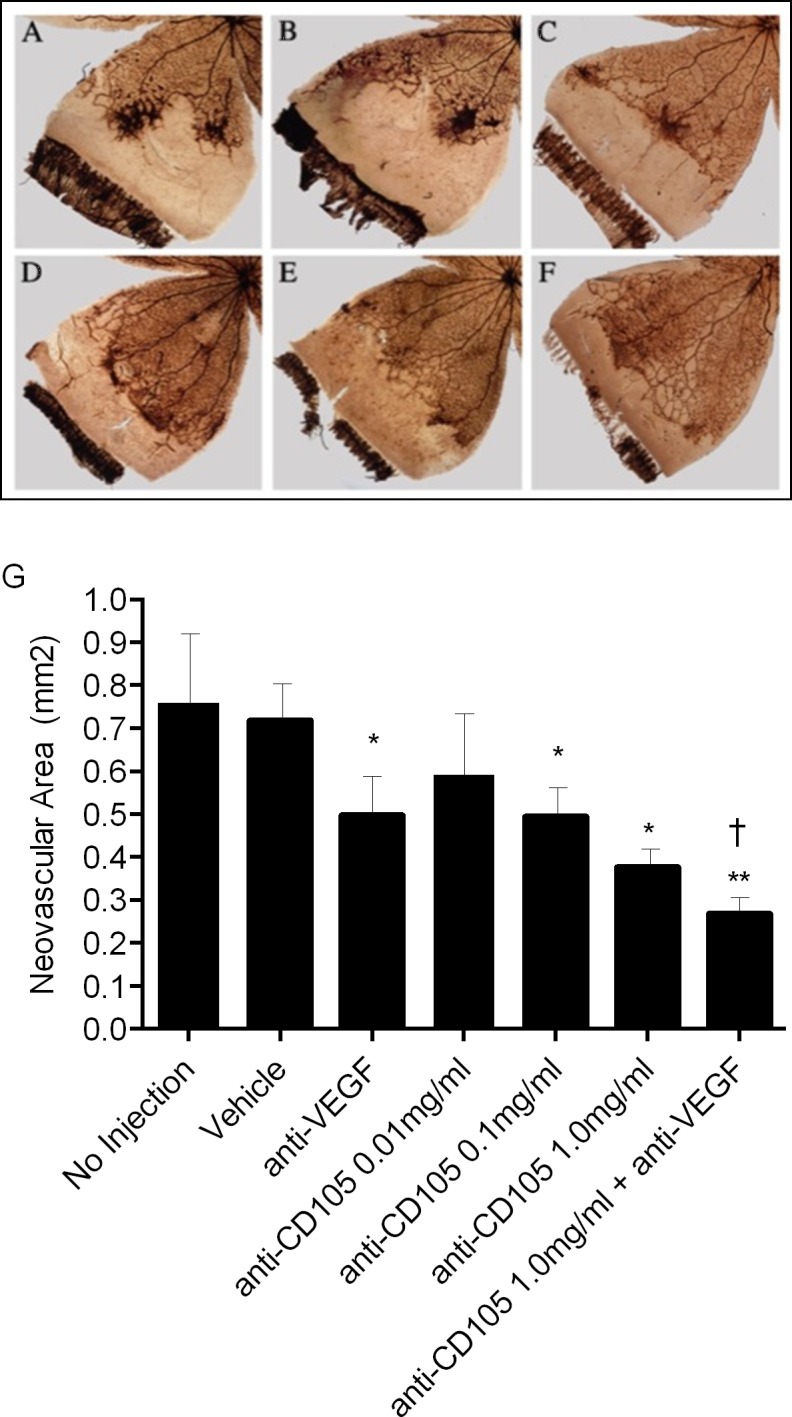
Comparison of representative retinal quadrants from eyes treated with anti-CD105. (A) No injection. (B) Vehicle. (C) Anti-VEGF, 1.0 mg/mL. (D) Anti-CD105, 0.1 mg/mL. (E) Anti-CD105, 1 mg/mL. (F) Anti-CD105 and anti-VEGF, 1.0 mg/mL. (G) The effect of anti-CD105 treatment on neovascular area in rat OIR. *P < 0.05. **P < 0.02, relative to vehicle. †P < 0.05, relative to anti-CD105 1.0 mg/mL.
Discussion
Our data support a role for endoglin in the promotion of preretinal NV like that associated with disease pathology in DR and ROP, among other conditions.29,43 In our initial in vitro experiments, endoglin immunoreactivity and protein levels were elevated in rat microvascular endothelial cells under hypoxic conditions compared to normoxic conditions. Endoglin levels were also increased in human retinal microvascular cells when placed in hypoxia. These findings were consistent with results we obtained from the rat OIR model, showing significantly increased endoglin, localized to the retinal endothelium, at postexposure times when the retina is incompletely vascularized and hypothetically hypoxic. Two early postexposure time points, 14(2) and 14(3), are significant in the rat OIR model, because this is the postexposure period when retinal VEGF is peaking and neovascular tufts are beginning to form.36 The immunohistochemical endoglin profiles in OIR rats at 14(2) and 14(3) revealed endoglin localization in the postcapillary venules near the peripheral extent of major veins, precisely matching the loci of incipient neovascular structures. Specifically, at the 14(2) time point, the elevated endoglin immunoreactivity appeared in advance of any identifiable neovascularization, while at 14(3) and 14(6) endoglin was closely colocalized with emerging and expanding neovascular lesions. The 14(6) time point is also significant because it is the peak of neovascular growth in this model. Since endoglin expression was elevated early and localized to future sites of neovascularization, we suggest that anti-endoglin staining may have promise as an early diagnostic biomarker with potential for in vivo imaging.44–47
In addition, proliferation assays were performed with anti-VEGF and anti-CD105 treatments on a 10% serum background in an effort to account for growth factors involved in neovascularization other than VEGF, while also being inclusive of VEGF. In a dose-dependent fashion, anti-CD105 treatment inhibited proliferation in RMECs under these conditions and anti-CD105 (100 μg/mL) was more effective at inhibiting proliferation than anti-VEGF at the same concentration. The combination treatment (anti-CD105 + anti-VEGF) significantly inhibited RMEC proliferation, at a level falling between those obtained using anti-CD105 at the highest concentration and anti-VEGF.
The analysis of neovascular area in OIR rats, revealed that endoglin blockade significantly inhibited neovascularization. This inhibition was dose-dependent, similar to our in vitro assays. Our findings also agree with other studies, in which mAbs against endoglin were administered systemically in experimental cancer models and showed reduced tumor vasculature.34,33,48–54 In OIR rats, administration of anti-VEGF by intravitreal injection resulted in a 32% reduction in preretinal NV (P < 0.05). Other studies testing neutralizing antibodies against VEGF in rat34 and mouse55 OIR have shown efficacies ranging from approximately 25% to 46%, respectively. The combined treatment of anti-VEGF and anti-CD105 also significantly inhibited neovascular tuft growth when compared with control conditions (P < 0.02). Moreover, this in vivo combination treatment (anti-CD105 + anti-VEGF) demonstrated greater efficacy than either antibody alone.
These in vivo data may appear to disagree with our in vitro data in which an additive effect of anti-CD105 and anti-VEGF on endothelial cell proliferation was not observed. However, proliferation is only one of several EC behaviors that are necessary for the genesis of neovascular structures; EC migration and differentiation are two others that may be significantly affected by endoglin signaling. Consistent with this notion, haploinsufficient-endoglin mice tested under OIR conditions showed that, even though retinal EC were hyperproliferative, a reduced neovascular response was observed.30 The haploinsufficient EC also demonstrated defective migration and capillary morphogenesis, two angiogenic cell behaviors that are crucial to neovascularization. Therefore, these data suggest that reduced cellular endoglin had a profound effect on angiogenic cell behaviors other than proliferation that affected the assemblage of neovascular structures.
We believe similar considerations may be applied to explain the differences between our in vitro proliferation assays and our in vivo efficacies against retinal neovascularization. Anti-CD105 therapy administered alone may prove useful in patients who are nonresponsive to anti-VEGF therapy or in those that experience adverse reactions.56 Conversely, smaller doses of each could be given in combination, potentially reducing unwanted side effects associated with either treatment. Future experiments will be performed to optimize and to determine the advantages/disadvantages of single versus combined therapies. Of particular interest will be whether anti-endoglin or combined anti-VEGF/anti-endoglin therapies will alleviate recurrent neovascularization. Administration of anti-VEGF to OIR rats has been associated with recurrent preretinal NV at later time points and this phenomenon has also been observed in human ROP.19 Therefore, future experiments testing the effects of anti-endoglin or combined anti-endoglin/anti-VEGF therapies, must address, and perhaps provide a potential resolution to recurrent preretinal NV.
Although hypoxia strongly induces endoglin and it is certainly a relevant stimulus in mouse OIR, we cannot rule out inflammation as a contributing, or even primary, stimulus for endoglin upregulation in our OIR experiments. It has been shown that inflammatory responses may play a key role in retinal angiogenesis. For example, in mouse OIR, retinal levels of the proinflammatory cytokine tumor necrosis factor α (TNFα), are increased and its genetic deletion protects against the pathologic neovascular response.57–61 To confirm endoglin induction by an inflammatory stimulus, we treated human retinal microvascular endothelial cells with 10 μg of the potent proinflammatory endotoxin, lipopolysaccharide, and observed increased endoglin levels compared with controls (data not shown). Additional studies will be required to address the specific role of inflammation and endoglin expression in both cell and animal models of angiogenesis.
In this study, we demonstrate that anti-CD105 therapy is efficacious against retinal NV. Based on the selective expression of CD105 in OIR retinas, we propose that endoglin has the potential to be used as a biomarker to identify areas of developing pathology.62 This therapy may allow for an earlier approach to address retinal NV, rather than treating at advanced stages of disease. Although endoglin shows promise in these regards, more studies are required before we determine if it is an ideal candidate to identify and target retinal sites in advance of or at very early stages of NV. These future studies must also address any potential side effects associated with anti-CD105 treatment.
Acknowledgements
Supported by National Institutes of Health Grants R01 EY07533 (JSP), P30 EY008126 (Core Grant in Vision Research), and F31 AG031036 (JSP/JMB); an Unrestricted Grant from Research to Prevent Blindness, Inc.; and a grant from the OneSight Foundation.
Disclosure: J.M. Barnett, None; S. Suarez, None; G.W. McCollum, None; J.S. Penn, None
References
- 1. Klagsbrun M. Regulators of angiogenesis: stimulators, inhibitors, and extracellular matrix. J Cell Biochem. 1991; 47: 199–200 [DOI] [PubMed] [Google Scholar]
- 2. Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992; 267: 10931–10934 [PubMed] [Google Scholar]
- 3. Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996; 87: 1153–1155 [DOI] [PubMed] [Google Scholar]
- 4. Risau W. Mechanisms of angiogenesis. Nature. 1997; 386: 671–674 [DOI] [PubMed] [Google Scholar]
- 5. Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003; 60: 107–114 [DOI] [PubMed] [Google Scholar]
- 6. Folkman J, Browder T, Palmblad J. Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost. 2001; 86: 23–33 [PubMed] [Google Scholar]
- 7. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003; 9: 653–660 [DOI] [PubMed] [Google Scholar]
- 8. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995; 1: 27–31 [DOI] [PubMed] [Google Scholar]
- 9. From the Centers for Disease Control and Prevention. Blindness caused by diabetes--Massachusetts, 1987-1994. JAMA. 1996; 276: 1865–1866 [PubMed] [Google Scholar]
- 10. Wells JA, Murthy R, Chibber R, et al. Levels of vascular endothelial growth factor are elevated in the vitreous of patients with subretinal neovascularisation. Br J Ophthalmol. 1996; 80: 363–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X, Bao S, Hambly BD, Gillies MC. Vascular endothelial growth factor-A: a multifunctional molecular player in diabetic retinopathy. Int J Biochem Cell Biol. 2009; 41: 2368–2371 [DOI] [PubMed] [Google Scholar]
- 12. Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007; 10: 133–140 [DOI] [PubMed] [Google Scholar]
- 13. Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. 2007; 18: 502–508 [DOI] [PubMed] [Google Scholar]
- 14. Abouammoh M, Sharma S. Ranibizumab versus bevacizumab for the treatment of neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2011; 22: 152–158 [DOI] [PubMed] [Google Scholar]
- 15. Do DV, Nguyen QD, Shah SM, et al. An exploratory study of the safety, tolerability and bioactivity of a single intravitreal injection of vascular endothelial growth factor Trap-Eye in patients with diabetic macular oedema. Br J Ophthalmol. 2009; 93: 144–149 [DOI] [PubMed] [Google Scholar]
- 16. Sang DN, D'Amore PA. Is blockade of vascular endothelial growth factor beneficial for all types of diabetic retinopathy? Diabetologia. 2008; 51: 1570–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wirostko B, Wong TY, Simo R. Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res. 2008; 27: 608–621 [DOI] [PubMed] [Google Scholar]
- 18. van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. JAMA. 2005; 293: 1509–1513 [DOI] [PubMed] [Google Scholar]
- 19. McCloskey M, Wang H, Jiang Y, Smith GW, Strange J, Hartnett ME. Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2013; 54: 2020–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu H, Zhang W, Xu Z, Caldwell RW, Caldwell RB, Brooks SE. Hyperoxia causes regression of vitreous neovascularization by downregulating VEGF/VEGFR2 pathway. Invest Ophthalmol Vis Sci. 2013; 54: 918–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004; 26: 943–954 [DOI] [PubMed] [Google Scholar]
- 22. Yourey PA, Gohari S, Su JL, Alderson RF. Vascular endothelial cell growth factors promote the in vitro development of rat photoreceptor cells. J Neurosci. 2000; 20: 6781–6788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lux A, Llacer H, Heussen FM, Joussen AM. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol. 2007; 91: 1318–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arias L, Caminal JM, Badia MB, Rubio MJ, Catala J, Pujol O. Intravitreal infliximab in patients with macular degeneration who are nonresponders to antivascular endothelial growth factor therapy. Retina. 2010; 30: 1601–1608 [DOI] [PubMed] [Google Scholar]
- 25. Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999; 274: 584–594 [DOI] [PubMed] [Google Scholar]
- 26. Davidson MK, Russ PK, Glick GG, Hoffman LH, Chang MS, Haselton FR. Reduced expression of the adherens junction protein cadherin-5 in a diabetic retina. Am J Ophthalmol. 2000; 129: 267–269 [DOI] [PubMed] [Google Scholar]
- 27. Lebrin F, Goumans MJ, Jonker L, et al. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004; 23: 4018–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Britten MB, Abolmaali ND, Assmus B, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003; 108: 2212–2218 [DOI] [PubMed] [Google Scholar]
- 29. Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002; 46: 3349–3360 [DOI] [PubMed] [Google Scholar]
- 30. Park S, Dimaio TA, Liu W, Wang S, Sorenson CM, Sheibani N. Endoglin regulates the activation and quiescence of endothelium by participating in canonical and non-canonical TGF-beta signaling pathways. J Cell Sci. 2013; 126: 1392–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu ZLF, Maring JA, van den Driesche S, et al. Endoglin is dispensable for vasculogenesis, but required for vascular endothelial growth factor-induced angiogenesis. PLoS One. 2014; 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maier JA, Delia D, Thorpe PE, Gasparini G. In vitro inhibition of endothelial cell growth by the antiangiogenic drug AGM-1470 (TNP-470) and the anti-endoglin antibody TEC-11. Anticancer Drugs. 1997; 8: 238–244 [DOI] [PubMed] [Google Scholar]
- 33. Takahashi N, Haba A, Matsuno F, Seon BK. Antiangiogenic therapy of established tumors in human skin/severe combined immunodeficiency mouse chimeras by anti-endoglin (CD105) monoclonal antibodies, and synergy between anti-endoglin antibody and cyclophosphamide. Cancer Res. 2001; 61: 7846–7854 [PubMed] [Google Scholar]
- 34. National Cancer Institute. Bevacizumab with or without anti-endoglin monoclonal antibody TRC105 in treating patients with recurrent glioblastoma multiforme. ClinicalTrials.gov number, NCT01648348. [Google Scholar]
- 35. Penn JS, Tolman BL, Henry MM. Oxygen-induced retinopathy in the rat: relationship of retinal nonperfusion to subsequent neovascularization. Invest Ophthalmol Vis Sci. 1994; 35: 3429–3435 [PubMed] [Google Scholar]
- 36. Lutty GA, McLeod DS. A new technique for visualization of the human retinal vasculature. Arch Ophthalmol. 1992; 110: 267–276 [DOI] [PubMed] [Google Scholar]
- 37. Penn JS, Henry MM, Wall PT, Tolman BL. The range of PaO2 variation determines the severity of oxygen-induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci. 1995; 36: 2063–2070 [PubMed] [Google Scholar]
- 38. Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci. 1993; 34: 576–585 [PubMed] [Google Scholar]
- 39. Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994; 36: 724–731 [DOI] [PubMed] [Google Scholar]
- 40. Barnett JM, McCollum GW, Penn JS. Role of cytosolic phospholipase A(2) in retinal neovascularization. Invest Ophthalmol Vis Sci. 2010; 51: 1136–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26: 859–870 [DOI] [PubMed] [Google Scholar]
- 42. Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007; 114: 2179–2182 [DOI] [PubMed] [Google Scholar]
- 43. Liu Y, Tian H, Blobe GC, Theuer CP, Hurwitz HI, Nixon AB. Effects of the combination of TRC105 and bevacizumab on endothelial cell biology [ published online ahead of print July 5, 2014]. Invest New Drugs. doi:10.1007/s10637-014-0129-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saad RS, Liu YL, Nathan G, Celebrezze J, Medich D, Silverman JF. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in colorectal cancer. Mod Pathol. 2004; 17: 197–203 [DOI] [PubMed] [Google Scholar]
- 45. Liu Y, Starr MD, Brady JC, et al. Modulation of circulating protein biomarkers following TRC105 (anti-endoglin antibody) treatment in patients with advanced cancer. Cancer Med. 2014; 3: 580–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abu El-Asrar AM, Nawaz MI, De Hertogh G, et al. The angiogenic biomarker endocan is upregulated in proliferative diabetic retinopathy and correlates with vascular endothelial growth factor. Curr Eye Res. 2014; 1–11 [DOI] [PubMed] [Google Scholar]
- 47. Rosen LS, Gordon MS, Robert F, Matei DE. Endoglin for targeted cancer treatment. Curr Oncol Rep. 2014; 16: 365 [DOI] [PubMed] [Google Scholar]
- 48. Tabata M, Kondo M, Haruta Y, Seon BK. Antiangiogenic radioimmunotherapy of human solid tumors in SCID mice using (125)I-labeled anti-endoglin monoclonal antibodies. Int J Cancer. 1999; 82: 737–742 [DOI] [PubMed] [Google Scholar]
- 49. Matsuno F, Haruta Y, Kondo M, Tsai H, Barcos M, Seon BK. Induction of lasting complete regression of preformed distinct solid tumors by targeting the tumor vasculature using two new anti-endoglin monoclonal antibodies. Clin Cancer Res. 1999; 5: 371–382 [PubMed] [Google Scholar]
- 50. Seon BK, Matsuno F, Haruta Y, Kondo M, Barcos M. Long-lasting complete inhibition of human solid tumors in SCID mice by targeting endothelial cells of tumor vasculature with antihuman endoglin immunotoxin. Clin Cancer Res. 1997; 3: 1031–1044 [PubMed] [Google Scholar]
- 51. Tsujie M, Tsujie T, Toi H, et al. Anti-tumor activity of an anti-endoglin monoclonal antibody is enhanced in immunocompetent mice. Int J Cancer. 2008; 122: 2266–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tsujie M, Uneda S, Tsai H, Seon BK. Effective anti-angiogenic therapy of established tumors in mice by naked anti-human endoglin (CD105) antibody: differences in growth rate and therapeutic response between tumors growing at different sites. Int J Oncol. 2006; 29: 1087–1094 [PubMed] [Google Scholar]
- 53. Uneda S, Toi H, Tsujie T, et al. Anti-endoglin monoclonal antibodies are effective for suppressing metastasis and the primary tumors by targeting tumor vasculature. Int J Cancer. 2009; 125: 1446–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tan GH, Huang FY, Wang H, Huang YH, Lin YY, Li YN. Immunotherapy of hepatoma with a monoclonal antibody against murine endoglin. World J Gastroenterol. 2007; 13: 2479–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gammons MV, Dick AD, Harper SJ, Bates DO. SRPK1 inhibition modulates VEGF splicing to reduce pathological neovascularization in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2013; 54: 5797–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Faruque LI, Lin M, Battistella M, et al. Systematic review of the risk of adverse outcomes associated with vascular endothelial growth factor inhibitors for the treatment of cancer. PLoS One. 2014; 9: e101145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gardiner TA, Gibson DS, de Gooyer TE, de la Cruz VF, McDonald DM, Stitt AW. Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am J Pathol. 2005; 166: 637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cudmore M, Ahmad S, Al-Ani B, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007; 115: 1789–1797 [DOI] [PubMed] [Google Scholar]
- 59. Middleton J, Americh L, Gayon R, et al. A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146. J Pathol. 2005; 206: 260–268 [DOI] [PubMed] [Google Scholar]
- 60. Rossi E, Sanz-Rodriguez F, Eleno N, et al. Endothelial endoglin is involved in inflammation: role in leukocyte adhesion and transmigration. Blood. 2013; 121: 403–415 [DOI] [PubMed] [Google Scholar]
- 61. Torsney E, Charlton R, Parums D, Collis M, Arthur HM. Inducible expression of human endoglin during inflammation and wound healing in vivo. Inflamm Res. 2002; 51: 464–470 [DOI] [PubMed] [Google Scholar]
- 62. Orbay H, Zhang Y, Valdovinos HF, et al. Positron emission tomography imaging of CD105 expression in a rat myocardial infarction model with (64)Cu-NOTA-TRC105. Am J Nucl Med Mol Imaging. 2013; 4: 1–9 [PMC free article] [PubMed] [Google Scholar]