Abstract
Mixed mode separation using a combination of micellar electrokinetic chromatography (MEKC) and polyelectrolyte multilayer (PEM) coatings is herein reported for the separation of achiral and chiral analytes. Many analytes are difficult to separate by MEKC and PEM coatings alone. Therefore, the implementation of a mixed mode separation provides several advantages for overcoming the limitations of these well-established methods. In this study, it was observed that achiral separations using MEKC and PEM coatings individually resulted in partial resolution of 8 very similar aryl ketones when the molecular micelle (sodium poly(N-undecanoyl-l-glycinate) (poly-SUG)) concentration was varied from 0.25% – 1.00% (w/v) and the bilayer number varied from 2 – 4. However, when mixed mode separation was introduced, baseline resolution was achieved for all 8 analytes. In the case of chiral separations, temazepam, aminoglutethimide, benzoin, benzoin methyl ether and coumachlor were separated using the three separation techniques. For chiral separations, the chiral molecular micelle, sodium poly(N-undecanoyl-l-leucylvalinate) (poly-l-SULV), was employed at concentrations of 0.25–1.50% (w/v) for both MEKC and PEM coatings. Overall, the results revealed partial separation with MEKC and PEM coatings individually. However, mixed mode separation enabled baseline separation of each chiral mixture. The separation of achiral and chiral compounds from different compound classes demonstrates the versatility of this mixed mode approach.
Keywords: Mixed mode, Molecular micelle, Open tubular capillary electrochromatography, Polyelectrolyte multilayer coatings, Micellar electrokinetic chromatography
1 Introduction
Chiral separations are important in the pharmaceutical, biomedical and environmental industries, primarily because the interactions and behaviors of individual enantiomers have not been fully explored. The importance of these studies is further magnified by the mass circulation of thousands of chiral drugs [1, 2]. Enantiomers of the same drug may have very different clinical effects in the human body since the body metabolizes these isomers through different pathways [3]. Many drugs exhibit dissimilar pharmacokinetic behavior. As a result, the United States Food and Drug Administration requires that the pharmacological and toxicological activity of each isomer be investigated and documented [4]. The development of methods to separate chiral analytes continues to garner much attention.
In order to achieve chiral separation, enantiomeric interactions must occur in a chiral environment. This is generally achieved through the use of a chiral selector or chiral discriminating agent able to differentially interact with each individual enantiomer. Several chiral selectors have been used in capillary electrophoresis (CE) separations; these include bile acids [5], crown ethers [6, 7], polysaccharides [8], proteins [9, 10], cyclodextrins [11, 12] and molecular micelles [14, 15]. Our group is among the pioneers in the use of molecular micelles for CE enantioseparations. Molecular micelles are prepared by polymerizing surfactants at sufficiently high concentrations for formation of micelles. These resulting polymers are thought to resemble a conventional micelle and are preferred over unpolymerized micelles because molecular micelles have no critical micelle concentration and, thus, can be used at concentrations below the CMC. In addition, the covalent bonds formed during the polymerization process eliminate the dynamic equilibrium between monomer and micelle. Experimental parameters such as pH and concentration of added organic solvent, known to disrupt the formation of conventional micelles, have been shown not to seriously damage molecular micellar interactions [1, 13, 14]. The addition of molecular micelles to a separation system may result in the resolution of a wide variety of compounds. For example, Rizvi et al. investigated the separation of the β-blockers, labetalol, and nadalol by using variations of the chiral selector, polyalkenoxy amino acid polymers [1]. Also, Shamsi et al. used CE and resolved several different compound classes by using the versatile chiral selector, poly (sodium N-undecanoyl-l-leucylvalinate) (poly-l-SULV) [14].
The technique of CE has emerged as one of the leading separation approaches because high separation efficiencies are achieved with relatively low consumption of analyte and chiral selector. Thus, CE has the added advantages of relatively simple method development and short analysis times [17, 18]. Micellar electrokinetic chromatography (MEKC) and capillary electrochromatography (CEC) are hybrids of CE which combine the benefits of electrophoresis and chromatography to separate both charged and neutral analytes [16–18, 26]. In MEKC, a pseudostationary phase is created by the introduction of a surfactant at a concentration above the CMC, to the mobile phase. Separation of both charged and neutral analytes are based on hydrophobic and ionic interactions of the analytes with micelles in the pseudostationary phase [20]. MEKC has been used to separate both achiral and chiral analytes [21–24]. Shamsi et al. [24] used poly-l-SULV to separate 58 chiral analytes with MEKC as the separation mode. The results indicted the wide selectivity of this molecular micelle.
The methodology of CEC combines both the selectivity of HPLC and the efficiency of CE [15, 27]. Separation is based on differences in electrophoretic mobilities and partitioning of the analytes into the stationary phase. Neutral analytes are separated through interactions with the stationary phase which is adsorbed to the capillary wall, while charged analytes are separated due to differences in charge and size as well as partitioning behavior [27]. CEC has shown great potential in the separation of both achiral and chiral analytes. The stationary phase can be prepared by several methods including adsorption, where the stationary phase can either be dynamically or physically adsorbed to the capillary wall to shield the negatively charged silanol groups with a layer of the coating material [15, 28, 29]. In one mode of CEC, open tubular capillary electrochromatography (OT-CEC), the stationary phase coated unto the capillary wall and the mobile phase, which flows through the column, is driven by the electroosmotic flow (EOF). Liu et al. used avidin, a basic protein, as the adsorbed stationary phase to separate a total of sixteen different enantiomers [7]. One widely used coating constructed by a physical adsorption process is a polyelectrolyte multilayer (PEM) coating.
A PEM coating is formed by alternately exposing the hydrophilic inner wall of a silica capillary first to cationic and then anionic polymers. The combination of each is called a bilayer and the mechanism of a PEM coating formation is via ion exchange that results in stable coatings [37]. PEM coatings are constructed using chiral cationic and anionic polymers and have been used to separate a number of chiral analytes. Rmaile and Schlenoff used the polymers poly-l-lysine and poly-l-glutamic acid, among others, to resolve chiral probes such as D- and L-ascorbic acid, 3-3(3,4-dihydroxyphenyl)-L- and D-alanine (DOPA), and a chiral viologen (a geometric isomer) [38]. In our laboratory, Kamande et al. used poly-l-lysine hydrobromide and poly (sodium N-undecanoyl-l-leucyl-alaninate) (poly-l-SULA) to separate three binaphthyl derivatives and two β-blockers [39]. We note that it is not necessary for both polymers to be chiral in order to separate chiral analytes. For example, Kapnissi et al. optimized several experimental parameters using PEMs generated with achiral poly (diallyldimethylammonium chloride) (PDADMAC) and chiral poly (sodium N-undecanoyl-l-leucyl-valinate) (poly-l-SULV) to resolve chiral analytes. In that study the authors created up to a 12 bilayer capillary using ionic liquids as additives [40].
In this paper, the influence of separation mode on the resolution of achiral and chiral analytes is investigated. The effects of bilayer number and polymer concentration using the molecular micelles poly(N-undecanoyl-l-glycinate) (poly-SUG) and (poly-l-SULV) are also studied.
Materials and methods
2.1 Chemicals
The cationic polymer, poly(diallyldimethylammonium chloride) (PDADMAC) was purchased from Sigma Chemical Company (St. Louis, MO). The chemicals used to synthesize surfactant monomers, N-hydroxysuccinimide, undecylenic acid, dicyclohexylcarbodiimide, and sodium bicarbonate were purchased from Fluka (Milwaukee, WI). The peptides leucine-valine and glycine were purchased from Bachem Bioscience, Inc. (King of Prussia, PA). Sodium hydroxide, ethyl acetate, and tetrahydrofuran were purchased from Sigma-Aldrich (Milwaukee, WI). Sodium phosphate dibasic, monobasic sodium phosphate, tris[hydroxymethyl]aminomethane, sodium borate, methanol, and acetone were purchased from Fisher Scientific (Fair Lawn, NJ). The achiral alkyl aryl ketones, acetophenone, propiophenone, butyrophenone, valerophenone, hexanophenone, heptanophenone, octanophenone, decanophenone, as well as the chiral analytes, temazepam, benzoin, aminoglutethimide, coumachlor, and benzoin methyl ether were also purchased from Sigma (St. Louis, MO.) All materials were used as received. The molecular structures of the analytes investigated are shown in Figure 1.
Figure 1.
Structures of analytes; A. Chiral analytes; B: Achiral aryl ketones
2.2 Instrumentation
A roll of fused silica capillary, with an internal diameter of 50µm was purchased from Polymicro Technologies (Phoenix, AZ). A piece of this material with a total length of 60 cm (50 cm effective length) was used for the experiments described in this manuscript. All separations were conducted using a Beckman P/ACE MDQ capillary electrophoresis system, equipped with a photodiode array detector (Fullerton, CA). Liquid coolant was used to maintain the temperature at 15°C. The applied voltage ranged from 15kV to 30kV. The achiral and chiral analytes were detected at 220nm and 254 nm. All analytes were injected using 0.5psi for 5s.
2.3 Synthesis of molecular micelles
The achiral molecular micelle, sodium poly(N-undecanoyl-l-glycinate) (poly-SUG) as well as the chiral dipeptide molecular micelle sodium poly(N-undecanoyl-L-leucylvalinate) (poly-l-SULV) were synthesized according to a procedure previously described by Wang and Warner [41]. Solutions containing 100 mM of the monomers were polymerized by use of a 60Co γ-ray irradiation source. The molecular structures of the molecular micelles and the cationic polymer used in this study are illustrated in Figure 2.
Figure 2.
A. Structural representation of (I) Poly-SUG and (II) Poly-l-SULV
B. Structural representation of PDADMAC.
2.4 Sample and buffer preparation
For achiral separations, the background electrolyte consisted of 100 mM Tris, pH 10, while 50 mM phosphate (25 mM monobasic/25 mM dibasic) pH 7.5 was used for chiral separations. In all experiments, 1M NaOH and 1M HCl were used to adjust the pH of the background electrolyte. Prior to use, all buffers were filtered using a 0.45 µm polypropylene nylon filter and sonicated for 15 minutes. Solutions of 8 alkyl aryl ketones (0.1 mg/mL) were dissolved in MeOH for the achiral investigations and 0.2 mg/mL solutions were prepared in 50:50 methanol/water for use in the chiral studies. The achiral cationic polymer, PDADMAC was used at 0.5% (w/v) in deionized water. Achiral and chiral anionic molecular micelles (poly-SUG and poly-l-SULV) were varied from concentrations of 0.25% (w/v) to 1.50% (w/v) for all separation studies.
2.5 Micellar Electrokinetic Chromatography (MEKC) Procedure
An untreated silica capillary is rinsed with 1M NaOH for 30 minutes to deprotonate the capillary wall followed by a 15 minute rinse with deionized water. The pseudostationary phase is created by the addition of varying concentrations (0.25% – 1.00% (w\v)) of molecular micelles to the background electrolyte. This phase is then flushed through the capillary for 5 minutes prior to analyte injection. The capillary is rinsed with 0.1M NaOH for 2 minutes, deionized H2O for 2 minutes and the micellar background electrolyte for 5 minutes between each run. All rinses are performed using the rinse function on the CE instrument.
2.5 Polyelectrolyte multilayer coating procedure
As in the MEKC procedure, a fused silica capillary is deprotonated with 1M NaOH for 30 minutes followed by a 15 minute deionized water rinse. A 20 minute rinse with the cationic polymer followed by a 5 minute rinse with deionized water initiates the first layer of the PEM coating. To complete the bilayer, the anionic polymer is rinsed through the column for 5 minutes, followed by a 5 minute deionized water rinse. All other bilayers were created with alternate 5 minute rinses of the cationic and anionic polymers. The number of bilayers was varied from 2 to 4. All rinses were performed using the rinse function of the CE instrument, with applied pressure of 20psi.
2.6 Mixed Mode Separation
The mixed mode separation method combines the MEKC and PEM coatings procedures. First, the PEM coatings are constructed with the desired number of bilayers needed for the study. Then, the molecular micelles are added to the mobile phase (MEKC) at desired concentrations and the various studies are completed.
3 Results and discussion
3.1 Effect of concentration of poly-l-SUG on the separation of 8 achiral alkyl aryl ketones using MEKC
To investigate the influence of molecular micelle concentration on the separation of 8 achiral alkyl aryl ketones using MEKC, five different mobile phases were prepared. The molecular micelle concentration was varied from 0.25% (w/v) to 1.00% (w/v) poly-SUG in 100 mM Tris at pH 10 (results shown in figure 3). At 0.25% (w/v), only the first four aryl ketones, acetophenone, propiophenone, butyrophenone, and valerophenone were resolved with moderate peak shape and efficiency. As the molecular micelle concentration increased to 1.00% (w/v), the resolution of the aryl ketones increased with a slight increase in migration time. The elution order of these analytes is indicated in the figure with the least hydrophobic, acetophenone, eluting first and the most hydrophobic, decanophenone, eluting last. All peaks were identified by spiking the concentration of one analyte at a time. At 1.00% (w/v) poly-SUG, the resolution of the first five analytes increased. However, there was only partial separation of the last three aryl ketones. The shapes and efficiencies of the latter peaks were due to the more hydrophobic aryl ketones being retained in the micellar phase longer than the aqueous phase. Baseline resolution of all 8 aryl ketones was not achieved using these parameters in MEKC.
Figure 3.
Influence of poly-SUG concentration on the separation of 8 aryl ketones using MEKC
Conditions: A: 0.25% (w/v) p-SUG; B: 0.5% (w/v) p-SUG; C: 0.75% (w/v) p-SUG; D: 1.00% (w/v) p-SUG Buffer: 100mM Tris, pH 10; Analyte concentration: 0.1mg/ml, Capillary Length: 57cm total (50 cm effective length); Capillary I.D.: 50 µm; Temperature: 15 °C; Voltage: 15kV, Injection: 5psi for 5s; Detection: 220nm; Analytes: 1. Acetophenone, 2. Propiophenone, 3. Butyrophenone, 4. Valerophenone, 5. Hexanophenone, 6. Heptanophenone, 7. Octanophenone, 8. Decanophenone
3.2 Effect of concentration and bilayer number of poly-l-SUG on the separation of 8 achiral alkyl aryl ketones using PEM coatings
The influence of the anionic molecular micelle concentration, poly-SUG, on the resolution of the 8 aryl ketones was investigated with PEM coatings. Different PEM coatings were constructed using 0.25% (w/v), 0.5% (w/v), and 1.00% (w/v) poly-SUG as the anionic layer. The cationic layer was held constant using 0.5% (w/v) PDADMAC. All concentrations of poly-SUG resulted in only partial separation of the aryl ketones (Data not shown). The optimum anionic polymer concentration was chosen to be 0.5% (w/v) poly-SUG since the highest resolution with a relatively short migration time was achieved. All subsequent experiments were performed at this concentration.
Three different PEM coatings were achieved using 0.5% (w/v) PDADMAC and 0.5% (w/v) poly-SUG consisting of 2, 3 and 4 bilayers (Figure 4). The influence of bilayer number on the separation of 8 aryl ketones was investigated. The column coated with 2 bilayers resulted in partial separation of the aryl ketones within 10 minutes. Even though the resolutions increased as the number of bilayers increased, there was still only partial separation (Rs < 1.5) of the ketones using the 3 bilayer system with an elution time of 14 minutes and 20 minutes for the 4 bilayer coating. It has been well established in PEM coatings that an increase in the number of bilayers results in increased interactions between the analyte and the coatings which in turn results in higher resolutions [39, 40]. As a result, it was concluded that baseline resolution of all 8 aryl ketones would not be possible using PEM coatings alone.
Figure 4.
Influence of bilayer number on the separation of 8 aryl ketones using PEM coatings
Conditions: A: 2 bilayers; B: 3 bilayers; C: 4 bilayers; Coating: 0.5% (w/v) PDADMAC and 0.5% (w/v) p-SUG; Buffer: 100 mM Tris, pH 10; Analyte concentration: 0.1 mg/ml, Capillary Length: 57 cm total (50 cm effective length); Capillary I.D.: 50 µm; Temperature: 15 °C; Voltage: 15 kV, Injection: 5 psi for 5 s; Detection: 220 nm; Analytes: 1. Acetophenone, 2. Propiophenone, 3. Butyrophenone, 4. Valerophenone, 5. Hexanophenone, 6. Heptanophenone, 7. Octanophenone, 8. Decanophenone
3.3 Effect of concentration of poly-l-SUG and voltage on the separation of 8 achiral alkyl aryl ketones using mixed mode separation technique
The use of MEKC and PEM coatings alone resulted in only partial separation of the 8 alkyl aryl ketones. Therefore, a different approach, i.e. mixed mode separation, was employed in order to achieve baseline resolution. In mixed mode separation, the analytes are able to partition into the stationary phase as well as the mobile phase. Increased interactions between analytes and molecular micelles results in higher resolution. In figure 5, each capillary was coated with 2 bilayers of 0.5% PDADMAC and 0.5% p-SUG (PEM coating), also, different concentrations of p-SUG were placed in the mobile phase. In figure 5A, 0.25% p-SUG in the mobile phase resulted in the separation in seven (7) of the eight (8) aryl ketones. As the concentration of the molecular micelle increased (figure 5B–5D), the resolution of the aryl ketones increased and all 8 ketones were baseline resolved. Also, higher concentrations of p-SUG provided longer migration times and higher peak efficiencies. The electrophoretic mobility of anionic molecular micelles is opposite to that of the EOF and the hydrophobic aryl ketones interact strongly with poly-SUG. Therefore, the analytes are retained in the column longer, hence, longer migration times. The optimum concentration of p-SUG was 0.75% (w/v) since the highest resolution in a reasonable migration time as well as higher peak efficiencies were obtained (figure 5C).
Figure 5.
Influence of poly-SUG concentration on the separation of 8 aryl ketones using mixed mode separation technique
Conditions: All PEM coatings were constructed using 2 bilayers of 0.5% PDADMAC and 0.5% p-SUG (A-D); A: MEKC: 0.25% (w/v) p-SUG; B: MEKC: 0.5% (w/v) p-SUG; C: MEKC: 0.75% (w/v) p-SUG; D: MEKC: 1.00% (w/v) p-SUG; Buffer: 100 mM Tris, pH 10; Analyte concentration: 0.1 mg/ml, Capillary Length: 57 cm total (50 cm effective length); Capillary I.D.: 50 µm; Temperature: 15 °C; Voltage: 15 kV, Injection: 5 psi for 5 s; Detection: 220 nm; Analytes: 1. Acetophenone, 2. Propiophenone, 3. Butyrophenone, 4. Valerophenone, 5. Hexanophenone, 6. Heptanophenone, 7. Octanophenone, 8. Decanophenone
The effect of voltage on the separation of 8 aryl ketones was also investigated. The voltage was varied from 15kV to 30 kV (results not shown). As the voltage increased, the viscosity of the electrolyte decreased and the analytes moved at a faster rate through the column. At these separation conditions shorter migration times (18 minutes) and higher peak efficiencies were obtained.
3.4 Effect of the separation mode on the resolution of the chiral benzodiazepine, temazepam
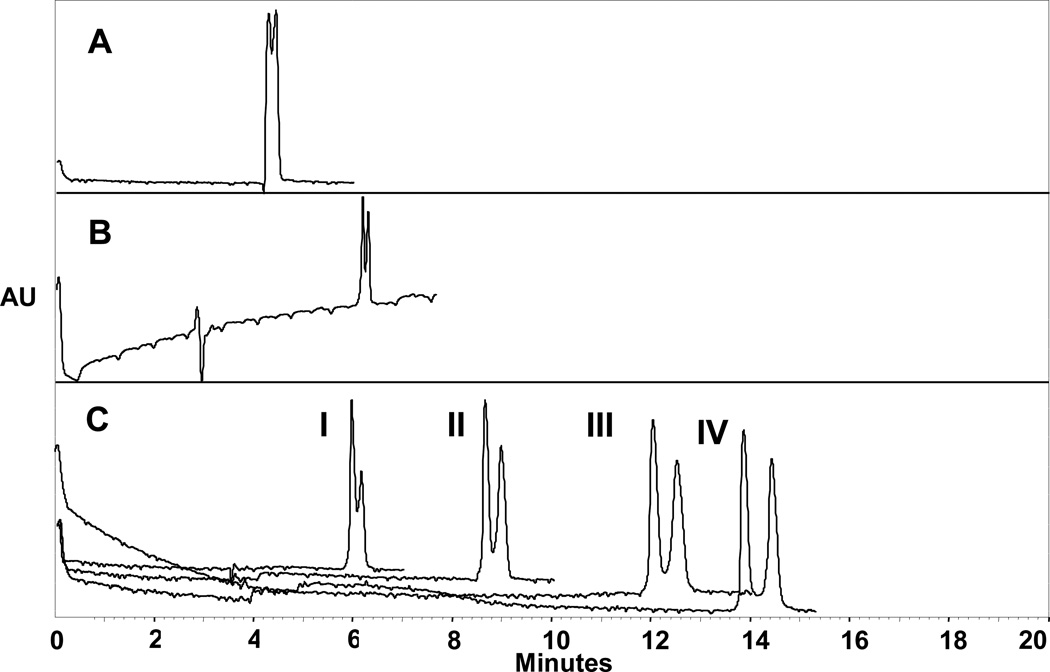
Due to the success of the mixed mode approach to the separation of achiral analytes, experiments were conducted to investigate its effect on chiral separation. Each of the 3 separation techniques were also applied to chiral analytes of different compound classes. The results obtained using each separation mode for temazepam is shown in figure 6. In figure 6A, PEM coatings were constructed with 2 bilayers of 0.5% (w/v) PDADMAC and 0.75% (w/v) poly-l-SULV. Only partial separation was achieved for temazepam (Rs = 0.39) in a migration time of 5 minutes. Next, the separation of temazepam was investigated using four concentrations (0.25 – 1.00% (w/v)) of poly-l-SULV. The technique used for this study was MEKC. In figure 6B, the highest resolution (Rs = 0.75) obtained when 1.00% (w/v) poly-l-SULV was used is illustrated. Examination of the electropherogram reveals an increase in resolution compared to PEM coatings as well as increases in peak efficiencies and migration times. However, baseline resolution could not be achieved by using either method alone. Therefore, the mixed mode separation approach was employed to fully resolve this compound. In figure 6C, the combination of 2 bilayers (PEM coating) on the capillary wall as well as varying concentrations (0.25 – 1.00% (w/v)) of poly-l-SULV in the mobile phase (MEKC) is illustrated. As seen in figure 6C–I, the resolution of temazepam increased slightly to 0.76 when 0.25% (w/v) was used. When the concentration of poly-l-SULV increased from 0.5% (w/v) to 1.00% (w/v) (Figure 6C-II–IV), the resolution of temazepam increased to 1.76. The increase in resolution is to due to the increased partitioning and interactions between temazepam and the chiral molecular micelle, poly-l-SULV which is located both in the stationary and mobile phases. These interactions include hydrophobic interactions between the molecular micelle core and the chiral analyte, electrostatic interactions, or hydrogen bonding between the analyte and head group of the molecular micelle (poly-l-SULV). In addition, several other interactions may occur due to ion-dipole bonds, Van der Waals forces and π-π interactions. The differences in these interactions results in the resolution of two isomers.
Figure 6.
Influence of separation mode on the resolution of temazepam
Conditions: A. PEM coatings: 2 bilayers of 0.5% (w/v) PDADMAC and 0.75% (w/v) p-SULV; B. MEKC: 1.00% (w/v) p-SULV; C: Mixed mode: PEM Coating: 2 bilayers of 0.5% (w/v) PDADMAC and 0.75% (w/v) p-SULV and MEKC: I. 0.25% (w/v); II. 0.5% (w/v); III. 0.75% (w/v); IV. 1.00% (w/v) poly-l-SULV; Buffer: 50 mM phosphate, pH 9.2; Analyte concentration: 0.2 mg/ml, Capillary Length: 57 cm total (50 cm effective length); Capillary I.D.: 50 µm; Temperature: 15 °C; Voltage: 30 kV, Injection: 5 psi for 5 s; Detection: 254 nm
3.5 Influence of the polymer concentration on the resolution of aminoglutethimide using mixed mode separation technique
Examination of previous results suggest superiority of the mixed mode separation technique as compared to MEKC or PEM coatings alone in terms of analyte resolution. In this study, the influence of polymer concentration in the mobile phase using the mixed mode technique was investigated. The PEM coatings were first constructed using 2 bilayers of 0.5% (w/v) PDADMAC and 0.75% (w/v) poly-l-SULV. Then, the concentration of poly-l-SULV in the mobile phase was varied from 0.25–1.5% (w/v). The results obtained are shown in figure 7. When 0.25% (w/v) poly-l-SULV was used, partial separation (Rs = 0.29) of aminoglutethimide was achieved within 10 minutes (Figure 7A). Figure 7B shows an increase in resolution (Rs = 0.75) when using 0.5% (w/v) poly-l-SULV in a similar elution time. As expected, when the concentration of poly-l-SULV in the mobile phase increased from 1.00- 1.50% (w/v) (Figure 7C–D), the resolution (Rs = 1.40 – 1.68) as well as the migration time (13 – 16 minutes) increased. The optimum condition for separation (baseline resolution) was achieved when 1.5% (w/v) poly-l-SULV was used. The presence of the molecular micelles both in the stationary and mobile phases plays a seminal role in increasing the polymer-analyte interactions, hence, increased resolution.
Figure 7.
Influence of poly-l-SULV concentration in the mobile phase on the resolution of aminoglutethimide using mixed mode separation technique
Conditions: All PEM coatings were constructed using 2 bilayers of 0.5% (w/v) PDADMAC and 0.75% (w/v) p-SULV (A-D). A. Mixed Mode: MEKC: 0.25% (w/v) p-SULV; B. Mixed Mode: MEKC: 0.5% (w/v) p-SULV; C. Mixed Mode:, MEKC: 1.00 %(w/v) p-SULV; D. Mixed Mode: MEKC: 1.50% (w/v) p-SULV Buffer: 50 mM phosphate, pH 7.5; Analyte concentration: 0.2 mg/ml, Capillary Length: 57 cm total (50 cm effective length); Capillary I.D.: 50 µm; Temperature: 15 °C; Voltage: 30 kV, Injection: 5 psi for 5 s; Detection: 254 nm
3.6 Optimum separation conditions of three chiral analytes (benzoin, benzoin methyl ether and coumachlor) using MEKC, PEM coatings, and mixed mode separation technique
Three additional chiral analytes were investigated to demonstrate the effectiveness of the mixed mode separation. Table I is a compilation of the separation conditions that achieved the highest resolution for 3 chiral analytes using each separation mode. Using MEKC, resolution values of 0.96, 0.78 and 1.26 were obtained for benzoin, benzoin methyl ether (BME) and coumachlor respectively. The separation of benzoin and coumachlor occurred in approximately 14 minutes, whereas BME had an elution time of 6.13 minutes. Since baseline resolution could not be achieved using MEKC, further experiments were performed using PEM coatings to determine its influence on the resolution of these analytes. However, the results obtained using PEM coatings were inferior to those of MEKC. Though less molecular micelles were consumed, both resolution and migration times decreased using coated capillaries. As a result, the mixed mode separation approach was implemented to overcome the limitations of each method when used separately. As shown in table I, the resolution of each chiral analyte increased using mixed mode separation and all three analytes were baseline resolved within 9 minutes. In addition, the capacity factors increased for all 3 analytes when the mixed mode separation was used and the selectivity attained was similar to those of MEKC. These values are great indicators of the effectiveness of these chiral columns.
Table 1.
Optimum separation conditions of three chiral analytes using MEKC, PEM coatings and mixed mode (MM) separation technique
| Separation Mode |
Analyte | [Poly-L- SULV] (%w/v) |
Volt (kV) |
EOF (min) |
MT1 | MT2 | Rs | K1 | K2 | α |
|---|---|---|---|---|---|---|---|---|---|---|
| MEKC | Benzoin | 1.00 | 15 | 7.45 | 13.22 | 13.58 | 0.96 | 0.77 | 0.82 | 1.06 |
| 3bilayers-PEM | Benzoin | 1.00 | 30 | 4.12 | 4.27 | 4.43 | 0.34 | 0.04 | 0.08 | 2.08 |
| 2bilayers-MM | Benzoin | 0.75/1.50 | 30 | 3.34 | 7.26 | 7.51 | 1.50 | 1.17 | 1.25 | 1.06 |
| MEKC | BME | 1.00 | 30 | 3.04 | 6.00 | 6.13 | 0.78 | 0.97 | 1.01 | 1.04 |
| 3bilayers-PEM | BME | 1.00 | 30 | 3.88 | 3.97 | 4.25 | 0.51 | 0.02 | 0.10 | 4.11 |
| 3bilayers-MM | BME | 0.75/1.50* | 30 | 3.24 | 8.19 | 8.43 | 1.51 | 1.53 | 1.60 | 1.05 |
| MEKC | Coumachlor | 1.00 | 15 | 7.41 | 12.84 | 3.18 | 1.26 | 0.73 | 0.78 | 1.06 |
| 3bilayers-PEM | Coumachlor | 0.75 | 30 | 4.20 | 6.13 | 6.80 | 0.82 | 0.46 | 0.62 | 1.35 |
| 3bilayes-MM | Coumachlor | 0.75/1.50* | 30 | 3.25 | 6.77 | 6.99 | 1.52 | 1.08 | 1.15 | 1.06 |
Conditions: All PEM coatings were constructed using 0.5% (w/v) PDADMAC. Temperature: 15 °C; Buffer: 50 mM phosphate, pH 7.5; Analyte concentration: 0.2 mg/ml, Capillary Length: 57 cm total (50 cm effective length); Capillary I.D.: 50 µm; Injection: 5 psi for 5 s; Detection: 254 nm;
0.75% (w/v) poly-l-SULV in the stationary phase (PEM coating) and 1.50% (w/v) poly-l-SULV in the mobile phase (MEKC).
4 Concluding Remarks
Mixed mode separation has been implemented to separate both achiral and chiral analytes. This method can be employed to separate analytes of various compound classes that are difficult to resolve using MEKC or PEM coatings alone. All results are indicative of an increase in resolution when mixed mode separation is used. Baseline resolution was achieved for 8 achiral aryl ketones as well as temazepam, aminoglutethimide, benzoin, benzoin methyl ether, and coumachlor. Increasing the molecular micelle concentration in the mobile phase resulted in increased chiral resolution. The separation conditions were optimized for the specific analytes investigated; however, mixed mode separation has the potential to be applied to a wide range of analytes. The selectivity of the system can be tailored by altering the molecular micelles used, the bilayer number, as well as cationic and anionic polymer concentrations, among others. Overall, this approach would be of great benefit for quick pharmaceutical screening as well as in areas that require the difficult separation of achiral or chiral analytes.
Acknowledgements
I.M. Warner acknowledges the National Institutes of Health (39844-13), the National Science Foundation (616824), and the Phillip W. West Endowment for the financial support of this research. The authors thank Dr. Bilal El-Zahab for helpful suggestions during the preparation of this manuscript.
Abbreviations
- PEM
polyelectrolyte multilayer
- OT-CEC
open tubular capillary electrochromatography
- PDADMAC
poly(diallyldimethylammonium chloride)
- poly-l-SUG
sodium poly(N-undecanoyl-l-glycinate)
- poly-l-SULV
sodium poly(N-undecanoyl-l-leucylvalinate)
- MM
mixed mode
References
- 1.Rivi SA, Akbay C, Shamsi SA. Electrophoresis. 2004;25:853–860. doi: 10.1002/elps.200305762. [DOI] [PubMed] [Google Scholar]
- 2.Caner H, Groner E, Levy L, Agranat I. Drug Discovery Today. 2004;9:105–110. doi: 10.1016/s1359-6446(03)02904-0. [DOI] [PubMed] [Google Scholar]
- 3.Terabe S, Otsuka K, Ichikawa K, Tsuchiya A, Ando T. Anal. Chem. 1984;56:111–113. [Google Scholar]
- 4.Stenson C. S. Chem. Eng. News. 2001 May 14;:45. [Google Scholar]
- 5.Terabe S, Shibata M, Miyashita Y. J.Chromatogr. A. 1989;480:403–411. [Google Scholar]
- 6.Verleysen K, Sabah S, Scriba G, Sandra P. Chromatographia. 1999;49:215–218. [Google Scholar]
- 7.Verleysen K, Sandra P. J. High Res. Chromatogr. 1999;22:33–38. [Google Scholar]
- 8.Nishi H, Izumoto S, Nakamura K, Nakai H, Sato T. Chromatographia. 1996;42:617–623. [Google Scholar]
- 9.Tanaka Y, Terabe S. J. Chromatogr. A. 1995;694:277–284. [Google Scholar]
- 10.Liu Z, Otsuka K, Terabe S. J. Sep. Sci. 2001;24:17–26. [Google Scholar]
- 11.Fanali S. Electrophoresis. 2009;30:S203–S210. doi: 10.1002/elps.200900056. [DOI] [PubMed] [Google Scholar]
- 12.Scriba GKE. J. Sep. Sci. 2008;31:1991–2011. doi: 10.1002/jssc.200800095. [DOI] [PubMed] [Google Scholar]
- 13.Mwongela S, Akbay C, Zhu X, Collins S, Warner IM. Electrophoresis. 2003;24:2940–2947. doi: 10.1002/elps.200305521. [DOI] [PubMed] [Google Scholar]
- 14.Shamsi SA, Valle BC, Billiot F, Warner IM. Anal. Chem. 2003;75:379–387. doi: 10.1021/ac020386r. [DOI] [PubMed] [Google Scholar]
- 15.Kamande MW, Kapnissi CP, Zhu X, Akbay C, Warner IM. Electrophoresis. 2003;24:945–951. doi: 10.1002/elps.200390137. [DOI] [PubMed] [Google Scholar]
- 16.Williams CC, Shamsi SA, Warner IM. In: Advances in Chromatography. Brown PR, Grushka E, editors. New York, NY: Marcel Dekker, Inc.; 1997. pp. 363–423. [PubMed] [Google Scholar]
- 17.Skoog DA, Holler JF, Nieman TA. In: Principles of Instrumental Analysis. 5th Edn. Sherman M, Bortel J, Messina F, editors. Orlando, Fl: Harcourt Brace College Publishers; 1998. [Google Scholar]
- 18.Heiger DN. In: High Performance Capillary Electrophoresis-An Introduction. 2nd Edn. Hupe P, Rozing G, Mcmanigill D, van de Goor T, editors. France: Hewlett-Packard Company; 1992. [Google Scholar]
- 19.Graul TW, Schlenoff JB. Anal. Chem. 1999;71:4007–4013. [Google Scholar]
- 20.Terabe S, Otsuka K, Ichikawa K, Tsuchiya A, Ando T. Anal. Chem. 1984;56:111–113. [Google Scholar]
- 21.Williams AA, Fakayode SO, Huang X, Warner IM. Electrophoresis. 2006;27:4127–4140. doi: 10.1002/elps.200600071. [DOI] [PubMed] [Google Scholar]
- 22.Akbay C, Gill NL, Warner IM. Electrophoresis. 2007;28:1752–1761. doi: 10.1002/elps.200600449. [DOI] [PubMed] [Google Scholar]
- 23.Rizvi SAA, Shamsi SA. Electrophoresis. 2007;28:1762–1778. doi: 10.1002/elps.200600483. [DOI] [PubMed] [Google Scholar]
- 24.Shamsi SA, Valle BC, Billiot F, Warner IM. Anal. Chem. 2003;75:379–387. doi: 10.1021/ac020386r. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Lucy CA. Electrophoresis. 2004;25:825–832. doi: 10.1002/elps.200305760. [DOI] [PubMed] [Google Scholar]
- 26.Kilar F. Electrophoresis. 2003;24:3908–3916. doi: 10.1002/elps.200305650. [DOI] [PubMed] [Google Scholar]
- 27.Cikalo MG, Bartle KD, Robson MM, Myers P, Euerby MR. Analyst. 1998;123:87R–102R. [Google Scholar]
- 28.Zou H, Ye M. Electrophoresis. 2000;21:4073–4095. doi: 10.1002/1522-2683(200012)21:18<4073::AID-ELPS4073>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Albarghouthi MN, Stein TM, Barron AE. Electrophoresis. 2003;24:1166–1175. doi: 10.1002/elps.200390150. [DOI] [PubMed] [Google Scholar]
- 30.Trammell BC, Ma L, Luo H, Jin D, et al. Anal. Chem. 2002;74:4634–4639. doi: 10.1021/ac020206d. [DOI] [PubMed] [Google Scholar]
- 31.Dai J, Baker GL, Bruening ML. Anal. Chem. 2006;78:135–140. doi: 10.1021/ac0513966. [DOI] [PubMed] [Google Scholar]
- 32.Matyska MT, Pesek JJ, Katrekar A. Anal. Chem. 1999;71:5508–5514. [Google Scholar]
- 33.Li W, Fries DP, Malik A. J. Chromatogr. A. 2004;1044:23–52. doi: 10.1016/j.chroma.2004.04.079. [DOI] [PubMed] [Google Scholar]
- 34.Yan W, Gao R, Zhang Z, Wang Q, Jiang CV, Yan C. J. Sep. Sci. 2003;26:1–6. [Google Scholar]
- 35.Pesek JJ, Matyska MT, Cho S. J. Chromatogr. A. 1999;845:237–246. doi: 10.1016/s0021-9673(99)00154-5. [DOI] [PubMed] [Google Scholar]
- 36.Constantin S, Freitag R. J. Sep. Sci. 2002;25:1245–1251. [Google Scholar]
- 37.Kapnissi CP, Akbay C, Schlenoff JB, Warner IM. Anal. Chem. 2002;74:2328–2335. doi: 10.1021/ac015733w. [DOI] [PubMed] [Google Scholar]
- 38.Rmaile HH, Schlenoff JB. J. Am. Chem. Soc. 2003;125:6602–6603. doi: 10.1021/ja035251x. [DOI] [PubMed] [Google Scholar]
- 39.Kamande MW, Zhu X, Kapnissi-Christodoulou C, Warner IM. Anal. Chem. 2004;76:6681–6692. doi: 10.1021/ac049313t. [DOI] [PubMed] [Google Scholar]
- 40.Kapnissi CP, Valle BC, Warner IM. Anal. Chem. 2003;75:6097–6104. doi: 10.1021/ac034452g. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Warner IM. Anal. Chem. 1994;66:3773–3776. [Google Scholar]