Abstract
This brief review gives an overview of newer developments in 18F-chemistry with the focus on small 18F-labelled molecules as intermediates for modular build-up syntheses. The short half-life (<2 h) of the radionuclide requires efficient syntheses of these intermediates considering that multistep syntheses are often time consuming and characterized by a loss of yield in each reaction step. Recent examples of improved synthesis of 18F-labelled intermediates show new possibilities for no-carrier-added ring-fluorinated arenes, novel intermediates for tri[18F]fluoromethylation reactions, and 18F-fluorovinylation methods.
1. Introduction
The positron emitter fluorine-18 is a commonly used radionuclide in molecular imaging with positron emission tomography (PET) due to its advantageous nuclear properties. Thus, it finds wide application as noninvasive, quantitative, and versatile modality in medical diagnosis, research, and drug development [1]. Fluorine-18 has a short half-life of 109.7 min which only allows time-limited syntheses and study protocols. The methods for introducing this short-lived radionuclide into organic molecules thus require fast chemistry, and it is desirable to introduce the 18F-label during the last possible synthetic step.
A further aspect is the stoichiometry of 18F-chemistry that differs from “cold” fluorinations. The radionuclide is produced in low (nano- to picomolar) amounts and its concentration in reaction mixtures is several orders of magnitude lower than the precursor concentration. Furthermore, the syntheses of the radiotracers have to be performed in closed, lead-shielded hot cells, which necessitates an easily applicable and remote-controlled process. Thus, besides the development of more efficient and flexible 18F-labelling methods new technological approaches have been examined, especially in the field of microfluidic chemistry [2–10]. The development of a reliable 18F-labelling technique together with an automatic synthesis module is a major prerequisite of routine production of 18F-labelled PET radiopharmaceuticals [11–13].
Methods for the introduction of [18F]fluorine into organic molecules can be divided into two groups, namely, direct and indirect. The direct method entails introduction of [18F]fluorine without changing the carbon skeleton structure of the molecule. However, in many cases this necessitates the protection of functional groups or requires other transformations like reduction or oxidation of functional groups after introduction of radiofluorine [14].
The indirect method involves build-up syntheses, that is, changing the carbon skeleton structure and starting from small molecules which themselves can be easily 18F-fluorinated by nucleophilic substitution. Such small 18F-labelled alkyl [15] or aryl [16] groups bear typically reactive functional groups for further transformation reactions. Those intermediates are used to synthesize more complex biological molecules which cannot be labelled with fluorine-18 due to mechanistic reasons or are not stable enough to tolerate direct 18F-fluorination conditions.
In the case of 18F-labelling of macromolecules like peptides, proteins, and antibodies, these small 18F-labelled intermediates are commonly called “prosthetic groups.” In the last decade progress has been made regarding the 18F-labelling of macromolecules [17, 18]. Besides the use of prosthetic groups several alternative methods have also been introduced, capable of using even mild and aqueous conditions, for example, chelated aluminum [19–21], boron- [22], and/or silicon-based [18F]fluoride acceptor groups [23–26]. The latter methods were also used for the synthesis of small molecules [27].
This review focuses on new developments regarding the use of small 18F-labelled intermediates for build-up syntheses of biologically active compounds. The 18F-labelling of macromolecules and the click chemistry approach are not considered. Those special topics of 18F-labelling can be found in other contributions to this issue [28–30].
2. 18F-Fluorinating Agents
As starting material for all chemical syntheses either aqueous [18F]fluoride or gaseous [18F]F2 is used, both of which are generally produced at a cyclotron via the 18O(p,n)18F nuclear reaction [31]. The nucleophilic [18F]fluoride ion is available in no-carrier-added (n.c.a.) form which allows the synthesis of radiotracers with high specific activity. In contrast, in-target produced [18F]F2 is available only in carrier-added (c.a.) form which leads to radiotracers with low specific activity.
Historically, for important radiopharmaceuticals like 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG) and 6-[18F]fluoro-L-dopa only electrophilic 18F-fluorination was available. Today this method is rarely used because of the need of carrier for [18F]F2 production. Thus, the use of electrophilic 18F-fluorination is limited to nontoxic compounds as well as to those that can be applied with a low specific activity. Also, since [18F]F2 is very reactive and 18F-labelled side products are formed, less reactive electrophilic 18F-agents were developed [32]. More recently, the synthesis of N-[18F]fluorobenzenesulfonimide (NFSi) was described, which is a highly stable, reactive and selective electrophilic 18F-labelling agent and allows the synthesis of 18F-labelled allylic fluorides and α-fluorinated ketones from allylsilanes and silyl enol ethers, respectively [33].
An alternative method using a “posttarget” synthesis of [18F]F2 leads to moderate specific activity of up to 24.7 GBq/μmol, starting from n.c.a. [18F]fluoride [34]. It was recently revisited for the radiosynthesis of [18F]selectfluor bis(triflate), the 18F-labelled form of (1-chloromethyl-4-fluorodiazonia-bicyclo[2.2.2]-octane bis-(tetrafluoroborate)), an easy to handle and stable electrophilic fluorinating reagent (cf. Figure 1) [35]. This reagent could successfully be used for the silver(I)-mediated 18F-fluorination of electron-rich arylstannane models and intermediates, as well as for the preparation of 6-[18F]fluoro-L-DOPA [36], albeit all with limited specific activity of 3.7 ± 0.9 GBq/μmol.
Figure 1.
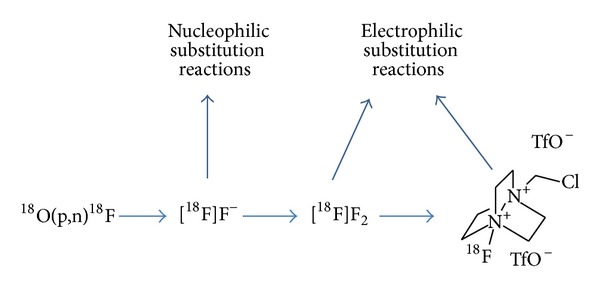
Nuclear reactions to produce fluorine-18 and the 18F-fluorinating agents [18F]fluoride, [18F]fluorine gas, and [18F]selectfluor bis(triflate).
3. Aliphatic Intermediates
Aliphatic 18F-fluorination is certainly the most prominent method for 18F-labelling [32], and important PET-radiotracers for clinical use are aliphatically 18F-labelled compounds which fulfill these requirements, for example, [18F]FDG, 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT), [18F]fluoro(m)ethylcholine, and O-2-[18F]fluoroethyl-L-tyrosine ([18F]FET) (cf. Figure 2) [15, 37, 38]. [18F]Fluoro(m)ethylcholine is an example for 18F-labelled endogenous compounds, whereas [18F]FDG and [18F]FLT are 18F-labelled deoxy derivatives of the corresponding endogenous substances. In all cases a proton is replaced by a fluorine atom without changing the carbon skeleton of the original compound. In contrast, [18F]FET is an example of an endogenous 18F-labelled compound where the introduction of the radionuclide is performed by an 18F-fluoroalkylation reaction. Here, the 18F-label is introduced into the molecule by addition of further C-atoms which means that the skeleton of the molecule is significantly changed. Other examples of this kind of reaction are the 18F-fluoroacylation and 18F-fluoroamidation reactions which are widely used for labelling of macromolecules [39], most often in aqueous solution.
Figure 2.
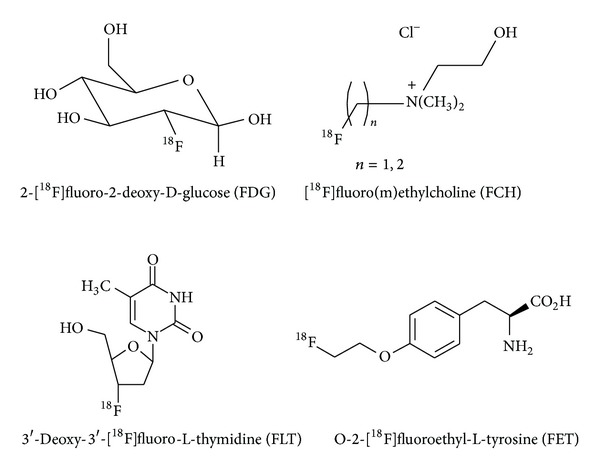
Important 18F-labelled radiotracers in clinical use.
3.1. Intermediates for Nucleophilic Substitution and Other Coupling Reactions
The synthesis of intermediates for 18F-fluoroalkylation is characterized by a two- or three-step procedure (cf. Figure 3) [40]. First, [18F]fluoride is introduced into a molecule using precursors containing a good leaving group. The 18F-labelled precursor is then isolated and purified before coupling with a further molecule.
Figure 3.
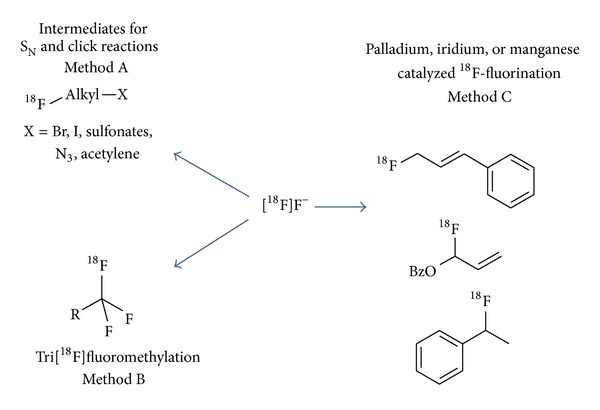
Pathways for aliphatic 18F-labelling intermediates starting from n.c.a. [18F]fluoride.
In the first step the [18F]fluoride has to be separated from the target water and activated for a nucleophilic substitution reaction. The standard conditions of these basic methods are described in several reviews [11, 32, 41]. A simplification of this approach was achieved by water removal on a strong anion-exchange resin [42] or by use of strong organic bases as additives replacing the inorganic bases or salts classically used in the resin eluent [43–46]. Instead of trapping on anion-exchange resins n.c.a. [18F]fluoride can also be separated by electrochemical methods which are useful to minimize the reaction volume especially for the use in microfluidic systems [47–51]. The use of mixtures of nonpolar tert-alcohols with acetonitrile as a reaction medium enhanced the reactivity of cesium[18F]fluoride or tetrabutylammonium [18F]fluoride and reduced the formation of typical by-products compared to those conventionally obtained only with dipolar aprotic solvents [52, 53].
Bromine and iodine and several sulfonate derivatives serve generally as leaving groups for a nucleophilic aliphatic radiofluorination [15, 40, 54, 55]. Alternatively, in the case of preparation of O-[18F]fluoromethylated aliphatic and aromatic ethers, the 1,2,3-triazolium triflate group serves as a very good nucleofuge for displacement by the [18F]fluoride ion [56].
The purification of 18F-fluorinating agents is performed by HPLC, solid phase extraction (SPE), or distillation. The main challenge is the complete separation of the 18F-labelled intermediate from the precursor which also would act as reaction partner in the following coupling step. This leads to unwanted side reactions which could lower the radiochemical yield (RCY) or necessitate a higher concentration of the precursor for the subsequent coupling reaction. A purification of the 18F-fluorinating agent via HPLC (or GC) is very effective and is often used [57–59], but it is more inconvenient for automatization [60, 61]. The use of SPE [62–64] or a distillation process for purification is principally easier to automate [40]. For instance, 1-bromo-3-(nitrobenzene-4-sulfonyloxy)-propane as starting precursor will be retained in the reaction vessel during the distillation process of 1-bromo-3-[18F]fluoropropane, due to its very high boiling point, thus eliminating the risk of formation of pseudocarrier [65]. In a few cases the direct coupling of the 18F-labelled intermediate was performed without former separation and purification [66].
Another possibility for simplified workup is the use of fluorous solid phase extraction (FSPE). A nucleophilic 18F-fluorination of fluorous-tagged precursors can easily be purified by FSPE regardless of the affinity of the untagged substrate for the stationary phase. FSPE-purified labelled compounds can then be used in subsequent reactions or more easily purified by HPLC before administration [67, 68]. A similar approach was performed using molecular imprinted polymers [69].
Coupling reactions of the 18F-fluorination agent with the desired target molecule are performed either by the use of a further leaving group, by the click chemistry approach [70], by Staudinger ligation [71–73], or by Pd(0) mediated reactions [74].
A series of arylsulfonates were prepared as nucleophile assisting leaving groups (NALG) in which the metal chelating unit is attached to the aryl ring by an ether linker. Under microwave irradiation and without the assistance of a cryptand, such as Kryptofix 2.2.2, primary substrates with selected NALGs led to a 2-3-fold improvement in radiofluorination yields over traditional leaving groups [75].
3.2. Tri[18F]fluoromethyl Group
The CF3 group has an electronegativity similar to that of oxygen [76] and is characterized by a large hydrophobic parameter as measured by the relative partition coefficient [77]. The trifluoromethyl group is an important pharmacophore present in many biologically active pharmaceutical and agrochemical drugs. The increased lipophilicity and a superior metabolic stability compared to that of the trifluoromethyl analogues often account for an improved activity profile [78]. Thus, radiolabelled trifluoromethyl groups are of potential interest to facilitate drug discovery. Earlier attempts to synthesize an 18F-labelled trifluoromethyl group were also characterized by low RCY and low specific activity due to decomposition of the target material [79–81].
The recently published developments can be divided in aliphatic and aromatic tri[18F]fluoromethylation reactions (cf. Figure 3, method B).
A novel, one-step method for nucleophilic radiosynthesis of aliphatic tri[18F]fluoromethyl groups using the n.c.a. [18F]fluoride ion under relatively mild conditions was developed by incorporation of the radiolabel by an equivalent nucleophilic addition of H[18F]F to the 1-tosyl-2,2-difluorovinyl group (cf. Figure 4). The tosylate function then serves as leaving group in a subsequent coupling step [82, 83]. The specific activity of the tri[18F]fluoromethylether was determined to be 86 MBq/nmol. The need of a double bond to achieve the addition of the [18F]fluoride limits this reaction to aliphatic tri[18F]fluoromethylations.
Figure 4.
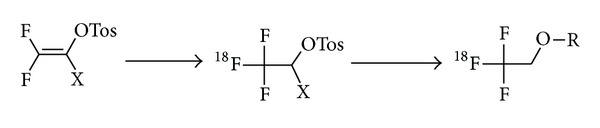
New aliphatic tri[18F]fluoromethylation.
Aromatic tri[18F]fluoromethyl groups were formerly synthesized using hardly accessible aromatic-CF2Br groups [84]. Two new approaches were published quite recently (cf. Figure 5). Both methods start with an aliphatic precursor which is first labelled with fluorine-18 and then coupled to the benzene ring. In a two-step procedure tri[18F]fluoromethane ([18F]fluoroform) available from difluoroiodomethane and [18F]fluoride [85] is coupled in a copper(I) mediated reaction to aromatic halides using potassium tert-butoxide as base. The RCY was determined up to 65% with a specific activity of up to 50 GBq/μmol [86]. This method has recently been improved performing a one-pot synthesis in the presence of copper(I)bromide, N,N-diisopropyl-N-ethylamine, and the corresponding iodoarene without separation of the [18F]fluoroform intermediate [87]. The RCYs of the desired tri[18F]fluoroarenes were determined with up to 90%, but no information on the specific activities was given.
Figure 5.
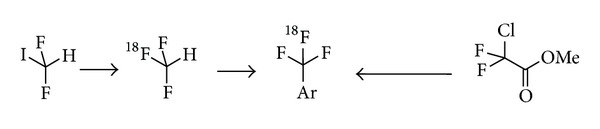
Aromatic tri[18F]fluoromethylation reactions.
An alternative method is used in a one-pot process. The trifluoromethylation agent [18F]CF3Cu, generated in situ from methyl chlorodifluoroacetate, CuI, TMEDA, and [18F]fluoride, is coupled to (hetero)aryl iodides in RCYs ranging from 17 to 87% [88]. A drawback of this procedure is still the relative low specific activity of 0.1 GBq/μmol exemplified so far only for 4-tri[18F]fluoromethyl nitrobenzene. However, the method enables an efficient tri-[18F]fluoromethylation of complex molecules like [18F]fluoxetine. N-Boc protected [18F]fluoxetine was readily prepared in 37% RCY. The subsequent N-Boc deprotection delivered [18F]fluoxetine with 95% yield. A more detailed review on the scope and limitations of the radiosynthesis of tri[18F]fluoromethyl groups is provided as part of this special issue [89].
3.3. Palladium, Managnese and Iridium Catalyzed 18F-Fluorovinylation
Transition metal catalyzed allylic substitution is a powerful method for carbon–carbon and carbon–heteroatom bond formation (cf. Figure 3 above, method C). These reactions encompass a wide variety of heteroatoms (N, O, and S) as nucleophiles [90]. In the field of 18F-chemistry a palladium catalyzed allylic fluorination reaction was developed and transferred to n.c.a. conditions yielding 18F-labelled cinnamyl fluoride starting from [18F]TBAF, cinnamyl methyl carbonate, [Pd (dba)2], and triphenylphosphine in anhydrous acetonitrile [91].
Further, a rapid allylic fluorination method utilizing trichloroacetimidates in conjunction with an iridium catalyst has been developed. The reaction is performed at room temperature without the need of inert gas atmosphere and relies on the Et3N·3HF reagent to provide branched allylic fluorides with complete regioselectivity. This high-yielding reaction can be carried out on a multigram scale and shows considerable functional group tolerance. The use of Kryptofix 2.2.2/K2CO3 allowed an incorporation of fluorine-18 within 10 min [92]. The RCY of allylic [18F]fluoride was determined to be 38%. A specific activity for the aforementioned reactions, however, was not reported.
A new method enables the facile n.c.a. 18F-labelling of aliphatic C–H bonds in benzylic position using manganese salen catalysts with RCY ranging from 20% to 72% within 10 min without the need for preactivation of the labelling precursor [93].
4. Aromatic and Heteroaromatic Intermediates
4.1. 18F-Labelled Aromatic and Heteroaromatic Intermediates by Classic Approaches
Historically, the use of the Balz-Schiemann or Wallach reaction was the first attempt to synthesize 18F-labelled aromatic rings starting from [18F]fluoride (cf. Figure 6, method A) [94, 95]. However, the thermal decomposition of the corresponding aryl diazonium salts and of the aryl triazenes is characterized by low RCY, a low specific radioactivity, and extensive by-product formation [95]. The use of tetrachloroborate or 2,4,6-triisopropylbenzenesulfonate as counterions led to improvements of the Balz-Schiemann reaction which enables the synthesis of [18F]fluoroarenes in 39% RCY at the n.c.a. level, exemplified for 4-[18F]fluorotoluene [96]. In a recently published study these nucleophilic 18F-labelling methods were reinvestigated using polymer bound aryl diazonium salts and aryl triazenes [97]. The solid phase supported de-diazofluorination using arenediazonium cations, ionically bound to a sulfonate functionalised ion exchange resin, was, however, not suitable for nucleophilic 18F-labelling of aromatic compounds, whereas the solid supported triazene yielded the 18F-labelled product in a reasonable RCY of 16%.
Figure 6.
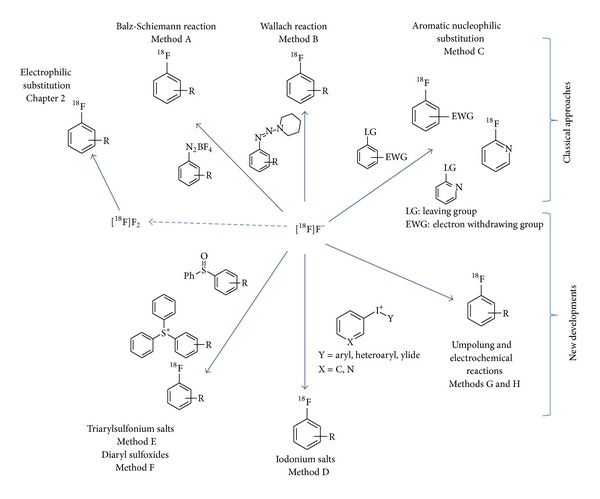
Pathways for aromatic 18F-labelling.
Most successful for the introduction of fluorine-18 into aromatic rings is the conventional aromatic nucleophilic substitution (SNAr) reaction using the [18F]fluoride anion to displace a suitable leaving group from an electron deficient benzene ring. As leaving groups serve halides, the nitro and the trimethylammonium function. The activation of the aromatic ring is usually achieved by suitable functional groups with a–M effect like the carbonyl, carboxyl, cyano, and nitro group [32]. These highly activating groups especially enable the efficient introduction of [18F]fluoride into aromatic rings to label small 18F-intermediates for build-up syntheses. The activating functionality is then converted by reduction, oxidation, or hydrolysis to nucleophilic groups for subsequent coupling reactions. The n.c.a. intermediates 4-[18F]fluoroaniline, 4-[18F]fluorobenzylamine [98, 99], 4-[18F]fluorobenzoic acid, or 4-[18F]fluorophenol (see Section 3.3), which are not directly achievable by a 18F-fluorination reaction, are obtained by these strategies (cf. Figure 7) [16, 100]. 4-[18F]Fluorobenzaldehyde is also used in multicomponent reactions to yield 18F-radiotracers with the label positioned on an aryl moiety, not susceptible to direct nucleophilic fluorination [101].
Figure 7.
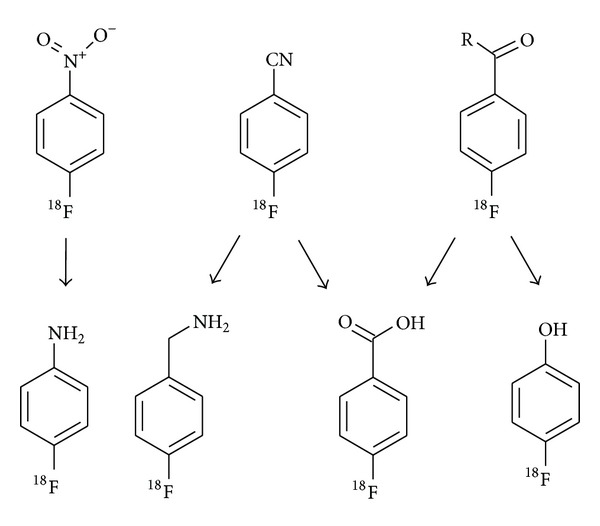
18F-labelled aromatic small molecules available by SNAr reactions used as intermediates and further important 18F-intermediates derived therefrom.
The azocarbonyl unit is a new group for activation of the arene ring by an SNAr mechanism. The aromatic core of phenylazocarboxylic esters is highly activated towards nucleophilic aromatic 18F-substitution (cf. Figure 8) [102]. This kind of compounds was converted in a radical arylation reaction into biaryl compounds or in substitutions at its carbonyl unit to produce azocarboxamides. Because of the high reactivity of the aryl radical, side products like [18F]fluorobenzene and 4-[18F]fluorophenol were also formed.
Figure 8.
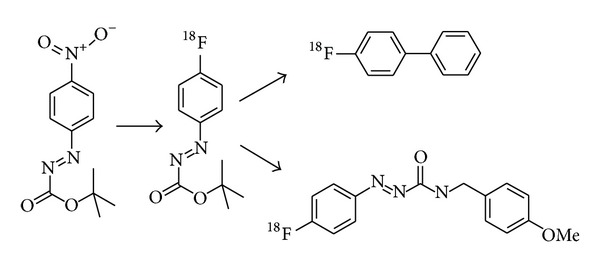
Radical arylation and substitution reactions of a 18F-labelled phenylazocarboxylic ester [102].
The conventional nucleophilic aromatic substitution reaction can principally be used for the n.c.a. 18F-labelling of aromatic rings in complex molecules [14]. However, the direct introduction of [18F]fluoride is often hampered by a lack of activation and further functional groups, especially those which have acidic protons. In the case of free amino, hydroxyl, or carboxylic acid functions the use of protecting groups is indispensable which have to be removed at the end of synthesis. Generally, the direct 18F-labelling of complex molecules enables the establishment of one-pot syntheses which is advantageous of being better introduced in a remote controlled synthesizer. In a multistep synthesis the intermediates have often to be purified (e.g., [103]) which hampers the installation in a synthesis module. Thus, one-pot syntheses are normally preferable over the build-up synthesis using several reactor vessels. There are exceptions to this rule, for example, when the build-up synthesis gives substantially higher RCYs [104].
In contrast to benzene, some heteroarenes like pyridine efficiently support the SNAr reaction and can directly be used to prepare 18F-labelled heteroarenes in the 2- or 4-position [105–107]. Because of its straightforward feasibility, this method was even applied for radiofluorination of complex structures containing an azabenzoxazole [108], a 1,3-thiazole [109], a fluoropurine [110], a pyridine [111–118], a quinolinol [119], or a pyrimidine moiety [120].
4.2. New Developments on Radiofluorination of Arenes
In general, the examination of new methods for 18F-labelling of arene rings focuses on the late stage introduction of [18F]fluorine into complex organic molecules without the need of any transformation reaction afterwards. This principally simplifies the establishment of 18F-labelling methods in fully automated, remotely controlled synthesis units. However, these new methods are also useful for the synthesis of small intermediates for build-up synthesis. The novel methods of two prominent ones, [18F]fluorophenol and [18F]fluoro-halobenzene, are separately described (see Sections 4.3 and 4.4).
4.2.1. Iodonium Salts (See Figure 6, Method D, and Figure 9)
Figure 9.
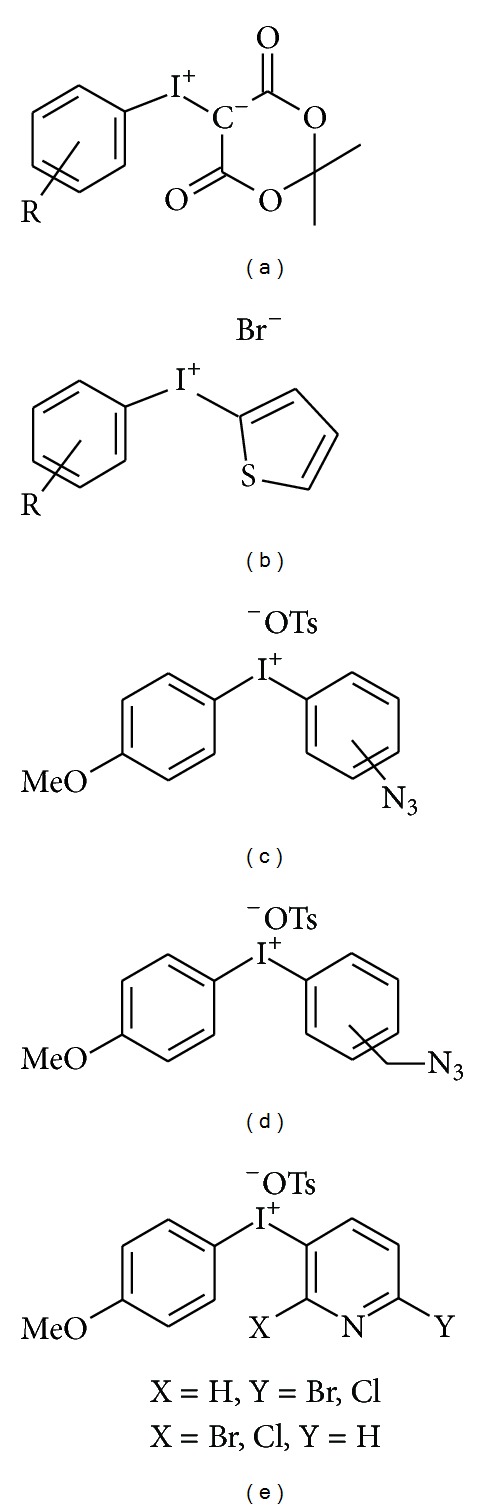
Structures of several iodonium salts for 18F-labelling: (a) iodonium ylide, (b) aryl(thienyl) iodonium salt, (c) [(azidomethyl)phenyl](4′-methoxyphenyl) iodonium tosylate, (d) [(azido)phenyl](4′-methoxyphenyl) iodonium tosylates, and (e) halopyridinyl-(4′-methoxyphenyl)iodonium tosylate.
The classical approach of n.c.a. nucleophilic aromatic 18F-substitution reactions is limited to activated arene rings. The use of diaryliodonium salts enables the introduction of n.c.a. [18F]fluoride into aromatic rings without further activation of strong electron withdrawing groups, which was first demonstrated in 1995 [121]. The reaction via an SNAr mechanism leads to n.c.a. [18F]fluoroarenes and the corresponding iodoarenes. The nucleophilic attack on the diaryliodonium salt occurs preferably at the more electron-deficient ring and a steric influence of substituents, especially of ortho-substituted, could be observed [95]. Further studies have recently been performed to examine the possibilities and limitations of this reaction, with a focus on the synthesis of ortho- and meta-substituted arenes and the use of microreactors [122–124].
An interesting aspect here is that the reaction of diaryliodonium salts with [18F]fluoride is feasible in the presence of water, however, depending on the substituents present on the arene ring. Iodonium salts bearing a para- or ortho-electron-withdrawing group (e.g., p-CN) reacted rapidly (~3 min) to give the expected major [18F]fluoroarene product in useful, albeit moderate radiochemical yields even when the solvent had a water content of up to 28%. Iodonium salts bearing electron-withdrawing groups in metaposition (e.g., m-NO2) or an electron-donating substituent (p-OMe) gave low radiochemical yields under the same conditions. The finding that [18F]fluoroarenes, that having an ortho-alkyl substituent or an ortho- or a para-electron withdrawing group, can be synthesized without the need to remove irradiated water or to add a cryptand, could be attractive in some radiotracer production settings, particularly as this method saves time, avoids any need for automated drying of cyclotron-produced [18F]fluoride, and also avoids substantial loss of radioactivity through adsorption onto hardware surfaces [125].
In order to control the attack of the [18F]fluoride ion on the diaryliodonium salts it is important that one arene ring be more electron-rich than the ring to be labelled with fluorine-18. Here, the use of symmetrically substituted diaryliodonium salts [126] or the use of aryl(heteroaryl) iodonium salts [127] is an alternative to direct the 18F-labelling to the desired ring. More recently, the use of aryiodonium ylides became of interest for this purpose. The electron-rich status of the ylides, made, for example, from dimedone (5,5-dimethylcyclohexane-1,3-dione), even enables the synthesis of electron-rich arenes in high RCY [128]. This type of precursor has recently been demonstrated to be even suitable for complex molecules [129].
Some special intermediates like azide-containing diaryliodonium salts bearing an azidomethyl group on one aryl ring and with a 4-methoxy group on the second one enable the synthesis of click-labelling synthons up to 50 % RCY, even in the presence of a high fraction of water in the reaction solvent [130].
Halopyridinyl-(4′-methoxyphenyl)iodonium tosylates were used to rapidly produce [18F]fluorohalopyridines and in useful RCYs, including the otherwise difficult to access 3-[18F]fluorohaloisomers [131].
4.2.2. Sulfur Activated Systems (See Figure 6 and Methods E and F)
Another newer method for the 18F-labelling of nonactivated aromatic compounds makes use of triarylsulfonium salts. The method is applicable to a wide range of substituted aryl systems including amides [132].
A new radiosynthetic method for producing n.c.a. [18F]fluoroarenes is based on the reactions of diaryl sulfoxides bearing electron-withdrawing paragroups with the [18F]fluoride ion. These reactions are relatively mild, rapid, and efficient. However, this reaction is limited to aromatic rings bearing an electron withdrawing function like the nitro, cyano, or trifluoromethyl group [133].
4.2.3. Umpolungs Reactions (See Figure 6 and Methods G and H)
New concepts to synthesize 18F-labelled aromatic rings try to achieve fluoride-derived electrophilic n.c.a. fluorination reagents by fluoride umpolung [134, 135]. A preliminary realization of this concept was achieved by using a n.c.a. [18F]fluoride capture by a Pd(IV) complex to form an electrophilic 18F-fluorination reagent followed by a subsequent n.c.a. 18F-fluorination of palladium aryl complexes [136, 137]. Another kind of palladium catalyzed fluoride activation enables the synthesis of 18F-labelled 1-[18F]fluoronaphthalene in 33% RCY but only in the presence of fluoride carrier [138]. Another advanced method for a transition metal catalyzed late-stage radiofluorination relies on a one-step oxidative 18F-fluorination using a nickel aryl complex and a strong oxidation agent [139].
[18F]Fluoride can also be introduced into organic molecules by electrochemical oxidative fluorination via an aryl cation that undergoes rearomatization by loss of a proton. Oxidation of benzene in an electrolysis cell, using Et3N·3HF and Et3N·HCl in acetonitrile as the electrolyte, gave c.a. [18F]fluorobenzene in 17% RCY [140] and [18F]fluorophenylalanine in 10.5% RCY with a specific activity of 1.2 GBq/mmol [141].
However, the aim of all these methods is the late stage 18F-fluorination of electron-neutral and electron-rich aromatic compounds to simplify the synthesis of radiotracers. Regarding the palladium and nickel reactions, the precursor synthesis is often complex, has to be carefully handled under inert atmosphere, and needs high synthetic experience. This method is far from ideal, given the many reagents and demanding reaction conditions necessary, which hamper to fulfill a “good manufacturing practice” (GMP) pharmaceutical production [142, 143]. Thus, although principally applicable, their limitation and complexity do not warrant usefulness for the syntheses of build-up intermediates, as there are more efficient methods available for those molecules.
4.3. N.c.a. 4-[18F]Fluorophenol
4-[18F]Fluorophenol is a versatile structural unit for the synthesis of more complex radiopharmaceuticals bearing a 4-[18F]fluorophenoxy moiety. Former syntheses of n.c.a. 4-[18F]fluorophenol were made either by a modified Balz-Schiemann reaction or by hydrolysis of a 4-[18F]fluorobenzene diazonium salt with radiochemical yields of only 10–15% and 15–33% within 35 and 60 min, respectively [144]. These methods required either the preparation of an anhydrous tetrachloroborate or a two-step synthesis from [18F]fluoroaniline and were not established for radiotracer production.
A more reliable preparation of n.c.a. 2- and 4-[18F]fluorophenol was achieved using the Baeyer-Villiger reaction on 18F-labelled benzaldehyde, acetophenone, or benzophenone derivatives. Total radiochemical yields of about 25% were received using m-chloroperbenzoic acid as oxidant in the presence of trifluoroacetic acid [145]. The Baeyer-Villiger reaction of 18F-labelled benzophenone derivatives containing further electron withdrawing groups yielded up to 65% of 4-[18F]fluorophenol within 60 min with a high radiochemical purity. However, a considerable disadvantage of this method is the somewhat cumbersome work-up of the aqueous reaction mixture in order to isolate the product for its further use [146]. The formation of 18F-labelled 4-phenol derivatives by Baeyer-Villiger oxidation was, for example, applied to the direct 18F-fluorination of 6-[18F]fluoro-L-dopa [147].
A novel radiochemical transformation by an oxidative 18F-fluorination of tert-butylphenols uses the concept of an aryl umpolung (cf. Figure 10) and is also applicable to other O-unprotected phenols. The reaction is performed at room temperature by applying a one-pot protocol and can also be performed in a commercially available microfluidic device [148].
Figure 10.
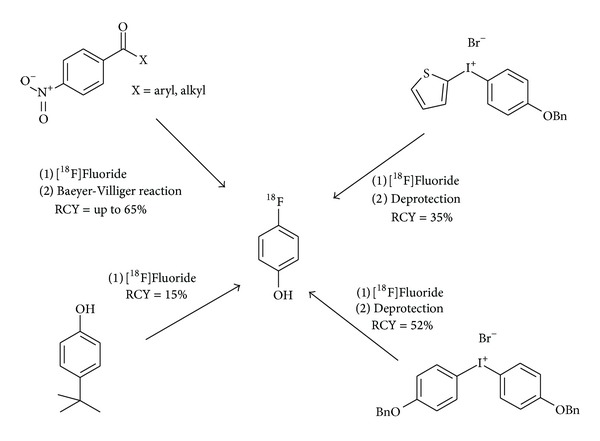
Methods for the synthesis of n.c.a. 4-[18F]fluorophenol.
Furthermore, aryl(thienyl) iodonium salts [149] and bis(4-benzyloxyphenyl) iodonium salts [150] have successfully been employed for the preparation of [18F]fluorophenol in a two-step procedure. Compared with the Baeyer Villiger method using benzophenone derivatives, this pathway saves 20 to 45 min of preparation time and delivers [18F]fluorophenol in an organic solution. So these methods are more useful for subsequent coupling reactions under anhydrous conditions. In contrast to the aryl umpolung reaction, the iodonium strategy, however, necessitates a deprotection step after the 18F-exchange.
4.4. N.c.a. 4-[18F]Fluorohalobenzene
Recently, the synthesis of 4-[18F]fluorohalobenzenes has comprehensively been described [151]; here a few further aspects are added. 1-Bromo-4-[18F]fluorobenzene or 4-[18F]fluoro-1-iodobenzene serves as intermediates for C–C coupling reactions using Grignard-, lithium- [152], or palladium-mediated reactions [151, 153]. In Figure 11 the most efficient routes for the synthesis of n.c.a. 4-[18F]fluorohalobenzenes are illustrated. The use of symmetrically substituted diaryliodonium salts enables an efficient one-step synthesis of n.c.a. 1-bromo-4-[18F]fluorobenzene [154] as well as n.c.a. 4-[18F]fluoro-1-iodobenzene [155]. For the latter, the precursor synthesis is more challenging and has recently been improved [156]. The precursor syntheses of iodophenylthienyliodonium bromide and 4-iodophenyliodonium-(5-[2,2-dimethyl-1,3-dioxane-4,6-dione]) ylide [157] are easier to perform and the latter gave up to 70% RCY of 4-[18F]fluoro-1-iodobenzene [158]. The most efficient method for the one-step synthesis of 4-[18F]fluoro-1-iodobenzene is, however, the use of triarylsulfonium salts [132, 159] which leads to 90% RCY. A challenge, when using iodonium salts as precursor for the synthesis of 4-[18F]fluorohalobenzenes, is the formation of other nonradioactive halobenzene derivatives which are normally not separated from the 18F-labelled product and thus could hamper the final product separation.
Figure 11.
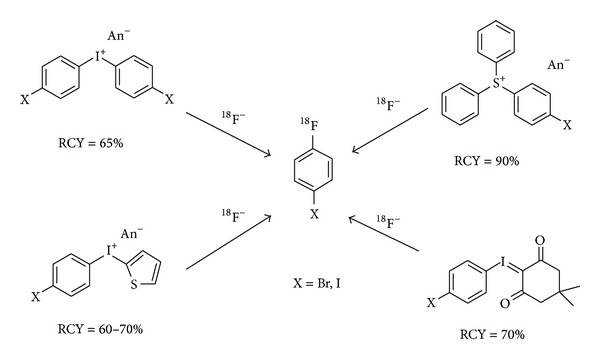
Most efficient one-step approaches for the n.c.a synthesis of [18F]fluoro-halobenzenes.
5. Conclusion
The lack of universally useful methods for direct n.c.a. radiofluorination of complex molecules causes the wide use of 18F-labelled intermediates for the build-up synthesis of radiotracers. Nevertheless, multistep build-up syntheses of 18F-labelled radiotracers are confronted with several fundamental challenges, which often hamper a remotely controlled, large scale production by this type of reactions. Time consuming separation steps and the use of moisture or even air sensitive reagents complicate the automation of these build-up syntheses. Their application is therefore limited to specialized laboratories with the suitable equipment and experienced staff. The use of build-up reactions then often enables the only way to achieve the synthesis of new radiotracers. Once proven that a radiotracer has the potential to be a useful radiopharmaceutical for molecular imaging, most often ways can be found to establish its routine production via an alternative, simpler synthetic concept and/or by optimisation. Here, the novel developments in umpolung reactions or the improvements in iodonium chemistry in 18F-labelling of arenes are promising methods, which might also be effective for the late-stage 18F-fluorination of complex precursors. However, their suitability for daily routine GMP-production of radiopharmaceuticals remains to be elucidated.
Acknowledgment
The author wishes to thank Professor Heinz H. Coenen for his critical discussions and helpful suggestions.
Conflict of Interests
The author declares that there is no conflict of interests regarding the publication of this paper.
References
- 1.Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chemical Reviews. 2008;108(5):1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 2.Lu SY, Pike VW. Micro-reactors for PET tracer labeling. In: Schubiger PA, Lehmann L, Friebe M, editors. PET Chemistry: the Driving Force in Molecular Imaging. Vol. 62. Berlin, Germany: Springer ; 2007. pp. 271–287. (Ernst Schering Research Foundation Workshop). [DOI] [PubMed] [Google Scholar]
- 3.Pascali G, Watts P, Salvadori PA. Microfluidics in radiopharmaceutical chemistry. Nuclear Medicine and Biology. 2013;40(6):776–787. doi: 10.1016/j.nucmedbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Liang SH, Collier TL, Rotstein BH, et al. Rapid microfluidic flow hydrogenation for reduction or deprotection of 18F-labeled compounds. Chemical Communications. 2013;49:8755–8757. doi: 10.1039/c3cc45166f. [DOI] [PubMed] [Google Scholar]
- 5.Lebedev A, Miraghaie R, Kotta K, et al. Batch-reactor microfluidic device: first human use of a microfluidically produced PET radiotracer. Lab on a Chip: Miniaturisation for Chemistry and Biology. 2013;13(1):136–145. doi: 10.1039/c2lc40853h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahl K, Schou M, Halldin C. Radiofluorination and reductive amination using a microfluidic device. Journal of Labelled Compounds and Radiopharmaceuticals. 2012;55(13):455–459. [Google Scholar]
- 7.Bouvet V, Wuest M, Tam P-H, Wang M, Wuest F. Microfluidic technology: an economical and versatile approach for the synthesis of O-(2-[18F]fluoroethyl)-l-tyrosine ([18F]FET) Bioorganic and Medicinal Chemistry Letters. 2012;22(6):2291–2295. doi: 10.1016/j.bmcl.2012.01.083. [DOI] [PubMed] [Google Scholar]
- 8.Bouvet VR, Wuest M, Wiebe LI, Wuest F. Synthesis of hypoxia imaging agent 1-(5-deoxy-5-fluoro-α-d-arabinofuranosyl)-2-nitroimidazole using microfluidic technology. Nuclear Medicine and Biology. 2011;38(2):235–245. doi: 10.1016/j.nucmedbio.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Rensch C, Jackson A, Lindner S, et al. Microfluidics: a groundbreaking technology for PET tracer production? Molecules. 2013;18(7):7930–7956. doi: 10.3390/molecules18077930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang BY, Jeong JM, Lee Y-S, Lee DS, Chung J-K, Lee MC. Facile calculation of specific rate constants and activation energies of 18F-fluorination reaction using combined processes of coat-capture-elution and microfluidics. Tetrahedron. 2011;67(13):2427–2433. [Google Scholar]
- 11.Cai L, Lu S, Pike VW. Chemistry with [18F]fluoride ion. European Journal of Organic Chemistry. 2008;(17):2853–2873. [Google Scholar]
- 12.Wadsak W, Mien L-K, Ettlinger DE, et al. 18F fluoroethylations: different strategies for the rapid translation of 11C-methylated radiotracers. Nuclear Medicine and Biology. 2007;34(8):1019–1028. doi: 10.1016/j.nucmedbio.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Amaraesekera B, Marchis PD, Bobinski KP, et al. High-pressure, compact, modular radiosynthesizer for production of positron emitting biomarkers. Applied Radiation and Isotopes. 2013;78:88–101. doi: 10.1016/j.apradiso.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Ermert J, Coenen HH. Nucleophilic 18F-fluorination of complex molecules in activated carbocyclic aromatic position. Current Radiopharmaceuticals. 2010;3(2):109–126. [Google Scholar]
- 15.Roeda D, Dollé F. Aliphatic nucleophilic radiofluorination. Current Radiopharmaceuticals. 2010;3(2):81–108. [Google Scholar]
- 16.Ermert J, Coenen HH. No-carrier-added [18F]fluorobenzene derivatives as intermediates for built-up radiosyntheses. Current Radiopharmaceuticals. 2010;3(2):127–160. [Google Scholar]
- 17.Kuhnast B, Dollé F. The challenge of labeling macromolecules with fluorine-18: three decades of research. Current Radiopharmaceuticals. 2010;3(3):174–201. [Google Scholar]
- 18.Smith TAD. Fluoride labelling of macromolecules in aqueous conditions: silicon and boroaryl-based [18F]fluorine acceptors, [18F]FDG conjugation and Al18F chelation. Journal of Labelled Compounds and Radiopharmaceuticals. 2012;55(8):281–288. [Google Scholar]
- 19.McBride WJ, Sharkey RM, Karacay H, et al. A novel method of 18F radiolabeling for PET. Journal of Nuclear Medicine. 2009;50(6):991–998. doi: 10.2967/jnumed.108.060418. [DOI] [PubMed] [Google Scholar]
- 20.Laverman P, McBride WJ, Sharkey RM, et al. A novel facile method of labeling octreotide with18F-fluorine. Journal of Nuclear Medicine. 2010;51(3):454–461. doi: 10.2967/jnumed.109.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBride WJ, D’Souza CA, Karacay H, Sharkey RM, Goldenberg DM. New lyophilized kit for rapid radiofluorination of peptides. Bioconjugate Chemistry. 2012;23(3):538–547. doi: 10.1021/bc200608e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Li Y, Lozada J, et al. Stoichiometric leverage: rapid 18F-aryltrifluoroborate radiosynthesis at high specific activity for click conjugation. Angewandte Chemie: International Edition. 2013;52(8):2303–2307. doi: 10.1002/anie.201208551. [DOI] [PubMed] [Google Scholar]
- 23.Schirrmacher R, Bradtmöller G, Schirrmacher E, et al. 18F-labeling of peptides by means of an organosilicon-based fluoride acceptor. Angewandte Chemie: International Edition. 2006;45(36):6047–6050. doi: 10.1002/anie.200600795. [DOI] [PubMed] [Google Scholar]
- 24.Mu L, Schubiger PA, Ametamey SM. [18F]fluorosilicon- and [18F]fluoroboron-based biomolecules for PET imaging. Current Radiopharmaceuticals. 2010;3(3):224–242. [Google Scholar]
- 25.Dialer LO, Selivanova SV, Müller CJ, et al. Studies toward the development of new silicon-containing building blocks for the direct 18F-labeling of peptides. Journal of Medicinal Chemistry. 2013;56:7552–7563. doi: 10.1021/jm400857f. [DOI] [PubMed] [Google Scholar]
- 26.Wängler B, Kostikov AP, Niedermoser S, et al. Protein labeling with the labeling precursor [18F]SiFA-SH for positron emission tomography. Nature Protocols. 2012;7(11):1964–1969. doi: 10.1038/nprot.2012.111. [DOI] [PubMed] [Google Scholar]
- 27.Joyard Y, Azzouz R, Bischoff L, et al. Synthesis of new 18F-radiolabeled silicon-based nitroimidazole compounds. Bioorganic and Medicinal Chemistry. 2013;21(13):3680–3688. doi: 10.1016/j.bmc.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 28.Kettenbach K, Schieferstein H, Ross T. 18F-Labeling using click cycloadditions. BioMed Research International. 2014;2014:16 pages. doi: 10.1155/2014/361329.361329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maschauer S, Prante O. Sweetening pharmaceutical radiochemistry by fluoroglycosylation: a short review. BioMed Research International. 2014;2014:16 pages. doi: 10.1155/2014/214748.214748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernard-Gauthier V, Wängler C, Schirrmacher E, et al. 18F-Labeled silicon-based fluoride acceptors-opportunities for novel positron emitting radiopharmaceuticals. doi: 10.1155/2014/454503. BioMed Research International. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qaim SM, Clark JC, Crouzel C, et al. PET radionuclide production. In: Stöcklin G, Pike VW, editors. Radiopharmaceuticals for Positron Emission Tomography: Methodological Aspects. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 15–26. [Google Scholar]
- 32.Coenen HH. Fluorine-18 labeling methods: features and possibilities of basic reactions. In: Schubiger PA, Lehmann L, Friebe M, editors. PET Chemistry: The Driving Force in Molecular Imaging. Vol. 62. Berlin, Germany: Springer; 2007. pp. 15–50. (Ernst Schering Research Foundation Workshop). [DOI] [PubMed] [Google Scholar]
- 33.Teare H, Robins EG, Årstad E, Luthra SK, Gouverneur V. Synthesis and reactivity of [18F]-N-fluorobenzenesulfonimide. Chemical Communications. 2007;(23):2330–2332. doi: 10.1039/b701177f. [DOI] [PubMed] [Google Scholar]
- 34.Bergman J, Johnström P, Haaparanta M, Solin O, Duelfer T, Stone-Elander S. Radiolabelling of 2-oxoquazepam with electrophilic 18F prepared from [18F]fluoride. Applied Radiation and Isotopes. 1995;46(10):1027–1034. [Google Scholar]
- 35.Teare H, Robins EG, Kirjavainen A, et al. Radiosynthesis and evaluation of [18F]Selectfluor bis(triflate) Angewandte Chemie: International Edition. 2010;49(38):6821–6824. doi: 10.1002/anie.201002310. [DOI] [PubMed] [Google Scholar]
- 36.Stenhagen ISR, Kirjavainen AK, Forsback SJ, et al. Fluorination of an arylboronic ester using [18F]selectfluor bis(triflate): application to 6-[18F]fluoro-l-DOPA. Chemical Communications. 2013;49(14):1386–1388. doi: 10.1039/c2cc38646a. [DOI] [PubMed] [Google Scholar]
- 37.Wester H-J. Nuclear imaging probes: from bench to bedside. Clinical Cancer Research. 2007;13(12):3470–3481. doi: 10.1158/1078-0432.CCR-07-0264. [DOI] [PubMed] [Google Scholar]
- 38.Coenen HH, Elsinga PH, Iwata R, et al. Fluorine-18 radiopharmaceuticals beyond [18F]FDG for use in oncology and neurosciences. Nuclear Medicine and Biology. 2010;37(7):727–740. doi: 10.1016/j.nucmedbio.2010.04.185. [DOI] [PubMed] [Google Scholar]
- 39.Wester HJ, Schottelius M. Fluorine-18 labeling of peptides and proteins. In: Schubiger AP, Lehmann L, Friebe M, editors. PET Chemistry: the Driving Force in Molecular Imaging. Vol. 62. 2007. pp. 79–111. (Ernst Schering Research Foundation Workshop). [DOI] [PubMed] [Google Scholar]
- 40.Zhang M-R, Suzuki K. [18F]Fluoroalkyl agents: synthesis, reactivity and application for development of PET ligands in molecular imaging. Current Topics in Medicinal Chemistry. 2007;7(18):1817–1828. doi: 10.2174/156802607782507448. [DOI] [PubMed] [Google Scholar]
- 41.Jacobson O, Chen X. PET designated flouride-18 production and chemistry. Current Topics in Medicinal Chemistry. 2010;10(11):1048–1059. doi: 10.2174/156802610791384298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wessmann SH, Henriksen G, Wester H-J. Cryptate mediated nucleophilic 18F-fluorination without azeotropic drying. NuklearMedizin. 2012;51(1):1–8. doi: 10.3413/Nukmed-0425-11-08. [DOI] [PubMed] [Google Scholar]
- 43.Lemaire CF, Aerts JJ, Voccia S, et al. Fast production of highly reactive no-carrier-added [18F] fluoride for the labeling of radiopharmaceuticals. Angewandte Chemie: International Edition. 2010;49(18):3161–3164. doi: 10.1002/anie.200906341. [DOI] [PubMed] [Google Scholar]
- 44.Aerts J, Voccia S, Lemaire C, et al. Fast production of highly concentrated reactive [18F] fluoride for aliphatic and aromatic nucleophilic radiolabelling. Tetrahedron Letters. 2010;51(1):64–66. [Google Scholar]
- 45.Mathiessen B, Jensen ATI, Zhuravlev F. Homogeneous nucleophilic radiofluorination and fluorination with phosphazene hydrofluorides. Chemistry. 2011;17(28):7796–7805. doi: 10.1002/chem.201100458. [DOI] [PubMed] [Google Scholar]
- 46.Mathiessen B, Zhuravlev F. Automated solid-phase radiofluorination using polymer-supported phosphazenes. Molecules. 2013;18:10531–10547. doi: 10.3390/molecules180910531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamacher K, Hirschfelder T, Coenen HH. Electrochemical cell for separation of [18F]fluoride from irradiated 18O-water and subsequent no carrier added nucleophilic fluorination. Applied Radiation and Isotopes. 2002;56(3):519–523. doi: 10.1016/s0969-8043(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 48.Reischl G, Ehrlichmann W, Machulla H-J. Electrochemical transfer of [18F] fluoride from [18O]water into organic solvents ready for labeling reactions. Journal of Radioanalytical and Nuclear Chemistry. 2002;254(1):29–31. [Google Scholar]
- 49.Saiki H, Iwata R, Nakanishi H, et al. Electrochemical concentration of no-carrier-added [18F]fluoride from [18O]water in a disposable microfluidic cell for radiosynthesis of 18F-labeled radiopharmaceuticals. Applied Radiation and Isotopes. 2010;68(9):1703–1708. doi: 10.1016/j.apradiso.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Wong R, Iwata R, Saiki H, Furumoto S, Ishikawa Y, Ozeki E. Reactivity of electrochemically concentrated anhydrous [18F]fluoride for microfluidic radiosynthesis of 18F-labeled compounds. Applied Radiation and Isotopes. 2012;70(1):193–199. doi: 10.1016/j.apradiso.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 51.Sadeghi S, Liang V, Cheung S, et al. Reusable electrochemical cell for rapid separation of [18F]fluoride from [18O]water for flow-through synthesis of 18F-labeled tracers. Applied Radiation and Isotopes. 2013;75:85–94. doi: 10.1016/j.apradiso.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong WK, Jeong H-J, Seok TL, Sohn M-H, Katzenellenbogen JA, Dae YC. Facile nucleophilic fluorination reactions using tert-alcohols as a reaction medium: significantly enhanced reactivity of alkali metal fluorides and improved selectivity. Journal of Organic Chemistry. 2008;73(3):957–962. doi: 10.1021/jo7021229. [DOI] [PubMed] [Google Scholar]
- 53.Sachin K, Jeong H-J, Lim ST, Sohn M-H, Chi DY, Kim DW. An efficient synthesis of ([18F]fluoropropyl)quinoline-5,8-diones by rapid radiofluorination-oxidative demethylation. Tetrahedron. 2011;67(10):1763–1767. [Google Scholar]
- 54.Priem T, Bouteiller C, Camporese D, Romieu A, Renard P-Y. Synthesis and reactivity of a bis-sultone cross-linker for peptide conjugation and [18F]-radiolabelling via unusual “double click” approach. Organic and Biomolecular Chemistry. 2012;10(5):1068–1078. doi: 10.1039/c1ob06600e. [DOI] [PubMed] [Google Scholar]
- 55.Löser R, Bergmann R, Frizler M, et al. Synthesis and radiopharmacological characterisation of a fluorine-18-labelled azadipeptide nitrile as a potential PET tracer for invivo imaging of cysteine cathepsins. ChemMedChem. 2013;8(8):1330–1344. doi: 10.1002/cmdc.201300135. [DOI] [PubMed] [Google Scholar]
- 56.Park C, Lee BS, Chi DY. High efficiency synthesis of F-18 fluoromethyl ethers: an attractive alternative for C-11 methyl groups in positron emission tomography radiopharmaceuticals. Organic Letters. 2013;15:4346–4349. doi: 10.1021/ol401792n. [DOI] [PubMed] [Google Scholar]
- 57.Jarkas N, Voll RJ, Goodman MM. Synthesis of a phenolic precursor and its efficient O-[18F]fluoroethylation with purified no-carrier-added [18F]2-fluoroethyl brosylate as the labeling agent. Journal of Labelled Compounds and Radiopharmaceuticals. 2013;56:539–543. doi: 10.1002/jlcr.3046. [DOI] [PubMed] [Google Scholar]
- 58.Majo VJ, Milak MS, Prabhakaran J, et al. Synthesis and in vivo evaluation of [18F]2-(4-(4-(2-(2-fluoroethoxy)phenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H) -dione ([18F]FECUMI-101) as an imaging probe for 5-HT1A receptor agonist in nonhuman primates. Bioorganic and Medicinal Chemistry. 2013;21(17):5598–5604. doi: 10.1016/j.bmc.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caillé F, Morley TJ, Tavares AAS, et al. Synthesis and biological evaluation of positron emission tomography radiotracers targeting serotonin 4 receptors in brain: [18F]MNI-698 and [18F]MNI-699. Bioorganic and Medicinal Chemistry Letters. 2013;23:6243–6247. doi: 10.1016/j.bmcl.2013.09.097. [DOI] [PubMed] [Google Scholar]
- 60.Zhang M-R, Tsuchiyama A, Haradahira T, Yoshida Y, Furutsuka K, Suzuki K. Development of an automated system for synthesizing 18F-labeled compounds using [18F]fluoroethyl bromide as a synthetic precursor. Applied Radiation and Isotopes. 2002;57(3):335–342. doi: 10.1016/s0969-8043(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 61.Riss PJ, Hoehnemann S, Piel M, Roesch F. Two-step radiosynthesis of [18F]FE-β-CIT and [18F]PR04.MZ. Journal of Labelled Compounds and Radiopharmaceuticals. 2013;56(7):356–359. doi: 10.1002/jlcr.3032. [DOI] [PubMed] [Google Scholar]
- 62.Rodnick ME, Brooks AF, Hockley BG, Henderson BD, Scott PJH. A fully-automated one-pot synthesis of [18F]fluoromethylcholine with reduced dimethylaminoethanol contamination via [18F]fluoromethyl tosylate. Applied Radiation and Isotopes. 2013;78:26–32. doi: 10.1016/j.apradiso.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franck D, Kniess T, Steinbach J, et al. Investigations into the synthesis, radiofluorination and conjugation of a new [18F]fluorocyclobutyl prosthetic group and its in vitro stability using a tyrosine model system. Bioorganic and Medicinal Chemistry. 2013;21(3):643–652. doi: 10.1016/j.bmc.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 64.Sun T, Tang G, Tian H, et al. Radiosynthesis of 1-[18F]fluoroethyl-L-tryptophan as a novel potential amino acid PET tracer. Applied Radiation and Isotopes. 2012;70(4):676–680. doi: 10.1016/j.apradiso.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 65.Klok RP, Klein PJ, Herscheid JDM, Windhorst AD. Synthesis of N-(3-[18F]fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl)nortropane ([18F]FP-β-CIT) Journal of Labelled Compounds and Radiopharmaceuticals. 2006;49(2):77–89. [Google Scholar]
- 66.Billaud EMF, Rbah-Vidal L, Vidal A, et al. Synthesis, radiofluorination, and in vivo evaluation of novel fluorinated and iodinated radiotracers for PET imaging and targeted radionuclide therapy of melanoma. Journal of Medicinal Chemistry. 2013;56:8455–8467. doi: 10.1021/jm400877v. [DOI] [PubMed] [Google Scholar]
- 67.Bejot R, Fowler T, Carroll L, et al. Fluorous synthesis of 18F radiotracers with the [18F]fluoride ion: nucleophilic fluorination as the detagging process. Angewandte Chemie: International Edition. 2009;48(3):586–589. doi: 10.1002/anie.200803897. [DOI] [PubMed] [Google Scholar]
- 68.Bejot R, Goggi J, Moonshi SS, Robins EG. A practical synthesis of [18F]FtRGD: an angiogenesis biomarker for PET. Journal of Labelled Compounds and Radiopharmaceuticals. 2013;56(2):42–49. doi: 10.1002/jlcr.3019. [DOI] [PubMed] [Google Scholar]
- 69.Smith GE, Bayoudh S, Perollier C, et al. Rapid purification of fluorine-18 containing synthons using molecularly imprinted polymer cartridges. Journal of Labelled Compounds and Radiopharmaceuticals. 2013;56, article S119 [Google Scholar]
- 70.Pretze M, Pietzsch D, Mamat C. Recent trends in bioorthogonal click-radiolabeling reactions using fluorine-18. Molecules. 2013;18(7):8618–8665. doi: 10.3390/molecules18078618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pretze M, Wuest F, Peppel T, Köckerling M, Mamat C. The traceless Staudinger ligation with fluorine-18: a novel and versatile labeling technique for the synthesis of PET-radiotracers. Tetrahedron Letters. 2010;51(49):6410–6414. [Google Scholar]
- 72.Carroll L, Boldon S, Bejot R, Moore JE, Declerck J, Gouverneur V. The traceless Staudinger ligation for indirect 18F-radiolabelling. Organic and Biomolecular Chemistry. 2011;9(1):136–140. doi: 10.1039/c0ob00564a. [DOI] [PubMed] [Google Scholar]
- 73.Mamat C, Franke M, Peppel T, Köckerling M, Steinbach J. Synthesis, structure determination, and (radio-)fluorination of novel functionalized phosphanes suitable for the traceless Staudinger ligation. Tetrahedron. 2011;67(25):4521–4529. [Google Scholar]
- 74.Doi H, Goto M, Suzuki M. Pd0-mediated rapid C-[18F]fluoromethylation by the cross-coupling reaction of a [18F]fluoromethyl halide with an arylboronic acid ester: novel method for the synthesis of a 18F-labeled molecular probe for positron emission tomography. Bulletin of the Chemical Society of Japan. 2012;85(11):1233–1238. [Google Scholar]
- 75.Lu S, Lepore SD, Song YL, et al. Nucleophile assisting leaving groups: a strategy for aliphatic 18F-fluorination. Journal of Organic Chemistry. 2009;74(15):5290–5296. doi: 10.1021/jo900700j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huheey JE. The electronegativity of groups. Journal of Physical Chemistry. 1965;69(10):3284–3291. [Google Scholar]
- 77.McClinton MA, McClinton DA. Trifluoromethylations and related reactions in organic chemistry. Tetrahedron. 1992;48(32):6555–6666. [Google Scholar]
- 78.Ma J-A, Cahard D. Asymmetric fluorination, trifluoromethylation, and perfluoroalkylation reactions. Chemical Reviews. 2004;104(12):6119–6146. doi: 10.1021/cr030143e. [DOI] [PubMed] [Google Scholar]
- 79.Prabhakaran J, Underwood MD, Parsey RV, et al. Synthesis and in vivo evaluation of [18F]-4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide as a PET imaging probe for COX-2 expression. Bioorganic and Medicinal Chemistry. 2007;15(4):1802–1807. doi: 10.1016/j.bmc.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 80.Majo VJ, Arango V, Simpson NR, et al. Synthesis and in vitro evaluation of [18F]BMS-754807: a potential PET ligand for IGF-1R. Bioorganic and Medicinal Chemistry Letters. 2013;23(14):4191–4194. doi: 10.1016/j.bmcl.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lasne MC, Perrio C, Rouden J, et al. Chemistry of beta-emitting compounds based on fluorine-18+ Topics in Current Chemistry. 2002;222:201–258. [Google Scholar]
- 82.Riss PJ, Aigbirhio FI. A simple, rapid procedure for nucleophilic radiosynthesis of aliphatic [18F]trifluoromethyl groups. Chemical Communications. 2011;47(43):11873–11875. doi: 10.1039/c1cc15342k. [DOI] [PubMed] [Google Scholar]
- 83.Riss PJ, Ferrari V, Brichard L, Burke P, Smith R, Aigbirhio FI. Direct, nucleophilic radiosynthesis of [18F]trifluoroalkyl tosylates: improved labelling procedures. Organic and Biomolecular Chemistry. 2012;10(34):6980–6986. doi: 10.1039/c2ob25802a. [DOI] [PubMed] [Google Scholar]
- 84.Hammadi A, Crouzel C. Synthesis of [18F]-(S)-fluoxetine: a selective serotonine uptake inhibitor. Journal of Labelled Compounds and Radiopharmaceuticals. 1993;33(8):703–710. [Google Scholar]
- 85.Van Der Born D, Herscheid JDM, Orru RVA, Vugts DJ. Efficient synthesis of [18F]trifluoromethane and its application in the synthesis of PET tracers. Chemical Communications. 2013;49(38):4018–4020. doi: 10.1039/c3cc37833k. [DOI] [PubMed] [Google Scholar]
- 86.van der Born D, Herscheid JDM, Vugts DJ. Aromatic trifluoromethylation using [18F]fluoroform. Journal of Labelled Compounds and Radiopharmaceuticals. 2013;56, article S2 [Google Scholar]
- 87.Rühl T, Rafique W, Lien VT, et al. Cu(i)-mediated 18F-trifluoromethylation of arenes: rapid synthesis of 18F-labeled trifluoromethylarenes. Chemical Communications. 2014;50:6056–6059. doi: 10.1039/c4cc01641f. [DOI] [PubMed] [Google Scholar]
- 88.Huiban M, Tredwell M, Mizuta S, et al. A broadly applicable [18F]trifluoro-methylation of aryl and heteroaryl iodides for PET imaging. Nature Chemistry. 2013;5:941–944. doi: 10.1038/nchem.1756. [DOI] [PubMed] [Google Scholar]
- 89.Lien VT, Riss P. Radiosynthesis of [18F]trifluoralkyl groups: scope and limitations. doi: 10.1155/2014/380124. BioMed Research International. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sundararaju B, Achard M, Bruneau C. Transition metal catalyzed nucleophilic allylic substitution: activation of allylic alcohols via p-allylic species. Chemical Society Reviews. 2012;41:4467–4483. doi: 10.1039/c2cs35024f. [DOI] [PubMed] [Google Scholar]
- 91.Hollingworth C, Hazari A, Hopkinson MN, et al. Palladium-catalyzed allylic fluorination. Angewandte Chemie: International Edition. 2011;50(11):2613–2617. doi: 10.1002/anie.201007307. [DOI] [PubMed] [Google Scholar]
- 92.Topczewski JJ, Tewson TJ, Nguyen HM. Iridium-catalyzed allylic fluorination of trichloroacetimidates. Journal of the American Chemical Society. 2011;133(48):19318–19321. doi: 10.1021/ja2087213. [DOI] [PubMed] [Google Scholar]
- 93.Huang X, Liu W, Ren H, et al. Late stage benzylic C-Hf with [18F]fluoride for PET imaging. Journal of the American Chemical Society. 2014;136(19):6842–6845. doi: 10.1021/ja5039819. [DOI] [PubMed] [Google Scholar]
- 94.Palmer AJ, Clark JC, Goulding RW. The preparation of fluorine 18 labelled radiopharmaceuticals. International Journal of Applied Radiation and Isotopes. 1977;28(1-2):53–65. doi: 10.1016/0020-708x(77)90160-0. [DOI] [PubMed] [Google Scholar]
- 95.Coenen HH, Ermert J. Direct nucleophilic 18F-fluorination of electron rich arenes: present limits of no-carrier-added reactions. Current Radiopharmaceuticals. 2010;3(3):163–173. [Google Scholar]
- 96.Knöchel A, Zwernemann O. Development of a no-carrier-added method for 18F-labelling of aromatic compounds by fluorodediazonation. Journal of Labelled Compounds and Radiopharmaceuticals. 1996;38(4):325–336. [Google Scholar]
- 97.Riss PJ, Kuschel S, Aigbirhio FI. No carrier-added nucleophilic aromatic radiofluorination using solid phase supported arenediazonium sulfonates and 1-(aryldiazenyl)piperazines. Tetrahedron Letters. 2012;53(14):1717–1719. [Google Scholar]
- 98.Koslowsky I, Mercer J, Wuest F. Synthesis and application of 4-[18F]fluorobenzylamine: a versatile building block for the preparation of PET radiotracers. Organic and Biomolecular Chemistry. 2010;8(20):4730–4735. doi: 10.1039/c0ob00255k. [DOI] [PubMed] [Google Scholar]
- 99.Way J, Wuest F. Fully automated synthesis of 4-[18F]fluorobenzylamine based on borohydride/NiCl2 reduction. Nuclear Medicine and Biology. 2013;40(3):430–436. doi: 10.1016/j.nucmedbio.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 100.Wuest F. Fluorine-18 labeling of small molecules: the use of 18F-labeled aryl fluorides derived from no-carrier-added [18F]fluoride as labeling precursors. (Ernst Schering Research Foundation workshop).PET Chemistry: The Driving Force in Molecular Imaging. 2007;64:51–78. doi: 10.1007/978-3-540-49527-7_3. [DOI] [PubMed] [Google Scholar]
- 101.Li L, Hopkinson MN, Yona RL, Bejot R, Gee AD, Gouverneur V. Convergent 18F radiosynthesis: a new dimension for radiolabelling. Chemical Science. 2011;2(1):123–131. [Google Scholar]
- 102.Fehler SK, Maschauer S, Höfling SB, et al. Fast and efficient 18F-labeling by [18F] fluorophenylazocarboxylic esters. Chemistry. 2014;20:370–375. doi: 10.1002/chem.201303409. [DOI] [PubMed] [Google Scholar]
- 103.Mühlhausen U, Ermert J, Coenen HH. Synthesis, labelling and first evaluation of [18F]R91150 as a serotonin 5-HT2A receptor antagonist for PET. Journal of Labelled Compounds and Radiopharmaceuticals. 2009;52(1):13–22. [Google Scholar]
- 104.Kügler F, Ermert J, Coenen HH. Labeling of benzodioxin piperazines with fluorine-18 as prospective radioligands for selective imaging of dopamine D4 receptors. Journal of Labelled Compounds and Radiopharmaceuticals. 2013;56:609–618. doi: 10.1002/jlcr.3074. [DOI] [PubMed] [Google Scholar]
- 105.Dollé F. [18F]Fluoropyridines: from conventional radiotracers to the labeling of macromolecules such as proteins and oligonucleotides. In: Schubiger PA, Lehmann L, Friebe M, editors. PET Chemistry: The Driving Force in Molecular Imaging. Vol. 62. 2007. pp. 113–157. [DOI] [PubMed] [Google Scholar]
- 106.Malik N, Solbach C, Voelter W, MacHulla H-J. Nucleophilic aromatic substitution by [18F]fluoride at substituted 2-nitropyridines. Journal of Radioanalytical and Nuclear Chemistry. 2010;283(3):757–764. [Google Scholar]
- 107.Malik N, Voelter W, MacHulla H-J, Solbach C. Radiofluorination of 2-fluoropyridines by isotopic exchange with [18F]fluoride. Journal of Radioanalytical and Nuclear Chemistry. 2011;287(1):287–292. [Google Scholar]
- 108.Hostetler ED, Sanabria-Bohórquez S, Fan H, Zeng Z, et al. [18F]Fluoroazabenzoxazoles as potential amyloid plaque PET tracers: synthesis and in vivo evaluation in rhesus monkey. Nuclear Medicine and Biology. 2011;38:1193–1203. doi: 10.1016/j.nucmedbio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 109.Siméon FG, Wendahl MT, Pike VW. The [18F]2-fluoro-1,3-thiazolyl moiety: an easily-accessible structural motif for prospective molecular imaging radiotracers. Tetrahedron Letters. 2010;51(46):6034–6036. doi: 10.1016/j.tetlet.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marchand P, Lorilleux C, Gilbert G, et al. Efficient radiosynthesis of 2-[18F]fluoroadenosine: a new route to 2-[18F]fluoropurine nucleosides. ACS Medicinal Chemistry Letters. 2010;1(6):240–243. doi: 10.1021/ml100055m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kügler F, Sihver W, Ermert J, et al. Evaluation of 18F-labeled benzodioxine piperazine-based dopamine D 4 receptor ligands: lipophilicity as a determinate of nonspecific binding. Journal of Medicinal Chemistry. 2011;54(24):8343–8352. doi: 10.1021/jm200762g. [DOI] [PubMed] [Google Scholar]
- 112.Rbah-Vidal L, Vidal A, Besse S, et al. Early detection and longitudinal monitoring of experimental primary and disseminated melanoma using [18F]ICF01006, a highly promising melanoma PET tracer. European Journal of Nuclear Medicine and Molecular Imaging. 2012;39(9):1449–1461. doi: 10.1007/s00259-012-2168-y. [DOI] [PubMed] [Google Scholar]
- 113.Fischer S, Hiller A, Smits R, et al. Radiosynthesis of racemic and enantiomerically pure (-)-[18F]flubatine-A promising PET radiotracer for neuroimaging of α4Β2 nicotinic acetylcholine receptors. Applied Radiation and Isotopes. 2013;74:128–136. doi: 10.1016/j.apradiso.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 114.Liu J, Zhu L, Plössl K, Lieberman BP, Kung HF. Synthesis and evaluation of novel N-fluoropyridyl derivatives of tropane as potential PET imaging agents for the dopamine transporter. Bioorganic and Medicinal Chemistry Letters. 2011;21(10):2962–2965. doi: 10.1016/j.bmcl.2011.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Swahn B-M, Sandell J, Pyring D, et al. Synthesis and evaluation of pyridylbenzofuran, pyridylbenzothiazole and pyridylbenzoxazole derivatives as18F-PET imaging agents for β-amyloid plaques. Bioorganic and Medicinal Chemistry Letters. 2012;22(13):4332–4337. doi: 10.1016/j.bmcl.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 116.Damont A, Boisgard R, Kuhnast B, et al. Synthesis of 6-[18F]fluoro-PBR28, a novel radiotracer for imaging the TSPO 18 kDa with PET. Bioorganic and Medicinal Chemistry Letters. 2011;21(16):4819–4822. doi: 10.1016/j.bmcl.2011.06.048. [DOI] [PubMed] [Google Scholar]
- 117.Hamill TG, Eng W, Jennings A, et al. The synthesis and preclinical evaluation in rhesus monkey of [18F]MK-6577 and [11C]CMPyPB glycine transporter 1 positron emission tomography radiotracers. Synapse. 2011;65(4):261–270. doi: 10.1002/syn.20842. [DOI] [PubMed] [Google Scholar]
- 118.Hostetler ED, Eng W, Joshi AD, et al. Synthesis, characterization, and monkey PET studies of [18F]MK-1312, a PET tracer for quantification of mGluR1 receptor occupancy by MK-5435. Synapse. 2011;65(2):125–135. doi: 10.1002/syn.20826. [DOI] [PubMed] [Google Scholar]
- 119.Vasdev N, Cao P, Van Oosten EM, et al. Synthesis and PET imaging studies of [18F]2-fluoroquinolin-8-ol ([18F]CABS13) in transgenic mouse models of Alzheimer’s disease. MedChemComm. 2012;3(10):1228–1230. [Google Scholar]
- 120.Tietz O, Sharma SK, Kaur J, et al. Synthesis of three 18F-labelled cyclooxygenase-2 (COX-2) inhibitors based on a pyrimidine scaffold. Organic and Biomolecular Chemistry. 2013;11:8052–8064. doi: 10.1039/c3ob41935e. [DOI] [PubMed] [Google Scholar]
- 121.Pike VW, Aigbirhio FI. Reactions of cyclotron-produced [18F]fluoride with diaryliodonium salts: a novel single-step route to no-carrier-added [18F]fluoroarenes. Journal of the Chemical Society, Chemical Communications. 1995;(21):2215–2216. [Google Scholar]
- 122.Chun J-H, Pike VW. Single-step syntheses of no-carrier-added functionalized [18F]fluoroarenes as labeling synthons from diaryliodonium salts. Organic and Biomolecular Chemistry. 2013;11(37):6300–6306. doi: 10.1039/c3ob41353e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chun J-H, Lu S, Pike VW. Rapid and efficient radiosyntheses of meta-substituted [18F]fluoroarenes from [18F]fluoride ion and diaryliodonium tosylates within a microreactor. European Journal of Organic Chemistry. 2011;(23):4439–4447. doi: 10.1002/ejoc.201100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chun J-H, Lu S, Lee Y-S, Pike VW. Fast and high-yield microreactor syntheses of ortho -substituted [18F]Fluoroarenes from reactions of [18F]Fluoride ion with diaryliodonium salts. Journal of Organic Chemistry. 2010;75(10):3332–3338. doi: 10.1021/jo100361d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chun J-H, Telu S, Lu S, Pike VW. Radiofluorination of diaryliodonium tosylates under aqueous-organic and cryptand-free conditions. Organic and Biomolecular Chemistry. 2013;11(31):5094–5099. doi: 10.1039/c3ob40742j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ermert J, Hocke C, Ludwig T, Gail R, Coenen HH. Comparison of pathways to the versatile synthon of no-carrier-added 1-bromo-4-[18F]fluorobenzene. Journal of Labelled Compounds and Radiopharmaceuticals. 2004;47(7):429–441. [Google Scholar]
- 127.Ross TL, Ermert J, Hocke C, Coenen HH. Nucleophilic 18F-fluorination of heteroaromatic iodonium salts with no-carrier-added [18F]fluoride. Journal of the American Chemical Society. 2007;129(25):8018–8025. doi: 10.1021/ja066850h. [DOI] [PubMed] [Google Scholar]
- 128.Satyamurthy N, Barrio JR. Preparing F-18 labeled aryl derivative, useful for preparation of F-18 labeled biomarkers, comprises replacing iodonium ylide group of benzene derivative containing iodonium ylide group with no-carrier-added F-18 fluoride ion. Patent. 2010;(WO2010117435-A2)
- 129.Cardinale J, Ermert J, Humpert S, et al. Iodonium ylides for one-step, no-carrier-added radiofluorination of electron rich arenes, exemplified with 4-(([18F]fluoro-phenoxy)-phenylmethyl)piperidine NET and SERT ligands. RSC Advances. 2014;4:17293–17299. [Google Scholar]
- 130.Chun J-H, Pike VW. Single-step radiosynthesis of “18F-labeled click synthons” from azide-functionalized diaryliodonium salts. European Journal of Organic Chemistry. 2012;(24):4541–4547. doi: 10.1002/ejoc.201200695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chun J-H, Pike VW. Selective syntheses of no-carrier-added 2- and 3-[18F] fluorohalopyridines through the radiofluorination of halopyridinyl(4′-methoxyphenyl)iodonium tosylates. Chemical Communications. 2012;48(79):9921–9923. doi: 10.1039/c2cc35005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mu L, Fischer CR, Holland JP, et al. 18F-radiolabeling of aromatic compounds using triarylsulfonium salts. European Journal of Organic Chemistry. 2012;(5):889–892. [Google Scholar]
- 133.Chun J-H, Morse CL, Chin FT, Pike VW. No-carrier-added [18F]fluoroarenes from the radiofluorination of diaryl sulfoxides. Chemical Communications. 2013;49(21):2151–2153. doi: 10.1039/c3cc37795d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tredwell M, Gouverneur V. 18F labeling of arenes. Angewandte Chemie: International Edition. 2012;51(46):11426–11437. doi: 10.1002/anie.201204687. [DOI] [PubMed] [Google Scholar]
- 135.Gouverneur V. Radiochemistry: flipping fluoride’s reactivity. Nature Chemistry. 2012;4(3):152–154. doi: 10.1038/nchem.1280. [DOI] [PubMed] [Google Scholar]
- 136.Lee E, Kamlet AS, Powers DC, et al. A fluoride-derived electrophilic late-stage fluorination reagent for PET imaging. Science. 2011;334(6056):639–642. doi: 10.1126/science.1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brandt JR, Lee E, Boursalian GB, et al. Mechanism of electrophilic fluorination with Pd(IV): fluoride capture and subsequent oxidative fluoride transfer. Chemical Science. 2014;5:169–179. doi: 10.1039/C3SC52367E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cardinale J, Ermert J, Kügler F, Helfer A, Brandt MR, Coenen HH. Carrier-effect on palladium-catalyzed, nucleophilic 18F-fluorination of aryl triflates. Journal of Labelled Compounds and Radiopharmaceuticals. 2012;55(12):450–453. [Google Scholar]
- 139.Lee E, Hooker JM, Ritter T. Nickel-mediated oxidative fluorination for PET with aqueous [18F] fluoride. Journal of the American Chemical Society. 2012;134(42):17456–17458. doi: 10.1021/ja3084797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reischl G, Kienzle GJ, Machulla H-J. Electrochemical radiofluorination. Part 2. Anodic monofluorination of substituted benzenes using [18F]fluoride. Applied Radiation and Isotopes. 2003;58(6):679–683. doi: 10.1016/s0969-8043(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 141.Kienzle GJ, Reischl G, Machulla H-J. Electrochemical radiofluorination. 3. Direct labeling of phenylalanine derivatives with [18F]fluoride after anodic oxidation. Journal of Labelled Compounds and Radiopharmaceuticals. 2005;48(4):259–273. [Google Scholar]
- 142.Långström B, Hartvig P. GMP: three letters with many interpretations: protection of patients or killing the clinical and research applications of PET? European Journal of Nuclear Medicine and Molecular Imaging. 2008;35(4):693–694. doi: 10.1007/s00259-007-0657-1. [DOI] [PubMed] [Google Scholar]
- 143.Elsinga PH. Present and future of PET-radiopharmaceuticals. Nuclear Medicine Review. 2012;15:C13–C16. [Google Scholar]
- 144.Barre L, Barbier L, Lasne MC. Investigation of possible routes to no-carrier-added 4-[18F]fluorophenol. Journal of Labelled Compounds and Radiopharmaceuticals. 1994;35:167–168. [Google Scholar]
- 145.Ekaeva I, Barre L, Lasne M-C, Gourand F. 2- and 4-[18F]Fluorophenols from Baeyer-Villiger Oxidation of [18F]Fluorophenylketones and [18F]Fluorobenzaldehydes. Applied Radiation and Isotopes. 1995;46(8):777–782. [Google Scholar]
- 146.Ludwig T, Ermert J, Coenen HH. 4-[18F]fluoroarylalkylethers via an improved synthesis of n.c.a. 4-[18F]fluorophenol. Nuclear Medicine and Biology. 2002;29(2):255–262. doi: 10.1016/s0969-8051(01)00302-x. [DOI] [PubMed] [Google Scholar]
- 147.Wagner FM, Ermert J, Coenen HH. Three-step, “one-pot” radiosynthesis of 6-fluoro-3,4-dihydroxy-l-phenylalanine by isotopic exchange. Journal of Nuclear Medicine. 2009;50(10):1724–1729. doi: 10.2967/jnumed.109.063297. [DOI] [PubMed] [Google Scholar]
- 148.Gao Z, Lim YH, Tredwell M, et al. Metal-free oxidative fluorination of phenols with [18F]fluoride. Angewandte Chemie: International Edition. 2012;51(27):6733–6737. doi: 10.1002/anie.201201502. [DOI] [PubMed] [Google Scholar]
- 149.Ross TL, Ermert J, Coenen HH. Synthesis of no-carrier-added 4-[18F]fluorophenol from 4-benzyloxyphenyl-(2-thienyl)iodonium bromide. Molecules. 2011;16(9):7621–7626. doi: 10.3390/molecules16097621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Helfer A, Castillo Meleán J, Ermert J, et al. Bis(4-benzyloxyphenyl)iodonium salts as effective precursors for the no-carrier-added radiosynthesis of 4-[18F]fluorophenol. Applied Radiation and Isotopes. 2013;82:264–267. doi: 10.1016/j.apradiso.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 151.Way J, Bouvet V, Wuest F. Synthesis of 4-[18F]fluorohalobenzenes and Palladium-mediated Cross-coupling Reactions for the Synthesis of F-18-labeled Radiotracers. Current Organic Chemistry. 2013;17:2138–2152. [Google Scholar]
- 152.Ermert J, Ludwig T, Gail R, Coenen HH. [18F]Fluorophenyl organometallics as intermediates of no-carrier-added 18F-fluoroarylation reactions. Journal of Organometallic Chemistry. 2007;692(19):4084–4092. [Google Scholar]
- 153.Gao Z, Gouverneur V, Davis BG. Enhanced aqueous Suzuki-Miyaura coupling allows site-specific polypeptide 18F-labeling. Journal of the American Chemical Society. 2013;135:13612–13615. doi: 10.1021/ja4049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ermert J, Hocke C, Ludwig T, Gail R, Coenen HH. Comparison of pathways to the versatile synthon of no-carrier-added 1-bromo-4-[18F]fluorobenzene. Journal of Labelled Compounds and Radiopharmaceuticals. 2004;47(7):429–441. [Google Scholar]
- 155.Wüst FR, Kniess T. Synthesis of 4-[18F]fluoroiodobenzene and its application in Sonogashira cross-coupling reactions. Journal of Labelled Compounds and Radiopharmaceuticals. 2003;46(8):699–713. [Google Scholar]
- 156.Cardinale J, Ermert J, Coenen HH. Convenient preparation of (4-iodophenyl)aryliodonium salts. Tetrahedron. 2012;68(22):4112–4116. [Google Scholar]
- 157.Cardinale J, Ermert J. Simplified synthesis of aryliodonium ylides by a one-pot procedure. Tetrahedron Letters. 2013;54(16):2067–2069. [Google Scholar]
- 158.Kügler F, Cardinale J, Kaufholz P, et al. Efficient radiosyntheses of 18F-fluorinated aromatic amines using innovative iodonium precursors. Journal of Nuclear Medicine, Meeting Abstract. 2012;53, abstract 185 [Google Scholar]
- 159.Way JD, Wuest F. Automated radiosynthesis of no-carrier-added 4-[18F]fluoroiodobenzene: a versatile building block in 18F radiochemistry. Journal of Labelled Compounds and Radiopharmaceuticals. 2014;57:104–109. doi: 10.1002/jlcr.3137. [DOI] [PubMed] [Google Scholar]


