Abstract
The endothelium is the orchestral conductor of blood vessel function. Pathological blood vessel formation (a process termed pathological angiogenesis) or the inability of endothelial cells (ECs) to perform their physiological function (a condition known as EC dysfunction) are defining features of various diseases. Therapeutic intervention to inhibit aberrant angiogenesis or ameliorate EC dysfunction could be beneficial in diseases such as cancer and cardiovascular disease, respectively, but current strategies have limited efficacy. Based on recent findings that pathological angiogenesis and EC dysfunction are accompanied by EC-specific metabolic alterations, targeting EC metabolism is emerging as a novel therapeutic strategy. Here, we review recent progress in our understanding of how EC metabolism is altered in disease and discuss potential metabolic targets and strategies to reverse EC dysfunction and inhibit pathological angiogenesis.
Keywords: angiogenesis, endothelial cell dysfunction, metabolism
Introduction
Blood vessels perform many functions that are critical for tissue homeostasis (Carmeliet, 2003). The endothelium, a single layer of endothelial cells (ECs) that lines the blood vessel lumen, controls vessel function. EC functions include the regulation of vascular tone and barrier, leukocyte trafficking, blood coagulation, nutrient and electrolyte uptake and neovascularization of hypoxic tissue, to name only a few (Cines et al, 1998; Pober et al, 2009; Potente et al, 2011). Many diseases are characterized by pathological blood vessel responses or formation. The inability of ECs to perform their physiological function (a condition termed EC dysfunction) contributes to cardiovascular disease and diabetes (Davignon & Ganz, 2004), whereas diseases such as cancer and age-related macula degeneration are characterized by new blood vessel formation (a process termed angiogenesis) (Carmeliet & Jain, 2011). Targeting ECs to prevent dysfunction or inhibit is potentially beneficial for a wide variety of diseases, but current treatment modalities, focusing primarily on growth factors, receptors, signaling molecules and others have limited efficacy or specificity (Bergers & Hanahan, 2008; Versari et al, 2009; Lee et al, 2012).
An emerging but understudied therapeutic target is EC metabolism. It has been long known that risk factors for cardiovascular disease (hypercholesterolemia, hypertension, dyslipidemia, diabetes, obesity and aging) cause EC-specific metabolic perturbations leading to EC dysfunction (Davignon & Ganz, 2004; Pober et al, 2009). Similarly, the links between EC metabolism and angiogenesis are apparent as angiogenic ECs migrate and proliferate in metabolically challenging environments such as hypoxic and nutrient-deprived tissue (Harjes et al, 2012). Moreover, the growth factor-induced switch from a quiescent to an angiogenic phenotype is mediated by important adaptations in EC energy metabolism (De Bock et al, 2013a,b; Schoors et al, 2014a,b). EC metabolic alterations are therefore not just innocent bystanders but mediate pathogenesis. In this review, we summarize existing data on the role of EC metabolism in mediating vascular disease and discuss how metabolism may be targeted for therapeutic benefit.
General endothelial metabolism
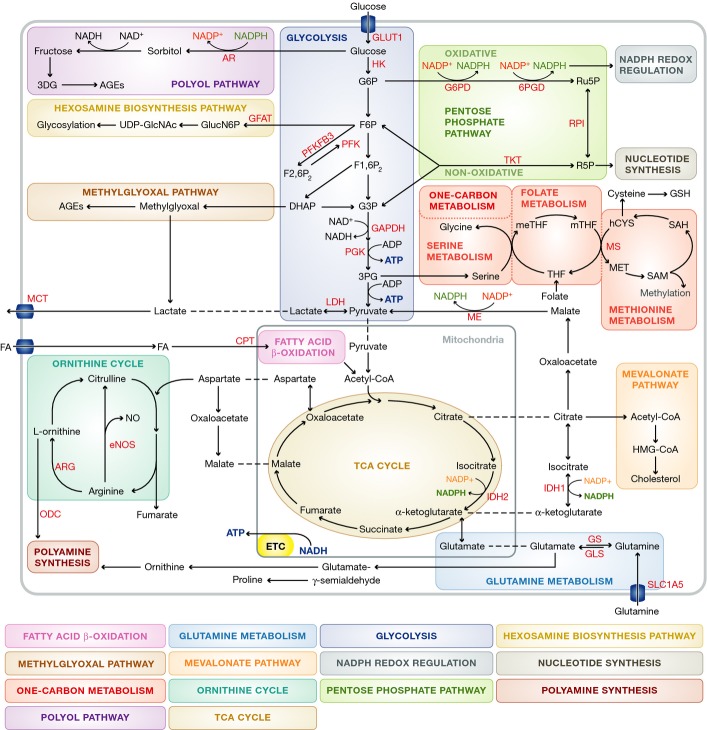
Despite their close proximity to oxygenated blood, ECs rely on glycolysis instead of oxidative metabolism for adenosine triphosphate (ATP) production (Parra-Bonilla et al, 2010; De Bock et al, 2013b). In fact, under physiological conditions, over 80% of ATP is produced by converting glucose into lactate (Fig 1). Less than 1% of glucose-derived pyruvate enters the mitochondria for oxidative metabolism through the tricarboxylic acid cycle (TCA) and subsequent ATP production via the electron transport chain (ETC) (Fig 1) (Culic et al, 1997; De Bock et al, 2013b). However, ECs retain the ability to switch to oxidative metabolism of glucose, amino acids and fatty acids in case of reduced glycolytic rates (Krutzfeldt et al, 1990; Dranka et al, 2010).
Figure 1. Overview of general EC metabolism.
For clarity, not all metabolites and enzymes of the depicted pathways are shown. Abbreviations: 3DG: 3-deoxyglucosone; 3PG: 3-phosphoglycerate; 6PGD: 6-phosphogluconate dehydrogenase; AGE: advanced glycation end-product; AR: aldose reductase; ARG: arginase; ATP: adenosine triphosphate; CPT: carnitine palmitoyltransferase; DHAP: dihydroxyacetone phosphate; eNOS: endothelial nitric oxide synthase; ETC: electron transport chain; F6P: fructose 6-phosphate; F1,6P2: fructose 1,6-bisphosphate; F2,6P2: fructose 2,6 bisphosphate; FA: fatty acid; G6P: glucose 6-phosphate; G6PD: glucose 6-phosphate dehydrogenase; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; GFAT: glutamine-6-phosphate amidotransferase; GlucN6P: glucosamine-6-phosphate; GLS: glutaminase; GLUT: glucose transporter; GS: glutamine synthetase; GSH: glutathione: hCYS: homocysteine; HMG-CoA: hydroxymethylglutaryl coenzyme A; IDH; isocitrate dehydrogenase; LDH: lactate dehydrogenase; MCT: monocarboxylate transporter; ME: malic enzyme; MET: methionine; meTHF: 5.10-methylene-tetrahydrofolate; mTHF: 5-methyltetrahydrofolate; MS: methionine synthetase; NAD: nicotinamide adenine dinucleotide; NADPH: nicotinamide adenine dinucleotide phosphate; NO: nitric oxide; ODC: ornithine decarboxylase; PFK1: phosphofructokinase-1 PFKFB3: 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3; PGK: phosphoglycerate kinase; ROS: reactive oxygen species; RPI: ribose-5-phosphate isomerase; SAH: S-adenosylhomocysteine: SAM: S-adenosylmethionine; TCA cycle: tricarboxylic acid cycle; THF: tetrahydrofolate; TKT: transketolase; UDP-GlcNAc: uridine diphosphate N-acetylglucosamine.
Glossary
1C metabolism
A complex metabolic network characterized by the transfer of carbon from serine/glycine for folate compound chemical reactions and involved in nucleotide, lipid and protein biosynthesis, redox homeostasis and production of methylation substrates.
Advanced glycation end products (AGEs)
Proteins or lipids that have been non-enzymatically glycated, often as a result of hyperglycemia and/or oxidative stress, that cause damaging intracellular and extracellular dysfunction.
Angiogenesis
Growth of new blood vessels from existing microvasculature.
Endothelium
Continuous inner lining of all vasculature composed of endothelial cells (ECs), which regulates physiological vascular function and angiogenesis.
EC dysfunction
Inability of endothelial cells to fulfill their physiological role as mediators of the blood barrier and vasotone.
Fatty acid oxidation
Metabolism of fatty acids in mitochondria into acetyl-CoA to fuel the TCA cycle.
Glycolysis
Anaerobic metabolism of glucose producing ATP and pyruvate
Glycosylation
A post-translational modification that enzymatically adds glycans, or oligosaccharides, to proteins and lipids.
Hexosamine biosynthesis pathway
Side pathway from glycolytic intermediate fructose 6-phosphate (F6P) that produces substrates for glycosylation.
Isoprenoid
Mevalonate pathway intermediates used for the production of cholesterol and as substrates for prenylation.
Metabolic flux
Flow of metabolites through a given metabolic pathway.
Metabolic flux analysis
Quantification of metabolic flux by tracing the fate of Isotope-labeled substrates.
Metabolism
The spectrum of organic and chemical cellular reactions dedicated to the production of energy and building blocks for general maintenance and functionality.
Methylglyoxal pathway
Glycolytic side pathway from dihydroxyacetone phosphate (DHAP) that results in production of methylglyoxal and/or AGEs.
Oxidative metabolism
Aerobic metabolic pathways that break down substrates through oxidation for energy production and biosynthesis.
Pentose phosphate pathway
Metabolic pathway important for redox homeostasis and biosynthesis which utilizes glucose-derived glucose-6-phosphate (G6P) for production of NADPH through its oxidative branch, and fructose 6-phosphate (F6P) and 3-phosphoglycerate (3PG) for nucleotide production in its non-oxidative branch.
Polyol pathway
Pathway implicated in diabetic endothelial dysfunction by reduction of glucose into sorbitol and then fructose to fuel production of AGEs.
Prenylation
Post-translational addition of isoprenoids such as farnesyl or geranyl–geranyl to a protein.
Quiescence
Cell state defined by a lack of activity.
Reactive nitrogen species
Highly reactive nitrogen-containing molecules that often interact with ROS, promote oxidative stress and reduce bioavailability of nitric oxide.
Reactive oxygen species (ROS)
Highly reactive molecules that contain oxygen (produced by aerobic metabolic processes) and are involved in normal cell homeostasis and signaling, but whose accumulation, termed oxidative stress, leads to cell damage.
Stalk cell
Endothelial cells that trail migratory tip cells and proliferate to extend growth of a new blood vessel during sprouting angiogenesis.
Tip cell
Migratory endothelial cells that lead spouting microvessels up a chemokine gradient during angiogenesis.
ECs lining peripheral tissue vessels or the blood brain barrier (BBB) express multiple members of the two major families of sugar transporters, that is, glucose transporters (GLUT) and sodium/glucose co-transporters (SGLTs), but the high-affinity GLUT1 is considered to be the main route of glucose uptake in ECs (Fig 1) (Mann et al, 2003; Gaudreault et al, 2004, 2008; Sahoo et al, 2014). Phosphorylation of intracellular glucose by hexokinase (HK) destines it for metabolic utilization, predominately by conversion to lactate via glycolysis (Fig 1) (Paik et al, 2005; De Bock et al, 2013b). Glycolytic intermediates also serve as precursors for biosynthetic pathways including the pentose phosphate pathway (PPP), hexosamine biosynthesis and glycogenesis (Fig 1, for an extensive review see (De Bock et al, 2013a,b)).
The PPP consists of oxidative and non-oxidative branches, and its overall flux is determined by the rate-limiting enzyme glucose-6-phosphate dehydrogenase (G6PD) (Fig 1). Partially regulated by VEGF signaling, G6PD destines glucose-6-phosphate (G6P) for utilization in the PPP (Pan et al, 2009). The oxidative branch of the PPP converts G6P into ribulose-5-phosphate (Ru5P) and produces NADPH from NADP+, thereby generating reducing power to maintain EC redox balance and biosynthetic reactions (Dobrina & Rossi, 1983; Jongkind et al, 1989; Spolarics & Spitzer, 1993; Spolarics & Wu, 1997; Vizan et al, 2009). The non-oxidative branch converts Ru5P into xylulose-5-phosphate (Xu5P) and ribose-5-phosphate (R5P), the latter is necessary for nucleotide biosynthesis (Pandolfi et al, 1995). However, PPP intermediates may also be converted back into glycolytic intermediates via the action of transketolase (TKT) and transaldolase. These reactions are reversible, allowing biosynthesis of macromolecules from glycolytic metabolites via the non-oxidative arm.
The hexosamine biosynthesis pathway starts with the conversion of the glycolytic intermediate fructose-6-phosphate (F6P) into glucosamine-6-phosphate (GlucN6P) (Fig 1). GlucN6P is then metabolized to uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), a key substrate for glycosylation reactions that control many aspects of EC function (Benedito et al, 2009; Laczy et al, 2009; Croci et al, 2014). The polyol pathway and methylglyoxal pathways are glycolysis side-pathways that are mostly known for their role in cardiovascular disease (Fig 1; see below) (Goldin et al, 2006).
Other metabolic pathways are less well characterized in ECs. Fatty acid (FA) oxidation (FAO) and glutamine oxidation have been implicated in replenishing the TCA cycle to produce ATP via oxidative phosphorylation (Fig 1) (Leighton et al, 1987; Hinshaw & Burger, 1990; Dagher et al, 1999, 2001; De Bock et al, 2013b). However, since ECs predominately rely on glucose metabolism to provide ATP, the energetic function of FAO and glutamine oxidation is not clear (De Bock et al, 2013b). FAs and amino acids can serve as precursors for biomass production, but such a role in ECs has not been demonstrated using isotope tracer labeling studies. FAO produces significant amounts of nicotinamide adenine dinucleotide phosphate (NADPH), which is an important co-factor in many biosynthetic reactions and essential to maintain redox balance. In addition, FAO generates acetyl-coA which is another important precursor for biomolecule production.
For example, acetyl-CoA is used, among other things, for the synthesis of cholesterol via the mevalonate pathway (Fig 1). Although endothelial cholesterol metabolism has been poorly studied, perturbations in cholesterol homeostasis are known to affect key EC functions such as intracellular signaling, inflammatory activation, nitric oxide synthesis and angiogenesis (Boger et al, 2000; Ivashchenko et al, 2010; Whetzel et al, 2010; Xu et al, 2010; Fang et al, 2013). ECs express all the cholesterol biosynthesis enzymes and the LDL receptor for extracellular uptake (Fig 1). These proteins are under transcriptional control of the sterol regulatory element binding protein (SREBP1 and -2) and liver X receptors (LXR) (Noghero et al, 2012). SREBP1 and LXRs inhibit cholesterol synthesis and absorption, whereas SREBP2 induces synthesis and inhibits cholesterol efflux via transcriptional repression of the ATP-binding cassette (ABC) transporter 1 ABCA1, which together with ABCG1 mediates cholesterol efflux from ECs (Hassan et al, 2006). Notably, endothelial SREBP2 also controls expression of arginine metabolism enzymes, although the physiological significance of this interaction between cholesterol and arginine metabolism remains to be determined (Zeng et al, 2004).
Arginine and glutamine are the best studied amino acids (AAs) in ECs. Arginine is a metabolite in the ornithine cycle and converted into citruline and nitric oxide (NO) by endothelial nitric oxide synthase (eNOS) (Fig 1) (Sessa et al, 1990). Alterations in arginine and eNOS metabolism are among the best-characterized causes of EC dysfunction and a prime therapeutic target (Leiper & Nandi, 2011). Glutamine is the most abundant AA in the peripheral blood and preferentially taken up by ECs via the solute carrier family 1 member 5 (SLC1A5) transporter (Fig 1) (Herskowitz et al, 1991; Pan et al, 1995). Glutamine-utilizing pathways are mainly biosynthetic and can be divided into those that utilize the γ-nitrogen (nucleotide biosynthesis, hexosamine biosynthesis, asparagine synthesis) and those that use the α-nitrogen or carbon backbone (DeBerardinis & Cheng, 2010). The latter reactions use glutamine-derived glutamate rather than glutamine itself and include glutathione (GSH) synthesis, anaplerotic refueling of the TCA cycle and biosynthesis of polyamines, proline and other non-essential AAs (NEAAs) (Fig 1) (DeBerardinis & Cheng, 2010).
Serine and glycine are especially interesting examples of glutamine / glutamate-derived NEAAs, not only because of their direct effects on ECs (Weinberg et al, 1992; Rose et al, 1999; Yamashina et al, 2001; Mishra et al, 2008; den Eynden et al, 2009; McCarty et al, 2009; Stobart et al, 2013), but also since their synthesis requires both the glutamate α-nitrogen and the glycolytic intermediate 3-phosphoglycerate (3PG) (Fig 1) (Locasale, 2013). Hence, serine and glycine metabolism integrates metabolic input from central carbon (glycolysis) and nitrogen (glutamine) metabolism. Moreover, the reversible interconversion of serine and glycine is directly coupled to one-carbon metabolism, intermediates of which are considered important targets to treat cardiovascular disease (Fig 1; see below) (Locasale, 2013). In fact, while EC metabolism is largely understudied, several of the above-mentioned metabolic pathways have been implicated as mediators of pathological angiogenesis or EC dysfunction.
EC metabolism in diseases characterized by angiogenesis and EC hyperproliferation
Cancer
Tumors need blood vessels to supply oxygen and detoxify waste products (Jain, 1987; Papetti & Herman, 2002; Welti et al, 2013). When tumors become too large to allow adequate diffusion of oxygen and nutrients from local vasculature they secrete pro-angiogenic growth factors to induce angiogenesis (Bergers & Benjamin, 2003). Pharmacological inhibition of growth factor signaling (primarily vascular endothelial growth factor (VEGF) signaling) is the only clinically approved anti-angiogenic strategy, but the benefits are limited as tumors acquire resistance within months after treatment initiation (Bergers & Hanahan, 2008; Carmeliet & Jain, 2011; Ebos & Kerbel, 2011; Welti et al, 2013). Escape from anti-angiogenic therapy is mediated by increased secretion of pro-angiogenic factors, activation of alternative angiogenic signaling pathways, recruitment of pro-angiogenic accessory cells and other mechanisms (Loges et al, 2010; Sennino & McDonald, 2012). A recent report indicated that glycosylation-dependent interactions of galectin-1 with VEGF receptor 2 (VEGFR2) could activate pro-angiogenic signaling even when the VEGF ligand is blocked (Fig 2A) (Croci et al, 2014). Hence, angiogenic signaling is robust and redundant, and inhibition of individual signaling molecules and growth factors can be overcome by escape mechanisms.
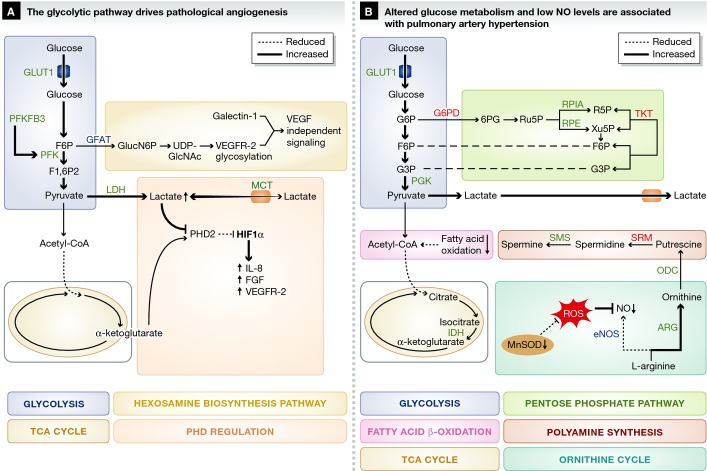
Figure 2. Metabolic pathways implicated in diseases characterized by pathological angiogenesis or hyperproliferative ECs.
(A) Angiogenic ECs rely on glycolysis, instead of oxidative metabolism, for ATP production and upregulate PFKFB3 to increase the conversion of glucose into lactate through glycolysis. Lactate is secreted and taken up through lactate transporters. High Lactate influx through MCT1 results in increased intracellular lactate levels that compete with α-ketoglutarate for PHD-2 binding, leading to HIF-1α stabilization and upregulation of pro-angiogenic genes. VEGFR-2 glycosylation is required for galectin-1-induced VEGF-independent signaling. (B) PAH ECs are metabolically characterized by high aerobic glycolysis and low oxidative metabolism. NO production through eNOS is impaired due to upregulation of arginase II and increased oxidative stress due to limited availability of MnSOD. In addition, several enzymes in the pentose phosphate pathway and polyamine biosynthesis pathway are differentially expressed in PAH ECs, but the importance of these findings remains to be determined (B). Green font / bold line: upregulated, red font / broken line: downregulated. For clarity, not all metabolites and enzymes of the depicted pathways are shown. Abbreviations: as in Fig 1. FGF: fibroblast growth factor; HIF: hypoxia-inducible factor; IL: interleukin; PHD: prolyl hydroxylase domain; R5P: ribose-5-phosphate; RPE: ribulose-5-phosphate 3-epimerase; RPIA: ribose-5-phosphate isomerase; Ru5P: ribulose-5-phosphate; SRM: spermidine synthase; VEGFR: vascular endothelial growth factor receptor; Xu5P: xylulose-5-phosphate.
The switch from a quiescent to an angiogenic phenotype (as occurs in cancer) is metabolically demanding and mediated by adaptations in EC metabolism (Fig 2A). While the changes in metabolic fluxes of ECs, freshly isolated from tumors, have not been characterized yet, ECs in tumors and inflamed tissues likely resemble highly activated ECs. Lactate dehydrogenase B (LDH-B) is upregulated in tumor endothelium, and VEGF signaling increases glycolytic flux by inducing GLUT1 and the glycolytic enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) (Fig 2A) (van Beijnum et al, 2006; Yeh et al, 2008; De Bock et al, 2013b). PFKFB3 catalyzes the synthesis of fructose-2,6-bisphosphate (F2,6P2), which is an allosteric activator of 6-phosphofructo-1-kinase (PFK-1) (Van Schaftingen et al, 1982). PFK-1 converts fructose-6-phosphate (F6P) to fructose-1,6-bisphosphate (F1,6P2) in the rate-limiting step of glycolysis. EC-specific PFKFB3 deletion diminishes retinal and hindbrain vascularization in mice, showing that increased glycolytic flux is required for growth factor-induced angiogenesis (De Bock et al, 2013b). Moreover, PFKFB3 overexpression in zebrafish drives EC specification into sprout forming tip cells, even in the presence of tip cell-inhibitory Notch signals that promote proliferating stalk elongating cells (De Bock et al, 2013b). Increased glycolysis not only provides energy for proliferation and biosynthesis, but also for locomotion. Specifically, PFKFB3 and other glycolytic enzymes co-localize with F-actin bundles in filopodia and lamellipodia to produce ATP needed for rapid actin remodeling, underlying locomotion and tip cell formation (De Bock et al, 2013b).
The important role of glycolysis in angiogenesis provides opportunities for therapeutic targeting. Indeed, pharmacological blockade with 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) or EC-specific genetic silencing of PFKFB3 inhibits tumor growth in vivo (Xu et al, 2014). In addition, 3PO inhibits glycolytic flux partially and transiently and has recently shown efficacy in reducing pathological angiogenesis in a variety of disease models (Schoors et al, 2014b; Xu et al, 2014). The systemic harm caused by inhibiting glycolysis is minimal, however, showing that even moderate, short-term impairment of glycolysis renders ECs more quiescent without overt detrimental side effects (Schoors et al, 2014b). The finding that partial and transient reduction of glycolysis may be sufficient to inhibit pathological angiogenesis provides a paradigm shift in our thinking about anti-glycolytic therapies, away from complete and permanent blockade of glycolysis, which can induce undesired adverse systemic effects.
Aside from serving as an energy source or building blocks for biosynthesis, glycolytic metabolites can also modulate angiogenesis by acting as bona fide signaling molecules. This is evidenced by the observation that glycolytic tumor cells secrete lactate, which is taken up by ECs through the monocarboxylate transporter 1 (MCT1) (Fig 2A) (Sonveaux et al, 2012). Instead of being metabolized, lactate induces HIF-1α activation leading to increased expression of VEGFR2 and bFGF (Sonveaux et al, 2012). Moreover, lactate competes with α-ketoglutarate for binding to the oxygen sensing prolyl hydroxylase-2 (PHD-2), resulting in diminished PHD-2 activity and subsequent hypoxia-inducible factor-1α (HIF-1α) stabilization (Fig 2A). Stabilized HIF-1α induces pro-angiogenic signaling pathways such as nuclear factor kappa-light-chain-enhancer of activated B-cells (NFkB)/interleukin 8 (IL-8) leading to increased angiogenesis (Fig 2A) (Hunt et al, 2007; Vegran et al, 2011; Sonveaux et al, 2012). Exploratory studies found that lactate induces angiogenesis in vivo and that pharmacological blockade of MCT1 inhibits angiogenesis and reduces tumor growth in mice (Sonveaux et al, 2012). Together, these data suggest an intricate relationship between classical pro-angiogenic signals such as VEGF, HIF-1α and hypoxia, and EC glucose metabolism. Targeting EC glucose metabolism to inhibit tumor angiogenesis is in its infancy as a therapeutic strategy, but recent evidence suggests its viability.
Pulmonary arterial hypertension
Idiopathic pulmonary arterial hypertension (PAH) is characterized by heightened pressure in pulmonary arteries caused by excessive EC proliferation and vascular dysfunction (Xu & Erzurum, 2011). Emerging evidence indicates that metabolic abnormalities underlie PAH (Fig 2B) (Sutendra & Michelakis, 2014; Zhao et al, 2014). In line with recent findings that glycolysis regulates angiogenesis, hyperproliferative PAH ECs rely on increased glycolytic flux and reduced oxygen consumption, which may be related to HIF-1α overexpression (Fig 2B) (Xu et al, 2007; Fijalkowska et al, 2010; Majmundar et al, 2010; Tuder et al, 2012). Human pulmonary ECs expressing mutated bone morphogenetic protein receptor 2 (BMPR2), which confers PAH, show altered expression of several glycolytic enzymes including GLUT1 and phosphoglycerate kinase 1 (PGK1). PAH ECs also show increased expression of enzymes of the PPP (R5P isomerase, Ru5P-3-epimerase) and polyamine biosynthesis pathway (ornithine decarboxylase (ODC), spermine synthase (SMS)). These metabolic changes may underlie the rapid proliferation of PAH ECs, since glycolysis, the PPP and mitogenic polyamines all promote cellular proliferation (Morrison & Seidel, 1995). However, the expression of other PPP and polyamine enzymes [G6PD, TKT, spermidine synthase (SRM)] is reduced—a finding that requires further explanation (Fig 2B) (Atkinson et al, 2002; Rudarakanchana et al, 2002; Long et al, 2006; Fessel et al, 2012). In addition, ECs isolated from EC-specific BMPR2 mutant mice show similarly increased expression of PGK1, indicating altogether that alterations in glycolysis as well as PPP likely underlie PAH (Majka et al, 2011).
In addition to alterations in glycolysis, idiopathic PAH ECs have fewer mitochondria and decreased mitochondrial metabolic activity (Xu et al, 2007). BMPR2 mutant ECs have reduced quantities of TCA cycle intermediates, reduced fatty acid oxidation and transcriptional reduction of several enzymes involved in fatty acid metabolism, including the rate-limiting enzyme of fatty acid oxidation carnitine palmitoyltransferase 1 (CPT1) (Fig 2) (Fessel et al, 2012). Together, these findings suggest reduced oxidative metabolism. Indeed, pharmacological inhibition of hyper-activated pyruvate dehydrogenase kinase (PDK), an enzyme that shunts glucose-derived pyruvate away from oxidative TCA metabolism, has shown therapeutic efficacy. However, whether these effects are mediated via ECs specifically remains to be determined (McMurtry et al, 2004). For unexplained reasons, PAH patients also show increased isocitrate dehydrogenase (IDH)-1 and IDH-2 serum activity, a finding that corroborates with the increased IDH activity observed in BPMR2 mutant ECs (Fessel et al, 2012). Still, the mechanisms that alter metabolic pathways in PAH ECs and the importance of some of these metabolic adaptations in the pathogenesis of PAH remain unclear.
Reduced nitric oxide (NO) levels are another hallmark of PAH ECs (Fijalkowska et al, 2010). Low NO levels may be related to the reduced levels of the mitochondrial antioxidant manganese superoxide dismutase (MnSOD) (Fijalkowska et al, 2010). Indeed, MnSOD increases NO availability by clearing superoxide anion, which inactivates NO to form peroxynitrite (Fig 2) (Masri et al, 2008). However, other factors likely contribute to the low NO levels in PAH ECs (Xu et al, 2004). Indeed, human PAH ECs express high levels of arginase II, which competes with endothelial nitric oxide synthetase (eNOS) for their common substrate L-arginine (Fig 2) (Xu et al, 2004). Inhibition of endothelial arginase II increases NO production in vitro, suggesting that arginase II can be targeted to prevent EC hyperproliferation and restore NO availability (Krotova et al, 2010). While the mechanisms that induce abnormal metabolic activity in PAH ECs are understudied, restoring NO may provide dual benefits in preventing excessive EC proliferation as well as restoring EC vasoactivity.
The metabolic adaptations in PAH (high glycolytic rates and reduced oxidative metabolism) are partly reminiscent of the metabolic profile of angiogenic ECs. It would be thus interesting to determine if reducing glycolysis by pharmacological blockade of PFKFB3 can reduce the hyperproliferative rate in PAH ECs. Alternatively, the beneficial effects of PDK inhibition in PAH to induce oxidative metabolism could also be beneficial to block angiogenesis by preventing the glycolytic switch in ECs. Indeed, PDK blockade with dichloroacetate inhibits angiogenesis in glioblastoma patients (Michelakis et al, 2010).
EC metabolism in diseases characterized by EC dysfunction
Diabetes
Diabetes is characterized by high blood glucose levels that affect EC metabolism and cause dysfunction (Fig 3A) (Blake & Trounce, 2013). Hyperglycemia induces peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α), an important regulator of metabolic gene expression and mitochondrial biogenesis (Puigserver et al, 1998; Herzig et al, 2001; Lin et al, 2002). PGC1α increases angiogenesis when expressed in heart and muscle cells (Arany et al, 2008; Patten et al, 2012). In contrast, diabetes-induced PGC-1α expression in ECs renders them less responsive to angiogenic factors and blunts angiogenesis (Sawada et al, 2014).
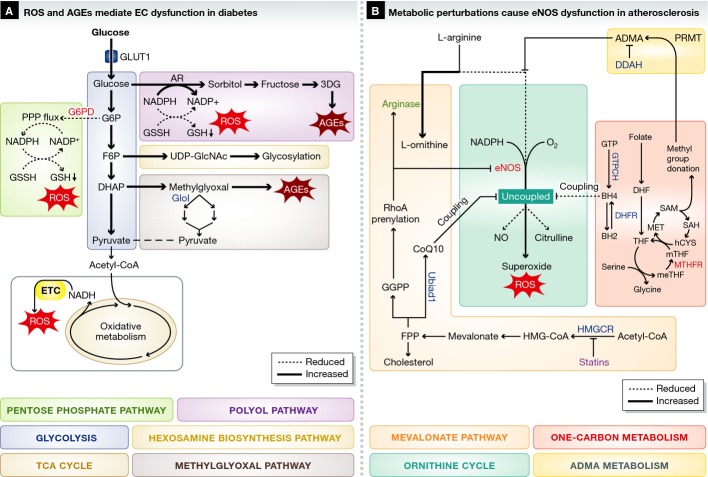
Figure 3. Metabolic pathways implicated in diseases characterized by EC dysfunction.
(A) High glucose levels in diabetes pushes glycolytic flux and cause ROS production and AGE formation. (B) Metabolic alterations that cause eNOS dysfunction mediate atherosclerosis pathogenesis. Asymmetric dimethylarginine (ADMA) competes with arginine for binding to eNOS. Arginase expression is increased and eNOS expression is decreased, leading to reduced eNOS activity. 1C metabolism and mevalonate metabolism provide eNOS coupling co-factors and inhibit ROS production. The mevalonate pathway also provides farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), required for GTPase prenylation. For clarity, not all metabolites and enzymes of the depicted pathways are shown. Green font / bold line: upregulated, red font / broken line: downregulated. Abbreviations: as in Figure 1. BH2: dihydrobiopterin; BH4: tetrahydrobiopterin; ADMA: asymmetric dimethylarginine; CoQ10: coenzyme Q10; DDAH: dimethylarginine dimethylaminohydrolase; DHF: dihydrofolate; DHFR: dihydrofolate reductase; FPP: farnesyl pyrophosphate; GGPP: geranylgeranyl pyrophosphate; GTP: Guanosine triphosphate; HMGCR: hydroxymethylglutaryl coenzyme A reductase; PRMT: protein arginine methyltransferase.
In addition to affecting gene expression, high glucose levels alter metabolism to induce the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which might be mediators of EC dysfunction (Fig 3) (Blake & Trounce, 2013). High glucose levels cause ECs to produce ROS via activation of NADPH-dependent oxidases (Inoguchi et al, 2003). In addition, hyperglycemia inhibits PPP flux by down-regulation of G6PD, the rate-limiting enzyme of the PPP. The PPP is an important source of intracellular NADPH, which is necessary to convert oxidized glutathione (GSSH) into reduced GSH, a critical ROS scavenger (Fig 3A) (Leopold et al, 2003; Zhang et al, 2012). Therefore, by reducing PPP flux, high glucose depletes NADPH levels and contributes to ROS accumulation (Goldin et al, 2006). Interestingly, G6PD overexpression restores redox homeostasis in high glucose cultured ECs (Leopold et al, 2003; Zhang et al, 2012). Some studies suggest that high glucose shifts the normally glycolytic EC metabolism toward oxidative metabolism and increased mitochondrial respiration (Fig 3). However, these results appear contextual, as other studies did not report such an induction of oxidative metabolism (Nishikawa et al, 2000; Koziel et al, 2012; Pangare & Makino, 2012; Dymkowska et al, 2014). While the precise effects on mitochondrial respiration require further study, hyperglycemia-induced mitochondrial ROS induces DNA breaks and thereby activates polyAPD-ribose polymerase (PARP-1) (Du et al, 2000, 2003; Nishikawa et al, 2000; Giacco & Brownlee, 2010; Blake & Trounce, 2013). PolyADP-ribosylation by PARP-1 inactivates GAPDH and stalls glycolysis, allowing accumulation of glycolytic metabolites (Du et al, 2003).
Accumulation of F6P increases the flux through the hexosamine biosynthesis pathway (HBP), which produces UDP-GlcNac, an important precursor of glycosylation reactions (Fig 3A) (Brownlee, 2001). While glycosylation is important for physiological EC function, hyperglycemia-induced protein glycosylation inhibits angiogenic functions (Du et al, 2001; Federici et al, 2002; Luo et al, 2008). Other glycolytic intermediates are diverted into the polyol and methylglyoxal pathways that produce damaging agents such as ROS and advanced glycation end products (AGEs) (Fig 3A) (Goldin et al, 2006). AGEs induce vascular dysfunction by altering extracellular matrix protein function and dysregulating cytokine expression (Yan et al, 2008). In addition, receptor of AGE (RAGE) binding by AGEs in vascular cells causes inflammation and reduced NO availability associated with vascular complications in diabetic patients (Bucala et al, 1991; Vlassara et al, 1995; Min et al, 1999; Wautier & Schmidt, 2004; Goldin et al, 2006; Manigrasso et al, 2014).
Excess glucose that cannot be metabolized by glycolysis enters the polyol pathway when converted into sorbitol by aldose reductase (AR) at the expense of NADPH, increasing ROS. Sorbitol is subsequently converted into fructose and the highly reactive 3-deoxyglucosone (3DG), which promotes the formation of AGEs (Fig 3A) (Kashiwagi et al, 1994; Oyama et al, 2006; Giacco & Brownlee, 2010; Sena et al, 2012; Yoshida et al, 2012). Transgenic overexpression of human AR in the Gendothelium of diabetic mice accelerates atherosclerosis formation and inhibition of endothelial AR reduces intracellular ROS, EC migration and proliferation (Obrosova et al, 2003; Tammali et al, 2011; Vedantham et al, 2011; Yadav et al, 2012). Methylglyoxal is another AGE precursor and produced from the glycolytic intermediates glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP). Methylglyoxal is detoxified by conversion into pyruvate via the multienzyme glyoxalase system, of which glyoxalase-I (GloI) is rate-limiting (Fig 3A) (Thornalley, 1993). Glyoxalase-I overexpression reverses hyperglycemia-induced angiogenesis defects in vitro and transgenic overexpression of glyoxalase-I in rats reduces vascular AGE formation and improves vasoreactivity (Brouwers et al, 2010, 2014) (Ahmed et al, 2008). Together, these observations indicate that targeting AR and glyoxalase might confer a therapeutic benefit in diabetic patients.
Atherosclerosis
Atherosclerosis is a chronic inflammatory process in the blood vessel wall leading to luminal narrowing and subsequent cardiovascular events (Hopkins, 2013). Systemic metabolic perturbations are among the most important risk factors of atherosclerosis. However, metabolic flux changes have not been studied in ECs isolated from atherosclerotic lesions, and the effects of atherosclerosis on central metabolism of ECs thus remains to be characterized. Nonetheless, EC metabolism is strongly associated with a key pathophysiological feature of atherosclerosis: reduced and uncoupled eNOS activity resulting in low NO bioavailability and high ROS production (Fig 3B) (Kawashima & Yokoyama, 2004). eNOS activity critically depends on the availability of L-arginine, co-factor tetrahydrobiopterin (BH4) (Fig 3B) and possibly co-enzyme Q10 (CoQ10) (Gorren et al, 2000; Crabtree et al, 2009a; Mugoni et al, 2013). If L-arginine, BH4 or CoQ10 become limited, eNOS no longer oxidizes L-arginine to form citrulline and NO, but instead produces ROS (a condition termed eNOS uncoupling) (Fig 3B) (Stroes et al, 1998; Mugoni et al, 2013). Targeting L-arginine and BH4 metabolism to increase eNOS activity in patients with cardiovascular disease is potentially beneficial, but available evidence indicates that the picture is more complex than initially anticipated.
Small-scale clinical trials indicate that administration of L-arginine to patients with coronary heart disease improves vasoresponsiveness, possibly by increasing NO production by eNOS (Lerman et al, 1998). Interestingly, however, intracellular and plasma arginine levels are sufficiently high to support NO biosynthesis via eNOS. Therefore, the benefits of L-arginine supplementation on elevating NO levels are not readily explained by increasing the supply of L-arginine; however, it is possible that L-arginine is compartmentalized in poorly interchangeable pools. Another possible explanation of the beneficial effects of L-arginine is competition with asymmetric methylated arginines, which bind and inhibit eNOS (Fig 3B) (Boger, 2004; Chen et al, 2013). More in detail, post-translational methylation of arginine residues in proteins by protein arginine methyltransferase (PRMT) results in the addition of up to two methyl groups to arginine. Protein turnover releases these post-translationally modified amino acids as asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA). The asymmetric dimethylarginines bind and uncouple eNOS resulting in increased ROS production and reduced NO availability (Fig 3B) (Dhillon et al, 2003; Leiper & Nandi, 2011). Hence by competing with ADMAs, supplemented L-arginine could maintain eNOS activity to produce NO (Bode-Boger et al, 2003). Additional potential interventions to reduce eNOS inhibition by ADMA include PRMT inhibition (to reduce arginine methylation) and activation of methylarginine catabolism by dimethylarginine dimethylaminohydrolase (DDAH) (Fig 3B) (Leiper & Nandi, 2011). Interestingly, DDAH1 is predominantly expressed in ECs and EC-specific deletion attenuates NO production and induces hypertension, indicating that ADMA scavenging by ECs is important to maintain homeostasis (Hu et al, 2009).
Because L-arginine is a substrate for both eNOS and arginase (Wu & Meininger, 1995), NO production depends on the relative expression levels of each enzyme (Fig 3) (Chang et al, 1998; Ming et al, 2004; Ryoo et al, 2008). Endothelial arginase expression is induced by many risk factors for cardiovascular disease, while reducing arginase expression restores NO production in vitro and improves vasodilatation in vivo (Ryoo et al, 2006, 2008; Thengchaisri et al, 2006; Romero et al, 2008). The activity of eNOS and arginase is regulated by the RhoA/ROCK signaling cascade. RhoA and Rock decrease eNOS expression, while RhoA also increases arginase activity (Fig 3B) (Laufs et al, 1998; Takemoto et al, 2002). For proper activation and localization to the cell membrane, RhoA must be prenylated (more specifically, geranylgeranylated) by geranylgeranyltransferase (GGT) using geranylgeranyl pyrophosphate (GGPP) as a substrate (Laufs & Liao, 1998). This isoprenoid is an intermediate of the mevalonate pathway, which produces cholesterol from acetyl-coA (Fig 3B). Blocking the mevalonate pathway by inhibiting HMG-coA reductase using statins lowers cholesterol synthesis and is clinically approved to prevent cardiovascular events in dyslipidemia patients. In addition, HMG-coA blockade also decreases geranylgeranyl production, which reduces RhoA activity and restores a more beneficial eNOS/arginase balance (Goldstein & Brown, 1990; Liao & Laufs, 2005). Interestingly, UBIAD1 was recently identified as a novel prenyltransferase that produces non-mitochondrial CoQ10 from farnesyl pyrophosphate (FPP), another isoprenoid produced in the mevalonate pathway (Fig 3) (Mugoni et al, 2013). CoQ10 is an important anti-oxidant with beneficial effects on EC function and hypothesized to be a novel co-factor required for eNOS coupling (Gao et al, 2012; Mugoni et al, 2013). Hence, in contrast to the above-mentioned beneficial effects, HMG-coA reductase inhibition might thus also have a less favorable effect by increasing ROS levels through reducing CoQ10 synthesis (Fig 3) (Mugoni et al, 2013).
In addition to CoQ10, eNOS requires BH4 as a co-factor. Reduced BH4 availability is found in patients at risk of atherosclerosis and promotes ROS production through eNOS uncoupling (Fig 3B) (Pieper, 1997; Stroes et al, 1997; Heitzer et al, 2000). Endothelial BH4 levels are maintained by de novo biosynthesis via the rate-limiting enzyme guanosine triphosphate cyclohydrolase I (GTPCH) and by a salvage pathway from dihydrobiopterin (BH2) via dihydrofolate reductase (DHFR) (Fig 3B) (Bendall et al, 2014). Insufficient levels of GTPCH and DHFR, important enzymes in GTP and folate metabolism, respectively, have been associated with reduced BH4 availability, endothelial dysfunction and cardiovascular disease in several preclinical models (Chalupsky & Cai, 2005; Crabtree et al, 2009b, 2011; Sugiyama et al, 2009; Kidokoro et al, 2013). Interestingly, DHFR not only regenerates active BH4 from oxidized inactive BH2 but is also a key enzyme in folate and one-carbon metabolism, intermediates of which in turn regulate BH4 biosynthesis and are associated with cardiovascular disease (Humphrey et al, 2008).
One-carbon (1C) metabolism centers around the ability of folate-derived co-enzymes to carry activated 1C units (Fig 3) (Tibbetts & Appling, 2010). DHFR catalyzes the formation of tetrahydrofolate (THF) from folate fueling 1C metabolism. THF accepts 1C units from serine to produce 5,10-methylene-THF (meTHF) and glycine. MeTHF is reduced to 5-methyl-THF (mTHF) by methylenetetrahydrofolate reductase (MTHFR) (Fig 3). Importantly, inactivating mutations in the MTHFR gene result in hyperhomocysteinemia, which decreases GTPCH and DHFR levels and may subsequently reduce BH4 levels (Bendall et al, 2014). Indeed, MTHFR mutations have been associated with cardiovascular disease, but the exact association is still controversial (Kelly et al, 2002; Klerk et al, 2002; Frederiksen et al, 2004; Yang et al, 2012). mTHF produced by MTHFR activity is required as a methyl donor in the methionine synthase (MS) catalyzed reaction that converts mTHF into THF (completing the folate cycle) and forms methionine (MET) from homocysteine (hCYS) (Fig 3B) (Locasale, 2013). Methionine is used to generate S-adenosylmethionine (SAM), which is an important methyl donor and plays a pivotal role in methylation of lysine and arginine residues in proteins (Fig 3B) (Leiper & Nandi, 2011). As discussed above, methylated arginine residues are emerging as important mediators of EC dysfunction. Moreover, SAM-mediated protein methylation produces S-adenosylhomocysteine, which is converted back into homocysteine. Homocysteine decreases the bioavailability of BH4 possibly through downregulation of GTPCH and DHFR, while BH4 supplementation alleviates homocysteine-induced EC dysfunction (Dhillon et al, 2003; Topal et al, 2004). Together, these findings suggest that dysregulation of endothelial 1C metabolism is involved in the pathogenesis of cardiovascular disease, but the exact mechanisms remain to be elucidated. Nonetheless, early clinical and preclinical studies have found that therapeutic targeting of 1C metabolism, for example, via folate supplementation lowers levels of homocysteinemia and increases BH4 regeneration from BH2 (Verhaar et al, 2002). However, large-scale clinical trials failed to show benefits of folate or BH4 supplementation to prevent cardiovascular disease (Clarke et al, 2010; Cunnington et al, 2012; Marti-Carvajal et al, 2013). These clinical and preclinical findings suggest that while L-arginine, folate, methionine, COQ10 and homocysteine metabolism are potential therapeutic targets, a more detailed understanding of how these pathways cause dysfunction is required to design more rational therapeutic agents.
EC metabolism in the pathogenesis of other diseases
EC metabolism is best characterized in the diseases discussed above. However, these represent only a minor fraction of the disorders in which pathological EC responses are presumably involved. Indeed, it is highly likely that EC metabolic alterations are also involved in the pathogenesis of other diseases such as ischemia, pre-eclampsia, vasculitis, vascular neoplasms and others although this has hardly been studied.
On the other hand, many of the EC metabolic alterations that lead to EC dysfunction are likely induced by cardiovascular risk factors such as those that characterize metabolic syndrome, hyperhomocysteinemia and hyperuricemia. For example, elevated serum uric acid (a breakdown product of purine nucleotides generated by xanthine oxidase with potent anti-oxidant activity) is common in patients with hypertension and may even be a root cause of EC dysfunction leading to cardiovascular disease (Feig et al, 2008). Interestingly, while uric acid has been described as major anti-oxidant in human plasma, ECs exposed to uric acid display increased ROS production creating a paradox that has not been resolved (Lippi et al, 2008; Sautin & Johnson, 2008). Regardless, in cardiovascular disease models uric acid reduces mitochondrial content, intracellular ATP and arginase activity (Zharikov et al, 2008; Sanchez-Lozada et al, 2012). In addition, uric acid inhibits NO production in ECs in vitro, and in vivo levels of serum nitrites (an indicator of NO production) are inversely proportional to serum uric acid concentrations (Khosla et al, 2005). Interestingly, ECs exposed to uric acid increase expression of AR and alter expression of several other proteins linked to metabolism (Zhang et al, 2014). These studies suggest that hyperuricemia induces EC dysfunction through metabolic alterations. Whether the same is true for other cardiovascular risk factors remains in question.
A broader characterization of EC metabolism in the future might reveal novel therapeutic targets in metabolic pathways that are generally not considered to be important in pathological EC function. Recent findings that endothelial cholesterol efflux to high-density lipoprotein regulates angiogenesis (Fang et al, 2013), and that EC-specific insulin receptor knock-out accelerates atherosclerotic plaque formation (Gage et al, 2013) point to a key role for EC metabolism in the pathogenesis of disease and indicate that many more yet to be identified non-traditional but potentially druggable metabolic enzymes, transporters and pathways may play a role in vascular disease.
Pending issues
The findings in this review suggest that blood vessel pathology is mediated, or at least characterized, by disease-specific alterations. However, at present, there are no studies that incorporate state-of-the-art metabolomics tools to characterize EC metabolism in disease. Metabolic profiling using isotope incorporation studies and metabolic flux analysis could greatly increase our understanding of the metabolic alterations that underlie EC pathology.
In vivo studies to characterize EC metabolism in animal models of human disease could provide highly relevant insight in disease-specific metabolic alterations. However, this requires isolation of ECs from diseased tissue, which at present poses technical and interpretational challenges for proper analysis of metabolism using advanced metabolomics methods.
Another pressing issue is the lack of studies characterizing metabolism in patient-derived tissue using either in or ex vivo models. The recent development of new protocols to isolate ECs from patient tissue offers the possibility to study metabolism in clinically relevant systems. Accordingly, such studies could greatly advance the identification of novel biomarkers and therapeutic targets in EC metabolism.
Therapeutic targeting of EC metabolism
Overall, it is clear that pathological blood vessel responses are associated with metabolic alterations in ECs. These metabolic adaptations are not just innocent bystanders, but in many cases mediate important aspects of disease. Increased EC glucose metabolism is emerging as a key feature of angiogenic and hyper-proliferative ECs. Targeting EC glucose metabolism has recently been shown as a viable strategy to curb pathological angiogenesis, but is still in its infancy (Schoors et al, 2014b). Recent technical and conceptual advances, however, now make it possible to perform comprehensive metabolic studies. These technical breakthroughs have led to a resurgent interest in targeting cell metabolism for therapeutic gains. As a proof of concept, targeting EC metabolism by pharmacological inhibition of the glycolytic enzyme PFKFB3 has shown recent success in inhibiting pathological angiogenesis (Fig 4) (De Bock et al, 2013b; Schoors et al, 2014b; Xu et al, 2014). These results, together with the observation that EC metabolism is altered in many diseases, suggest that EC metabolism is an attractive and viable but understudied therapeutic target.
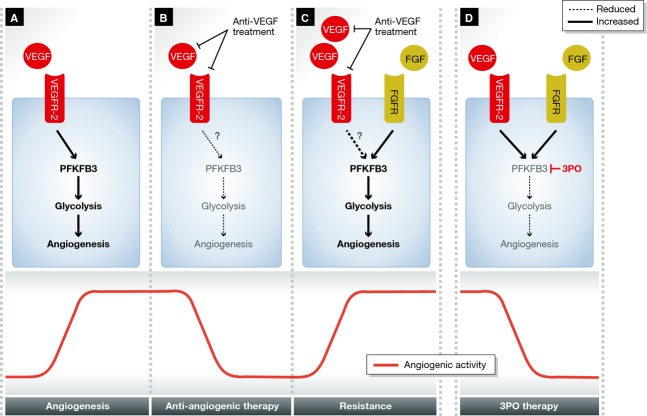
Figure 4. Targeting EC metabolism as an alternative to targeting growth factors in angiogenesis.
(A) Vascular endothelial growth factor (VEGF) induces 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) and increases glycolytic flux, required for angiogenesis. (B) Anti-VEGF treatment reduces glycolytic flux and angiogenesis. (C) Increased growth factor signaling through alternative pathways, in this case fibroblast growth factor (FGF), mediates resistance to anti-angiogenic therapy. (D) Pharmacological targeting of PFKFB3 with (3PO) reduces angiogenesis irrespective of growth factor signaling and is therefore possibly less prone to resistance. Abbreviations: as in Figure 1. 3PO: 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one; FGF: fibroblast growth factor.
Acknowledgments
We apologize for not being able to cite the work of all other studies related to this topic because of space restrictions. The authors gratefully acknowledge Massimo M. Santoro and Richard C. Cubbon for their valuable comments that helped improve the manuscript. J.G. is a PhD student supported by a BOF fellowship from the University of Leuven. The work of P.C. is supported by a Federal Government Belgium grant (IUAP P7/03), long-term structural Methusalem funding by the Flemish Government, grants from the Research Foundation Flanders (FWO), the Foundation of Leducq Transatlantic Network (ARTEMIS), Foundation against Cancer, an European Research Council (ERC) Advanced Research Grant (EU-ERC269073) and the AXA Research Fund.
For more information
Author website: http://www.vrc-lab.be/peter-carmeliet
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ahmed U, Dobler D, Larkin SJ, Rabbani N, Thornalley PJ. Reversal of hyperglycemia-induced angiogenesis deficit of human endothelial cells by overexpression of glyoxalase 1 in vitro. Ann N Y Acad Sci. 2008;1126:262–264. doi: 10.1196/annals.1433.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- van Beijnum JR, Dings RP, van der Linden E, Zwaans BM, Ramaekers FC, Mayo KH, Griffioen AW. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108:2339–2348. doi: 10.1182/blood-2006-02-004291. [DOI] [PubMed] [Google Scholar]
- Bendall JK, Douglas G, McNeill E, Channon KM, Crabtree MJ. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal. 2014;20:3040–3077. doi: 10.1089/ars.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R, Trounce IA. Mitochondrial dysfunction and complications associated with diabetes. Biochim Biophys Acta. 2013;1840:1404–1412. doi: 10.1016/j.bbagen.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Bode-Boger SM, Muke J, Surdacki A, Brabant G, Boger RH, Frolich JC. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vas Med. 2003;8:77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, Bode-Boger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res. 2000;87:99–105. doi: 10.1161/01.res.87.2.99. [DOI] [PubMed] [Google Scholar]
- Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr. 2004;134:2842S–2847S. doi: 10.1093/jn/134.10.2842S. discussion 2853S. [DOI] [PubMed] [Google Scholar]
- Brouwers O, Niessen PM, Haenen G, Miyata T, Brownlee M, Stehouwer CD, De Mey JG, Schalkwijk CG. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010;53:989–1000. doi: 10.1007/s00125-010-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers O, Niessen PM, Miyata T, Ostergaard JA, Flyvbjerg A, Peutz-Kootstra CJ, Sieber J, Mundel PH, Brownlee M, Janssen BJ, et al. Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia. 2014;57:224–235. doi: 10.1007/s00125-013-3088-5. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Investig. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CI, Liao JC, Kuo L. Arginase modulates nitric oxide production in activated macrophages. Am J Physiol. 1998;274:H342–H348. doi: 10.1152/ajpheart.1998.274.1.H342. [DOI] [PubMed] [Google Scholar]
- Chen F, Lucas R, Fulton D. The subcellular compartmentalization of arginine metabolizing enzymes and their role in endothelial dysfunction. Front Immunol. 2013;4:184. doi: 10.3389/fimmu.2013.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, Bonaa KH, Spence JD, Nygard O, Jamison R, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170:1622–1631. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- Crabtree MJ, Hale AB, Channon KM. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radical Biol Med. 2011;50:1639–1646. doi: 10.1016/j.freeradbiomed.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, Channon KM, Alp NJ. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009a;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem. 2009b;284:28128–28136. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci DO, Cerliani JP, Dalotto-Moreno T, Mendez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, Toscano MA, Caramelo JJ, Garcia-Vallejo JJ, Ouyang J, et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- Culic O, Gruwel ML, Schrader J. Energy turnover of vascular endothelial cells. Am J Physiol. 1997;273:C205–C213. doi: 10.1152/ajpcell.1997.273.1.C205. [DOI] [PubMed] [Google Scholar]
- Cunnington C, Van Assche T, Shirodaria C, Kylintireas I, Lindsay AC, Lee JM, Antoniades C, Margaritis M, Lee R, Cerrato R, et al. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation. 2012;125:1356–1366. doi: 10.1161/CIRCULATIONAHA.111.038919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher Z, Ruderman N, Tornheim K, Ido Y. The effect of AMP-activated protein kinase and its activator AICAR on the metabolism of human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;265:112–115. doi: 10.1006/bbrc.1999.1635. [DOI] [PubMed] [Google Scholar]
- Dagher Z, Ruderman N, Tornheim K, Ido Y. Acute regulation of fatty acid oxidation and amp-activated protein kinase in human umbilical vein endothelial cells. Circ Res. 2001;88:1276–1282. doi: 10.1161/hh1201.092998. [DOI] [PubMed] [Google Scholar]
- Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- De Bock K, Georgiadou M, Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013a;18:634–647. doi: 10.1016/j.cmet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013b;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Cheng T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon B, Badiwala MV, Maitland A, Rao V, Li SH, Verma S. Tetrahydrobiopterin attenuates homocysteine induced endothelial dysfunction. Mol Cell Biochem. 2003;247:223–227. doi: 10.1023/a:1024146501743. [DOI] [PubMed] [Google Scholar]
- Dobrina A, Rossi F. Metabolic properties of freshly isolated bovine endothelial cells. Biochim Biophys Acta. 1983;762:295–301. doi: 10.1016/0167-4889(83)90084-8. [DOI] [PubMed] [Google Scholar]
- Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radical Biol Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Investig. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymkowska D, Drabarek B, Podszywalow-Bartnicka P, Szczepanowska J, Zablocki K. Hyperglycaemia modifies energy metabolism and reactive oxygen species formation in endothelial cells in vitro. Arch Biochem Biophys. 2014;542:7–13. doi: 10.1016/j.abb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Eynden JV, Ali SS, Horwood N, Carmans S, Brone B, Hellings N, Steels P, Harvey RJ, Rigo JM. Glycine and glycine receptor signalling in non-neuronal cells. Front Mol Neurosci. 2009;2:9. doi: 10.3389/neuro.02.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Choi SH, Baek JS, Liu C, Almazan F, Ulrich F, Wiesner P, Taleb A, Deer E, Pattison J, et al. Control of angiogenesis by AIBP-mediated cholesterol efflux. Nature. 2013;498:118–122. doi: 10.1038/nature12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. New Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessel JP, Hamid R, Wittmann BM, Robinson LJ, Blackwell T, Tada Y, Tanabe N, Tatsumi K, Hemnes AR, West JD. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm Circ. 2012;2:201–213. doi: 10.4103/2045-8932.97606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, et al. Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol. 2010;176:1130–1138. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen J, Juul K, Grande P, Jensen GB, Schroeder TV, Tybjaerg-Hansen A, Nordestgaard BG. Methylenetetrahydrofolate reductase polymorphism (C677T), hyperhomocysteinemia, and risk of ischemic cardiovascular disease and venous thromboembolism: prospective and case-control studies from the Copenhagen City Heart Study. Blood. 2004;104:3046–3051. doi: 10.1182/blood-2004-03-0897. [DOI] [PubMed] [Google Scholar]
- Gage MC, Yuldasheva NY, Viswambharan H, Sukumar P, Cubbon RM, Galloway S, Imrie H, Skromna A, Smith J, Jackson CL, et al. Endothelium-specific insulin resistance leads to accelerated atherosclerosis in areas with disturbed flow patterns: a role for reactive oxygen species. Atherosclerosis. 2013;230:131–139. doi: 10.1016/j.atherosclerosis.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221:311–316. doi: 10.1016/j.atherosclerosis.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Gaudreault N, Scriven DR, Moore ED. Characterisation of glucose transporters in the intact coronary artery endothelium in rats: GLUT-2 upregulated by long-term hyperglycaemia. Diabetologia. 2004;47:2081–2092. doi: 10.1007/s00125-004-1583-4. [DOI] [PubMed] [Google Scholar]
- Gaudreault N, Scriven DR, Laher I, Moore ED. Subcellular characterization of glucose uptake in coronary endothelial cells. Microvasc Res. 2008;75:73–82. doi: 10.1016/j.mvr.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Gorren AC, Bec N, Schrammel A, Werner ER, Lange R, Mayer B. Low-temperature optical absorption spectra suggest a redox role for tetrahydrobiopterin in both steps of nitric oxide synthase catalysis. Biochemistry. 2000;39:11763–11770. doi: 10.1021/bi0007775. [DOI] [PubMed] [Google Scholar]
- Harjes U, Bensaad K, Harris AL. Endothelial cell metabolism and implications for cancer therapy. Br J Cancer. 2012;107:1207–1212. doi: 10.1038/bjc.2012.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HH, Denis M, Krimbou L, Marcil M, Genest J. Cellular cholesterol homeostasis in vascular endothelial cells. Can J Cardiol. 2006;22(Suppl B):35B–40B. doi: 10.1016/s0828-282x(06)70985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- Herskowitz K, Bode BP, Block ER, Souba WW. Characterization of L-glutamine transport by pulmonary artery endothelial cells. Am J Physiol. 1991;260:L241–L246. doi: 10.1152/ajplung.1991.260.4.L241. [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Hinshaw DB, Burger JM. Protective effect of glutamine on endothelial cell ATP in oxidant injury. J Surg Res. 1990;49:222–227. doi: 10.1016/0022-4804(90)90123-j. [DOI] [PubMed] [Google Scholar]
- Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93:1317–1542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- Hu X, Xu X, Zhu G, Atzler D, Kimoto M, Chen J, Schwedhelm E, Luneburg N, Boger RH, Zhang P, et al. Vascular endothelial-specific dimethylarginine dimethylaminohydrolase-1-deficient mice reveal that vascular endothelium plays an important role in removing asymmetric dimethylarginine. Circulation. 2009;120:2222–2229. doi: 10.1161/CIRCULATIONAHA.108.819912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc. 2008;83:1203–1212. doi: 10.4065/83.11.1203. [DOI] [PubMed] [Google Scholar]
- Hunt TK, Aslam RS, Beckert S, Wagner S, Ghani QP, Hussain MZ, Roy S, Sen CK. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal. 2007;9:1115–1124. doi: 10.1089/ars.2007.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, et al. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003;14:S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- Ivashchenko CY, Bradley BT, Ao Z, Leiper J, Vallance P, Johns DG. Regulation of the ADMA-DDAH system in endothelial cells: a novel mechanism for the sterol response element binding proteins, SREBP1c and -2. Am J Physiol Heart Circ Physiol. 2010;298:H251–H258. doi: 10.1152/ajpheart.00195.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- Jongkind JF, Verkerk A, Baggen RG. Glutathione metabolism of human vascular endothelial cells under peroxidative stress. Free Radical Biol Med. 1989;7:507–512. doi: 10.1016/0891-5849(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A, Asahina T, Ikebuchi M, Tanaka Y, Takagi Y, Nishio Y, Kikkawa R, Shigeta Y. Abnormal glutathione metabolism and increased cytotoxicity caused by H2O2 in human umbilical vein endothelial cells cultured in high glucose medium. Diabetologia. 1994;37:264–269. doi: 10.1007/BF00398053. [DOI] [PubMed] [Google Scholar]
- Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- Kelly PJ, Rosand J, Kistler JP, Shih VE, Silveira S, Plomaritoglou A, Furie KL. Homocysteine, MTHFR 677C–>T polymorphism, and risk of ischemic stroke: results of a meta-analysis. Neurology. 2002;59:529–536. doi: 10.1212/wnl.59.4.529. [DOI] [PubMed] [Google Scholar]
- Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Kidokoro K, Satoh M, Channon KM, Yada T, Sasaki T, Kashihara N. Maintenance of endothelial guanosine triphosphate cyclohydrolase I ameliorates diabetic nephropathy. J Am Soc Nephrol. 2013;24:1139–1150. doi: 10.1681/ASN.2012080783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR 677C–>T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288:2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- Koziel A, Woyda-Ploszczyca A, Kicinska A, Jarmuszkiewicz W. The influence of high glucose on the aerobic metabolism of endothelial EA.hy926 cells. Pflugers Arch. 2012;464:657–669. doi: 10.1007/s00424-012-1156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotova K, Patel JM, Block ER, Zharikov S. Endothelial arginase II responds to pharmacological inhibition by elevation in protein level. Mol Cell Biochem. 2010;343:211–216. doi: 10.1007/s11010-010-0515-5. [DOI] [PubMed] [Google Scholar]
- Krutzfeldt A, Spahr R, Mertens S, Siegmund B, Piper HM. Metabolism of exogenous substrates by coronary endothelial cells in culture. J Mol Cell Cardiol. 1990;22:1393–1404. doi: 10.1016/0022-2828(90)90984-a. [DOI] [PubMed] [Google Scholar]
- Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, Chen YF, Darley-Usmar V, Oparil S, Chatham JC. Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system. Am J Physiol Heart Circ Physiol. 2009;296:H13–H28. doi: 10.1152/ajpheart.01056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- Lee R, Channon KM, Antoniades C. Therapeutic strategies targeting endothelial function in humans: clinical implications. Curr Vasc Pharmacol. 2012;10:77–93. doi: 10.2174/157016112798829751. [DOI] [PubMed] [Google Scholar]
- Leighton B, Curi R, Hussein A, Newsholme EA. Maximum activities of some key enzymes of glycolysis, glutaminolysis, Krebs cycle and fatty acid utilization in bovine pulmonary endothelial cells. FEBS Lett. 1987;225:93–96. doi: 10.1016/0014-5793(87)81137-7. [DOI] [PubMed] [Google Scholar]
- Leiper J, Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discovery. 2011;10:277–291. doi: 10.1038/nrd3358. [DOI] [PubMed] [Google Scholar]
- Leopold JA, Zhang YY, Scribner AW, Stanton RC, Loscalzo J. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arterioscler Thromb Vasc Biol. 2003;23:411–417. doi: 10.1161/01.ATV.0000056744.26901.BA. [DOI] [PubMed] [Google Scholar]
- Lerman A, Burnett JC, Jr, Higano ST, McKinley LJ, Holmes DR., Jr Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–2128. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Lippi G, Montagnana M, Franchini M, Favaloro EJ, Targher G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin Chim Acta. 2008;392:1–7. doi: 10.1016/j.cca.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes & cancer. 2010;1:12–25. doi: 10.1177/1947601909356574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res. 2006;98:818–827. doi: 10.1161/01.RES.0000215809.47923.fd. [DOI] [PubMed] [Google Scholar]
- Luo B, Soesanto Y, McClain DA. Protein modification by O-linked GlcNAc reduces angiogenesis by inhibiting Akt activity in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:651–657. doi: 10.1161/ATVBAHA.107.159533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majka S, Hagen M, Blackwell T, Harral J, Johnson JA, Gendron R, Paradis H, Crona D, Loyd JE, Nozik-Grayck E, et al. Physiologic and molecular consequences of endothelial Bmpr2 mutation. Respir Res. 2011;12:84. doi: 10.1186/1465-9921-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab. 2014;25:15–22. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- Marti-Carvajal AJ, Sola I, Lathyris D, Karakitsiou DE, Simancas-Racines D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2013;1:CD006612. doi: 10.1002/14651858.CD006612.pub3. [DOI] [PubMed] [Google Scholar]
- Masri FA, Comhair SA, Dostanic-Larson I, Kaneko FT, Dweik RA, Arroliga AC, Erzurum SC. Deficiency of lung antioxidants in idiopathic pulmonary arterial hypertension. Clin Transl Sci. 2008;1:99–106. doi: 10.1111/j.1752-8062.2008.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty MF, Barroso-Aranda J, Contreras F. The hyperpolarizing impact of glycine on endothelial cells may be anti-atherogenic. Med Hypotheses. 2009;73:263–264. doi: 10.1016/j.mehy.2008.12.021. [DOI] [PubMed] [Google Scholar]
- McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31ra34. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- Min C, Kang E, Yu SH, Shinn SH, Kim YS. Advanced glycation end products induce apoptosis and procoagulant activity in cultured human umbilical vein endothelial cells. Diabetes Res Clin Pract. 1999;46:197–202. doi: 10.1016/s0168-8227(99)00094-7. [DOI] [PubMed] [Google Scholar]
- Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, Hayoz D, Ruffieux J, Rusconi S, Montani JP, et al. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004;110:3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- Mishra RC, Tripathy S, Desai KM, Quest D, Lu Y, Akhtar J, Gopalakrishnan V. Nitric oxide synthase inhibition promotes endothelium-dependent vasodilatation and the antihypertensive effect of L-serine. Hypertension. 2008;51:791–796. doi: 10.1161/HYPERTENSIONAHA.107.099598. [DOI] [PubMed] [Google Scholar]
- Morrison RF, Seidel ER. Vascular endothelial cell proliferation: regulation of cellular polyamines. Cardiovasc Res. 1995;29:841–847. [PubMed] [Google Scholar]
- Mugoni V, Postel R, Catanzaro V, De Luca E, Turco E, Digilio G, Silengo L, Murphy MP, Medana C, Stainier DY, et al. Ubiad1 is an antioxidant enzyme that regulates eNOS activity by CoQ10 synthesis. Cell. 2013;152:504–518. doi: 10.1016/j.cell.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Noghero A, Perino A, Seano G, Saglio E, Lo Sasso G, Veglio F, Primo L, Hirsch E, Bussolino F, Morello F. Liver X receptor activation reduces angiogenesis by impairing lipid raft localization and signaling of vascular endothelial growth factor receptor-2. Arterioscler Thromb Vasc Biol. 2012;32:2280–2288. doi: 10.1161/ATVBAHA.112.250621. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Minchenko AG, Vasupuram R, White L, Abatan OI, Kumagai AK, Frank RN, Stevens MJ. Aldose reductase inhibitor fidarestat prevents retinal oxidative stress and vascular endothelial growth factor overexpression in streptozotocin-diabetic rats. Diabetes. 2003;52:864–871. doi: 10.2337/diabetes.52.3.864. [DOI] [PubMed] [Google Scholar]
- Oyama T, Miyasita Y, Watanabe H, Shirai K. The role of polyol pathway in high glucose-induced endothelial cell damages. Diabetes Res Clin Pract. 2006;73:227–234. doi: 10.1016/j.diabres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Paik JY, Lee KH, Ko BH, Choe YS, Choi Y, Kim BT. Nitric oxide stimulates 18F-FDG uptake in human endothelial cells through increased hexokinase activity and GLUT1 expression. J Nucl Med. 2005;46:365–370. [PubMed] [Google Scholar]
- Pan M, Wasa M, Ryan U, Souba W. Inhibition of pulmonary microvascular endothelial glutamine transport by glucocorticoids and endotoxin. JPEN J Parenter Enteral Nutr. 1995;19:477–481. doi: 10.1177/0148607195019006477. [DOI] [PubMed] [Google Scholar]
- Pan S, World CJ, Kovacs CJ, Berk BC. Glucose 6-phosphate dehydrogenase is regulated through c-Src-mediated tyrosine phosphorylation in endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:895–901. doi: 10.1161/ATVBAHA.109.184812. [DOI] [PubMed] [Google Scholar]
- Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995;14:5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangare M, Makino A. Mitochondrial function in vascular endothelial cell in diabetes. J Smooth Muscle Res. 2012;48:1–26. doi: 10.1540/jsmr.48.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–C970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- Parra-Bonilla G, Alvarez DF, Al-Mehdi AB, Alexeyev M, Stevens T. Critical role for lactate dehydrogenase A in aerobic glycolysis that sustains pulmonary microvascular endothelial cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L513–L522. doi: 10.1152/ajplung.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–338. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper GM. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J Cardiovasc Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]
- Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose ML, Madren J, Bunzendahl H, Thurman RG. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis. 1999;20:793–798. doi: 10.1093/carcin/20.5.793. [DOI] [PubMed] [Google Scholar]
- Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, Morrell NW. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet. 2002;11:1517–1525. doi: 10.1093/hmg/11.13.1517. [DOI] [PubMed] [Google Scholar]
- Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, Shoukas A, Romer LH, Berkowitz DE. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006;99:951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, et al. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008;102:923–932. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Aurich MK, Jonsson JJ, Thiele I. Membrane transporters in a human genome-scale metabolic knowledgebase and their implications for disease. Front Physiol. 2014;5:91. doi: 10.3389/fphys.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lozada LG, Lanaspa MA, Cristobal-Garcia M, Garcia-Arroyo F, Soto V, Cruz-Robles D, Nakagawa T, Yu MA, Kang DH, Johnson RJ. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121:e71–e78. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides, Nucleotides Nucleic Acids. 2008;27:608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada N, Jiang A, Takizawa F, Safdar A, Manika A, Tesmenitsky Y, Kang KT, Bischoff J, Kalwa H, Sartoretto JL, et al. Endothelial PGC-1alpha mediates vascular dysfunction in diabetes. Cell Metab. 2014;19:246–258. doi: 10.1016/j.cmet.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoors S, Cantelmo AR, Georgiadou M, Stapor PC, Wang X, Wong BW, Bifari F, Quaegebeur A, Decimo I, Schoonjans L, et al. Incomplete and transitory decrease of glycolysis: a new paradigm for anti-angiogenic therapy? Cell cycle. 2014a;13:16–22. doi: 10.4161/cc.27519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquiere B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014b;19:37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Sena CM, Matafome P, Crisostomo J, Rodrigues L, Fernandes R, Pereira P, Seica RM. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol Res. 2012;65:497–506. doi: 10.1016/j.phrs.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat Rev. 2012;12:699–709. doi: 10.1038/nrc3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa WC, Hecker M, Mitchell JA, Vane JR. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: L-glutamine inhibits the generation of L-arginine by cultured endothelial cells. Proc Natl Acad Sci USA. 1990;87:8607–8611. doi: 10.1073/pnas.87.21.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, Moon EJ, Dhup S, Danhier P, Frerart F, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE. 2012;7:e33418. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolarics Z, Spitzer JJ. Augmented glucose use and pentose cycle activity in hepatic endothelial cells after in vivo endotoxemia. Hepatology. 1993;17:615–620. doi: 10.1002/hep.1840170415. [DOI] [PubMed] [Google Scholar]
- Spolarics Z, Wu JX. Role of glutathione and catalase in H2O2 detoxification in LPS-activated hepatic endothelial and Kupffer cells. Am J Physiol. 1997;273:G1304–G1311. doi: 10.1152/ajpgi.1997.273.6.G1304. [DOI] [PubMed] [Google Scholar]
- Stobart JL, Lu L, Anderson HD, Mori H, Anderson CM. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2013;110:3149–3154. doi: 10.1073/pnas.1215929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroes E, Hijmering M, van Zandvoort M, Wever R, Rabelink TJ, van Faassen EE. Origin of superoxide production by endothelial nitric oxide synthase. FEBS Lett. 1998;438:161–164. doi: 10.1016/s0014-5793(98)01292-7. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Levy BD, Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J Biol Chem. 2009;284:12691–12700. doi: 10.1074/jbc.M809295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- Tammali R, Reddy AB, Srivastava SK, Ramana KV. Inhibition of aldose reductase prevents angiogenesis in vitro and in vivo. Angiogenesis. 2011;14:209–221. doi: 10.1007/s10456-011-9206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, Kuo L. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol. 2006;26:2035–2042. doi: 10.1161/01.ATV.0000233334.24805.62. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. The glyoxalase system in health and disease. Mol Aspects Med. 1993;14:287–371. doi: 10.1016/0098-2997(93)90002-u. [DOI] [PubMed] [Google Scholar]
- Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- Topal G, Brunet A, Millanvoye E, Boucher JL, Rendu F, Devynck MA, David-Dufilho M. Homocysteine induces oxidative stress by uncoupling of NO synthase activity through reduction of tetrahydrobiopterin. Free Radical Biol Med. 2004;36:1532–1541. doi: 10.1016/j.freeradbiomed.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:260–266. doi: 10.1164/rccm.201108-1536PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E, Lederer B, Bartrons R, Hers HG. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982;129:191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Vedantham S, Noh H, Ananthakrishnan R, Son N, Hallam K, Hu Y, Yu S, Shen X, Rosario R, Lu Y, et al. Human aldose reductase expression accelerates atherosclerosis in diabetic apolipoprotein E-/- mice. Arterioscler Thromb Vasc Biol. 2011;31:1805–1813. doi: 10.1161/ATVBAHA.111.226902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- Verhaar MC, Stroes E, Rabelink TJ. Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:6–13. doi: 10.1161/hq0102.102190. [DOI] [PubMed] [Google Scholar]
- Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32(Suppl 2):S314–S321. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizan P, Sanchez-Tena S, Alcarraz-Vizan G, Soler M, Messeguer R, Pujol MD, Lee WN, Cascante M. Characterization of the metabolic changes underlying growth factor angiogenic activation: identification of new potential therapeutic targets. Carcinogenesis. 2009;30:946–952. doi: 10.1093/carcin/bgp083. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Fuh H, Donnelly T, Cybulsky M. Advanced glycation endproducts promote adhesion molecule (VCAM-1, ICAM-1) expression and atheroma formation in normal rabbits. Mol Med. 1995;1:447–456. [PMC free article] [PubMed] [Google Scholar]
- Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Varani J, Johnson KJ, Roeser NF, Dame MK, Davis JA, Venkatachalam MA. Protection of human umbilical vein endothelial cells by glycine and structurally similar amino acids against calcium and hydrogen peroxide-induced lethal cell injury. Am J Pathol. 1992;140:457–471. [PMC free article] [PubMed] [Google Scholar]
- Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123:3190–3200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetzel AM, Sturek JM, Nagelin MH, Bolick DT, Gebre AK, Parks JS, Bruce AC, Skaflen MD, Hedrick CC. ABCG1 deficiency in mice promotes endothelial activation and monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2010;30:809–817. doi: 10.1161/ATVBAHA.109.199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Meininger CJ. Impaired arginine metabolism and NO synthesis in coronary endothelial cells of the spontaneously diabetic BB rat. Am J Physiol. 1995;269:H1312–H1318. doi: 10.1152/ajpheart.1995.269.4.H1312. [DOI] [PubMed] [Google Scholar]
- Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Dang Y, Ren YR, Liu JO. Cholesterol trafficking is required for mTOR activation in endothelial cells. Proc Natl Acad Sci USA. 2010;107:4764–4769. doi: 10.1073/pnas.0910872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Erzurum SC. Endothelial cell energy metabolism, proliferation, and apoptosis in pulmonary hypertension. Compr Physiol. 2011;1:357–372. doi: 10.1002/cphy.c090005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, An X, Guo X, Habtetsion TG, Wang Y, Xu X, Kandala S, Li Q, Li H, Zhang C, et al. Endothelial 6-Phosphofructo-2-Kinase/Fructose-2, 6-Bisphosphatase, Isoform 3 Plays a Critical Role in Angiogenesis. Arterioscler Thromb Vasc Biol. 2014;34:1231–1239. doi: 10.1161/ATVBAHA.113.303041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav UC, Srivastava SK, Ramana KV. Prevention of VEGF-induced growth and tube formation in human retinal endothelial cells by aldose reductase inhibition. J Diabetes Complications. 2012;26:369–377. doi: 10.1016/j.jdiacomp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashina S, Konno A, Wheeler MD, Rusyn I, Rusyn EV, Cox AD, Thurman RG. Endothelial cells contain a glycine-gated chloride channel. Nutr Cancer. 2001;40:197–204. doi: 10.1207/S15327914NC402_17. [DOI] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285–293. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bailey L, Clarke R, Flanders WD, Liu T, Yesupriya A, Khoury MJ, Friedman JM. Prospective study of methylenetetrahydrofolate reductase (MTHFR) variant C677T and risk of all-cause and cardiovascular disease mortality among 6000 US adults. Am J Clin Nutr. 2012;95:1245–1253. doi: 10.3945/ajcn.111.022384. [DOI] [PubMed] [Google Scholar]
- Yeh WL, Lin CJ, Fu WM. Enhancement of glucose transporter expression of brain endothelial cells by vascular endothelial growth factor derived from glioma exposed to hypoxia. Mol Pharmacol. 2008;73:170–177. doi: 10.1124/mol.107.038851. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Wei D, Nomura W, Izawa S, Inoue Y. Reduction of glucose uptake through inhibition of hexose transporters and enhancement of their endocytosis by methylglyoxal in Saccharomyces cerevisiae. J Biol Chem. 2012;287:701–711. doi: 10.1074/jbc.M111.322222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Liao H, Liu Y, Lee TS, Zhu M, Wang X, Stemerman MB, Zhu Y, Shyy JY. Sterol-responsive element-binding protein (SREBP) 2 down-regulates ATP-binding cassette transporter A1 in vascular endothelial cells: a novel role of SREBP in regulating cholesterol metabolism. J Biol Chem. 2004;279:48801–48807. doi: 10.1074/jbc.M407817200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yang Z, Zhu B, Hu J, Liew CW, Zhang Y, Leopold JA, Handy DE, Loscalzo J, Stanton RC. Increasing glucose 6-phosphate dehydrogenase activity restores redox balance in vascular endothelial cells exposed to high glucose. PLoS One. 2012;7:e49128. doi: 10.1371/journal.pone.0049128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hong Q, Huang Z, Xue P, Lv Y, Fu B, Chen X, Wu D. ALDR enhanced endothelial injury in hyperuricemia screened using SILAC. Cell Physiol Biochem. 2014;33:479–490. doi: 10.1159/000358628. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Peng J, Lu C, Hsin M, Mura M, Wu L, Chu L, Zamel R, Machuca T, Waddell T, et al. Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS ONE. 2014;9:e88727. doi: 10.1371/journal.pone.0088727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharikov S, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, Patel J. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295:C1183–C1190. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]