Abstract
In mammals, the meiotic cell cycle of oocytes starts during embryogenesis and then pauses. Much later, in preparation for fertilization, oocytes within preovulatory follicles resume meiosis in response to luteinizing hormone (LH). Before LH stimulation, the arrest is maintained by diffusion of cyclic (c)GMP into the oocyte from the surrounding granulosa cells, where it is produced by the guanylyl cyclase natriuretic peptide receptor 2 (NPR2). LH rapidly reduces the production of cGMP, but how this occurs is unknown. Here, using rat follicles, we show that within 10 min, LH signaling causes dephosphorylation and inactivation of NPR2 through a process that requires the activity of phosphoprotein phosphatase (PPP)-family members. The rapid dephosphorylation of NPR2 is accompanied by a rapid phosphorylation of the cGMP phosphodiesterase PDE5, an enzyme whose activity is increased upon phosphorylation. Later, levels of the NPR2 agonist C-type natriuretic peptide decrease in the follicle, and these sequential events contribute to the decrease in cGMP that causes meiosis to resume in the oocyte.
Keywords: Guanylyl cyclase, Luteinizing hormone, Meiosis, Natriuretic peptide receptor, Ovarian follicle, Phosphorylation
INTRODUCTION
Oocytes in mammalian preovulatory follicles are held in meiotic prophase arrest by cyclic (c)GMP that is produced in the granulosa cells surrounding the oocyte, which then diffuses into the oocyte through gap junctions (Norris et al., 2009; Vaccari et al., 2009). Luteinizing hormone (LH), which is released from the anterior pituitary during each reproductive cycle, acts on a G-protein coupled receptor in the outer granulosa cells of the follicle (Bortolussi et al., 1977, Rajagopalan-Gupta et al., 1998) (see Fig. 1A), to relieve the inhibition of the prophase-to-metaphase transition. LH acts by lowering the levels of cGMP in the granulosa cells, thus reducing cGMP in the oocyte. Although this regulatory system is best understood in mice (Norris et al., 2009; Vaccari et al., 2009; Zhang et al., 2010; Kawamura et al., 2011; Robinson et al., 2012; Tsuji et al., 2012; Geister et al., 2013), there is evidence that similar mechanisms operate in other mammals (Törnell et al., 1991; Peng et al., 2013; Hiradate et al., 2014; Zhang et al., 2014), including women (Kawamura et al., 2011; Liu et al., 2014). The LH-induced resumption of the meiotic cell cycle leads into a series of events by which the oocyte matures to become a fertilizable egg (Conti et al., 2012; Clift and Schuh, 2013; Holt et al., 2013; Mehlmann, 2013; Hunzicker-Dunn and Mayo, 2014).
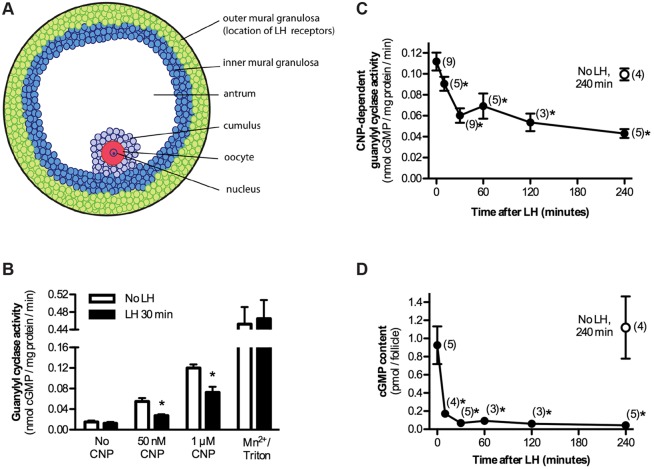
Fig. 1.
Rapid decrease in follicle NPR2 guanylyl cyclase activity and cGMP content in response to LH signaling. (A) Diagram showing the cell layers of a rat ovarian follicle. (B) Guanylyl cyclase activity in follicle membranes in the presence or absence of CNP. Membranes were prepared from follicles with or without treatment with LH for 30 min. Each point shows the mean±s.e.m. for four separate membrane preparations, each assayed in triplicate. In the presence of 50 nM or 1 μM CNP, 30 min of treatment with LH caused a decrease in activity to ∼50% of the initial level. 1% Triton X-100 and 5 mM MgCl2 was used as a control condition that fully activates the enzyme (see text). (C) Time course of the LH-induced decrease in NPR2 activity, as determined in the presence of 1 μM CNP. The open circle represents measurements from follicle membranes incubated for 240 min in the absence of LH. Each point shows the mean±s.e.m. for (n) separate membrane preparations, each assayed in triplicate. (D) Time course of the LH-induced decrease in follicle cGMP content. Error bars are not visible for some points because they are smaller than the data point markers. C,D, *measurements that were significantly different (P<0.05) from the control without LH. Measurements at 30 and 240 min after LH exposure were not significantly different.
As a consequence of the initial cGMP decrease in the mural granulosa cells, cGMP diffuses out of the oocyte into the surrounding granulosa cells, and the decrease in cGMP in the oocyte re-initiates the meiotic cell cycle (Norris et al., 2009; Vaccari et al., 2009). The direct effect of the cGMP decrease in the oocyte is to relieve the inhibition of the cAMP phosphodiesterase PDE3A, which is competitively inhibited by cGMP. Thus, the cGMP reduction causes the levels of cAMP to decrease. cAMP, which is produced in the oocyte (Mehlmann et al., 2002, 2004; Horner et al., 2003; Hinckley et al., 2005; Ledent et al., 2005), maintains meiotic prophase arrest through activation of protein kinase A (PKA) (Bornslaeger et al., 1986; Kovo et al., 2006). PKA activity inhibits the CDC25B phosphatase and stimulates the WEE1B and MYT1 kinases, and this keeps the CDK1 kinase that controls the prophase-to-metaphase transition phosphorylated and inactive (Conti et al., 2012; Holt et al., 2013; Mehlmann, 2013). When LH signaling reduces oocyte levels of cGMP, and in turn cAMP, meiosis resumes.
cGMP is produced in the granulosa cells of mice by natriuretic peptide receptor 2 (NPR2), also known as guanylyl cyclase-B (Zhang et al., 2010; Kawamura et al., 2011; Robinson et al., 2012; Tsuji et al., 2012; Geister et al., 2013). NPR2 appears to be the only relevant guanylyl cyclase in mouse follicles because follicle-enclosed oocytes in the ovaries of mice with non-functional NPR2 resume meiosis spontaneously (Zhang et al., 2010; Tsuji et al., 2012; Geister et al., 2013) and an inactivating mutation in NPR2 reduces the cGMP content of the follicle to an undetectable level (Geister et al., 2013). Gene expression analysis and enzyme assays have indicated that other guanylyl cyclases are present at levels much lower than those of NPR2 (Robinson et al., 2012). NPR2 is located throughout the granulosa cells of the follicle, in both the mural granulosa and the cumulus cells directly surrounding the oocyte; it is not expressed in the oocyte (Gutkowska et al., 1999; Zhang et al., 2010). The agonist of NPR2, C-type natriuretic peptide (CNP, released from a precursor protein that is encoded by the Nppc gene) is produced only in the mural granulosa cells (Zhang et al., 2010).
NPR2 is a single transmembrane-spanning enzyme that is activated by the binding of CNP to its extracellular domain (Potter et al., 2006; Potter, 2011). In order for the CNP activation signal to be transmitted to the catalytic domain, the juxtamembrane intracellular region of NPR2 must be phosphorylated on some combination of five serine residues and two threonine residues that have been identified as regulatory (Potter, 1998; Potter and Hunter, 1998; Yoder et al., 2010, 2012). However, unlike many growth factor receptors, NPR2 phosphorylation is not increased upon binding to its agonist CNP (Potter, 1998). Thus, there are at least two separate mechanisms by which signaling pathways could increase or decrease the guanylyl cyclase activity of NPR2 – changing the amount of CNP or changing the level of receptor phosphorylation.
LH signaling is known to decrease the amount of CNP in rat and mouse ovaries (Jankowski et al., 1997; Kawamura et al., 2011; Robinson et al., 2012; Liu et al., 2014) and in human and porcine follicular fluid (Kawamura et al., 2011; Zhang et al., 2014); the decrease in the levels of CNP is associated with a decrease in Nppc mRNA (Kawamura et al., 2011; Tsuji et al., 2012; Liu et al., 2014). However, in the mouse ovary, where the kinetics are best characterized, the CNP decrease is first detected at 2 h (Robinson et al., 2012; Liu et al., 2014), whereas the decrease in cGMP is detected at 15 to 20 min (Norris et al., 2010; Liu et al., 2014). Guanylyl cyclase activity in mouse follicle membranes decreases to approximately half of the basal level at 20 min after LH application, and this is independent of any change in CNP (Robinson et al., 2012; Liu et al., 2014). Cultured human granulosa cells also show a rapid decrease in cGMP production, measured in the presence of a constant concentration of CNP (Liu et al., 2014).
The mechanism underlying this early decrease in guanylyl cyclase activity is unknown. Here, we show that the rapid reduction in NPR2 activity in rat follicles in response to LH signaling is caused by the dephosphorylation of NPR2, which is mediated by a process that requires the activity of the protein phosphatases of the phosphoprotein phosphatase (PPP) family, the most likely candidates being PPP1, PPP2 and/or PPP6. The rapid dephosphorylation of NPR2 is accompanied by a rapid phosphorylation of the cGMP phosphodiesterase PDE5 (also known as PDE5A), an enzyme whose activity is increased upon phosphorylation. Later, CNP levels decrease in the follicle, and these sequential events contribute to the decrease in cGMP that causes meiosis to resume in the oocyte.
RESULTS
LH signaling reduces NPR2 activity and cGMP content in rat ovarian follicles
Previous studies demonstrating an LH-induced decrease in guanylyl cyclase activity in ovarian follicles have been conducted using mice (Robinson et al., 2012), but the amount of protein that can be obtained from mouse follicles is small. We therefore tested whether a similar regulatory system operates in rats, from which an order of magnitude more follicle protein per animal can be obtained, making analysis of changes in phosphorylation feasible.
To test whether LH causes a decrease in NPR2 guanylyl cyclase activity in rat follicles, and to investigate the time course of the decrease as a basis for subsequent mechanistic studies, isolated preovulatory rat follicles were incubated for various times with or without LH. Because NPR2 is located in the plasma membrane, the follicles were then homogenized to obtain a crude membrane fraction. The membranes were assayed for guanylyl cyclase activity, with and without the NPR2 agonist CNP; CNP-dependent activity indicates the activity of NPR2 (Fig. 1B,C). By 30 min after LH exposure, the CNP-dependent guanylyl cyclase activity decreased to ∼50% of the initial level and stayed at this reduced level for at least 4 h, without any additional change (Fig. 1B,C). Approximately 40% of the decrease to the plateau level had occurred by 10 min (Fig. 1C). No change in CNP-dependent guanylyl cyclase activity was seen in follicles incubated for 4 h without LH (Fig. 1C). The cGMP content of the follicle also decreased rapidly in response to LH – most of the change had occurred by 10 min (Fig. 1D).
The LH-induced decrease in CNP-dependent guanylyl cyclase activity was not explained by a reduction in the affinity of NPR2 for CNP because the fraction by which the activity decreased in response to LH, when it was measured in the presence of 50 nM CNP, was similar to that measured in the presence of 1 μM CNP (Fig. 1B). LH had no effect on guanylyl cyclase activity when it was measured in the presence of 1% Triton X-100 and 5 mM MnCl2 (Fig. 1B), a condition that activates guanylyl cyclase activity independently of natriuretic peptide and phosphorylation and that is indicative of guanylyl cyclase protein levels (Potter and Hunter, 1999; Abbey and Potter, 2003). Because the predominant guanylyl cyclase in ovarian follicles is NPR2, this indicates that the LH-induced decrease in CNP-stimulated activity is not due to a decrease in the amount of NPR2 protein.
LH signaling causes rapid dephosphorylation of NPR2
To investigate whether the rapid LH-induced decrease in NPR2 activity correlated with a decrease in NPR2 phosphorylation, we first considered the use of 32PO4 metabolic labeling. However, because the expression level of NPR2 in native tissues is low, this approach has only been feasible for overexpressing cells (Potter, 1998). The NPR2 fraction of the total cell protein in ovarian follicles is ∼5% of that in the 3T3 cells which had been transfected with NPR2 and used in previous 32PO4 labeling studies, based on the relative levels of enzyme activity (Potter, 1998). To obtain the same amount of NPR2 protein used in the transfected 3T3 cell studies, this would have required ∼10 mg of follicle membrane protein, corresponding to dissection of follicles from ∼30 rats, for a single gel lane. Likewise, detection of changes in phosphorylation by mass spectrometry (e.g. Cargnello et al., 2012) would require much more NPR2 protein than is practical to obtain from rat follicles.
We then investigated whether an LH-induced shift in NPR2 migration could be detected using standard SDS-PAGE, as a previous study showed that dephosphorylation results in a small shift in the electrophoretic migration of the closely related guanylyl cyclase natriuretic peptide receptor 1 (NPR1) (Potter and Garbers, 1992). In standard SDS-PAGE gels, NPR2 from rat follicles migrated as a predominant band at the same position as rat NPR2 from stably transfected HEK-293T cells, at the typically observed size of ∼130 kDa (Potter, 1998). The fuzziness of this band is primarily due to multiple glycosylation sites (Koller et al., 1993). There was also a minor band at ∼115 kDa, which is thought to represent the polypeptide chain that has not been post-translationally modified (Koller et al., 1993; Potter, 1998) (Fig. 2A). However, treatment with LH did not result in a consistent shift in the electrophoretic migration of NPR2 under the conditions that we used (Fig. 2A).
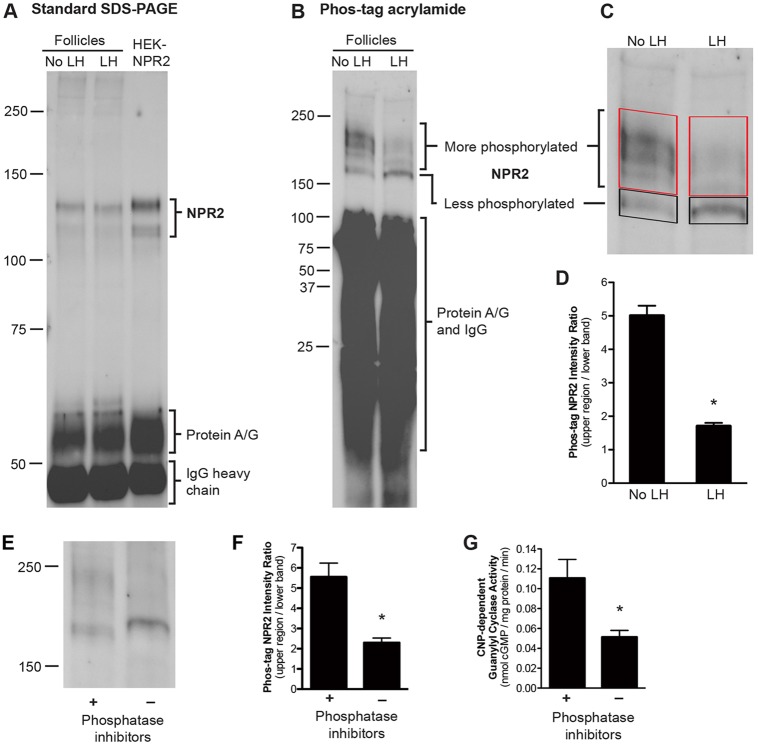
Fig. 2.
Rapid dephosphorylation of NPR2 in follicle membranes in response to LH signaling. (A) A western blot of a standard SDS-PAGE gel that had been probed with NPR2 antiserum; for each lane, NPR2 was immunoprecipitated from 145 μg of membrane protein from follicles with or without treatment with LH for 20 min, or from 10 μg of membrane protein from HEK-293T cells that stably expressed NPR2. (B) A western blot of an SDS-PAGE gel containing 25 μM Phos-tag acrylamide that had been probed with NPR2 antiserum; NPR2 was immunoprecipitated from 175 μg of membrane protein from follicles with or without treatment with LH for 30 min. Molecular weight standards are shown for reference, but do not indicate relative molecular mass on a Phos-tag gel. (C) Method for quantifying the LH-induced shift in NPR2 migration on Phos-tag gels. Boxes show the upper region (more phosphorylated) and lower band (less phosphorylated) for which background-corrected total immunostaining intensity was measured. The upper region was defined as the region extending from just above the lower band to the top of the dense zone near the 250-kDa marker. (D) Ratios of the intensity of the upper region to that of the lower band; mean±s.e.m. for 20 blots similar to that shown in B. (E-G) Validation of the shift in Phos-tag gel migration as an indicator of NPR2 dephosphorylation. Follicle membranes were incubated at 30°C for 30 min, with or without phosphatase inhibitors (see supplementary Materials and Methods). In the absence of phosphatase inhibitors, NPR2 was dephosphorylated (E). (F) Quantification of the shift in NPR2 migration caused by dephosphorylating conditions (mean±s.e.m. for four experiments). (G) Dephosphorylating conditions resulted in a decrease in NPR2 guanylyl cyclase activity (mean±s.e.m. for three experiments). *P<0.05.
As an alternative approach to investigate whether LH exposure led to dephosphorylation of NPR2, we used gels including Phos-tag acrylamide and MnCl2. In these gels, phosphorylated proteins transiently interact with the Mn2+-Phos-tag complex, which retards their migration relative to less-phosphorylated or non-phosphorylated forms (Kinoshita et al., 2006, 2012; McTague et al., 2012; Yu et al., 2013). NPR2 was purified from the membranes by using sequential immunoprecipitation and fractionation on gels containing Phos-tag acrylamide and was visualized by western blotting. In these gels, NPR2 from untreated follicles migrated as multiple bands, indicating the presence of multiple phosphorylated forms of the enzyme (Fig. 2B; see also Fig. 3A, Fig. 4A). Although molecular weight standards are not accurate indicators of relative molecular mass on a Phos-tag gel, they are useful as descriptive markers; the NPR2 bands migrated in a region that extended from above the 150 kDa marker to approximately the 250 kDa marker.
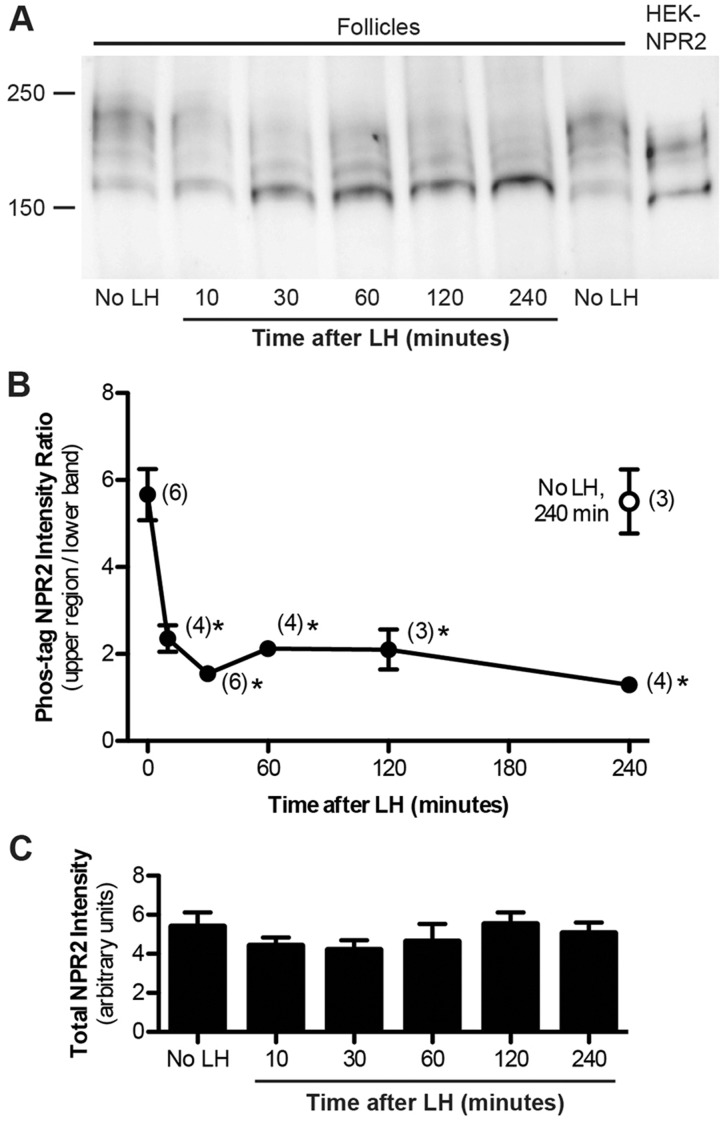
Fig. 3.
Time course of the LH-induced dephosphorylation of NPR2. (A) Follicles were incubated with LH for various times. Crude membranes were then isolated and used for immunoprecipitation of NPR2 (from 190 μg of membrane protein per lane), Phos-tag gel electrophoresis and immunoblotting for NPR2. Samples from follicles without treatment with LH were prepared in parallel, at 30 min and 240 min after starting the incubation. The regions used for quantification are shown in supplementary material Fig. S3A. The right-hand lane sample was prepared using the same methods, instead using 10 μg of membrane protein from HEK-293T cells that stably expressed NPR2. (B) Time course of the LH-induced dephosphorylation of NPR2, quantified as shown in Fig. 2C. The open circle represents measurements from follicle membranes incubated for 240 min in the absence of LH. Each point shows the mean±s.e.m. for the number of experiments shown in parentheses. *P<0.05, measurements that were significantly different from the control without LH. Measurements at 30 and 240 min after LH exposure were not significantly different. (C) The total amount of NPR2 immunoreactive material in follicle membranes did not change during the first 4 h after treatment with LH. Measurements were made from the same set of blots used for B, by drawing a box around the entire NPR2 region of the blot (the sum of the red and black boxes shown in Fig. 2C).
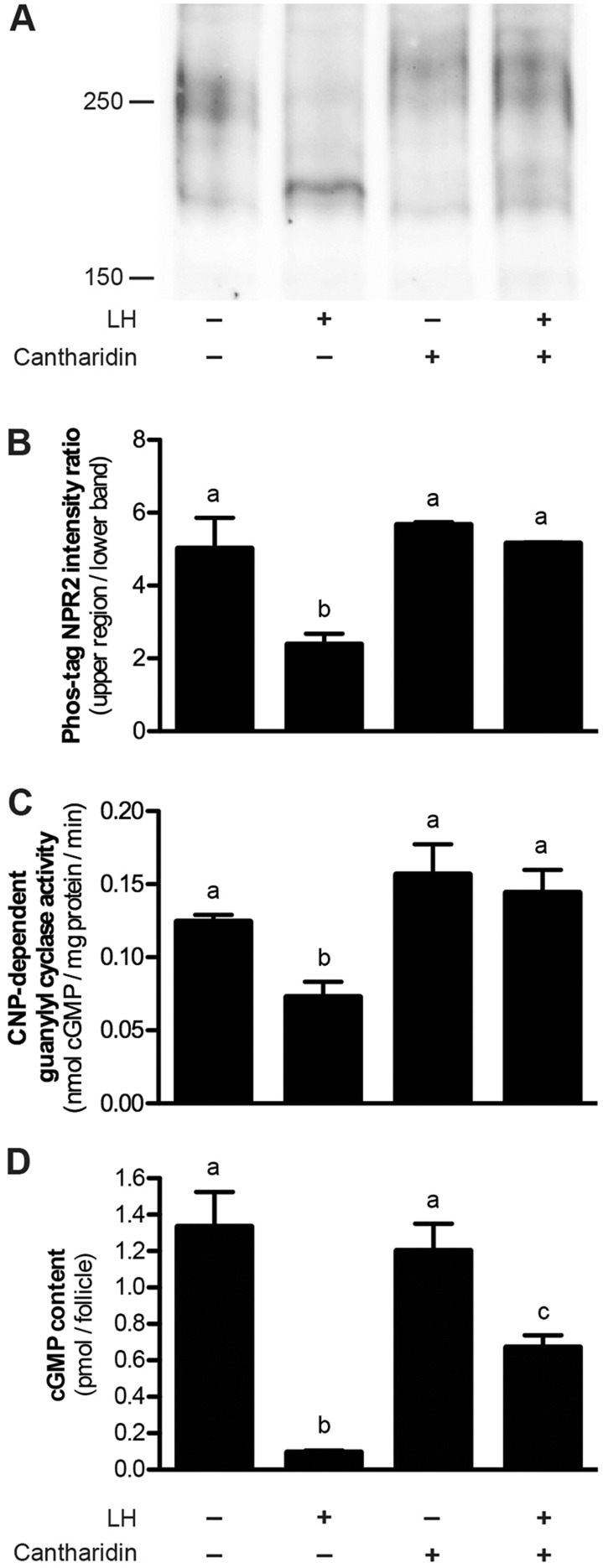
Fig. 4.
Inhibition of the LH-induced dephosphorylation and inactivation of NPR2 and decrease in cGMP content upon the treatment of follicles with the PPP-family phosphatase inhibitor cantharidin. (A) Follicles were incubated with or without 100 µM cantharidin for 1 h, then with or without LH for 30 min. Crude membranes were isolated and used for immunoprecipitation, Phos-tag gel electrophoresis and immunoblotting for NPR2. In the presence of cantharidin, the ratio of NPR2 in the upper region and lower band did not change in response to LH, indicating that cantharidin inhibited the LH-induced dephosphorylation of NPR2. The regions used for quantification are shown in supplementary material Fig. S3B. (B) A graph showing the results of three experiments similar to that shown in A (mean±s.e.m.). (C) Membranes from follicles treated with or without cantharidin followed by LH, as described above, were assayed for NPR2 guanylyl cyclase activity. The graph shows the results from three experiments. (D) Follicles treated with or without cantharidin followed by LH, as described above, were assayed for cGMP content. The graph shows the results from seven experiments. Values not indicated by the same letter are significantly different (P<0.05).
Controls that were performed using pre-immune serum (supplementary material Fig. S1), and co-migration of the immunoreactive bands from follicles with those from a HEK cell line that stably expressed NPR2 (Fig. 2A, Fig. 3A), validated the specificity of the antibody. The relative amount of NPR2 staining in a sample of follicle membranes was 2-7% of that in a sample from overexpressing HEK cells (normalized to the amount of membrane protein loaded per lane), and this corresponded closely to the relative amount of NPR2 activity (2-4% of that reported for this cell line, Robinson and Potter, 2011; Robinson and Potter, 2012), further confirming the antibody specificity. The use of Phos-tag gel migration as an indicator of the phosphorylation state of NPR2 was validated by showing that incubation of follicle membranes under conditions that promoted or inhibited phosphatase activity resulted in faster or slower migrating forms of NPR2 (Fig. 2E-G).
Exposure of the follicles to LH dramatically compressed the majority of the NPR2 into a predominant lower band, indicating dephosphorylation (Fig. 2B; see also Fig. 3A, Fig. 4A). The shift in migration was quantified by measuring the total amount of NPR2 immunostaining in the upper region relative to that in the region of the lower band (Fig. 2C,D). Most of the decrease in the relative amount of immunoreactive material in the upper region had occurred by 10 min after LH exposure, and a minimum level was reached at 30 min (Fig. 3A,B). The dephosphorylation persisted for at least 4 h (Fig. 3A,B), as did the reduction of NPR2 activity (Fig. 1C).
No decrease in NPR2 phosphorylation occurred in follicles that had been incubated for 4 h in the absence of LH (Fig. 3A,B). When LH was applied for 30 min and then washed out, the NPR2 dephosphorylation and activity measured 4 h later were the same as when LH was present continuously (two independent experiments, data not shown). Based on measurements of the total immunoreactive protein, no change in the total amount of NPR2 protein was detected when comparing membranes from follicles with or without treatment with LH (Fig. 3C). This conclusion is also supported by our earlier finding that LH did not affect the amount of guanylyl cyclase activity measured in the presence of Triton X-100 and MnCl2 (Fig. 1B).
Most of the decrease in NPR2 phosphorylation had occurred by 10 min after application of LH (Fig. 3B), whereas only approximately 40% of the decrease in guanylyl cyclase activity had occurred by this time, compared with that measured at 30 min (Fig. 1C). The cause of the delay between the decreases in NPR2 phosphorylation and enzyme activity is unknown, but one possibility is that dephosphorylation of a particular serine or threonine residue that is crucial for the activity decrease occurs late in a series of dephosphorylation events, all of which are detected by the Phos-tag method. Consistent with this hypothesis, the individual phosphorylation sites in NPR2 have varying effects on the activity of the enzyme (Potter and Hunter, 1998; Yoder et al., 2012).
The LH-induced decrease in NPR2 activity is prevented by inhibiting NPR2 dephosphorylation with PPP-family protein phosphatase inhibitors
To test whether preventing the LH-induced dephosphorylation of NPR2 inhibits the decrease in NPR2 activity, and to begin to distinguish which of the approximately 30 known serine-threonine phosphatase catalytic subunits are required for dephosphorylation of NPR2, we treated the follicles with specific phosphatase inhibitors. Serine-threonine phosphatases belong to two main families – PPP or PPM (protein phosphatase Mg2+ or Mn2+ dependent); in addition, there is a smaller family (FCP) whose only known function is to dephosphorylate RNA polymerase II (Cohen, 2004; Swingle et al., 2007). Among these, only the PPP family is inhibited by the natural toxins cantharidin and okadaic acid (Swingle et al., 2007; Pereira et al., 2011). PPM family phosphatases (also known as PP2C) are not inhibited by 100 μM cantharidin (Li et al., 1993) or by 10 μM okadaic acid (Wang et al., 1995).
Pre-incubation of follicles for 1 h with cantharidin (100 μM) or okadaic acid (10 μM) prevented the LH-induced dephosphorylation and decrease in guanylyl cyclase activity of NPR2 (Fig. 4; supplementary material Fig. S2). These results indicate that the activity of PPP family phosphatases is required for the dephosphorylation of NPR2 in response to LH and that dephosphorylation is required for the LH-induced decrease in NPR2 activity. Among the PPP family phosphatases, our results with cantharidin argue against an important function for PPP3 (also known as PP2B or calcineurin) because PPP3 is insensitive to 100 μM cantharidin (Honkanen, 1993; Pereira et al., 2011).
These toxin results do not distinguish between other PPP family phosphatases because PPP1, PPP2, PPP4, PPP5 and PPP6 are all inhibited by the concentrations of cantharidin and okadaic acid that we applied to the follicles (Swingle et al., 2007; Pereira et al., 2011). Other toxins with greater specificities for particular PPP-family phosphatases (fostriecin, cytostatin and rubratoxin) were also tested and found to have no consistent effects under the conditions used (data not shown). However, these results were not definitive because we could not determine whether significant amounts of these toxins penetrated into the cytoplasm of the follicle cells.
The LH-induced decrease in follicle cGMP content is attenuated by inhibiting the decrease in NPR2 guanylyl cyclase activity
To determine the effect of preventing the LH-induced decrease in NPR2 guanylyl cyclase activity on the LH-induced cGMP decrease, we measured the cGMP content of follicles that had been pre-treated with 100 μM cantharidin for 1 h and then treated for 30 min with LH. Without cantharidin pre-treatment, LH caused cGMP content to decrease to 7% of the control level (Fig. 1D and Fig. 4D). Cantharidin pre-treatment attenuated the LH-induced decrease – cGMP content only decreased to 56% of the control level (Fig. 4D).
The effect of phosphatase inhibition on the LH-induced decrease in cGMP content is consistent with the conclusion that the LH-induced decrease in NPR2 phosphorylation and guanylyl cyclase activity contributes to the cGMP decrease. However, the finding that phosphatase inhibition did not completely prevent the LH-induced cGMP decrease, whereas it did completely prevent the LH-induced decrease in NPR2 phosphorylation and activity (Fig. 4A-C), indicates that LH must also induce another change in the follicle, such as an increase in cGMP phosphodiesterase activity. The finding that most of the cGMP decrease had occurred by the 10 min time point after treatment with LH (Fig. 1D), whereas only 40% of the NPR2 activity decrease had occurred by this time point (Fig. 1C), also suggests that LH induces an increase in cGMP phosphodiesterase activity in parallel with the decrease in NPR2 guanylyl cyclase activity.
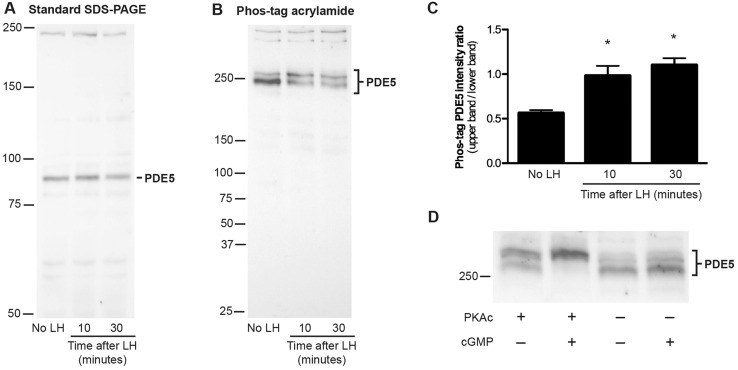
LH signaling causes phosphorylation of the cGMP phosphodiesterase PDE5
An important component of the cGMP phosphodiesterase activity in mouse granulosa cells is contributed by PDE5 (Vaccari et al., 2009). PDE5 activity is stimulated by phosphorylation (Corbin et al., 2000; Rybalkin et al., 2002); therefore, we examined whether LH signaling increased PDE5 phosphorylation in rat follicles. In gels without Phos-tag, PDE5 migrated as a band at ∼90 kDa, and treating follicles with LH did not change this migration (Fig. 5A). However, in Phos-tag-containing gels, PDE5 migrated as two separate bands, and treatment of the follicles with LH for 10 or 30 min increased the fraction of PDE5 in the upper band, indicating phosphorylation (Fig. 5B,C). PDE5 remained phosphorylated until at least 4 h after stimulation with LH (data not shown). In vitro phosphorylation of PDE5 in follicle lysates with the catalytic subunit of PKA also caused a shift of PDE5 to the upper band, confirming that the LH-induced shift was due to phosphorylation (Fig. 5D).
Fig. 5.
Rapid phosphorylation of PDE5 in follicles in response to LH signaling. (A,B) Western blots of proteins from follicles with or without 10 or 30 min of treatment with LH, which were probed with an antibody against PDE5. (A) Standard SDS-PAGE gel, 30 µg protein per lane; (B) SDS-PAGE gel containing 25 μM Phos-tag acrylamide, 20 µg protein per lane. Molecular weight standards are shown for reference, but do not indicate relative molecular mass on a Phos-tag gel. (C) Ratios of the intensity of the upper band to that of the lower band; mean±s.e.m. for four blots similar to that shown in B. (D) Phos-tag gel separation and immunoblotting for PDE5 in follicle lysates that had been incubated with or without the catalytic subunit of PKA (PKAc). Incubations were performed with or without 10 μM cGMP (see supplementary Materials and Methods); cGMP binds to an allosteric site on PDE5 and is required for PKAc to phosphorylate PDE5 (Corbin et al., 2000; Rybalkin et al., 2002). In the presence of PKAc and cGMP, the migration of PDE5 was retarded, indicating phosphorylation.
Although the effect of LH on the cGMP phosphodiesterase activity of PDE5 in rat follicles is unknown, the rapid phosphorylation of PDE5 in response to LH is expected to increase PDE5 activity based on in vitro studies (Corbin et al., 2000; Rybalkin et al., 2002). An increase in PDE5 activity could contribute to the LH-induced cGMP decrease in the follicle, acting in parallel with the decrease in the production of cGMP that results from the dephosphorylation of the NPR2 guanylyl cyclase.
Analysis of PPP family phosphatase gene expression in granulosa cells
To distinguish the PPP-family phosphatases that might contribute to the dephosphorylation of NPR2, we investigated which of these are expressed in granulosa cells. The rat genome contains 13 PPP-family genes, encoding seven subfamilies of PPP-family phosphatases (Pereira et al., 2011). We detected mRNA encoding all of these phosphatases, although the fractions of those encoding PPP4, PPP5 and PPP7 were each <2% of the total (Fig. 6). mRNA encoding PPP1, PPP2, PPP3 and PPP6 constituted 61%, 11%, 19% and 6% of the total, respectively. Although the amount of mRNA is not directly proportional to the amount of the protein they encode, and although the localization of a particular phosphatase could affect its functional significance, these measurements point to a role for PPP1, PPP2, PPP3 and/or PPP6, rather than PPP4, PPP5 or PPP7. Because our earlier findings with cantharidin argue against a function of PPP3 in the LH-induced decrease in NPR2 activity, PPP1, PPP2 and/or PPP6 are the phosphatases that are most likely to be important in this signaling cascade.
Fig. 6.
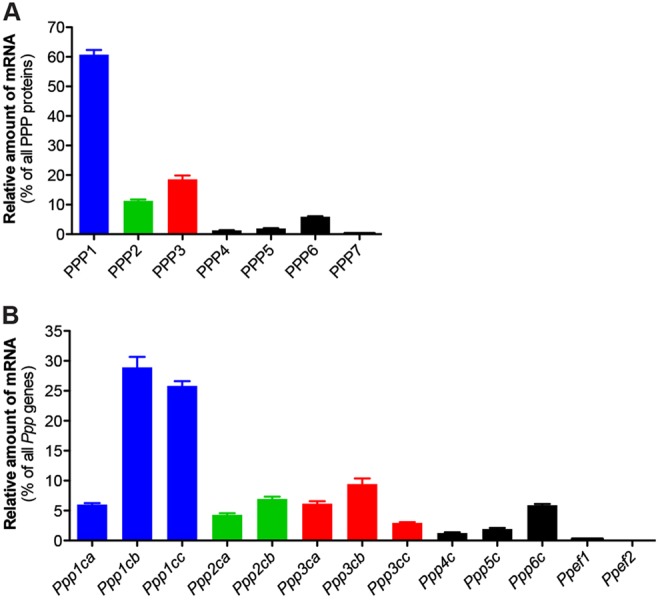
Relative amounts of each PPP phosphatase catalytic subunit mRNA in isolated granulosa cells. (A) The graph shows the mean±s.e.m. for four independent preparations of granulosa cell RNA. The PPP1 bar shows the total for mRNA encoded by Ppp1ca, Ppp1cb and Ppp1cc. The PPP2 bar shows the total for Ppp2ca and Ppp2cb. The PPP3 bar shows the total for Ppp3ca, Ppp3cb and Ppp3cc. The PPP7 bar shows the total for Ppef1 and Ppef2. (B) Data for each individual gene.
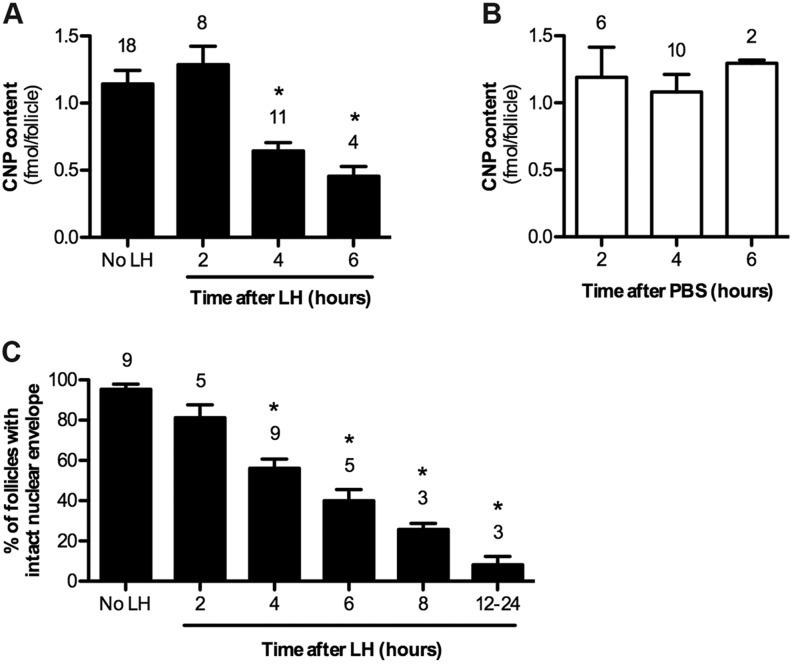
LH signaling gradually reduces the follicle content of the NPR2 agonist CNP, contributing to the decrease in cGMP production that triggers resumption of meiosis
The guanylyl cyclase assays described above were performed using membranes in the presence of a saturating concentration of CNP (1 μM), so the observed decreases in NPR2 activity occurred independently of any changes in CNP. However, previous studies in several species have shown that on a time scale of hours after hormone injection to activate LH receptors in vivo, CNP levels decrease (see Introduction). To integrate our findings regarding the dephosphorylation of NPR2 with this previous knowledge of another component of the regulatory system, we directly compared the kinetics of the decrease of CNP levels in the same experimental system that we had used for the phosphorylation studies. CNP content, as measured using ELISA, did not change during the initial 2 h after the application of LH to follicles, but by 4 h, CNP levels had decreased to 56% of the baseline level (Fig. 7A). CNP content in the follicles did not change during parallel incubations without LH (Fig. 7B). The LH-induced CNP decrease would further decrease NPR2 activity, beginning 2-4 h after the initial dephosphorylation. However, the magnitude of this subsequent activity decrease is unknown because the ELISA measurements detect the CNP peptide, as well as its biologically inactive precursor protein.
Fig. 7.
Time course of the decrease in the CNP content of follicles, and the resumption of meiosis in response to LH signaling. (A) A time course of the decrease in CNP content of follicles that had been treated with LH. (B) There was no change in the CNP content of follicles that had been incubated for 2-6 h without LH. (C) A time course of nuclear envelope breakdown in follicles that had been treated with LH. Values indicate the mean±s.e.m. for the indicated number of experiments (above each bar). A total of 386 follicles were examined for C. A,C, the ‘no LH’ data combine results from follicles that had been incubated without LH for times corresponding to the times of LH exposure. *P<0.05, measurements that are significantly different from controls without LH.
Because the significance of the decrease in guanylyl cyclase activity is to reduce cGMP and re-initiate meiosis in the oocyte, we also examined the time course of nuclear envelope breakdown (the first morphologically detectable event in the resumption of meiosis) following the addition of LH to isolated follicles in our culture system. The time by which 50% of the oocytes had undergone nuclear envelope breakdown was ∼4 h, with a marginally significant decrease observed at 2 h; almost all oocytes had resumed meiosis 12-24 h after LH exposure (Fig. 7C). This time course is similar to that previously observed using a slightly different follicle culture system or after injection of LH into rats (Tsafriri, 1985). The effect of phosphatase inhibitors on LH-induced resumption of meiosis could not be determined because these inhibitors also act on phosphatases in the oocyte, causing meiosis to resume independently of LH (Rime and Ozon, 1990).
DISCUSSION
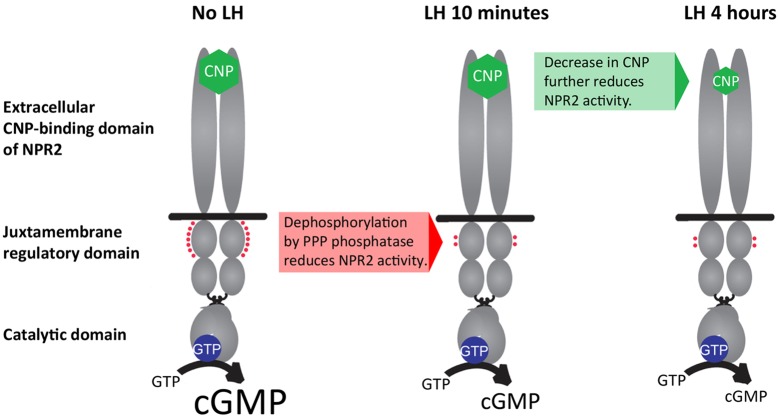
The results described here show that LH signaling decreases guanylyl cyclase activity in rat ovarian follicles by two sequential processes: rapid dephosphorylation of NPR2, detectable at 10 min after treatment with LH, and a slower decrease in the NPR2 agonist CNP, detectable at 4 h after treatment with LH (Fig. 8). Phosphorylation of the cGMP phosphodiesterase PDE5 at 10 min after LH exposure indicates that, in parallel, PDE5 activity may be rapidly increased. This would also contribute to the LH-induced cGMP decrease in the follicle, together with the decrease in the production of cGMP that results from the dephosphorylation of the NPR2 guanylyl cyclase. The decrease of cGMP in the follicle leads to the resumption of meiosis in the oocyte.
Fig. 8.
LH signaling in rat ovarian follicles decreases NPR2 guanylyl cyclase activity by way of a rapid dephosphorylation of regulatory sites followed by a slower decrease of the levels of the agonist CNP. The functional domains of the homodimeric transmembrane protein NPR2 are shown in gray. Binding of CNP (green) to the extracellular domain and phosphorylation of seven juxtamembrane regulatory sites (red) both increase the catalytic activity of the enzyme. LH signaling acts by way of a PPP-family phosphatase to dephosphorylate some of these sites; dephosphorylation occurs by 10 min and persists for at least 4 h. By 4 h after LH exposure, the CNP content of the follicle decreases. Both of these changes decrease guanylyl cyclase activity, contributing to the decrease in cGMP that restarts meiosis.
In addition, LH induces other changes in the follicle that would further decrease cGMP levels in the oocyte. One such change is a delayed decrease in cGMP generation by the inner granulosa (cumulus) cells of mouse follicles, detected 2-3 h after LH receptor stimulation, measured in the presence of a constant concentration of CNP (Robinson et al., 2012; Liu et al., 2014). Another factor that could contribute to the decrease in oocyte cGMP levels is that LH causes a transient decrease in gap junction permeability between the granulosa cells (Granot and Dekel, 1994; Sela-Abramovich et al., 2005; Norris et al., 2008). However, at least in mouse follicles, gap junction closure is not essential for the cGMP decrease (Norris et al., 2009), although it could be a ‘fail-safe’ mechanism if other mechanisms are defective.
How activation of the LH receptor is coupled to dephosphorylation of NPR2, and how dephosphorylation results in inactivation of NPR2, are unknown. The LH receptor activates Gs, as well as other G-proteins (Rajagopalan-Gupta et al., 1998; Hunzicker-Dunn and Mayo, 2014), and through one or more of these G-proteins, LH signaling must activate phosphatases and/or inactivate kinases, or possibly change the conformation of NPR2 such that it is a better or worse substrate for either enzyme. Although the kinases involved have not been identified, our results indicate that the phosphatases that are important for this dephosphorylation belong to the PPP family, and not to the PPM or FCP families. Among the PPP family, our findings using cantharidin argue against an important function for PPP3 (PP2B or calcineurin). Because PPP3 is activated by Ca2+, this conclusion is consistent with the finding that deletion of Gαq/11 in mouse granulosa cells, and the resulting prevention of LH-induced inositol phosphate accumulation, does not inhibit meiotic progression in response to LH (Breen et al., 2013). Taken together with our gene expression analysis, PPP1, PPP2 and/or PPP6 emerge as the most likely candidates for mediating the LH-induced dephosphorylation of NPR2.
Studies of rat granulosa cells in culture have shown that LH activation of Gs leads to the PKA-dependent activation of PPP2 and that PPP2 is associated with the MAP2D A-kinase anchoring protein (Flynn et al., 2008). One possible scenario, although not the only one, is that NPR2 could also be associated with MAP2D, such that it might be dephosphorylated by PPP2 in response to LH-Gs-PKA signaling. Because NPR2 contains multiple phosphorylation sites, and the sequences surrounding these sites differ, the regulation of other phosphatases (and kinases) might also be important in initiating or maintaining the dephosphorylation and inactivation of NPR2 in response to LH.
In particular, EGF receptor signaling is required for part of the LH-induced decrease in the cGMP content and the resumption of meiosis in mouse follicles; how much of the cGMP decrease depends on EGF receptor kinase activity is variable in different studies (Park et al., 2004; Vaccari et al., 2009; Norris et al., 2010; Hsieh et al., 2011; Liu et al., 2014). The release of EGF receptor ligands from the outer mural granulosa cell layers where the LH receptors are located provides a mechanism for paracrine signaling to cells in the interior of the follicle. In rats, the EGF receptor dependence of the LH-induced cGMP decrease has not been determined, but EGF receptor kinase activity is required over a period of hours for LH-induced resumption of meiosis (Ashkenazi et al., 2005; Reizel et al., 2010).
Signaling through various other hormones and growth factors also reduces the guanylyl cyclase activity of NPR2 in other cells, for example, vasopressin (Abbey and Potter, 2002), PDGF (Chrisman and Garbers, 1999; Abbey and Potter, 2003), lysophosphatidic acid (Abbey and Potter, 2003; Potthast et al., 2004), sphingosine-1-phosphate (Abbey-Hosch et al., 2005) and thyrotropin-releasing hormone (Thompson et al., 2014). In studies of sphingosine-1-phosphate acting on cultured fibroblasts that overexpress NPR2 (Abbey-Hosch et al., 2005), there is a correlation between dephosphorylation and the decrease in guanylyl cyclase activity. However, LH signaling in the ovarian follicle is the first example of a physiological pathway that is mediated by such a mechanism.
Other developmental processes that are regulated by the activity of NPR2 and the closely related natriuretic peptide receptor NPR1 could be controlled similarly. In particular, bone growth is affected by mutations in Npr2 or the Nppc gene that encodes CNP; increased NPR2 activity results in longer bones, whereas decreased activity results in shorter bones (Chusho et al., 2001; Tamura et al., 2004; Yasoda et al., 2004; Olney, 2006; Miura et al., 2012; Geister et al., 2013). Natriuretic peptide receptors also function in the development of the nervous system (Ter-Avetisyan et al., 2014) and heart (Becker et al., 2014). Some of the actions of growth factors and hormones that affect chondrocyte differentiation, axon bifurcation and cardiomyocyte proliferation might involve the regulation of natriuretic peptide receptor phosphorylation and/or levels of natriuretic peptides, as seen for LH-mediated regulation of meiosis in the ovary.
MATERIALS AND METHODS
Isolation and culture of rat ovarian follicles
Ovaries were obtained from 25- to 26-day-old CD-Sprague-Dawley rats (Charles River Laboratories); procedures were approved by the animal care committee of the University of Connecticut Health Center. Rats were injected with 12 U of equine chorionic gonadotropin 48 h before ovary collection to stimulate follicle growth and LH receptor expression. Preovulatory follicles, 700-900 μm in diameter, were dissected from the ovaries and cultured as previously described for mouse follicles (Norris et al., 2008) with some modifications (see supplementary Materials and Methods).
Cantharidin was obtained from Tocris Bioscience (R&D Systems) and prepared as a 50 mM stock in dimethylsulfoxide (DMSO). Okadaic acid was obtained from LC Laboratories and was prepared as a 1 mM stock in DMSO.
Preparation of crude membranes from rat follicles and measurement of guanylyl cyclase activity
Crude membranes were prepared from rat follicles, and guanylyl cyclase assays were conducted as previously described for mouse follicles (Robinson and Potter, 2011; Robinson et al., 2012). cGMP production was measured after a 9-min assay period and was approximately linear over this period (Robinson et al., 2012). Basal activity without CNP was subtracted from the activity in the presence of CNP to obtain the CNP-dependent activity. Additional details are described in the supplementary Materials and Methods.
Immunoprecipitation, Phos-tag acrylamide gel electrophoresis and western blotting
Immunoprecipitation was used to purify the low-abundance NPR2 protein from rat follicle membranes. NPR2 was immunoprecipitated by incubation with a rabbit polyclonal antiserum (6328) made against the ten C-terminal amino acids of NPR2 (Abbey and Potter, 2002). Phosphorylated forms of NPR2 were separated by electrophoresis on SDS-PAGE gels made with 6% acrylamide that had been co-polymerized with 25 μM Phos-tag-acrylamide (WAKO Chemicals) and 100 μM MnCl2. Blots were probed with the 6328 antiserum. Phosphorylated forms of PDE5 were separated from follicle lysates, using Phos-tag gels as described for NPR2. The blots were probed with an affinity-purified rabbit polyclonal antibody made against a C-terminal sequence from human PDE5 (catalog no. 2395, Cell Signaling Technology). Additional details, as well as protocols for the generation of dephosphorylated NPR2 and phosphorylated PDE5 controls, are described in the supplementary Materials and Methods.
Images were analyzed using ImageJ software. As an indicator of changes in NPR2 phosphorylation, the intensity of the immunostaining was measured within boxes surrounding the upper region and the lower band (see Fig. 2C; supplementary material Fig. S3). The background intensity of each lane was collected from a box below the NPR2 signal and subtracted. The background-corrected intensity of the upper region was then divided by that of the lower band. This ratio method corrects for variability in the amount of protein that was immunoprecipitated and loaded in each lane. As a measure of total immunoreactive protein, we added the intensity of the upper region and the lower band and then subtracted the background intensity.
ELISA measurements of cGMP and CNP in follicles
The cGMP and CNP contents of rat follicles were measured as previously described (Norris et al., 2009; Robinson et al., 2012) by using ten follicles per sample and ELISA kits from Enzo Life Sciences (no. ADI-900-014 for cGMP) and Phoenix Pharmaceuticals (no. FEK-012-03 for CNP). Data were analyzed using Prism software (GraphPad).
Measurement of relative amounts of phosphatase mRNAs in granulosa cells
RNA was extracted from mural granulosa cells, mRNAs were reverse transcribed using random hexamers, and quantitative TaqMan analysis was performed as previously described (Robinson et al., 2012). Primer sequences are listed in supplementary material Table S1.
Statistics
Differences between a single treatment and control were analyzed by paired t-test using Prism software. Other data were analyzed by either repeated measures ANOVA using Prism (where sample sizes between groups were equal) or by repeated measures mixed models in IBM SPSS (v. 21.0). Post-hoc t-tests were corrected for multiple comparisons by the Holm–Bonferroni method (Holm, 1979). P-values<0.05 were considered to indicate a significant difference.
Supplementary Material
Acknowledgements
We thank Marco Conti, Jackie Corbin, Nava Dekel, John Eppig, Michael Goldberg, Mary Hunzicker-Dunn, Eiji Kinoshita, Lisa Mehlmann, Matthew Movsesian, Dieter Müller, Viacheslav Nikolaev and Rachael Norris for helpful discussions.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
All authors performed experiments or data analysis and contributed to the development of the concepts. J.R.E., L.R.P. and L.A.J. prepared the manuscript, and all authors edited the manuscript prior to submission.
Funding
This work was supported by National Institutes of Health grants [R37HD014939, R01GM098309 and T32AR050938]; and by the Fund for Science. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.112219/-/DC1
References
- Abbey, S. E. and Potter, L. R. (2002). Vasopressin-dependent inhibition of the C-type natriuretic peptide receptor, NPR-B/GC-B, requires elevated intracellular calcium concentrations. J. Biol. Chem. 277, 42423-42430 10.1074/jbc.M206686200 [DOI] [PubMed] [Google Scholar]
- Abbey, S. E. and Potter, L. R. (2003). Lysophosphatidic acid inhibits C-type natriuretic peptide activation of guanylyl cyclase-B. Endocrinology 144, 240-246 10.1210/en.2002-220702 [DOI] [PubMed] [Google Scholar]
- Abbey-Hosch, S. E., Smirnov, D. and Potter, L. R. (2005). Differential regulation of NPR-B/GC-B by protein kinase C and calcium. Biochem. Pharmacol. 70, 686-694 10.1016/j.bcp.2005.04.034 [DOI] [PubMed] [Google Scholar]
- Ashkenazi, H., Cao, X., Motola, S., Popliker, M., Conti, M. and Tsafriri, A. (2005). Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology 146, 77-84 10.1210/en.2004-0588 [DOI] [PubMed] [Google Scholar]
- Becker, J. R., Chatterjee, S., Robinson, T. Y., Bennett, J. S., Panakova, D., Galindo, C. L., Zhong, L., Shin, J. T., Coy, S. M. and Kelly, A. E.et al. (2014). Differential activation of natriuretic peptide receptors modulates cardiomyocyte proliferation during development. Development 141, 335-345 10.1242/dev.100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornslaeger, E. A., Mattei, P. and Schultz, R. M. (1986). Involvement of cAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev. Biol. 114, 453-462 10.1016/0012-1606(86)90209-5 [DOI] [PubMed] [Google Scholar]
- Bortolussi, M., Marini, G. and Dal Lago, A. (1977). Autoradiographic study of the distribution of LH(HCG) receptors in the ovary of untreated and gonadotrophin-primed immature rats. Cell Tissue Res. 183, 329-342 10.1007/BF00220640 [DOI] [PubMed] [Google Scholar]
- Breen, S. M., Andric, N., Ping, T., Xie, F., Offermans, S., Gossen, J. A. and Ascoli, M. (2013). Ovulation involves the luteinizing hormone-dependent activation of Gq/11 in granulosa cells. Mol. Endocrinol. 27, 1483-1491 10.1210/me.2013-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello, M., Tcherkezian, J., Dorn, J. F., Huttlin, E. L., Maddox, P. S., Gygi, S. P. and Roux, P. P. (2012). Phosphorylation of the eukaryotic translation initiation factor 4E-transporter (4E-T) by c-Jun N-Terminal Kinase promotes stress-dependent P-body assembly. Mol. Cell. Biol. 32, 4572-4584 10.1128/MCB.00544-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisman, T. D. and Garbers, D. L. (1999). Reciprocal antagonism coordinates C-type natriuretic peptide and mitogen-signaling pathways in fibroblasts. J. Biol. Chem. 274, 4293-4299 10.1074/jbc.274.7.4293 [DOI] [PubMed] [Google Scholar]
- Chusho, H., Tamura, N., Ogawa, Y., Yasoda, A., Suda, M., Miyazawa, T., Nakamura, K., Nakao, K., Kurihara, T. and Komatsu, Y.et al. (2001). Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc. Natl. Acad. Sci. USA 98, 4016-4021 10.1073/pnas.071389098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift, D. and Schuh, M. (2013). Restarting life: fertilization and the transition from meiosis to mitosis. Nat. Rev. Mol. Cell Biol. 14, 549-562 10.1038/nrm3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, T. W. (2004). Overview of protein serine/threonine phosphatases. In Protein Phosphatases (ed. Arino J. and Alexander D. R.), pp. 1-20 Berlin: Springer-Verlag. [Google Scholar]
- Conti, M., Hsieh, M., Zamah, A. M. and Oh, J. S. (2012). Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 356, 65-73 10.1016/j.mce.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin, J. D., Turko, I. V., Beasley, A. and Francis, S. H. (2000). Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur. J. Biochem. 267, 2760-2767 10.1046/j.1432-1327.2000.01297.x [DOI] [PubMed] [Google Scholar]
- Flynn, M. P., Maizels, E. T., Karlsson, A. B., McAvoy, T., Ahn, J.-H., Nairn, A. C. and Hunzicker-Dunn, M. (2008). Luteinizing hormone receptor activation in ovarian granulosa cells promotes protein kinase A-dependent dephosphorylation of microtubule-associated protein 2D. Mol. Endocrinol. 22, 1695-1710 10.1210/me.2007-0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geister, K. A., Brinkmeier, M. L., Hsieh, M., Faust, S. M., Karolyi, I. J., Perosky, J. E., Kozloff, K. M., Conti, M. and Camper, S. A. (2013). A novel loss-of-function mutation in Npr2 clarifies primary role in female reproduction and reveals a potential therapy for acromesomelic dysplasia, Maroteaux type. Hum. Mol. Genet. 22, 345-357 10.1093/hmg/dds432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot, I. and Dekel, N. (1994). Phosphorylation and expression of connexin-43 ovarian gap junction protein are regulated by luteinizing hormone. J. Biol. Chem. 269, 30502-30509. [PubMed] [Google Scholar]
- Gutkowska, J., Jankowski, M., Sairam, M. R., Fujio, N., Reis, A. M., Mukaddam-Daher, S. and Tremblay, J. (1999). Hormonal regulation of natriuretic peptide system during induced oocyte follicular development in the rat. Biol. Reprod. 61, 162-170 10.1095/biolreprod61.1.162 [DOI] [PubMed] [Google Scholar]
- Hinckley, M., Vaccari, S., Horner, K., Chen, R. and Conti, M. (2005). The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev. Biol. 287, 249-261 10.1016/j.ydbio.2005.08.019 [DOI] [PubMed] [Google Scholar]
- Hiradate, Y., Hoshino, Y., Tanemura, K. and Sato, E. (2014). C-type natriuretic peptide inhibits porcine oocyte meiotic resumption. Zygote 22, 372-377 10.1017/S0967199412000615 [DOI] [PubMed] [Google Scholar]
- Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65-70. [Google Scholar]
- Holt, J. E., Lane, S. I. R. and Jones, K. T. (2013). The control of meiotic maturation in mammalian oocytes. Curr. Top. Dev. Biol. 102, 207-226 10.1016/B978-0-12-416024-8.00007-6 [DOI] [PubMed] [Google Scholar]
- Honkanen, R. E. (1993). Cantharidin, another natural toxin that inhibits the activity of serine/threonine protein phosphatases types 1 and 2A. FEBS Lett. 330, 283-286 10.1016/0014-5793(93)80889-3 [DOI] [PubMed] [Google Scholar]
- Horner, K., Livera, G., Hinckley, M., Trinh, K., Storm, D. and Conti, M. (2003). Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev. Biol. 258, 385-396 10.1016/S0012-1606(03)00134-9 [DOI] [PubMed] [Google Scholar]
- Hsieh, M., Thao, K. and Conti, M. (2011). Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS ONE 6, e21574 10.1371/journal.pone.0021574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzicker-Dunn, M. and Mayo, K. (2014). Gonadotropin signaling in the ovary. In Knobil and Neill's Physiology of Reproduction, 4th edn (ed. Neill J. D.). San Diego, CA: Elsevier (in press). [Google Scholar]
- Jankowski, M., Reis, A. M., Mukaddam-Daher, S., Dam, T. V., Farookhi, R. and Gutkowska, J. (1997). C-type natriuretic peptide and the guanylyl cyclase receptors in the rat ovary are modulated by the estrous cycle. Biol. Reprod. 56, 59-66 10.1095/biolreprod56.1.59 [DOI] [PubMed] [Google Scholar]
- Kawamura, K., Cheng, Y., Kawamura, N., Takae, S., Okada, A., Kawagoe, Y., Mulders, S., Terada, Y. and Hsueh, A. J. W. (2011). Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum. Reprod. 26, 3094-3101 10.1093/humrep/der282 [DOI] [PubMed] [Google Scholar]
- Kinoshita, E., Kinoshita-Kikuta, E., Takiyama, K. and Koike, T. (2006). Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics 5, 749-757 10.1074/mcp.T500024-MCP200 [DOI] [PubMed] [Google Scholar]
- Kinoshita, E., Kinoshita-Kikuta, E. and Koike, T. (2012). Phos-tag affinity electrophoresis for protein kinase profiling. In Protein Kinase Technologies (ed. Mukai H.), pp. 13-34 New York: Humana Press. [Google Scholar]
- Koller, K. J., Lipari, M. T. and Goeddel, D. V. (1993). Proper glycosylation and phosphorylation of the type A natriuretic peptide receptor are required for hormone-stimulated guanylyl cyclase activity. J. Biol. Chem. 268, 5997-6003. [PubMed] [Google Scholar]
- Kovo, M., Kandli-Cohen, M., Ben-Haim, M., Galiani, D., Carr, D. W. and Dekel, N. (2006). An active protein kinase A (PKA) is involved in meiotic arrest of rat growing oocytes. Reproduction 132, 33-43 10.1530/rep.1.00824 [DOI] [PubMed] [Google Scholar]
- Ledent, C., Demeestere, I., Blum, D., Petermans, J., Hamalainen, T., Smits, G. and Vassart, G. (2005). Premature ovarian aging in mice deficient for Gpr3. Proc. Natl Acad. Sci. USA 102, 8922-8926 10.1073/pnas.0503840102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.-M., MacKintosh, C. and Casida, J. E. (1993). Protein phosphatase 2A and its [3H]cantharidin/[3H]endothall thioanhydride binding site: inhibitor specificity of cantharidin and ATP analogues. Biochem. Pharmacol. 46, 1435-1443 10.1016/0006-2952(93)90109-A [DOI] [PubMed] [Google Scholar]
- Liu, X., Xie, F., Zamah, A. M., Cao, B. and Conti, M. (2014). Multiple pathways mediate luteinizing hormone regulation of cGMP signaling in the mouse ovarian follicle. Biol. Reprod. 91, 1-11 10.1095/biolreprod.113.116814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTague, J., Amyotte, N., Kanyo, R., Ferguson, M., Chick, C. L. and Ho, A. K. (2012). Different signaling mechanisms are involved in the norepinephrine-stimulated TORC1 and TORC2 nuclear translocation in rat pinealocytes. Endocrinology 153, 3839-3849 10.1210/en.2012-1315 [DOI] [PubMed] [Google Scholar]
- Mehlmann, L. M. (2013). Signaling for meiotic resumption in granulosa cells, cumulus cells, and oocyte. In Oogenesis (ed. Coticchio G., Albertini D. F. and De Santis L.), pp. 171-182 London: Springer-Verlag. [Google Scholar]
- Mehlmann, L. M., Jones, T. L. Z. and Jaffe, L. A. (2002). Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science 297, 1343-1345 10.1126/science.1073978 [DOI] [PubMed] [Google Scholar]
- Mehlmann, L. M., Saeki, Y., Tanaka, S., Brennan, T. J., Evsikov, A. V., Pendola, F. L., Knowles, B. B., Eppig, J. J. and Jaffe, L. A. (2004). The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 306, 1947-1950 10.1126/science.1103974 [DOI] [PubMed] [Google Scholar]
- Miura, K., Namba, N., Fujiwara, M., Ohata, Y., Ishida, H., Kitaoka, T., Kubota, T., Hirai, H., Higuchi, C. and Tsumaki, N.et al. (2012). An overgrowth disorder associated with excessive production of cGMP due to a gain-of-function mutation of the natriuretic peptide receptor 2 gene. PLoS ONE 7, e42180 10.1371/journal.pone.0042180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, R. P., Freudzon, M., Mehlmann, L. M., Cowan, A. E., Simon, A. M., Paul, D. L., Lampe, P. D. and Jaffe, L. A. (2008). Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135, 3229-3238 10.1242/dev.025494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, R. P., Ratzan, W. J., Freudzon, M., Mehlmann, L. M., Krall, J., Movsesian, M. A., Wang, H., Ke, H., Nikolaev, V. O. and Jaffe, L. A. (2009). Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136, 1869-1878 10.1242/dev.035238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, R. P., Freudzon, M., Nikolaev, V. O. and Jaffe, L. A. (2010). Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction 140, 655-662 10.1530/REP-10-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney, R. C. (2006). C-type natriuretic peptide in growth: a new paradigm. Growth Horm. IGF Res. 16, 6-14 10.1016/j.ghir.2006.03.016 [DOI] [PubMed] [Google Scholar]
- Park, J.-Y., Su, Y.-Q., Ariga, M., Law, E., Jin, S.-L. C. and Conti, M. (2004). EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303, 682-684 10.1126/science.1092463 [DOI] [PubMed] [Google Scholar]
- Peng, J.-Y., Xin, H.-Y., Han, P., Zhao, H.-B., Bai, L., An, X.-P. and Cao, B.-Y. (2013). Identification and gene expression analyses of natriuretic peptide system in the ovary of goat (Capra hircus). Gene 524, 105-113 10.1016/j.gene.2013.04.054 [DOI] [PubMed] [Google Scholar]
- Pereira, S. R., Vasconcelos, V. M. and Antunes, A. (2011). The phosphoprotein phosphatase family of Ser/Thr phosphatases as principal targets of naturally occurring toxins. Crit. Rev. Toxicol. 41, 83-110 10.3109/10408444.2010.515564 [DOI] [PubMed] [Google Scholar]
- Potter, L. R. (1998). Phosphorylation-dependent regulation of the guanylyl cyclase-linked natriuretic peptide receptor B: dephosphorylation is a mechanism of desensitization. Biochemistry 37, 2422-2429 10.1021/bi972303k [DOI] [PubMed] [Google Scholar]
- Potter, L. R. (2011). Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases. Pharmacol. Ther. 130, 71-82 10.1016/j.pharmthera.2010.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, L. R. and Garbers, D. L. (1992). Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J. Biol. Chem. 267, 14531-14534. [PubMed] [Google Scholar]
- Potter, L. R. and Hunter, T. (1998). Identification and characterization of the major phosphorylation sites of the B-type natriuretic peptide receptor. J. Biol. Chem. 273, 15533-15539 10.1074/jbc.273.25.15533 [DOI] [PubMed] [Google Scholar]
- Potter, L. R. and Hunter, T. (1999). A constitutively “phosphorylated” guanylyl cyclase-linked atrial natriuretic peptide receptor mutant is resistant to desensitization. Mol. Biol. Cell 10, 1811-1820 10.1091/mbc.10.6.1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, L. R., Abbey-Hosch, S. and Dickey, D. M. (2006). Natriuretic peptides, their receptors, and cyclic monophosphate-dependent signaling functions. Endocr. Rev. 27, 2974-2985 10.1210/er.2005-0014 [DOI] [PubMed] [Google Scholar]
- Potthast, R., Abbey-Hosch, S. E., Antos, L. K., Marchant, J. S., Kuhn, M. and Potter, L. R. (2004). Calcium-dependent dephosphorylation mediates the hyperosmotic and lysophosphatidic acid-dependent inhibition of natriuretic peptide receptor-B/guanylyl cyclase-B. J. Biol. Chem. 279, 48513-48519 10.1074/jbc.M408247200 [DOI] [PubMed] [Google Scholar]
- Rajagopalan-Gupta, R. M., Lamm, M. L. G., Mukherjee, S., Rasenick, M. M. and Hunzicker-Dunn, M. (1998). Luteinizing hormone/choriogonadotropin receptor-mediated activation of heterotrimeric guanine nucleotide binding proteins in ovarian follicular membranes. Endocrinology 139, 4547-4555. [DOI] [PubMed] [Google Scholar]
- Reizel, Y., Elbaz, J. and Dekel, N. (2010). Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol. Endocrinol. 24, 402-411 10.1210/me.2009-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rime, H. and Ozon, R. (1990). Protein phosphatases are involved in the in vivo activation of histone H1 kinase in mouse oocyte. Dev. Biol. 141, 115-122 10.1016/0012-1606(90)90106-S [DOI] [PubMed] [Google Scholar]
- Robinson, J. W. and Potter, L. R. (2011). ATP potentiates competitive inhibition of guanylyl cyclase A and B by the staurosporine analog, Go6976: reciprocal regulation of ATP and GTP binding. J. Biol. Chem. 286, 33841-33844 10.1074/jbc.M111.273565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J. W. and Potter, L. R. (2012). Guanylyl cyclases A and B are asymmetric dimers that are allosterically activated by ATP binding to the catalytic domain. Sci. Signal. 5, ra65 10.1126/scisignal.2003253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J. W., Zhang, M., Shuhaibar, L. C., Norris, R. P., Geerts, A., Wunder, F., Eppig, J. J., Potter, L. R. and Jaffe, L. A. (2012). Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic GMP decrease that promotes resumption of meiosis in oocytes. Dev. Biol. 366, 308-316 10.1016/j.ydbio.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalkin, S. D., Rybalkina, I. G., Feil, R., Hofmann, F. and Beavo, J. A. (2002). Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J. Biol. Chem. 277, 3310-3317 10.1074/jbc.M106562200 [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich, S., Chorev, E., Galiani, D. and Dekel, N. (2005). Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 146, 1236-1244 10.1210/en.2004-1006 [DOI] [PubMed] [Google Scholar]
- Swingle, M., Ni, L. and Honkanen, R. E. (2007). Small molecule inhibitors of ser/thr protein phosphatases: specificity, use and common forms of abuse. Methods Mol. Biol. 365, 23-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, N., Doolittle, L. K., Hammer, R. E., Shelton, J. M., Richardson, J. A. and Garbers, D. L. (2004). Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc. Natl. Acad. Sci. USA 101, 17300-17305 10.1073/pnas.0407894101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Avetisyan, G., Rathjen, F. G. and Schmidt, H. (2014). Bifurcation of axons from cranial sensory neurons is disabled in the absence of Npr2-induced cGMP signaling. J. Neurosci. 34, 737-747 10.1523/JNEUROSCI.4183-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, I. R., Mirczuk, S. M., Smith, L., Lessey, A. J., Simbi, B., Sunters, A., Baxter, G. F., Lipscomb, V. J., McGonnell, I. M. and Wheeler-Jones, C. P.et al. (2014). Homologous and heterologous desensitization of guanylyl cyclase-B signaling in GH3 somatolactotropes. Cell Tissue Res. 355, 425-436 10.1007/s00441-013-1763-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnell, J., Billig, H. and Hillensjö, T. (1991). Regulation of oocyte maturation by changes in ovarian levels of cyclic nucleotides. Human. Reprod. 6, 411-422. [DOI] [PubMed] [Google Scholar]
- Tsafriri, A. (1985). The control of meiotic maturation in mammals. In Biology of Fertilization, Vol. 1 (ed. Metz C. B. and Monroy A.), pp. 221-252 Orlando: Academic Press. [Google Scholar]
- Tsuji, T., Kiyosu, C., Akiyama, K. and Kunieda, T. (2012). CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol. Reprod. Dev. 79, 795-802 10.1002/mrd.22114 [DOI] [PubMed] [Google Scholar]
- Vaccari, S., Weeks, J. L., Hsieh, M., Menniti, F. S. and Conti, M. (2009). Cyclic GMP signaling is involved in the Luteinizing Hormone-dependent meiotic maturation of mouse oocytes. Biol. Reprod. 81, 595-604 10.1095/biolreprod.109.077768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Santini, F., Qin, K. and Huang, C. Y. (1995). A Mg2+-dependent, Ca2+-inhibitable serine/threonine protein phosphatase from bovine brain. J. Biol. Chem. 270, 25607-25612 10.1074/jbc.270.43.25607 [DOI] [PubMed] [Google Scholar]
- Yasoda, A., Komatsu, Y., Chusho, H., Miyazawa, T., Ozasa, A., Miura, M., Kurihara, T., Rogi, T., Tanaka, S. and Suda, M.et al. (2004). Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat. Med. 10, 80-86 10.1038/nm971 [DOI] [PubMed] [Google Scholar]
- Yoder, A. R., Stone, M. D., Griffin, T. J. and Potter, L. R. (2010). Mass spectrometric identification of phosphorylation sites in guanylyl cyclase A and B. Biochemistry 49, 10137-10145 10.1021/bi101700e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder, A. R., Robinson, J. W., Dickey, D. M., Andersland, J., Rose, B. A., Stone, M. D., Griffin, T. J. and Potter, L. R. (2012). A functional screen provides evidence for a conserved, regulatory, juxtamembrane phosphorylation site in guanylyl cyclase A and B. PLoS ONE 7, e36747 10.1371/journal.pone.0036747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, F.-X., Zhang, Y., Park, H. W., Jewell, J. L., Chen, Q., Deng, Y., Pan, D., Taylor, S. S., Lai, Z.-C. and Guan, K.-L. (2013). Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 27, 1223-1232 10.1101/gad.219402.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M., Su, Y.-Q., Sugiura, K., Xia, G. and Eppig, J. J. (2010). Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330, 366-369 10.1126/science.1193573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., Yang, Y., Liu, W., Chen, Q., Wang, H., Wang, X., Zhang, Y., Zhang, M. and Xia, G. (2014). Brain natriuretic peptide and C-type natriuretic peptide maintain porcine oocyte meiotic arrest. J. Cell. Physiol. (in press). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.