Summary
Radiation therapy is often considered the treatment of choice for low-grade gliomas. However, given the long-term effects of radiation on the developing brain, the appropriate use of radiation therapy in pediatric patients remains controversial. The purpose of this study was to evaluate progression-free survival (PFS) of pediatric low-grade glioma patients treated with radiation therapy. Data were obtained through a retrospective chart review of patients treated between 1991 and 2008 from a single tertiary care center in the midwest. The study population consisted of 17 patients, of whom 8 (47%) had tumor recurrence after radiation therapy. The median follow-up time was 8.2 years, with a range of 2.3 to 17.2 years. The median age at diagnosis was 5.4 years, and the median age at radiation therapy was 9.4 years. The 3- and the 10-year PFS were 69% ± 11.7% and 46% ± 13.3%, respectively. A significant difference in PFS was seen when comparing brainstem tumors with hypothalamic/optic pathway tumors (P = 0.019). Differences in PFS based on the age at diagnosis, the extent of initial surgery, and indication for radiation therapy were not significant. A larger multicenter study is needed to better assess PFS in these patients.
Keywords: pediatric low-grade gliomas, radiation therapy low-grade astrocytoma
Pediatric low-grade gliomas (LGGs) are a heterogenous set of tumors comprising approximately 40% of all childhood primary central nervous system neoplasms.1 The 2 most common LGGs in children are pilocytic astrocytoma (WHO grade I) and diffuse fibrillary astrocytoma (WHO grade II). Other less common LGGs include pilomyxoid astrocytomas, pleomorphic xanthoastrocytomas, gangliogliomas, desmoplastic neuroepithelial tumors, oligodendrogliomas, and subependymal giant cell astrocytomas.1,2 These tumors occur most commonly in the cerebellum, but are also found in the cerebrum, the deep midline supratentorial region, the hypothalamus, the optic pathway, and the brainstem.1,3
In most institutions, gross total resection (GTR) is the initial therapeutic approach to cerebral hemisphere or cerebellar tumors when feasible. In a recent Children’s Oncology Group study, GTR was associated with an 8-year progression-free survival (PFS) of 93% and an overall survival (OS) of >99%.4 However, for tumors located in the deep midline supratentorial region, the optic pathway/hypothalamus, and the brainstem, surgery is generally restricted to partial resection or biopsy.3,5 Although OS still remains high, PFS is significantly decreased in tumors that are resected incompletely.3,6,7 Chemotherapy and/or radiation therapy are often used as adjuvant therapies for incompletely resected tumors and are most often reserved for residual tumors that are symptomatic or demonstrate progression by imaging and/or clinical symptoms.3 Immediate postoperative radiation or chemotherapy outside of this setting has not been shown to improve outcomes.3,8,9
Radiation therapy has significant long-term complications including cognitive, focal, neurological, endocrine, visual, and auditory dysfunction.10–13 The severity of complications is most often dependent on the age at the time of radiation and the location of the tumor. Merchant et al11 demonstrated an average 8-point decrease in IQ 5 years after receiving radiation for LGGs. This degree of decrease was dependent on the age at radiation.11 Adult LGG patients who have undergone radiation therapy have also been found to have cognitive deficits.14 These deficits were not initially found at a mean of 6 years, but were seen at a mean of 12 years from radiation.14 Multiple chemotherapy regimens have been used to treat progressive LGGs with 3- to 5-year PFS ranging between 30% and 71%.15–23 Even though chemotherapy sometimes does not cure LGGs, it often delays tumor growth and postpones the use of radiotherapy, thus sparing the deleterious effects of irradiation on a developing brain. However, the appropriate use of radiation therapy in these patients continues to remain controversial as there is no clear consensus among clinicians.
The purpose of our study was to evaluate the outcome of pediatric LGG patients treated with radiation therapy at our institution. The primary outcome was PFS. LGGs are considered to be biologically similar in nature, but are heterogenous with respect to their location and resectability. Our institutional practices changed over time with regard to the indication for radiation. We therefore wanted to determine whether the PFS was impacted by whether the patient had a progressive tumor or whether the tumor was treated prophylactically after radiation. Our secondary outcomes were to assess differences in outcome on the basis of the age at diagnosis, the tumor location, the histologic grade, the extent of initial surgery, and the indication for radiation therapy. Owing to the small scale of this study and the heterogenous locations of tumors and ages of patients, functional outcomes and late effects were not assessed.
MATERIALS AND METHODS
We conducted a retrospective cohort study of LGG patients aged 21 years and below, who were subsequently treated with radiation therapy at Saint Louis University and/or Cardinal Glennon Children’s Medical Center between 1991 and 2008. Databases from the Division of Pediatric Oncology and Division of Radiation-Oncology were used to identify the patients. Data extracted from clinical records included age at diagnosis, sex, tumor location (cerebellar, midline/hypothalamic/optic pathway, hemispheric, brainstem), histologic grade, stage (metastatic vs. nonmetastatic), age at initial surgery, extent of initial surgery (none, biopsy, partial resection, GTR), chemotherapy regimens, NF status (yes/no), age at the time of radiation, radiation type, and dose.
Diagnosis of grade I or grade II LGG were biopsy proven except for tumors located in the hypothalamus or the optic pathway. The number and the type of chemotherapy regimens before and after radiation therapy were obtained. Indication for radiation therapy was divided into 2 categories: adjuvant therapy given postoperatively after initial diagnosis and salvage therapy for disease progression/recurrence.
Time to last follow-up and the disease status were also collected. PFS was defined as the time to first disease progression, or recurrence, after radiation therapy. The Kaplan-Meier method was used to estimate probabilities of PFS and OS. Data were stratified by age at diagnosis, tumor location, histologic grade, extent of initial surgery, and indication for radiation therapy. Groups were compared using the log-rank test. All statistical analyses were performed using the SPSS v12 statistical program. The study was approved by the Saint Louis University Institutional Review Board.
RESULTS
Between 1991 and 2008, 109 patients were diagnosed with LGGs at our institution. Among these patients, 21 (19.2%) were treated with radiation therapy. Out of the 21 patients, 4 were excluded from our study, limiting the study population to 17 patients. Two were excluded as they had received radiation therapy at an outside institution, 1 patient transferred care to another institution only 9 months after receiving radiation, whereas the remaining patient had an incomplete medical record and therefore was excluded.
Among the study patients, 71% were male patients and 59% were < 5 years old at the time of diagnosis. Grade I gliomas were more common (59%) than grade II tumors. Gliomas needing radiation were more commonly located in the brainstem (35%) and the hypothalamus/optic pathway/midline region (35%). None of the patients included in the study had neurofibromatosis. Two patients had metastasis in their disease course: 1 before and 1 after receiving radiation therapy. Clinical characteristics of the study population are summarized in Table 1.
TABLE 1.
Clinical Characteristics of the Study Population
| Characteristics | n (%) |
|---|---|
| Sex | |
| Male | 12 (71) |
| Female | 5 (29) |
| Age at diagnosis (y) | |
| < 5 | 10 (59) |
| ≥ 5 | 7 (41) |
| Tumor location | |
| Brainstem | 6 (35) |
| Midline/hypothalamus/optic pathway | 6 (35) |
| Cerebellum | 3 (18) |
| Cerebrum | 2 (12) |
| Histologic grade | |
| Grade I | 10 (59) |
| Grade II | 7 (41) |
| Neurofibromatosis type 1 status | |
| Yes | 0 (0) |
| No | 17 (100) |
| Metastasis | |
| Yes | 2 (12) |
| No | 15 (88) |
Surgery was limited to either a partial resection (59%) or biopsy (41%); no patient with a gross tumor resection qualified for the study. The timing and the modality of radiation therapy were determined by the treating physician. A total of 35% received radiation therapy postoperatively after initial diagnosis, whereas the remaining 65% received radiation therapy for disease progression/recurrence. Two patients received Gamma Knife radiation (14 to 16 Gy in 1 fraction), 2 received CyberKnife radiation (21 Gy in 3 fractions and 26 Gy in 5 fractions), whereas the remaining 13 received conformal radiation therapy (CRT), with doses ranging from 50 to 54 Gy. Of the 6 patients who received adjuvant radiation therapy, one 11.6-year-old patient had CRT with a residual left optic nerve tumor and vision loss, one 9.9-year-old patient received Gamma Knife for a midline suprasellar tumor, 2 children aged 4.6 and 8 years, respectively, with a brainstem tumor received CRT, one 14-year-old patient with a symptomatic cerebellar tumor was treated with CyberKnife therapy, and one 11-year-old patient with a grade II symptomatic temporal lobe tumor was treated with CRT. Four patients (24%) were < 5 years old at the time of radiation. Only 4 patients (24%) received radiation therapy without any prior chemotherapy use. Of the patients who received chemotherapy before radiation, the number of chemotherapy regimens ranged from 1 to 4. Treatment characteristics are summarized in Table 2.
TABLE 2.
Treatment Characteristics of the Study Population
| Characteristics | n (%) |
|---|---|
| Extent of initial surgery | |
| Biopsy | 7 (41) |
| Partial resection | 10 (59) |
| Gross total resection | 0 (0) |
| Indication for radiation therapy | |
| Adjuvant therapy postoperatively | 6 (35) |
| Tumor recurrence/progression | 11 (65) |
| Age at radiation therapy (y) | |
| < 5 | 4 (24) |
| ≥ 5 and < 10 | 6 (35) |
| ≥ 10 | 7 (41) |
| Type of radiation therapy | |
| Conformal radiation therapy | 13 (76) |
| CyberKnife | 2 (12) |
| Gamma Knife | 2 (12) |
| Chemotherapy before radiation therapy | |
| Yes | 13 (76) |
| No | 4 (24) |
| No. CT regimens when given before RT | |
| 1 | 7 (54) |
| 2 | 4 (31) |
| 3 | 1 (8) |
| 4 | 1 (8) |
CT indicates chemotherapy; RT, radiation therapy.
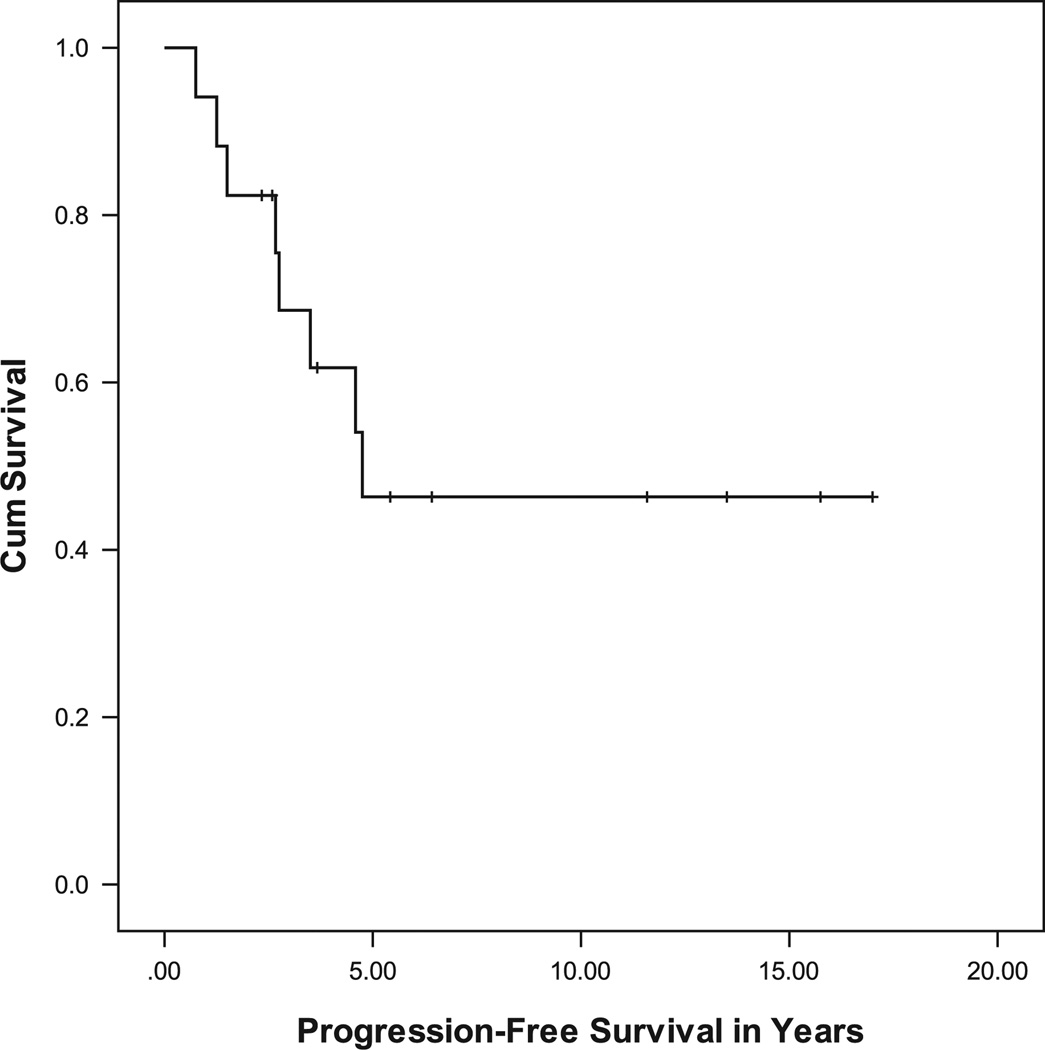
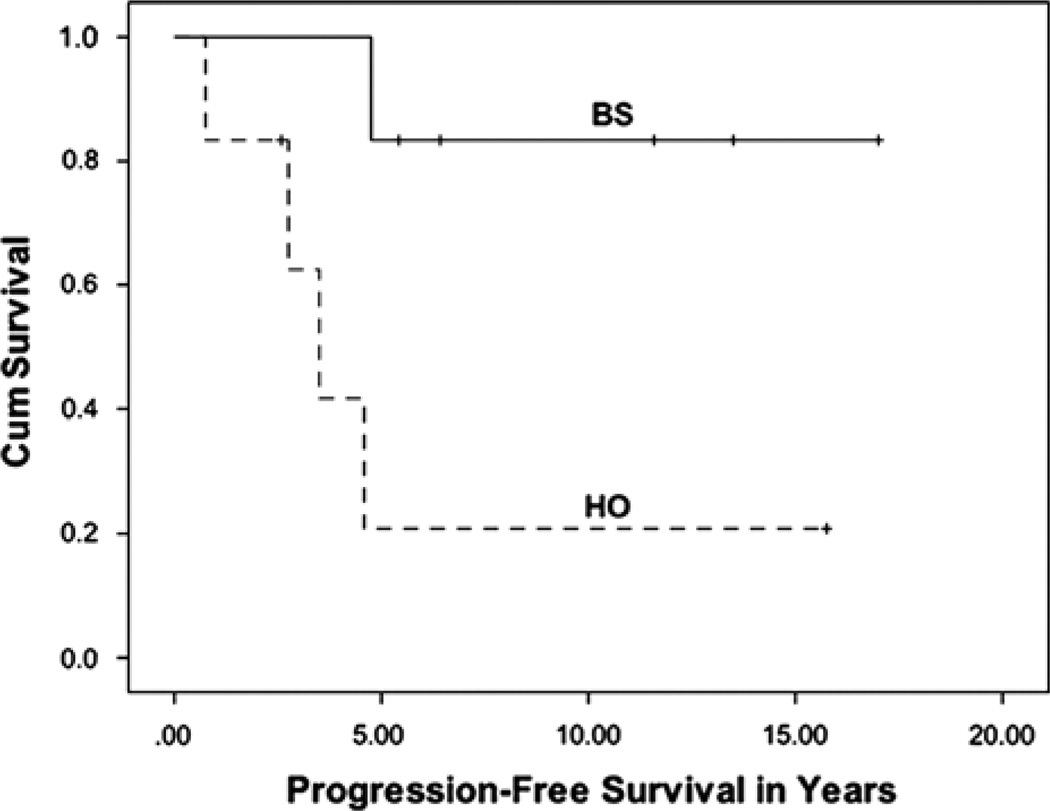
Recurrence was seen in 8 of the 17 patients (47%). The median follow-up period was 8.2 years, with a range of 2.3 to 17.2 years. The median age at diagnosis was 5.4 years, and the median age at radiation therapy was 9.4 years. The 3- and the 10-year OS were 100%, whereas the 3- and the 10-year PFS were 69% ± 11.7% and 46% ± 13.3%, respectively (Fig. 1). Among the various subgroups, a trend (P = 0.054) was seen in PFS on the basis of the tumor location. A significant difference (P = 0.019) was seen when comparing brainstem tumors with hypothalamic/optic pathway tumors (Fig. 2). There was no difference in the PFS or the indication for treatment for optic pathway tumors that extended outside of the optic tract compared with those that did not. Differences in PFS based on the age at diagnosis, the histology, the extent of initial surgery, and indications for radiation therapy were not significant. Of note, 2 patients received radiation therapy twice in the course of their disease, one of whom had a tumor recurrence 10 years after the second radiation treatment.
FIGURE 1.
Progression-free survival for all study patients.
FIGURE 2.
Progression-free survival for patients with brainstem (BS) tumors and hypothalamic/optic pathway (HO) tumors.
DISCUSSION
The indication and the timing for radiation therapy for pediatric LGG patients has remained controversial given the potential long-term effects of irradiation to the developing brain. Studies have clearly demonstrated that patients treated with radiation at a young age are at risk for endocrine dysfunction, cerebrovascular disease, and neurocognitive and hearing deficits.10,12,13,24 In our study, the median age at the time of radiation therapy was 9.4 years. Only 24% of the patients who received radiation therapy were below 5 years of age, with none receiving radiation when below 3 years of age. A total of 41% were 10 years and above at the time of radiation. This suggests that our general practice has been to delay radiation therapy if possible. The practice of initial treatment with radiation therapy decreased throughout the study period as the use of chemotherapy as the first-line treatment became more frequent, especially in younger children.
Lack of consensus regarding the optimal use of radiation therapy is due in part to the absence of randomized prospective trials. Although a randomized prospective phase 3 trial (POG 9130/CCG 9891/INT 0128 trial) was designed to evaluate neurosurgical and radiotherapeutic treatments of children with LGGs, practitioner bias forced early closure of this trial.9 Hence, most of our data regarding the use of radiation therapy in these patients have been obtained from previous retrospective studies.5,7,9,10,13,25 A large, prospective German study (HIT)-LGG-1996 included 138 patients who received radiation therapy, with 5- and 10-year PFS of 65% and 62%, respectively, with a median follow-up of 8.3 years (range, 0 to 13.5 y) from the end of therapy.26 Risk factors for tumor progression after radiotherapy included age < 1 year, diencephalic syndrome, metastatic disease at diagnosis, and a nonpilocytic grade I/nondiffuse grade II histology on univariate analysis; all but diencephalic syndrome were increased risk on multivariate analysis. The 3-year PFS and the median time to follow-up are similar to our study; however, the 10-year PFS was superior to ours. The (HIT)-LGG-1996 separated patients into 3 arms on the basis of the treatment after initial surgery: observation, chemotherapy, and radiation therapy. Thus, all patients in the radiation therapy arm had radiation therapy as the first line of treatment after surgery. In our study, the 3 patients who progressed more than 3 years after radiation were all below 36 months of age at diagnosis and had at least 2 chemotherapy regimens before radiation therapy. Although the numbers are too small to demonstrate statistical significance, it is possible those diagnosed at a young age and requiring multiple treatment regimens may be at risk for late recurrences.
Given the chronic nature of these tumors, a long follow-up period is necessary. In our study, the median follow-up period after radiation therapy was 8.2 years, with a range of 2.3 to 17.2 years. A total of 41% of these patients were analyzed at 10 years after radiation therapy. The 3- and the 10-year PFS were 69% ± 11.7% and 46% ± 13.3%, respectively. Previous studies have shown 3- to 5-year PFS ranging from 48% to 87% and a 10-year PFS ranging from 43% to 74%.8,9,24,27,28 The 3- and the 10-year OS in our study were 100%. Previous studies have shown the OS in LGG patients treated with radiation therapy to be between 90% and 98% at 5 years and between 74% and 96% at 10 years.9,24,27 In the prospective series of 78 pediatric LGG patients treated with CRT, Merchant et al24 reported 5- and 10-year PFS of 87.4% ± 4.4% and 74.3% ± 15.4%, respectively, and OS of 98.5% ± 1.6% and 95.9% ± 5.8%, respectively. Although they did not find a statistically significant difference in the outcome for the grade of tumors, only 4 patients in the study had grade II tumors. In addition, 13 patients (17%) had NF-1, none of which progressed. NF-1 is associated with a better prognosis and longer PFS, which may contribute to the increased overall PFS in this study.
In our study, we classified the use of radiation therapy into 2 categories—adjuvant therapy given postoperatively after initial diagnosis and salvage therapy for tumor recurrence/progression. A total of 35% received radiation therapy postoperatively, whereas 65% received radiation therapy after tumor recurrence/progression. We did not find any significant differences in OS and PFS among the 2 groups. Although some studies have suggested that postoperative adjuvant radiation therapy improves PFS, it does not necessarily translate into improved OS.3,7,13 Oh et al7 demonstrated that when GTR was not achieved, adjuvant radiation therapy improved the PFS (89% vs. 49%, P < 0.003) but not the OS. Other studies have shown no improvement in PFS with immediate postoperative irradiation.8,9 Mishra et al9 looked at the role of upfront radiation therapy specifically for incompletely resected pediatric WHO grade II LGGs, and found no significant difference in the PFS and the OS between those who did and did not receive radiation at diagnosis. This suggests that radiation can be delayed until the time of progression, as in our study.
Only 24% of the patients did not receive chemotherapy before irradiation in our study. The number of chemotherapy regimens ranged from 1 to 4, with 46% of these patients receiving >1 regimen. Scheinemann et al28 demonstrated high feasibility and low mortality of repeated chemotherapy treatment for progressive LGGs. They concluded that the relatively benign nature of LGG justifies the implementation of second-line modality at progression.28 The 3- to 5-year PFS ranged between 30% and 71% for various chemotherapy regimens.15–21,23,29,30 The most common initial regimen used is carboplatin and vincristine (CV), with a 3-year PFS of 68%.3,30 Results of the randomized clinical trial comparing CV with 6-thioguanine, procarbazine, dibromodulcitol, lomustine (CCNU), and vincristine (TCPV) demonstrated a 5-year EFS of 39% ± 4% for CV and 52% ± 5% for TCPV.29 Thus, the 3-year PFS for chemotherapy is similar to that of radiation therapy. A longer PFS cannot be compared as a 10-year PFS for chemotherapy is not available currently.
There was a lower 3-year PFS in hypothalamic/optic pathway tumors after radiation therapy when compared with brainstem tumors. Four of the 6 hypothalamic/optic pathway tumors recurred after radiation, whereas only 1 of the 6 brainstem tumors recurred. This suggests that brainstem tumors have a more favorable long-term response to radiation therapy; however, our sample size was small. Ohata et al7 similarly demonstrated inferior outcomes in hypothalamic/optic pathway tumors in PFS (39% vs. 76%) compared with other locations when treated with surgery, chemotherapy, and/or radiation. Differences in outcomes in terms of the age at diagnosis, the histologic grade, and the extent of initial surgery (biopsy vs. partial resection) were not significant in our study. Of note, no patient with a gross tumor resection qualified for the study, reiterating that GTR should be the goal when it can be achieved with an acceptable functional outcome.3–7,9
This was a single-institutional study that was limited by its small sample size and retrospective nature. Unidentified sources of selection bias must be considered, which a retrospective study cannot control well. In our study, 4 patients were not treated with fractionated CRT. Two patients received CyberKnife radiation and 2 received Gamma Knife radiation. The use of these 2 techniques is practitioner dependent, and most previous studies are limited to CRT use.
CONCLUSIONS
The treatment of LGGs remains a challenge for clinicians. Although radiation therapy is an effective treatment for LGGs, it does not provide long-term control of these tumors in some patients as shown in our study. A multidisciplinary approach using surgery, chemotherapy, and radiation therapy is needed, especially when GTR is not possible. Delaying radiation therapy by treating patients with chemotherapy seems appropriate, knowing the long-term side effects of radiation to the developing brain. Given our small sample size, it was difficult to find any significant differences in the outcome for patient, tumor, or treatment characteristics. Larger multicenter studies are needed to better assess for PFS in LGG patients treated with radiation therapy.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Giannini C, Scheithauer BW. Classification and grading of low-grade astrocytic tumors in children. Brain Pathol. 1997;7:785–798. doi: 10.1111/j.1750-3639.1997.tb01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YH, Lachuer J, Mittelbronn M, et al. Alterations in the RB1 pathway in low-grade diffuse gliomas lacking common genetic alterations. Brain Pathol. 2011;21:645–651. doi: 10.1111/j.1750-3639.2011.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24:1397–1408. doi: 10.1177/0883073809342005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wisoff JH, Sandford RA, Heier LA, et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery. 2011;68:1546–1555. doi: 10.1227/NEU.0b013e318214a66e. [DOI] [PubMed] [Google Scholar]
- 5.Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children’s Research Hospital. J Clin Oncol. 1997;15:2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong GT, Conklin HM, Huang S, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13:223–234. doi: 10.1093/neuonc/noq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh KS, Hung J, Robertson PL, et al. Outcomes of multi-disciplinary management in pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2011;81:e481–e488. doi: 10.1016/j.ijrobp.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Fisher PG, Tihan T, Goldthwaite PT, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51:245–250. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 9.Mishra KK, Puri DR, Missett BT, et al. The role of up-front radiation therapy for incompletely resected pediatric WHO grade II low-grade gliomas. Neuro Oncol. 2006;8:166–174. doi: 10.1215/15228517-2005-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benesch M, Lackner H, Sovinz P, et al. Late sequela after treatment of childhood low-grade gliomas: a retrospective analysis of 69 long-term survivors treated between 1983 and 2003. J Neurooncol. 2006;78:199–205. doi: 10.1007/s11060-005-9091-z. [DOI] [PubMed] [Google Scholar]
- 11.Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merchant TE, Hua CH, Shukla H, et al. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51:110–117. doi: 10.1002/pbc.21530. [DOI] [PubMed] [Google Scholar]
- 13.Pollack IF, Classen D, al-Shboul Q, et al. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg. 1995;82:536–547. doi: 10.3171/jns.1995.82.4.0536. [DOI] [PubMed] [Google Scholar]
- 14.Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8:810–818. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 15.Fouladi M, Hunt DL, Pollack IF, et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children’s Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98:1243–1252. doi: 10.1002/cncr.11637. [DOI] [PubMed] [Google Scholar]
- 16.Heath JA, Turner CD, Poussaint TY, et al. Chemotherapy for progressive low-grade gliomas in children older than ten years: the Dana-Farber experience. Pediatr Hematol Oncol. 2003;20:497–504. doi: 10.1080/08880010390232709. [DOI] [PubMed] [Google Scholar]
- 17.Lafay-Cousin L, Sung L, Carret AS, et al. Carboplatin hypersensitivity reaction in pediatric patients with low-grade glioma: a Canadian Pediatric Brain Tumor Consortium experience. Cancer. 2008;112:892–899. doi: 10.1002/cncr.23249. [DOI] [PubMed] [Google Scholar]
- 18.Lee MJ, Ra YS, Park JB, et al. Effectiveness of novel combination chemotherapy, consisting of 5-fluorouracil, vincristine, cyclophosphamide and etoposide, in the treatment of low-grade gliomas in children. J Neurooncol. 2006;80:277–284. doi: 10.1007/s11060-006-9185-2. [DOI] [PubMed] [Google Scholar]
- 19.Massimino M, Spreafico F, Cefalo G, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20:4209–4216. doi: 10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- 20.Mishra KK, Squire S, Lamborn K, et al. Phase II TPDCV protocol for pediatric low-grade hypothalamic/chiasmatic gliomas: 15-year update. J Neurooncol. 2010;100:121–127. doi: 10.1007/s11060-010-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moghrabi A, Friedman HS, Ashley DM, et al. Phase II study of carboplatin (CBDCA) in progressive low-grade gliomas. Neurosurg Focus. 1998;4:e3. doi: 10.3171/foc.1998.4.4.6. [DOI] [PubMed] [Google Scholar]
- 22.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 23.Perilongo G. Considerations on the role of chemotherapy and modern radiotherapy in the treatment of childhood low grade glioma. J Neurooncol. 2005;75:301–307. doi: 10.1007/s11060-005-6754-8. [DOI] [PubMed] [Google Scholar]
- 24.Merchant TE, Kun LE, Wu S, et al. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27:3598–3604. doi: 10.1200/JCO.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher BJ, Bauman GS, Leighton CE, et al. Low-grade gliomas in children: tumor volume response to radiation. Neurosurg Focus. 1998;4:e5. doi: 10.3171/foc.1998.4.4.8. [DOI] [PubMed] [Google Scholar]
- 26.Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14:1265–1284. doi: 10.1093/neuonc/nos202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher BJ, Bauman GS, Leighton CE, et al. Low-grade gliomas in children: tumor volume response to radiation. J Neurosurg. 1998;88:969–974. doi: 10.3171/jns.1998.88.6.0969. [DOI] [PubMed] [Google Scholar]
- 28.Scheinemann K, Bartels U, Tsangaris E, et al. Feasibility and efficacy of repeated chemotherapy for progressive pediatric low-grade gliomas. Pediatr Blood Cancer. 2011;57:84–88. doi: 10.1002/pbc.22917. [DOI] [PubMed] [Google Scholar]
- 29.Kim YH, Pierscianek D, Mittelbronn M, et al. TET2 promoter methylation in low-grade diffuse gliomas lacking IDH1/2 mutations. J Clin Pathol. 2011;64:850–852. doi: 10.1136/jclinpath-2011-200133. [DOI] [PubMed] [Google Scholar]
- 30.Packer RJ, Lange B, Ater J, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11:850–856. doi: 10.1200/JCO.1993.11.5.850. [DOI] [PubMed] [Google Scholar]