Abstract
Nuclear receptors are transcription factors that regulate networks of target genes in response to small molecules. There is a strong bias in our knowledge of these receptors because they were mainly characterized in classical model organisms, mostly vertebrates. Therefore, the evolutionary origins of specific ligand-receptor couples still remain elusive. Here we present the identification and characterization of a retinoic acid receptor (RAR) from the mollusk Nucella lapillus (NlRAR). We show that this receptor specifically binds to DNA response elements organized in direct repeats as a heterodimer with retinoid X receptor. Surprisingly, we also find that NlRAR does not bind all-trans retinoic acid or any other retinoid we tested. Furthermore, NlRAR is unable to activate the transcription of reporter genes in response to stimulation by retinoids and to recruit coactivators in the presence of these compounds. Three-dimensional modeling of the ligand-binding domain of NlRAR reveals an overall structure that is similar to vertebrate RARs. However, in the ligand-binding pocket (LBP) of the mollusk receptor, the alteration of several residues interacting with the ligand has apparently led to an overall decrease in the strength of the interaction with the ligand. Accordingly, mutations of NlRAR at key positions within the LBP generate receptors that are responsive to retinoids. Altogether our data suggest that, in mollusks, RAR has lost its affinity for all-trans retinoic acid, highlighting the evolutionary plasticity of its LBP. When put in an evolutionary context, our results reveal new structural and functional features of nuclear receptors validated by millions of years of evolution that were impossible to reveal in model organisms.
Among all transcription factors, nuclear receptors (NRs) are unusual in that their ability to modulate gene transcription is regulated by the binding of a ligand (1). We have a good understanding of how these receptors are able to regulate transcription after ligand binding. However, our view of NR action is strongly biased towards vertebrate models and towards mammals in particular. Therefore, key questions on the evolutionary origin of NRs and of their ligands still remain elusive. In particular, the evolutionary elaboration of specific ligand-receptor couples is still unclear (2, 3). Several observations have suggested that, in contrast to what was initially expected, a given ligand-receptor couple may not be stable in evolutionary time and that changes in ligand binding specificity was quite frequent during animal diversification (4). Estrogen receptor (ER) orthologs from several protostome species, for example, have been shown to be insensitive to estradiol, as is the case for mollusk ERs (5, 6). In addition, the presence of the bona fide vertebrate ER ligand, 17β-estradiol, in nonchordate species has been questioned (7–9). Similarly, thyroid hormone receptors (TRs), present in basal chordates, have been shown to recognize a different ligand than vertebrate TRs (5, 10). These examples clearly suggest that the presence of the ortholog of a given NR gene does not necessarily imply similar molecular and biochemical functions.
Retinoic acid (RA) is a morphogen derived from vitamin A that controls key processes during vertebrate development. In particular, it controls the patterning of the anteroposterior axis and the differentiation of various cell types, such as neurons or hematopoietic cells. RA is synthesized from retinol and can be found in the form of three principal isomers, all-trans RA (ATRA), which is the major biologically active compound in vertebrates, 13-cis RA (13cRA), and 9-cis RA (9cRA), which has been proposed as a ligand for retinoid X receptor (RXR), but whose in vivo presence is still debated (11). Like other NRs, retinoic acid receptor (RAR) and RXR proteins consist of two main domains, the DNA-binding domain (DBD) and the ligand-binding domain (LBD). RAR and RXR regulate the transcription of their target genes by forming heterodimers that bind specific response elements called retinoic acid response element (RAREs) in the regulatory regions of target genes (12, 13). These elements consists of two copies of the consensus sequence (A/G)G(G/T)(G/T)(G/C)A organized as direct repeats (DRs) and separated by a variable number of nucleotides (12, 13). In vertebrates, RAR/RXR heterodimers recognize DR1 (one nucleotide spacer), DR2, and DR5 elements (14–17), whereas the RXR homodimer recognizes almost exclusively DR1 (16). Recent genome-wide analyses of RAREs using chromatin immunoprecipitation followed by high-throughout sequencing have revealed that other elements, such as DR8 sequences, are also recognized by RAR/RXR (15).
Little is known about RA signaling outside of chordates, and evidence for biological functions of RA in nonchordate invertebrates is scarce (18). However, some studies have shown that RA might have a physiological role in mollusks. In Lymnaea stagnalis, both ATRA and 9cRA are involved in neuronal differentiation (19, 20). In Thais clavigera and Nucella lapillus, the injection of 9cRA causes abnormalities in sex organ development (21, 22). Moreover, in several species, treatment of embryos with ATRA affects eye formation (23). However, these results are based on treatments with exogenous RA and do not prove that endogenous retinoids are indeed present and active in these animals. There is some evidence of the presence of endogenous retinoids, such as ATRA and 9cRA, in mollusks (20, 24, 25). In addition, it was demonstrated that an RXR from the mollusk Biomphalaria glabrata can bind 9cRA and activate transcription in response to this compound (26). Therefore, these data suggests that RA might have a biological function in mollusks.
Using an in silico approach, key elements of the RA machinery, such as RAR and RXR, have been identified in the genomes of nonchordate species, including lophotrochozoans, indicating that the RA signaling pathway may be present and functional in these species (18). For example, rar genes have been identified in ambulacrarians, hemichordates, annelids, and mollusks (27–31). Furthermore, a rar from the mollusk T. clavigera was recently cloned and characterized. However, the protein encoded by this gene was unable to activate transcription in the presence of RA (32). The available data therefore suggest, but do not unambiguously prove, that RA signaling may be active in nonchordates, such as mollusks. Therefore, detailed characterizations of lophotrochozoan RARs are needed to gain insights into the evolutionary origin of retinoid signaling in metazoans.
Here we describe a novel RAR from the gastropod mollusk N. lapillus. We show that this N. lapillus RAR (NlRAR) is able to heterodimerize with N. lapillus RXR (NlRXR) and bind specific DNA sequences based on the classical direct repeat RARE consensus. Ligand-binding and transactivation assays reveal that NlRAR does not bind to and is not activated by ATRA or other retinoids, despite the presence of these compounds in N. lapillus tissues. Analysis of the ligand-binding pocket of NlRAR revealed a series of amino acid changes that may explain a reduction of its affinity for the bona fide vertebrate ligands. Indeed, the mutation of these positions in the NlRAR LBD retrieves ATRA and 9cRA binding and activation of the mutated receptor. Taken together, our results show that, even though N. lapillus possesses the machinery necessary for RA signaling, this signaling pathway might be functional in a different manner in mollusks, when compared with vertebrates or even other lophotrochozoans. This finding thus highlights the plasticity of the LBD in terms of ligand binding and reveals an unexpected flexibility of RARs to respond to novel physiological and/or biochemical contexts.
Materials and Methods
Cloning of N. lapillus rar and plasmid constructs
Total RNA was extracted from N. lapillus prehatched juveniles and adult tissues and reverse transcribed. A N. lapillus rar cDNA, 1101 bp long encoding a protein of 367 amino acids, was cloned using a PCR strategy (Ref. 22 and Supplemental Materials). Genes encoding the full-length receptors (RAR and RXR) were cloned into the pSG5 vector and a flag tag was added at the N terminus. The LBDs of the receptors were cloned into pG4MpolyII (33) to produce GAL4-LBD fusions. Full-length RAR and RXR protein sequences from different species (see Supplemental Materials) were aligned with Seaview (34), and the tree was calculated using PhyML with the JTT model and optimized invariable sites and 1000 bootstrap replicates (34). Gaps were treated as unknown characters.
Electrophoretic mobility shift assays (EMSAs)
In vitro translation of tagged NlRAR and NlRXR labeled with 35S-methionine was performed using the TNT wheat germ extract system (Promega). The EMSAs were performed as previously described (26) and details are listed in the Supplemental Materials.
Ligands and peptides
ATRA, 9cRA, 13cRA, and TTNPB were purchased from Sigma-Aldrich. Stock solutions were prepared in ethanol at 10−2 M. The fluorescent peptides NCOR ID1, NCOR ID2, and SRC-1 NR2 were purchased from EZBiolab. The fluorescent SMRT ID1 peptide was obtained from Neosystem, and the fluorescent SMRT ID2 peptide was synthesized by Dr. J. F. Guichou (Centre National de la Recherche Scientifique, Unité Mixte de Recherche 5048, Montpellier, France). Their sequences are listed in the Supplemental Materials.
Transactivation assays in human embryonic kidney (HEK) 293T cells
HEK 293T cells were maintained in DMEM supplemented with 10% fetal calf serum (Invitrogen). Transfection and treatments were performed as previously described (35) with 60 ng of total DNA, and the final concentration for the ligands were between 10−7 and 10−9 M.
Limited proteolysis assays (LPAs)
LPAs were performed as previously described (35) using the receptors translated in vitro using the TNT-coupled reticulocyte lysate system (Promega) and labeled with 35S-methionine. The final concentrations tested for each ligand were between 10−5 and 10−7 M.
Ligand-binding assays
The LBD of the human HsRARα and NlRAR were cloned into pGEM-4T (Amersham Biosciences), containing a glutathione-S-transferase tag. The fusion proteins were purified, and each protein was then incubated with 10 nM all-trans-[11,12-3H] RA (PerkinElmer) with increasing concentrations of nonlabeled ATRA (from 1 to 1000 nM). After an incubation at 4°C for 1 hour, hydroxyapatite was added to each tube. Hydroxyapatite-bound radioactivity was determined by liquid scintillation counting.
Fluorescence anisotropy measurements
The NlRAR LBD was cloned into the pET-32a vector. Expression and purification of the LBDs of NlRAR and of the human HsRARα were performed as previously described (36). Florescence anisotropy assays were performed using a Safire2 microplate reader (TECAN) as described in (36).
Three-dimensional (3D) modeling of RAR LBDs and mutagenesis
The NlRAR model was constructed using the modeling metaserver @TOME 2 (37) and the crystal structure of the LBD of the human RARα (3KMR) as a template. Mutants were constructed by PCR-assisted, site-directed mutagenesis using the Pfu DNA polymerase (Promega). The DpnI enzyme (New England Biolabs) was used to remove the parental DNA template.
Tissue retinoid determination
Tissues were stored at −80°C until assayed. They were homogenized and extracted as previously described (38). RA isomers were quantified using liquid chromatography-tandem mass spectrometry on an AB Sciex 5500 QTRAP in MRM mode using APCI in positive ion mode as previously described (38, 39). Retinol and retinyl esters (REs) were quantified by high-performance liquid chromatography with ultraviolet detection on a Waters Acquity as previously described (38, 40).
Results
Identification of a RAR ortholog in the mollusk N. lapillus
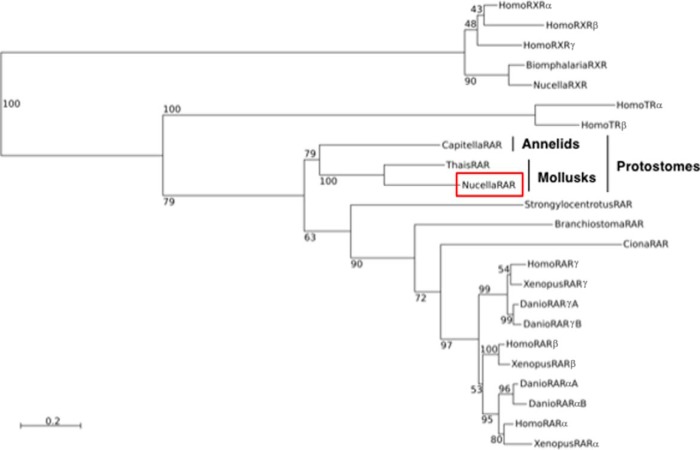
We cloned a short fragment of a gene encoding RAR from N. lapillus using a RT-PCR strategy (GenBank accession number KJ410131). This rar clone contains an open reading frame of 1101 bp that encodes a 367-amino acid-long protein, called NlRAR. The predicted NlRAR protein is characterized by the overall domain structure of NRs: a DBD with conserved zinc finger motifs important for DNA binding and a LBD that contains the main features present in other RARs implicated in ligand binding, dimerization with RXR, and recognition of transcriptional coactivators and corepressors (Supplemental Figure 1). Amino acid alignments indicate that the NlRAR DBD shares approximately 81%–83% sequence similarity with chordate RARs and 93% with the DBD of the recently identified RAR from the mollusk T. clavigera. Concerning the LBD, the identity levels are 51%–54% with the chordate RARs and 68% with T. clavigera RAR. In a phylogenetic tree, the NlRAR sequence is placed close to the T. clavigera RAR and is associated with another protostome RAR from the annelid Capitella teleta (Figure 1). As expected, these protostome RARs are an early offshoot of the RAR branch, clearly suggesting that a unique RAR ortholog was present in the last common ancestor of all bilaterian animals.
Figure 1.
Phylogenetic position of NlRAR. Phylogenetic tree of the RAR cloned from N. lapillus. Numbers on each branch are bootstrap support values in percentage of 1000 replicates. The tree was rooted with the RXR sequences. The species listed are the following: Branchiostoma floridae, Biomphalaria glabrata, Capitella teleta, Ciona intestinalis, Danio rerio, Homo sapiens, Nucella lapillus, Strongylocentrotus purpuratus, Thais clavigera, and Xenopus tropicalis.
It is interesting to compare the conservation of specific sequence features between NlRAR and other RARs (Supplemental Figure 1). Within the DBD, the proximal box, which is involved in determining the DNA binding specificity (41), and the terminal box (T-box), which is required for the heterodimerization with RXR on DR2 elements (42, 43), are 100% conserved, suggesting that the NlRAR recognizes the classical RARE core sequences as a heterodimeric binding partner with RXR. In contrast, the distal box, which plays a role in the heterodimerization of RAR and RXR on DR5 elements (44), is more divergent (Supplemental Figure 1).
The NlRAR also shares the general NR organization with 12 helices within the LBD (45). However, the NlRAR LBD is characterized by a significant amount of amino acid differences compared with the chordate RARs. For example, of the 24 amino acids that have been shown to interact with ATRA in human RARγ (Supplemental Figure 1) (46), seven are different in NlRAR. Among these divergent residues, four are conserved between the RARs from T. clavigera and N. lapillus, suggesting that this sequence signature may be a conserved feature of mollusks. Nonetheless, this low degree of sequence conservation of key residues in the ligand-binding pocket (LBP) between mollusk and chordate RARs suggests that the ligand binding properties of mollusk RARs, and thus of NlRAR, might be different from those of chordates RARs.
NlRAR and NlRXR are able to recognize specific DNA sequences
To characterize the DNA binding properties of NlRAR, we performed EMSA experiments using as a partner the previously described N. lapillus RXR (22) (Figure 2). This RXR gene encodes two isoforms, NlRXRa and NlRXRb, which differ by a small insertion in the T-box of NlRXRb. Because NlRXRa is the major isoform, we used this isoform for our experiments.
Figure 2.
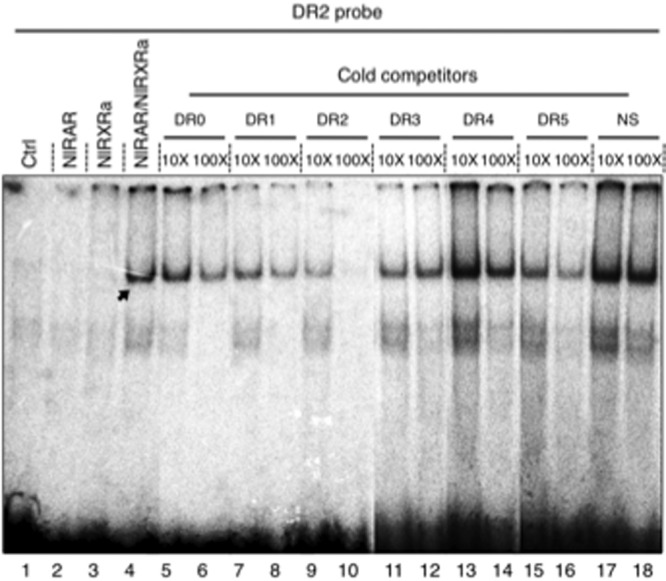
DNA recognition by NlRAR and NlRXRa. Binding of in vitro synthesized NlRAR/NlRXRa heterodimers to a radioactive DR2 probe. Control lanes corresponding to unprogrammed reticulocytes (lane 1), NlRAR alone (lane 2), and NlRXR alone (lane 3). The NlRAR/NlRXRa heterodimers very strongly bind the radioactive DR2 probe (lane 4). Cold competitors correspond to 10-fold (×10) or 100-fold (×100) excess of unlabeled oligonucleotides (DR0 to DR5) (lanes 5–16). A nonspecific element (NS) was used as a negative control (lanes 17–18).
Binding was assessed on consensus DR elements as defined in vertebrates (Figure 2 and Supplemental Figure 2). Using a radiolabeled DR2 probe, no binding of NlRAR alone could be detected (Figure 2). In contrast, when NlRXRa was added, the NlRAR/NlRXRa heterodimer strongly binds the DR2 element (Figure 2). This binding to the labeled DR2 probe is lost in presence of a large excess (10- or 100-fold) of unlabeled DR1, DR2, and DR5 (Figure 2). In contrast, the use of unlabeled DR3 or DR4 as well as a nonspecific unlabeled probe added in excess was not able to compete for binding (Figure 2). Similar results were obtained, with a radiolabeled DR5 probe (Supplemental Figure 2A). Therefore, we conclude that the NlRAR/NlRXRa heterodimer is able to bind specific sequences of DNA, in particular DR1, DR2, or DR5 elements, but not DR3 or DR4 sequences. As shown in Supplemental Figure 2B, NlRXRa is able to bind as a homodimer to DR1 elements. In contrast, the NlRXRb isoform shows impaired DNA binding both as a homodimer and as a heterodimer with NlRAR. Interestingly, in T. clavigera, similar RXR isoforms have been observed, and it has been shown that the T-box insertion reduces the transcriptional activity of the receptor (47).
Taken together, these results show that N. lapillus possesses a RAR/RXR heterodimer that recognizes a set of RAREs very similar to the ones recognized by chordate RAR/RXR heterodimers.
NlRAR does not bind ATRA and other retinoids, but NlRXR binds 9cRA
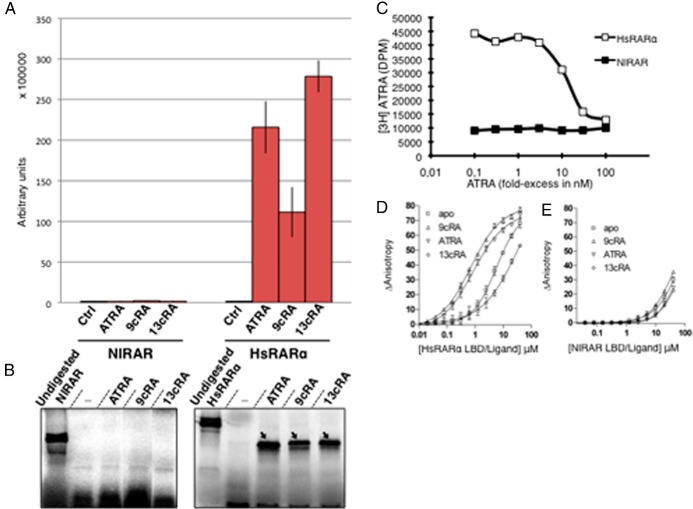
To obtain insights into the ligand binding properties of the RAR and RXR from N. lapillus, we tested the ability of both NlRAR and NlRXR to bind to different retinoids and to activate transcription in a ligand-dependent manner (Figure 3 and Supplemental Figures 3–5). Initially, the ability of NlRAR to activate transcription in mammalian cells was assessed using transient transfection with GAL4-LBD-RAR fusion constructs. We observed that NlRAR was not able to activate the transcription of the luciferase reporter with any of the ligands tested (ATRA, 9cRA, 13cRA, retinol, and retinal) at doses varying from 10−7 to 10−5 M (Figure 3A and Supplemental Figure 3A, data not shown). In contrast, all of these compounds stimulated the transcriptional activity of human RARα (Figure 3A and Supplemental Figure 3A).
Figure 3.
NlRAR does not bind or activate transcription in the presence retinoids. A, The ability of NlRAR and human RARα (HsRARα) to activate transcription of a luciferase reporter gene construct was tested in transfected HEK 293T cells in the presence of 10−5 M of ATRA, 9cRA, and 13cRA. The GAL4 DNA binding domain alone was used as a negative control (Ctrl). B, The binding affinity of both receptors for ATRA, 9cRA, and 13cRA was tested by limited proteolysis assays. Ligands were used at 10−5 M, and digested protein in the presence of ethanol was used as a negative control (lane −). Protected bands are indicated by arrows. C, Competitive binding assay with 10 nM 3H-labeled ATRA and increasing concentrations of unlabeled ligand. The human RARα (HsRARα) was used as a positive control. D and E, Titration of fluorescein-labeled SRC-1 NR2 peptide by the ligand-binding domains of human RARα (HsRARα) (D) and NlRAR (E) in the absence (apo) or presence (9cRA, ATRA, and 13cRA) of different ligands.
This inability of NlRAR to activate transcription, in the presence of a ligand, may be due to the fact that we tested a mollusk protein in mammalian cells, even if the regions of the LBD implicated in coactivator binding are relatively well-conserved in NlRAR. We nonetheless tested the direct binding of different ligands to NlRAR using LPAs. This method allows the in vitro assessment of the conformational change after the ligand binding to the receptor (35, 48). Using this technique, we observed that the LBD of NlRAR was not protected from proteolysis by ATRA (at final concentrations ranging from 10−7 to 10−5 M) or by any other retinoid tested (9cRA, 13cRA, retinol, and retinal) (Figure 3B and Supplemental Figure 3B).
To further confirm these results, we performed a competitive binding assay using 3H-labeled ATRA and increasing concentrations of unlabeled ligand. We observed that the addition of increasing concentrations of unlabeled ATRA decreased the human RARα-bound radioactivity (Figure 3C). The unlabeled competitor displaced 50% of the binding at a concentration of approximately 10 nM, which is in general agreement with the known affinity of RARα for ATRA. In contrast, NlRAR yielded only residual radioactivity levels, and the addition of increasing concentrations of unlabeled ATRA produced no reduction in radioactivity, suggesting that the weak binding observed in this assay is nonspecific (Figure 3C).
In addition, we investigated the ability of NlRAR to recruit coactivators by analyzing the titration of a fluorescein-labeled SRC-1 NR2 peptide by NlRAR in the presence of different retinoids (ATRA, 9cRA, and 13cRA) (Figure 3, D and E). As expected, we observed that the affinity of the human RARα for the SRC-1 NR2 peptide increases in the presence ATRA and 9cRA. When human RARα is analyzed in the presence of 13cRA, the receptor does not recruit the SRC-1 NR2 peptide. For the mollusk receptor, we observed no binding of the SRC-1 NR2 peptide in the presence of any of the compounds tested (Figure 3E). In contrast, we observed that the NlRAR LBD interacts efficiently with corepressor-derived peptides (Supplemental Figure 4A) and that none of the ligands tested is able to dissociate this interaction, suggesting that NlRAR could act as a constitutive repressor (Supplemental Figure 4C). As expected, the dissociation of the corepressor peptides is observed with human RARα, irrespective of which ligand is used (Supplemental Figure 4B). Because the N. lapillus RAR seems to behave like a transcriptional repressor, we tested whether this receptor has a competitive effect on transactivation mediated by a human RAR. As shown in Supplemental Figure 5, this is effectively the case: the introduction of increasing amounts of NlRAR shuts down the RA-dependent transactivation, mediated by human RAR, of a RARE-containing reporter gene. Altogether, these results show that NlRAR is not able to bind ATRA, as well as other classical retinoids, and that in the presence of these retinoids, NlRAR does not activate target gene transcription.
Contrasting the results we obtained for NlRAR, we observed that, as expected, NlRXR is able to activate the transcription of the luciferase reporter gene in a dose-dependent manner in the presence of 9cRA and other rexinoids (Supplemental Figure 6A) (49, 50).
Specific amino acid differences are responsible for the loss of NlRAR ligand binding
As shown above, the novel RAR identified in N. lapillus is unable to bind the different retinoids tested and to activate transcription in response to these compounds. Given that the sequence of the NlRAR LBD is globally relatively well conserved, with 52%–54% amino acid identity when compared with vertebrate RARs, which is in the range of the conservation between vertebrate ERα and ERβ, both of which bind to and are activated by estrogens (51), the incapacity of RA binding by NlRAR is very likely not due to a general divergence of the LBD. This finding indicates that alterations of specific amino acids inside the NlRAR LBP strongly reduce the affinity for ATRA. Indeed, in human receptors, it was shown that the mutation of a small number of residues involved in the binding to RA can abolish ligand binding and transactivation (52, 53). Sequence alignments and protein modeling thus revealed that, of the 24 amino acids known to be in contact with the ligand in vertebrate RARs, seven are different in NlRAR (T154 in helix 1, V183 and A190 in helix 3, M224 in helix 5, A242 in the β-turn, L361 in the loop linking helix 11 and helix 12, and V365 in helix 12). These seven amino acid differences might thus be responsible for the reduction of the affinity for retinoids that we identified in NlRAR (Figure 4A and Supplemental Figure 1).
Figure 4.
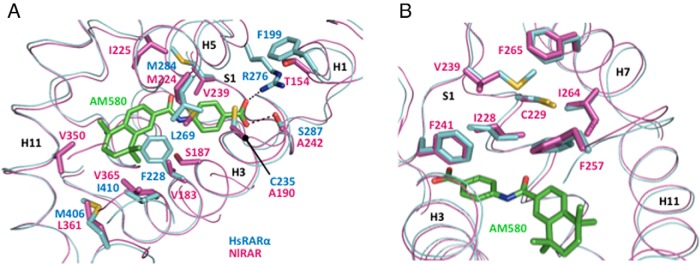
Model of the RAR ligand-binding pocket. A and B, Superimposition of the model of the NlRAR LBP (magenta) on the crystal structure of the human RARα (HsRARα) LBP (light blue) bound to the RARα-selective agonist AM580 (green). The NlRAR model was constructed using the modeling meta-server @TOME 2 (37) and the crystal structure of the HsRARα LBD (PDB code 3KMR) as a template. Divergent residues as well as NlRAR amino acids corresponding to HsRARα-specific residues are indicated.
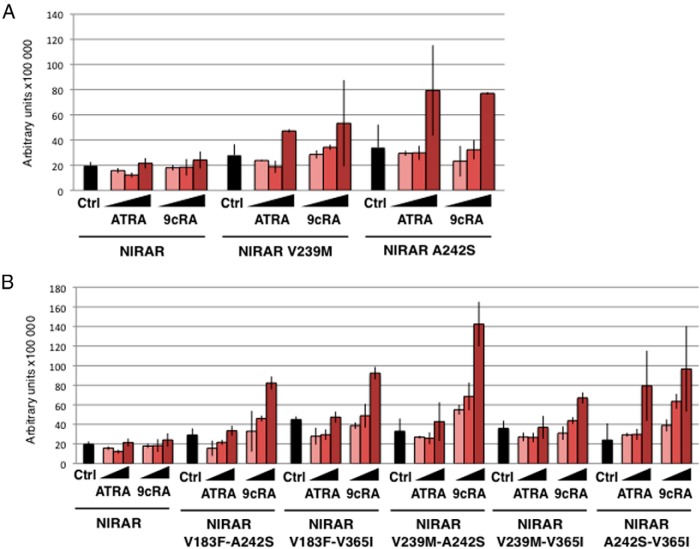
The impact on ligand binding of three amino acid differences (V183F, A242S, and V365I) was evaluated by replacing these residues in NlRAR by the corresponding amino acids present in human RARα (Supplemental Figure 1). In addition, we mutated V239 in the β-strand S1 into the corresponding human RARα methionine residue (Supplemental Figure 1). Although this residue does not establish a direct contact with the ligand in human RARs, the presence in NlRAR of a valine residue at this position (a significantly smaller amino acid) might destabilize the hydrophobic network located in close proximity of the ligand (Figure 4B). We first assessed the ability of the different GAL4-LBD-mutant-RAR constructs to activate transcription in the presence of different retinoids. Of the four single mutants tested, two, V239M and A242S, activated transcription in the presence of high doses of ATRA and 9cRA (Figure 5A), but not in the presence of the synthetic ligand TTNPB (Supplemental Table 1). This difference is very likely due to the higher adaptability and flexibility of natural retinoids when compared with the constrained and rigid structures of synthetic ones. Along these lines, 3D modeling demonstrated that the A242S mutation restores an important hydrogen bond between the receptor and the carboxylate moiety of the retinoids (Figure 4A). The transactivation assays further revealed that the other two single mutants (V183F and V365I) could not activate transcription (Supplemental Table 1).
Figure 5.
Specific mutations recover retinoid binding of NlRAR. The ability of single (A) and double (B) amino acid mutants of NlRAR to activate transcription of a luciferase reporter gene construct was tested in transfected HEK 293T cells in the presence of increasing concentrations (10−7, 10−6, and 10−5 M) of ATRA and 9cRA. The GAL4 DNA binding domain alone was used as a negative control (Ctrl). The nature of the amino acid mutation is indicated for each mutant.
To confirm these results and extend the analysis of the LBP, we also constructed five double mutants (V183F-A242S, V183F-V365I, V239M-A242S, V239M-V365I, and A242S-V365I) (Supplemental Figure 1). Interestingly, all the double mutants can be activated by 9cRA and two of them (V239M-A242S and A242S-V365I) can additionally be activated by ATRA (Figure 5B). Again, all the synthetic compounds tested were inactive (Supplemental Table 1). We further observed that the level of receptor activation by ATRA and 9cRA greatly varies according to the mutations introduced in NlRAR. The double mutant combining the two single mutants that restored activity (V239M plus A242S) strongly activates transcription in the presence of 9cRA and only weakly in the presence of ATRA. The double mutant A242S plus V365I is activated more efficiently by both ATRA and 9cRA than the corresponding single mutants. We also observed that the combination of the two single mutants that did not yield a transcriptional activation in the presence of retinoids (V183F plus V365I) gave rise to a mutant receptor that is activated by 9cRA. These data suggest that a single mutation might be sufficient to restore at least a weak retinoid binding capacity in NlRAR and indicate that a combination of mutations within the LBP might potentiate each other to further increase the affinity of NlRAR for retinoid binding. The LBP of NlRAR is thus unable to bind ATRA and other retinoids due to a series of specific mutations with additive effects that incrementally altered the affinity for retinoids. Importantly, this also indicates that mutations of amino acids that are not in direct contact with the ligand may also affect ligand binding, as observed with NlRAR V239M.
Endogenous retinoids in adult tissues
Analysis of mollusk genomes has revealed the presence of homologs of genes encoding enzymes responsible for RA synthesis, such as aldh1 and aldh8 (28). We therefore studied the retinoids endogenously present in N. lapillus adults by tandem mass spectrometry (39). We were able to detect ATRA, 13cRA, retinol, and retinyl esters, but not 9cRA. Concentrations of 13cRA were much higher than those of ATRA: 90.0 ± 6.7 pmol/g tissue compared with 12.8 ± 0.9 pmol/g tissue, respectively (Figure 6A). In addition, retinyl ester concentrations were at 2.8 ± 0.3 nmol/g tissue and those of retinol at 0.18 ± 0.01 nmol/g tissue (Figure 6B). This shows that ATRA is present in adult tissues, but at levels much lower than 13cRA, which seems to be the most abundant RA isomer. Interestingly, as in most human and mouse tissues, 9cRA seems to be absent from the tissues we tested.
Figure 6.
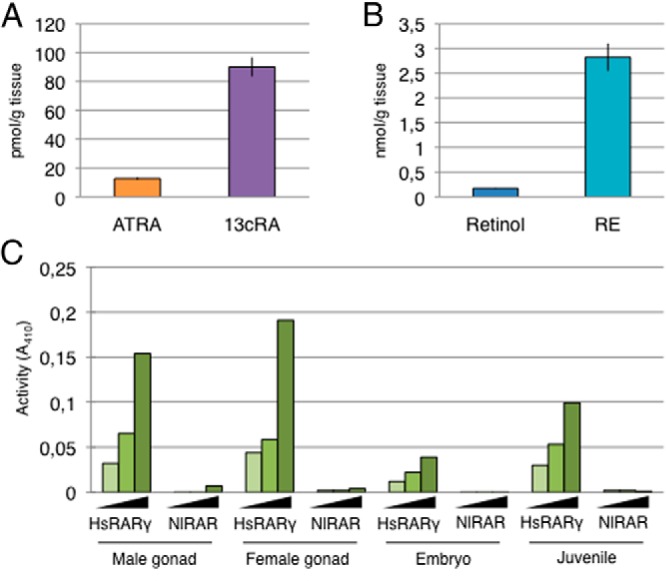
Retinoids are endogenously present in N. lapillus adults. A, Quantification of ATRA and 13cRA in adults of the mollusk N. lapillus. Quantities are expressed in picomoles per gram of tissue and data are mean ± SEM (n = 22). B, Quantification of retinol and retinyl esters (RE) in adults of the mollusk N. lapillus. Quantities are expressed in nanomoles per gram of tissue and data are mean ± SEM (n = 22). C, Ability of retinoid extracts from the mollusk N. lapillus (from male gonad, female gonad, embryo, or juvenile) to activate human RARγ (HsRARγ) or N. lapillus RAR (NlRAR).
Considering that NlRAR is unable to bind the different retinoids tested and to activate transcription, we assessed whether extracts from N. lapillus can activate NlRAR or one of its vertebrate homologs. We observed that, although N. lapillus extracts of male and female gonads, embryos, and juveniles can effectively activate the human RARγ, which confirms the presence of active retinoids in these extracts, NlRAR is not activated by any of these extracts. This strongly suggests that NlRAR is nonresponsive to the retinoids contained in the N. lapillus tissue extracts (Figure 6C).
Finally, we assessed the expression of the N. lapillus rar gene in different adult tissues and observed that it is expressed in a variety of tissues and organs with the highest levels found in the kidney (Supplemental Figure 7).
Discussion
Very little is known about the evolutionary origin of the RA signaling pathway, which was believed until recently to be chordate specific. Here, we describe a novel RAR homolog, cloned from the mollusk N. lapillus. The molecular characterization of this mollusk RAR showed that it is capable of binding DNA on classical RAREs as a heterodimer with RXR. In contrast, NlRAR is unable to bind ATRA as well as other retinoids and to activate transcription or recruit coactivators in response to these compounds. We explain this absence of binding by specific changes in the LBP of NlRAR as the reversion of some of these sites results in a mutated NlRAR that can be activated by retinoids.
Evolution of RARs in metazoan animals
Despite its unambiguous phylogenetic association with RARs from other metazoan phyla, NlRAR is unable to bind ATRA or to activate transcription in response to retinoids. This result is likely representative of the general situation in mollusks (or at least in gastropods) because the RAR from the gastropod T. clavigera is also not responsive to ATRA, hence mirroring the situation we describe for NlRAR (32). The fact that Thais and Nucella both contain a conserved RAR despite the ancient divergence of these two taxa (about 100 million years) (54, 55) clearly indicates that these genes are functional: two conserved genes separated by such a very long period of time must have a, possibly even conserved, function.
Given the phylogenetic relationships of bilaterian animals, the presence of RAR in lophotrochozoans suggests that the evolutionary origin of this receptor is more ancient than previously thought and that a RAR was already present in Urbilateria, the last common ancestor of all bilaterians (28). We propose that the urbilaterian RAR was effectively a liganded receptor and thus capable of binding retinoids and that this capacity was secondarily lost in gastropod mollusks, because the RAR homolog of the annelid worm Platynereis dumerilii might bind to and be activated by ATRA (Figure 7). Therefore, the most parsimonious scenario is that the activity of the urbilaterian RAR was regulated by retinoids, such as ATRA, and that, specifically in the mollusk lineage, RAR became unresponsive to retinoids (Figure 7).
Figure 7.
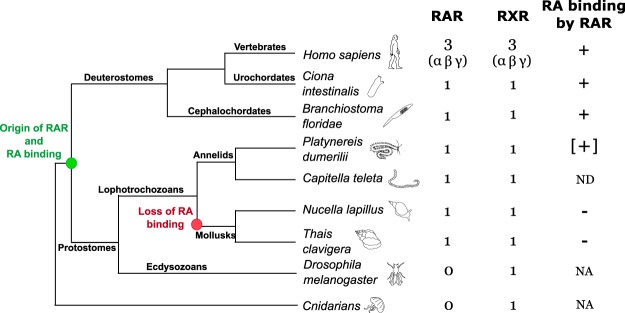
Inferred evolutionary history of RARs and their ability to bind retinoids in bilaterian animals. The origin of RAR and retinoid/RA binding coincides with the evolutionary diversification of bilaterian animals (green circle). The ability of RAR to bind RA was lost in the mollusk lineage (red circle). NA, not applicable, ND, not determined. The data from the annelid Platynereis dumerilii are based on unpublished observations by J.G.-M., M.S., and V.L. (brackets).
Loss of RA binding in mollusk RAR
The loss of binding to retinoids observed in NlRAR might be explained by amino acid differences in the LBP of N. lapillus RAR and its vertebrate homologs. The 3D structures of the LBPs reveal several key residues that are contacting the ligand in human RARα that are different in NlRAR. We observed that mutations at two positions (V239 and A242), when changed to the human amino acid (to a methionine and to a serine, respectively), result in receptors that recover some retinoid binding activity. Mutations in the NlRAR LBP have an additive effect with the accumulation of vertebrate-like mutations leading to a higher retinoid-dependent transactivation activity. Altogether, these observations suggest that the retinoid binding to NlRAR was lost because of the accumulation of several mutations associated with the LBP, with each individual mutation probably having only minor effects on the ligand binding capacity of the receptor. Therefore, the loss of retinoid binding by N. lapillus RAR was likely not sudden, but incremental, resulting from the accumulation of individual mutations over time.
It is interesting to note that, albeit likely not functioning as a ligand binding site (at least for retinoids), the LBDs of the N. lapillus and T. clavigera RAR are reasonably well conserved, with 68% of sequence identity after approximately 100 million years of divergence (54, 55). Furthermore, 3D modeling of the T. clavigera RAR LBD revealed that the loss of ligand-binding activity in this receptor is probably not due to changes of the same sites as in the N. lapillus RAR. For example, C321 in the T. clavigera RAR replaces an arginine residue present in the C-terminal part of helix 5 in vertebrate RARs. This arginine is crucial for forming a stabilizing salt bridge with the acidic function of retinoids. Another important difference between vertebrate RARs and the T. clavigera RAR is the replacement of a cysteine residue contacting the ligand by a smaller glycine residue (G280 in the T. clavigera RAR). Both of these amino acids are not conserved between the RARs of T. clavigera and N. lapillus, and the C321 of T. clavigera RAR even corresponds to an arginine in N. lapillus, as is the case in vertebrates. These findings reinforce the notion that the loss of retinoid binding of mollusk RARs occurred incrementally by the accumulation of several mutations, which might have taken place independently in different mollusk lineages.
To better understand the loss of retinoid binding of the N. lapillus and T. clavigera RAR, we assessed which alterations to the RAR sequence are specific to the mollusk lineage; for this, we reconstructed the ancestral sequences of the mollusk and protostome lineages (nodes 29 and 31, respectively, Supplemental Figure 8). We hence identified 46 substitutions that are specific to the two mollusk RARs, of which 43 are in the LBD. In vertebrates, only two of these are in direct contact with the ligand: the threonine/serine 148 in helix 1 and the valine 365 in helix 12 (Supplemental Figure 8). These sites represent fundamental differences that distinguish the mollusk RARs from all other known RAR sequences and are thus likely to have occurred early in the divergence of the mollusk lineage, at the onset of the reduction of the affinity for retinoids of the mollusk RARs. This scenario for the stepwise loss of retinoid binding of the mollusk RARs is very similar to the mechanisms proposed for the acquisition of substrate specificity of the duplicated vertebrate steroid receptors (2).
Retinoids in mollusks
Our analysis of the retinoid content of N. lapillus revealed the presence, at relatively high levels, of ATRA and 13cRA and the apparent absence of 9cRA. In addition, we also detected retinol and retinyl esters in N. lapillus. These findings are somewhat different from previous retinoid measurements in N. lapillus, given that these previous reports were unable to detect retinol and retinyl esters in N. lapillus tissues (25). Also, these authors did not observe retinyl ester synthesis, when retinol was injected into the adult, suggesting that N. lapillus does not have the ability to store retinoids in the form of retinyl esters. In addition, in this previous study, 9cRA was detected in N. lapillus tissues, albeit at very low levels (25). These apparent discrepancies may be linked to the fact that the authors used isolated tissues of N. lapillus, whereas we used extracts prepared from complete animals.
Importantly, though, there is one common feature between these results: the detection of high levels of ATRA and 13cRA (25). These data thus suggest a paradoxical reality: elevated concentrations of active retinoids are present in N. lapillus, but the putative cognate receptor, NlRAR, is unable to bind these compounds. It is possible that the biologically active ligand recognized by NlRAR is a different molecule, maybe even a different retinoid. The fact that the N. lapillus and T. clavigera RAR LBD exhibit 68% sequence identity and that at least some positions of the LBP are conserved between the two species (V228, M269, and V410) may suggest that a common ligand for these receptors remains to be identified (54, 55), even if extracts from N. lapillus were unable to activate NlRAR, in contrast to human RARγ. The fact that we detected ATRA and 13cRA in N. lapillus tissues and that mollusk genomes contain orthologs of the enzymes implicated in RA metabolism (28, 27, 28, 31) favors the notion that retinoids have a specific function in members of this taxon. Studies of the biological functions of NlRAR are needed to obtain additional insights into the mechanisms of its activity and possible activation by a ligand.
Conclusion
Our findings illustrate the plasticity of the LBD of nuclear receptors by illustrating the lineage-specific loss of retinoid binding capacity by RARs. The characterization of NR orthologs from various different animals has revealed that classical liganded vertebrate NRs, such as RARs, TRs, or ERs, are evolutionarily much more ancient than expected, but also that the presence of these genes in a given genome does not automatically translate into an evolutionarily conserved function. Our work also illustrates that the characterization of NRs from alternative model systems can shed new light on our understanding of the evolution of endocrine systems that might have never been obtained from work on model organisms.
Acknowledgments
We thank Marie Sémon for help with sequence analyses, Laurent Guéguen for help with the reconstruction of ancestral sequences, and Guillaume Holzer for performing the competition experiments.
V.L. is supported by the Centre National de la Recherche Scientifique, the ENS de Lyon, and the MENRT. M.S. is supported by the Centre National de la Recherche Scientifique and by Grant ANR-11-JSV2-002-01 awarded by the Agence Nationale de la Recherche. L.F.C.C. and M.M.S. are supported by the FCT (Grants PTDC/MAR/105199/2008 and PTDC/MAR/115199/2009). J.-I.N. is supported by the KAKENHI (Grant 21590143). M.K. received support from the National Institute of Allergy and Infectious Diseases Contract HHSN272202000046C and from the University of Maryland, School of Pharmacy, Mass Spectrometry Center (Grant SOP1841-IQB2014).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATRA
- all-trans RA
- 9cRA
- 9-cis RA
- 13cRA
- 13-cis RA
- 3D
- three-dimensional
- DBD
- DNA-binding domain
- DR
- direct repeat
- ER
- estrogen receptor
- HEK
- human embryonic kidney
- LBD
- ligand-binding domain
- LBP
- ligand-binding pocket
- LPA
- limited proteolysis assay
- NlRAR
- RAR from the mollusk Nucella lapillus
- NlRXR
- RXR from the mollusk Nucella lapillus
- NR
- nuclear receptor
- RA
- retinoic acid
- RAR
- retinoic acid receptor
- RARE
- retinoic acid response element
- RXR
- retinoid X receptor
- T-box
- terminal box
- TR
- thyroid hormone receptor.
References
- 1. Laudet V, Gronemeyer H. The nuclear receptor facts book. San Diego: Academic Press; 2002. [Google Scholar]
- 2. Harms MJ, Eick GN, Goswami D, et al. Biophysical mechanisms for large-effect mutations in the evolution of steroid hormone receptors. Proc Natl Acad Sci USA. 2013;110:11475–11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bridgham JT, Keay J, Ortlund EA, Thornton JW. Vestigialization of an allosteric switch: genetic and structural mechanisms for the evolution of constitutive activity in a steroid hormone receptor. PLoS Genet. 2014;10:e1004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lecroisey C, Laudet V, Schubert M. The cephalochordate amphioxus: a key to reveal the secrets of nuclear receptor evolution. Brief Funct Genomics. 2012;11:156–166. [DOI] [PubMed] [Google Scholar]
- 5. Paris M, Pettersson K, Schubert M, et al. An amphioxus orthologue of the estrogen receptor that does not bind estradiol: insights into estrogen receptor evolution. BMC Evol Biol. 2008;8:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. [DOI] [PubMed] [Google Scholar]
- 7. Markov GV, Tavares R, Dauphin-Villemant C, Demeneix BA, Baker ME, Laudet V. Independent elaboration of steroid hormone signaling pathways in metazoans. Proc Nat Acad Sci USA. 2009;106:11913–11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scott AP. Do mollusks use vertebrate sex steroids as reproductive hormones? Part I: critical appraisal of the evidence for the presence, biosynthesis and uptake of steroids. Steroids. 2012;77:1450–1468. [DOI] [PubMed] [Google Scholar]
- 9. Scott AP. Do mollusks use vertebrate sex steroids as reproductive hormones? II. Critical review of the evidence that steroids have biological effects. Steroids. 2013;78:268–281. [DOI] [PubMed] [Google Scholar]
- 10. Paris M, Escriva H, Schubert M, et al. Amphioxus postembryonic development reveals the homology of chordate metamorphosis. Curr Biol. 2008b;18:825–830. [DOI] [PubMed] [Google Scholar]
- 11. Kane MA. Analysis, occurrence, and function of 9-cis-retinoic acid. Biochim Biophys Acta. 2012;1821:10–20. [DOI] [PubMed] [Google Scholar]
- 12. Balmer JE, Blomhoff R. A robust characterization of retinoic acid response elements based on a comparison of sites in three species. J Steroid Biochem Mol Biol. 2005;96:347–354. [DOI] [PubMed] [Google Scholar]
- 13. Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 14. Durand B, Saunders M, Leroy P, Leid M, Chambon P. All-trans and 9-cis retinoic acid induction of CRABPII transcription is mediated by RAR-RXR heterodimers bound to DR1 and DR2 repeated motifs. Cell. 1992;71:73–85. [DOI] [PubMed] [Google Scholar]
- 15. Moutier E, Ye T, Choukrallah M-A, et al. Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J Biol Chem. 2012;287:26328–26341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2000;80:1021–1054. [DOI] [PubMed] [Google Scholar]
- 17. Smith WC, Nakshatri H, Leroy P, Rees J, Chambon P. A retinoic acid response element is present in the mouse cellular retinol binding protein I (mCRBPI) promoter. EMBO J. 1991;10:2223–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gutierrez-Mazariegos J, Schubert M, Laudet V. Evolution of retinoic acid receptors and retinoic acid signaling. Subcell Biochem. 2014;70:55–73. [DOI] [PubMed] [Google Scholar]
- 19. Dmetrichuk JM, Carlone RL, Spencer GE. Retinoic acid induces neurite outgrowth and growth cone turning in invertebrate neurons. Dev Biol. 2006;294:39–49. [DOI] [PubMed] [Google Scholar]
- 20. Dmetrichuk JM, Carlone RL, Jones TRB, Vesprini ND, Spencer GE. Detection of endogenous retinoids in the molluskan CNS and characterization of the trophic and tropic actions of 9-cis retinoic acid on isolated neurons. J Neurosci. 2008;28:13014–13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishikawa J, Mamiya S, Kanayama T, Nishikawa T, Shiraishi F, Horiguchi T. Involvement of the retinoid X receptor in the development of imposex caused by organotins in gastropods. Environ Sci Technol. 2004;38:6271–6276. [DOI] [PubMed] [Google Scholar]
- 22. Castro FC, Lima D, Machado A, et al. Imposex induction is mediated through the retinoid X receptor signalling pathway in the neogastropod Nucella lapillus. Aquat Toxicol. 2007;85:57–66. [DOI] [PubMed] [Google Scholar]
- 23. Creton R, Zwaan G, Dohmen R. Specific developmental defects in mollusks after treatment with retinoic acid during gastrulation. Dev Growth Differ. 1993;35:357–364. [DOI] [PubMed] [Google Scholar]
- 24. Gesto M, Castro LF, Reis-Henriques MA, Santos MM. Retinol metabolism in the mollusk Osilinus lineatus indicates an ancient origin for retinyl ester storage capacity. PLoS One. 2012;7:e35138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gesto M, Castro LF, Santos M. Differences in retinoid levels and metabolism among gastropod lineages: imposex-susceptible gastropods lack the ability to store retinoids in the form of retinyl esters. Aquat Toxicol. 2013;142–143:96–103. [DOI] [PubMed] [Google Scholar]
- 26. Bouton D, Escriva H, de Mendonça RL, et al. A conserved retinoid X receptor (RXR) from the mollusk Biomphalaria glabrata transactivates transcription in the presence of retinoids. J Mol Endocrinol. 2005;34:567–582. [DOI] [PubMed] [Google Scholar]
- 27. Albalat R, Cañestro C. Identification of Aldh1a, Cyp26 and RAR orthologs in protostomes pushes back the retinoic acid genetic machinery in evolutionary time to the bilaterian ancestor. Chem Biol Interact. 2009;178:188–196. [DOI] [PubMed] [Google Scholar]
- 28. Campo-Paysaa F, Marlétaz F, Laudet V, Schubert M. Retinoic acid signaling in development: tissue-specific functions and evolutionary origins. Genesis. 2008;46:640–656. [DOI] [PubMed] [Google Scholar]
- 29. Cañestro C, Postlethwait JH, Gonzàlez-Duarte R, Albalat R. Is retinoic acid genetic machinery a chordate innovation? Evol Dev. 2006;8:394–406. [DOI] [PubMed] [Google Scholar]
- 30. Marlétaz F, Holland L, Laudet V, Schubert M. Retinoic acid signaling and the evolution of chordates. Int J. 2006;2:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simões-Costa MS, Azambuja AP, Xavier-Neto J. The search for non-chordate retinoic acid signaling: lessons from chordates. J Exp Zool B Mol Dev Evol. 2008;310:54–72. [DOI] [PubMed] [Google Scholar]
- 32. Urushitani H, Katsu Y, Ohta Y, Shiraishi H, Iguchi T, Horiguchi T. Cloning and characterization of the retinoic acid receptor-like protein in the rock shell, Thais clavigera. Aquat Toxicol. 2013;142–143:403–413. [DOI] [PubMed] [Google Scholar]
- 33. Allenby G, Bocquel MT, Saunders M, et al. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci USA. 1993;90:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–224. [DOI] [PubMed] [Google Scholar]
- 35. Escriva H, Bertrand S, Germain P, et al. Neofunctionalization in vertebrates: the example of retinoic acid receptors. PLoS Genet. 2006;2:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. le Maire A, Teyssier C, Erb C, et al. A unique secondary-structure switch controls constitutive gene repression by retinoic acid receptor. Nat Struct Mol Biol. 2010;17:801–807. [DOI] [PubMed] [Google Scholar]
- 37. Pons JL, Labesse G. @TOME-2: a new pipeline for comparative modeling of protein-ligand complexes. Nucleic Acids Res. 2009;37:W485–W491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kane MA, Napoli JL. Quantification of endogenous retinoids (Chapter 1). Retinoids: Methods and Protocols. In: Sun H, Travis GH, eds. Methods in Molecular Biology. Totowa: Humana Press; 2010;652:1–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem. 2008;80:1702–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kane MA, Folias AE, Napoli JL. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal Biochem. 2008;378:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perlmann T, Rangarajan PN, Umesono K, Evans RM. Determinants for selective RAR and TR recognition of direct repeat HREs. Genes Dev. 1993;7:1411–1422. [DOI] [PubMed] [Google Scholar]
- 42. Wilson TE, Paulsen RE, Padgett KA, Milbrandt J. Participation of non-zinc finger residues in DNA binding by two nuclear orphan receptors. Science. 1992;256:107–110. [DOI] [PubMed] [Google Scholar]
- 43. Zechel C, Shen XQ, Chen JY, Chen ZP, Chambon P, Gronemeyer H. The dimerization interfaces formed between the DNA binding domains of RXR, RAR and TR determine the binding specificity and polarity of the full-length receptors to direct repeats. EMBO J. 1994;13:1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zechel C, Shen XQ, Chambon P, Gronemeyer H. Dimerization interfaces formed between the DNA binding domains determine the cooperative binding of RXR/RAR and RXR/TR heterodimers to DR5 and DR4 elements. EMBO J. 1994;13:1414–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–964. [DOI] [PubMed] [Google Scholar]
- 46. Renaud J, Rochel N, Ruff M, Vivat V. Crystal structure of the RAR-γ ligand-binding domain bound to all-trans retinoic acid. Nature. 1995;378:681–689. [DOI] [PubMed] [Google Scholar]
- 47. Urushitani H, Katsu Y, Ohta Y, Shiraishi H, Iguchi T, Horiguchi T. Cloning and characterization of retinoid X receptor (RXR) isoforms in the rock shell, Thais clavigera. Aquat Toxicol. 2011;103:101–111. [DOI] [PubMed] [Google Scholar]
- 48. Leid M. Ligand-induced alteration of the protease sensitivity of retinoid X receptor α. J Biol Chem. 1994;269:14175–14181. [PubMed] [Google Scholar]
- 49. Egea PF, Mitschler A, Moras D. Molecular recognition of agonist ligands by RXRs. Mol Endocrinol. 2002;16:987–997. [DOI] [PubMed] [Google Scholar]
- 50. Nahoum V, Pérez E, Germain P, et al. Modulators of the structural dynamics of the retinoid X receptor to reveal receptor function. Proc Nat Acad Sci USA. 2007;104:17323–17328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ostrowski J, Hammer L, Roalsvig T, Pokornowski K, Reczek PR. The N-terminal portion of domain E of retinoic acid receptors α and β is essential for the recognition of retinoic acid and various analogs. Proc Natl Acad Sci USA. 1995;92:1812–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tate BF, Grippo JF. Mutagenesis of the ligand binding domain of the human retinoic acid receptor α identifies critical residues for 9-cis-retinoic acid binding. J Biol Chem. 1995;270:20258–20263. [DOI] [PubMed] [Google Scholar]
- 54. Barco A, Claremont M, Reid DG, et al. A molecular phylogenetic framework for the Muricidae, a diverse family of carnivorous gastropods. Mol Phylogenet Evol. 2010;56:1025–1039. [DOI] [PubMed] [Google Scholar]
- 55. Claremont M, Vermeij GJ, Williams ST, Reid DG. Global phylogeny and new classification of the Rapaninae (Gastropoda: Muricidae), dominant molluscan predators on tropical rocky seashores. Mol Phylogenet Evol. 2013;66:91–102. [DOI] [PubMed] [Google Scholar]