Abstract
Uteroferrin (UF) is a progesterone-induced acid phosphatase produced by uterine glandular epithelia in mammals during pregnancy and targeted to sites of hematopoiesis throughout pregnancy. The expression pattern of UF is coordinated with early fetal hematopoietic development in the yolk sac and then liver, spleen, and bone to prevent anemia in fetuses. Our previous studies suggested that UF exerts stimulatory impacts on hematopoietic progenitor cells. However, the precise role and thereby the mechanism of action of UF on hematopoiesis have not been investigated previously. Here, we report that UF is a potent regulator that can greatly enhance fetal erythropoiesis. Using primary fetal liver hematopoietic cells, we observed a synergistic stimulatory effect of UF with erythropoietin and other growth factors on both burst-forming unit-erythroid and colony-forming unit-erythroid formation. Further, we demonstrated that UF enhanced erythropoiesis at terminal stages using an in vitro culture system. Surveying genes that are crucial for erythrocyte formation at various stages revealed that UF, along with erythropoietin, up-regulated transcription factors required for terminal erythrocyte differentiation and genes required for synthesis of hemoglobin. Collectively, our results demonstrate that UF is a cytokine secreted by uterine glands in response to progesterone that promotes fetal erythropoiesis at various stages of pregnancy, including burst-forming unit-erythroid and colony-forming unit-erythroid progenitor cells and terminal stages of differentiation of hematopoietic cells in the erythroid lineage.
During mammalian pregnancy, both mother and fetus experience hematopoietic stress due to a significantly increased demand for blood cell formation, especially differentiation of red blood cells (RBCs). The maternal plasma volume and erythrocytes increase more than 30% during pregnancy to ensure a sufficient supply of oxygen and removal of carbon dioxide from the conceptus (fetus and placenta). Establishment of the hematopoietic system is a crucial process for successful embryogenesis and conceptus development (1, 2). To facilitate coping with hematopoietic stress during pregnancy, a variety of cytokines are produced by both maternal and fetal organs (3–5). However, many of them have not been well documented with respect to their roles in the hematopoietic regulatory networks.
In our previous studies with pregnant pigs, we discovered that a progesterone-induced acid phosphatase, uteroferrin (UF) (also known as tartrate-resistant acid phosphatase and phosphatase, acid, type 5, tartrate resistant), is secreted by uterine glandular epithelium in response to progesterone, transported across the placental areolae, targeted to reticuloendothelial cells of fetal liver by its high mannose carbohydrate moiety, taken up by the reticuloendothelial cells by receptor-mediated endocytosis, and delivers iron for synthesis of hemoglobin (6–11). Therefore, one major function of UF during pregnancy in pigs is to transport iron between the maternal uterus and the fetal-placental unit, and its iron is essential for fetal erythropoiesis. UF is also known to be secreted by human placenta and rodent spleen, and the alignment of amino acid sequences for UF reveals that it is a conserved protein with high amino acid homology among humans, mice, and pigs (Figure 1) (12).
Figure 1.
Comparison of amino acid sequences for human, mouse, and swine UF. Amino acid sequences were obtained from the National Center for Biotechnology Information (NCBI) database. The alignment was analyzed with the Multiple Sequence Alignment of the Clustal Omega algorithm. Shaded areas show identical amino acids, colons show functionally conserved amino acid substitutions, and periods show semiconserved amino acid substitutions among human, mouse, and swine UF. Numbers on the right refer to the last amino acid on each line.
Importantly, UF also has hematopoietic growth factor activity. Using in vitro colony-forming unit (CFU) assays, UF was found to promote differentiation of hematopoietic progenitor cells from swine and human bone marrow as evidenced by greater increase in CFU activities than that stimulated by erythropoietin (Epo) or granulocyte-macrophage (GM) colony-stimulating factor alone (13, 14). Intravenous injection of UF also significantly increases total numbers of hematopoietic progenitor cells in bone marrow and peripheral blood of neonatal piglets that are differentiated into CFU-granulocyte, erythrocyte, monocyte, megakaryocyte (GEMM), burst-forming unit-erythroid (BFU-E) and CFU-GM (13). Additionally, UF increases the frequency of colonies derived from human bone marrow cluster of differentiation (CD)34+CD33− hematopoietic progenitors (13). These observations suggest a potential hematopoietic enhancing effect of UF in humans and pigs, especially preferential stimulatory effects on erythrocyte formation. However, the distinct function of UF in regulating hematopoiesis with the respect to RBC development, particularly its role in erythroid lineage cell fate determination and subsequent terminal differentiation stages of erythropoiesis, has not been investigated.
Here, we adopted various analytical systems to evaluate the function of UF in regulating erythropoiesis using primary fetal cells from mice. Our results demonstrate that UF enhances erythropoiesis by stimulating progenitor cells formation and promotes terminal differentiation of erythroblasts into reticulocytes by regulating expression of important transcription factors, stimulating heme biosynthesis, and increasing hemoglobinization.
Materials and Methods
Mice
Male and female C57BL/6J mice 6 weeks of age were used for breeding. All mice were maintained on a 12-hour light, 12-hour dark cycle and fed ad libitum. The morning of detection of the vaginal plug was taken as day 0.5 of gestation. The embryos were collected from pregnant mice on embryonic day (E)13.5, and fetal liver cells were isolated. All animal experiments followed protocols that were reviewed and approved by the Institutional Animal Care and Use Committee of Texas A&M University (ID, 2010-114).
Colony-forming assay
Fetal liver cells isolated from E13.5 mice were mixed with BD IMag antimouse TER119 (lymphocyte antigen 76) particles-magnetic diameter (20 μL of particles per 107cells; BD Biosciences), and TER119− cells were purified following the manufacturer's instructions. To evaluate the hematopoietic effects of UF on myeloid progenitor cells, 2 × 105 fetal liver TER119− cells isolated from E13.5 mice were plated in MethoCult GF3434 (StemCell Technologies) in the presence of Epo (3 U/mL), stem cell factor (SCF) (50 ng/mL), IL-3 (10 ng/mL), and IL-6 (10 ng/mL). UF was supplemented into the medium at 5 ng/mL. The CFU-erythroid (CFU-E) colonies were counted after 2 days of culture, whereas CFU-GEMM, BFU-E, and CFU-GM colonies were scored after 10 days of culture on the basis of their morphology.
Erythroid differentiation in vitro
TER119− cells were seeded in fibronectin-coated wells at 2 × 105 cells/mL. On day 1, TER119− cells were cultured in Iscove's modified Dulbecco's medium containing 15% fetal bovine serum, 1% detoxified BSA, 200-μg/mL holo-transferrin, 10-μg/mL recombinant human insulin, 2mM L-glutamine and 2-U/mL Epo, 10−4M β-mercaptoethanol, and 2mM L-glutamine. On day 2, TER119− cells were cultured in Iscove's modified Dulbecco's medium containing 20% fetal bovine serum, 10−4M β-mercaptoethanol, and 2mM L-glutamine. Highly purified porcine UFs were added to the medium to achieve final concentrations of 5, 10, or 50 ng/mL.
Immunostaining and flow cytometry analysis
Unless specified, antibodies were obtained from eBioscience. Cells were simultaneously immunostained with allophycocyanin (APC)-conjugated anti-TER119 and fluorescein isothiocyanate (FITC)-conjugated anti-CD71 antibodies to identify differentiated erythroblasts. DRAQ5 (deep red/low infrared fluorescent flow cytometric probe) dye was used to distinguish nucleated and nonnucleated cells (15). Propidium iodide was added to exclude dead cells from analysis. Cells were then analyzed using an Accuri C6 Flow Cytometer System (BD Biosciences). Data were analyzed using Flowjo software or Accuri C6 software (BD Biosciences).
Cytospin preparations and histological staining
After being centrifuged onto slides, cells were air dried and fixed in −20°C methanol for 5 minutes and then stained with May-Grünewald-Giemsa according to the manufacture's recommendations (Sigma). All images were captured using an Axioplan 2 microscope with the AxioCam HR digital camera and AxioVision 4 software (Zeiss).
Quantitative RT-PCR (qRT-PCR) analysis
Total RNAs were extracted by following the TRIzol extraction protocol according to the manufacturer's instructions. Gene expression analysis was performed using a iScript One-Step RT-PCR kit with SYBR Green (Bio-Rad) on Bio-Rad CFX384 (Bio-Rad). The data presented correspond to the mean of 2−ΔΔCt from 4 independent experiments after being normalized to β-actin. The gene-specific primers used for qRT-PCR are listed in Supplemental Table 1.
Data and statistical analysis
Results are expressed as mean ± SEM. Each data point derived from qRT-PCR assays represents an average of 2 technical replicates, and data were average over independently replicated experiments (n = 4 independently collected samples). The statistical analysis was performed using one-way ANOVA followed by Bonferroni's post hoc test or Student's t test using GraphPad Prism version 6.0 software. P < .05 was considered statistically significant.
Results
UF enhances BFU-E and CFU-E formation from fetal liver cells
To evaluate the hematopoietic effects of UF on myeloid progenitor cells, we performed colony-forming assays using murine fetal liver cells. Our results demonstrated that UF significantly enhanced formation of BFU-E and CFU-E in the presence of Epo, SCF, IL-3, and IL-6 (Figure 2). However, UF alone did not support colony formation of fetal liver hematopoietic progenitors. Colonies derived from other myeloid progenitors, including CFU-GEMM and CFU-GM, were not different in the presence or absence of UF (Figure 2). These results suggest that UF enhances colony forming abilities of erythropoietic precursors.
Figure 2.
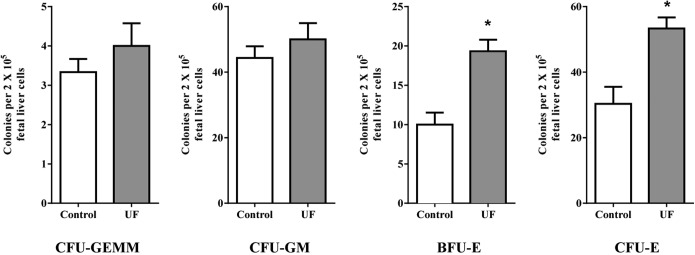
Stimulatory effects of UF on differentiation of hematopoietic progenitor cells. In the CFU assay, 2 × 105 murine fetal liver cells were plated in the semisolid methylcellulose medium in the presence of SCF, IL-3, IL-6, and Epo, and UF was supplemented into the medium. The CFU-Es were counted after 2 days of culture, whereas CFU-GEMM, BFU-E, and CFU-GM were counted after 10 days of culture. Data are presented as mean ± SEM; n = 4. *, P < .05.
UF stimulates erythrocyte differentiation in a dose-dependent manner
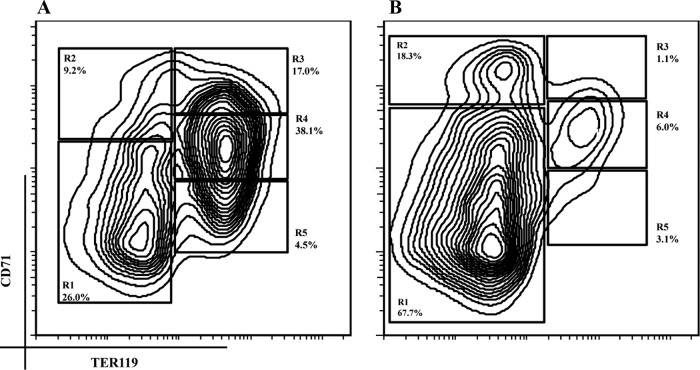
Next, we evaluated the potential effects of UF on erythropoiesis at later stages. We adopted a well-established 2-day in vitro differentiation model that mimics in vivo erythroid cell development during terminal erythropoiesis. The erythroblast-specific surface marker, TER119, is detectable from the proerythroblast stage throughout subsequent differentiation stages of erythroblasts (16, 17). CD71 is the transferrin receptor that is highly expressed in proerythroblasts and gradually decreased with differentiation of erythroblast (15, 18). With dynamic changes in the expression levels of CD71 and TER119 during erythropoiesis, it allows for step-by-step monitoring of the progression of CFU-E progenitor cells through the multiple steps of their terminal differentiation (15, 18–21). As shown in Figure 3A, murine fetal liver cells double-labeled for TER119 and CD71 were divided mainly into 5 distinct cell populations (R1–R5), which correspond morphologically to the consecutive stages of erythroblast differentiation that occurs in vivo. The R1–R5 cell populations represent predominately primitive progenitor cells, proerythroblasts and early basophilic erythroblasts, early and late basophilic erythroblasts, polychromatophilic and orthochromatophilic erythroblasts, late orthochromatophilic erythroblasts, and reticulocytes, respectively (15, 18, 20, 21).
Figure 3.
Purification of fetal liver TER119− cells. Mouse fetal liver cells (A) or TER119− cells (B) were freshly isolated from livers of E13.5 embryos and double-labeled with a FITC-conjugated anti-CD71 monoclonal antibody (mAb) and an APC-conjugated anti-TER119 mAb. Dead cells (propidium iodide-positive) and debris (low forward scatter) were excluded from analysis. The relative number of cells from each region of R1 to R5 is indicated as the percentage of all viable cells and shown on each plot.
To determine whether UF stimulates terminal erythrocyte formation, we depleted the TER119+ erythroid cells that represent differentiated erythrocytes. In addition, mature RBCs were lysed. The purity of the TER119− population was confirmed using flow cytometry as shown in Figure 3B (>85% of total live cells were in stages R1 and R2).
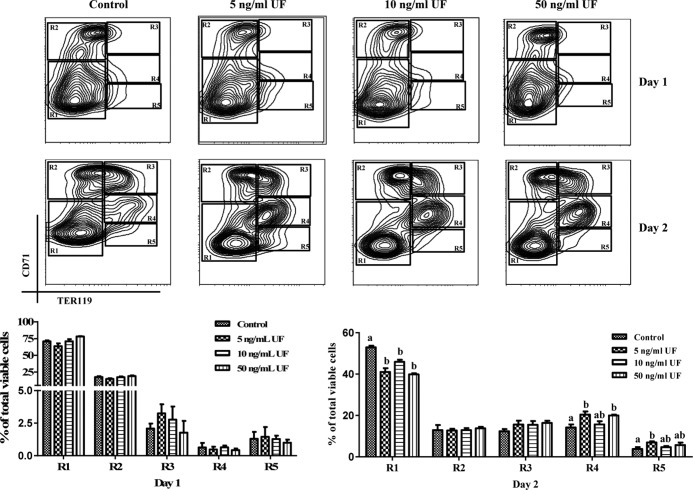
During day 1 of culture, Epo-dependent CFU-E progenitor cells differentiated into R2 and R3 cell populations that are mainly in the early differentiation stages for erythroblasts (Figure 4) (18, 20, 21). In the presence of UF, we observed similar populations of differentiated erythroblasts as for the control after day 1 of culture (Figure 4). At the end of 2 days of culture, erythroblasts further differentiated and migrated into the R3–R5 populations (Figure 4) (18, 20, 21). These results indicate that hematopoietic progenitor cells underwent successful differentiation through the terminal stages of erythropoiesis in this in vitro differentiation model. Flow cytometry analysis revealed that treatments with 5- or 50-ng/mL UF increased the population of cells that further differentiated into R4 erythroblast stage as compared with the control cells after 2 days of culture (Figure 4). In addition, 5-ng/mL UF treatment induced a greater population of R5 erythroblasts as compared with the control cells (Figure 4). Thus, these results suggest that UF stimulates the differentiation of erythroblasts.
Figure 4.
Dose-dependent effects of UF on terminal stages of erythropoiesis. TER119− cells were cultured in vitro for 1 day on fibronectin-coated plates in medium containing Epo and transferrin. Epo and transferrin were removed from culture at the end of day 1. UF was added into medium at either 0 (control), 5, 10, or 50 ng/mL. Cells were double-labeled with FITC-conjugated anti-CD71 monoclonal antibody (mAb) and APC-conjugated anti-TER119 mAb and analyzed by flow cytometry. After day 1 of culture, TER119− cells had differentiated into R2 and R3 cells. After 2 days culture, erythroblasts had differentiated into R3, R4, and R5 cells. Data are presented as mean ± SEM; n = 4. Means with different superscript letters are significantly different (P < .05).
UF enhances erythroblasts differentiation into reticulocytes
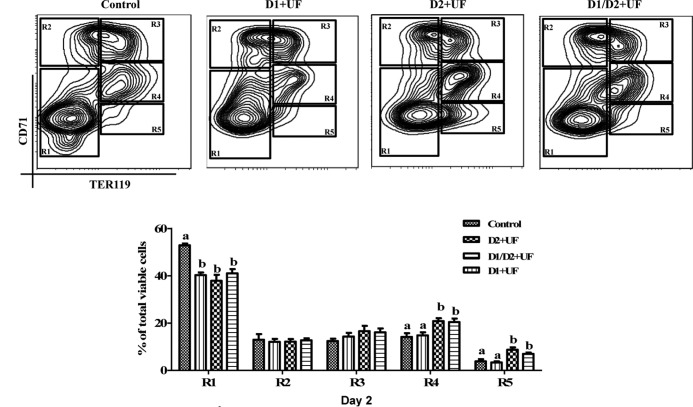
To identify the precise stage of erythroblast differentiation affected by UF, TER119− cells were treated with 5-ng/mL UF on day 1 only, day 2 only, or on both days 1 and 2 of culture. Flow cytometry analysis indicated that cells treated with UF only in day 1 of culture displayed similar stages of differentiation to that of the control after 2 days of culture (Figure 5). Intriguingly, UF supplementation only on day 2 or both days 1 and 2 induced a greater population of R4 and R5 cells, compared with the control (Figure 5). In addition, there was no significant different between these 2 UF treatments with respect to the R3–R5 cell populations (Figure 5).
Figure 5.
UF enhances later stages of terminal differentiation of erythrocytes. TER119− cells were treated with 5-ng/mL UF only on day 1 of culture (D1+UF), only on day 2 of culture (D2+UF), or on both days 1 and 2 of culture (D1/D2+UF). TER119− cells without UF treatment served as the control. At the end of day 2 of culture, cells were double-labeled with FITC-conjugated anti-CD71 monoclonal antibody (mAb) and APC-conjugated anti-TER119 mAb and analyzed by flow cytometry. Data are presented as mean ± SEM; n = 4. Means with different superscript letters are significantly different (P < .05).
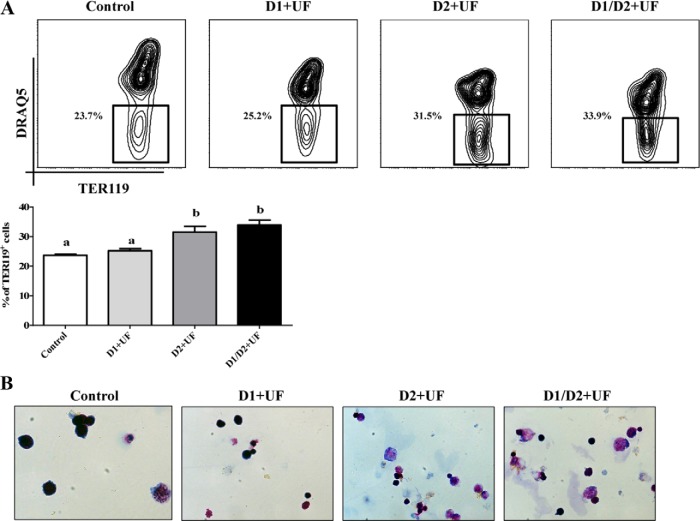
We further showed that cells treated with UF in day 2 or both days 1 and 2 had a greater population of enucleated TER119+ cells (TER119+DRAQ5−) compared with the control (Figure 6A). However, supplementation of UF only in day 1 of culture resulted in similar populations of enucleated cells as that for the control (Figure 6, A and B). Consistently, the May-Grünewald-Giemsa staining images showed that UF treatments only on day 2 or on days 1 and 2 of culture elevated the formation of enucleated reticulocyte in this in vitro differentiation model (Figure 6B). These results indicate that UF enhances differentiation of erythoid lineage cells in the later stages of terminal differentiation of erythrocytes.
Figure 6.
Enhanced differentiation of erythroblast into reticulocyte by UF. After 2 days in culture, cells were conjugated with anti-TER119 magnetic particles, and TER119+ cells were isolated using a magnet. A, Reticulocytes were characterized based on their being enucleated. DRAQ5 dye was used to distinguish nucleated and nonnucleated cells. B, May-Grünewald staining of TER119+ cells. Data are presented as mean ± SEM; n = 4. Means with different superscript letters are significantly different (P < .05).
UF induces important transcription factors and genes regulating erythropoiesis
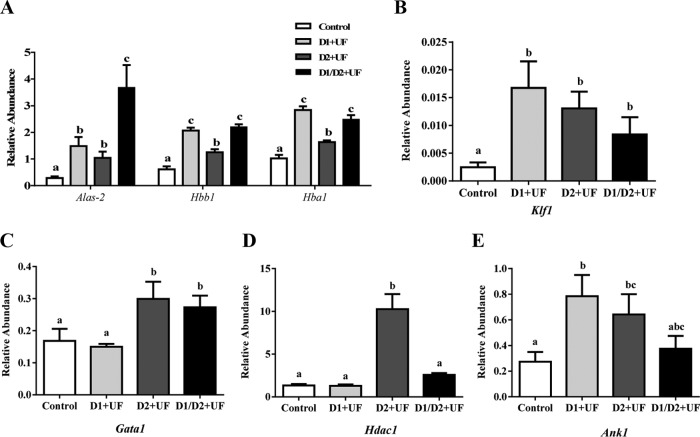
The terminal stages of erythroblast differentiation involve substantial changes, such as chromatin condensation, enucleation, and hemoglobinization (22–24). The results from qRT-PCR analysis indicated that expression of genes involved in the biosynthesis of heme and production of hemoglobin, such as δ-aminolevulinate synthase 2 (Alas2), hemoglobin-α locus 1 (Hba1), and hemoglobin-β locus 1 (Hbb1) were significantly greater in response to UF at the end of 2 days of culture (Figure 7A), and UF increased expression of the transcription factor Krüppel-like factor 1 (Klf1) (Figure 7B). Another key transcription factor, gata-binding protein 1 (Gata1), was up-regulated in the presence of UF on day 2 and in cells treated with UF on both days 1 and 2 as compared with control cultures (Figure 7C). In addition, cells treated with UF only on day 2 of culture exhibited increased expression of histone deacetylase 1 (Hdac1) (Figure 7D). Supplementation of UF only on day 1 or only on day 2 of culture induced the expression of ankyrin 1 (Ank1) in erythroid cells (Figures 7E).
Figure 7.
UF-induced gene expression during the terminal stages of erythropoiesis. A, Expression of Alas2, Hbb1, and Hba1 genes related to heme biosynthesis and hemoglobin synthesis was determined using qRT-PCR. B and C, Increases in expression of transcription factors Gata1 and Klf1, respectively, based on qRT-PCR results. D and E, Effects of UF on expression of Hdac1 and ankyrin 1 (Ank1) as determined using qRT-PCR. D1+UF indicates that UF was added to medium only on day 1 of culture; D2+UF indicates that UF was added to medium only on day 2 of culture; D1/D2+UF indicates that UF was added to medium on both days 1 and 2 of culture. Data are presented as mean ± SEM; n = 4. Means with different superscript letters are significantly different (P < .05).
Discussion
In this study, we used an established in vitro 2-day erythroblast differentiation model that mimics the 4–5 terminal divisions of erythroid progenitor cells in vivo (15, 18–21). Cells were double-labeled with nonerythroid-specific CD71 and erythroid-specific TER119 to track stages of differentiation during terminal differentiation. Briefly, TER119− cells include those that are highly Epo-dependent CFU-E progenitor cells undergoing the first 2 divisions and differentiating into erythroblasts during day 1 of culture. At the end of day 2 of culture, reticulocytes were derived from the terminally differentiated erythroblasts that involve chromatin condensation, hemoglobinization, and enucleation of the cells (22, 23). This system allows step-wise investigation of mechanisms governing terminal erythropoiesis in a quantitative manner using flow cytometry (19). Our results indicated that CFU-E progenitor cells developed into R2 and R3 cells after 1 day in culture in the presence of Epo and transferrin and that those cells subsequently differentiated into R3–R5 cells at the end of day 2 of culture, which demonstrated that CFU-E progenitor cells cultured in this model undergo normal progression of differentiation through the terminal stages of erythropoiesis.
Results of previous studies suggested that UF is a potential hematopoietic growth factor that stimulates hematopoietic stem cell (HSC) to differentiate into myeloid progenitor cells (13, 14). In this study, the CFU assays revealed that mouse fetal liver HSCs treated with UF gave rise to more lineage-determined BFU-E and CFU-E progenitor cells, which supports results of stimulatory effects of UF on HSCs to differentiate into erythroid cells as observed in previous in vivo studies with neonatal pigs (14). However, the precise molecular mechanism whereby UF stimulates hematopoietic progenitor cell CFU activities was not elucidated previously. Using the in vitro erythroid progenitor differentiation model, results of the present study revealed that UF did not induce differentiation of CFU-E progenitor cells into erythroblasts during day 1 of culture. However, flow cytometry analysis revealed production of more enucleated reticulocytes in response to UF treatment at the end of day 2 of culture. In addition, erythroblasts treated with UF only on day 2 of culture generated a similar population of reticulocytes to that in response to treating cells with UF on both days 1 and 2 of culture. These results suggest that UF has stimulatory effects during the period when erythroblasts are differentiating into reticulocytes. In addition, there was a dose-dependent effect of UF on terminal differentiation of erythroblasts into reticulocytes with 5-ng/mL UF stimulating greater production of reticulocytes by the end of day 2 of culture as compared with effects of 10 and 50-ng/mL UF.
Various growth factors, such as Epo, play critical roles in the process of erythropoiesis. In this study, we did not observe that UF alone had hematopoietic growth factor activity regarding differentiation of erythroblasts in the absence of Epo and other growth factors (data not shown). This suggests that UF exerts synergistic or additive stimulatory effects when acting in concert with Epo and other growth factors to increase erythropoiesis.
The terminal differentiation of erythroid cells is controlled by a transcriptional regulatory network governing important changes in expression of genes involved with enzymes for synthesis of hemoglobin and transcription factors regulating final differentiation of erythroid cells (22, 23). In this study, the expression of genes related to hemoglobin synthesis, including Hbb1 (25) and Hba1 (26), as well as Alas2 (27), were up-regulated by UF at the end of day 2 of culture. These findings suggest that the stimulatory effects of UF on terminal stages of erythropoiesis is likely due in part to its delivery of iron for synthesis of hemoglobin. The transcription factor Gata1 (28), which controls proliferation and maturation of erythroid cells, was also induced by UF, as was Hdac1 (29), which forms a complex with Gata1 to regulate chromatin changes during the terminal stage of erythropoiesis. In addition, the expression of transcription factor Klf1 (30) was increased by UF treatment during differentiation of erythroblast into reticulocytes. Thus, these results suggest that UF increases production of reticulocytes from erythroblasts by up-regulating expression of genes for key transcription factors.
UF has been isolated from: 1) uteri of pigs, ewes, mares, and cows under the influence of progesterone; 2) spleens of pigs, humans, cattle, rats, and mice; 3) chondrocytes of patients with osteoporosis; 4) human placentae; and 5) spleens of humans with hairy cell leukemia and Gaucher's disease (6, 10, 31–33). The biochemical properties of human and porcine UF are very similar and have 82% nucleotide sequence identity (12). Molecular mechanisms regulating transcriptional and translational events responsible for synthesis and secretion of UF in normal and diseased tissues are unclear, and mechanisms whereby overexpression of UF in adults is associated with pathologies such as osteoporosis and hairy cell leukemia remain unclear. However, there is evidence for a link between UF and secreted phosphoprotein 1 (SPP1) that may account for some of the known pathologies. For example, SPP1 preferentially binds integrin heterodimers (ITGA9:ITGB1 and ITGA5:ITGB1), as well as CD44 on HSCs to mediate adhesion, self-renewal, quiescence, and/or differentiation of HSCs (34, 35). Sumitomo et al (36) conclude that mediator complex subunit 1 in stromal cells supports hematopoietic stem and progenitor cells and that SPP1 is the direct target of mediator complex subunit 1 responsible for this support of hematopoietic stem and progenitor cells. UF can affect the phosphorylation status of SPP1, which may be a mechanism whereby it influences erythropoiesis (37, 38). For example, hyperphosphorylation of SPP1 due to mutations in UF increases production of interferon-α, skeletal defects, systemic autoimmunity, and impairs erythropoiesis (39–42).
In conclusion, results of this study indicate that UF is a hematopoietic growth factor that increases BFU-E and CFU-E progenitor cells and promotes terminal stages of differentiation of cells in the erythroid lineage during erythropoiesis.
Acknowledgments
We thank Xiaoling Zhu of Texas A&M University for her assistance in isolating RNA and Deborah J. Kovar of LARR of Texas A&M University (TAMU) for animal care.
This work was supported by the American Heart Association (13PRE17050104 to W.Y.), American Diabetes Association (1-13-JF-59 to B.Z.) and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK 1R01DK098662 to B.Z.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Alas2
- δ-aminolevulinate synthase 2
- APC
- allophycocyanin
- BFU-E
- burst-forming unit-erythroid
- CD
- cluster of differentiation
- CFU
- colony-forming unit
- CFU-E
- CFU-erythroid
- DRAQ5
- deep red/low infrared fluorescent flow cytometric probe
- E
- embryonic day
- Epo
- erythropoietin
- FITC
- fluorescein isothiocyanate
- Gata1
- gata-binding protein 1
- GM
- granulocyte-macrophage
- GEMM
- granulocyte, erythrocyte, monocyte, megakaryocyte
- Hba1
- hemoglobin-α locus 1
- Hbb1
- hemoglobin-β locus 1
- Hdac1
- histone deacetylase 1
- HSC
- hematopoietic stem cell
- Klf1
- Krüppel-like factor 1
- qRT-PCR
- quantitative RT-PCR
- RBC
- red blood cell
- SCF
- stem cell factor
- SPP1
- secreted phosphoprotein 1
- TER119
- lymphocyte antigen 76
- UF
- uteroferrin.
References
- 1. Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71(suppl 5):1280S–1284S. [DOI] [PubMed] [Google Scholar]
- 2. Scholl TO, Reilly T. Anemia, iron and pregnancy outcome. J Nutr. 2000;130(suppl 2S):443S–447S. [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharyya S, Lin J, Linzer DI. Reactivation of a hematopoietic endocrine program of pregnancy contributes to recovery from thrombocytopenia. Mol Endocrinol. 2002;16(6):1386–1393. [DOI] [PubMed] [Google Scholar]
- 4. Zhou B, Lum HE, Lin J, Linzer DI. Two placental hormones are agonists in stimulating megakaryocyte growth and differentiation. Endocrinology. 2002;143(11):4281–4286. [DOI] [PubMed] [Google Scholar]
- 5. Zhou B, Kong X, Linzer DI. Enhanced recovery from thrombocytopenia and neutropenia in mice constitutively expressing a placental hematopoietic cytokine. Endocrinology. 2005;146(1):64–70. [DOI] [PubMed] [Google Scholar]
- 6. Ketcham CM, Baumbach GA, Bazer FW, Roberts RM. The type 5, acid phosphatase from spleen of humans with hairy cell leukemia. Purification, properties, immunological characterization, and comparison with porcine uteroferrin. J Biol Chem. 1985;260(9):5768–5776. [PubMed] [Google Scholar]
- 7. Baumbach GA, Saunders PT, Bazer FW, Roberts RM. Uteroferrin has N-asparagine-linked high-mannose-type oligosaccharides that contain mannose 6-phosphate. Proc Natl Acad Sci USA. 1984;81(10):2985–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen TT, Bazer FW, Cetorelli JJ, Pollard WE, Roberts RM. Purification and properties of a progesterone-induced basic glycoprotein from the uterine fluids of pigs. J Biol Chem. 1973;248(24):8560–8566. [PubMed] [Google Scholar]
- 9. Saunders PT, Renegar RH, Raub TJ, et al. The carbohydrate structure of porcine uteroferrin and the role of the high mannose chains in promoting uptake by the reticuloendothelial cells of the fetal liver. J Biol Chem. 1985;260(6):3658–3665. [PubMed] [Google Scholar]
- 10. Roberts RM, Raub TJ, Bazer FW. Role of uteroferrin in transplacental iron transport in the pig. Fed Proc. 1986;45(10):2513–2518. [PubMed] [Google Scholar]
- 11. Michel FJ, Fliss MF, Bazer FW, Simmen RC. Characterization and developmental expression of binding sites for the transplacental iron transport protein, uteroferrin, in fetal hematopoietic tissues. Biol Neonate. 1992;61(2):82–91. [DOI] [PubMed] [Google Scholar]
- 12. Padua MB, Lynch VJ, Alvarez NV, et al. ACP5 (uteroferrin): phylogeny of an ancient and conserved gene expressed in the endometrium of mammals. Biol Reprod. 2012;86(4):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bazer FW, Worthington-White D, Fliss MF, Gross S. Uteroferrin: a progesterone-induced hematopoietic growth factor of uterine origin. Exp Hematol. 1991;19(9):910–915. [PubMed] [Google Scholar]
- 14. Laurenz JC, Hadjisavas M, Schuster D, Bazer FW. The effect of uteroferrin and recombinant GM-CSF on hematopoietic parameters in normal female pigs (Sus scrofa). Comp Biochem Physiol B Biochem Mol Biol. 1997;118(3):579–586. [DOI] [PubMed] [Google Scholar]
- 15. Fraser ST, Isern J, Baron MH. Maturation and enucleation of primitive erythroblasts during mouse embryogenesis is accompanied by changes in cell-surface antigen expression. Blood. 2007;109(1):343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kina T, Ikuta K, Takayama E, et al. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Brit J Haematol. 2000;109(2):280–287. [DOI] [PubMed] [Google Scholar]
- 17. Vannucchi AM, Paoletti F, Linari S, et al. Identification and characterization of a bipotent (erythroid and megakaryocytic) cell precursor from the spleen of phenylhydrazine-treated mice. Blood. 2000;95(8):2559–2568. [PubMed] [Google Scholar]
- 18. Chen K, Liu J, Heck S, Chasis JA, An X, Mohandas N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci USA. 2009;106(41):17413–17418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koulnis M, Pop R, Porpiglia E, Shearstone JR, Hidalgo D, Socolovsky M. Identification and analysis of mouse erythroid progenitors using the CD71/TER119 flow-cytometric assay. J Vis Exp. 2011;54:e2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102(12):3938–3946. [DOI] [PubMed] [Google Scholar]
- 21. Socolovsky M, Murrell M, Liu Y, Pop R, Porpiglia E, Levchenko A. Negative autoregulation by FAS mediates robust fetal erythropoiesis. PLoS Biol. 2007;5(10):e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hattangadi SM, Wong P, Zhang L, Flygare J, Lodish HF. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood. 2011;118(24):6258–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ji P, Murata-Hori M, Lodish HF. Formation of mammalian erythrocytes: chromatin condensation and enucleation. Trends Cell Biol. 2011;21(7):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ji P, Yeh V, Ramirez T, Murata-Hori M, Lodish HF. Histone deacetylase 2 is required for chromatin condensation and subsequent enucleation of cultured mouse fetal erythroblasts. Haematologica. 2010;95(12):2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki Y, Shimizu T, Sakai H, et al. Model mice for Presbyterian hemoglobinopathy (Asn(β108)–>Lys) confer hemolytic anemia with altered oxygen affinity and instability of Hb. Biochem Biophys Res Commun. 2002;295(4):869–876. [DOI] [PubMed] [Google Scholar]
- 26. Pászty C, Mohandas N, Stevens ME, et al. Lethal α-thalassaemia created by gene targeting in mice and its genetic rescue. Nat Genet. 1995;11(1):33–39. [DOI] [PubMed] [Google Scholar]
- 27. Harigae H, Nakajima O, Suwabe N, et al. Aberrant iron accumulation and oxidized status of erythroid-specific δ-aminolevulinate synthase (ALAS2)-deficient definitive erythroblasts. Blood. 2003;101(3):1188–1193. [DOI] [PubMed] [Google Scholar]
- 28. Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci USA. 1996;93(22):12355–12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang T, Jian W, Luo Y, et al. Acetylation of histone deacetylase 1 regulates NuRD corepressor complex activity. J Biol Chem. 2012;287(48):40279–40291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siatecka M, Bieker JJ. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood. 2011;118(8):2044–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dixon SN, Gibbons RA. Proteins in the uterine secretions of the cow. J Reprod Fertil. 1979;56(1):119–127. [DOI] [PubMed] [Google Scholar]
- 32. Zavy MT, Roberts RM, Bazer FW. Acid phosphatase and leucine aminopeptidase activity in the uterine flushings of non-pregnant and pregnant gilts. J Reprod Fertil. 1984;72(2):503–507. [DOI] [PubMed] [Google Scholar]
- 33. Bazer FW, Roberts RM. Biochemical aspects of conceptus–endometrial interactions. J Exp Zool. 1983;228(2):373–383. [DOI] [PubMed] [Google Scholar]
- 34. Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106(4):1232–1239. [DOI] [PubMed] [Google Scholar]
- 35. Stier S, Ko Y, Forkert R, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201(11):1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sumitomo A, Ishino R, Urahama N, et al. The transcriptional mediator subunit MED1/TRAP220 in stromal cells is involved in hematopoietic stem/progenitor cell support through osteopontin expression. Mol Cell Biol. 2010;30(20):4818–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Means RT, Jr, Krantz SB. Inhibition of human erythroid colony-forming units by interferons α and β: differing mechanisms despite shared receptor. Exp Hematol. 1996;24(2):204–208. [PubMed] [Google Scholar]
- 38. Tarumi T, Sawada K, Sato N, et al. Interferon-α-induced apoptosis in human erythroid progenitors. Exp Hematol. 1995;23(12):1310–1318. [PubMed] [Google Scholar]
- 39. Behrens TW, Graham RR. TRAPing a new gene for autoimmunity. Nat Genet. 2011;43:90–91. [DOI] [PubMed] [Google Scholar]
- 40. Briggs TA, Rice GI, Daly S, et al. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet. 2011;43:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hayman AR, Jones SJ, Boyde A, et al. Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disrupted endochondral ossification and mild osteopetrosis. Development. 1996;122:3151–3162. [DOI] [PubMed] [Google Scholar]
- 42. Hayman AR, Cox TM. Tartrate-resistant acid phosphatase knockout mice. J Bone Miner Res. 2003;18:1905–1907. [DOI] [PubMed] [Google Scholar]