Abstract
Purpose
To determine the association of single nucleotide polymorphisms (SNPs) of the thrombospondin 1 (THBS1) gene with development of chronic ocular surface inflammation (keratoconjunctivitis) after refractive surgery.
Design
Retrospective cohort study.
Participants
Active duty U.S. Army soldiers (n = 143) who opted for refractive surgery.
Methods
Conjunctival impression cytology samples collected from participants before the surgery were used to harvest DNA for genotyping 5 THBS1 SNPs (rs1478604, rs2228262, rs2292305, rs2228262, and rs3743125) using the Sequenom iPLEX Gold platform (Sequenom, San Diego, CA). Samples collected after surgery were used to harvest RNA for gene expression analysis by real-time polymerase chain reaction (PCR). Participants were followed for 1 year after surgery to monitor the status of keratoconjunctivitis.
Main Outcome Measures
Genetic basis of the development of chronic keratoconjunctivitis after refractive surgery.
Results
Carriers of minor alleles of 3 SNPs each were found to be more susceptible to developing chronic keratoconjunctivitis (rs1478604: odds ratio [OR], 2.5; 95% confidence interval [CI], 1.41–4.47; P = 2.5×10−3; rs2228262 and rs2292305: OR, 1.9; 95% CI, 1.05–3.51; P = 4.8×10−2). Carriers of the rs1478604 minor allele expressed significantly reduced levels of thrombospondin 1 (TSP1) (P = 0.042) and increased levels of an inflammatory cytokine associated with keratoconjunctivitis, interleukin-1 β (P = 0.025), in their ocular surface epithelial cells compared with homozygous major allele controls.
Conclusions
Genetic variation in the THBS1 gene that results in decreased expression of the encoded glycoprotein TSP1 in ocular surface epithelial cells significantly increases the susceptibility to develop chronic ocular surface inflammation after refractive surgery. Further investigation of THBS1 SNPs in a larger sample size is warranted.
Because refractive surgeries are minimally invasive, they are a widely used form of vision correction to treat myopia, hyperopia, and astigmatism. An estimated 1.5 million procedures are performed per year.1 During refractive surgery, disruption of the ocular surface structures, especially the innervations, leads to dry eye. Although this condition is transient in some individuals, it develops into a chronic form in many others.2–4 Dry eye is the most common complication observed after refractive surgery, with 40% to 60% of patients reported to develop this condition.3,5,6 Dry eye affects tear composition, with consequential ocular discomfort and visual disturbances, and is accompanied by inflammation of the ocular surface. A chronic inflammatory condition of the ocular surface results in damage to the ocular surface with decreased corneal and conjunctival integrity and a loss of mucin-secreting goblet cells.7 In general, dry eye is multifactorial in cause, with risk factors other than refractive surgery, such as age,8 hormonal changes,9 dry environments,10 contact lens wear,11 use of medications (e.g., antihistamines and diuretics), and systemic autoimmune disorders.8 Not all individuals exposed to these risk factors develop dry eye; however, the role of genetic factors has remained unaddressed, presumably because of the diversity of risk factors and the lack of a consensus regarding diagnostic criteria and classification of dry eye conditions until recently (2007 Report of the Dry Eye Workshop).7
Dry eye due to a failure of tear secretion by the lacrimal glands is referred to as “aqueous tear-deficient dry eye,” which is further subclassified on the basis of autoimmune pathogenesis as Sjögren’s syndrome dry eye (SSDE) and non-SSDE. Disrupted corneal innervations during refractive surgery interrupt the stimulation of the lacrimal gland, resulting in aqueous tear-deficient dry eye. Such dry eye normally is a self-resolving condition that improves with the recovery of corneal sensitivity, but in some it progresses into a chronic condition often lasting more than 6 months. Further classification of dry eye after refractive surgery remains unclear, possibly because of presumed nonautoimmune pathology underlying both self-resolving and chronic dry eye conditions. Although clinical manifestation of chronic ocular surface inflammation after refractive surgery resembles that seen in SSDE, the evidence of autoimmune pathology in the former remains to be established in human subjects. In murine studies, however, ocular surface stress has been reported to induce dry eye with an autoimmune pathology,12–15 but it is not clear whether these studies are in essence describing SSDE. Therefore, on the basis of the current understanding of the pathogenesis, dry eye after refractive surgery may be considered non-SSDE; however, growing evidence from murine studies suggests a potential autoimmune pathogenic mechanism underlying the resultant chronic inflammatory condition of the ocular surface.
Although the pathology underlying chronic dry eye after refractive surgery remains unclear, we sought to evaluate a potential genetic association of this condition as a way to shed some light on its pathogenesis. To address the challenge of the etiological heterogeneity of dry eye, in this study we chose a distinct causative factor, such as refractive surgery, to determine the genetic predisposition of healthy individuals who undergo this procedure to develop chronic dry eye. Thus, our study design helped avoid any influence of other factors that may contribute to the development of dry eye.
Deficiency of thrombospondin 1 (TSP1) in mice results in the spontaneous development of a chronic dry eye condition associated with autoimmune Sjögren’s syndrome.16 Murine ocular surface inflammation bears a striking resemblance to that reported in human dry eye when taking into consideration various diagnostic parameters. These include altered tear quality, lacrimal gland dysfunction, disruption of the ocular surface barrier, reduced goblet cell density, and expression of inflammatory markers in the ocular surface tissue. A large multidomain glycoprotein, TSP1 is produced by activated platelets and many other cell types, including epithelial cells of the ocular surface.17,18
On the basis of the significant role of TSP1 identified in the regulation of dry eye pathogenesis, we chose the thrombospondin 1 (THBS1) gene as a candidate gene, located on human chromosome 15, to begin evaluation of whether genetic variants of this gene in human subjects increase their susceptibility to developing dry eye. Polymorphisms in the THBS1 gene encoding this molecule have been associated with altered calcium binding capacity, reduced plasma levels, and increased risk of myocardial infarction in humans.19–21 In this study, we evaluated the association of previously identified single nucleotide polymorphisms (SNPs), selected to represent different functional domains of TSP1 and regulatory areas of the THBS1 gene, with the development of dry eye after refractive surgery. Our results indicate a significant association between THBS1 polymorphism with dry eye and provide the first such evidence for a potential genetic basis for this condition.
Methods
Subjects
This research followed the tenets of the Declaration of Helsinki. The institutional review board of the Department of the Army approved the study protocol. All patients gave written informed consent.
A total of 143 active duty U.S. Army soldiers wearing glasses or contact lenses for myopia with or without astigmatism were initially included in this study. Subjects aged 21 to 40 years were recruited from those who requested refractive surgery. The selected subjects had manifest refractive spherical equivalent of up to −10.00 diopters (D) at the spectacle plane with refractive cylinder up to 3.00 D, with best spectacle-corrected visual acuity of ≥ 20/20 in both eyes and demonstrated refractive stability (neither the spherical nor the cylindrical portion of the refraction changed >0.50 D during the 12-month period immediately preceding the baseline examination) as confirmed by clinical records. These subjects also were willing to fill out dry eye questionnaires (McMonnies) and to return for follow-up visits 1, 3, and 7 days and 1, 3, 6, and 12 months after their surgery. Patients were excluded from the study who were pregnant, had previous surgery or trauma to the study eye, had severe dry eye as reflected by Schirmer test with anesthesia of 0, had any active ophthalmic disease, or had a history of medical conditions or medications that may impair healing.
Subjects underwent 1 of the 2 refractive surgeries: LASIK or photorefractive keratotomy. Comprehensive eye examinations were performed before the surgery; 1, 3, and 7 days immediately after the procedure and 1, 3, 6, and 12 months after surgery. Clinical assessments included measurement of tear production using the Schirmer test, determination of tear film stability using sodium fluorescein tear break-up time, evaluation of ocular surface integrity with Rose Bengal staining, McMonnies dry eye questionnaire, and impression cytology procedure. Impression cytology represents a noninvasive or minimally invasive alternative to biopsy of the ocular surface epithelium22 and was performed before the surgery and repeated 3 months after surgery. Specifically, 2 conjunctival impression cytologies were obtained from the nasal interpalpebral and inferior palpebral conjunctiva of each patient, using a circular disc of cellulose acetate filter pressed onto the conjunctival tissue for 2 seconds.
According to the diagnostic criteria shown in Table 1, each subject was given a score at each postoperative visit (at 1, 3, 6, and 12 months) as follows: 0 = normal, 1 = intermediate, and 2 = dry eye. After 12 months, all visits were tallied to categorize overall dry eye status. The “control” group in this study includes normal subjects with a score of 0 at the 6-month visit and 0 or 1 at all other visits, that is, no visits with a score of 2; all the others formed the “dry eye” group, which included subjects with chronic dry eye (intermediate and dry eye) that persisted beyond 6 months.
Table 1.
Diagnostic Criteria for Dry Eye after Refractive Surgery
| Dry Eye
|
||
|---|---|---|
| Control (Normal) | Intermediate | Dry Eye |
| ✓No symptoms | ✓No Symptoms | ✓Symptoms |
| ✓No punctal plugs | ✓No punctal plugs | ✓Punctal plugs |
| ✓No SPK | ✓No SPK | ✓SPK |
| ✓RB score = 0 | ✓0 ≤ RB score ≤ 1 | ✓RB score >1 ✓Or combination of 2 of the following: |
| ✓TBUT ≥10 | ✓5 ≤ TBUT <10 | TBUT <5 |
| ✓Schirmer tear test ≥10 | ✓5 ≤ Schirmer <10 | Schirmer tear test <5 |
| ✓McMonnies questionnaire: | ✓McMonnies questionnaire: | McMonnies questionnaire: score >20 |
| ✓Male patients score ≤10 | ✓Male patients 10 < score ≤20 | |
| ✓Female patients score ≤15 | ✓Female patients 15 < score ≤20 | |
RB = Rose Bengal; SPK = superficial punctate keratitis; TBUT = tear break-up time.
Punctal plugs are a device inserted into the tear duct to prevent drainage of liquid from the eye. SPK is the death of small groups of cells on the surface of the cornea (usually pinpoint).
Single Nucleotide Polymorphism Selection
The candidate gene of this study, THBS1, encodes a glycoprotein, TSP1, with multiple functional domains (Fig 1). Each domain has been characterized with a distinct function, for example, binding of latent transforming growth factor (TGF)- β and CD36 is associated with type I repeats in domain I and calcium binding is associated with domain III.23 To search for genomic variants that may confer a risk of dry eye, we reviewed SNPs identified in the THBS1 region (Chr15:37660572-37676959). In a 16.39-kbp-long portion of THBS1 that spans mRNA positions 42–4403 and covers exons coding for all the TSP1 domains in addition to noncoding 5′- and 3′-untranslated regions (UTRs), 21 SNPs have been identified and registered in the Phase II HapMap database for 4 populations (Yoruba in Ibadan, Nigeria [YRI]; Japanese in Tokyo, Japan [JPT]; Han Chinese in Beijing, China [CHB]; CEPH [Utah residents with ancestry from northern and western Europe; CEU]).24 We performed pairwise tagging in Haploview (r2 = 0.8) for the available SNPs in HapMap populations (www.hapmap.org, accessed March 22, 2013) and selected tagged SNPs from each. Two SNPs (rs1478604 and rs2228262) were consistently tagged in all the populations. We then selected SNPs that represented different functional domains of TSP1 and were in high linkage disequilibrium (LD) with the 2 tagged SNPs in at least 2 HapMap populations.25 A total of 5 SNPs, including 2 tagged SNPs, as indicated in Table 2 and Figure 1, were used in this study. Global minor allele frequencies of all the selected SNPs were >0.01.
Figure 1.
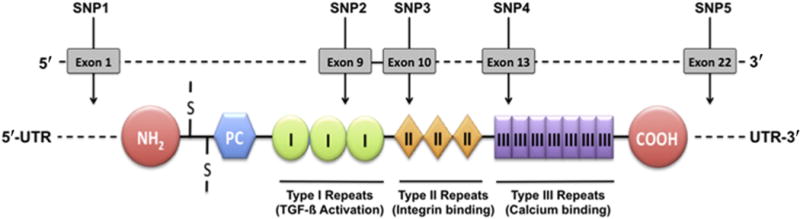
Location of genotyped THBS1 polymorphisms. COOH = globular C-terminal domain; NH2 = globular N-terminal domain; PC = procollagen homology domain; S-S = interchain disulfide bonds; SNP = single nucleotide polymorphism; TGF-β = transforming growth factor-β; UTR = untranslated region; I = type I repeats; II = type II repeats; III = type III repeats.
Table 2.
Description and Location of the Analyzed THBS1 Polymorphisms
| mRNA
|
Protein
|
|||||||
|---|---|---|---|---|---|---|---|---|
| SNP | Reference SNP | MAF | Function | Position | Allele change | Position | Residue change | Location |
| SNP1 | rs1478604 | 0.41 | UTR-5 | 42 | T → C | NA | NA | UTR-5 |
| SNP2 | rs2228261 | 0.241 | Synonymous | 1589 | AAC → AAT | 470 | N → N | Type I repeats |
| SNP3 | rs2292305 | 0.253 | Missense | 1746 | ACA → GCA | 523 | T → A | Type II repeats |
| SNP4 | rs2228262 | 0.053 | Missense | 2278 | AAT → AGT | 700 | N → S | Type III repeats |
| SNP5 | rs3743125 | 0.163 | UTR-3 | 3970 | G → A | NA | NA | UTR-3 |
MAF = minor allelic frequency; NA = not applicable; SNP = single nucleotide polymorphism; UTR = untranslated region.
Genotyping
Genomic DNA was obtained from conjunctival impression cytology (CIC) samples of 143 patients before refractive surgery, using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA). Total DNA concentration was measured with the Quant-iT dsDNA kit (Invitrogen, Eugene, OR). Samples that generated adequate DNA (5–10 ng/μ1) (133 samples) were genotyped for 5 THBS1 SNPs using the Sequenom iPLEX Gold platform (Sequenom, San Diego, CA) at the Molecular Genetics core of Children’s Hospital, Boston, and Harvard Medical School. The SNP ID (dbSNP), the genomic and protein positions, and the nucleotide exchanges of the genotyped THBS1 SNPs are shown in Table 2. To control for possible confounding by population stratification, a panel of 90 ancestry-informative markers (AIMs) was screened in all samples.26
Quality Control
We genotyped 5 selected SNPs (SNP1–5) in 133 individuals. Samples with a genotype rate <85% were excluded (n = 58). The numbers of samples excluded from the control and dry eye groups did not differ significantly (P > 0.05; odds ratio [OR], 1.5; confidence interval [CI], 0.6–3.6). A final set of 16 controls and 59 subjects with dry eye, with a genotyping rate of 98%, was analyzed further. This quality-control step did not result in any change in overall population characteristics in the 2 groups (Table 3, available at www.aaojournal.org). All but 1 SNP were in Hardy–Weinberg equilibrium (HWE) (SNP1–4, P > 0.3; SNP5 P = 0.0023). Therefore, SNP5 was excluded from further association analysis. As an additional quality control, a sex SNP was genotyped and matched with the demographic data. All the sex genotypes matched with the corresponding demographic information of the individuals.
Real-time Polymerase Chain Reaction
Total RNA was isolated from CIC samples of 27 subjects 3 months after refractive surgery (15 healthy controls and 12 patients with dry eye), using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Contaminating DNA was eliminated by digestion with RNase-Free DNase Set (Qiagen). The extracted RNA was reverse-transcribed to cDNA with the SuperScript Vilo cDNA Kit (Invitrogen, Eugene, OR) following the manufacturer’s protocol. Real-time polymerase chain reaction (PCR) assay was performed on the Eppendorf Realplex2 system (Eppendorf AG, Hamburg, Germany) using SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) to determine relative quantitative expression levels of TSP1 and interleukin (IL)-1β genes. TSP1 primers (F-5′-GGACTCTGACGGCGATGGTC-3′ and R-5′-ATCGGCGGAAATCGGTCTC-3′), IL-1β primers (F-5′-CGGCATCCAGCTACGAATC-3′ and R-5′-CCATGGCCACAACAACTGAC-3′), and β-actin primers (F-5′-CGGGAAATCGTGCGTGAC-3′ and R-5′-GTGGCCATCTCTTGCTCGAA-3′) were used. Amplification reactions were set up in triplicate with the following thermal profile: 95°C for 3 minutes, 40 cycles at 95°C for 10 seconds, 56°C for 10 seconds, and 72°C for 10 seconds. To verify the specificity of the amplification reaction, a melting curve analysis was performed. The fluorescence signal generated at each cycle was analyzed using the system software. The threshold cycle values were used to determine relative quantitation of gene expression, with ß-actin as a reference gene.
Statistical Analysis
THBS1 polymorphisms in healthy patients and patients with dry eye after surgery were analyzed using gPLINK software version 1.027 (http://pngu.mgh.harvard.edu/~purcell/plink/) and GraphPad Prism version 5.0 for Mac (GraphPad Software, La Jolla, CA). To control for possible population stratification, 90 AIMs were genotyped, and data were analyzed against HapMap populations CEU, JPT/CHB, and YRI using Structure software version 2.3.428 (http://pritchardlab.stanford.edu/structure.html). Allelic frequency divergence was compared between and within the populations, and the genomic inflation factor lambda (λ) was determined. Departure from HWE also was evaluated. Exclusion criteria included deviation from HWE at P < 0.01 and sample genotyping rate <85%.
Allele and genotyping frequencies were compared using the Fisher exact test to estimate the significance of the association with dry eye after surgery. The ORs, 95% CIs, and frequencies for indicated genetic models were calculated, and P < 0.05 was considered significant. Considering high LD among 3 SNPs with significant associations, no Bonferroni correction was applied to avoid false-negative associations.
Haploview 4.129 (http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/haploview) was used to study LD. Coefficients of LD (D) of allele frequencies for SNPs were compared using the CI method of Gabriel et al30 to infer haplotype blocks.
Student t test was used to determine significant differences in mRNA expression levels. Data were expressed as mean ± standard error of the means. Differences were considered to be significant when P ≤ 0.05.
Results
Study Population
In total, 143 active duty U.S. Army soldiers opting for refractive surgery formed the study population. All of these individuals underwent refractive surgery and were examined for the presence of dry eye using diagnostic criteria as indicated in Table 1. Individuals who did not develop dry eye formed the control group, and others, including those with intermediate and chronic dry eye, were included in the dry eye group (Fig 2). The mean age of individuals in both groups was 29 years, with a comparable male to female ratio (dry eye 1.95 vs. controls 1.7). At the end of the study period, the Schirmer test, a routine clinical test used to evaluate aqueous tear production, indicated reduced levels in the dry eye group compared with those in controls (P < 0.01) (Table 4).
Figure 2.
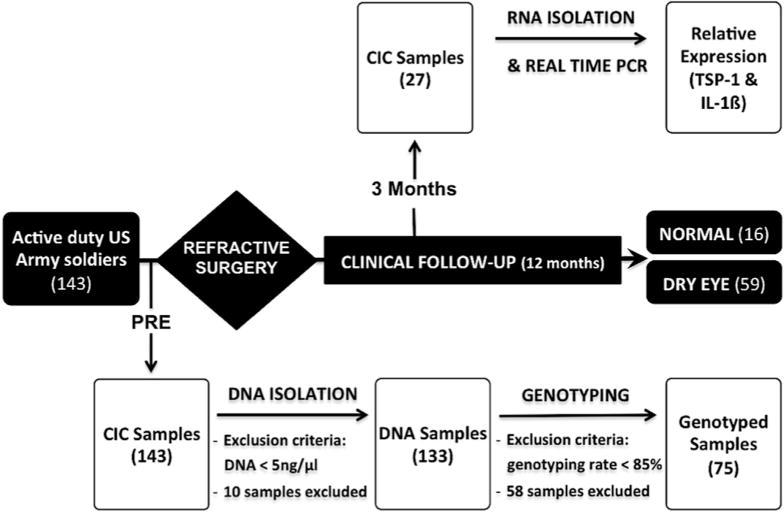
Study design. A total of 143 subjects underwent refractive surgery and were examined during 12 months for the presence of dry eye. Individuals who did not develop dry eye formed the “control” group (normal; n = 16), and the others were included in the “dry eye” group (dry eye; n = 59). Genomic DNA and RNA were obtained from conjunctival impression cytology (CIC) samples before (PRE) and 3 months after refractive surgery for genotyping of thrombospondin 1(TSP-1) single nucleotide polymorphisms (SNPs) and real-time polymerase chain reaction (PCR) analysis of TSP1 and interleukin (IL)-1β.
Table 4.
Characteristics of Study Population
| Characteristics | Control | Dry Eye |
|---|---|---|
| Patients, n (%) | 16 (21.3%) | 59 (78.7%) |
| Age (years) | 28.9±5.6 | 29.4±5.0 |
| Female/male, n | 6/10 | 20/39 |
| Schirmer test with anesthesia (mm)† | 29.00±7.00 | 17.84±10.76* |
| Rose Bengal staining† | 0.00±0 | 0.11±0.36 |
| TBUT (sec)† | 15.00±6.60 | 13.35±8.73 |
| McMonnies questionnaire† | 7.07±4.57 | 9.19±3.76 |
SD = standard deviation; TBUT = tear break-up time.
Data are mean ± SD unless otherwise indicated.
P < 0.05.
Clinical assessments 12 months after refractive surgery.
To rule out any influences of population stratification on the genetic analysis, we included a set of 90 AIMs in genotyping to infer genetic ancestry of the population under investigation.26 Comparison of AIM genotypes in our study population with established HapMap populations identified ancestry to be approximately 51% CEU, 32% YRI, and 17% JPT/CHB, which appeared to be consistent with the latest (FY11) active duty Army demographic profile (published by the Department of the Army at http://www.armyg1.army.mil/hr/demographics.asp, accessed March 1, 2013). The allelic frequency divergence (variance of log likelihood = 287.4) and low genomic inflation factor lambda for the study population (λ = 0.44) suggested an absence of significant population stratification. Furthermore, allelic frequencies of analyzed SNPs in this study closely matched those reported in the corresponding HapMap populations (Table 5, available at www.aaojournal.org) indicating their comparable distribution.25 Likewise, the LD pattern of the 4 analyzed SNPs in the study population (Fig 3) resembled those detected in HapMap populations (Fig 4, available at www.aaojournal.org).25
Figure 3.
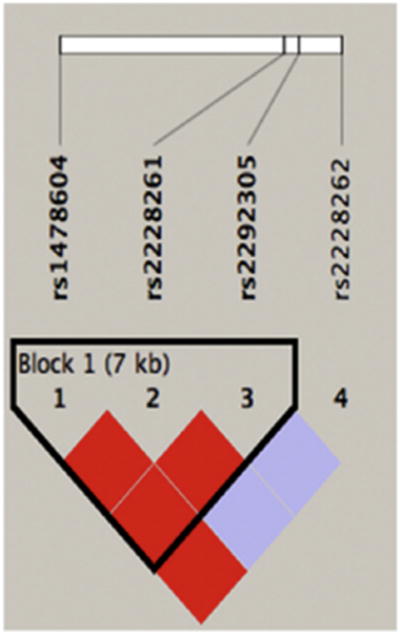
The linkage disequilibrium (LD) pattern of genotyped THBS1 polymorphisms. The LD coefficient (D′)–based pairwise LD map of 4 single nucleotide polymorphisms genotyped in this study, created with Haploview software version 4.1 (http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/haploview), is shown. Darker shades of red indicate higher D′. A haplotype block is outlined and was defined using the method of Gabriel et al.30
Association of THBS1 Variants with the Development of Post-refractive Dry Eye
Allelic and genotype frequencies of 4 SNPs from the THBS1 gene were assessed for their association with the development of dry eye after refractive surgery. As shown in Table 6, the frequency of the SNP1 minor allele was significantly increased in individuals diagnosed with dry eye compared with the individuals in the control group (P = 0.03), with an OR of 2.83 (95% CI, 1.21–7.16). Frequencies of the remaining 3 SNPs did not differ significantly between the groups. This result suggests that individuals with the SNP1 minor allele are susceptible to developing dry eye after undergoing refractive surgery compared with those expressing the major allele. Analysis of genotypic frequencies yielded significant associations with the dominant model, where frequencies of the minor allele carriers of SNP1, 2, or 3 were significantly increased in the dry eye group compared with the control group. Specifically, the frequency of SNP1 C minor allele carriers (CT+CC) in patients with dry eye was 69% versus 47% in the control group (P = 0.0025; OR, 2.5; 95% CI, 1.4–4.5). The frequencies of minor allele carriers of SNP2 (CT+TT) and SNP3 (AG+GG) were 39% in patients with dry eye compared with 25% in controls (P = 0.048; OR, 1.9; 95% CI, 1.1–3.5). No significant association was observed in SNP4 because the frequency of the minor allele carriers (AG+GG) was 19% in both groups (P = 1.0; OR, 1.0; 95%, CI 0.49–2.03). The SNP5 was excluded from analysis because of departure from HWE. The association of minor allele carriers of SNP1 was the strongest with the development of dry eye after refractive surgery. These results suggest that the presence of only 1 minor allele is sufficient to increase the risk of dry eye. Taken together, these data indicate statistically significant evidence for association between the THBS1 polymorphism and the development of dry eye after refractive surgery.
Table 6. THBS1.
Polymorphism Analysis in Patients with Dry Eye after Refractive Surgery
| THBS1 Polymorphism | Dominant Model | Allele Model | ||
|---|---|---|---|---|
| SNP1 | TT | CT+CC | T positive | C positive |
| No. (%) of controls (n = 15) | 8 (53) | 7 (47) | 23 (76) | 7 (23) |
| No. (%) of patients with DE (n = 54) | 17 (31) | 37 (69) | 58 (54) | 50 (46) |
| OR (95% CI) | 2.51 (1.41–4.47) | 2.83 (1.21–7.16) | ||
| P value | 0.0025* | 0.035* | ||
| SNP2 | CC | CT+TT | C positive | T positive |
| No. (%) of controls (n = 16) | 12 (75) | 4 (25) | 28 (88) | 4 (13) |
| No. (%) of patients with DE (n = 59) | 36 (61) | 23 (39) | 90 (76) | 28 (24) |
| OR (95% CI) | 1.91 (1.05–3.51) | 2.178 (0.70–6.74) | ||
| P value | 0.048* | NS | ||
| SNP3 | AA | AG+GG | A positive | G positive |
| No. (%) of controls (n = 16) | 12 (75) | 4 (25) | 28 (88) | 4 (13) |
| No. (%) of patients with DE (n = 59) | 36 (61) | 23 (39) | 90 (76) | 28 (24) |
| OR (95% CI) | 1.92 (1.05–3.51) | 2.178 (0.70–6.74) | ||
| P value | 0.048* | NS | ||
| SNP4 | AA | AG+GG | A positive | G positive |
| No. (%) of controls (n = 16) | 13 (81) | 3 (19) | 29 (91) | 3 (9) |
| No. (%) of patients with DE (n = 59) | 48 (81) | 11 (19) | 106 (90) | 12 (10) |
| OR (95% CI) | 1.0 (0.49–2.03) | 1.1 (0.29–4.10) | ||
| P value | NS (1) | NS | ||
| SNP5 | Excluded (HWE P < 0.05) | |||
CI = confidence interval; DE = dry eye; HWE = Hardy–Weinberg equilibrium; NS = not significant; OR = odds ratio; SNP = single nucleotide polymorphism; THBS1 = thrombospondin 1 gene.
P < 0.05, Fisher exact test.
Linkage Disequilibrium and Haplotype Analysis
To locate disease-associated sequence variation, we estimated LD with Haploview analysis. The pairwise LD structure was constructed with 4 SNPs genotyped (all with minor allele frequencies >0.01 and conforming to HWE). Coefficients of LD (D) were generated applying the CI method of Gabriel et al.30 As shown in Figure 3, SNP1, 2, and 3 fall in 1 LD block, with SNP2 and SNP3 in perfect LD (D = 1). We then examined haplotypes based on these 3 SNPs (Table 7). Although a trend of increased frequencies of haplotypes carrying minor alleles of SNP1 and 2 (CTG and CCA, respectively) was detected in patients with dry eye compared with control subjects (0.237 vs. 0.125 and 0.214 vs. 0.133, respectively), the differences did not reach statistical significance. However, the frequency of the TCA haplotype representing major alleles of the 3 SNPs was significantly increased among control subjects (0.742) compared with patients with dry eye (0.549), suggesting a potential protective role of this haplotype in the development of dry eye after refractive surgery.
Table 7.
Haplotype Frequencies of the Thrombospondin 1 Gene Polymorphisms in Patients with Dry Eye after Refractive Surgery
| Haplotype | Frequency | Case Frequency | Control Frequency | Chi-square | P Value |
|---|---|---|---|---|---|
| TCA | 0.59 | 0.549 | 0.742 | 3.895 | 0.0484* |
| CTG | 0.213 | 0.237 | 0.125 | 1.891 | 0.1691 |
| CCA | 0.197 | 0.214 | 0.133 | 1.05 | 0.3056 |
P < 0.05.
Allele-Specific Expression of Thrombospondin 1 and Its Correlation with Inflammation
We previously reported spontaneous development of ocular surface inflammation (dry eye) in mice deficient in TSP1.16 Therefore, we hypothesized that similarly reduced TSP1 expression in humans may result in ocular surface inflammation. In our study, CIC samples collected 3 months after refractive surgery provided ocular surface epithelial cells from each individual that could be evaluated for TSP1 expression. We harvested RNA from CIC samples and assessed TSP1 levels in a real-time PCR assay. We also assessed expression of an inflammatory marker associated with dry eye: IL-1β. We noted significantly reduced expression of TSP1 in SNP1 minor allele carriers (CT+CC) compared with the individuals expressing the major allele (TT) (Fig 5A). This reduced TSP1 expression also was accompanied by significantly increased expression of IL-1β (Fig 5B). These results confirm our observations from the association study that a SNP in a regulatory 5′-UTR of THBS1 correlates with a lowered expression of TSP1 in ocular surface epithelial cells and with increased expression of an inflammatory marker. Our results strongly support the association of THBS1 polymorphism with the development of ocular surface inflammation as seen in dry eye.
Figure 5.
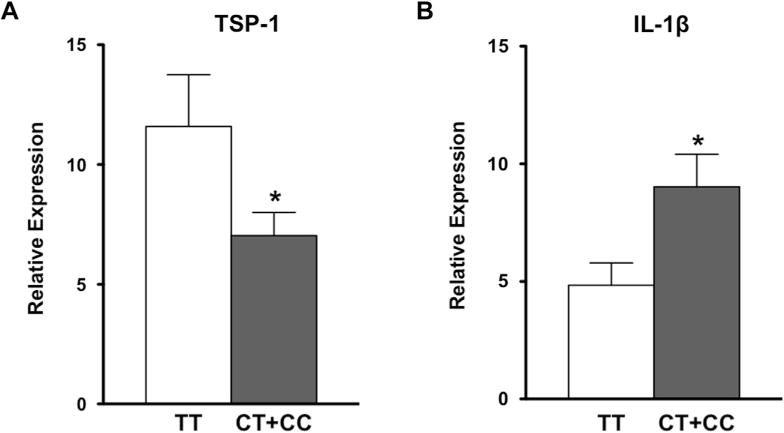
Reduced thrombospondin 1 (TSP-1) levels and increased inflammation are detectable in single nucleotide polymorphisms (SNP1) minor allele carriers. RNA was harvested from conjunctival impression cytology samples and analyzed using SYBR Green (Applied Biosystems, Carlsbad, CA) real-time polymerase chain reaction assay to determine levels of TSP-1 (A) and interleukin (IL)-1β (B). Expression of TSP-1 in SNP1 minor allele carriers (CC+CT) compared with the major genotype (TT) was significantly reduced, whereas expression of inflammatory cytokine IL-1β was significantly increased. *P < 0.05.
Discussion
The present study showed the minor allele C and genotypes CT and CC of SNP1 (rs1478604) in the THBS1 gene are likely associated with the development of dry eye after refractive surgery in a group of active duty U.S. Army soldiers. The strength of both of these associations was an OR >2.5. Moreover, the frequency of a haplotype carrying major alleles of 3 SNPs was significantly higher in the control group than in the dry eye group. We also detected significantly reduced expression of TSP1 in the ocular surface epithelia of individuals with the SNP1 minor allele. These individuals also expressed significantly elevated levels of an inflammatory cytokine, IL-1β, associated with dry eye.
TSP1 is expressed by many cell types including ocular surface epithelia.17,18,31 Binding sites for many receptors and intracellular and extracellular molecules have been identified in different domains of TSP1.17 As such, many diverse functions are attributed to TSP1 depending on the receptors engaged by this molecule and the cells expressing them. In the eye, damaged corneal epithelial cells are reported to use TSP1 in corneal wound repair.32,33 A significant role of TSP1 in the regulation of ocular inflammatory angiogenesis and lymphangiogenesis is documented.34,35 In murine studies, we have reported TSP1-dependent regulation of ocular antigen-presenting cells and inflammatory responses mediated by them.36–38 There are several studies that demonstrated TSP1-dependent regulation of inflammatory responses by human dendritic cells.39,40 These reports in combination with our reported dry eye pathology in TSP1-deficient mice strongly suggest a potential role for TSP1 in the regulation of ocular surface inflammatory conditions such as dry eye.
Study Limitations
Our study design allowed for a clear assessment of dry eye resulting from a distinct factor, such as refractive surgery. However, given the high prevalence of dry eye 6 months after refractive surgery (60%–90%),2–4 achieving a 1:1 sample-to-control ratio with a 1-year follow-up was not possible. By taking into account this limitation and considering that no such study has been reported as yet, several stringent quality-control measures were applied to minimize potential confounding factors and to increase the probability of its replication in a future larger study. The control group in our study includes individuals who underwent refractive surgery similar to the experimental dry eye group. In addition to this, we identified a candidate gene with a known sequence and well-characterized structure of the protein it encodes. This information helped in the selection of SNPs from TSP1 domains with known associations with cellular function.
Genetic variation in the 5′-UTR region of DNA with regulatory components has a strong potential to influence the transcription and therefore possible expression of a gene. In this study, the 5′-UTR location of SNP1 appears to alter transcription of THBS1; we noted a significant decline in the expression of TSP1 mRNA in ocular surface epithelia derived from individuals genotyped with minor allele of SNP1 (C). The lowest expression of TSP1 was detected in 1 subject homozygous for this minor allele (data not shown). This change in TSP1 expression was accompanied by increased expression of an inflammatory marker, IL-1β, associated with dry eye. These results implicate a regulatory role of TSP1 in the ocular surface inflammatory condition.
The location of 2 SNPs included in this study (SNP2 and 3) in the structural domains of TSP1 (type 1 and 2 repeats, respectively) corresponds with the functions of TSP1 relevant to the dry eye condition. That is, although activation of immunosuppressive TGF-ß is associated with type 1 repeats,41 the binding of TSP1 to the epidermal growth factor receptor is associated with type 2 repeats.42 A disruption in type 1 repeats can interfere with the availability of anti-inflammatory TGF-ß to counter inflammation and wound healing43 at the ocular surface. Likewise, disruption in type 2 repeats may interfere with epidermal growth factor receptor signaling of mucin-secreting goblet cells of the ocular surface.44,45 A marginally significant increase in genotype frequencies of both SNP2 and 3 in subjects with dry eye suggested a trend that is in line with the functions associated with the corresponding TSP1 regions. Identical frequencies of these 2 SNPs also are consistent with a perfect LD between them.
We consider this study an exploratory assessment of the genetic basis underlying a commonly encountered complication after refractive surgery: dry eye. Our results in this study strongly support a potential genetic basis for the dry eye condition. On the basis of the intensity of dry eye as determined by clinical scoring, 72% of subjects from the dry eye group were classified with an intermediate level and the remaining 28% were classified with a higher intensity of chronic inflammation. Such a distribution of subjects clearly supports variability of the disease, whereas incomplete disease penetrance is noted in the case of significantly associated SNPs. Both of these observations are consistent with the multifactorial cause of a complex trait disease such as dry eye. Furthermore, in the control group, subjects heterozygous for SNP1 (47%) and for SNP2 and SNP3 (25% each) remained unaffected, indicating their carrier phenotype.
In conclusion, in determining candidacy for refractive surgeries, systemic autoimmune diseases such as rheumatoid arthritis and Sjögren’s syndrome are considered contraindications because of the possibility of exacerbating any ocular inflammation associated with these diseases. However, the potential risk of precipitating autoimmunity by exposing ocular surface autoantigens in an inflammatory response such as dry eye remains unknown. Our results in this study substantiate our findings from murine studies suggesting that reduced TSP1 expression promotes a proinflammatory phenotype of tissue antigen-presenting cells and disrupts immune regulation that follows normal wound healing, leading to chronic inflammatory disease. In TSP1-deficient mice, this chronic inflammation progresses to age-related autoimmunity resulting in autoimmune Sjögren’s syndrome.16 Therefore, long-term studies of individuals with chronic dry eye (after refractive surgery) are warranted. This is especially needed because younger populations opt to undergo refractive surgeries at an age long before the average onset of systemic autoimmune conditions. Furthermore, it was recently reported that approximately 12% of patients with dry eye develop autoimmune Sjögren’s syndrome or systemic autoimmune disease,46 suggesting dry eye as a potential early event or symptom of a systemic condition. In the context of such possibilities, screening individuals for susceptibility to developing dry eye before refractive surgeries will help improve postsurgery management and prevent possible long-term risks. In this regard, our study presents THBS1 variants as a potential genetic biomarker to identify individuals at risk of developing chronic dry eye.
Supplementary Material
Acknowledgments
Funding: Department of Defense Grant W81XWH11-1-0477 and National Eye Institute grant EY015472. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or the U.S. Government.
Abbreviations and Acronyms
- AIM
ancestry-informative marker
- CI
confidence interval
- CIC
conjunctival impression cytology
- D
diopters
- HWE
Hardy–Weinberg equilibrium
- IL
interleukin
- LD
linkage disequilibrium
- OR
odds ratio
- PCR
polymerase chain reaction
- SNP
single nucleotide polymorphism
- SSDE
Sjögren’s syndrome dry eye
- TGF-ß
transforming growth factor-beta
- THBS1
thrombospondin 1
- TSP1
thrombospondin 1
- UTR
untranslated region
Footnotes
Presented at: the Association of Research in Vision and Ophthalmology, May 5–9, 2013, Seattle, Washington.
Financial Disclosure(s):
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Kojima T, Ongucci T, Hallak J, Azar D. Peer-Reviewed Literature. In: Alio JL, Azar DT, editors. Management of Complications in Refractive Surgery. Berlin, Heidelberg: Springer-Verlag; 2008. pp. 329–50. [Google Scholar]
- 2.Hovanesian JA, Shah SS, Maloney RK. Symptoms of dry eye and recurrent erosion syndrome after refractive surgery. J Cataract Refract Surg. 2001;27:577–84. doi: 10.1016/s0886-3350(00)00835-x. [DOI] [PubMed] [Google Scholar]
- 3.De Paiva CS, Chen Z, Koch DD, et al. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. 2006;141:438–45. doi: 10.1016/j.ajo.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Toda I, Asano-Kato N, Komai-Hori Y, Tsubota K. Dry eye after laser in situ keratomileusis. Am J Ophthalmol. 2001;132:1–7. doi: 10.1016/s0002-9394(01)00959-x. [DOI] [PubMed] [Google Scholar]
- 5.Yu EY, Leung A, Rao S, Lam DS. Effect of laser in situ keratomileusis on tear stability. Ophthalmology. 2000;107:2131–5. doi: 10.1016/s0161-6420(00)00388-2. [DOI] [PubMed] [Google Scholar]
- 6.Salomao MQ, Ambrosio R, Jr, Wilson SE. Dryeyeassociated with laser in situ keratomileusis: mechanical microkeratome versus femtosecond laser. J Cataract Refract Surg. 2009;35:1756–60. doi: 10.1016/j.jcrs.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 8.Moss SE, Klein R, Klein BE. Incidence of dry eye in an older population. Arch Ophthalmol. 2004;122:369–73. doi: 10.1001/archopht.122.3.369. [DOI] [PubMed] [Google Scholar]
- 9.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–26. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 10.Uchiyama E, Aronowicz JD, Butovich IA, McCulley JP. Increased evaporative rates in laboratory testing conditions simulating airplane cabin relative humidity: an important factor for dry eye syndrome. Eye Contact Lens. 2007;33:174–6. doi: 10.1097/01.icl.0000252881.04636.5e. [DOI] [PubMed] [Google Scholar]
- 11.Uchino M, Schaumberg DA, Dogru M, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology. 2008;115:1982–8. doi: 10.1016/j.ophtha.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjogren’s Syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–7. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 13.Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–8. [PubMed] [Google Scholar]
- 14.Barabino S, Shen L, Chen L, et al. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005;46:2766–71. doi: 10.1167/iovs.04-1326. [DOI] [PubMed] [Google Scholar]
- 15.Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32:19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turpie B, Yoshimura T, Gulati A, et al. Sjogren’s syndromelike ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol. 2009;175:1136–47. doi: 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol [serial online] 2011;3:a009712. doi: 10.1101/cshperspect.a009712. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3179333/. Accessed January 20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekiyama E, Nakamura T, Cooper LJ, et al. Unique distribution of thrombospondin-1 in human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2006;47:1352–8. doi: 10.1167/iovs.05-1305. [DOI] [PubMed] [Google Scholar]
- 19.Zwicker JI, Peyvandi F, Palla R, et al. The thrombospondin-1 N700S polymorphism is associated with early myocardial infarction without altering von Willebrand factor multimer size. Blood. 2006;108:1280–3. doi: 10.1182/blood-2006-04-015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topol EJ, McCarthy J, Gabriel S, et al. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation. 2001;104:2641–4. doi: 10.1161/hc4701.100910. [DOI] [PubMed] [Google Scholar]
- 21.Hannah BL, Misenheimer TM, Pranghofer MM, Mosher DF. A polymorphism in thrombospondin-1 associated with familial premature coronary artery disease alters Ca2+ binding. J Biol Chem. 2004;279:51915–22. doi: 10.1074/jbc.M409632200. [DOI] [PubMed] [Google Scholar]
- 22.Calonge M, Diebold Y, Saez V, et al. Impression cytology of the ocular surface: a review. Exp Eye Res. 2004;78:457–72. doi: 10.1016/j.exer.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm. 2011;2011:296069. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorisson GA, Smith AV, Krishnan L, Stein LD. The International HapMap Project Web site. Genome Res. 2005;15:1592–3. doi: 10.1101/gr.4413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–96. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 26.Hoggart CJ, Parra EJ, Shriver MD, et al. Control of confounding of genetic associations in stratified populations. Am J Hum Genet. 2003;72:1492–504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–59. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 30.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 31.Hiscott P, Seitz B, Schlotzer-Schrehardt U, Naumann GO. Immunolocalisation of thrombospondin 1 in human, bovine and rabbit cornea. Cell Tissue Res. 1997;289:307–10. doi: 10.1007/s004410050877. [DOI] [PubMed] [Google Scholar]
- 32.Matsuba M, Hutcheon AE, Zieske JD. Localization of thrombospondin-1 and myofibroblasts during corneal wound repair. Exp Eye Res. 2011;93:534–40. doi: 10.1016/j.exer.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uno K, Hayashi H, Kuroki M, et al. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem Biophys Res Commun. 2004;315:928–34. doi: 10.1016/j.bbrc.2004.01.146. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Cursiefen C, Barabino S, et al. Novel expression and characterization of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1) by conjunctival cells. Invest Ophthalmol Vis Sci. 2005;46:4536–40. doi: 10.1167/iovs.05-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cursiefen C, Maruyama K, Bock F, et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med. 2011;208:1083–92. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masli S, Turpie B, Streilein JW. Thrombospondin orchestrates the tolerance-promoting properties of TGFbetatreated antigen-presenting cells. Int Immunol. 2006;18:689–99. doi: 10.1093/intimm/dxl006. [DOI] [PubMed] [Google Scholar]
- 37.Ng TF, Turpie B, Masli S. Thrombospondin-1-mediated regulation of microglia activation after retinal injury. Invest Ophthalmol Vis Sci. 2009;50:5472–8. doi: 10.1167/iovs.08-2877. [DOI] [PubMed] [Google Scholar]
- 38.Saban DR, Bock F, Chauhan SK, et al. Thrombospondin-1 derived from APCs regulates their capacity for allosensitiza-tion. J Immunol. 2010;185:4691–7. doi: 10.4049/jimmunol.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doyen V, Rubio M, Braun D, et al. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J Exp Med. 2003;198:1277–83. doi: 10.1084/jem.20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grimbert P, Bouguermouh S, Baba N, et al. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25− T cells in response to inflammation. J Immunol. 2006;177:3534–41. doi: 10.4049/jimmunol.177.6.3534. [DOI] [PubMed] [Google Scholar]
- 41.Schultz-Cherry S, Chen H, Mosher DF, et al. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270:7304–10. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- 42.Calzada MJ, Annis DS, Zeng B, et al. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J Biol Chem. 2004;279:41734–43. doi: 10.1074/jbc.M406267200. [DOI] [PubMed] [Google Scholar]
- 43.Nor JE, Dipietro L, Murphy-Ullrich JE, et al. Activation of latent TGF-beta1 by thrombospondin-1 is a major component of wound repair. Oral Biosci Med. 2005;2:153–61. [PMC free article] [PubMed] [Google Scholar]
- 44.Gu J, Chen L, Shatos MA, et al. Presence of EGF growth factor ligands and their effects on cultured rat conjunctival goblet cell proliferation. Exp Eye Res. 2008;86:322–34. doi: 10.1016/j.exer.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodges RR, Bair JA, Carozza RB, et al. Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Exp Eye Res. 2012;103:99–113. doi: 10.1016/j.exer.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liew MS, Zhang M, Kim E, Akpek EK. Prevalence and predictors of Sjogren’s syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br J Ophthalmol. 2012;96:1498–503. doi: 10.1136/bjophthalmol-2012-301767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


