Abstract
BACKGROUND & AIMS
The molecular mechanisms of lymphoproliferation associated with the disruption of interferon (IFN) signaling and chronic hepatitis C virus (HCV) infection are poorly understood. Lymphomas are extrahepatic manifestations of HCV infection; we sought to clarify the molecular mechanisms of these processes.
METHODS
We established interferon regulatory factor-1– null (irf-1−/−) mice with inducible and persistent expression of HCV structural proteins (irf-1/CN2 mice). All the mice (n = 900) were observed for at least 600 days after Cre/loxP switching. Histologic analyses, as well as analyses of lymphoproliferation, sensitivity to Fas-induced apoptosis, colony formation, and cytokine production, were performed. Proteins associated with these processes were also assessed.
RESULTS
Irf-1/CN2 mice had extremely high incidences of lymphomas and lymphoproliferative disorders and displayed increased mortality. Disruption of irf-1 reduced the sensitivity to Fas-induced apoptosis and decreased the levels of caspases-3/7 and caspase-9 messenger RNA species and enzymatic activities. Furthermore, the irf-1/CN2 mice showed decreased activation of caspases-3/7 and caspase-9 and increased levels of interleukin (IL)-2, IL-10, and Bcl-2, as well as increased Bcl-2 expression, which promoted oncogenic transformation of lymphocytes. IL-2 and IL-10 were induced by the HCV core protein in splenocytes.
CONCLUSIONS
Disruption of IFN signaling resulted in development of lymphoma, indicating that differential signaling occurs in lymphocytes compared with liver. This mouse model, in which HCV expression and disruption of IFN signaling synergize to promote lymphoproliferation, will be an important tool for the development of therapeutic agents that target the lymphoproliferative pathway.
More than 175 million people worldwide are infected with hepatitis C virus (HCV), which is a positive-strand RNA virus that infects both hepatocytes and peripheral blood mononuclear cells.1–4 Chronic hepatitis infection can lead to hepatitis, cirrhosis, hepatocellular carcinoma, and lymphoproliferative diseases, such as B-cell non-Hodgkin's lymphomas and mixed cryoglobulinemia.5–10 The current therapy for chronic HCV infection involves treatment with type I interferon (IFN) and derivatives of IFN, such as pegylated IFN.11 Treatment with type I IFN is associated with regression of lymphoma in patients with hepatitis C.12 However, more than 50% of HCV-infected individuals are resistant to treatment, which indicates that the inhibition of IFN signal transduction facilitates the persistent expression of HCV proteins by hepatocytes.
Transgenic mice that express the HCV core protein have been established using a promoter derived from hepatitis B virus,13 whereas mice that express structural or complete viral proteins have been established using promoters derived from the albumin gene.14 These mice are immunotolerant to the transgene and do not develop hepatic inflammation, although they do develop age-related hepatic steatosis and hepatocellular carcinomas. We also developed a transgenic mouse model in which the HCV complementary DNA, including viral genes that encode the core, E1, E2, and NS2 proteins, was conditionally expressed by the Cre/loxP system (CN2 mice).15 The conditional expression of HCV proteins protected mice from Fas-mediated lethal acute liver failure by inhibiting cytochrome c release from the mitochondria.16 However, the expression of HCV in these mice was usually lost after 21 days. Therefore, an animal model of persistent HCV protein expression is required to examine the effects of chronic HCV infection in vivo.
IFN signaling mediates tumor suppressor effects and antiviral responses and is regulated by key transcription factors of the interferon-regulatory factor (IRF) protein family, including Irf-1, -2, -3, -7, and -9. Targeted disruption of irf-1 results in aberrant lymphocyte development and a marked reduction in the number of CD8+ T cells in the peripheral blood, spleen, and lymph nodes.17 In addition, natural killer cell development is impaired in irf-1−/− mice.18 The mechanisms by which HCV infection induces IFN resistance and influences the development of lymphomas are poorly understood. Therefore, in the present study, we established an irf-1−/− CN2 mouse model of persistent HCV expression, which allows investigation of the effects of HCV on lymphatic tissue tumor development.
Materials and Methods
Animal Experiments
Wild-type (WT), CN2, irf-1−/−, and Mx1-cre mice were maintained in conventional animal housing under specific pathogen-free conditions. AxCANCre and Ax-CAw1 were obtained from Dr Izumu Saito (University of Tokyo).15 To elicit Fas-induced liver damage, adult mice were injected intravenously with 10 μg of purified hamster monoclonal antibody against mouse Fas (clone Jo2; BD Biosciences, San Diego, CA) in 200 μL of phosphate-buffered saline. All animal experiments were performed according to the guidelines of the Tokyo Metropolitan Institute of Medical Science or Kumamoto University Subcommittee for Laboratory Animal Care. The protocol was approved by an institutional review board. Detailed procedures, including induction of the HCV transgene by poly(I:C) in CN2-29 Mx1-Cre mice, are described in Supplementary Materials and Methods.
Measurements of Caspase Activities
The cytosolic splenocyte fractions were isolated as described,16 and the detailed procedures are described in the Supplementary Material and Methods.
Lentiviral Vectors and Infection
Isolated splenocytes from WT or irf-1−/− mice (total of 107 cells) were infected with recombinant lentiviruses that express HCV core, E1, E2, NS2, lacZ, and empty vector, respectively. One day after infection, cells were selected with puromycin (final concentration of 1 μg/ mL). After 5 days of puromycin selection, viable cells were examined.
Baculovirus Expression and Purification of HCV Core, E1, and E2 Proteins
The E1 and E2 sequences from a genotype 1a isolate (strain H77)19 and a genotype 1b isolate (strain HC-J4),20 without the C-terminal transmembrane domains but containing the His6 tag at the C terminus, were cloned into a transfer vector (pBlueBacHis2; Invitrogen, Carlsbad, CA). The expression of recombinant core, E1, and E2 proteins in insect cells and their purification have been described previously.21
Results
Viral Protein Expression and Disruption of irf-1 Synergistically Increase the Development of Lymphoproliferative Disorders
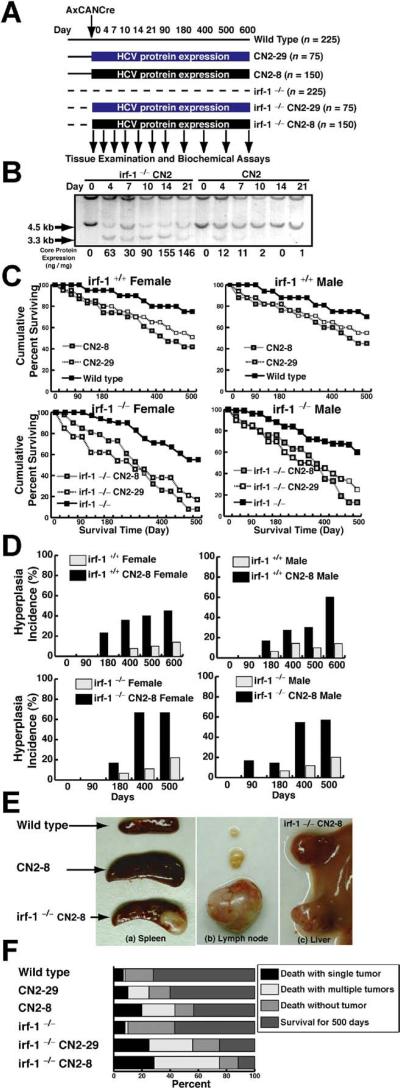
To clarify the in vivo effects of HCV protein expression, we examined the survival of mice that carry the CN2 transgene (CN2-8, CN2-29).15 The experimental design is shown in Figure 1A (total number of mice, 900). Without Cre/loxP switching, the animals that carry the HCV transgene (CN2-8 and CN2-29: core, E1, E2, and NS2 proteins) appeared healthy and developed normally.15 All of the transgene carriers were observed for at least 600 days after Cre/loxP switching (Figure 1A). Administration of a recombinant adenovirus that expresses cre (AxCANCre) induced the efficient recombination of CN2 transgenes in the hepatocytes from CN2 and irf-1−/− CN2 mice (Figure 1B). Recombination produced the floxed CN2 transgene (3.3 kilobases) and was completed within 4–7 days; it diminished before day 21 in CN2 mice but persisted in irf-1−/− CN2 mice. The expression of core protein in the hepatocytes of CN2 mice peaked on day 7 and decreased to an undetectable level by day 21 (Supplementary Figure 1A). The expression of core protein in hepatocytes coincided with a high level of inflammation, as determined by measurements of serum alanine aminotransferase activity (Supplementary Figure 1A and data not shown). The HCV core protein was detected in CN2-8 mice 4–14 days after the administration of Ax-CANCre, and disruption of irf-1 ensured core protein expression for more than 500 days (Supplementary Figure 1A and 1B). Therefore, irf-1 disruption allowed efficient and persistent expression of HCV proteins. HCV core protein gene expression was confirmed by reverse-transcription polymerase chain reaction (PCR) of livers, splenocytes, and peripheral blood monocytes (Supplementary Figure 1C). AxCANCre administration to the transgenic mouse induced the efficient expression of HCV transgenes in lymphocytes and splenocytes (Supplementary Figure 1C).
Figure 1.
Disruption of irf-1 enhances oncogenic potential in combination with HCV transgene expression. (A) Experimental design for the animal model. Transgenic mice and their nontransgenic littermates (10–14 weeks of age) were administered the Cre-expressing adenovirus (AxCANCre) and killed after 4, 7, 10, 14, 21, 90, 120, 400, 500, or 600 days. (B) Southern blot analysis of hepatocyte DNA from mice derived by crossing irf-1−/− and HCV-transgenic (CN2) mice. Genomic DNA samples from WT (+/+) and CN2 mouse hepatocytes were digested with XbaI and subjected to Southern blot analysis using a radio-labeled genomic flanking probe to determine the rate of recombination of the HCV transgene construct (3.3-kilobase fragment). Disruption of irf-1 allows persistent expression of HCV proteins. The effects of HCV protein expression on the survival rates of male and female irf-1−/− and irf-1+/+ CN2 mice are shown. (C) Kaplan–Meier survival curves for WT mice, irf-1−/− mice, CN2 transgenic mouse strains 8 and 29, and irf-1−/− CN2-8 and CN-29 mice following infection with a recombinant adenovirus that expresses cre (AxCANCre). (D) HCV protein expression enhances hyperplasia in male and female CN2 and irf-1−/− CN2 mice. The occurrence of hyperplasia was monitored every 7 days for 600 days following the administration of AxCANCre. (E) Spleens (a) and lymph nodes (b) from age-matched WT, CN2, and irf-1−/− CN2 mice 500 days after the administration of AxCANCre. (c) Liver from the same irf-1−/− CN2 mouse (developing severe lymphadenopathy and splenomegaly) following the administration of AxCANCre. (F) The cause of death in CN2 transgenic mice with hyperplasias. Mice of each genotype (n = 150) were monitored up to day 600 after the administration of AxCANCre, and necropsies were performed to determine the number of tumors. Tumors included thymomas, splenomas, lymphomas, and hepatocellular carcinomas.
The survival rate of WT mice injected with the creadenovirus (AxCANCre) (Figure 1C) or control adenovirus (AxCAw1) (data not shown) was higher than that of the transgenic mice (CN2-8 and CN2-29), which excludes the possibility that the recombinant adenovirus affected the results. More than 75% of the WT mice injected with AxCANCre survived to day 500, whereas the HCV-expressing mice had lower survival rates. The irf-1−/− CN2-8 and irf-1−/− CN2-29 strains had even lower survival rates, indicating that persistent HCV protein expression in combination with irf-1 disruption significantly decreases survival (Figure 1C).
Lymphoproliferative Disorders Are Accelerated With Age and Level of Viral Protein Expression
To determine the mechanism underlying the increased mortality caused by persistent HCV protein expression in irf-1−/− CN2 mice, we examined the kinetics of dysplasia (Figure 1D). Strikingly, 67% of the female irf-1−/− CN2 mice and 70% of the male irf-1−/− CN2 mice developed tumors 400 days after the administration of AxCANCre. Some of the irf-1−/− CN2 mice developed hyperplasia of the lymph nodes, and these tumors developed much earlier than the tumors in their irf-1+/+ or CN2 counterparts (Figure 1D). Aberrant cell proliferation developed randomly among the male and female carrier animals between day 180 and day 600. On day 400 after Cre/loxP switching, the average weights of the spleens of the WT, CN2, and irf-1−/− CN2 mice were 90, 160, and 310 mg, respectively. The disruption of irf-1 aggravated the HCV-induced spontaneous proliferative disturbances in lymphatic tissues. The number of CN2 mice that died with at least one tumor and the number of tumors per mouse were significantly increased by the ablation of irf-1 (Figure 1F). Although the type of hyperplasia did not differ significantly between the irf-1−/− CN2 mice and their irf-1+/+ CN2 siblings, the time to onset of tumori-genesis differed dramatically (Figure 1D and 1F), indicating that age is a significant factor in the promotion of lymphomagenesis by HCV proteins.
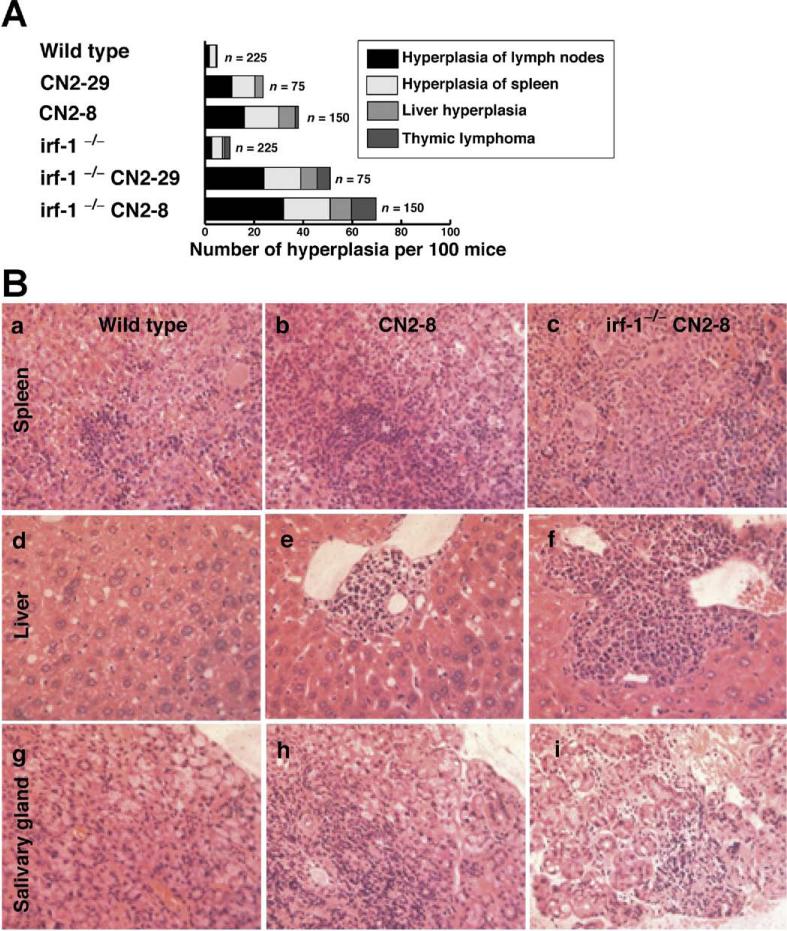
A significant percentage of the mice that expressed the HCV core protein (irf-1−/− CN2 mice) developed polyclonal lymphoid growth disturbances, including splenomegaly, expanded lymph nodes, adenocarcinoma in the abdomen or leg, and lymphoma of the liver or Peyer's patches (Figure 2A). In contrast, hepatocytes with abundant expression of HCV proteins rarely developed into hepatocellular carcinomas. H&E staining of splenomegalic tissue revealed extensive hyperplasia of the white pulp zones, in which the cortical zones contained lym phoid follicles and scattered germinal centers, although mitotic figures were rarely observed (Figure 2B and data not shown). These results indicate that persistent expression of HCV proteins frequently induces lymphoproliferative disorders in addition to liver hyperplasia, which is consistent with the phenotype of patients with hepato-cellular carcinoma.3,4,9
Figure 2.
Disruption of irf-1 aggravates lymphocyte infiltration in combination with HCV trans-gene expression. (A) Histologic analysis of spontaneous proliferative disturbances in the CN2 transgenic mice. Of the 900 mice injected with AxCANCre, 25 of 75 (33%) CN2-29, 47 of 150 (31%) CN2-8, 29 of 75 (39%) irf-1−/− CN2-29, and 62 of 150 (41%) irf-1−/− CN2-8 mice developed proliferative disturbances. Data shown are from the same cohort of mice analyzed in Figure 1F. (B) H&E-stained tissue sections of (a–c) spleens, (d–f) livers, and (g–i) salivary glands from age-matched WT, CN2, and irf-1−/− CN2 mice after the administration of AxCANCre.
Abnormal T-Cell and B-Cell Proliferation in HCV Transgenic Mice
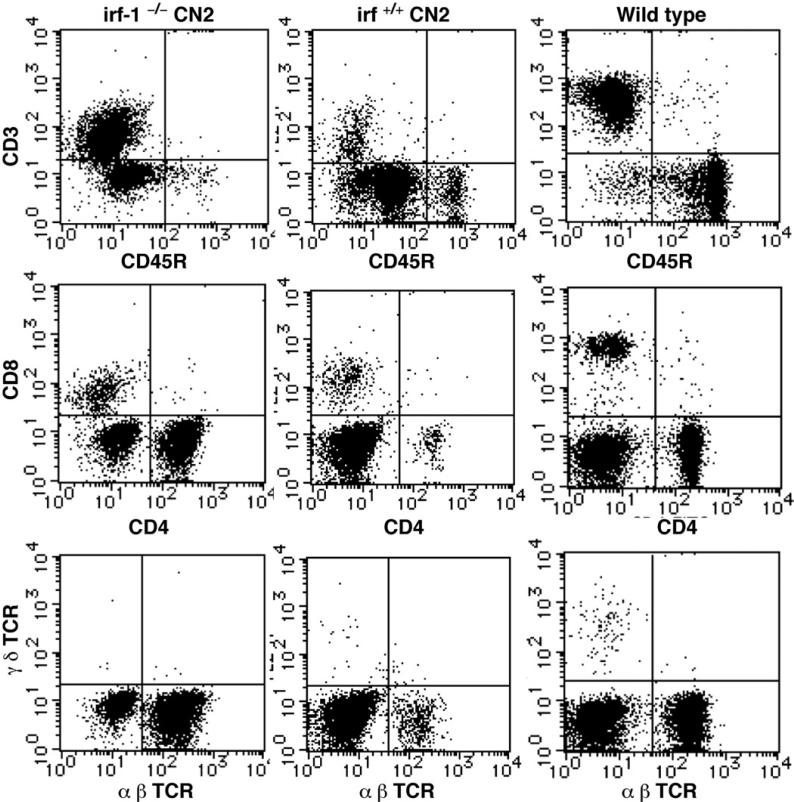
To characterize the disruption of lymphocyte proliferation due to HCV protein expression in the transgenic mice, we used flow cytometry to determine the ratio of T cells to B cells by staining with antibodies directed against CD3, CD45R, CD4, CD8, and the T-cell receptor. The average ratio of T cells to B cells in the lymph nodes and spleens of CN2 mice was significantly higher than that in the WT mice. The majority of the CD3+ lymphocytes and a few CD8+ lymphocytes expressed CD4 on their surfaces. The proliferating cells were mainly CD4+ T cells, although some were CD45R+B cells (Figure 3 and data not shown). The irf-1−/− CN2 mice also developed B-cell lymphomas (data not shown). These results confirm that HCV protein expression induces lymphoproliferative disorders that involve excessive expansion of both T and B cells. In irf-1−/− CN2 mice, the cell population that was negative for T-cell receptor (α, β, γ, and δ isoforms) staining was smaller than that in the other mice.
Figure 3.
HCV expression and irf-1 ablation affect the lymphocyte population. T-cell and B-cell proliferation in irf-1+/+ CN2 mice, irf-1−/− CN2 mice, and WT mice. CD3+, CD45R+, CD4+, CD8+, and T-cell receptor–positive cells from age-matched irf-1−/− CN2, irf-1+/+ CN2, and WT mice with hyperplasia were analyzed by fluorescence-activated cell sorting. Lymphocytes were prepared from CN2-8 and WT littermates at the age of 16 months, after administration of AxCANCre for 400 days.
Inhibition of Fas-Induced Apoptosis Owing to Disruption of irf-1 Leads to Persistent Expression of HCV in Transgenic Mouse Livers
The Fas ligand is essential for the development of hepatitis via cytotoxic T-lymphocyte–mediated cell killing.22 Therefore, we determined the sensitivities of irf-1 −/− hepatocytes to Fas-induced apoptosis. The irf-1−/− mice and WT littermates were injected intravenously with a monoclonal antibody against Fas. The disruption of irf-1 inhibited Fas-induced apoptosis, presumably by decreasing the levels of caspase-6 and -7 messenger RNA (mRNA; Supplementary Figure 2). These results suggest that the reduced expression of effector caspases delays Fas-mediated apoptosis in irf-1−/− mice and abrogates the elimination of HCV-expressing cells in vivo.
Stable Expression of HCV Proteins Induces Lymphoproliferative Diseases
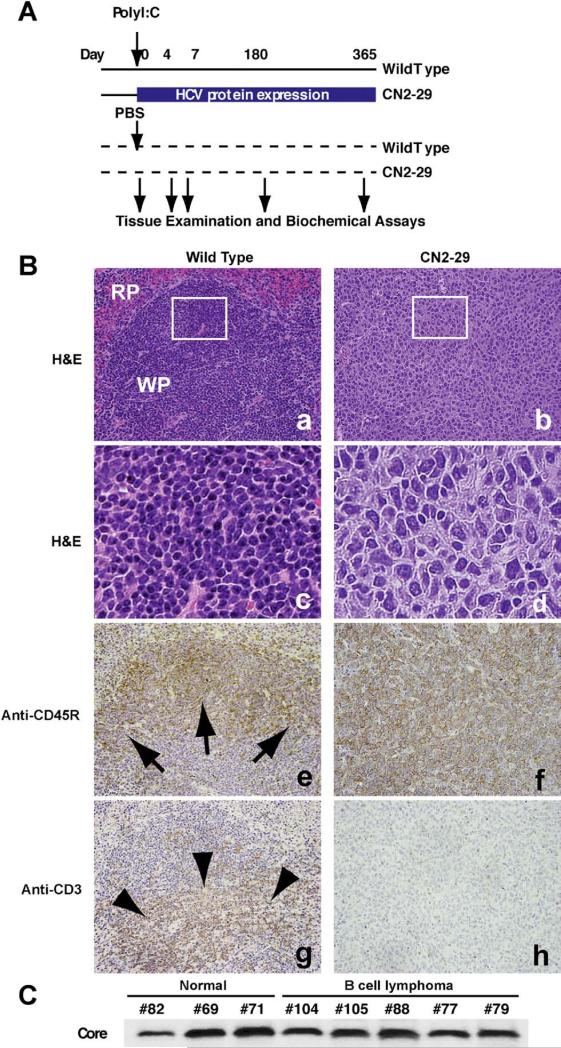
To confirm that HCV proteins induce lymphoproliferation without the adenoviral vector system, switching of the expression of HCV proteins was conducted using the Mx promoter–driven cre recombinase with poly(I:C) induction (Figure 4A). The Mx promoter is active in hepatocytes as well as in hematopoietic cells. We crossed CN2 mice with Mx1-Cre transgenic mice; CRE recombinase was expressed from the IFN-inducible Mx1 promoter. Injection of the Mx1-Cre/CN2-29 mice with poly (I:C) induced IFN production and efficiently induced the generation of CN2 gene products in hematopoietic cells (mainly in Kupffer cells and lymphocytes), liver, and spleen but not in most other tissues. At 7 days after induction of viral proteins, HCV core proteins were detected in both hepatocytes and hematopoietic cells (data not shown). After 180 days, almost 40% of the CN2(-29) mice developed lymphomas, whereas the WT mice did not (Figure 4B). The neoplastic cells were larger than lymphocytes, and their nuclei were irregular, round, oval, elongated, and polygonal. HCV core protein expression was confirmed by immunoblotting (Figure 4C), and increases in the levels of interleukin (IL)-2, IL-10, and IL-12 were observed (data not shown). The hematopoietic marker CD45R was detected in the lymphoproliferative regions and spleens (Figure 4B). The efficiency of expression switching was confirmed by both the HCV transgene copy numbers and protein expression using quantitative PCR and immunoblotting, respectively (Supplementary Figure 3). These results further validate that sustained expression of HCV proteins induces lymphoproliferation.
Figure 4.
Stable expression of HCV viral proteins induces lymphoproliferative diseases. (A) Switching of the expression of HCV proteins was conducted using the Mx promoter–driven cre recombinase with poly(I:C) induction. The Mx promoter is active in hepatocytes as well as in hematopoietic cells. We crossed CN2 mice with Mx1-Cre transgenic mice; Cre recombinase was expressed from the IFN-inducible Mx1 promoter. Injection of Mx1-Cre/CN2-29 mice with poly(I:C) induces IFN production and efficiently induces the expression of CN2 gene products in hematopoietic cells (mainly in Kupffer cells and lymphocytes), livers, and spleens but not in most other tissues. (B) The white pulp (WP) and red pulp (RP) comprise the components of the spleen in WT mice. The neoplastic cells replace the normal structures, such as the white pulp and red pulp. (c and d) The neoplastic cells are larger than lymphocytes (c), and the nuclei are irregular, round, oval, elon-gated, and polygonal (d). (e and g) The white pulp in WT mice consists of both a B-cell–rich area (arrows, e) and T-cell– rich area (arrowheads, g). (f and h) The neoplastic cells show staining for the B-cell marker CD45R, thereby supporting the diagnosis of B-cell lymphoma (f), while they do not show staining for the T-cell marker CD3 (h). Frames c and d are higher-magnification views of the white box areas in a and b, respectively. (C) Core protein expression was confirmed by immunoblotting.
Increased IL-2, IL-10, and IL-12 Levels in HCV Transgenic Mice
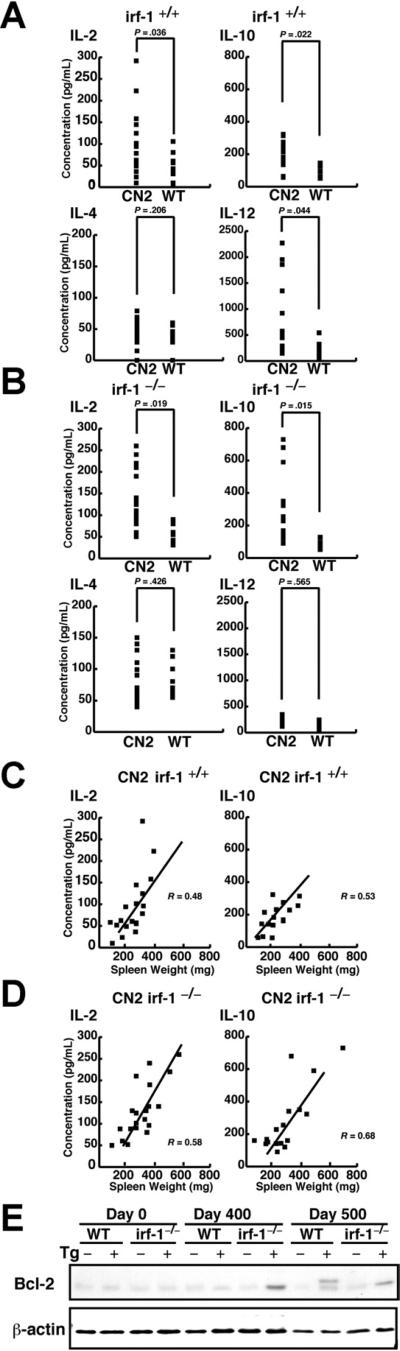
To study the mechanisms of HCV-induced lymphoproliferative diseases, we measured the serum IL-2, IL-4, IL-10, and IL-12 levels in the CN2 transgenic mice and their WT littermates (Figure 5A). The serum IL-4 concentration did not differ significantly between the CN2 and WT mice following injection with AxCANCre. However, the CN2 mice had significantly increased levels of serum IL-2, IL-10, and IL-12. Notably, the CN2 mice with proliferative disturbances in the lymph nodes and spleen had dramatically elevated levels of these cytokines, suggesting that altered cytokine production is involved in aberrant lymphocyte proliferation or differentiation in CN2 mice. In contrast, the irf-1−/− CN2 mice did not show elevated levels of serum IL-12 but had significantly higher levels of serum IL-2 and IL-10 compared with irf-1−/− mice (Figure 5B). Thus, the disruption of irf-1 abrogates the increase in IL-12 level but augments the increases in the levels of IL-2 and IL-10 in CN2 mice. These results indicate that IL-2 and IL-10 play key roles in the induction of the lymphoproliferative phenotype in irf-1−/− CN2 mice.
Figure 5.
HCV protein expression alters the cytokine profile. (A) The serum IL-2, IL-4, IL-10, and IL-12 levels in irf-1+/+ CN2 (Tg+) and irf-1+/+ WT mice were measured by enzyme-linked immunosorbent assay. (B) The serum IL-2, IL-4, IL-10, and IL-12 levels in irf-1−/− CN2 (Tg+) and irf-1−/− WT mice were measured by enzyme-linked immunosorbent assay. The P values are based on comparisons of the mean cytokine concentrations. (C and D) Relationship between the IL-2 or IL-10 concentration in the serum and the spleen weights of (C) CN2irf-1+/+ or (D) CN2irf1−/− mice with progressive lymphoproliferation. The numbers of points in the graphs correspond to the numbers of tested animals. (E) Bcl-2 protein levels in the lymph nodes of irf-1+/+ (WT) and irf-1−/− transgenic (CN2) (Tg+) and WT mice on days 0, 400, and 500 after the administration of AxCANCre. Bcl-2 migrates at 26 kilodaltons. β-Actin was used as a loading control.
To verify the relationship between the weights of the lymph organs and the cytokine levels, the correlation coefficients were calculated according to Pearson (Figure 5C and 5D). Whereas spleen weight did not markedly influence the increase in IL-4 level (data not shown), a significant positive correlation was found between spleen weight and increased IL-2 and IL-10 levels in CN2 gene-expressing mice on the irf-1−/− background (R = 0.58, P < .05, and R = 0.68, P < .05, respectively) (Figure 5D). With respect to the serum levels of IL-2 and IL-10, a less intensive but significant positive correlation was found between the cytokine levels and spleen weights of CN2 gene-expressing mice on the irf-1+/+ background (R = 0.43, P < .05, and R = 0.53, P < .05, respectively) (Figure 5C). These results indicate that IL-2 and IL-10 are involved in lymphoproliferation in viral protein-expressing mice.
Aberrant Expression of Bcl-2 in Expanded Lymph Nodes of CN2 Mice
Bcl-2 immunoglobulin transgenic mice develop follicular lymphoproliferation23 due to the inability of various stimuli to induce apoptosis in these mice.24 Therefore, to examine whether HCV causes dysregulation of Bcl-2 in lymphoid tissues, we examined the expression of Bcl-2 (Figure 5E). Lymph nodes collected from irf-1−/− CN2 mice 400 days after the administration of AxCANCre showed elevated levels of Bcl-2. Immunoblot analysis revealed that a doublet for Bcl-2 (26 and 28 kilodaltons) appeared in some samples 500 days after AxCANCre administration, suggesting the presence of phosphorylated and nonphosphorylated Bcl-2.25
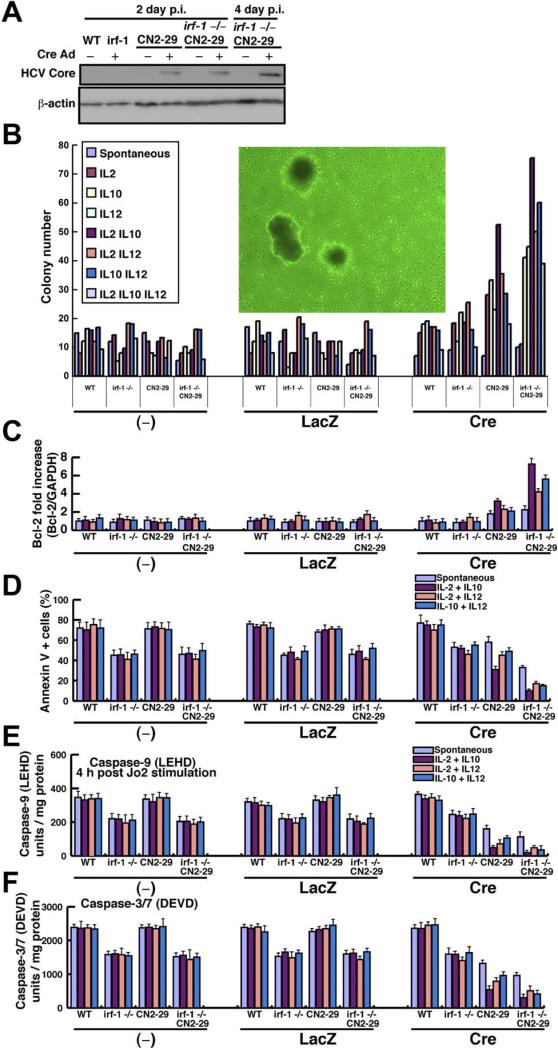
Combination Cytokine Treatment Enhances Splenocyte Colony Formation in Synergy With Viral Protein Expression
To determine whether aberrant cytokine profiles contribute to lymphocyte transformation, a colony formation assay was performed using the methylcellulose method. Mouse splenocytes were infected with adenoviruses that expressed the cre DNA recombinase or lacZ control. Expression of HCV core proteins was induced by cre-adenovirus infection of the splenocytes (Figure 6A). Colony counting was performed at postinfection day 28 (Figure 6B). Combined treatment with IL-2 and IL-10 greatly enhanced colony formation, especially in the splenocytes of HCV transgenic mice (CN2-29, irf-1−/−
Figure 6.
Lymphocyte transformation by aberrant cytokines and inhibition of apoptotic signaling. (A) Expression of the HCV core protein (21 kilodaltons) in irf-1+/+ (WT) and irf-1−/− transgenic (CN2-29) and WT mice 2 or 4 days postinfection (p.i.) with AxCANCre (multiplicity of infection, 1.0). β-Actin was used as a loading control. (B) Colony formation assay for splenocytes from irf-1+/+ (WT) and irf-1−/− WT or transgenic (CN2-29) mice in the absence or presence of the indicated cytokine and infected with mock, LacZ, and Cre adenoviruses. The inset shows an image of the colonies generated from the irf-1−/− CN2 splenocytes (original magnification 10×). (C) Quantification, by quantitative reverse-transcription PCR of Bcl-2 mRNA relative to control glyceraldehyde-3-phosphate dehydrogenase mRNA in the splenocytes of irf-1+/+ (WT) and irf-1−/− or transgenic (CN2-29) mice treated with the indicated cytokines and infected with mock, LacZ, and cre adenoviruses. (D) Apoptosis measured by Annexin V fluorescence-activated cell sorting analysis of splenocytes from irf-1+/+ (WT) and irf-1−/− or transgenic (CN2-29) mice treated with the indicated cytokines and infected with the mock, LacZ, and cre adenoviruses. (E and F) The caspase-9 and caspase-3/7 enzymatic activities in splenocytes from irf-1+/+ (WT) and irf-1−/− or transgenic (CN2-29) mice treated with the indicated cytokines were measured using a substrate cleavage assay after infection with the mock, LacZ, and Cre adenoviruses. Caspase-9 activity was measured 4 hours after injection of the anti-Fas monoclonal antibody (Jo2). LEHD, substrate for caspase-9; DEVD, substrate for caspase-3/7. Vertical bars are SD and were determined using the Student t test.
CN2-29). The addition of IL-12 suppressed colony formation induced by combined treatment with IL-2 and IL-10. In the irf-1−/− background, treatment with IL-2 plus IL-10 or IL-2 plus IL-12 greatly enhanced colony formation. To determine whether enhanced colony formation correlated with cytokine-induced Bcl-2 expression, the Bcl-2 mRNA levels in the splenocytes were quantified (Figure 6C). Because IL-2 enhances T-lymphocyte proliferation and transformation,26 it is of particular interest that treatment with IL-2 plus IL-10 resulted in marked increases in both lymphocyte transformation and the Bcl-2 mRNA levels upon HCV transgene expression. These results indicate that dysregulated cytokine expression, disruption of irf-1, and HCV transgene expression synergistically enhance splenocyte transformation.
Cytokine Treatment and HCV Transgene Expression Synergistically Inhibit Fas-Mediated Apoptosis
To determine whether cytokines inhibit Fas-induced apoptosis, we treated the splenocytes from transgenic and WT mice with cytokines and then measured Fas-induced apoptosis by Annexin V staining and fluorescence-activated cell sorting, and we also assayed caspase enzymatic activity (Figure 6D and 6E). IL-10 treatment in the presence of IL-2 greatly inhibited Fas-induced apoptosis. Furthermore, irf-1 disruption made the splenocytes resistant to Fas-induced apoptosis in the presence of IL-2, IL-10, and/or IL-12. In particular, IL-2 plus IL-10 treatment produced the strongest inhibition of Fas-induced apoptosis. These cytokines also up-regulated the Bcl-2 mRNA levels in splenocytes, which indicates that IL-2, IL-10, and/or IL-12 up-regulate bcl-2 expression, which subsequently inhibits Fas-induced apoptosis. This result is consistent with reports that IL-10 and/or IL-2 treatment induce bcl-2 in B or T lymphocytes.10,27 Caspase-3/7 activity was correlated with the level of bcl-2 expression (Figure 6C and 6F). These results indicate that aberrant cytokine expression and disruption of IFN signaling affect bcl-2 expression, which is associated with the inhibition of caspase expression.
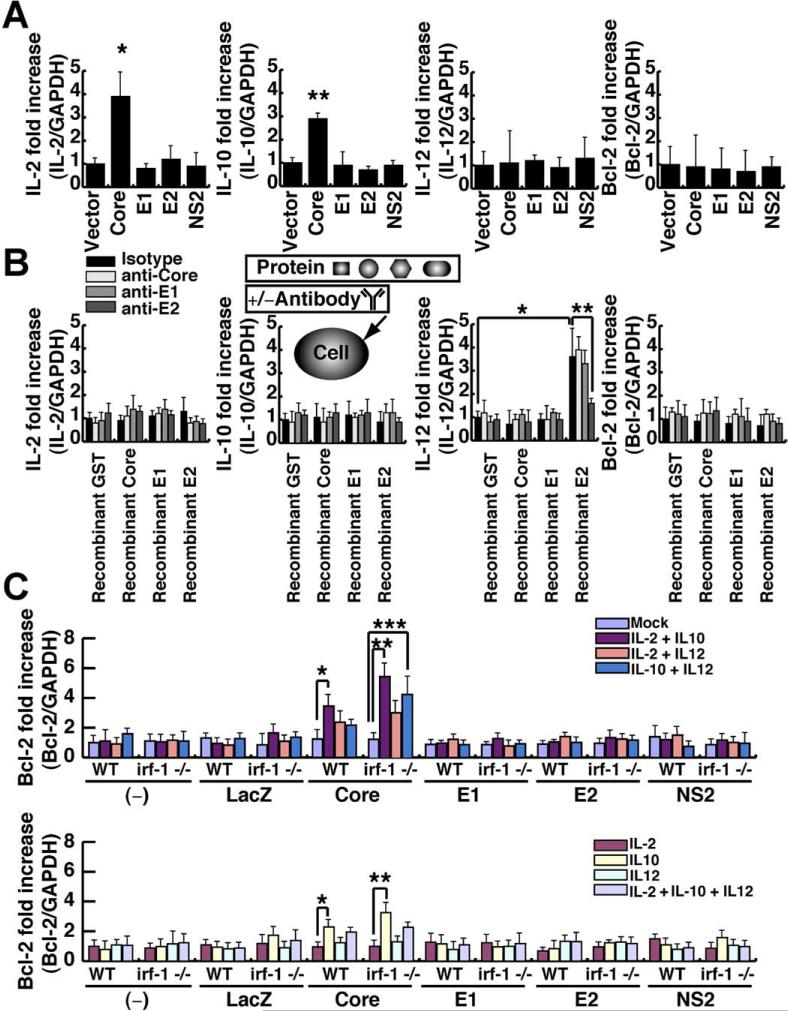
HCV Core and E2 Proteins Mediate IL-2, IL-10, and IL-12 Expression
To determine which viral protein is responsible for cytokine expression, individual viral proteins were stably expressed in splenocytes using recombinant lenti-viruses that express the HCV core, E1, E2, NS2, and lacZ. Each gene expression profile was confirmed by reverse-transcription PCR (Supplementary Figure 4). Only the HCV core protein induced IL-2 and IL-10 (Figure 7A). To determine whether extracellular viral proteins trigger cytokine expression, recombinant viral proteins were added to the cells. Only the viral envelope protein E2 induced IL-12 (Figure 7B). These results indicate that the HCV core and E2 proteins are responsible for IL-2, IL-10, and IL-12 expression.
Figure 7.
Induction of IL-2 and IL-10 by HCV core and IL-12 by E2 and of Bcl-2 by HCV core plus cytokines. (A) Individual viral proteins were stably expressed in splenocytes using recombinant lentiviruses that expressed the HCV core, E1, E2, NS2, and lacZ. Each gene expression profile was determined by quantitative reverse-transcription PCR. (B) E2 binding induces IL-12 in Raji cells, as determined by quantitative reverse-transcription PCR. Cells were treated with HCV core, E1, E2 (genotypes 1a and 1b), or glutathione S-transferase proteins, and the cytokine and bcl2 cellular RNA levels were examined using quantitative reverse-transcription PCR. (C) Quantification by quantitative reverse-transcription PCR of Bcl-2 mRNA relative to control glyceralde-hyde-3-phosphate dehydrogenase mRNAin splenocytes from irf-1+/+ (WT) and irf-1−/− WT or irf-1−/− mice treated with the indicated cytokines and infected with lentiviruses that express mock, core, E1, E2, NS2, and LacZ. Individual viral proteins were stably expressed using lentiviral vectors, and the cells were tested for Bcl-2 expression.
HCV Core and IL-10 Induce Bcl-2 Expression
To determine whether viral protein expression and cytokine stimulation synergistically induce Bcl-2 expression, individual viral proteins were stably expressed using lentiviral vectors, and the cells were tested for Bcl-2 expression. Core protein expression and IL-10 stimulation induced Bcl-2, while the other proteins did not (Figure 7C). Interestingly, the combination of IL-2 and IL-12 only induced Bcl-2 in the irf-1−/− background, while triple stimulation (IL-2, IL-10, and IL-12) did not induce Bcl-2 (Figure 7C). These results indicate that complex signaling networks induce Bcl-2 in the presence of viral nucleocapsid proteins.
Discussion
The present study shows that Bcl-2 levels, cytokine levels, aging, and inflammation enhance the development of lymphoproliferative disorders caused by HCV proteins (Supplementary Figure 5). Disruption of irf-1 enables the persistent expression of HCV protein, leading to lymphoproliferative diseases owing to reduced apoptosis (ie, lower levels of caspase-1, -6, and -7 expression). HCV CN2 transgenic (Tg+) mice are resistant to Fas-induced apoptosis due to the inhibition of cytochrome c release from mitochondria.16 Mice with disruption of irf-1 have several defects of their innate and adaptive immunity, such as lineage-specific defects in thymocyte development; immature T cells can develop into mature CD4+ cells but not into CD8+ T cells.18,28 IRF-1 controls the positive and negative selection of CD8+ thymocytes.29 IRF-1 is required for the development of the Th1-type immune response, and its absence leads to the induction of the Th2-type immune response.18,30 Because the number of natural killer cells is dramatically reduced in irf-1−/− mice,18 this defect may cause the marked increase in viral protein expression and the inhibition of tumor surveil-lance mechanisms, leading to the development of non-Hodgkin's lymphoma. Expression of the IL-12 p40 subunit is defective in irf-1−/− mice.18
Lymphomagenesis may require the additional genetic instability provided by HCV-induced hypermutation (2-hit model). Important questions are raised regarding the lymphoproliferative mechanisms of lymphomas in HCV-infected patients (B-cell malignancies predominate). Hy-permutation of the immunoglobulin genes in B cells induced by HCV infection is the cause of the lymphomagenesis seen in HCV infection,21,31 and this model may provide more direct insights into lymphoma production, because HCV-induced hypermutation causes genetic instability and causes chromosomal aberrations, possibly resulting in neoplastic transformation.32 In addition, the antiapoptotic phenotype generated by sustained viral protein expression may enhance the survival of lymphocytes and inhibit activation-induced cell death to turn off the activated lymphocytes. The dysregulated cytokine profiles and sustained lymphocyte survival may alter the fates of regulatory T cells and dendritic cells.33
In conclusion, the present study shows that the conditional expression of HCV proteins induces inflammation and lymphoproliferative disorders, which are enhanced by irf-1 disruption. Therefore, IRF-1–inducible genes probably play essential roles in suppressing HCV-induced lymphoma and in eliminating HCV protein-expressing cells. Our transgenic mice provide evidence that the overexpression of apoptosis-related proteins, including Bcl-2, and/or aberrant cytokine production are primary events in HCV-induced lymphoproliferation. It is interesting to note that lymphoproliferation was dominant over liver tumor development in the present study. Approximately 40% of the CN2-29Mx1Cre mice developed B-cell lymphomas, while 5% of the mice developed liver tumors. Further molecular analyses will enlighten the differential signaling pathways between hepatocytes and lymphocytes and increase our understanding of the differences between lymphomagenesis and liver tumor development caused by HCV.
Supplementary Material
Acknowledgments
The authors thank Prof Tadatsugu Taniguchi for his scholarly support of this study; Kazuaki Inoue and Kentaro Tomita for their advice on histology; Yutaka Amako, Isao Maruyama, and Kohsuke Tanaka for technical assistance; and Mitsugu Takahashi for breeding the transgenic mice.
Funding
Supported in part by a research fellowship from the Japan Society for the Promotion of Science; a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a grant from the Ministry of Health, Labour and Welfare of Japan; and the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation of Japan. This project was also supported by National Institutes of Health research grants P50AA11999, 5P30DK048522-13, and CA108302.
Abbreviations used in this paper
- IFN
interferon
- IL
interleukin
- IRF
interferon-regulatory factor
- PCR
polymerase chain reaction
- WT
wildtype
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Supplementary Data
To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2009.03.061.
References
- 1.Saito I, Miyamura T, Ohbayashi A, et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990;87:6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonetti RG, Camma C, Fiorello F, et al. Hepatitis C virus infection as a risk factor for hepatocellular carcinoma in patients with cirrhosis. A case-control study. Ann Intern Med. 1992;116:97–102. doi: 10.7326/0003-4819-116-2-97. [DOI] [PubMed] [Google Scholar]
- 3.Ferri C, Monti M, La Civita L, et al. Infection of peripheral blood mononuclear cells by hepatitis C virus in mixed cryoglobulinemia. Blood. 1993;82:3701–3704. [PubMed] [Google Scholar]
- 4.Silvestri F, Pipan C, Barillari G, et al. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood. 1996;87:4296–4301. [PubMed] [Google Scholar]
- 5.Rui L, Goodnow CC. Lymphoma and the control of B cell growth and differentiation. Curr Mol Med. 2006;6:291–308. doi: 10.2174/156652406776894563. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich CF, Lee JH, Herrmann G, et al. Enlargement of perihepatic lymph nodes in relation to liver histology and viremia in patients with chronic hepatitis C. Hepatology. 1997;26:467–472. doi: 10.1002/hep.510260230. [DOI] [PubMed] [Google Scholar]
- 7.Ascoli V, Lo Coco F, Artini M, et al. Extranodal lymphomas associated with hepatitis C virus infection. Am J Clin Pathol. 1998;109:600–609. doi: 10.1093/ajcp/109.5.600. [DOI] [PubMed] [Google Scholar]
- 8.De Vita S, De Re V, Sansonno D, et al. Gastric mucosa as an additional extrahepatic localization of hepatitis C virus: viral detection in gastric low-grade lymphoma associated with autoimmune disease and in chronic gastritis. Hepatology. 2000;31:182–189. doi: 10.1002/hep.510310127. [DOI] [PubMed] [Google Scholar]
- 9.Mele A, Pulsoni A, Bianco E, et al. Hepatitis C virus and B-cell non-Hodgkin lymphomas: an Italian multicenter case-control study. Blood. 2003;102:996–999. doi: 10.1182/blood-2002-10-3230. [DOI] [PubMed] [Google Scholar]
- 10.Cohen SB, Crawley JB, Kahan MC, et al. Interleukin-10 rescues T cells from apoptotic cell death: association with an upregulation of Bcl-2. Immunology. 1997;92:1–5. doi: 10.1046/j.1365-2567.1997.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlotsky JM. The nature of interferon-alpha resistance in hepatitis C virus infection. Curr Opin Infect Dis. 2003;16:587–592. doi: 10.1097/00001432-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Levine AM, Shimodaira S, Lai MM. Treatment of HCV-related mantle-cell lymphoma with ribavirin and pegylated interferon alfa. N Engl J Med. 2003;349:2078–2079. doi: 10.1056/NEJM200311203492121. [DOI] [PubMed] [Google Scholar]
- 13.Moriya K, Fujie H, Shintani Y, et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 14.Lerat H, Honda M, Beard MR, et al. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology. 2002;122:352–365. doi: 10.1053/gast.2002.31001. [DOI] [PubMed] [Google Scholar]
- 15.Wakita T, Taya C, Katsume A, et al. Efficient conditional trans-gene expression in hepatitis C virus cDNA transgenic mice mediated by the Cre/loxP system. J Biol Chem. 1998;273:9001–9006. doi: 10.1074/jbc.273.15.9001. [DOI] [PubMed] [Google Scholar]
- 16.Machida K, Tsukiyama-Kohara K, Seike E, et al. Inhibition of cytochrome c release in Fas-mediated signaling pathway in transgenic mice induced to express hepatitis C viral proteins. J Biol Chem. 2001;276:12140–12146. doi: 10.1074/jbc.M010137200. [DOI] [PubMed] [Google Scholar]
- 17.Yokota T, Oritani K, Takahashi I, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 18.Taki S, Sato T, Ogasawara K, et al. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity. 1997;6:673–679. doi: 10.1016/s1074-7613(00)80443-4. [DOI] [PubMed] [Google Scholar]
- 19.Yanagi M, Purcell RH, Emerson SU, et al. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc Natl Acad Sci U S A. 1997;94:8738–8743. doi: 10.1073/pnas.94.16.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanagi M, St Claire M, Shapiro M, et al. Transcripts of a chimeric cDNA clone of hepatitis C virus genotype 1b are infectious in vivo. Virology. 1998;244:161–172. doi: 10.1006/viro.1998.9092. [DOI] [PubMed] [Google Scholar]
- 21.Machida K, Cheng KT, Pavio N, et al. Hepatitis C virus E2-CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol. 2005;79:8079–8089. doi: 10.1128/JVI.79.13.8079-8089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo Y, Sung VM, Machida K, et al. Hepatitis C virus infects T cells and affects interferon-gamma signaling in T cell lines. Virology. 2007;361:161–173. doi: 10.1016/j.virol.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 23.McDonnell TJ, Deane N, Platt FM, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 24.Lacronique V, Mignon A, Fabre M, et al. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- 25.Ito T, Deng X, Carr B, et al. Bcl-2 phosphorylation required for anti-apoptosis function. J Biol Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 26.Stern JB, Smith KA. Interleukin-2 induction of T-cell G1 progression and c-myb expression. Science. 1986;233:203–206. doi: 10.1126/science.3523754. [DOI] [PubMed] [Google Scholar]
- 27.Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93:424–428. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taniguchi T, Ogasawara K, Takaoka A, et al. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 29.Penninger JM, Sirard C, Mittrucker HW, et al. The interferon regulatory transcription factor IRF-1 controls positive and negative selection of CD8[H11001] thymocytes. Immunity. 1997;7:243–254. doi: 10.1016/s1074-7613(00)80527-0. [DOI] [PubMed] [Google Scholar]
- 30.Lohoff M, Ferrick D, Mittrucker HW, et al. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity. 1997;6:681–689. doi: 10.1016/s1074-7613(00)80444-6. [DOI] [PubMed] [Google Scholar]
- 31.Machida K, Cheng KT, Sung VM, et al. Hepatitis C virus induces a mutator phenotype: enhanced mutations of immunoglobulin and protooncogenes. Proc Natl Acad Sci U S A. 2004;101:4262–4267. doi: 10.1073/pnas.0303971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machida K, Kondo Y, Huang JY, et al. Hepatitis C virus (HCV)-induced immunoglobulin hypermutation reduces the affinity and neutralizing activities of antibodies against HCV envelope protein. J Virol. 2008;82:6711–6720. doi: 10.1128/JVI.02582-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolganiuc A, Paek E, Kodys K, et al. Myeloid dendritic cells of patients with chronic HCV infection induce proliferation of regulatory T lymphocytes. Gastroenterology. 2008;135:2119–2127. doi: 10.1053/j.gastro.2008.07.082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.