Dear Sir
In genetics, the term ‘mosaicism’ describes the situation in which groups of cells have a different genetic composition to other cells in an organism. Somatic gene rearrangements due to multiplication or deletion of genes (copy number variation) and/or sections of chromosomes can lead to mosaicism.
The presence of multiple copies of the alpha-synuclein gene (SNCA) is known to be associated with Parkinson’s disease (PD) and the severity of symptoms increases with the number of copies of the gene [1]. While the features of PD associated with duplication of SNCA are usually (but not always) typical of the condition [2–3], patients with triplicate copies have atypical features, including rapidly evolving symptoms, severe cognitive impairment, limited response to levodopa, more severe symptoms of dementia and more frequent urinary incontinence [2]. A lack of studies of mosaicism in PD illustrates that the prevalence and mechanism of these rearrangements have not yet been elucidated.
Two unrelated cases with early-onset PD presented in our clinic and underwent extended testing to investigate the presence of SNCA mosaicism. Here, we report genetic and immmunohistochemical findings and describe the clinical phenotype for each case.
Case 1: A 40-year-old man, whose paternal grandfather had PD and died at the age of 60, first presented with dorsal pain and gait disorders, secondary to rigidity and bradykinesia of the lower left leg at the age of 32. By the age of 34, he had developed dizziness, orthostatic hypotension, urinary disorders, as well as bradykinesia, and rigidity affecting both sides of his body. By age 35, cognitive decline was evident and by the time he came to our clinic, he was completely dependent on others for his care. The patient was unresponsive to medication for PD.
Case 2: A 23-year-old male, with no family history of PD, was diagnosed with PD at the age of 18. He initially presented with dystonic posturing and tremors in his left foot. Within a few months, this had progressed to micrographia and bradykinesia and resting tremor in his upper left limb. He subsequently developed autonomic failure, moderate cognitive decline, and behavior disorders (rage episodes, panic attacks, and hallucinations). He responded positively to treatment, but developed end-dose impairment and peak-dose dyskinesia. He also developed impulse control disorders – secondary to treatment with dopamine agonists, resulting in punding behaviors (e.g. disassembling guitars, computers and his car).
Both patients’ legal representatives provided informed consent for their inclusion in the study on their behalf. The study was approved by the ethics committee of the Hospital de Clínicas José de San Martín, University of Buenos Aires, Argentina. The genetic ancestry of both patients was investigated using Realtime PCR- HRM [4] and SNaPShot® followed by STRUCTURE 2.3.4 [5]. Case 1’s mitochondrial gene pool indicated Native American origin (mtDNA Haplogroup C, Y – Haplogroup R1b1a2) whilst autosomal ancestry information markers indicated a strong European ethnicity (99.2%). Case 2 was similar, with a Native American mitochrondial gene pool (mtDNA Haplogroup B; Y- Haplogroup I1) and autosomal markers showing European ethnicity (95.8%).
Sample cells were taken from the buccal cavity using a standard oral swab. These were screened for multiplication of the 4q22.1 locus of SNCA using multiplex ligation-dependent probe amplification (MLPA) and fluorescence in-situ hybridisation (FISH). FISH was chosen because it provides a rapid, quantitative test for detecting mutations. Cloning experiments would also have provided quantitative results, but would have been much slower and more labor intensive.
The SALSA MLPA P051-C1 Parkinson 1 probemix kit (MRC, Holland) was selected because it contains probes for five SNCA exons, as well as probes for all the exons for DJ1, PARK2 and PINK1. FISH was conducted using rhodamine-labelled SNCA probes at 4q22.1 (BAC RP11-614o7, 151kb) and 4q21.3 (BAC-RP11-711j3, 192kb [control]). Both probes were supplied by CHORI (CA, USA). Samples were considered duplicated/triplicated if they had three or four FISH probe signals, respectively, in greater than 20% of interphase cells scored in 100 interphase nuclei examined.
Both patients were screened for the presence of the point mutation A30P in the SNCA gene, as well as dose mutations for the 2a,3,4,5,6, 7b exons of this gene. No exon dosage rearrangements were detected in SNCA or other relevant PD genes using MLPA. FISH results indicated few or no rearrangements (i.e. ≤4 FISH probe signals in >20% of interphase cells scored) [6] in peripheral leucocytes from either case, but of the 57 oral mucosal cells 21% showed triplication and 61% duplication of SNCA in case 1 and 43% showed duplication in case 2 (Figures 1A and 1D). Mutations in PINK1, PARK2, and DJ1 were not found and were excluded as causes for the patients’ symptoms.
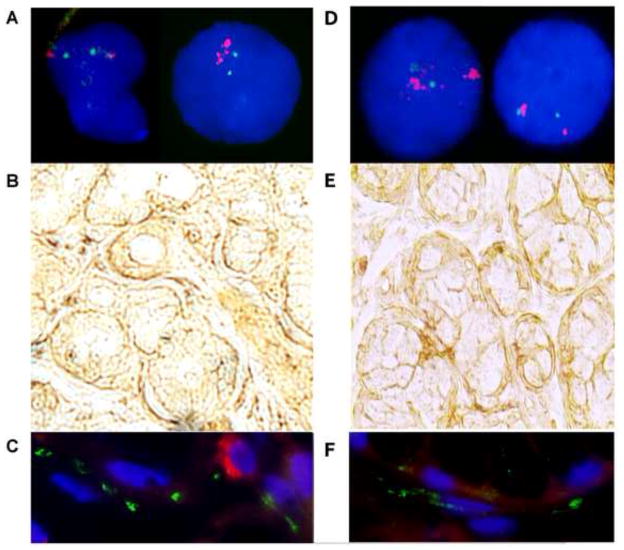
Figure 1. Results of extended testing for presence of alpha-synuclein gene (SNCA) mosaicism in cases 1 and 2.
Panels 1A and 1D:
FISH analysis was performed on interphase chromosomes with rhodamine-labelled SNCA BAC RP11-614. Panel 1A shows cells from the oral mucosa with (right) and without (left) amplification of the SNCA locus. Panel 1D shows cells from the oral mucosa without (right) and with (left) amplification of the SNCA locus. Nuclei were counterstained with DAPI. The images corresponding to case 1 and case 2 have been included in panels 1A and 1D, respectively.
Panels 1B and 1E: (100X) Immunohistochemistry findings in minor salivary glands using the peroxidase method with antibodies for the detection of SNCA.
Abundant SNCA immunoreactive profiles were detected in the periacinar space.
Panels 1C and 1F: (400X) Immunofluorescence findings in minor salivary glands.
The technique was performed using antibodies against α-SYN (1:100, rabbit polyclonal antibody, catalogue number 18-272-196445, GeneWay Biotech., Inc, San Diego, CA, USA) detected through a secondary antibody coupled to fluorescein isothiocyanate (FITC; green) and human neurofilament (NF) protein (1:300, mouse monoclonal antibody; clone 2F11, Dako, Denmark) detected through a secondary antibody coupled to rhodamine (red). The nuclei were counterstained using 4′,6-diamidino-2-phenylindole (DAPI; blue). Abundant α-SYN immunoreactive profiles were detected in the labial salivary gland in both cases. The α-SYN profiles were concentrated in the periacinar space, which contains the autonomic nerves supplying the gland.
The presence of cytokeratins is indicative of ectodermal cells. In order to prove that the cells we analysed for duplications and triplications were ectodermal in origin, samples from the oral mucosa were fixed with Carnoy (methanol:acetic acid, 3:1) and incubated with anti-keratin antibodies (1:300) for 1 hour in a Cytokeratin Cocktail, AE1, AE3 (Cell Marque, Rocklin, CA). This panel is only positive in mature ectodermic cells. Samples were then stained with peroxidase (ABC Kit Vecstastain, Vector Laboratories, Burlingame, CA) plus diaminobenzidine, and 4′,6-diamidino-2-phenylindole (DAPI). Cells containing cytokeratin stained positive.
Figure 2 shows that oral mucosal cells, stained with peroxidase and diaminobenzidine, overlapped fluorescent nuclear staining with DAPI. Two cells stained positive for cytokeratin and one was negative. Staining was observed in 60% of the 124 counted cells. All the cells that presented amplifications, as measured by the FISH technique for the SNCA gene, stained positive for cytokeratins, and, are thus of ectodermic origin.
Figure 2. Oral mucosal cells treated with anti-keratin antibodies and stained with peroxidase, diaminobenzidine and DAPI to determine their developmental origin. The images correspond to case 1.
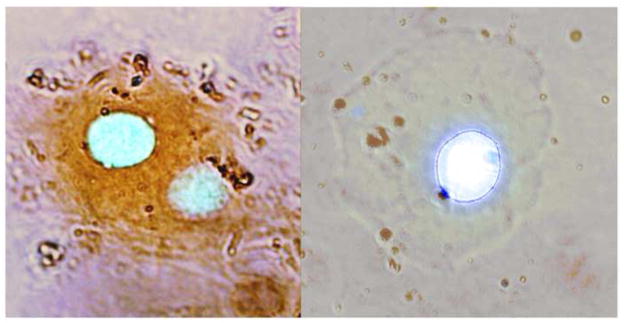
Positive staining in the left-hand photograph indicates that these two cells contain cytokeratin and hence originated in the ectoderm. The cell on the right does not express cytokeratin and consequently, it is not possible to confirm its ectodermal origin.
Immunohistochemical studies were performed on samples from the minor salivary glands using a rabbit polyclonal SNCA antibody [7]. Intense SNCA immunoreactive profiles were obtained for cells from the salivary glands of both patients (Figures 1B, 1C, 1E and 1F).
Case 1 displays the clinical phenotype classically associated with SNCA triplications (see above); however, this is, as far as we know, the first report in which normal cells have been found along with cells duplicated and triplicated for SNCA.
As previously mentioned, symptoms associated with duplication are similar to those of idiopathic PD. As such, Case 2’s young age (typical age of onset of PD is 35–77 years) and the early finding of cognitive impairment, are highly unusual; however, these atypical signs have previously been described [8].
The negative results obtained with MLPA are not entirely surprising; van Veghel-Plandsoen and colleagues have previously reported that MLPA is less sensitive than FISH for detecting low-grade mosaicism [9]. Failure to detect low levels of gene triplications may explain the otherwise exceptional findings of dementia in patients in whom only SNCA duplications are found.
Our decision to subject cells of the oral mucosa to analysis also may have facilitated identification of low level mosaicism. Usually, only lymphocytes from peripheral tissues are examined for SNCA rearrangements. Like cells from the central nervous system, oral mucosal cells are derived from the ectoderm and it is therefore interesting that duplicated/triplicated signals were obtained in these cells.
Our findings suggest that, before excluding the involvement of SNCA rearrangements in early onset PD with severe autonomic and early cognitive decline, the spectrum of evaluations should be extended to include more sensitive FISH analysis and immunohistochemical studies, as well as subjecting cells of ectodermal origin, (such as those of the oral mucosa) to analysis. Further studies are still needed to evaluate the potential role of these mosaicisms in the etiology of early-onset parkinsonism.
Table 1.
Comparison of features of parkinsonism in two cases with alpha-synuclein gen (SNCA) mosaicism
| Patient 1 | Patient 2 | |
|---|---|---|
| Age (years) | 40 | 23 |
| Age at onset (years) | 32 | 18 |
| Disease duration (years) | 8 | 5 |
| Initial symptoms | Dorsal pain/left lower limb bradykinesia | Left foot dystonic posture/bradykinesia |
| Tremor | − | + |
| Bradykinesia | +++ | +++ |
| Rigidity | +++ | + |
| Postural instability | ++ | − |
| Freezing of gait | +++ | + |
| Fluctuations | No | Wearing off |
| Disautonomy | +++ | +++ |
| Behavioral disorders | +++ | +++ |
| Dementia | +++ | + |
| MoCA | 3/30 | 14/30 |
| Antiparkinsonian response | − | +++ |
| Hyposmia | Negative | Present |
| Constipation | Present | Present |
| RBD | Present | Negative |
| UPDRS III | 36 | 23 |
| Hoehn and Yahr staging scale | IV | II |
| Brain MRI | Normal | Normal |
| Family history of Parkinson’s disease | Yes | No |
| Multiplication of SNCA (FISH in leucocytes) | 6.5% of the evaluated cells (negative) | 0% of the evaluated cells (negative) |
| Multiplication of SNCA (FISH in oral mucosae) | 75% of the evaluated cells | 42.6% of the evaluated cells |
| Type of rearrangement (SNCA) | Duplication/triplication | Duplication |
MoCA, Montreal Cognitive Assessment; RBD, rapid eye movement behavior disorder; UPDRS, Unified Parkinson’s Disease Rating Scale; MRI, magnetic resonance imaging; FISH, fluorescence in-situ hybridisation
Acknowledgments
This work was supported by the Parkinson’s Disease Foundation, R01 NS065070 from the National Institutes of Health and the Latin American Science and Technology Development Program (CYTED) through the RIBERMOV (210RT0390) network. Editorial support, funded by the authors, was provided by MedSense Ltd. (High Wycombe, UK).
References
- 1.Matsumoto L, Takuma H, Tamaoka A, Kurisaki H, Date H, Tsuji S, et al. CpG demethylation enhances alpha synuclein expression and affects the pathogenesis of Parkinson’s disease. PLoS One. 2010;5:e15522. doi: 10.1371/journal.pone.0015522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibanez P, Lesage S, Janin S, Lohmann E, Durif F, Destée A, et al. α-synuclein gene rearrangements in dominantly inherited parkinsonism. Arch Neurol. 2009;66:102–8. doi: 10.1001/archneurol.2008.555. [DOI] [PubMed] [Google Scholar]
- 3.Obi T, Nishioka K, Ross OA, Terada T, Yamazaki K, Sugiura A, et al. Clinicopathologic study of a SNCA gene duplication patient with Parkinson disease and dementia. Neurology. 2008;70:238–41. doi: 10.1212/01.wnl.0000299387.59159.db. [DOI] [PubMed] [Google Scholar]
- 4.Zuccarelli G, Alechine E, Caputo M, Bobillo C, Corach D, Sala A. Rapid screening for Native American mitochondrial and Y-chromosome haplogroups detection in routine DNA analysis. Forensic Sci Int Genet. 2011;5:105–108. doi: 10.1016/j.fsigen.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Corach D, Lao O, Bobillo C, van Der Gaag K, Zuniga S, Vermeulen M, et al. Inferring continental ancestry of Argentineans from autosomal, Y-chromosomal and mitochondrial DNA. Ann Hum Genet. 2010;74(1):65–76. doi: 10.1111/j.1469-1809.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 6.Ross OA, Braithwaite AT, Skipper LM, Kachergus J, Hulihan MM, Middleton FA, et al. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Ann Neurol. 2008;63:743–50. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cersósimo MG, Perandones C, Micheli FE, Raina GB, Beron AM, Nasswetter G, et al. Alpha-synuclein immunoreactivity in minor salivary gland biopsies of Parkinson’s disease patients. Mov Disord. 2011;26:188–90. doi: 10.1002/mds.23344. [DOI] [PubMed] [Google Scholar]
- 8.Ahn T-B, Kim SY, Kim JY, Park S-S, Lee DS, Min HJ, et al. α-synuclein gene is present in sporadic Parkinson disease. Neurology. 2008;70:43–9. doi: 10.1212/01.wnl.0000271080.53272.c7. [DOI] [PubMed] [Google Scholar]
- 9.van Veghel-Plandsoen MM, Wouters CH, Kromosoeto JN, den Ridder-Klünnen MC, Halley DJ, van den Ouweland AM. Multiplex ligation-depending probe amplification is not suitable for detection of low-grade mosaicism. Eur J Hum Genet. 2011;19:1009–12. doi: 10.1038/ejhg.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]