Abstract
High plasma levels of estradiol (E2) are associated with use of a place memory system over a response memory system. We examined whether infusing estradiol into the medial prefrontal cortex (mPFC) or anterior cingulate cortex (AC) could affect memory system bias in female rats. We also examined the ultrastructural distribution of estrogen receptor (ER)-α, ERβ, and G protein-coupled estrogen receptor 1 (GPER1) in the mPFC of female rats as a mechanism for the behavioral effects of E2 in the mPFC. Each rat was infused bilaterally with either E2 (0.13 μg) or vehicle into the mPFC or AC. The majority of E2 mPFC rats used place memory. In contrast, the majority of mPFC vehicle rats and AC E2 or vehicle rats used response memory. These data show that mPFC E2 rapidly biases females to use place memory. Electron microscopic analysis demonstrated that ERα, ERβ, and GPER1 are localized in the mPFC, almost exclusively at extranuclear sites. This is the first time that GPER1 has been localized to the mPFC of rats and the first time that ERα and ERβ have been described at extranuclear sites in the rat mPFC. The majority of receptors were observed on axons and axon terminals, suggesting that estrogens alter presynaptic transmission in the mPFC. This provides a mechanism via which ERs could rapidly alter transmission in the mPFC to alter PFC-dependent behaviors, such as memory system bias. The discrete nature of immunolabeling for these membrane-associated ERs may explain the discrepancy in previous light microscopy studies.
Several strategies can be used when animals are solving a maze to obtain a reward. One is response memory, which involves specific motor responses required to obtain a reward (1), meaning a rat will learn to always turn left or right in a maze. Alternately, place memory refers to the use of distal cues around the maze to orient to a location; these cues are compiled into a cognitive map that is used to navigate in the maze (1). White and McDonald (2) proposed that that response and place memory strategies are supported by two independently functioning parallel memory systems. The hippocampal system is thought to process information about environmental cues, as is done when using place memory. The second memory system is the dorsal striatal system, which is believed to support stimulus-response learning; in this form of learning an animal performs a habitual response when presented with a stimulus, as is done when using response memory. In some cases the effectiveness of one system can be increased by disabling the other (2).
In females these memory systems are sensitive to fluctuations in the levels of estrogens (for review see reference 3 and 4). When estrogen levels are high, female rats are biased toward the use of place memory (5–8). In contrast, when estrogen levels are low, female rats are biased toward the use of response memory (5–8). Injection of 17β-estradiol (E2) into the dorsal hippocampus has been found to improve place learning, whereas injections of E2 into the dorsal striatum impair response learning (9). These results suggest increases in systemic estrogens bias females toward the use of place memory due to estrogens acting in the hippocampus. Lower levels of estrogens are associated with a bias toward the use of response memory.
The hippocampus and the dorsal striatum have reciprocal projections to the medial prefrontal cortex (mPFC), so it is possible that this area may influence the outputs of these two systems (2). Neurons in the prelimbic and infralimbic regions of the mPFC are activated in response to a switch from place to response memory but not in response to changes in behavioral or task contingencies (10). These findings suggest that the mPFC plays a role in determining whether place or response memory will be used. Additionally, estrogens may contribute to mPFC control of memory system bias; ovariectomized (OVX) female rats with low E2 replacement switch from a response to place memory when dopamine D1 or D2 receptor antagonists are infused into the mPFC, whereas females administered high doses of E2 used a place memory, regardless of dopamine antagonist administration (Quinlan MG, et al, unpublished observation). Such results support a role for the mPFC in the E2-induced bias toward the use of place memory.
The mechanisms by which estrogens could alter neurotransmission in the mPFC to elicit these effects on multiple memory systems remain unclear. Estrogens act by binding at both nuclear and membrane-associated estrogen receptors (ERs) to elicit rapid changes in cell firing and slower alterations in protein production. In terms of the classical ERs, previous research has yielded contradictory results on the distribution of these receptors in the mPFC. Most studies demonstrate little ERα immunoreactivity (IR) in the mPFC of rats (12, 13) and low levels of ERβ-IR (13) and mRNA (14) in the mPFC of female rats. However, other studies demonstrate moderate levels of ERα-IR in the mPFC of rats (15, 16) and moderate levels of ERβ mRNA in the mPFC of mice (17). These studies observe these ERs exclusively at nuclear sites in the mPFC. One possible reason for the discrepancy in previous findings is that light microscopy, unlike electron microscopy (EM), is not sensitive enough to detect ERs at cell membranes in the mPFC. This assertion is supported by the finding that ultrastructural analysis detects abundant ERα immunoreactivity at the cell membrane in the primate prefrontal cortex (16), which is not observed with light microscopy in rodents (12–16). However, further research is needed to clarify whether ERα and ERβ are localized to the extranuclear sites in the mPFC of female rats and to investigate if the most recently discovered estrogen receptor, G protein-coupled estrogen receptor 1 (GPER1), is also present in the rat mPFC.
This experiment was conducted to determine whether estrogens act in the mPFC to bias female rats toward the use of place memory when solving an appetitive task. Here OVX female rats administered chronic low E2 replacement were implanted with bilateral cannulae in the mPFC or the anterior cingulate cortex (AC). The AC was selected as a control brain region because it is immediately dorsal to the mPFC, so any effect of E2 could be attributed to the mPFC and not E2 diffusion to dorsal regions along the cannula tract. All rats received microinfusions of E2 and vehicle, in counterbalanced order, immediately prior to navigating a maze task that could be completed using either place or response memory. Additionally, tissue from the mPFC of female rats in the diestrous phase of the cycle was immunolabeled for ERα, ERβ, and GPER1 and was examined using EM.
Materials and Methods
Experiment 1: mPFC and memory systems bias
Animals
This experiment used 32 female Sprague Dawley rats (Charles River) that weighed 240–260 g on arrival. Rats were pair housed until surgery, after which they were individually housed. The colony room was maintained on a reverse 12-hour light cycle, with lights off at 9:00 am. Standard lab chow and water were available ad libitum until the start of the experiment when food restriction began. The procedures used in the experiment were approved by the Concordia University Animal Care Committee in accordance with the guidelines of the Canadian Council on Animal Care.
Surgery and hormone administration
Surgeries were conducted as described previously (7, Quinlan MG, et al, unpublished observation). Cannulae were implanted (Plastics One) for microinfusion of E2 or cyclodextrin vehicle. For the mPFC group, coordinates from bregma were as follows: anterior-posterior = +3.1 mm, medial-lateral = ± 1.5 mm at 15°, and dorsal-ventral = −3.0 mm from skull surface. For the AC group, the stereotaxic coordinates were as follows: anterior-posterior = +3.1, medial-lateral = ±1.35 at 15°, and dorsal-ventral = −1.5. During the same procedure, rats were ovariectomized via a single lumbar incision and implanted sc at the nape of the neck with a SILASTIC brand capsule (Dow Corning) containing 5% E2 (Sigma Chemical Co) in cholesterol (Sigma). These implants have been shown to produce low E2 plasma levels, similar to levels observed during the diestrous phase of the estrous cycle (18). This low plasma level of E2 has previously been associated with a response memory bias (7, 8). After the procedure, the rats were allowed 1 week to recover before training began.
E2 encapsulated in cyclodextrin and the cyclodextrin vehicle were dissolved in artificial cerebrospinal fluid immediately before the testing session began. Drugs were infused bilaterally using injectors that extended 1 mm beyond the end of the cannulae. Infusions were 1 minute at a rate of 0.5 μL/min, after which the injectors were left in place for another minute while the drug diffused. This dose of E2 has been shown to have behavioral effects when infused into the brain (19).
Apparatus, modified plus maze
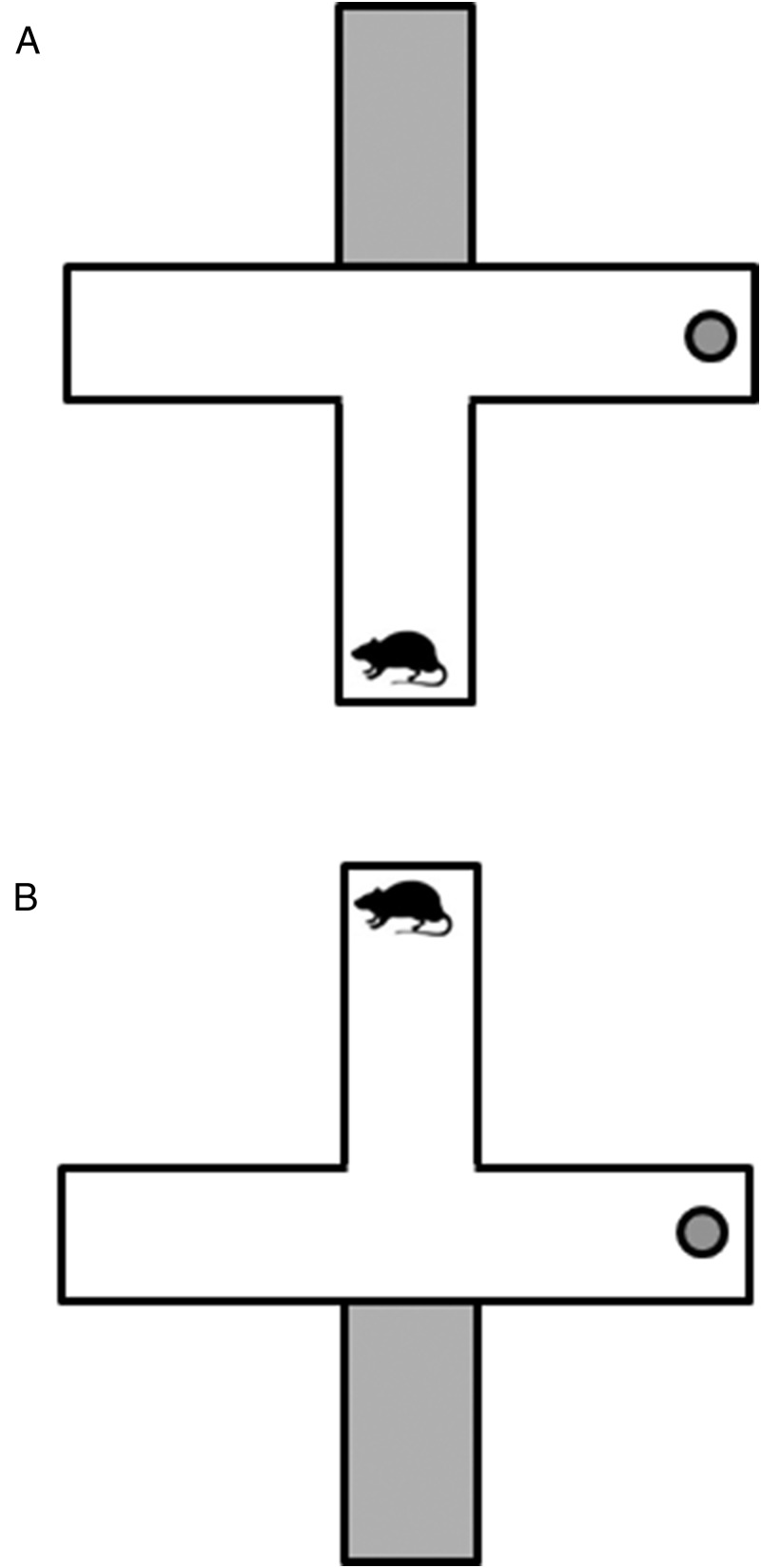
Training was conducted using a modified plus maze, as previously described (4, 7, 8, Quinlan MG, et al, unpublished observation). During training trials, access to the probe start arm was blocked off, resulting in a T-shaped maze (Figure 1A), and prior to testing the experimenter unblocked the probe arm and blocked the start arm, creating a T-maze 180° in orientation relative to the training T-maze (Figure 1B). At the end of each goal arm, there was a bowl for the food reward (Kellogg's Froot Loops), and Froot Loops crumbs were placed under the arms to mask any odor cues. There were extramaze cues around the room to facilitate the navigation of the maze, and testing took place under illumination from 20-W lights above each goal arm.
Figure 1.
A, Maze orientation during training trials. B, Maze orientation during probe trial used to determine what memory system is being used to navigate the maze.
Procedure
The training and testing phases of the experiment have been described extensively elsewhere (7, 8). Briefly, rats were food restricted and maintained at 90% of their free-feeding weight and trained to find a Fruit Loops that was consistently located in one of two goal arms. Each rat received 10 training trials daily; during each trial the rat was placed in the start arm and permitted to enter either of the goal arms. The intertrial interval was 10–60 seconds. Rats were trained daily until reaching the criterion, which was 8 of 10 correct trials for 3 consecutive days.
The day after the rats attained the criterion, either E2 encapsulated in cyclodextrin (5.44 μg/mL: 5% 17β-estradiol, 95% cyclodextrin) or cyclodextrin vehicle (5.16 μg/mL) was infused bilaterally into the mPFC or AC. All rats were tested under both treatment conditions (E2 and vehicle), and order effects were controlled by counterbalancing. Immediately after the infusion, each rat underwent 10 trials; rats underwent testing only if they remained at criterion for these trials. After the 10th trial, the maze was inverted for testing. There were 10–20 minutes between the infusion and the probe trial. During the probe trial, if the rat entered the goal arm that had been baited during the training phase, they were considered to be using place memory. In contrast, if the rat entered the opposite goal arm, thereby making the same directional turn as during training, the rat was considered to be using response memory. After the first probe trial, rats were retrained until they reached criterion, which took a minimum of 3 days of training. Then they were infused again with either E2 or vehicle, whichever one was not administered prior to the first probe trial, and underwent a second probe trial.
Histology
After behavioral testing, rats were infused with methylene blue to mark cannula placements, and then they were decapitated. Their brains were removed, flash frozen in isopentane, and stored at −80°C. Brains were sliced coronally on a cryostat at 40 μm and mounted on slides for confirmation of placements.
Statistical analysis
This experiment is a within-subject experimental design with treatment (E2 or vehicle) as the within factor. Because the dependent variable is categorical, nonparametric statistical techniques were used to determine whether there was a significant difference in memory system use under E2 and vehicle treatments. A McNemar test was used to compare the proportion of rats using each strategy after the infusions of the two compounds in the mPFC and AC groups. An odds ratio (OR) and a logit d were computed to provide an estimate of effect size for each McNemar analysis (20).
Experiment 2: ultrastructural analysis of ERs in the mPFC
Animals
Six adult female Sprague Dawley rats from Charles River Laboratories, approximately 225–250 g on arrival, were pair housed with ad libitum access to food and water and with 12-hour light, 12-hour dark cycles, with lights on at 6:00 am. Tissue from rats in the diestrous phase of the estrous cycle was analyzed for these experiments. Rats in the diestrous phase were used because this phase of the cycle corresponds to the low E2 replacement administered in the behavioral experiment. All procedures were in accordance with the National Institutes of Health guidelines and approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee. The rats used in these experiments are the same as those used by Williams et al (21) and Almey et al (18).
Antisera
For ERα identification, a rabbit polyclonal antiserum (AS409) produced against the full peptide for the native rat ERα was supplied by S. Hayashi (Saitama Cancer Center Research Institute, Japan). The specificity of this antibody has previously been demonstrated by binding to 3H-estradiol, immunoblots, and preadsorption controls (22–24). For localization of ERβ, a rabbit polyclonal antiserum produced against a peptide sequence in the C terminus of ERβ the mouse was used (Z8P; Zymed Laboratories) (14). This antibody has been shown to be specific for ERβ by Western blot analysis (∼60 kDa), double labeled with mRNA using in situ hybridization, preadsorption control, and absence of labeling in fixed brain sections from ERβ knockout mice (14, 25). Moreover, ERβ immunoreactivity colabels with green fluorescent protein in Esr2 transgenic mice (26). Finally, to visualize GPER1, this experiment used a rabbit polyclonal antiserum generated against a synthetic peptide, CAVIPDSTEQSDVRFSSAV (Multiple Peptide Systems) derived from the C terminus of the human GPER1 receptor, which was supplied by one of the authors (E.F.) (27). The specificity of this antibody has been shown on Western blots and in preadsorption controls (28, 29).
Tissue preparation
Rats were perfused, and brains were prepared for immunolabeling of mPFC tissue (Figure 2F) as described previously (18, 30). Additionally, in all experiments that involved immunolabeling for ERα or ERβ, a tissue section containing the ventromedial and arcuate nuclei of the hypothalamus was included in the immunohistochemical procedure as a positive control. Abundant ERα and ERβ labeling are present in these regions (13), so the success of immunolabeling could be confirmed prior to processing the mPFC for EM.
Figure 2.
Light microscopic examination of ER localization in the mPFC. A, Light nuclear IR for ERα was observed. B, Dense ERα-IR in the ventromedial hypothalamus. C, Light ERβ-IR was observed in the mPFC. D, Dense nuclear ERβ-IR in the hypothalamus. E, Dense extranuclear GPER1-IR is detected in the neuropil. F, A coronal schematic depicting the area of the mPFC (gray trapezoid) analyzed by EM.
Immunohistochemical labeling and tissue fixation and embedding
Free-floating tissue sections containing the mPFC from three of the six rats were each processed for immunohistochemical localization of ERα, ERβ, or GPER1. Briefly, sections were incubated in antirabbit ERα (1:10 000 dilution), ERβ (1:2000), or GPER1 (1:1000) for 24 hours at room temperature and 4 days at 4°C in 0.1% BSA in Tris-buffered saline (TBS). Sections were then incubated in the following: 1) biotinylated donkey antirabbit immunoglobulin (IgG; diluted 1:400; Jackson ImmunoResearch Laboratories, Inc) in 0.5% BSA in TBS, 30 minutes; 2) avidin-biotin complex (Vector Laboratories), 30 minutes; and 3) 3,3-diaminobenzidine (Aldrich) and H2O2 in TBS, 6–7 minutes. After immunolabeling, tissue sections were fixed in osmium, embedded in plastic, and sectioned and collected on grids as described previously (18).
Sections through the mPFC were examined under a Philips CM10 EM with an AMT digital camera. The subcellular distribution of each ER was examined in two sections per rat; a 5832-μm2 area of each section was counted in each section and categorized as dendrites, dendritic spines, axons, axon terminals, or glia using established criteria (31) [also see Almey et al (18) for a specific description of profiles identification]. The total number of labeled profiles was averaged across the three rats. Tissue selected for analysis was taken from a depth of 0.2–1.5 μm from the plastic-tissue interface, and only samples thin sectioned evenly across the plastic tissue interface were included in analyses. Soma were not included in the quantification analyses because they frequently occupy more than half of the area analyzed, reducing the overall number of ER-IR profiles observed. Final photomicrographs were generated from digital images, in which the brightness and contrast were adjusted using GIMP 2.8. Figures were assembled in Microsoft PowerPoint 2013.
Results
Experiment 1: mPFC and memory systems bias
Histology
The study began with 32 rats, but three rats were eliminated because they never reached the criterion, so 29 rats were included in the final analysis (n =14 for mPFC, n =15 for AC). Cannula placements in the mPFC and the AC of these remaining rats were within the target brain regions (Figure 3A and B).
Figure 3.
Percentage of rats that used a place or response strategy after the microinfusions of E2 or vehicle in the medial prefrontal cortex (A) and the anterior cingulate cortex (B). The number of rats per group is shown on the bar, and cannula placements are shown in the image beside the graph.
Behavior
After a microinfusion of E2 into the mPFC, 86% of the rats used place memory and 14% used response memory; after the microinfusions of vehicle to the mPFC, 29% of rats used place memory, whereas 71% used response memory (Figure 3A). This difference in memory use after the E2 and cyclodextrin infusions was statistically significant (McNemar test, P = .008), demonstrating that E2 administered directly to the mPFC of female rats induced a bias toward the use of a place memory. The OR indicates that the use of place memory was 15 times higher after an infusion of E2 than it was after an infusion of vehicle. The logit d effect size was 1.56, which demonstrates that this is a large effect (20).
In contrast to the findings after the microinfusions into the mPFC, E2 or vehicle infusions into the AC elicited comparable behavioral effects. When E2 was infused to the AC, 13% of the rats used a place memory and 87% of the rats used response memory; similarly, when vehicle was infused into the AC, 20% of rats used place memory, whereas 80% of rats used response memory (Figure 3B). Analysis with a McNemar test revealed no significant difference in memory use after the infusions of E2 or cyclodextrin to the AC. The OR was 0.62, indicating that there was close to an equal chance of rats using a place and response strategy in the E2 and vehicle groups. The logit d effect size, was 0.28, which is a small effect (20).
Experiment 2: ultrastructural analysis of ERs in the mPFC
Light microscopy
By light microscopy, no nuclear or extranuclear ERα or ERβ labeling was observed in the mPFC (Figure 2, A and C). However, abundant nuclei containing ERα-IR and ERβ-IR were seen in the ventromedial and arcuate regions of the hypothalamus, indicating that immunohistochemistry was successful (Figure 2, B and D). In contrast, GPER1-IR was observed in the cytoplasm, but not nuclei, of the perikarya throughout the mPFC (Figure 2E).
ERα, ERβ, and GPER1 are observed primarily at presynaptic sites in the mPFC
Estrogen receptor-α
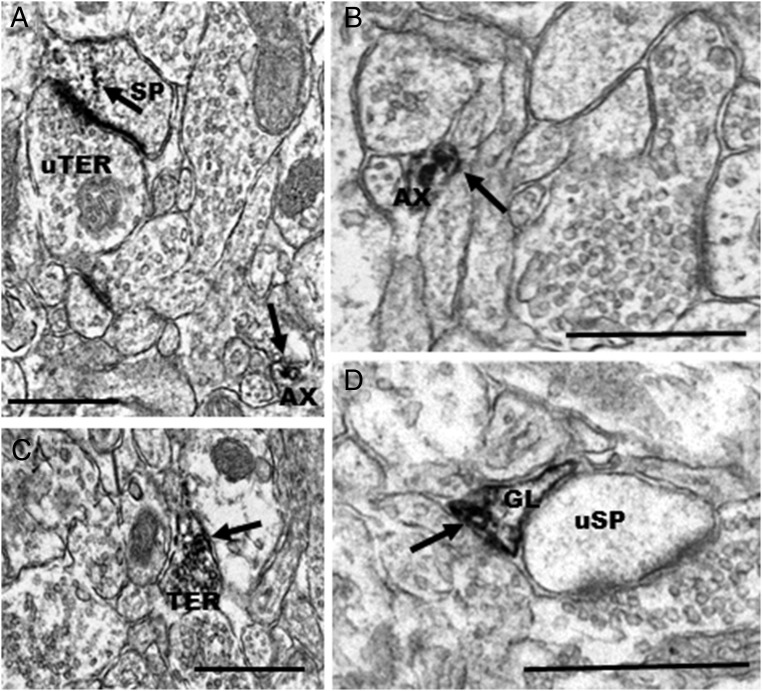
At the ultrastructural level, ERα-IR was present in all types of neuronal processes and glia in the mPFC (Figure 4). Semiquantitative analysis (Table 1) demonstrated that most ERα-IR was in axons (41.7%) and axon terminals (28.8%). In axons (<0.15 μm in diameter), ERα-IR was typically discrete and was affiliated with the plasma membrane or clusters of small vesicles (Figure 4, A and C). Axon terminals had cross-sectional diameters that ranged from approximately 0.3–0.8 μm and contained numerous small synaptic vesicles (SSVs) and occasionally mitochondria but no dense-core vesicles. In terminals, ERα-IR was found in clusters around SSVs (Figure 4A), at the plasma membrane, and occasionally associated with mitochondrial membranes. In addition to presynaptic sites, some ERα-IR labeling was observed in dendritic shafts (∼8.3%) and dendritic spines (6.7%). In the dendritic shafts, peroxidase reaction product was often affiliated with the plasma membranes and microtubules (Figure 4D) and was occasionally observed at the mitochondrial membranes. In dendritic spines, ERα-IR accumulated in the spine head and was observed on the plasma membrane, particularly near the postsynaptic density. ERα-IR was found at both pre- and postsynaptic profiles forming asymmetric synapses. Occasionally, ERα-IR axon terminals synapsed onto ERα-IR spines. Finally, ERα-IR was observed in glial profiles (14.4%; Figure 4B).
Figure 4.
Electron micrographs showing examples of profiles containing ERα-IR in the mPFC. These photomicrographs show IR for ERα in (A) an axon (AX) and in a terminal (TER), in which IR is observed at small synaptic vesicles and on the membrane of a mitochondrion (mit) (B), ERα-IR associated with the membrane of a glial cell (GL) (C), ERα-IR filling an axon (AX), and IR for ERα in a dendrite, observed at the plasma membrane and associated with microtubules (D). Bar, 500 nm.
Table 1.
Percentage of Total IR Profiles and Number of IR Profiles and the Corresponding SE, Observed in a 5832-μm2 Area of the Medial Prefrontal Cortex, Averaged Across Rats
| Receptor | Axons |
Terminals |
Dendrites |
Spines |
Glia |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | n ± SEM | % | n ± SEM | % | n ± SEM | % | n ± SEM | % | n ± SEM | % | n ± SEM | |
| ERα | 41.7 | 53.67 ± 4.1 | 28.8 | 37.00 ± 2.5 | 8.3 | 10.67 ± 0.3 | 6.7 | 8.67 ± 0.9 | 14.5 | 18.67 ± 3.2 | 100 | 128.67 ± 4.3 |
| ERβ | 25.3 | 17.00 ± 1.5 | 33.2 | 22.33 ± 2.3 | 8.4 | 5.67 ± 1.2 | 9.4 | 6.33 ± 0.9 | 18.3 | 12.33 ± 2.0 | 100 | 67.33 ± 2.3 |
| GPER1 | 36.7 | 103.7 ± 6.2 | 29.3 | 82.7 ± 4.8 | 12.9 | 36.33 ± 2.3 | 6.0 | 17.00 ± 2.3 | 14.4 | 40.7 ± 2.0 | 100 | 282.3 ± 9.2 |
Estrogen receptor-β
At the ultrastructural level, ERβ-IR was observed almost exclusively at extranuclear sites in neuronal and glia profiles (Figure 5 and Table 1). ERβ-IR was most commonly localized in axons (28.8%) and axon terminals (29.9%). In axons, ERβ-IR was typically found throughout the profiles (Figure 5B). In axon terminals, ERβ-IR was observed in clusters of reaction product associated with SSV and was sometimes affiliated with the plasma membrane (Figure 5C). ERβ-IR was also in dendrites (9.6%) and dendritic spines (10.7%). ERβ-IR reaction product filled dendritic profiles but often was densest near the plasma membrane. In dendritic spines ERβ-IR typically accumulated in the spine head and was frequently observed at the cell membrane near the synapse (Figure 5A). ERβ-IR was occasionally observed in the perikarya in which it was observed at the plasma membrane and associated with organelles (not shown). ERβ-IR was observed in terminals and dendritic spines that formed asymmetric synapses, but ERβ-IR terminals were not observed forming synapses with ERβ-IR spines. ERβ-IR also was frequently observed in glia profile (20.9%; Figure 5D).
Figure 5.
Electron micrographs showing examples of profiles containing IR for ERβ in the mPFC. These photomicrographs show ERβ in (A) an axon (AX) and in a dendritic spine (SP) that forms an asymmetrical synapse with an unlabeled terminal (uTER) (B), ERβ-IR filling an axon profile (AX) (C), ERβ-IR associated with vesicles and the plasma membrane of an axon terminal (TER), and (D) GPER1 in a glial cell that is in apposition to an unlabeled dendritic spine (uSP). Bar, 500 nm.
G protein-coupled estrogen receptor-1
Immunoperoxidase labeling for GPER-1 was observed throughout the mPFC (Figure 6 and Table 1). Like ERα and ERβ, most GPER1-IR was presynaptic: axons and axon terminals accounted for 36.7% and 29.3% of the GPER1-labeled profiles, respectively. In axons, GPER1-IR was usually discrete and often associated with the plasma membrane or small clusters of vesicles (Figure 6, C and D). In axon terminals GPER1-IR was most commonly clustered on groups of SSVs or at the plasma membrane (Figure 6A). GPER1-IR also was observed at postsynaptic sites: dendritic shafts constituted 12.8% of total GPER1-labeled profiles, and dendritic spines constituted 6.2% of the total IR profiles. In dendritic shafts, GPER1-IR was typically associated with the plasma membrane but also was affiliated with microtubules (Figure 6D) and mitochondrial membranes. In dendritic spines, GPER1-IR peroxidase reaction product accumulated in the spine head and was associated with the plasma membrane, sometimes in the perisynaptic zones (Figure 6B). Neuronal perikarya with GPER1-IR were also observed. Labeling was exclusively in the cytoplasm in which it was discretely affiliated with endoplasmic reticulum, mitochondria, and the plasma membrane (Figure 6C). Although GPER1-IR was observed both pre- and postsynaptically and was often observed close to the synapse in terminals and spines, it was rare for GPER1-IR terminals to synapse onto GPER1-IR spines. Finally, GPER1-IR was observed in glia profiles (14.4%); the labeling in glial cells was discrete and was observed at the plasma membrane.
Figure 6.
Electron micrographs showing examples of profiles containing IR for GPER1 in the mPFC. These photomicrographs show GPER1-IR associated with (A) small synaptic vesicles in a terminal (TER) that is adjacent to an unlabeled terminal (uTER) and spine (uSP) that form an asymmetric synapse, (B) GPER1-IR associated with the plasma membrane of a dendritic spine that is forming a synapse with an unlabeled terminal (uTER) (C), GPER1 in a soma (SOM) in which it is localized to the endoplasmic reticulum and the membrane of mitochondria (mit) and IR for GPER1 in two axons (AX) (D), and GPER1 in an axon (AX) and in a dendrite (DEN), where it is associated with microtubules and the cell membrane. Bar, 500 nm.
Discussion
An infusion of E2 to the mPFC, but not the AC, of female rats rapidly induces a bias toward the use of place memory to solve an appetitive task. Ultrastructural analyses demonstrate that ERα, ERβ, and GPER1 are all present, almost exclusively at extranuclear sites in the mPFC, providing a mechanism via which E2 in the mPFC could rapidly alter memory system use.
Experiment 1: mPFC and memory systems bias
Without additional intracortical E2 administration, rats in both the mPFC and AC groups had chronic low E2 serum levels, via sc implants, that are typically associated with a bias toward the use of response memory (5, 6, 8). After the injection of the vehicle, cyclodextrin, into either the mPFC or the AC, rats still predominantly used a response strategy (71% and 80%, respectively). This finding agrees with previous studies, which show that response memory was used by 73% of OVX female rats with SILASTIC brand capsules (Dow Corning) maintaining a low level of E2 (8) and 71% of female rats in the estrus phase of the cycle when E2 is low (6).
Estrogens in the mPFC bias female rats towards use of place memory
Interestingly, E2 administered directly to the mPFC biases female rats toward the use of place memory, which offers new insight into how systemic E2 influences multiple memory systems. In this experiment 86% of rats that received an infusion of E2 to the mPFC used a place strategy, which provides strong evidence that systemic E2 is acting, at least in part, in the mPFC to elicit this bias toward place strategy use. This finding parallels previous research demonstrating that high serum levels of E2 are associated with a bias toward the use of place memory (6, 8). The infusion of E2 to the AC did not alter memory system use, with 87% rats using response memory after an infusion of E2. Because E2 administration to the AC has no effect on multiple memory system use, this suggests that E2-induced changes in place memory use are specific to the mPFC and do not generalize to other regions of the frontal cortex. However, it is recognized here that estrogens also act in the hippocampus and striatum to influence memory system bias (9, 19). Because there are reciprocal connections between the regions, there may be interactions between the E2 effects in the mPFC, striatum, and hippocampus, but this remains unclear. Additionally, the time between the microinfusion of E2 and testing was approximately 10 minutes, so the effects of E2 in the mPFC on memory system bias are likely be nongenomic, resulting from binding at membrane-associated E2 receptors.
When the results of the present study are considered in the context of previous research, it seems possible that E2 in the mPFC influences strategy use by altering dopamine transmission in this region. Dopamine projections to the mPFC originate from the ventral tegmental area and to a lesser extent, from the substantia nigra (32). Dopamine transmission in the mPFC is influenced by E2, such that higher serum levels of E2 are associated with lower basal levels of dopamine in the mPFC (33) and lower levels of dopamine in mPFC homogenate (34, 35). Additionally, findings from this laboratory indicate that female rats with low levels of E2 switch from a response memory to a place memory when administered either a D1 or D2 receptor antagonist, either systemically (8) or directly into the mPFC (Quinlan MG, et al, unpublished observations). This experiment parallels such findings because infusions of E2 to the mPFC also induce a switch from response to place strategy in female rats with low systemic levels of E2. Together these findings are consistent with a model of strategy use in which changes of dopamine transmission in the mPFC, either via presynaptic E2-induced inhibition of dopamine availability or via dopamine receptor antagonist, bias rats toward the use of place strategy.
Experiment 2: ultrastructural analysis of ERs in the mPFC
These experiments demonstrated that, at the ultrastructural level, ERα, ERβ, and GPER1 are localized to extranuclear sites in neuronal and glial profiles in the mPFC of female rats. Although ERs are observed at all neuronal profiles, most ERs are observed on axons and terminals, suggesting that estrogens alter transmission in the mPFC via presynaptic mechanisms. Additionally, when results for the three ERs are considered together it is clear that GPER1 is the most common ER in the mPFC of female rats because GPER1 is twice as abundant as ERα and 4 times more abundant than ERβ (see Table 1). This implies that the effects of E2 in the mPFC occur predominantly through binding at GPER1, although binding at ERα and ERβ would also affect the transmission in the mPFC. These microscopy results contribute to an explanation of how E2 rapidly alters the transmission in the mPFC to affect memory system bias.
Methodological considerations
To determine whether ERα, ERβ, and GPER1 are found in the mPFC, the present study used an immunoperoxidase label and preembedding methods, which result in excellent cellular morphology that allows for discrete subcellular localization of antigens (36). To ensure that any differences in the number of labeled profiles were not due to differences in antibody penetration or sample size, all tissue samples analyzed for quantification were identical in size and taken from near the plastic/tissue interface. This methodology tends to underestimate the absolute number of peroxidase-labeled profiles (36). IR for ERα, ERβ, and GPER1 are discrete, so the absence of ER labeling within cellular profiles does not demonstrate that these profiles lack ERs. Thus, the quantification analyses presented here are conservative and likely underestimate the actual numbers of profiles containing these ERs.
ERα, ERβ, and GPER1 are observed exclusively at extranuclear sites in the mPFC
ERα, ERβ, and GPER1 in the mPFC are exclusively localized to extranuclear sites and are observed at the plasma membrane in all types of neuronal profiles and glial cells. This finding contrasts some previous light microscopy and in situ hybridization studies that observe little ERα and ERβ or observe ERα and ERβ exclusively at nuclear sites (12–14). However, this study is in agreement with other research that demonstrates immunolabeling for ERα in the mPFC of monkeys and rats (15, 16) and moderate levels of ERβ mRNA in the mPFC of mice (17). The difference between the present study and previous studies is likely because of the greater sensitivity and resolution of EM; in this experiment, light microscopy was not sufficient to observe any extranuclear ERα or ERβ, but EM allowed for the detection of discrete membrane-associated ERα and ERβ-IR in the mPFC. To our knowledge this is the first time GPER1 has been localized to the mPFC of the rat. At the ultrastructural level, GPER1 is observed at the plasma membrane and in the cytoplasm of various neuronal profiles, corresponding to previous research examining the distribution of GPER1 (37–39).
All ERs are predominantly localized to presynaptic sites in the mPFC
At the ultrastructural level, the highest proportions of ERα, ERβ, and GPER1-IR profiles are in axons and terminals, which parallels previous findings in primates (16). This indicates that estrogens in the mPFC likely alter neurotransmission via presynaptic mechanisms, such as vesicle formation, immobilization, and/or release of neurotransmitter from the terminal. ERs observed in axons might reflect transportation of these receptors from the soma to the terminal, but these receptors may also have effects on protein transport or the transduction of electrochemical signals (40, 41). Additionally, the presynaptic receptors observed in terminals may be important in the local control of transmitter release because estrogens have been shown to decrease dopamine availability in the mPFC (33). ER-IR is observed exclusively at extranuclear sites in the mPFC, which is in congruence with previous findings that have localized this receptor to extranuclear sites in other brain regions, such as the hippocampus and dorsal striatum of rodents (18, 24) and the prefrontal cortex of primates (16). Binding at these receptors on the plasma membrane could rapidly alter transmission in the mPFC, which provides a possible mechanism for estrogens' rapid effects on multiple memory system bias.
In addition to ERs being localized to presynaptic sites, they were also observed at postsynaptic sites, on dendrites and dendritic spines, indicating that estrogens in the mPFC also have some post synaptic effects on transmission in the mPFC. Although ERβ was most prevalent in presynaptic profiles, it is interesting that 11% of ERβ was localized to dendritic spines, and this labeling in spines was often dense (Figure 3D), whereas ERα and GPER1 labeling in spines was only half of that observed with ERβ (∼6%). This suggests that binding at ERβ may also have postsynaptic effects in the mPFC, such as altering the cell permeability to ions or affecting the activity of second messenger cascades.
ERs are localized to glia and to mitochondrial membranes
GPER1, ERα, and ERβ are all localized to the plasma membrane of glial cells and mitochondrial membranes in the mPFC. This parallels observations from our previous study examining ER distribution in the dorsal striatum (18). Estrogens are known to mediate glial-induced neuroprotection (42, 43), in part through binding at GPER1 (43). Thus, the localization of all three ERs to glia contributes to an explanation of how estrogens affect glial-mediated neuroprotection. Estrogens have also been implicated in mitochondrial functioning and cellular metabolism (44, 45). The observation of ERs on mitochondrial membranes provides a mechanism for estrogen-induced alterations in cellular metabolism. Additionally, GPER1 is observed at the endoplasmic reticulum in the mPFC, paralleling findings in COS7, HEC50, and Chinese hamster ovary cell cultures (46), and the hippocampal formation (11, 37). GPER1 is likely localized to this organelle because regulatory steps in the biosynthesis of GPER1 occur at the endoplasmic reticulum (39).
These ultrastructural findings contribute to an explanation of the mechanisms via which E2 in the mPFC biases female rats toward the use of place memory. ERs in the mPFC were observed almost exclusively at extranuclear sites, indicating that estrogens would have rapid effects on neurotransmission in the mPFC. This corresponds to the behavioral findings presented here, that E2 acts rapidly in the mPFC to induce a bias toward the use of place memory to navigate an environment. Most ERα, ERβ, and GPER1 are observed on axons and terminals in the mPFC, and it is possible these axons and terminals are dopaminergic. It is hypothesized that the E2-induced shift toward the use of place memory is caused by E2-altering dopaminergic transmission in the mPFC. This would provide a mechanism via which systemic estrogens could alter dopamine availability to affect many behaviors, including multiple memory system bias. Dual-labeling studies should be conducted to determine whether these ERs are in fact localized to dopaminergic neurons in the mPFC.
Acknowledgments
This work was supported by a discovery grant from the Natural Sciences and Engineering Research Council of Canada (to W.G.B.) and National Institutes of Health Grants DA08259, HL096571, HL098351 (to T.A.M.), and P01AG016765 (to T.A.M.; program principal investigator, John Morrison, Mt Sinai School of Medicine, New York New York). The Centre for Studies in Behavioral Neurobiology is a Groupe de Recherché funded by the Fonds de Recherche du Québec Santé.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AC
- anterior cingulate cortex
- E2
- estradiol
- EM
- electron microscopy
- ER
- estrogen receptor
- GPER1
- G protein-coupled estrogen receptor 1
- GPER1R
- G protein-coupled estrogen receptor 1 receptor
- IR
- immunoreactivity
- mPFC
- medial prefrontal cortex
- OR
- odds ratio
- OVX
- ovariectomized
- SSV
- small synaptic vesicle
- TBS
- Tris-buffered saline.
References
- 1. Tolman EC, Ritchie BF, Kalish D. Studies in spatial learning: orientation and the short-cut. J Exp Psychol. 1946;36:13–24. [DOI] [PubMed] [Google Scholar]
- 2. White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. [DOI] [PubMed] [Google Scholar]
- 3. Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. [DOI] [PubMed] [Google Scholar]
- 4. Hussain D, Hoehne A, Woodside B, Brake WG. Reproductive experience modifies the effects of estradiol on learning and memory bias in female rats. Horm Behav. 2013;63:418–423. [DOI] [PubMed] [Google Scholar]
- 5. Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. [DOI] [PubMed] [Google Scholar]
- 6. Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. [DOI] [PubMed] [Google Scholar]
- 7. Quinlan MG, Almey A, Caissie M, LaChappelle I, Radiotis G, Brake WG. Estradiol and striatal dopamine receptor antagonism influence memory system bias in the female rat. Neurobiol Learn Mem. 2013;106:221–229. [DOI] [PubMed] [Google Scholar]
- 8. Quinlan MG, Hussain D, Brake WG. Use of cognitive strategies in rats: the role of estradiol and its interaction with dopamine. Horm Behav. 2008;53:185–191. [DOI] [PubMed] [Google Scholar]
- 9. Zurkovsky L, Serio SJ, Korol DL. Intra-striatal estradiol in female rats impairs response learning within two hours of treatment. Horm Behav. 2011;60:470–477. [DOI] [PubMed] [Google Scholar]
- 10. Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. J Neurosci. 2009;29:7208–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. [DOI] [PubMed] [Google Scholar]
- 12. Cardona-Gomez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:751–760. [DOI] [PubMed] [Google Scholar]
- 13. Kritzer MF. Regional, laminar, and cellular distribution of immunoreactivity for ER α and ER β in the cerebral cortex of hormonally intact, adult male and female rats. Cereb Cortex. 2002;12:116–128. [DOI] [PubMed] [Google Scholar]
- 14. Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- 15. Montague D, Weickert CS, Tomaskovic-Crook E, Rothmond DA, Kleinman JE, Rubinow DR. Oestrogen receptor α localisation in the prefrontal cortex of three mammalian species. J Neuroendocrinol. 2008;20:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. Synaptic estrogen receptor-α levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30:12770–12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitra SW, Hoskin E, Yudkovitz J, et al. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology. 2003;144:2055–2067. [DOI] [PubMed] [Google Scholar]
- 18. Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology. 2012;153:5373–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klein R. Beyond Significance Testing: Reforming Data Analysis Methods in Behavioural Research. Washington DC: American Psychological Association; 2004. [Google Scholar]
- 21. Williams TJ, Torres-Reveron A, Chapleau JD, Milner TA. Hormonal regulation of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiol Learn Mem. 2011;95:206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okamura H, Yamamoto K, Hayashi S, Kuroiwa A, Muramatsu M. A polyclonal antibody to the rat oestrogen receptor expressed in Escherichia coli: characterization and application to immunohistochemistry. J Endocrinol. 1992;135:333–341. [DOI] [PubMed] [Google Scholar]
- 23. Alves SE, Weiland NG, Hayashi S, McEwen BS. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J Comp Neurol. 1998;391:322–334. [PubMed] [Google Scholar]
- 24. Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal α estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 25. Creutz LM, Kritzer MF. Estrogen receptor-β immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol. 2002;446:288–300. [DOI] [PubMed] [Google Scholar]
- 26. Milner TA, Thompson LI, Wang G, et al. Distribution of estrogen receptor β containing cells in the brains of bacterial artificial chromosome transgenic mice. Brain Res. 2010;1351:74–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. [DOI] [PubMed] [Google Scholar]
- 28. Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res. 2011;1379:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. [DOI] [PubMed] [Google Scholar]
- 30. Milner TA, Waters B, Robinson D, Pierce JP. Degenerating processes identified by electron microscopic immunocytochemical methods. Methods Mol Biol. 2011;793:23–59. [DOI] [PubMed] [Google Scholar]
- 31. Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System. 3rd ed New York: Oxford University Press; 1991. [Google Scholar]
- 32. Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. [DOI] [PubMed] [Google Scholar]
- 33. Dazzi L, Seu E, Cherchi G, Barbieri PP, Matzeu A, Biggio G. Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology. 2007;32:892–901. [DOI] [PubMed] [Google Scholar]
- 34. Dupont A, Dussault JH, Rouleau D, et al. Effect of neonatal thyroid deficiency on the catecholamine, substance P, and thyrotropin-releasing hormone contents of discrete rat brain nuclei. Endocrinology. 1981;108:2039–2045. [DOI] [PubMed] [Google Scholar]
- 35. Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. [DOI] [PubMed] [Google Scholar]
- 36. Leranth C, Pickel VM. Electron microscopic preembedding double-immunostaining methods. In: Heimer I, Zaborsky I, eds. Neuroanatomical Tract Tracing Methods II: Recent Progress. New York: Plenum; 1989. [Google Scholar]
- 37. Matsuda K, Sakamoto H, Mori H, et al. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett. 2008;441:94–99. [DOI] [PubMed] [Google Scholar]
- 38. Filardo EJ, Graeber CT, Quinn JA, et al. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res. 2006;12:6359–6366. [DOI] [PubMed] [Google Scholar]
- 39. Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verdier D, Lund JP, Kolta A. GABAergic control of action potential propagation along axonal branches of mammalian sensory neurons. J Neurosci. 2003;23:2002–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cheung DW. Synaptic transmission in the guinea-pig vas deferens: the role of nerve action potentials. Neuroscience. 1990;37:127–134. [DOI] [PubMed] [Google Scholar]
- 42. Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: implications for neuroprotection. Biochim Biophys Acta. 2010;1800:1106–1112. [DOI] [PubMed] [Google Scholar]
- 43. Liu SB, Han J, Zhang N, Tian Z, Li XB, Zhao MG. Neuroprotective effects of oestrogen against oxidative toxicity through activation of G-protein-coupled receptor 30 receptor. Clin Exp Pharmacol Physiol. 2011;38:577–585. [DOI] [PubMed] [Google Scholar]
- 44. Razmara A, Sunday L, Stirone C, et al. Mitochondrial effects of estrogen are mediated by estrogen receptor α in brain endothelial cells. J Pharmacol Exp Ther. 2008;325:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Araujo GW, Beyer C, Arnold S. Oestrogen influences on mitochondrial gene expression and respiratory chain activity in cortical and mesencephalic astrocytes. J Neuroendocrinol. 2008;20:930–941. [DOI] [PubMed] [Google Scholar]
- 46. Otto C, Rohde-Schulz B, Schwarz G, et al. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. [DOI] [PubMed] [Google Scholar]