Abstract
Heterochromatin protein 1 (HP1) proteins are chromatin-bound transcriptional regulators. While their chromodomain binds histone H3 methylated on lysine 9, their chromoshadow domain associates with the H3 histone fold in a region involved in chromatin remodeling. Here, we show that phosphorylation at histone H3 threonine 45 and serine 57 within this latter region differentially affects binding of the three mammalian HP1 isoforms HP1α, HP1β and HP1γ. Both phosphorylation events are dependent on the activity of the DYRK1A kinase that antagonizes HP1-mediated transcriptional repression and participates in abnormal activation of cytokine genes in Down’s syndrome-associated megakaryoblastic leukemia.
Subject Categories: Chromatin, Epigenetics, Genomics & Functional Genomics; Transcription; Molecular Biology of Disease
Keywords: chromatin silencing, cytokine, epigenetic, histone code, inflammation
Introduction
HP1 proteins (HP1α, HP1β, and HP1γ in mammals) are archetypal examples of transcriptional regulators functioning in both stable ‘silencing’ and short-term repression of inducible genes involved in development, cell differentiation, cell cycle, and immune response (see for example 1–4).
Each HP1 isoform contains two structured regions—the chromo and chromoshadow domains (CD and CSD, respectively). The CD associates with the tail of histone H3 methylated on lysine 9 (H3K9), and with the tail of histone H1.4 methylated on lysine 26. The CSD is a dimerization domain and an interaction surface for numerous molecular partners of HP1, most of which contain a PXVXL motif (for a recent review, see 5).
The CSD of HP1α also interact with histone H3 within the first helix of its histone fold, a region we refer to as the ShaDock 6–8. This region contains a variant of the PXVXL motif (PXXVXL) required for the HP1α binding 6. In our hands, the ShaDock also associates with the more euchromatic HP1γ isoform and with the SWI/SNF catalytic subunit Brg1, via overlapping but non-identical sequences 6.
The binding of proteins to the ShaDock is a regulated mechanism. In particular, the ATPase activity of Brg1 facilitates HP1 binding, and HP1 proteins use this property to detect and arrest chromatin remodeling 6. Inversely, binding of HP1α to the ShaDock is disrupted by phosphorylation at H3Y41 by the kinase JAK2 8.
In the present study, we have investigated whether further phosphorylation events within the ShaDock would differentially affect the binding of the three HP1 isoforms and Brg1. We find that phosphorylation at H3T45 and at H3S57 by the chromosome 21-encoded kinase DYRK1A (for a review, see 9) antagonizes HP1 chromatin binding and its subsequent transcriptional repression at a series of genes involved in stress response.
Results and Discussion
H3 ShaDock phosphorylation differentially affects binding of HP1 proteins
We first reexamined the affinity for the H3 ShaDock of HP1α, HP1β, HP1γ, and a region of Brg1 located between the helicase-like domain and the bromodomain of this SWI/SNF catalytic subunit (ΔBrg1 – aa 1223–1420) 6. When expressed as GST fusions, these proteins associated with full-length recombinant histone H3, confirming the interaction of Brg1 with the histone and indicating that all three HP1 isoforms bind H3 independently of the H3K9me3 modification (Fig 1A,B).
Figure 1. Phospho-mimic mutations in the histone H3 ShaDock modifies binding of HP1 isoforms.
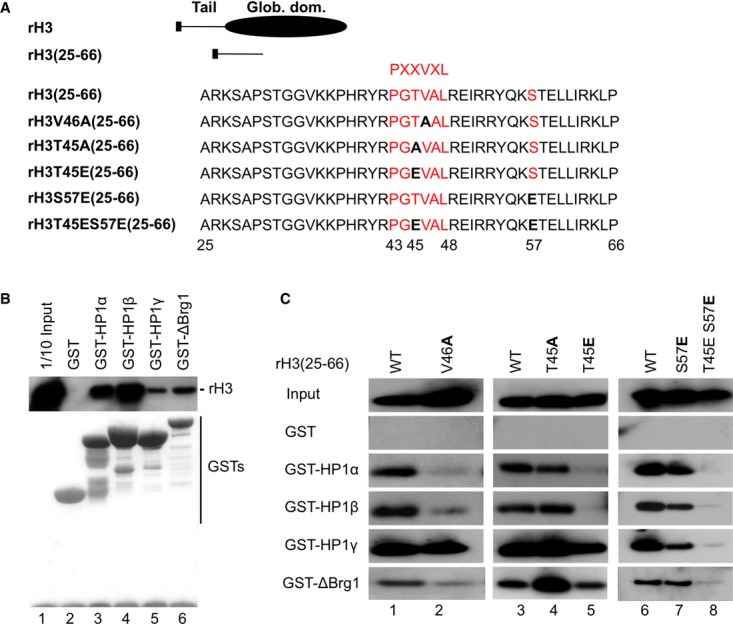
A Diagram of the B10-tagged recombinant histone H3 (rH3) and the rH3(25-66) peptide.
B Indicated GST fusions bound to agarose beads were incubated with rH3 then washed. Western blotting with anti-B10 antibody revealed retained proteins. The bottom panel shows the GST fusion proteins stained with Ponceau S.
C GST fusions bound to agarose beads were incubated with the indicated rH3(25-66) peptides and washed. As in (B), retained peptides were revealed with anti-B10 antibody.
Source data are available online for this figure.
Binding of HP1α, HP1β, and ΔBrg1 to a histone H3 fragment spanning from aa 25 to 66 (rH3(25-66)) was sensitive to a V46A mutation within the PXXVXL motif of the H3 ShaDock, while binding of HP1γ was unaffected (Fig 1A,C, lanes 1 and 2). The effect of this mutation on the interaction of Brg1 illustrates the overlap of its binding site with that of the HP1 proteins and explains in part why SWI/SNF and HP1 proteins cannot be recruited simultaneously to histone H3 6. We also note that earlier studies used H3 fragments shorter than the rH3(25-66) in their interaction experiments, possibly explaining why only interaction of H3 with HP1α was detected then 8.
We next used the rH3(25-66) fragment to test the impact of phospho-mimic mutations on the binding of the HP1 isoforms and ΔBrg1. A H3T45E mutation inside the PXXVXL motif (PXT(45)VXL) abolished binding of HP1α and HP1β, while binding of HP1γ and ΔBrg1 was moderately decreased (Fig 1A,C, lane 5). Under the same conditions, a H3T45A mutation had no effect on the binding of the HP1 proteins, while it seemed to strengthen that of ΔBrg1 (Fig 1A,C, lane 4).
We also examine H3S57 within a KS(57)T sequence identical to that present downstream of H3K9 (K(9)ST). A H3S57E phospho-mimic mutation reduced HP1γ binding to an intermediate level, while a combined H3S57E/H3T45E mutation had a much stronger affect and also reduced binding of ΔBrg1 (Fig 1A,C, lanes 7 and 8).
These data suggested that H3T45 phosphorylation controls binding of the HP1α and HP1β isoforms, while dual H3T45/H3S57 phosphorylation might be required to efficiently interfere with the binding of the more euchromatic HP1γ and Brg1.
H3T45 and H3S57 are phosphorylated by DYRK1A
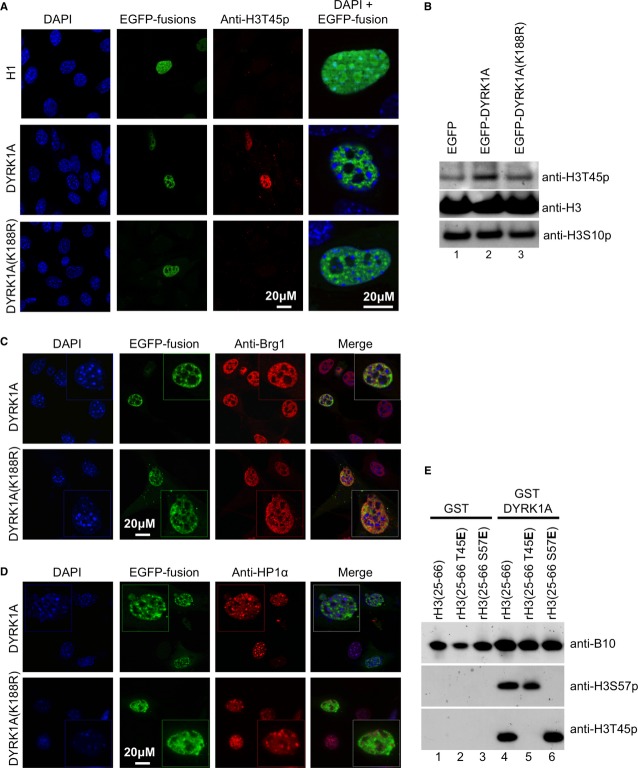
In an earlier study, the kinase DYRK1A was shown to phosphorylate H3T45 in vitro 10. Forced expression of an EGFP-DYRK1A fusion protein in NIH3T3 mouse fibroblasts similarly caused H3T45 phosphorylation, indicating that DYRK1A can also phosphorylate H3 at this position in vivo. The nuclear signal was widespread and co-distributed with EGFP-DYRK1A (Fig 2A). No H3T45p signal was observed when using EGFP-H1 or a mutant DYRK1A with reduced catalytic activity (EGFP-DYRK1A(K188R)). An increased level of H3T45p was also observed when extracts from wild-type DYRK1A-expressing cells were analyzed by Western blots (Fig 2B).
Figure 2. DYRK1A phosphorylates histone H3.
A NIH3T3 cells were transfected with expression vectors for either EGFP-histone H1, EGFP-DYRK1A, or mutant EGFP-DYRK1A(K188R) fusion proteins. After 48h, cells were fixed and indirectly labeled with anti-H3T45p antibody. DNA was stained with DAPI.
B Total protein extracts from NIH3T3 cells transfected with the indicated expression vectors were immunoblotted with indicated antibodies.
C, D NIH3T3 cells were transfected with expression vectors for either EGFP-DYRK1A or mutant EGFP-DYRK1A(K188R) fusion proteins as in (A). After 48 h, cells were fixed and indirectly labeled with anti-Brg1 (C) or anti-HP1α (D) antibody. DNA was stained with DAPI. In each panel, inset shows an EGFP-positive nucleus magnified 2-fold.
E WT or indicated mutant rH3(25–66) peptides were incubated with either purified GST or GST-DYRK1A fusion proteins and detected with the indicated antibodies.
Source data are available online for this figure.
Interestingly, EGFP-DYRK1A labeling, in addition to being excluded from nucleoli, was absent from foci of dense pericentromeric heterochromatin. In that sense, it showed a distribution similar to that of Brg1, while it was excluded from regions enriched in HP1α (Fig 2C,D – Note also that a diffuse HP1α signal is present outside the foci of dense pericentromeric heterochromatin). The sub-nuclear localization of DYRK1A was independent of its kinase activity.
To investigate whether DYRK1A was also involved in phosphorylation of H3S57, we generated an anti-H3S57p antibody (Supplementary Fig S1A). Using this reagent and the commercially available anti-H3T45p, we observed that recombinant GST-DYRK1A phosphorylated both H3T45 and H3S57 in vitro (Fig 2E, lane 4). The respective signals were abolished by H3T45E or H3S57E mutations, confirming the specificity of the antibodies (Fig 2E, lanes 5 and 6). The sequence surrounding H3S57 is very divergent from the R-P-X-S/T-P consensus DYRK1A phosphorylation sites, but variant phosphorylation sites have been described in other proteins 11,12. In this context, it is interesting that the MSK1 kinase down-stream of the MAP kinase pathway can similarly phosphorylate both H3T45 and H3S57 in vitro 13. Global increase in levels of H3S57p was observed in mitotic cells (identified by H3S10p labeling) but not upon EGFP-DYRK1A overexpression (Supplementary Fig S1B–C). We here speculate that the intra-nucleosomal position of H3S57 may limit its accessibility for DYRK1A in interphase.
DYRK1A shares target genes with HP1 proteins
The exclusion of DYRK1A from silent pericentromeric heterochromatin and the negative effect H3T45 and H3S57 phospho-mimics on HP1 binding to histone H3 in vitro prompted us to explore the global impact of DYRK1A on gene expression. We created DYRK1A-depleted HeLa cells using siRNAs and analyzed the effect on the transcriptome with exon arrays. On these arrays, a total of 1068 genes were down-regulated more than 1.5-fold (P < 0.05), while only 28 genes were up-regulated (Supplementary Fig S2A). These data that were validated by RT-qPCR on a large series of genes with two different DYRK1A siRNAs (Supplementary Table S1) strongly suggested that DYRK1A predominantly functions as a transcriptional activator or an anti-repressor.
Several genes up-regulated by DYRK1A depletion were targets of transcription factors negatively regulated by DYRK1A-mediated phosphorylation 14–16, including the nuclear factor of activated T cells (NFAT) targets EGR2 17 and ID2 18, and the p53 target BTG2 19. Genes down-regulated by DYRK1A depletion were largely inducible genes involved in cellular responses to stimuli and immune system processes (Supplementary Fig S2B). In particular, DYRK1A affected the expression of several components of the MAPK signaling pathway (Supplementary Fig S2C). Another important series of genes was directly regulated by the transcription factor NF-κB (Supplementary Table S2). Along the same lines, DYRK1A appeared to be a positive regulator of genes involved in ubiquitination, a process essential for degradation of I-κB and the subsequent activation of NF-κB (Supplementary Fig S2D). Altogether, these observations suggested that DYRK1A might have a major function in the response to extracellular stressors.
To investigate a potential antagonism between DYRK1A and HP1 proteins in transcriptional regulation, we focused on a series of NF-κB-regulated genes including IL8, TNFα, IL6, IL1α, IFI44, TNFAIP6, and IL7R. These genes were either previously described as recruiting HP1 proteins to their promoters 1,4,6,20,21 or appeared in an earlier array analysis as regulated by HP1γ 22. RT-qPCR data revealed negative regulation of these genes upon DYRK1A depletion, confirming the array data. In these experiments, TGFβ gene expression remained unchanged and was chosen as a negative control gene for the rest of our study (Fig 3A,B).
Figure 3. Antagonistic effects of DYRK1A and HP1 proteins on inducible genes.
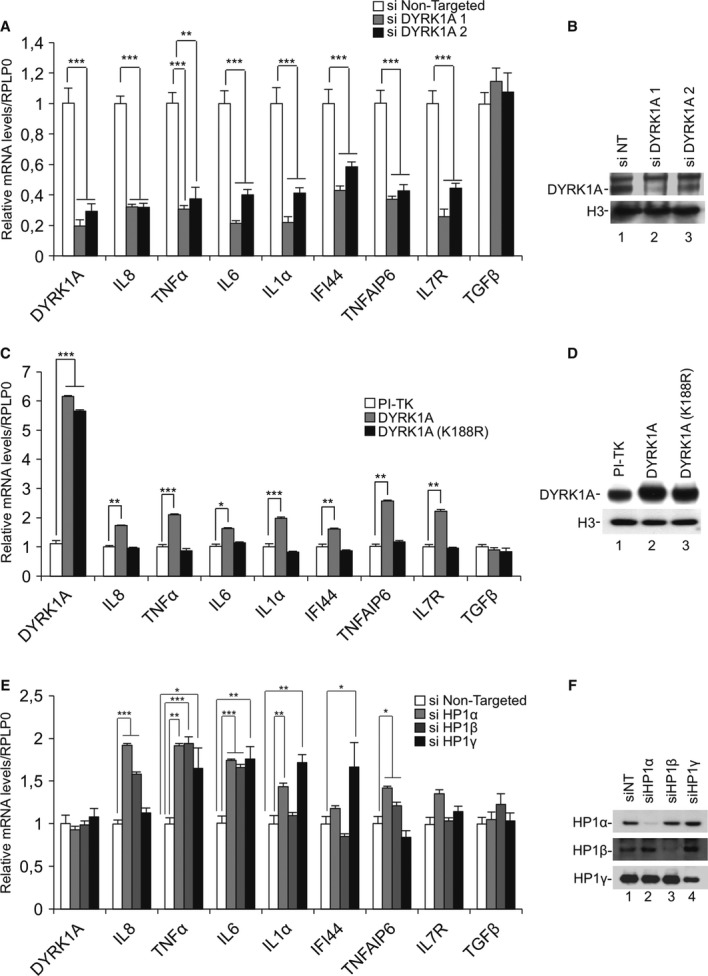
A HeLa cells were transfected with the indicatedsiRNAs. After 48 h, levels of mRNA for the indicated genes were measured by RT-qPCR.
B Total protein extracts from a representative experimentwere immunoblotted with indicated antibodies.
C Expression of WT DYRK1A or mutant DYRK1A(K188R) was induced by ponasterone in engineered HEK293 cells. After 48 h, levels of mRNA for the indicated genes were measured by RT-qPCR.
D Total protein extracts from a representative experimentwere immunoblotted with indicated antibodies.
E HeLa cells were transfected with the indicatedsiRNAs. After 48 h, levels of mRNA for the indicated genes were measured by RT-qPCR.
F Total protein extracts from a representative experimentwere immunoblotted with indicated antibodies. Data in histograms are presented relative to levels of Ribosomal Protein, Large, P0 (RPLP0) mRNA considered as invariant in the experimental conditions.
Data information: Data are means ± s.e.m. from three independent experiments. Significance of the differences was estimated using Student’s t-test. ***P < 0.001, 0.001 < **P < 0.01, 0.01 < *P < 0.05.
We also conducted the reverse experiment using a HEK293-derived cell line harboring inducible expression constructs for either WT DYRK1A or the DYRK1A(K188R) mutant with reduced kinase activity. Consistent with the depletion experiments, overexpression of WT DYRK1A but not DYRK1A(K188R) resulted in activation of these NF-κB-dependent genes (Fig 3C,D). This experiment also established that DYRK1A-mediated transcriptional activation is dependent on its kinase activity.
Finally, transcription of each investigated gene was induced upon depletion of at least one HP1 family member (Fig 3E,F). TGFβ expression remained unchanged under these conditions.
These observations show that DYRK1A and HP1 proteins share several target genes involved in the NF-κB response and have opposite effects on their expression.
DYRK1A phosphorylates histone H3 at inducible gene promoters and interferes with HP1 recruitment
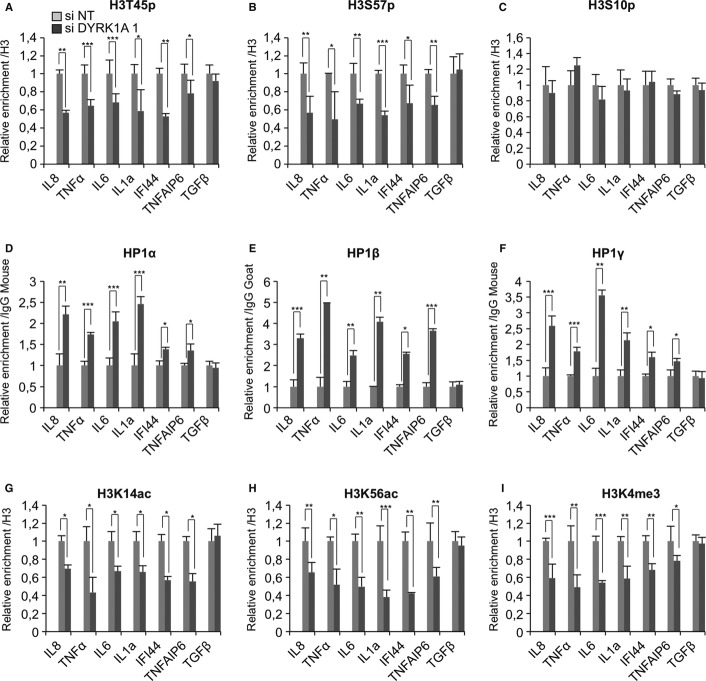
We investigated whether the impact of DYRK1A on the expression of the NF-κB-regulated genes was directly linked to histone H3 phosphorylation and decreased HP1 recruitment. Chromatin immunoprecipitation (ChIP) experiments with anti-DYRK1A antibodies established the presence of this protein at the promoters of IL8, TNFα, IL6, IL1α, IFI44, and TNFAIP6, but not TGFβ (Supplementary Fig S3A). At these promoters, DYRK1A recruitment was reduced upon its depletion with siRNAs, verifying the specificity of the ChIP assay. Reduced DYRK1A levels also resulted in decreased levels of H3T45p, confirming that this kinase phosphorylates histone H3 at this position in vivo (Fig 4A). Levels of H3S57p were also reduced, while levels of phospho-H3S10 (H3S10p) used as a control remained essentially unaffected (Fig 4B,C). Together with the in vitro data, these observations strongly suggest that H3S57 is a substrate of DYRK1A.
Figure 4. Depletion of DYRK1A antagonizes open chromatin marks at inducible genes.
A–I HeLa cells were transfected with indicated siRNAs. Accumulation of the indicated proteins or histone modifications at the promoter of the listed genes were evaluated by ChIP analysis followed by qPCR. Data are normalized to the values obtained with either non-immune IgGs or anti-histone H3 antibodies as indicated. Data shown are means ± s.e.m. from three independent experiments. In each panel, significance of the differences was estimated using Student’s t-test. ***P < 0.001, 0.001 < **P < 0.01, 0.01 < *P < 0.05.
Importantly, upon DYRK1A depletion, we detected a systematic increase in recruitment of the three HP1 proteins to the investigated promoters (Fig 4D–F). These observations corroborated the in vitro HP1-H3 interaction data and provided further evidence for the negative effect of DYRK1A kinase activity on HP1 chromatin binding. They also bring the first link between phosphorylation at H3T45 and H3S57 and transcriptional activity.
To further characterize the impact of DYRK1A on the local chromatin structure, we examined several additional histone modifications after depletion of this kinase. At all promoters except that of TGFβ, we observed decreased levels of histone acetylation as ascertained by ChIP with a pan anti-acetyl histone antibody (Supplementary Fig S3B). Decreased acetylation was more specifically detected at H3K14 and H3K56 (Fig 4G,H). Acetylation at this latter position is associated with transcriptional activation in both yeast 23–25 and mammalian cells 26–28 and possibly linked to H3S57 phosphorylation during transcriptional elongation 29.
Levels of H3K4 trimethylation, another marker of active chromatin, were also reduced (Fig. 4I). In contrast, levels of the repressive H3K9 tri-methylation (H3K9me3) mark were mostly unchanged and, in one case, up-regulated (Supplementary Fig S3C). This was interesting because it suggested the hypothesis that neutralization of the ShaDock binding activity of the HP1 proteins impacts their H3K9me-binding activity. This is consistent with a recent study showing that the CSD of HP1α participate in recognition of H3K9me 30. Finally, we observed that depletion of DYRK1A resulted in reduced levels of Brg1 at the examined promoters, further illustrating a closing of the chromatin structure in the absence of the kinase (Supplementary Fig S3D). This observation also suggests that the binding of Brg1 to the ShaDock is only accessory for its recruitment to promoters.
Anti-inflammatory effect of DYRK1A inhibition in leukemia cells
Our expression data strongly suggested a role for DYRK1A in the regulation of immune genes. Consistent with this, we found DYRK1A mRNA to be abundant in thymus, peripheral blood mononuclear cells (PBMCs), T-cell-derived Jurkat cells, and promyelocytic leukemia HL60 cells (Fig 5A). Online resources (Gene Enrichment Profiler) further substantiated this enrichment of DYRK1A in the immune system 31.
Figure 5. DYRK1A in leukemia cells.
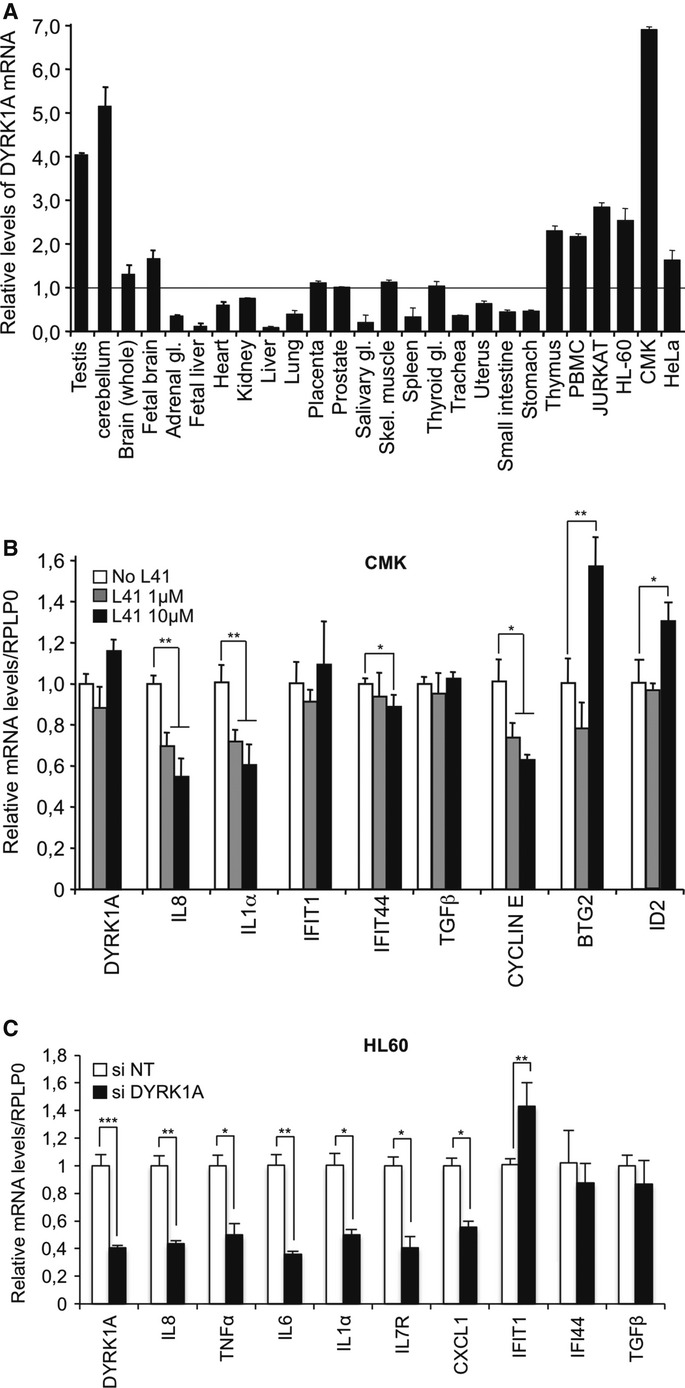
A Total RNA from 22 human tissues and the indicated cell lines were assayed by RT-qPCR for levels of DYRK1A mRNA. Values were normalized to levels 18S RNA in each sample. Data shown are means ± s.e.m. from two independent experiments. The level of mRNA detected in prostate was similar to the mean of all levels and was chosen as a reference (set to 1).
B CMK cells were cultured in the presence of 0, 1, or 10 μM DYRK1A inhibitor L41. After 72 h, levels of mRNA for the indicated genes were measured by RT-qPCR. Data shown are means ± s.e.m. from three independent experiments. Significance of the differences was estimated using Student’s t-test (***P < 0.001, 0.001 < **P < 0.01, 0.01 < *P < 0.05).
C HL-60 cells were transfected with indicated siRNAs. After 48 h, levels of mRNA for the indicated genes were measured by RT-qPCR. Data shown are means ± s.e.m. from two independent experiments. Significance was estimated as in (B).
Children with Down’s syndrome (DS) are at increased risk of developing acute megakaryoblastic leukemia, a hematological disorder frequently associated with pro-inflammatory cytokinemia 32. An earlier study showed that DYRK1A modulates megakaryoblastic expansion via phosphorylation of NFAT 33. To investigate whether DYRK1A also plays a role in the cytokinemia, we tested the effect of the specific DYRK1A inhibitor L41 34 on the megakaryoblastic cell line CMK. These experiments revealed a dose-dependent decrease of IL8 and IL1α expression in the presence of the inhibitor (Fig 5B). Other cytokines regulated by DYRK1A in HeLa cells (see above) were not expressed at detectable levels in the CMK cells. Treatment with L41 also reduced expression of cyclin E, a regulator of the G1-S transition during the cell cycle. In contrast, L41 had no clear effect on TGFβ and interferon-inducible genes, while it caused activation of two genes negatively regulated by DYRK1A (BTG2 and ID2). The CMK cells were refractory to siRNA-mediated knockdown of DYRK1A. We therefore verified the impact of DYRK1A activity on IL8 and IL1α in HL60 cells. In these experiments, IL8 and IL1α, and also TNFα, IL6, IL7R, and CXCL1 showed reduced expression upon depletion of DYRK1A, while, as in CMK cells, interferon-inducible genes and TGFβ were unchanged or moderately induced (Fig 5C). These observations suggest that targeting DYRK1A may allow reducing autocrine inflammatory signaling in myeloid leukemias.
Conclusions
In the present study, we show that phosphorylation at H3T45 and H3S57 is linked to reduced binding of HP1 proteins to chromatin and we suggest that phosphorylation of these residues has influence on the competition between HP1 proteins and Brg1 for association with the H3 ShaDock. This domain seems to function as a hub for protein interactions regulated by a very sophisticated code of histone modifications independently of H3K9 methylation.
While phosphorylation of the ShaDock may involve several kinases, including PKCδ 35 or MSK1 13, we showed that DYRK1A, mostly studied for its role in DS, positively regulates a number of immune gene promoters subject to HP1-mediated transcriptional repression. In fact, we found that DYRK1A positively regulates a very large number of inducible genes at which it may be required for maintenance of basal-level transcription. Thus, a major role of DYRK1A may be to counteract HP1-mediated repression and prevent silencing (see Model in Supplementary Fig S3E). Very generally, we suggest that DYRK1A is part of the machinery sustaining permanently open chromatin on rapidly induced genes.
Materials and Methods
Antibodies and chemicals
Anti-H3S57p antibody was produced in rabbits using a KLH-coupled peptide with the following sequence: AcNH-YQKpSTELLIRKLC-CONH2. Anti-DYRK1A (Sigma ref: WH0001859M1), anti-H3T45p (ab26127), anti-H3 (ab1791), anti-H3Y41p (ab26310), anti-H3K9me3 (ab8898), anti-HP1α (ChIP: 1H5; immunoblots: 2G9), anti-HP1β (ChIP: ab40828; immunoblots: 1G9), HP1γ (ChIP: 42s2; immunoblots: 1G6), and anti-Brg1 (2E12) were used for Western blotting and ChIP experiments. Cellular phosphatases were inhibited by treatment with 20 nM Calyculin A (Calbiochem 208851).
Real-time PCR
Levels of mRNA were quantified by real-time PCR (qPCR) after reverse transcription with 200 U of M-MLV reverse transcriptase according to the manufacturer’s recommendations (Invitrogen). Reactions were carried out with SYBR Green Kit Brilliant II reagents (Agilent) on an Mx3000 qPCR machine (Stratagene) according to manufacturer recommendations. Primers are listed in Supplementary Table S3. The fold change was estimated by an average of three independents experiments. Significance of the differences was estimated using Student’s t-test with a threshold at 5%.
Human exon array analysis
Labeled cDNA was synthesized from 200 ng total RNAs using NuGEN Applause WT-Amp Plus ST systems then hybridized to Affymetrix HuExon 1.0 ST GeneChips. Chips were scanned with an Affymetrix GeneChip Scanner 3000.
Accession codes
Exon array data can be accessed on GEO (code: GSE 43259).
Acknowledgments
We thank L Meijer, JP Bazureau, and F Carreaux for providing Leucettine L41, W Becker and F Ito for the gift of DYRK1A cDNA, E Allemand, E Batsché, S El Messaoudi-Aubert and O Mauger for valuable discussion. SMJ received fellowships from the Ministry of Research (MENRT), and Fondation ARC. Supporting grants included Fondation pour la Recherche Médicale (FRM), Fondation ARSEP, and REVIVE – Investissement d’Avenir.
Author contributions
Suk Min Jang and Saliha Azebi have carried out experiments and participated in the conception and the design of these experiments, their analysis, and their interpretation. They have also participated in the writing of the article. Guillaume Soubigou has carried out genome-wide exon array analysis and participated in the analysis of the generated data. Christian Muchardt participated in the conception and the design of the experiments, their analysis, and their interpretation. He has written the article, obtained the funding, and carries the overall responsibility for the data.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
Supplementary information for this article is available online: http://embor.embopress.org
References
- Sharma P, Azebi S, England P, Christensen T, Møller-Larsen A, Petersen T, Batsché E, Muchardt C. Citrullination of histone H3 interferes with HP1-mediated transcriptional repression. PLoS Genet. 2012;8:e1002934. doi: 10.1371/journal.pgen.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan RS, Zueva E, Cammas F, Schreiber HA, Masson V, Belz GT, Roche D, Maison C, Quivy J-P, Almouzni G, et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature. 2012;487:249–253. doi: 10.1038/nature11173. [DOI] [PubMed] [Google Scholar]
- Thorne JL, Ouboussad L, Lefevre PF. Heterochromatin protein 1 gamma and IκB kinase alpha interdependence during tumour necrosis factor gene transcription elongation in activated macrophages. Nucleic Acids Res. 2012;40:7676–7689. doi: 10.1093/nar/gks509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood A, Hon GC, Jin F, Henry RE, Espinosa JM, Ren B. CBX3 regulates efficient RNA processing genome-wide. Genome Res. 2012;22:1426–1436. doi: 10.1101/gr.124818.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg JC, Elgin SCR. HP1a: a structural chromosomal protein regulating transcription. Trends Genet. 2014;30:103–110. doi: 10.1016/j.tig.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne M, Eskeland R, Azebi S, Saint-André V, Jang SM, Batsché E, Fan H-Y, Kingston RE, Imhof A, Muchardt C. Interaction of HP1 and Brg1/Brm with the globular domain of histone H3 is required for HP1-mediated repression. PLoS Genet. 2009;5:e1000769. doi: 10.1371/journal.pgen.1000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richart AN, Brunner CIW, Stott K, Murzina NV, Thomas JO. Characterization of chromoshadow domain-mediated binding of heterochromatin protein 1α (HP1α) to histone H3. Journal of Biological Chemistry. 2012;287:18730–18737. doi: 10.1074/jbc.M111.337204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W. Recent insights into the function of DYRK1A. FEBS J. 2011;278:222. doi: 10.1111/j.1742-4658.2010.07953.x. [DOI] [PubMed] [Google Scholar]
- Himpel S, Tegge W, Frank R, Leder S, Joost HG, Becker W. Specificity determinants of substrate recognition by the protein kinase DYRK1A. J Biol Chem. 2000;275:2431–2438. doi: 10.1074/jbc.275.4.2431. [DOI] [PubMed] [Google Scholar]
- de Graaf K, Czajkowska H, Rottmann S, Packman LC, Lilischkis R, Lüscher B, Becker W. The protein kinase DYRK1A phosphorylates the splicing factor SF3b1/SAP155 at Thr434, a novel in vivo phosphorylation site. BMC Biochem. 2006;7:7. doi: 10.1186/1471-2091-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurat AV, Dietrich AD. Phosphorylation of Ser640 in muscle glycogen synthase by DYRK family protein kinases. J Biol Chem. 2004;279:2490–2498. doi: 10.1074/jbc.M301769200. [DOI] [PubMed] [Google Scholar]
- Winter S, Simboeck E, Fischle W, Zupkovitz G, Dohnal I, Mechtler K, Ammerer G, Seiser C. 14-3-3 proteins recognize a histone code at histone H3 and are required for transcriptional activation. EMBO J. 2008;27:88–99. doi: 10.1038/sj.emboj.7601954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Oh Y, Yoo L, Jung M-S, Song W-J, Lee S-H, Seo H, Chung KC. Dyrk1A phosphorylates p53 and inhibits proliferation of embryonic neuronal cells. Journal of Biological Chemistry. 2010;285:31895–31906. doi: 10.1074/jbc.M110.147520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arron JR, Winslow MM, Polleri A, Chang C-P, Wu H, Gao X, Neilson JR, Chen L, Heit JJ, Kim SK, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- da Costa Martins PA, Salic K, Gladka MM, Armand A-S, Leptidis S, el Azzouzi H, Hansen A, Coenen-de Roo CJ, Bierhuizen MF, van der Nagel R, et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol. 2010;12:1220–1227. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- Rengarajan J, Mittelstadt PR, Mages HW, Gerth AJ, Kroczek RA, Ashwell JD, Glimcher LH. Sequential involvement of NFAT and Egr transcription factors in FasL regulation. Immunity. 2000;12:293–300. doi: 10.1016/s1074-7613(00)80182-x. [DOI] [PubMed] [Google Scholar]
- Egusa H. Doi M, Saeki M, Fukuyasu S, Akashi Y, Yokota Y, Yatani H, Kamisaki Y The small molecule harmine regulates NFATc1 and Id2 expression in osteoclast progenitor cells. Bone. 2011;49:264–274. doi: 10.1016/j.bone.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Rouault JP, Falette N, Guéhenneux F, Guillot C, Rimokh R, Wang Q, Berthet C, Moyret-Lalle C, Savatier P, Pain B, et al. Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nat Genet. 1996;14:482–486. doi: 10.1038/ng1296-482. [DOI] [PubMed] [Google Scholar]
- El Gazzar MA, El Mezayen R, Nicolls MR, Dreskin SC. Thymoquinone attenuates proinflammatory responses in lipopolysaccharide-activated mast cells by modulating NF-kappaB nuclear transactivation. Biochim Biophys Acta. 2007;1770:556–564. doi: 10.1016/j.bbagen.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Yoza BK, McCall CE. Facultative heterochromatin formation at the IL-1 beta promoter in LPS tolerance and sepsis. Cytokine. 2011;53:145–152. doi: 10.1016/j.cyto.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-André V, Batsché E, Rachez C, Muchardt C. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat Struct Mol Biol. 2011;18:337–344. doi: 10.1038/nsmb.1995. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhang K, Grunstein M. Acetylation in histone H3 globular domain regulates gene expression in yeast. Cell. 2005;121:375–385. doi: 10.1016/j.cell.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Schneider J, Bajwa P, Johnson FC, Bhaumik SR, Shilatifard A. Rtt109 is required for proper H3K56 acetylation: a chromatin mark associated with the elongating RNA polymerase II. J Biol Chem. 2006;281:37270–37274. doi: 10.1074/jbc.C600265200. [DOI] [PubMed] [Google Scholar]
- Williams SK, Truong D, Tyler JK. Acetylation in the globular core of histone H3 on lysine-56 promotes chromatin disassembly during transcriptional activation. Proc Natl Acad Sci USA. 2008;105:9000–9005. doi: 10.1073/pnas.0800057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459:113–117. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S, Kim S-J, Sandal B, Lee S-M, Gao B, Zhang DD, Fang D. The type III histone deacetylase Sirt1 protein suppresses p300-mediated histone H3 lysine 56 acetylation at Bclaf1 promoter to inhibit T cell activation. Journal of Biological Chemistry. 2011;286:16967–16975. doi: 10.1074/jbc.M111.218206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo KA, Bauchmann MK, Baumann AP, Donahue CJ, Thiede MA, Hayes LS, Etages des SAG. Fraenkel E. Genome-wide profiling of H3K56 acetylation and transcription factor binding sites in human adipocytes. PLoS ONE. 2011;6:e19778. doi: 10.1371/journal.pone.0019778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam A, Logie C. Histone H3 serine 57 and lysine 56 interplay in transcription elongation and recovery from S-phase stress. PLoS ONE. 2010;5:e10851. doi: 10.1371/journal.pone.0010851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y, Watanabe M, Kawakami T, Jayasinghe CD, Otani J, Kikugawa Y, Shirakawa M, Kimura H, Nishimura O, Aimoto S, et al. Hinge and Chromoshadow of HP1α Participate in Recognition of K9 Methylated Histone H3in Nucleosomes. Journal of Molecular Biology. 2013;425:54–70. doi: 10.1016/j.jmb.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Benita Y, Cao Z, Giallourakis C, Li C, Gardet A, Xavier RJ. Gene enrichment profiles reveal T-cell development, differentiation, and lineage-specific transcription factors including ZBTB25 as a novel NF-AT repressor. Blood. 2010;115:5376–5384. doi: 10.1182/blood-2010-01-263855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A, Hayashi Y, Ogasawara M, Park M-J, Katoh M, Minakami H, Kitoh T, Kojima S, Kawa K, Kimura H. Pro-inflammatory cytokinemia is frequently found in Down syndrome patients with hematological disorders. Leuk Res. 2007;31:1199–1203. doi: 10.1016/j.leukres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Malinge S, Bliss-Moreau M, Kirsammer G, Diebold L, Chlon T, Gurbuxani S, Crispino JD. Increased dosage of the chromosome 21 ortholog Dyrk1a promotes megakaryoblastic leukemia in a murine model of Down syndrome. J Clin Invest. 2012;122:948–962. doi: 10.1172/JCI60455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debdab M, Carreaux F, Renault S, Soundararajan M, Fedorov O, Filippakopoulos P, Lozach O, Babault L, Tahtouh T, Baratte B, et al. Leucettines, a class of potent inhibitors of cdc2-like kinases and dual specificity, tyrosine phosphorylation regulated kinases derived from the marine sponge leucettamine B: modulation of alternative pre-RNA splicing. J Med Chem. 2011;54:4172–4186. doi: 10.1021/jm200274d. [DOI] [PubMed] [Google Scholar]
- Hurd PJ, Bannister AJ, Halls K, Dawson MA, Vermeulen M, Olsen JV, Ismail H, Somers J, Mann M, Owen-Hughes T, et al. Phosphorylation of histone H3 Thr-45 is linked to apoptosis. J Biol Chem. 2009;284:16575–16583. doi: 10.1074/jbc.M109.005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.