Abstract
Background
Evaluation of physical activity is integral to the assessment of daily physical function and a potential objective outcome measure for clinical trials. We evaluated the feasibility of using pedometers to measure physical activity in adolescents and adults with cystic fibrosis (CF) and assessed the responsiveness of its measurement to changes in health state.
Methods
Participants were recruited through two CF clinics in Seattle, WA. Subjects were instructed to use their pedometer for at least one ill and two well periods (each lasting 7 days). Step rate was calculated as steps per hour of use. Daily symptoms were also recorded using the CF Respiratory Symptom Diary (CFRSD). Generalized estimating equation linear regression was used to compare mean step rate between health states and by self-reported symptom category.
Results
We enrolled 30 CF patients with a mean (±SD) age of 22 (±7) years and a mean forced expiratory volume in 1 second (FEV1) of 57% (±25%) predicted. The mean period step rate increased from 397 (95% CI 324 – 497) steps/hour when ill to 534 (95% CI 413 – 654) steps/hour when well (p=0.015). Pedometer-recorded step rate also correlated with self-reported physical activity items on the CFRSD.
Conclusion
Step rate measured with a pedometer correlates significantly with changes in health status and self-reported activity, and could be used as an outcome measure in CF.
1. Introduction
Over the past decade, there has been tremendous growth in the number of medications and devices in the research and development pipeline for the treatment of cystic fibrosis [1]. One of the many challenges with clinical trial design is developing clinical outcome measures that efficiently demonstrate efficacy, thus improving the chance of successful U.S. Food and Drug Administration (FDA) registration approval [2].
Measures of clinical efficacy should be based on how a patient “feels, functions, or survives” [2]. The FDA considers patient-reported outcomes (PROs) to be the most direct approach to collecting symptom data [3]. As physical activity is an integral component of function, questionnaires have been developed to measure self-reported physical activity [4]. Physical activity questionnaires may demonstrate limited reliability and validity however because they can be susceptible to recall and response biases (e.g., perceived desirability of a given response) and may provide very rough estimates of physical activity with significant measurement error [5]. One potential solution is to complement the use of such instruments with actual monitoring of physical activity.
Physical activity monitors that track movement, such as pedometers and accelerometers, offer the advantage of direct measurement of physical activity and are considered more reliable than self-report questionnaires [6]. Although accelerometers capture more detailed information on physical activity than pedometers, pedometers are more practical as they are simpler to use, and more affordable [7]. Physical activity monitors have been studied extensively in the preventative health arena to quantify physical activity levels before and after health promotion interventions targeted at the physically inactive [8]. Physical activity monitors have also been studied in the CF population as physical fitness and activity levels have been previously shown to influence prognosis [9]. Although numerous studies have evaluated accelerometers in the CF population [10-21], to our knowledge, no studies have evaluated pedometers to detect changes in health state (e.g. ‘ill’ or exacerbation state defined by an increase in symptoms prompting initiation of antibiotics versus ‘well’ or stable state). Ability to differentiate between health states with an objectively derived measure such as pedometer-recorded step rate is appealing since it has the potential to be used as a functional endpoint in clinical trials.
This study had three aims. First, we wanted to evaluate the feasibility of using pedometers to monitor step counts in CF patients. Second, we wanted to determine if pedometer-recorded step rates could be used to discriminate between ‘ill’ and ‘well’ health states in CF patients. Third, we wanted to examine the relationship between pedometer-recorded step rates and self-reported symptoms/physical activity levels using the CFRSD [22]. We hypothesized that CF patients would tolerate pedometers; pedometer-recorded step rates would change significantly within patients between ‘ill’ and ‘well’ health states, and finally would be significantly correlated with self-reported symptoms and physical activity.
2. Methods
2.1. Participants
Individuals were recruited based on convenience and availability sampling from two CF clinics in Seattle, WA, the University of Washington Adult CF Clinic and Seattle Children's Hospital CF Clinic. The study population was primarily recruited for a study designed to compare 7-day and repeated 24-hour recall of symptoms of cystic fibrosis using the CFRSD [23]. A subgroup of this sample with pedometer recordings was used for this ancillary study as described below.
Although children 2 years or older were recruited for the primary study subjects who were ≥ 12 years old and had a confirmed diagnosis of CF with genetic and/or sweat chloride testing were included in this analysis [24]. Similar to the primary study patients were excluded if they had previously undergone solid organ transplantation. As one of the primary aims of this ancillary study was to compare step rates between health states, patients were also excluded if they did not have pedometer data from at least one ‘ill’ and one ‘well’ period. Patient characteristics, including age, gender, ethnicity and lung function as measured by the forced expiratory volume in 1 second (FEV1) percent-predicted were documented at enrollment.
2.2. Procedures
Following enrollment, study participants were instructed on how to wear a New-Lifestyles℗ Digi-Walker Pedometer (Model SW-401). This pedometer model was chosen for its proven accuracy and reliability [25]. Participants were requested to wear the pedometer during all waking hours for a total of 21 days, which comprised of two ‘well’ periods each of 7 days duration and one ‘ill’ period of 7 days. Participants recorded start/stop times of usage and step counts on these days. Enrolled patients were requested to contact the study coordinator when they were beginning to feel ‘ill’ based on symptoms. An ‘ill’ period was defined by the presence of pulmonary symptoms warranting oral and/or intravenous antibiotics following clinical evaluation. To adjust daily step counts for hours of actual pedometer usage we calculated a daily step rate, expressed as steps per hour. A mean step rate was also calculated for each individual when ill and well; data from the two well periods were combined to generate this value for the well period. Additional analyses employed daily data and repeated measures regression outlined below.
Feasibility of pedometer use was evaluated by analyzing: 1) the number of well periods of use per individual (note: ill periods were not analyzed for feasibility as this was dependent upon becoming ill during the study); 2) the number of days of use per period when ill and well; 3) the number of hours of use per day when ill and well; 4) the number of hours of use for adolescents (12-18 years) and adults (19 years and older); 5) the number of hours of use on weekdays and weekends; and 6) the variation of day-to-day measurements within an ill or well period as outlined in the statistical analysis below.
In addition to documentation of pedometer data, patients completed the 16-item CFRSD on a daily basis using a web-based questionnaire. The CFRSD has been described in detail elsewhere [22] and captures the patient-reported impact of CF along 3 domains: respiratory symptoms, emotional impact, and activity impact. Our study compared individual mean step rates based on the presence or absence of specific respiratory symptom items, emotional impact items, and activity impact items.
The Institutional Review Boards of the University of Washington and Children's Hospital and Regional Medical Center (CHRMC) approved the study and enrolled patients provided informed consent.
2.3. Statistical analyses
All descriptive statistics were presented as means ± standard deviations and ranges where appropriate. Simple linear regression analysis was used to examine the relationship between individual baseline lung function expressed as FEV1 percent-predicted and mean step rate during the well state. Correlation of daily step rates for an individual within a health state (ill or well) was determined by calculating the intraclass correlation coefficient (ICC). (Note: calculation of ICC was to quantify the day-to-day variation in step rates within a given health state, and not to evaluate test reliability of the pedometer itself). Differences in day-to-day step rates within a 7-day period were also analyzed using one-way repeated measures ANOVA.
Mean daily step rates were compared between ill and well periods using general estimating equation linear regression with the Huber-White estimator of variance to account for within-subject correlation. The same approach was used to compare mean daily step rates by yes or no response to the CFRSD items. A sensitivity analysis was conducted using pedometer-recorded step counts (as opposed to step rates) to determine whether adjusting for hours of device use influenced the results. All statistical testing was two-sided and performed at the 5% significance level using STATA 10.0 software.
3. Results
3.1. Patient Characteristics
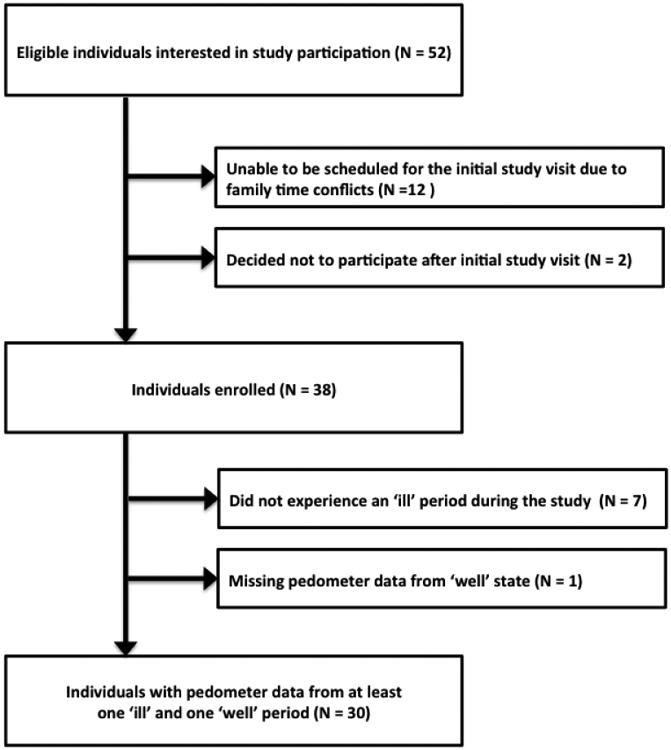
Fifty-two eligible CF patients were interested in primary study participation. Twelve eligible patients were excluded because they could not be scheduled for the initial study visit due to family time conflicts and 2 decided not to participate following the initial study visit but the specific reason for not participating was not declared. Out of the 38 individuals recruited for the primary study, 7 individuals did not experience an ill period during the study and 1 individual did not use the pedometer during at least one well period, with the result that 30 participants had pedometer data from at least one ill and one well period and were subsequently included in the analysis (Figure 1).
Figure 1. Study Population.
At the time of enrollment, participants were predominantly above the age of 19 years (70%), female (57%), and white (93%) with a mean (± SD) percent-predicted FEV1 of 57 (± 25) (Table 1).
Table 1. Baseline Patient Characteristics.
| Patient Characteristics (N=30) | N | % |
|---|---|---|
| Female gender | 17 | 57% |
| White ethnicity | 25 | 93%* |
| Age in years, mean (SD) and range | 22 (7) | 12-38 |
| Age group (years) | ||
| 12 to 18 | 9 | 30% |
| 19+ | 21 | 70% |
| FEV1% predicted, mean (SD) and range | 57 (25) | 25-107 |
Denominator 27 since 3 missing ethnicity values
3.2. Aim 1: To evaluate the feasibility of using pedometers to measure step counts
Twenty-seven of 30 participants used the pedometer during two well periods as recommended in the study protocol, resulting in an adherence rate of 90%. Participants used the pedometer for a mean (± SD) of 5.4 (± 1.0) out of 7 days during ill periods and 5.2 (± 1.3) out of 7 days during well periods, with an overall adherence rate of 76%. Pedometer recordings by day of week was distributed fairly evenly across the week, with a low of 11% of total recordings from Mondays and a high of 18% of total recordings from Fridays. The fairly even distribution of pedometer recordings throughout the week did not differ by health state (ill versus well: chi-square p-value = 0.76) or age group (12 to ≤ 18 years versus 19 to 38 years: chi-square p-value = 0.97). The mean number of hours of use per day was slightly lower during ill compared to well periods, with 11.7 (± 3.1) hours of use during ill periods and 12.2 (± 3.0) hours of use during well periods, but the difference was not statistically significant (mean difference: 0.5 hours; 95% CI for mean difference: -0.1 to 1.1 hours). The mean number of hours of use per day was similar for adolescents (12 to 18 years) and adults (19 years and older) with a mean difference of 0.1 hours (95% CI for mean difference: -0.6 to 0.7). The mean number of hours of use per day was slightly higher on weekdays with 12.1 (± 3.1) hours of daily use compared to weekends with 11.7 (± 3.1) hours of daily use, but once again the difference was not statistically significant (mean difference: 0.5 hours; 95% CI for mean difference: -0.2 to 1.1) and did not differ by health state (mean difference when ill: 0.4 hours; 95% CI for mean difference when ill: -0.6 to 1.5 versus mean difference when well: 0.4 hours; 95% CI for mean difference when well: -0.4 to 1.2). The intraclass correlation coefficient for daily mean step rate measurements within a given health state for an individual was fair, with a coefficient of 0.43 when ill and 0.47 when well. Furthermore, the step rate did not differ significantly day-to-day within a given 7-day period when analyzed using one-way repeated measures ANOVA (ill period p=0.24; well period p=0.55).
3.3. Aim 2: To determine if pedometer-recorded step rates differ for individuals based on health status (ill vs. well)
The mean pedometer-recorded step count was 4589 during the ill state and 6246 during the well state, with a mean difference of 1647 steps per day (95% CI for mean difference: 331 to 2983 steps per day). Similarly, the mean pedometer-recorded step rate increased by 35% or 137 (95% CI 27 to 247) steps per hour during the well state compared to the ill state (p=0.015). Despite this difference, mean step rate decreased for 12 of 30 individuals during the well state compared to the ill state. For these 12 individuals, the mean step rate decreased by 93 (95% CI 51 to 135) steps per hour. These 12 individuals were more likely to be 19 years or older and have worse lung function compared to those individuals whose mean step rate increased from ill to well, but the difference in lung function was not statistically significant (Table 2).
Table 2. Baseline Patient Characteristics By Direction of Change in Mean Step Rate from Ill to Well.
| Change in Individual Mean Step Rate from Ill to Well | |||
|---|---|---|---|
| Patient Characteristics | Increased (N=18) | Decreased (N=12) | p-value |
| Age group (years) | |||
| 12 to 18 | 8 | 1 | 0.03 |
| 19+ | 10 | 11 | |
| Female gender | 56% | 58% | 0.89 |
| FEV1 % pred - mean (SD) | 63 (27) | 49 (19) | 0.11 |
| Ill during 1st recording period | 56% | 50% | 0.78 |
3.4. Aim 3: To explore the relationship between pedometer-recorded step rate and self-reported symptoms based on the CFRSD
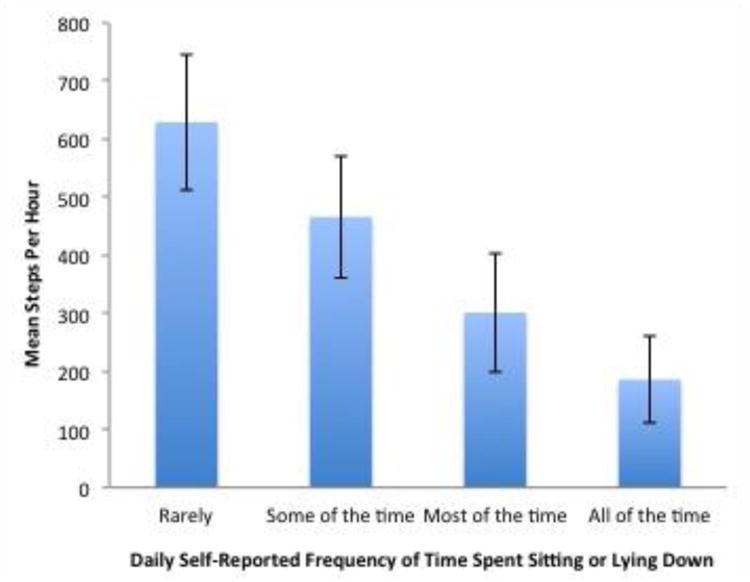
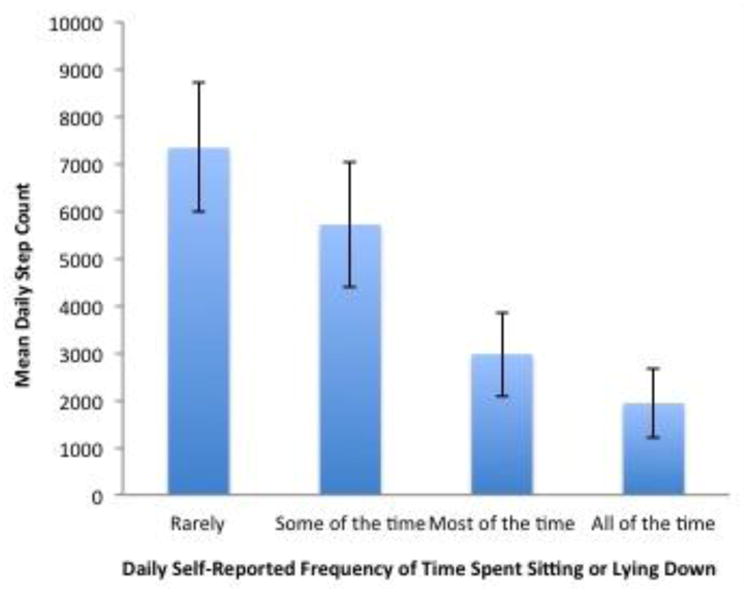
Mean step rate decreased significantly with the presence of the following self-reported respiratory symptoms: difficulty breathing, cough, chest tightness, and feeling tired (Table 3). Mean step rate also decreased significantly with the presence of the following emotional impact items: feeling worried, feeling cranky, and feeling frustrated. Presence of productive cough, wheezing, fever, chills, feeling sad, and difficulty sleeping did not significantly change step rates. Individuals that missed work or school due to illness and/or reported a reduction in usual activity levels had lower mean step rates. Individuals that reported a greater amount of time spent sitting or lying down also had lower mean step rates (Figure 2). In a sensitivity analysis, the relationship between daily step counts (as opposed to step rates) and self-reported symptoms was explored. These results were similar to our primary analysis (Appendix Table 1, Appendix Figure 1).
Table 3. Mean Step Rate by CF Respiratory Symptom Diary Response.
| Mean Steps Per Hour by CFRSD Response | |||
|---|---|---|---|
| CFRSD Item* | No | Yes | Difference (95% CI) |
| Symptom Items | |||
| Difficulty Breathing | 549 | 346 | -203 (-98 to -307) |
| Cough | 647 | 462 | -184 (-43 to -326) |
| Cough Up Mucus | 524 | 459 | -65 (+42 to -172) |
| Chest Tightness | 529 | 378 | -151 (-74 to -229) |
| Wheeze | 490 | 444 | -46 (+7 to -99) |
| Tired | 544 | 388 | -155 (-63 to -247) |
| Fever | 482 | 470 | -12 (+146 to -171) |
| Chills | 486 | 434 | -52 (+17 to -120) |
| Emotional Items | |||
| Feeling Worried | 494 | 332 | -162 (-85 to -239) |
| Feeling Cranky | 499 | 360 | -139 (-77 to -300) |
| Feeling Sad | 489 | 413 | -76 (+15 to -167) |
| Feeling Frustrated | 498 | 369 | -129 (-48 to -211) |
| Activity Items | |||
| Difficulty Sleeping | 469 | 532 | 63 (+258 to -132) |
| Missed Work or School | 541 | 249 | -292 (-82 to -501) |
| Reduced Usual Activity | 545 | 287 | -257 (-95 to -420) |
This table includes 15 of 16 CFRSD items with a binary response
Items highlighted in bold are statistically significant
Figure 2. Relationship Between Mean Step Rate and Self-Reported Frequency of Time Spent Sedentary.
3.5. Subgroup analyses of mean step rate by age and gender
When well, the mean step rate was 377 (95% CI 34 to 720) steps per hour higher for adolescents (12 to 18 years) compared to adults (19 years and older). Although males had slightly higher mean step rates compared to females when well, this difference was not statistically significant (mean difference: 78 steps per hour; 95% CI for mean difference -193 to 349).
3.6. Relationship between baseline lung function and mean step rate
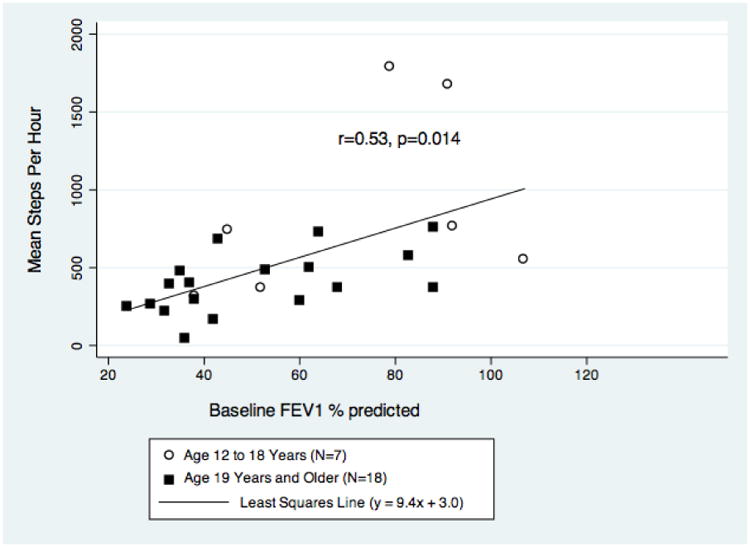
Additional analyses evaluated the relationship between disease severity based on FEV1 percent-predicted and mean step rate when well. On average, the mean step rate during the well state increased by 9.4 (95% CI 2.1-16.7) steps per hour with every 1-unit increase in baseline FEV1 percent-predicted (r=0.53; p=0.014). The linear relationship is described by the following regression equation: mean step rate = 9.4*(FEV1 % predicted) + 3.0. The variability in mean step rate increased at higher levels of baseline lung function (Figure 3).
Figure 3. Relationship Between Baseline FEV1 % predicted and Mean Step Rate When Well.
4. Discussion
Although numerous studies have examined the use of accelerometers in CF patients [10-21], to our knowledge this is the first study to examine the feasibility of using pedometers in CF patients and to evaluate their use during changes in health status. Pedometers offer several practical advantages over accelerometers as they are easier to use and much more affordable. Based on the results of this small study, the use of pedometers to monitor step counts in CF adolescents and adults during a 7-day ill or well period appears to be feasible.
Pedometer-recorded step counts for CF adolescents and adults when ‘well’ was just over 6000 steps per day and this is about 50% lower than values reported in the literature for healthy adolescents and young adults. Expected values for healthy adolescents and young adults range between 9000-13000 steps per day, depending on age and sex [26-28]. Although the underlying reasons for reduced pedometer-recorded step counts for stable CF individuals in our study are likely complex and multifactorial, it can be partly explained by the reduced lung function and respiratory symptoms of our study sample. We demonstrated lower mean step rates among individuals with lower baseline lung function (Figure 3) and in the presence of specific respiratory symptoms such as dyspnea, cough and chest tightness (Table 3). Other physiologic factors that may have contributed to the reduced step counts but not analyzed in our study include skeletal muscle dysfunction and poor nutritional status [20, 29, 30]. Non-physiologic factors such as high treatment burden and differences in the type of physical activity CF individuals engage in (e.g. rowing, biking, resistance training) may also explain the lower daily step counts for CF individuals relative to the general population [31-33].
Pedometer-recorded step rates were 35% higher in the well state relative to the ill state, suggesting that pedometers can be used to detect changes in physical activity levels with changes in health status. However, this finding needs to be interpreted with caution as 12 of 30 (40%) individuals actually had a decrease in their pedometer-recorded step rate when well compared to ill (as noted by the high standard deviation of the mean step rates). We found no evidence that this was due to reactivity bias due to the novelty of using a pedometer for the first time during the ill state [34]. We suspect that individuals whose pedometer-recorded step rates paradoxically increased when they were ill compared to well may have held jobs that were sedentary in nature and therefore time off from work due to illness may have freed up more time for exercise. Although we do not have records of employment to substantiate this, 11 of 12 individuals that experienced a paradoxical increase in step rate when ill were above the age of 19 years and therefore were more likely to be employed. Alternative but less likely explanations for why these individuals may have increased their step rates when ill compared to well include: 1) increased emphasis on walking/exercise during illness to facilitate airway clearance; 2) change in the type of physical activity from intense exercises such as rowing or resistance training (not captured by a pedometer) when well to less intense exercises such as walking when ill; and 3) increasing trips to the doctor's office during periods of illness.
As expected, pedometer-recorded step rates decreased significantly with self-reported symptoms of difficulty breathing, cough, chest tightness, and feeling tired. There was no significant decrement in pedometer-recorded step rates with symptoms of wheeze, fever and chills, but these symptoms were uncommon occurring in less than 10% of patients, likely resulting in under-powering of this analysis. Pedometer-recorded step rates decreased with symptoms such as feeling worried, cranky or frustrated, suggesting that an unstable emotional state can adversely affect physical function. More likely, a tumultuous emotional state may be the result of bothersome respiratory symptoms, which ultimately limits physical activity.
This study provides preliminary validation for activity-related questionnaire items in the CFRSD. Individuals that self-reported reductions in usual activity or increasing time spent sitting or lying down had lower pedometer-recorded step rates. In light of these findings, the CFRSD does not appear to be adversely influenced by recall or response bias. As the widespread use of pedometers to monitor physical activity levels in CF patients outside of the context of a clinical trial is unlikely, data supporting the validation of a questionnaire like the CFRSD is important.
Our study is subject to limitations. First, participants were not blinded to daily pedometer step counts, as the device step count was not concealed. Knowledge of the daily pedometer step count could have influenced the response to questionnaire items, especially those related to activity. Second, the generalizability of our feasibility findings to study subjects under 12 years of age is questionable as pedometers might be perceived as toys, and therefore the pedometer readings might be unreliable due to improper use. Future studies will need to assess the feasibility of pedometer use in this patient age group. Third, although we chose the most accurate pedometer available on the market based on clinical studies, pedometers are inherently prone to measurement error when used in free-living situations, as steps can erroneously be recorded with any movement of the hip and may be less accurate at faster walking speeds [35]. Lastly, although we could quantify the number of steps taken with pedometers we could not determine: 1) distance walked as we did not have individual stride length; 2) cadence or intensity of walking; 3) the amount of time spent walking at different intensity levels; 4) the timing and intensities of other types of exercise that do not involving walking and therefore may not be measured by pedometers; and 5) whether the device was adhered to during all waking hours.
In conclusion, the use of pedometers to measure daily step counts in adolescent and adult CF patients is feasible and the recordings can be used to detect changes in step rates with changes in health status and self-reported symptoms. Pedometer-recorded step rates also correlate with activity-related items from the CF Respiratory Symptom Diary. The use of pedometers to more objectively monitor physical activity as an endpoint in clinical trials appears promising but warrants further study.
Acknowledgments
Funding for this study was provided in part by the Cystic Fibrosis Foundation, through a research grant (GOSS05A0) and the Leroy Matthew's Physician Scientist Award, and by grants from NIH (RR-00037-39) and NIH/NHLBI (K23 HL 72017). BSQ was supported by the University of British Columbia Clinical Investigator Program and British Columbia Lung Association Fellowship Award.
Sources of support: Funding of this study was provided in part by the Cystic Fibrosis Foundation. BSQ was supported by a British Columbia Lung Association Fellowship Award.
Appendix
Appendix Figure 1. Relationship Between Mean Step Count and Self-Reported Frequency of Time Spent Sedentary.
Appendix Table 1. Mean Step Count by CF Respiratory Symptom Diary Response.
| Mean Step Count by CFRSD Response | |||
|---|---|---|---|
| CFRSD Item* | No | Yes | Difference (95% CI) |
| Symptom Items | |||
| Difficulty Breathing | 6387 | 4082 | -2305 (-1130 to -3480) |
| Cough | 6399 | 5527 | -871 (-93 to -1649) |
| Cough Up Mucus | 5742 | 5555 | -188 (+1110 to -1485) |
| Chest Tightness | 6234 | 4282 | -1952 (-1086 to -2817) |
| Wheeze | 5700 | 5238 | -463 (+261 to -1187) |
| Tired | 6291 | 4614 | -1678 (-684 to -2672) |
| Fever | 5636 | 5252 | -385 (+1641 to -2410) |
| Chills | 5672 | 4993 | -679 (+221 to -1580) |
| Emotional Items | |||
| Feeling Worried | 5783 | 3604 | -2179 (-1472 to -2886) |
| Feeling Cranky | 5824 | 4202 | -1622 (-805 to -2438) |
| Feeling Sad | 5675 | 5093 | -583 (+852 to -2017) |
| Feeling Frustrated | 5818 | 4261 | -1557 (-440 to -2674) |
| Activity Items | |||
| Difficulty Sleeping | 6362 | 5429 | 934 (+3673 to -1806) |
| Missed Work or School | 6379 | 2802 | -3577 (-838 to -6317) |
| Reduced Usual Activity | 6421 | 3133 | -3288 (-1257 to -5319) |
This table includes 15 of 16 CFRSD items with a binary response
Items highlighted in bold are statistically significant
Footnotes
Author's contributions: Conception and design: CHG, MLA, RLG, DLP, TCE, AG, SM. Analysis and interpretation: BSQ, CHG. Drafting the manuscript for important intellectual content: all authors.
Data from this manuscript was presented at the American Thoracic Society 2011 International Conference on May 15, 2011. Denver, Colorado.
Conflicts of interest statement: The authors report no financial or personal relationships that could inappropriately influence this study.
References
- 1.Cystic Fibrosis Foundation. Dare to Dream. Cystic Fibrosis Foundation 2009 Annual Report. Drug Development Pipeline. 2009 [Google Scholar]
- 2.Mayer-Hamblett N, Ramsey BW, Kronmal RA. Advancing outcome measures for the new era of drug development in cystic fibrosis. Proc Am Thorac Soc. 2007;4:370–7. doi: 10.1513/pats.200703-040BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. Guidance for industry patient reported outcome measures: use in medical product development to support labeling claims. Department of Health and Human Services; Rockville, MD: 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hay JA, Cairney J. Development of the Habitual Activity Estimation Scale for Clinical Research: A Systematic Approach. Pediatr Exerc Sci. 2006;18:193–202. [Google Scholar]
- 5.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassett DR, Jr, Cureton AL, Ainsworth BE. Measurement of walking distance -Questionnaire versus pedometer. Med Sci Sports Exerc. 2000;35:1018–23. doi: 10.1097/00005768-200005000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Tudor-Locke C, McClain JJ, Hart TL, Sisson SB, Washington TL. Expected values for pedometer-determined physical activity in youth. Res Q Exerc Sport. 2009;80:164–74. doi: 10.1080/02701367.2009.10599550. [DOI] [PubMed] [Google Scholar]
- 8.Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. Jama. 2007;298:2296–304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 9.Nixon PA, Orenstein DM, Kelsey SF, Doershuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. N Engl J Med. 1992;327:1785–8. doi: 10.1056/NEJM199212173272504. [DOI] [PubMed] [Google Scholar]
- 10.Wells GD, Wilkes DL, Schneiderman-Walker J, Elmi M, Tullis E, Lands LC, et al. Reliability and validity of the habitual activity estimation scale (HAES) in patients with cystic fibrosis. Pediatr Pulmonol. 2008;43:345–53. doi: 10.1002/ppul.20737. [DOI] [PubMed] [Google Scholar]
- 11.Dwyer TJ, Alison JA, McKeough ZJ, Elkins MR, Bye PT. Evaluation of the SenseWear activity monitor during exercise in cystic fibrosis and in health. Respir Med. 2009;103:1511–7. doi: 10.1016/j.rmed.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Selvadurai HC, Blimkie CJ, Cooper PJ, Mellis CM, Van Asperen PP. Gender differences in habitual activity in children with cystic fibrosis. Arch Dis Child. 2004;89:928–33. doi: 10.1136/adc.2003.034249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tejero Garcia S, Giraldez Sanchez MA, Cejudo P, Quintana Gallego E, Dapena J, Garcia Jimenez R, et al. Bone health, daily physical activity, and exercise tolerance in patients with cystic fibrosis. Chest. 2011;140:475–81. doi: 10.1378/chest.10-1508. [DOI] [PubMed] [Google Scholar]
- 14.Hebestreit H, Kieser S, Rudiger S, Schenk T, Junge S, Hebestreit A, et al. Physical activity is independently related to aerobic capacity in cystic fibrosis. Eur Respir J. 2006;28:734–9. doi: 10.1183/09031936.06.00128605. [DOI] [PubMed] [Google Scholar]
- 15.Fournier C, Bosquet L, Leroy S, Perez T, Neviere R, Wallaert B. Measurement of daily physical activity in patients with cystic fibrosis. Rev Mal Respir. 2005;22:63–9. doi: 10.1016/s0761-8425(05)85437-3. [DOI] [PubMed] [Google Scholar]
- 16.Selvadurai HC, Blimkie CJ, Meyers N, Mellis CM, Cooper PJ, Van Asperen PP. Randomized controlled study of in-hospital exercise training programs in children with cystic fibrosis. Pediatr Pulmonol. 2002;33:194–200. doi: 10.1002/ppul.10015. [DOI] [PubMed] [Google Scholar]
- 17.Janz KF, C SI, Barr RN, Kelly JM. Monitoring Exercise in Children and Adolescents with Cystic Fibrosis: Validation of the CSA Accelerometer. Cardiopulmonary Physical Therapy Journal. 1995;6:3–8. [Google Scholar]
- 18.Beghin L, Michaud L, Loeuille GA, Wizla-Derambure N, Sayah H, Sardet A, et al. Changes in lung function in young cystic fibrosis patients between two courses of intravenous antibiotics against Pseudomonas aeruginosa. Pediatr Pulmonol. 2009;44:464–71. doi: 10.1002/ppul.21017. [DOI] [PubMed] [Google Scholar]
- 19.Beghin L, Gottrand F, Michaud L, Vodougnon H, Wizla-Derambure N, Hankard R, et al. Energetic cost of physical activity in cystic fibrosis children during Pseudomonas aeruginosa pulmonary exacerbation. Clin Nutr. 2005;24:88–96. doi: 10.1016/j.clnu.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Troosters T, Langer D, Vrijsen B, Segers J, Wouters K, Janssens W, et al. Skeletal muscle weakness, exercise tolerance and physical activity in adults with cystic fibrosis. Eur Respir J. 2009;33:99–106. doi: 10.1183/09031936.00091607. [DOI] [PubMed] [Google Scholar]
- 21.Selvadurai HC, Allen J, Sachinwalla T, Macauley J, Blimkie CJ, Van Asperen PP. Muscle function and resting energy expenditure in female athletes with cystic fibrosis. Am J Respir Crit Care Med. 2003;168:1476–80. doi: 10.1164/rccm.200303-363OC. [DOI] [PubMed] [Google Scholar]
- 22.Goss CH, Edwards TC, Ramsey BW, Aitken ML, Patrick DL. Patient-reported respiratory symptoms in cystic fibrosis. J Cyst Fibros. 2009;8:245–52. doi: 10.1016/j.jcf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Bennett AV, Patrick DL, Lymp JF, Edwards TC, Goss CH. Comparison of 7-day and repeated 24-hour recall of symptoms of cystic fibrosis. J Cyst Fibros. 2010;9:419–24. doi: 10.1016/j.jcf.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassett DR, Jr, Ainsworth BE, Leggett SR, Mathien CA, Main JA, Hunter DC, et al. Accuracy of five electronic pedometers for measuring distance walked. Med Sci Sports Exerc. 1996;28:1071–7. doi: 10.1097/00005768-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Sequeira MM, Rickenbach M, Wietlisbach V, Tullen B, Schutz Y. Physical activity assessment using a pedometer and its comparison with a questionnaire in a large population survey. Am J Epidemiol. 1995;142:989–99. doi: 10.1093/oxfordjournals.aje.a117748. [DOI] [PubMed] [Google Scholar]
- 27.Vincent SD, Pangrazi RP, Raustorp A, Tomson LM, Cuddihy TF. Activity levels and body mass index of children in the United States, Sweden, and Australia. Med Sci Sports Exerc. 2003;35:1367–73. doi: 10.1249/01.MSS.0000079024.40014.91. [DOI] [PubMed] [Google Scholar]
- 28.Strycker LA, Duncan SC, Chaumeton NR, Duncan TE, Toobert DJ. Reliability of pedometer data in samples of youth and older women. Int J Behav Nutr Phys Act. 2007;4:4. doi: 10.1186/1479-5868-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boucher GP, Lands LC, Hay JA, Hornby L. Activity levels and the relationship to lung function and nutritional status in children with cystic fibrosis. Am J Phys Med Rehabil. 1997;76:311–5. doi: 10.1097/00002060-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Sahlberg ME, Svantesson U, Thomas EM, Strandvik B. Muscular strength and function in patients with cystic fibrosis. Chest. 2005;127:1587–92. doi: 10.1378/chest.127.5.1587. [DOI] [PubMed] [Google Scholar]
- 31.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros. 2009;8:91–6. doi: 10.1016/j.jcf.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nixon PA, Orenstein DM, Kelsey SF. Habitual physical activity in children and adolescents with cystic fibrosis. Med Sci Sports Exerc. 2001;33:30–5. doi: 10.1097/00005768-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Orenstein DM, Higgins LW. Update on the role of exercise in cystic fibrosis. Curr Opin Pulm Med. 2005;11:519–23. doi: 10.1097/01.mcp.0000181476.92810.07. [DOI] [PubMed] [Google Scholar]
- 34.Rowe DA, Mahar MI, Raedeke TD, Lore J. Measuring physical activity in children with pedometers: Reliability, reactivity, and replacement of missing data. Pediatr Exerc Sci. 2004;16:343–54. [Google Scholar]
- 35.Melanson EL, Knoll JR, Bell ML, Donahoo WT, Hill JO, Nysse LJ, et al. Commercially available pedometers: considerations for accurate step counting. Prev Med. 2004;39:361–8. doi: 10.1016/j.ypmed.2004.01.032. [DOI] [PubMed] [Google Scholar]


