Abstract
With current effective antiretroviral treatment, the spectrum of morbidity and mortality during chronic HIV disease has shifted away from AIDS defining clinical events. Persistent abnormalities in coagulation appear to contribute to excess risk for a broad spectrum of non-AIDS defining complications, including, but not limited to, venous and arterial thrombotic disease. Mechanisms specific to HIV disease, antiretroviral therapy, and lifestyle or behavioral factors contribute to a pro-coagulant state, in part, through increased tissue factor activity coupled with a paradoxical decline in the anti-coagulant response. Alterations in coagulation biology in the context of HIV disease appear to be largely a consequence of persistent systemic immune activation, micro- and macro-vascular disease, and, potentially, impaired hepatic synthesis of coagulation factors. The clinical consequences of HIV-related changes in coagulation biology, the degree to which they are unique to HIV disease, and whether they can be mitigated through adjunct treatments, remains a focus of current research.
Coagulation and Disease Risk in the Era of Effective Antiretroviral Treatment
Current antiretroviral therapy (ART) treatment now effectively and durably suppresses HIV replication. The well-described immune recovery and improved survival associated with effective ART has also motivate recent research that expands our understanding of the hematologic complications of HIV disease. The classic hematologic complications described early in the HIV/AIDS treatment era largely constituted thrombocytopenia, anemia, and neutropenia attributable, in part, to increased consumption, concurrent infections, bone marrow suppression and/or ART drug toxicity (e.g., zidovudine) [1]. More widespread use of less toxic ART has reduced cytopenias among an HIV population that is now largely treated with corresponding viral suppression where access to antiretroviral therapy is present [2, 3].
The spectrum of HIV-related clinical disease risk now more commonly includes non-AIDS defining disease such as atherosclerotic cardiovascular disease (CVD) and cancer, and rates for these diseases are typically higher than for uninfected populations [4-6]. A series of recent epidemiologic studies have demonstrated that inflammatory and coagulation biomarkers are both elevated with HIV infection and strongly predict risk for a broad spectrum of non-AIDS defining end-organ diseases (e.g., CVD, cancer, and liver and renal disease) [7-14]. Plasma levels of the coagulation biomarker D-dimer are approximately 50% elevated, and the increase clinical risk associated with elevated levels are more extreme, among treated HIV positive patients with viral suppression when compared with uninfected controls [7, 8, 13, 15]. Furthermore, the increased risk for subsequent CVD and all-cause mortality associated with elevated D-dimer levels is robust, is present over 5-8 years of follow-up, and is not present for AIDS-defining events [16, 17].
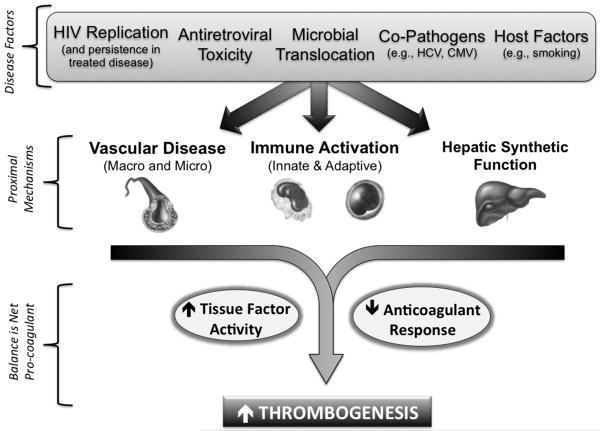
Although both venous and arterial thrombotic diseases as well as cancer appear to be increased in treated HIV disease, these complications still occur only at modest rates and may take years to manifest clinically [5, 6, 11, 17-20]. The focus of this review is then to explore the pro-coagulant mechanisms during chronically treated HIV disease (Figure 1), given the potential implications for excess long-term non-AIDS defining morbidity and mortality.
Figure 1. Pro-Coagulant Mechanisms During Chronic HIV Disease.
Presented is a conceptual model whereby factors specific to HIV itself, antiretroviral therapy, and lifestyle contribute to a pro-coagulant state. In addition, permanent damage to the immune system during chronic HIV disease despite effective ART treatment also contributes, including: a) decreased integrity of the mucosal lymphatic barrier, with corresponding endotoxemia, and b) a loss of T-cell regulatory function combined with excess antigen stimulus from co-pathogens. This contributes to a state of persistent immune activation (characterized by both T-cell and monocyte abnormalities), injury to endothelial surfaces with micro-vascular dysfunction, premature atherosclerosis, and potentially, impaired hepatic synthesis of coagulation factors. The end-result may increased thrombogenesis due to both an up-regulation of tissue factor activity, as well as alterations in extrinsic pathway factor levels with notable declines in the anticoagulant response (e.g., antithrombin and protein C).
Persistent Systemic Inflammation
Inflammation, in general terms, modulates thrombotic responses by up-regulating procoagulants, down-regulating anticoagulants and suppressing fibrinolysis [21]. Viral replication may non-specifically activate coagulation via cell injury and apoptosis leading to tissue factor activation, and, potentially, through altered hepatic production of coagulation factors [21-26]. Tissue damage, with associated release of cytokines and cellular products (e.g., histones) can also activate endothelial surfaces to increase TF expression, reduce endogenous anti-coagulant signals, and promote leukocyte infiltration [21, 27-30]. Conversely, thrombin itself can feedback and stimulate innate immune responses via protease-activated receptor 1 (PAR-1) signaling, leading to inflammatory cytokine production and enhanced interferon mediated anti-viral responses [31, 32]. Furthermore, thrombin has been shown to enhance the adaptive immune response by activating memory CD8+ T-cell subsets from HIV patients (also via PAR-1 signaling), and facilitating their migration to sites of tissue injury [33]. These data highlight the intrinsic interconnectedness of coagulation biology with the immunopathogenesis of inflammatory conditions such as HIV disease.
Immune dysfunction, activation of lymphocytes and monocytes, and elevated levels of inflammatory cytokines are hallmarks of untreated HIV infection. Most of these immunologic abnormalities improve with effective ART, but remain impaired compared to HIV uninfected persons [8, 34, 35]. The precise mechanisms driving high level immune activation during treated HIV disease are not entirely clear, but appear to involve both a persistent anti-HIV response (even with HIV RNA at low levels) and a more generalized immune activation (e.g., cytokine release) [36]. Specifically, HIV disease factors that may contribute to excess inflammation include: a) excess burden of co-pathogens such as CMV and other herpes viruses; b) HIV-mediated destruction of mucosal epithelium in the gut, which may lead to chronic translocation of bacterial products even after ART-related immune recovery; c) loss of key regulatory cells such as Th17 and perhaps T regulatory cells d) insulin resistance and metabolic abnormalities; and e) residual HIV replication during apparently effective ART [37-44]. Importantly, the consequences of these factors for innate immunity (e.g., monocyte activation in blood) has received increasing attention due the potential contribution to CVD and other non-AIDS disease risk [11, 13, 14, 35, 45-47]. Ultimately, the implication is that in the current era treated HIV disease is characterized by a state of persistent systemic inflammation with corresponding activation of coagulation.
Activation of Tissue Factor Pathways
Blood thrombosis is primarily initiated and perpetuated by local activation of tissue factor (e.g., the extrinsic pathway). Circulating TF exists on cell surfaces (e.g., activated monocytes), as soluble cell-free TF in plasma (where it is largely inactive in coagulation), and on cell-derived microparticles or vesicles released from activated or apoptotic cells that contain functionally active proteins from the parent cell [48]. Though TF-positive microparticles constitute only a small fraction of circulating TF, they likely represent a functionally active form of TF given their origin from activated cells (e.g., released form monocytes in response to endotoxemia) [48, 49]. We have demonstrated that ART treatment is associated with reductions in microparticle TF pro-coagulant activity, when compared to untreated HIV infection [50]. Furthermore, among ART-treated patients with viral suppression, microparticle TF pro-coagulant activity is associated with D-dimer levels [50].
Funderburg and colleagues have conducted a series of sentinel studies characterizing TF expression on monocytes among HIV infected patients [26, 46]. They reported that monocyte TF expression is increased among HIV infected versus uninfected persons, and was correlated directly with HIV viral load [26]. In that study, monocyte TF expression also correlated with D-dimer levels and with soluble CD14 (sCD14), a monocyte inflammatory marker and co-receptor for bacterial lipopolysaccharide (LPS) [26]. HIV pathogenesis studies postulate that increased translocation of microbial products across intestinal surfaces, a consequence of permanent damage to the mucosal lymphatic tissue, may contribute to monocyte activation, tissue factor expression, and pathogenic hypercoagulation [40, 42, 51]. Funderburg and colleagues when on to demonstrate that among treated HIV positive patients with suppressed viral loads, LPS levels were associated with increases in activated monocyte phenotypes (CD14++/CD16+) that also expressed TF to a greater degree when compared to the classical monocyte phenotype (CD14++/CD16−) [46]. This pattern of monocyte activation and TF expression was not present for HIV uninfected controls but was apparent among patients with acute coronary syndrome [46]. In another retrospective case-control study, soluble TF levels were elevated among HIV-infected patients preceding a CVD event, versus controls who did not have a CVD event [12]. However, to date, studies have been unable to fully assess whether increases in functionally active TF account for the epidemiologic associations between D-dimer and clinical risk [8, 9, 12, 13].
Finally, injury to vascular surfaces is well known to stimulate a number of pro-thrombotic mechanisms, including expression of TF [29, 52]. Within the context of macro-vascular disease, TF is typically expressed on vascular smooth muscles cells and macrophages within atherosclerotic plaques [53-55]. Atherosclerotic vascular disease, including coronary heart disease events, occur more frequently with HIV infection due to reasons that are multifactorial and have been reviewed elsewhere [5, 56-58]. Although TF is not typically expressed on inner endothelial surfaces under normal conditions, injury and damage to the endothelium can lead to TF expression throughout the microvasculature [29, 52, 59]. HIV infection is also associated with micro-vascular dysfunction, and HIV studies have reported associations between D-dimer levels and impaired small vessel elasticity, endothelial dysfunction, and biomarkers of endothelial activation [60-63]. Ultimately, the degree to which HIV-mediated injury and dysfunction of endothelial surfaces up-regulates TF-mediated coagulation is unclear, and more likely involves multiple mechanisms that influence coagulation biology.
Altered Composition of Coagulation Factors
We recently studied the influence of HIV replication and ART treatment specifically on the composition of extrinsic (TF) pathway coagulation factors [64]. In addition to the expected increases in some pro-coagulants (e.g., factor VIII and von Willebrand factor) as a consequence of systemic inflammation, we also observed that untreated HIV replication led to declines in all major anticoagulants (e.g., antithrombin, protein C and protein S) as well as other pro-coagulants dependent on hepatocyte function (e.g., fibrinogen, prothrombin, and factor VII). Through computational modeling to estimate thrombin generation based on the composition of extrinsic pathway factors developed by Brummel-Ziedins and colleagues, we demonstrated the net effect of HIV replication was to increase coagulation potential. Initiation of HIV treatment with ART subsequently led to reciprocal changes, including reduced inflammation (e.g., factor VIII levels), improved hepatocyte production of anticoagulants (e.g., antithrombin, protein C and protein S), and declines in thrombin generation potential.
A recent review on the coagulopathy of liver disease highlights data that declining liver function initially results in a pro-coagulant state until later end-stage disease, where the almost complete lack of coagulation factors results in hemorrhage [65]. Data from HIV studies also support a hypothesized HIV-mediated reduction in hepatocyte synthetic function. Levels of C-reactive protein (CRP), an acute phase reactant released by hepatocytes, were modestly higher among HIV mono-infected individuals and actually lower among those with HIV/HCV co-infection, when compared to uninfected controls [66]. In addition, while IL-6 and D-dimer levels decline with initiation of ART, CRP levels fail to improve or actually increase in some studies [67, 68]. Finally, HIV-1 appears to have the capacity to replicate in hepatocytes [69-71], and the FIB-4 index, a non-invasive estimate of hepatic fibrosis, correlates with the degree of HIV replication[72, 73]. In summary, recent observations and experimental data suggest that HIV infection may have a pro-coagulant effect, in part, through its effects on hepatocyte synthesis of coagulation factors. A critical unanswered question is whether these HIV-related changes in the profile of coagulation factors has direct long-term implications for disease pathogenesis.
Platelet Activation
Platelets provide an additional link between HIV-mediated inflammation and hypercoagulation, as they are activated at sites of infection or injury and interact with monocytes, lymphocytes and endothelial cells [74-76]. HIV-1 binds, and is highly associated, with platelets in the blood, which has been postulated to facilitate clearance and/or dissemination of the virus [77, 78]. Among patients with untreated HIV infection, thrombocytopenia is a classic hematologic abnormality that worsens with advancing HIV disease but typically normalizes with ART treatment [79].
When compared to uninfected persons, platelets from HIV positive persons demonstrate greater activation, chemokine release, and reactivity to epinephrine [80, 81]. Even after effective ART treatment with viral suppression, levels of platelet microparticles remain elevated, and more frequently express CD62P and TF, when compared with uninfected controls [82, 83]. Finally, platelet-monocyte complexes are another measure of platelet activation that has been shown to be more frequent among HIV infected versus uninfected persons [84]. In contrast, a recent study showed that untreated HIV infection was associated with paradoxical reductions in functional measures of platelet aggregation and delayed clot initiation despite having elevated D-dimer levels [85].
Data on platelet toxicity related to specific antiretroviral medications is limited, though exposure to abacavir has been associated with platelet hyperactivity in 2 studies providing a potential biologic explanation for the increased risk for myocardial infarction reported with this medication [86-88]. To some degree, methodological challenges in assessing platelet activation and function have limited data that characterize the effects from HIV infection, and the clinical implications, in the context of untreated and treated disease.
Treatment Strategies to Reduce Hypercoagulation in Treated HIV Disease
The need for adjunct disease modifying treatment strategies (given concurrently with ART) to target excess inflammation and coagulation activation is an area of active research that has been recently reviewed [89, 90]. A major challenge to conducting initial trials evaluating the potential benefits of candidate treatments is that intermediate measures of inflammation and/or coagulation have not been validated as surrogate markers in this population. Most preliminary studies to date have leveraged off-target properties of established cardioprotective medications such as aspirin, statins, and angiotensin receptor blockers [91-93]. In addition to the well-established anti-platelet benefits, aspirin has broad anti-inflammatory properties and has demonstrated potential to reduce T-cell activation, monocyte activation, and platelet activation in a pilot study of treated HIV patients [92]. The effects of rosuvastatin were recently studied in a randomized trial of 147 ART-treated HIV positive patients with evidence of increased immune activation at baseline [93]. In this study, rosuvastatin reduced TF expression on activated monocyte phenotypes and plasma markers of monocyte activation, but did not reduce D-dimer levels. The implications of these treatment effects for clinical risk and other aspects of coagulation biology are not yet clear.
No large-scale clinical trials are underway specifically studying an anticoagulant medication as primary prevention for ART-treated HIV positive patients. The new generation of oral anticoagulant medications (e.g., direct inhibitors to thrombin and factor Xa) would represent a highly novel approach to target hypercoagulation during chronic HIV disease, but would face significant challenges. Bleeding risks are predictable and become a major deterrent when considering a treatment strategy of primary prevention over decades for a population where clinical event rates remain modest (even if in excess of the general population). Furthermore, significant drug-drug interactions with antiretrovirals exist through hepatic metabolism via CPY-3A4 pathway (e.g., the factor Xa inhibitors such as rivaroxaban) as well as via inhibition of P-glycoprotein transportation.
Conclusion
Recent data have demonstrated that the coagulation system may be persistently activated during chronic HIV infection despite effective ART treatment. Alterations in coagulation biology associated with HIV specific factors (whether due to antiretroviral toxicity, the virus itself, or permanent damage to the immune system) are similar to that described in other states of chronic inflammation, and are characterized by a concurrent up-regulation of tissue factor pathways and reduction in the anti-coagulant response. While the increase in pro-coagulant potential may be modest, epidemiologic associations with plasma D-dimer levels suggest increased coagulation may contributed to excess risk across a broad spectrum of non-AIDS defining clinical diseases that manifests over years [7, 9, 12, 13, 17, 20, 64]. However, elevated D-dimer levels during chronic HIV infection may also reflect the convergence of multiple pathways and overall disease burden. For example, the profile of HIV-related changes to inflammatory and coagulation factors are similar to changes seen with aging and frailty in the general population (e.g., IL-6, D-dimer, von Willebrand factor, factor VIII, and factor VII) [7, 8, 64, 94]. Future research should focus on understanding the similarities and differences in altered coagulation biology during treated HIV disease compared to other disease states, as well as the clinical consequences and potential prevention strategies.
Acknowledgments
Drs. Russell Tracy, Nigel Key and Kathleen Brummel-Ziedins for collaboration and truly stimulating dialog that has shaped this discussion.
Footnotes
Disclosures: none
References
- 1.Sloand E. Hematologic complications of HIV infection. AIDS reviews. 2005;7(4):187–196. [PubMed] [Google Scholar]
- 2.Choi SY, Kim I, Kim NJ, Lee SA, Choi YA, Bae JY, Kwon JH, Choe PG, Park WB, Yoon SS, et al. Hematological manifestations of human immunodeficiency virus infection and the effect of highly active anti-retroviral therapy on cytopenia. The Korean journal of hematology. 2011;46(4):253–257. doi: 10.5045/kjh.2011.46.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dombrowski JC, Kitahata MM, Van Rompaey SE, Crane HM, Mugavero MJ, Eron JJ, Boswell SL, Rodriguez B, Mathews WC, Martin JN, et al. High Levels of Antiretroviral Use and Viral Suppression Among Persons in HIV Care in the United States, 2010. J Acquir Immune Defic Syndr. 2013;63(3):299–306. doi: 10.1097/QAI.0b013e3182945bc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A, Gatell J, Rakhmanova A, Johnson M, Kirk O, et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr. 2010;55(2):262–270. doi: 10.1097/QAI.0b013e3181e9be6b. [DOI] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013;173(8):614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 7.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuhaus J, Jacobs DR, Jr., Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tien PC, A.I. C, A.R. Z, C B, Scherzer R, Bacchetti P, Shlipak M, Grunfeld C. Inflammation and mortality in HIV-infected adults: analysis of the FRAM Study cohort; 17th Conference on Retroviruses and Opportunistic Infections (CROI); 2010; Abstract #725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51(3):268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, Natarajan V, Rehm C, Hadigan C, Sereti I. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. Aids. 2010;24(10):1509–1517. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, Bryant KJ, Goetz M, Tracy R, Oursler KA, Rimland D, et al. HIV Status, Burden of Comorbid Disease and Biomarkers of Inflammation, Altered Coagulation and Monocyte Activation. Clin Infect Dis. 2012 doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folsom AR, Delaney JA, Lutsey PL, Zakai NA, Jenny NS, Polak JF, Cushman M. Associations of factor VIIIc, D-dimer, and plasmin-antiplasmin with incident cardiovascular disease and all-cause mortality. Am J Hematol. 2009;84(6):349–353. doi: 10.1002/ajh.21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D, Skoutelis A, Goetz MB, Phillips AN, Group ISfMoATS Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. 2009;200(6):973–983. doi: 10.1086/605447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grund B, Baker J, Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, Cohen C, Phillips A, Lundgren J, Neaton J, et al. Combined effect of interleukin-6 and D-dimer on the risk of serious non-AIDS conditions: data from 3 prospective cohorts; 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA USA. 2013. [Google Scholar]
- 18.Fultz SL, McGinnis KA, Skanderson M, Ragni MV, Justice AC. Association of venous thromboembolism with human immunodeficiency virus and mortality in veterans. Am J Med. 2004;116(6):420–423. doi: 10.1016/j.amjmed.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Klein SK, Slim EJ, de Kruif MD, Keller TT, ten Cate H, van Gorp EC, Brandjes DP. Is chronic HIV infection associated with venous thrombotic disease? A systematic review. The Netherlands journal of medicine. 2005;63(4):129–136. [PubMed] [Google Scholar]
- 20.Crum-Cianflone NF, Weekes J, Bavaro M. Review: thromboses among HIV-infected patients during the highly active antiretroviral therapy era. AIDS Patient Care STDS. 2008;22(10):771–778. doi: 10.1089/apc.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie. 2010;30(1):5–6. 8–9. [PubMed] [Google Scholar]
- 22.Key NS, Vercellotti GM, Winkelmann JC, Moldow CF, Goodman JL, Esmon NL, Esmon CT, Jacob HS. Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc Natl Acad Sci U S A. 1990;87(18):7095–7099. doi: 10.1073/pnas.87.18.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24(6):1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 24.Antoniak S, Boltzen U, Riad A, Kallwellis-Opara A, Rohde M, Dorner A, Tschope C, Noutsias M, Pauschinger M, Schultheiss HP, et al. Viral myocarditis and coagulopathy: increased tissue factor expression and plasma thrombogenicity. Journal of molecular and cellular cardiology. 2008;45(1):118–126. doi: 10.1016/j.yjmcc.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Sales PC, Williams BR, Silva AM. Regulation of double-stranded RNA dependent protein kinase expression and attenuation of protein synthesis induced by bacterial toll-like receptors agonists in the absence of interferon. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2012;32(10):495–504. doi: 10.1089/jir.2012.0019. [DOI] [PubMed] [Google Scholar]
- 26.Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, Luciano AA, Stevens W, Rodriguez B, Brenchley JM, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115(2):161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blann AD. Endothelial cell activation, injury, damage and dysfunction: separate entities or mutual terms? Blood Coagul Fibrinolysis. 2000;11(7):623–630. doi: 10.1097/00001721-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Jaffe EA. Cell biology of endothelial cells. Human pathology. 1987;18(3):234–239. doi: 10.1016/s0046-8177(87)80005-9. [DOI] [PubMed] [Google Scholar]
- 29.Prydz H, Pettersen KS. Synthesis of thromboplastin (tissue factor) by endothelial cells. Haemostasis. 1988;18(4-6):215–223. doi: 10.1159/000215809. [DOI] [PubMed] [Google Scholar]
- 30.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 31.Antoniak S, Owens AP, 3rd, Baunacke M, Williams JC, Lee RD, Weithauser A, Sheridan PA, Malz R, Luyendyk JP, Esserman DA, et al. PAR-1 contributes to the innate immune response during viral infection. J Clin Invest. 2013;123(3):1310–1322. doi: 10.1172/JCI66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levi M, van der Poll T, ten Cate H, van Deventer SJ. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest. 1997;27(1):3–9. doi: 10.1046/j.1365-2362.1997.570614.x. [DOI] [PubMed] [Google Scholar]
- 33.Hurley A, Smith M, Karpova T, Hasley RB, Belkina N, Shaw S, Balenga N, Druey KM, Nickel E, Packard B, et al. Enhanced effector function of CD8(+) T cells from healthy controls and HIV-infected patients occurs through thrombin activation of protease-activated receptor 1. J Infect Dis. 2013;207(4):638–650. doi: 10.1093/infdis/jis730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, Hsue P, Emu B, Krone M, Lampiris H, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, Landay AL, Jaworowski A, Crowe SM. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. Aids. 2012;26(7):843–853. doi: 10.1097/QAD.0b013e328351f756. [DOI] [PubMed] [Google Scholar]
- 36.Deeks SG. HIV Infection, Inflammation, Immunosenescence, and Aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatano H, Jain V, Hunt PW, Lee TH, Sinclair E, Do TD, Hoh R, Martin JN, McCune JM, Hecht F, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2013;208(1):50–56. doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 39.Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F, Hsue P, McCune JM, Deeks SG. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5(1):e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estes J, Baker JV, Brenchley JM, Khoruts A, Barthold JL, Bantle A, Reilly CS, Beilman GJ, George ME, Douek DC, et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis. 2008;198(4):456–464. doi: 10.1086/590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 43.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, Dong T, Chesney G, Waters A, Easterbrook P, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2(2):E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, Tracy R, Corey L, Deeks SG. Valganciclovir reduces T cell activation in treated HIV infection. JID. 2011 doi: 10.1093/infdis/jir060. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, Simon DI, Costa MA, Rodriguez B, Sieg SF, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120(23):4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, Cheng WJ, Paukovics G, Palmer CS, Novak RM, et al. Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One. 2013;8(1):e55279. doi: 10.1371/journal.pone.0055279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Key NS, Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Semin Thromb Hemost. 2010;36(8):865–875. doi: 10.1055/s-0030-1267040. [DOI] [PubMed] [Google Scholar]
- 49.Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege V, Hedman H, Freyssinet JM. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. Journal of immunology. 1994;153(7):3245–3255. [PubMed] [Google Scholar]
- 50.Baker JV, Huppler Hullsiek K, Bradford RL, Prosser R, Tracy RP, Key NS. Circulating Levels of Tissue Factor Microparticle Procoagulant Activity Are Reduced With Antiretroviral Therapy and Are Associated With Persistent Inflammation and Coagulation Activation Among HIV-Positive Patients. J Acquir Immune Defic Syndr. 2013;63(3):367–371. doi: 10.1097/QAI.0b013e3182910121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandrea I, Cornell E, Wilson C, Ribeiro RM, Ma D, Kristoff J, Xu C, Haret-Richter GS, Trichel A, Apetrei C, et al. Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood. 2012;120(7):1357–1366. doi: 10.1182/blood-2012-03-414706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sagripanti A, Carpi A. Antithrombotic and prothrombotic activities of the vascular endothelium. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2000;54(2):107–111. doi: 10.1016/S0753-3322(00)88861-7. [DOI] [PubMed] [Google Scholar]
- 53.Annex BH, Denning SM, Channon KM, Sketch MH, Jr., Stack RS, Morrissey JH, Peters KG. Differential expression of tissue factor protein in directional atherectomy specimens from patients with stable and unstable coronary syndromes. Circulation. 1995;91(3):619–622. doi: 10.1161/01.cir.91.3.619. [DOI] [PubMed] [Google Scholar]
- 54.Marmur JD, Thiruvikraman SV, Fyfe BS, Guha A, Sharma SK, Ambrose JA, Fallon JT, Nemerson Y, Taubman MB. Identification of active tissue factor in human coronary atheroma. Circulation. 1996;94(6):1226–1232. doi: 10.1161/01.cir.94.6.1226. [DOI] [PubMed] [Google Scholar]
- 55.Moreno PR, Bernardi VH, Lopez-Cuellar J, Murcia AM, Palacios IF, Gold HK, Mehran R, Sharma SK, Nemerson Y, Fuster V, et al. Macrophages, smooth muscle cells, and tissue factor in unstable angina. Implications for cell-mediated thrombogenicity in acute coronary syndromes. Circulation. 1996;94(12):3090–3097. doi: 10.1161/01.cir.94.12.3090. [DOI] [PubMed] [Google Scholar]
- 56.Triant VA. Cardiovascular Disease and HIV Infection. Curr HIV/AIDS Rep. 2013 doi: 10.1007/s11904-013-0168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker JV, Lundgren J. Cardiovascular implications of untreated HIV infection. European Heart Journal. 2011 doi: 10.1093/eurheartj/ehq483. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Minno G, Coppola A, Mancini FP, Margaglione M. Homocysteine, platelet function and thrombosis. Haematologica. 1999;(84 Suppl EHA-4):61–63. [PubMed] [Google Scholar]
- 60.Hileman CO, Longenecker CT, Carman TL, Milne GL, Labbato DE, Storer NJ, White CA, McComsey GA. Elevated D-dimer is independently associated with endothelial dysfunction: a cross-sectional study in HIV-infected adults on antiretroviral therapy. Antivir Ther. 2012;17(7):1345–1349. doi: 10.3851/IMP2297. [DOI] [PubMed] [Google Scholar]
- 61.Baker J, Quick H, Hullsiek KH, Tracy R, Duprez D, Henry K, Neaton JD. Interleukin-6 and d-dimer levels are associated with vascular dysfunction in patients with untreated HIV infection. HIV Med. 2010;11(9):608–609. doi: 10.1111/j.1468-1293.2010.00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solages A, Vita JA, Thornton DJ, Murray J, Heeren T, Craven DE, Horsburgh CR., Jr. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006;42(9):1325–1332. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baker JV, Duprez D, Rapkin J, Hullsiek KH, Quick H, Grimm R, Neaton JD, Henry K. Untreated HIV infection and large and small artery elasticity. J Acquir Immune Defic Syndr. 2009;52(1):25–31. doi: 10.1097/qai.0b013e3181b02e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baker JV, Brummel-Ziedins K, Neuhaus J, Duprez D, Cummins N, Dalmau D, DeHovitz J, Lehmann C, Sullivan A, Woolley I, et al. HIV Replication Alters the Composition of Extrinsic Pathway Coagulation Factors and Increases Thrombin Generation. JAHA. 2013 doi: 10.1161/JAHA.113.000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. The New England journal of medicine. 2011;365(2):147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 66.Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, Tien P, Bacchetti P, Scherzer R, Grunfeld C, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. Journal of acquired immune deficiency syndromes. 2008;48(2):142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palella FJ, Jr., Gange SJ, Benning L, Jacobson L, Kaplan RC, Landay AL, Tracy RP, Elion R. Inflammatory biomarkers and abacavir use in the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study. AIDS. 2010;24(11):1657–1665. doi: 10.1097/QAD.0b013e3283389dfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Melbourne K, Ha B, Brown TT, Bloom A, Fedarko N, et al. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. AIDS. 2012;26(11):1371–1385. doi: 10.1097/QAD.0b013e328354f4fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, Ho DD. Identification and quantitation of HIV-1 in the liver of patients with AIDS. Aids. 1992;6(1):65–70. doi: 10.1097/00002030-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Vlahakis SR, Villasis-Keever A, Gomez TS, Bren GD, Paya CV. Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4. J Infect Dis. 2003;188(10):1455–1460. doi: 10.1086/379738. [DOI] [PubMed] [Google Scholar]
- 71.Xiao P, Usami O, Suzuki Y, Ling H, Shimizu N, Hoshino H, Zhuang M, Ashino Y, Gu H, Hattori T. Characterization of a CD4-independent clinical HIV-1 that can efficiently infect human hepatocytes through chemokine (C-X-C motif) receptor 4. Aids. 2008;22(14):1749–1757. doi: 10.1097/QAD.0b013e328308937c. [DOI] [PubMed] [Google Scholar]
- 72.Blackard JT, Welge JA, Taylor LE, Mayer KH, Klein RS, Celentano DD, Jamieson DJ, Gardner L, Sherman KE. HIV mono-infection is associated with FIB-4 - A noninvasive index of liver fibrosis - in women. Clin Infect Dis. 2011;52(5):674–680. doi: 10.1093/cid/ciq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mendeni M, Foca E, Gotti D, Ladisa N, Angarano G, Albini L, Castelnuovo F, Carosi G, Quiros-Roldan E, Torti C. Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without hepatitis C and B infection. Clin Infect Dis. 2011;52(9):1164–1173. doi: 10.1093/cid/cir071. [DOI] [PubMed] [Google Scholar]
- 74.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8(11):1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 75.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost. 2003;1(9):1897–1905. doi: 10.1046/j.1538-7836.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 76.Torre D, Pugliese A. Platelets and HIV-1 infection: old and new aspects. Curr HIV Res. 2008;6(5):411–418. doi: 10.2174/157016208785861140. [DOI] [PubMed] [Google Scholar]
- 77.Pugliese A, Savarino A, Cantamessa C, Torre D. Influence of fibronectin on HIV-1 infection and capability of binding to platelets. Cell Biochem Funct. 1996;14(4):291–296. doi: 10.1002/cbf.693. [DOI] [PubMed] [Google Scholar]
- 78.Lee TH, Stromberg RR, Heitman JW, Sawyer L, Hanson CV, Busch MP. Distribution of HIV type 1 (HIV-1) in blood components: detection and significance of high levels of HIV-1 associated with platelets. Transfusion. 1998;38(6):580–588. doi: 10.1046/j.1537-2995.1998.38698326338.x. [DOI] [PubMed] [Google Scholar]
- 79.Servais J, Nkoghe D, Schmit JC, Arendt V, Robert I, Staub T, Moutschen M, Schneider F, Hemmer R. HIV-associated hematologic disorders are correlated with plasma viral load and improve under highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28(3):221–225. doi: 10.1097/00042560-200111010-00003. [DOI] [PubMed] [Google Scholar]
- 80.Satchell CS, Cotter AG, O’Connor EF, Peace AJ, Tedesco AF, Clare A, Lambert JS, Sheehan GJ, Kenny D, Mallon PW. Platelet function and HIV: a case-control study. Aids. 2010;24(5):649–657. doi: 10.1097/QAD.0b013e328336098c. [DOI] [PubMed] [Google Scholar]
- 81.Holme PA, Muller F, Solum NO, Brosstad F, Froland SS, Aukrust P. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J. 1998;12(1):79–89. doi: 10.1096/fasebj.12.1.79. [DOI] [PubMed] [Google Scholar]
- 82.Corrales-Medina VF, Simkins J, Chirinos JA, Serpa JA, Horstman LL, Jy W, Ahn YS. Increased levels of platelet microparticles in HIV-infected patients with good response to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;54(2):217–218. doi: 10.1097/QAI.0b013e3181c8f4c9. [DOI] [PubMed] [Google Scholar]
- 83.Mayne E, Funderburg NT, Sieg SF, Asaad R, Kalinowska M, Rodriguez B, Schmaier AH, Stevens W, Lederman MM. Increased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expression. J Acquir Immune Defic Syndr. 2012;59(4):340–346. doi: 10.1097/QAI.0b013e3182439355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh MV, Davidson DC, Kiebala M, Maggirwar SB. Detection of circulating platelet-monocyte complexes in persons infected with human immunodeficiency virus type-1. Journal of virological methods. 2012;181(2):170–176. doi: 10.1016/j.jviromet.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haugaard AK, Lund TT, Birch C, Ronsholt F, Troseid M, Ullum H, Gerstoft J, Johansson PI, Nielsen SD, Ostrowski SR. Discrepant coagulation profile in HIV-infection: elevated D-dimer but impaired platelet aggregation and clot initiation. Aids. 2013 doi: 10.1097/01.aids.0000432462.21723.ed. [DOI] [PubMed] [Google Scholar]
- 86.Baum PD, Sullam PM, Stoddart CA, McCune JM. Abacavir increases platelet reactivity via competitive inhibition of soluble guanylyl cyclase. Aids. 2011;25(18):2243–2248. doi: 10.1097/QAD.0b013e32834d3cc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Satchell CS, O’Halloran JA, Cotter AG, Peace AJ, O’Connor EF, Tedesco AF, Feeney ER, Lambert JS, Sheehan GJ, Kenny D, et al. Increased platelet reactivity in HIV-1-infected patients receiving abacavir-containing antiretroviral therapy. J Infect Dis. 2011;204(8):1202–1210. doi: 10.1093/infdis/jir509. [DOI] [PubMed] [Google Scholar]
- 88.Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Dabis F, De Wit S, Law M, D’Arminio Monforte A, Friis-Moller N, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371(9622):1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Butler SL, Valdez H, Westby M, Perros M, June CH, Jacobson JM, Levy Y, Cooper DA, Douek D, Lederman MM, et al. Disease-modifying therapeutic concepts for HIV in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2011;58(3):297–303. doi: 10.1097/QAI.0b013e31822ccfcc. [DOI] [PubMed] [Google Scholar]
- 90.Rajasuriar R, Khoury G, Kamarulzaman A, French MA, Cameron PU, Lewin SR. Persistent immune activation in chronic HIV infection: do any interventions work? Aids. 2013 doi: 10.1097/QAD.0b013e32835ecb8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baker JV, Huppler Hullsiek K, Prosser R, Duprez D, Grimm R, Tracy RP, Rhame F, Henry K, Neaton JD. Angiotensin converting enzyme inhibitor and HMG-CoA reductase inhibitor as adjunct treatment for persons with HIV infection: a feasibility randomized trial. PLoS One. 2012;7(10):e46894. doi: 10.1371/journal.pone.0046894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Brien M, Montenont E, Hu L, Nardi MA, Valdes V, Merolla M, Gettenberg G, Cavanagh K, Aberg JA, Bhardwaj N, et al. Aspirin Attenuates Platelet Activation and Immune Activation in HIV-1-Infected Subjects on Antiretroviral Therapy: A Pilot Study. J Acquir Immune Defic Syndr. 2013;63(3):280–288. doi: 10.1097/QAI.0b013e31828a292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McComsey G, Jiang Y, Debanne S, Clagett B, Robinson J, Labbato D, Storer N, Lederman M, Funderburg NT. Effect of statins on immune activation and inflammation in HIV+ subjects on ART: a randomized placebo controlled trial; 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA USA. 2013. [Google Scholar]
- 94.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]