Abstract
The preimplantation embryo is particularly vulnerable to environmental perturbation, such that nutritional and in vitro stresses restricted exclusively to this stage may alter growth and affect long-term metabolic health. This is particularly relevant to the over 5 million children conceived by in vitro fertilization (IVF). We previously reported that even optimized IVF conditions reprogram mouse postnatal growth, fat deposition, and glucose homeostasis in a sexually dimorphic fashion. To more clearly interrogate the metabolic changes associated with IVF in adulthood, we used nontargeted mass spectrometry to globally profile adult IVF- and in vivo-conceived liver and gonadal adipose tissues. There was a sex- and tissue-specific effect of IVF on adult metabolite signatures indicative of metabolic reprogramming and oxidative stress and reflective of the observed phenotypes. Additionally, we observed a striking effect of IVF on adult sexual dimorphism. Male-female differences in metabolite concentration were exaggerated in hepatic IVF tissue and significantly reduced in IVF adipose tissue, with the majority of changes affecting amino acid and lipid metabolites. We also observed female-specific changes in markers of oxidative stress and adipogenesis, including reduced glutathione, cysteine glutathione disulfide, ophthalmate, urate, and corticosterone. In summary, embryo manipulation and early developmental experiences can affect adult patterns of sexual dimorphism and metabolic physiology.
The Developmental Origins of Health and Disease (DOHaD) hypothesis holds that embryonic and fetal adaptation to suboptimal uterine environments can predispose a series of metabolic diseases in adulthood, including cardiovascular disease, diabetes, hypertension, and stroke (1). Preimplantation development has been recognized as a window of notable environmental sensitivity, and many animal studies have reported that nutritional, oxidative, and in vitro stresses restricted exclusively to this period are sufficient to alter developmental growth and metabolic trajectories, leading to pathologies such as hypertension, dyslipidemia, and β-cell dysfunction in adulthood (2–4).
This is of particular relevance to the over 5 million children conceived using assisted reproductive technologies such as in vitro fertilization (IVF). Because the eldest IVF individuals are only in their mid-30s, the relationship between preimplantation embryo manipulation and adult-onset metabolic pathologies is elusive, although modest changes in growth kinetics, fasting glucose, blood pressure, vascular function, and fat deposition have been reported in IVF adolescents (5–8). To address this controversy, several mouse models of IVF have been developed and used to demonstrate that even clinically optimized IVF conditions are sufficient to reprogram adult metabolism (9–11). Our group has shown that female animals in particular exhibit latent overgrowth, increased fat accumulation and fasting glucose levels, and impaired insulin secretion in response to stimulatory levels of glucose. However, these mice are physiologically indistinguishable from controls until approximately 17 weeks of age (Supplemental Figure 1). In contrast, male animals display no overt phenotype (9).
Sex-based differences are present throughout most mammalian physiologies, behaviors, diseases, and phenotypes, arising from a variety of immunological, hormonal, genetic, and epigenetic mechanisms (12). Sexual dimorphism is particularly common to several metabolic hallmarks in adulthood, including glucose homeostasis, insulin sensitivity, β-cell function, and adipose tissue depots, and therefore can influence disease susceptibility and progression (13). Because up to one-third of transcripts are differentially expressed between males and females by the blastocyst stage (14), it is not surprising that developmental programming frequently exhibits sex bias, although this phenomenon is poorly understood (15).
Recent advances in metabolomics technology have permitted comprehensive and systematic analyses of the biochemical fingerprints within cells and tissues, thus providing an immediate compendium of cellular metabolic processes (16). Our group previously performed serum profiling in adult IVF- and control-conceived female mice and identified several biomarkers of both insulin resistance and impaired glucose handling similarly noted in metabolomics-based investigations into diabetes and obesity (9). These results have led us to further interrogate the metabolic changes associated with IVF in adulthood, as well as dissociate its sex-specific physiological phenotypes in liver and gonadal fat. These tissues were selected for their role in metabolism and insulin sensitivity and because our data have highlighted adipose tissue as a locus of developmental reprogramming and sex-specific phenotypic variation.
Materials and Methods
Animals
All animals were maintained according to institutional regulations, under a constant 12-hour light, 12-hour dark cycle with ad libitum access to water and standard chow (23% protein, 22% fat, and 55% carbohydrate, number 5058; PicoLab). Beginning at 24 weeks, all animals were placed on a high-fat diet (20% protein, 60% fat, and 20% carbohydrate, number D12492; Research Diets, Inc) (17) until time of death at 30 weeks.
IVF, embryo culture, and transfer
IVF, embryo culture, and embryo transfer experiments were performed as previously described (9). Briefly, C57BL/6J females aged 6–8 weeks were injected with 5-IU pregnant mare's serum gonadotropin followed 46–48 hours later by 5-IU human chorionic gonadotropin (hCG) to induce superovulation. Thirteen to fifteen hours after hCG administration, cumulous-oocyte-complexes were isolated from ampullae and incubated 4–6 hours in human tubal fluid medium (MR-070-D; Millipore) with capacitated (1 h) cauda epididymal sperm from C57BL/6J males. Fertilized zygotes were washed and cultured to the blastocyst stage in potassium simplex optimization medium (KSOM, containing amino acids and 0.2mM pyruvate, 10mM lactate, 0.2mM glucose, and 1mM glutamine) (MR-106-D; Millipore) (18), at 37°C under Ovoil (10029; Vitrolife) with 5% CO2 and 5% O2 in a modular humidified chamber. To generate postimplantation cohorts, pseudopregancy was induced by mating naturally cycling CF-1 females to vasectomized CD-1 males, confirmed by the presence of a copulation plug the next morning (considered d 0.5). Late-cavitating blastocysts were transferred to the uterine horns of recipients on day 2.5 of pseudopregnancy. For control experiments, C57BL/6J female mice were superovulated as described above and mated to C57BL/6J males overnight. Embryonic day 3.5 blastocysts (96 h after hCG administration) were flushed from the oviducts and transferred immediately to the uterine horns of CF-1 recipients, thus controlling for litter size and the embryo transfer procedure. Only animals derived from litters of 5–7 pups were used in this study. Recipient animals had similar weight at the time of transfer and gained the same amount of weight during pregnancy.
Metabolomic profiling
Nonfasted animals were killed by CO2 exposure followed by cervical dislocation in the morning, and tissues were harvested from animals generated by 5 separate IVF and 5 control experiments. At least 3 independent cohorts contributed to each analysis of tissue and sex. Estrous cycle was monitored using vaginal smear. Immediately after collection, whole liver (24 samples; n = 6 for each sex and conception condition) and gonadal fat (29 samples; n = 7 IVF and 7 control females, n = 10 IVF and 5 control males) samples were snap frozen for unbiased metabolomic profiling by Metabolon, Inc, as described in detail elsewhere (19, 20). Briefly, samples underwent a series of organic and aqueous extractions optimized for small molecule recovery and were then split into equal parts for gas chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry analyses. For the latter platform, samples were again divided for profiling in both positive (acidic) and negative (basic) ionization modes.
Bioinformatics and statistics
Mass spectrometry profiles were processed using software developed by Metabolon, Inc (21). Peaks were called against a library of 2500 named biochemicals comprised of amino acids, lipids, carbohydrates, nucleotides, peptides, vitamins, cofactors, and xenobiotics. For statistical interpretation of detected metabolites, ANOVA contrasts were performed to identify biochemicals that differed significantly between 1) the in vivo and IVF conception conditions and 2) males vs females, with two-way ANOVA analyses to describe biochemicals exhibiting a significant interaction between sex and conception parameters. For all comparisons, P < .05 was considered significant. Unsupervised Pearson correlations were used to evaluate the relationship between metabolite concentrations and both percent adiposity and fasting glucose levels at time of death. For moderately or strong coefficient values (defined as r > 0.6), additional correlation analyses were conducted with segregation by sex, conception condition, or both.
Heat maps were generated using GENE-E software developed by the Broad Institute (available at http://www.broadinstitute.org/cancer/software/GENE-E/). Heat maps depict the fold-change difference in metabolite concentration between mean IVF and control values, or the z-score (calculated as z = (x − μ)/σ; where x = the individual scaled metabolite value for an animal, μ = the mean value of the metabolite for the defined population, and σ = the SD of that population) comparing either metabolite concentrations between male and female animals, or individual control and IVF values to their respective population means.
The web-based metabolomic data processing tool MetaboAnalyst was used for tissue metabolite data analysis (22, 23). Detailed methodology may be found at http://www.metaboanalyst.ca. Metabolite set enrichment analysis (MSEA) was conducted on metabolite data mapped according to Human Metabolome Database (HMDB) or Kyoto Encyclopedia of Genes and Genomes (KEGG) identifiers, using the metabolite pathway associated metabolite set library (currently 88 entries).
Results
We conducted global metabolomics profiling of tissues harvested from mice produced in a previous study of IVF to model the DOHaD hypothesis (9). Briefly, mice were generated by IVF under conditions considered optimal for mouse embryo culture and reflective of current IVF clinical practices (KSOM with amino acids and 5% O2 tension) (24). As a control, in vivo-produced blastocysts were isolated 96 hours after fertilization for transfer to recipients (flushed blastocyst group), thus accounting for superovulation, litter size, and the embryo transfer procedure (10). To probe the consequences of nutritional stress, all animals were placed on a high-fat diet beginning at 24 weeks of age until time of death at 30 weeks (6 wk total), at which point liver and gonadal adipose tissues were harvested for experiments. Body weight was similar between IVF and control mice up through 16 weeks, at which point IVF females showed a statistical increase in body weight (before administration of the high-fat diet) that lasted through 28 weeks. At time of death, there were no significant changes in body weight or weight-standardized organ sizes between the 2 groups (Supplemental Figure 1). Dual-energy x-ray absorptiometry at 8, 16, 21, and 28 weeks revealed that IVF females had initially lower percent adiposity but then statistically surpassed control levels of body fat by 21 weeks of age. These weight and fat percent findings might be partially explained by the fact that IVF females consumed more food than controls at 7 and 20 weeks but not at 28 weeks.
Metabolic sexual dimorphism in adult fat tissue of control mice
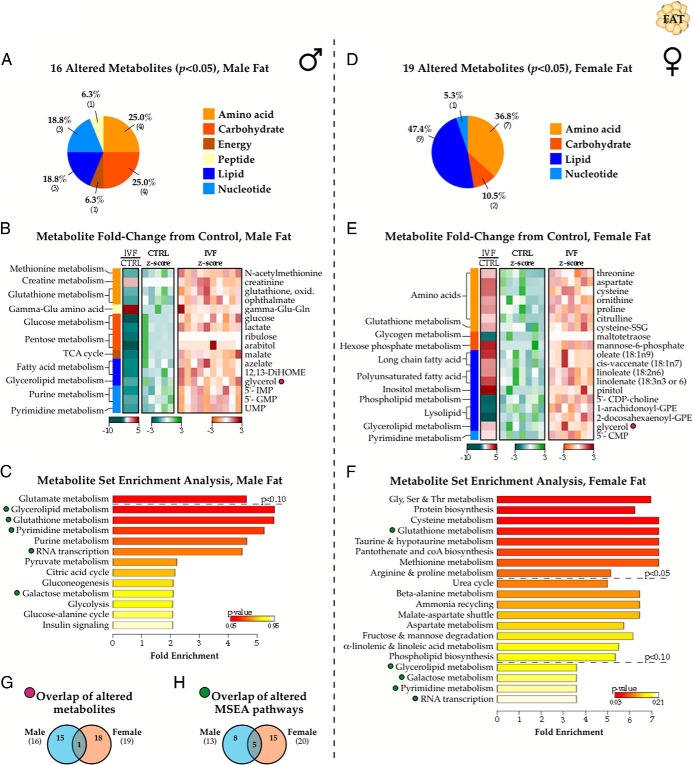
Unbiased metabolomic investigation of IVF and control fat samples (n = 10 male and 7 female IVF, n = 5 male and 7 female control) identified a total of 231 endogenous biochemicals comprising all major metabolic groups (Figure 1A). A complete list of relative metabolite concentrations may be found in Supplemental Table 1. Pearson correlations between metabolite concentrations and percent adiposity or fasting glucose levels at time of death revealed no significant relationships. Due to the sex-biased effect of IVF on adult metabolism (9, 10), we first compared profiles between control males and females and observed significant sexual dimorphism for 57 metabolites (24.7%, P < .05) (Figure 1B). The dataset was particularly enriched for small molecules involved in lipid and amino acid metabolism. Males exhibited broad increases in metabolite concentration relative to females (Figure 1C). We performed MSEA to determine whether any biologically meaningful pathways were overrepresented by the altered metabolites (25), which showed that metabolites involved in glycerolipid metabolism, the urea cycle, and sphingolipid metabolism exhibited the most significant sexual dimorphism in control samples (Figure 1D).
Figure 1.
Metabolic sexual dimorphism in adult adipose tissue. A, Nontargeted mass spectrometry profiling of 29-week IVF and control fat tissue (n = 5 male and 7 female control animals; 10 IVF males and 7 IVF females; 29 animals total) identified 231 named metabolites comprising all major metabolic groups. B, The concentrations of 57 metabolites (24.7%) were significantly different between males and females in control samples (P < .05), consisting predominantly of lipid and amino acid derivatives. C, The level of each biochemical in each sample is represented as the number of SDs above or below the mean level of that biochemical (z-score). Apart from succinylcarnitine and 3-dehydrocarnitine, sexually dimorphic metabolites displayed uniformly increased concentrations in males. D, Summary plot for MSEA, where pathways are ranked by Bonferroni-corrected P value with hatched lines depicting P value cutoffs. CMP, cytidine monophosphate; DiHOME, hydroxyoctadec-9(Z)-enolate; GPI, glycerophosphoinositol; HODE, hydroxyoctadecadienoic acid.
Sex-specific effect of IVF on the adult fat metabolome
In males, 16 metabolites were significantly altered between IVF and control fat samples (P < .05, 2 molecules increased and 14 decreased), and 9 approached significance (.05 < P < .1, 0 increased and 9 decreased) (Figure 2, A and B). This included a dramatic reduction in levels of the glycolytic metabolites glucose and lactate, the pentose phosphate pathway metabolites ribulose and arabitol, as well as the nucleotide precursors inosine 5′-monophosphate, GMP, and uridine monophosphate, suggesting a decreased shunting of glycolytic intermediates through the pentose phosphate pathway toward nucleotide synthesis. MSEA highlighted an involvement of the altered metabolites with these pathways, although the associations were not significant after post hoc correction (Figure 2C).
Figure 2.
Effect of IVF on the adult fat metabolome. A, Categorical distribution of the 16 metabolites significantly altered in IVF males from controls. B, Heat map depicting fold-change in metabolite concentration between IVF and control metabolite values in male fat samples, including z-distribution of individual control (blue) and IVF (red) values relative to their respective population means. C, MSEA summary of Bonferroni-corrected pathways associated with the metabolite changes. D–F, Same as A–C but for the 19 metabolites altered in female IVF fat samples. G and H, Venn diagrams showing overlap in altered metabolites (G and purple symbols) and MSEA-identified pathways (H and green symbols) between male and female IVF cohorts. CDP, cytidine diphospho; CMP, cytidine monophosphate; Glu-Gln, glutamylglutamine; IMP, inosine monophosphate; SSG, glutathione disulfide; UMP, uridine monophosphate.
Comparatively, female IVF fat tissue differed from female controls by 19 metabolites (P < .05, 15 increased and 4 decreased), and 21 showed a trend toward significance (.05 < P < .1, 13 increased and 8 decreased) (Figure 2, D and E). Concentrations of several amino acids were increased in IVF mice, including urea cycle intermediates. Multiple long-chain (18C) fatty acids were increased, whereas levels of the glycerophosphoethanolamines (GPEs) 1-arachidonoyl-GPE and 2-docosahexaenoyl-GPE were decreased. Further, decreased maltotetraose could reflect changes in glycogenolysis. There was also evidence of oxidative stress in female IVF fat, evidenced by depleted levels of glutathione (GSH) (P = .088) (Supplemental Table 1) and a corresponding increase in its oxidized form cysteine-glutathione disulfide (CySS) (P = .042). MSEA showed significant alterations to many amino acid and protein synthesis pathways, as well as a trend toward changes in the malate-aspartate shuttle, urea cycle, ammonia recycling, and fructose/mannose degradation (Figure 2F).
The effect of IVF on adult fat metabolite composition was strikingly sex specific: glycerol was the only metabolite significantly different in IVF vs control samples for both males and females. However, the changes occurred in different directions (1.8-fold decrease from control males, 1.8-fold increase in females) (Figure 2G). Additionally, between the 13 MSEA-identified pathways enriched in males and 20 in females, only 5 pathways were altered in both sexes (Figure 2, H and green symbols in C and F).
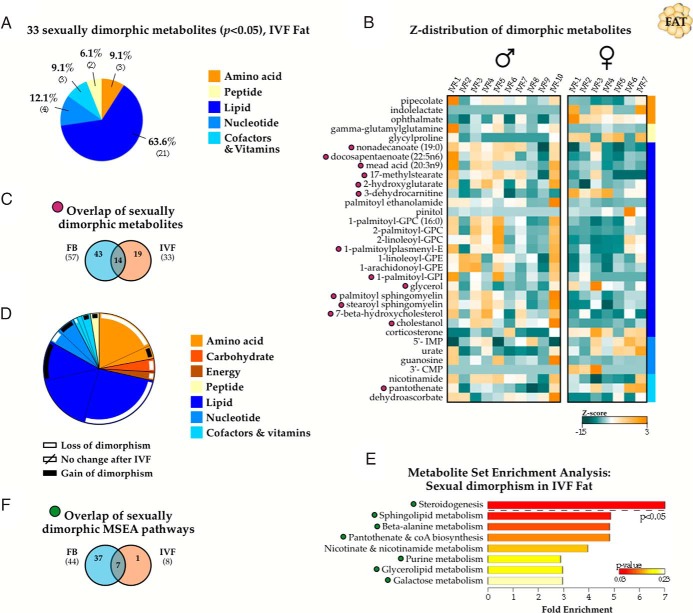
Reduced sexual dimorphism in IVF adult fat tissue
We next investigated the effect of IVF on metabolic sexual dimorphism and observed a striking depletion in the number of metabolites that differed in concentration between IVF male and IVF female fat samples. Compared with the 24.7% (57 of 231 metabolites) dimorphism in controls, only 33 (14.3%) showed significant male-female differences (P < .05), predominantly for metabolites involved in lipid metabolism (Figure 3, A and B). Only 14 metabolites retained significant sexual dimorphism between the control and IVF cohorts (purple symbols), indicating that male-female differential concentration was lost for 43 metabolites and gained for 19 (Figure 3C). Of these, sexual dimorphism in amino acid and lipid molecules were the most affected by IVF (Figure 3D). MSEA additionally showed that only steroidogenesis was different between sexes (Figure 3E). Overall, there was a dramatic reduction in IVF metabolic sexual dimorphism in gonadal fat tissue (Figure 3, F and green symbols).
Figure 3.
Reduced sexual dimorphism in IVF fat tissue. A, A total of 33 metabolites (14.3%) were sexually dimorphic (P < .05 between males and females) in fat samples from IVF animals (n = 10 males and 7 females). B, Z-score normalized expression of the IVF-associated sexually dimorphic metabolites, with purple symbols indicating metabolites retaining male vs female differences in both IVF and control samples. C, MSEA categorization showing a significantly decreased number of dimorphic pathways, with green symbols marking pathways with retained dimorphism from controls. D and F, Overlap of sexually dimorphic metabolites (D and purple symbols) and MSEA-identified pathways (F and green symbols) comparing control and IVF cohorts. E, Categorical distribution of the metabolites displaying altered sexual dimorphism. The white bars indicate metabolites with lost male vs female significance in IVF, and black bars show the percentage of metabolites acquiring significant male vs female concentrations in IVF compared with controls. For example, of the 41 lipid-categorized metabolites displaying male-female differences in at least 1 conception condition (dark blue section), 21 are no longer dimorphic in IVF tissues (black bar), 12 maintain sexual dimorphism in both control and IVF groups, and 8 exhibit dimorphism only in IVF samples (white bar). CMP, cytidine monophosphate; E, ethanolamine; GPC, glycerophosphocholine; GPI, glycerophosphoinositol; IMP, inosine monophosphate.
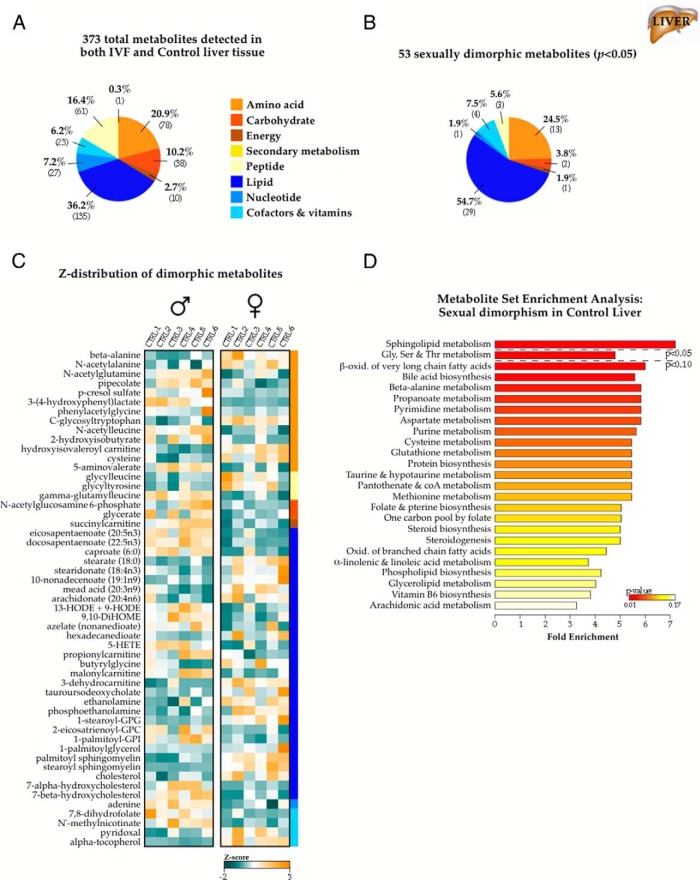
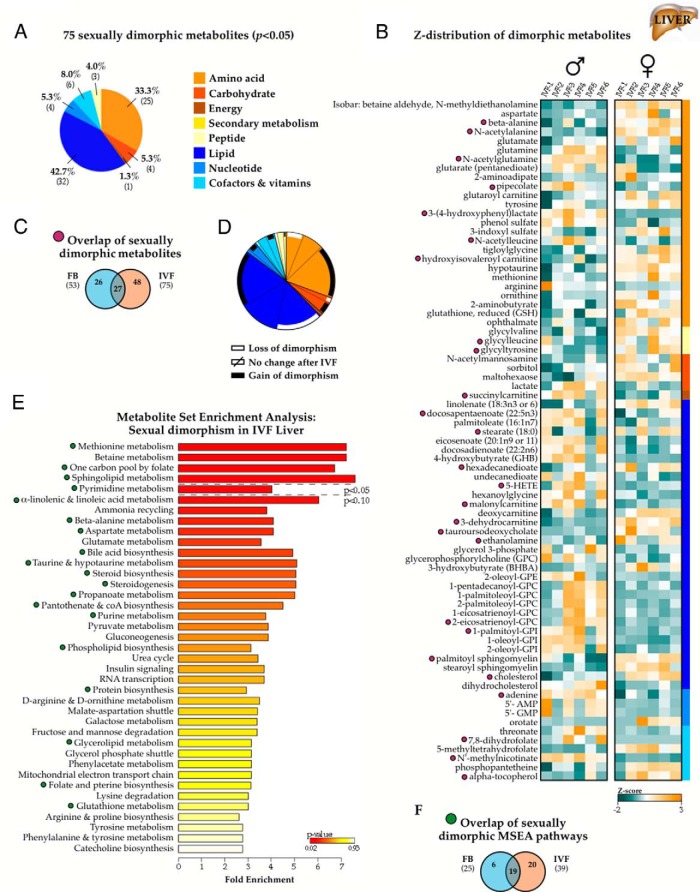
Sex-specific effect of IVF on the adult liver metabolome
We additionally profiled liver samples (n = 6 per sex and per conception condition) and detected a total of 373 endogenous biochemicals (Figure 4A), of which 53 (14.2%) exhibited significant male-female differences in concentration. As with the control fat tissue, dimorphic metabolites were predominantly comprised of lipid and amino acid derivatives (Figure 4B), but increases or decreases in metabolite levels were metabolite-specific (Figure 4C). MSEA classified sphingolipid metabolism, and glycine, serine, and threonine metabolism as the pathways most affected by sex differences (Figure 4D). Pearson correlations did not revealed any significant relationships between metabolite concentrations and percent adiposity or fasting glucose levels at time of death. Only levels of pyridoxal (one of the 3 forms of vitamin B6) displayed both a significant sex bias and a high correlation with fasting glucose levels and its hepatic levels (r = 0.67, P = .008).
Figure 4.
Metabolic sexual dimorphism in adult liver tissue. A, Nontargeted mass spectrometry profiling of 29-week IVF and control livers (n = 6 per sex and conception condition; 24 animals total) identified 373 named biochemicals comprising all major metabolic groups. B, Categorical distribution of the 53 metabolites with significantly different concentrations between males and females in control samples (P < .05). C, Z-distribution of the sexually dimorphic metabolites in control samples. Of note, concentrations of metabolites were not uniformly changed in one sex vs the other, as observed in fat tissue. Instead, there are more segmented and pathway-specific changes. D, MSEA summary with pathways ranked by Bonferroni-corrected P value. GPC, glycerophosphocholine; GPG, glycerophosphoglycerol; GPI, glycerophosphoinositol.
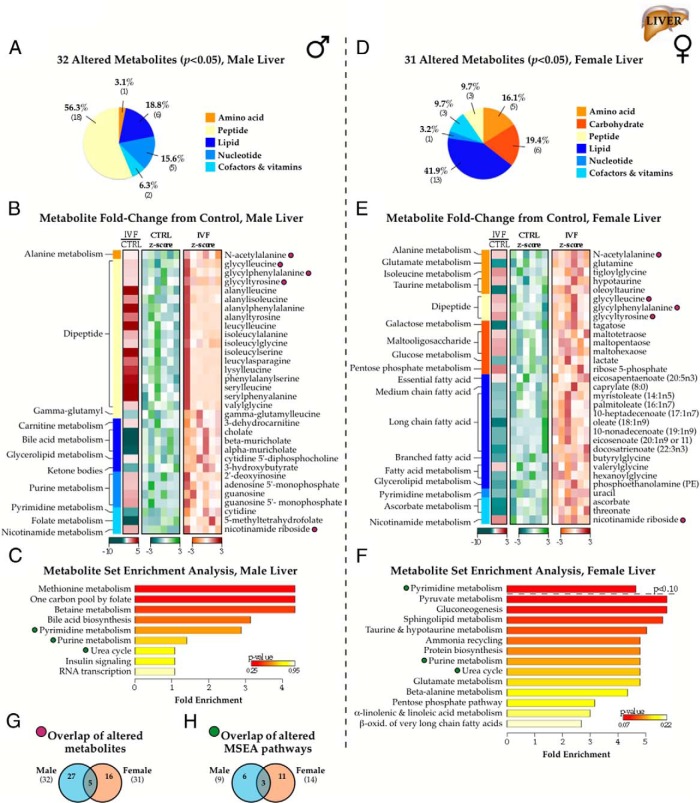
Investigation of IVF liver tissue revealed significant changes in levels of 32 metabolites between IVF and control male livers (P < .05, 25 molecules increased and 7 decreased), with an additional 30 approaching significance (.05 < P < .1, 20 increased and 10 decreased) (Figure 5A). A complete list of relative metabolite concentrations may be found in Supplemental Table 2. The most striking change was a broad incorporation of dipeptides into IVF livers, particularly for leucine, alanine, glycine, and isoleucine-based dipeptides (Figure 5B). There was also a strong depletion of the bile acid metabolites cholate, β-muricholate, and α-muricholate. Other notable decreases in male IVF livers relative to controls included the ketone body 3-hydroxybutyrate and the active form of folic acid, 5-methyltetrahydrofolate. Comparative increases consisted of 3-dehydrocarnitine, the nicotinamide adenine dinucleotide precursor nicotinamide riboside, as well as the purine metabolites 5′AMP, 5′GMP, and guanosine. None of the changes were associated with any significant MSEA pathways (Figure 5C).
Figure 5.
Effect of IVF on the adult liver metabolome. A, Categorical distribution of the 32 metabolites significantly altered in IVF males from controls. B, Heat map depicting fold-change in metabolite concentration between IVF and control metabolite values in male liver samples, including z-distribution of individual control (blue) and IVF (red) values relative to their respective population means. C, MSEA summary of Bonferroni-corrected pathways associated with the metabolite changes. D–F, Same as A–C but for the 31 metabolites altered in female IVF liver samples. G and H, Venn diagrams showing overlap in altered metabolites (G and purple symbols) and MSEA-identified pathways (H and green symbols) between male and female IVF cohorts.
In contrast, female IVF livers differed from female controls by 31 metabolites (P < .05, 13 increased and 18 decreased), and 32 showed a trend toward significance (.05 < P < .1, 12 increased and 20 decreased). These were enriched for fatty acid metabolites (Figure 5, D and E) including long-chain fatty acids and acylglycines; this in conjunction with a strong depletion of glutamine in all IVF samples may reflect changes in mitochondrial fatty acid catabolism. Increased glycogen intermediates maltotetraose, maltopentaose, and maltohexaose suggests an increase in liver glycogen breakdown in female IVF mice, which supports the changes in concentrations of lactate and ribose-5-phosphate that indicate an alternative fate for glucose. None of the altered metabolites were significantly associated with any metabolic pathways after post hoc correction, although MSEA did identify changes in gluconeogenesis, the pentose phosphate pathway, and long-chain fatty acid β-oxidation, among others (Figure 5F).
As was observed in the fat, the IVF-associated changes were relatively sex specific. Five metabolites were significantly different in IVF vs control samples for both males and females, including N-acetylalanine, the dipeptides glycylleucine, glycylphenylalanine and glycyltyrosine and nicotinamide riboside (Figure 5, G and purple symbols in B and D). MSEA identified 3 altered pathways in both sexes, although the changes were not significant (Figure 5, H and green symbols in C and F).
Exaggerated sexual dimorphism in the IVF adult liver
In contrast to the IVF fat tissue, sexual dimorphism in IVF livers was increased compared with controls. A total of 75 metabolites (20.1% vs 14.2% in controls) displayed significant male-female differences in concentration, similarly enriched for lipid and amino acid metabolites (Figure 6, A and B). Relative to control samples, 26 metabolites lost significant dimorphic concentrations (P > .05), sex differences were maintained in 27 molecules, and 43 new metabolites exhibited significantly different male-female concentrations (Figure 6, C and purple symbols). Of these, sexual dimorphism was particularly increased for amino acid metabolites and altered in lipids, with dimorphic concentrations shifting away from sterol and fatty acid metabolism and increasing for glycerophosphocholines and other lysolipids (Figure 6, B and D). MSEA processing revealed more significant dimorphism for methionine, 1-carbon folate, and pyrimidine metabolism, as well as novel dimorphic pathways compared with control samples, including betaine metabolism, ammonia recycling, glutamate metabolism, and other nonsignificant glucose handling pathways (Figure 6, E and F and green symbols).
Figure 6.
Exaggerated sexual dimorphism in IVF liver tissue. A, A total of 75 metabolites (20.1%) exhibited sexually dimorphic concentrations (P < .05 between males and females) in liver samples from IVF animals (n = 6 males and 6 females). B, Z-score normalized expression of the IVF-associated sexually dimorphic metabolites, with purple symbols indicating metabolites retaining male vs female differences in both IVF and control samples. C, Categorization of enriched pathways in the metabolite set, with green symbols marking pathways with retained dimorphism from controls. D and F, Overlap of sexually dimorphic metabolites (D and purple symbols) and MSEA-identified pathways (F and green symbols) comparing control and IVF liver cohorts. E, Categorical distribution of the metabolites displaying altered sexual dimorphism, with white and black bars indicating metabolites with lost or acquired significant male-female differences, respectively, in IVF vs control livers, as discussed in Figure 3E. GPC, glycerophosphocholine; GPI, glycerophosphoinositol.
Taken together, these results demonstrate a sex- and tissue-specific effect of IVF on adult metabolism.
Discussion
Preimplantation development has been recognized as a window of notable environmental sensitivity, and several animal studies have demonstrated that nutritional, oxidative, and in vitro stresses restricted exclusively to this period are sufficient to predispose growth and metabolic pathologies (2, 11, 26). Specifically, our group has shown that both stressful and optimized IVF conditions can reprogram adult mouse growth, fat deposition, and glucose homeostasis in a sexually dimorphic fashion (9, 10). We therefore used metabolomics to compare the biochemical profiles and uncouple the sex specificity of adult mouse IVF- with in vivo-conceived liver and gonadal adipose tissues.
One of the novel findings of our study is an expanded description of naturally occurring metabolite differences between males and females in liver and adipose tissues. This is important, because most published metabolomics-based investigations are either restricted to one sex or not stratified by sex. Of note, unsupervised hierarchical clustering revealed no correlation between metabolite profile and estrous cycle at time of death. It is well known that sex differences between males and females vastly affect mammalian phenotypes, behaviors, and disease through a variety of hormonal, immunological, and genetic mechanisms. We observed discordant sterol metabolism, redox state, mobilization, and oxidation of fatty acids between the 2 sexes. Similar findings have been reported after comparison of murine liver and adipose transcriptomes between males and females (27). In the liver, 14% of detected metabolites (53 of 373) were differentially concentrated between the sexes, compared with 24% (57 of 231) in fat, suggesting that fat tissue is a preferential locus of sexual dimorphism. Indeed, fat mass is largely divergent between sexes (28) and is a location of sexually dimorphic transcriptional changes in response to nutritional reprogramming (29). Although we are not aware of any analogous human metabolomics-based studies in these tissues, serum metabolites are markedly different between the sexes (30), although it is undocumented which tissue(s) contribute to these dissimilarities. Interestingly, apart from succinylcarnitine and 3-dehydrocarnitine, concentrations of the sexually dimorphic metabolites in fat tissue were uniformly increased in males and decreased in females (Figure 1C); by comparison, the sex bias in liver tissue was more segmented and network specific (Figure 4C). The significant male-female differences observed in control tissues highlights the importance of controlling for sex in metabolic investigations.
A second important finding is the demonstration of a tissue- and sex-specific effect of IVF on the adult metabolome. We did not observe uniform or consistent patterns of change between genders or across tissues suggestive of an “IVF fingerprint.” One possible explanation is that IVF male and female blastocysts are differentially affected by the environment they encounter during early development. Subsequently, the additional numerous and complex developmental steps occurring within developing liver or adipose tissue could be further altered in accordance with new, tissue-specific developmental cues. The net result would be each tissue adopting a unique and sex-specific metabolic signature of the developmental stress encountered, rather than a singly uniform pattern. Indeed, male-female disparities are apparent even before gonadal formation and are therefore partially independent of sex hormone quality and quantity. For example, differential expression of several X-linked transcripts, including the metabolic genes glucose-6-phosphate dehydrogenase (G6pd) or phosphoglycerate kinase (Pgk), may be observed as early as the preimplantation embryo stages (31). Moreover, up to one-third of transcripts are differentially expressed by sex in the blastocyst, in particular for glucose and protein metabolic pathways (14). This indicates that the preimplantation embryo is poised already to differentially respond to environmental changes in a sex-specific fashion, which may explain the frequent sex bias observed in various models of DOHaD and metabolic reprogramming (32).
Overall, we observed a striking effect of IVF on adult metabolic sexual dimorphism, which was increased in IVF liver and decreased in IVF adipose tissue (Supplemental Figure 2). As with controls, fat was more susceptible to change: only 24.6% of metabolites (14 of 57) (Figure 3C) exhibiting sex bias in control tissues maintained that dimorphism after IVF, compared with 50.9% (27 of 53) (Figure 6C) preserved dimorphism between control and IVF liver samples. Most the changes occurred in lipid and amino acid metabolites (Figures 3 and 6). Male-female differences in amino acid concentrations were almost completely abrogated in IVF adipose tissue (Figure 3B), whereas IVF livers displayed a shift toward increased dimorphism in these metabolites, particularly for compounds involved in glutamine, lysine, taurine metabolism, and the urea cycle (Figure 6B). Sexually dimorphic concentrations of glycerolipids and lysolipids were increased in both tissues, with IVF females displaying significantly lower levels than males. This is particularly relevant, because these female IVF animals display similarly strong decreases in serum concentrations of both glycero- and lysolipids (9), which has also been observed in metabolomics-based analyses of impaired fasting glucose (33) and diet-induced obesity (34).
We next investigated whether the metabolites differentially measured between IVF and in vivo tissues could be used as biomarkers to predict chronic disease susceptibility, as our physiologic studies indicate that IVF mice are predisposed to glucose intolerance (9, 10). Indeed, we uncovered several biochemicals present in IVF tissues that have been linked with metabolic diseases. Levels of the purine metabolites AMP, GMP, adenosine, and guanosine were increased in IVF livers, more so in males. AMP is the principal activator of AMP kinase, which functions in regulating energy metabolism and glucose homeostasis in the liver and other tissues (35). Specifically, activated AMP kinase conserves cellular resources by promoting ATP-generating mechanisms and inhibiting anabolic pathways. The elevation of multiple supporting metabolites suggests that increased AMP and GMP generation is not derived exclusively from energy-requiring cellular reactions involving ATP and GTP, respectively, but additionally through de novo synthesis and salvage pathways. These pathways support growth and proliferation through the provision of nucleotides needed for RNA and DNA synthesis, which may represent another connection between broad metabolic reprogramming and the disruption of glucose handling in mice conceived by IVF.
Next, aggregate comparison of all IVF with control liver samples (males and females) showed a striking depletion of several bile acids and salts, with males more severely affected (Figure 5, B and E, and Supplemental Table 2). Bile acids have a reciprocal relationship with both glucose and insulin (36), and it has been demonstrated that impaired bile acid synthesis and subsequent reduction in bile acid pool size significantly decreases energy expenditure and contributes to the pathogenesis of obesity and diabetes (37). Changes in bile acid metabolism may therefore be directly connected to perturbed glucose handling in IVF mice, a hypothesis supported by our group's previously reported microarray analysis of IVF livers and observation of transcriptional changes associated with bile acid biosynthesis (altered expression of hepatic Akr1c4, Baat, and Cyp27a1 in IVF mice) and diabetes mellitus signaling (including Casp9, Cycs, Fcer1g, HlaB, HlaC, Ikbkb, Il1rap, Irf1, and Nfkb2) (9).
Additional examples of synergy between IVF transcriptional and biochemical profiles with metabolic disease include the prominent increase in dipeptide concentration present in male IVF livers coupled with hepatic misexpression of protein ubiquitination pathway genes (Cul1, Cul2, Dnajb1, Dnajb4, Dnajb9, Dnajb14, Dnajc19, Dnajc21, HlaB, HlaC, Pan2, Psmc6, Psmd12, Sugt1, Tap1, Ube2l3, Uchl3, Usp16, and Usp46) (9). Dipeptides can regulate protein ubiquitination (38), the activity of which affects hepatic lipid production, insulin resistance, and secretion (39). Separately, altered expression of genes involved in mitochondrial function (Casp9, Cox6c, Cox7a2, Cycs, Gpx4, Ndufa5, Ndufb4, Ndufb6, Ndufs4, and Uqcrb) (9) in conjunction with decreased levels of long-chain fatty acids, glutamine, lactate, and increased ribose-5-phosphate in IVF female livers may reflect changes in mitochondrial activity and the use of alternative anabolic branches, such as the shunting of glucose through the pentose phosphate pathway.
Under the particular conception conditions used in our study, female animals are predisposed to increased fat accumulation, and both males and females show fat-exclusive maintenance of epigenetic alterations present in IVF blastocysts (9). This data suggest that adipose tissue is a locus of sex- and tissue-specific changes associated with IVF. Because fat is a primary driver of metabolic dysfunction (40), it is possible that the acquired sex bias in IVF tissues contributes to the sex-specific metabolic phenotypes. Comparison of IVF adipose metabolic profiles points to altered redox homeostasis, with female-specific increases in ophthalmate, CySS, urate and corticosterone. GSH is the primary source of antioxidant reducing power in animals, and both ophthalmate and CySS are formed under oxidative stress of GSH. Further, urate and corticosterone can induce proinflammatory signaling and increase the production of reactive oxygen species in adipocytes and other tissues (41–43). The relationship between adipogenesis and redox state is complex, and emerging evidence suggests that adipogenesis is accelerated by oxidizing conditions. For example, reactive oxygen species and antioxidant activity show parallel increases with fat accumulation through adipogenic transcription factor-dependent mechanisms (44–46). It is therefore possible that the female-specific oxidization in IVF fat tissue is in part responsible for the increased adiposity in these animals. Fitting with this hypothesis are the reported transcriptional changes associated with the production of reactive oxygen species in fat from IVF females (9).
A few factors in this study merit acknowledgment. Our control group was designed to specifically test the impact of IVF and embryo culture, while removing variables such as superovulation and the embryo transfer procedure. Therefore, comparison of control male and female animals may not accurately depict the natural sexual dimorphism present in adult tissues. Separately, white adipose tissue depots can vary by adipocyte size, protein composition, gene expression, and response to gonadal hormones (47, 48), such that our evaluation of gonadal fat represents a focused analysis of sexual dimorphism and conception impact on metabolism, and cannot necessarily be extrapolated to other adipose depots. The analysis could additionally be limited by outlying data points. For example, one of the male liver samples contributed extreme values; we consequently displayed the fold-change and z-score data in heat map form to evaluate variability. Metabolomics technology has not yet achieved total metabolite coverage, thus creating an intrinsic and unavoidable bias toward known compounds. This study should therefore be regarded as hypothesis generating, rather than providing a cause-effect relationship. Because a number of mechanisms may contribute to the observed changes in metabolite pool size, future studies must focus on transporter activity and enzyme kinetics to better describe the causes of the metabolite flux, as well as which aspects of IVF metabolic signatures are relevant to the underlying etiology of the outward phenotypes.
In summary, in accordance with the DOHaD hypothesis, our data support that preimplantation embryo development is a particularly sensitive environmental period and that in vitro culture can induce permanent changes to adult metabolism and energy use. Comparison of the IVF metabolic and transcriptional signatures indicate several areas of overlap, thus establishing a relationship between molecular alterations and physiological phenotype. It remains unclear why females specifically are susceptible to a more severe metabolic phenotype and increased evidence of oxidative stress, but this may be related to the particular IVF conditions and/or the significant sexual dimorphism already present in early embryos. Future studies should expand the metabolic analysis in additional tissues and further investigate sexual dimorphic epigenetic differences. This study underscores the importance of continued and sex-specific follow-up of IVF-conceived offspring beyond early postnatal life.
Acknowledgments
We thank Kirk Pappan at Metabolon, Inc for his help processing the metabolomics data. In memoriam David Barker.
This work was supported by the National Institute of Child Health and Human Development Grant RO1:HD 062803-01A1 and by an American Diabetes Association grant (P.F.R.). S.K.F. was supported by the National Institute of Health Training Fellowship 5T32DK007418-32. R.K.S. was supported by the California Institute for Regenerative Medicine Grant TB1-01194.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CySS
- cysteine-glutathione disulfide
- DOHaD
- Developmental Origins of Health and Disease
- GPE
- glycerophosphoethanolamine
- GSH
- glutathione
- hCG
- human chorionic gonadotropin
- IVF
- in vitro fertilization
- MSEA
- metabolite set enrichment analysis.
References
- 1. Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. [DOI] [PubMed] [Google Scholar]
- 2. Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. [DOI] [PubMed] [Google Scholar]
- 3. Fernández-Gonzalez R, Moreira P, Bilbao A, et al. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci USA. 2004;101:5880–5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernández-Gonzalez R, Moreira PN, Pérez-Crespo M, et al. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008;78:761–772. [DOI] [PubMed] [Google Scholar]
- 5. Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008;93:1682–1688. [DOI] [PubMed] [Google Scholar]
- 6. Ceelen M, van Weissenbruch MM, Roos JC, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J Clin Endocrinol Metab. 2007;92:3417–3423. [DOI] [PubMed] [Google Scholar]
- 7. Ceelen M, van Weissenbruch MM, Prein J, et al. Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8–18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod. 2009;24:2788–2795. [DOI] [PubMed] [Google Scholar]
- 8. Scherrer U, Rimoldi SF, Rexhaj E, et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012;125:1890–1896. [DOI] [PubMed] [Google Scholar]
- 9. Feuer SK, Liu X, Donjacour A, et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology. 2014;155:1956–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donjacour A, Liu X, Lin W, Simbulan R, Rinaudo PF. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model. Biol Reprod. 2014;90:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rexhaj E, Paoloni-Giacobino A, Rimoldi SF, et al. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J Clin Invest. 2013;123:5052–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol. 2009;304:8–18. [DOI] [PubMed] [Google Scholar]
- 13. Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(suppl 1):60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc Natl Acad Sci USA. 2010;107:3394–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilbert JS, Banek CT. Sex differences in the developmental programming of adult disease. INTECH Open Access Publisher; 2012. [Google Scholar]
- 16. Kaddurah-Daouk R, Kristal BS, Weinshilboum RM. Metabolomics: a global biochemical approach to drug response and disease. Annu Rev Pharmacol Toxicol. 2008;48:653–683. [DOI] [PubMed] [Google Scholar]
- 17. Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lawitts JA, Biggers JD. Culture of preimplantation embryos. Methods Enzymol. 1993;225:153–164. [DOI] [PubMed] [Google Scholar]
- 19. Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81:6656–6667. [DOI] [PubMed] [Google Scholar]
- 20. Lawton KA, Berger A, Mitchell M, et al. Analysis of the adult human plasma metabolome. Pharmacogenomics. 2008;9:383–397. [DOI] [PubMed] [Google Scholar]
- 21. Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform. 2010;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37:W652–W660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2.0–a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012;40:W127–W133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwarzer C, Esteves TC, Araúzo-Bravo MJ, et al. ART culture conditions change the probability of mouse embryo gestation through defined cellular and molecular responses. Hum Reprod. 2012;27:2627–2640. [DOI] [PubMed] [Google Scholar]
- 25. Xia J, Wishart DS. MSEA: a web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 2010;38:W71–W77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Banrezes B, Sainte-Beuve T, Canon E, Schultz RM, Cancela J, Ozil JP. Adult body weight is programmed by a redox-regulated and energy-dependent process during the pronuclear stage in mouse. PLoS One. 2011;6:e29388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang X, Schadt EE, Wang S, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21:415–430. [DOI] [PubMed] [Google Scholar]
- 29. Guo C, Li C, Myatt L, Nathanielsz PW, Sun K. Sexually dimorphic effects of maternal nutrient reduction on expression of genes regulating cortisol metabolism in fetal baboon adipose and liver tissues. Diabetes. 2013;62:1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mittelstrass K, Ried JS, Yu Z, et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011;7:e1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wrenzycki C, Lucas-Hahn A, Herrmann D, Lemme E, Korsawe K, Niemann H. In vitro production and nuclear transfer affect dosage compensation of the X-linked gene transcripts G6PD, PGK, and Xist in preimplantation bovine embryos. Biol Reprod. 2002;66:127–134. [DOI] [PubMed] [Google Scholar]
- 32. Bermejo-Alvarez P, Rizos D, Lonergan P, Gutierrez-Adan A. Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease. Reproduction. 2011;141:563–570. [DOI] [PubMed] [Google Scholar]
- 33. Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, Ong CN. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metab. 2013;98:E1060–E1065. [DOI] [PubMed] [Google Scholar]
- 34. Kim HJ, Kim JH, Noh S, Hur HJ, et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res. 2011;10:722–731. [DOI] [PubMed] [Google Scholar]
- 35. Viollet B, Guigas B, Leclerc J, et al. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf). 2009;196:81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li T, Francl JM, Boehme S, et al. Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J Biol Chem. 2012;287:1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watanabe M, Horai Y, Houten SM, et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J Biol Chem. 2011;286:26913–26920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Du F, Navarro-Garcia F, Xia Z, Tasaki T, Varshavsky A. Pairs of dipeptides synergistically activate the binding of substrate by ubiquitin ligase through dissociation of its autoinhibitory domain. Proc Natl Acad Sci USA. 2002;99:14110–14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wing SS. The UPS in diabetes and obesity. BMC Biochem. 2008;9(suppl 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. [DOI] [PubMed] [Google Scholar]
- 41. Baldwin W, McRae S, Marek G, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60:1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–C596. [DOI] [PubMed] [Google Scholar]
- 43. Zafir A, Banu N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 2009;12:167–177. [DOI] [PubMed] [Google Scholar]
- 44. Calzadilla P, Sapochnik D, Cosentino S, et al. N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int J Mol Sci. 2011;12:6936–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vigilanza P, Aquilano K, Baldelli S, Rotilio G, Ciriolo MR. Modulation of intracellular glutathione affects adipogenesis in 3T3-L1 cells. J Cell Physiol. 2011;226:2016–2024. [DOI] [PubMed] [Google Scholar]
- 46. Imhoff BR, Hansen JM. Differential redox potential profiles during adipogenesis and osteogenesis. Cell Mol Biol Lett. 2011;16:149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sackmann-Sala L, Berryman DE, Munn RD, Lubbers ER, Kopchick JJ. Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity. 2012;20:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fuente-Martín E, Argente-Arizón P, Ros P, Argente J, Chowen JA. Sex differences in adipose tissue: it is not only a question of quantity and distribution. Adipocyte. 2013;2:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]