We have demonstrated for the first time the safety and feasibility of intrapericardial delivery of microencapsulated xenogeneic mesenchymal stem cells with fused x-ray and MR imaging guidance for the treatment of cardiac disease in nonimmunosuppressed animals to monitor and track cell retention.
Abstract
Purpose
To assess intrapericardial delivery of microencapsulated, xenogeneic human mesenchymal stem cells (hMSCs) by using x-ray fused with magnetic resonance (MR) imaging (x-ray/MR imaging) guidance as a potential treatment for ischemic cardiovascular disease in an immunocompetent swine model.
Materials and Methods
All animal experiments were approved by the institutional animal care and use committee. Stem cell microencapsulation was performed by using a modified alginate-poly-l-lysine-alginate encapsulation method to include 10% (wt/vol) barium sulfate to create barium-alginate microcapsules (BaCaps) that contained hMSCs. With x-ray/MR imaging guidance, eight female pigs (approximately 25 kg) were randomized to receive either BaCaps with hMSCs, empty BaCaps, naked hMSCs, or saline by using a percutaneous subxiphoid approach and were compared with animals that received empty BaCaps (n = 1) or BaCaps with hMSCs (n = 2) by using standard fluoroscopic delivery only. MR images and C-arm computed tomographic (CT) images were acquired before injection and 1 week after delivery. Animals were sacrificed immediately or at 1 week for histopathologic validation. Cardiac function between baseline and 1 week after delivery was evaluated by using a paired Student t test.
Results
hMSCs remained highly viable (94.8% ± 6) 2 days after encapsulation in vitro. With x-ray/MR imaging, successful intrapericardial access and delivery were achieved in all animals. BaCaps were visible fluoroscopically and at C-arm CT immediately and 1 week after delivery. Whereas BaCaps were free floating immediately after delivery, they consolidated into a pseudoepicardial tissue patch at 1 week, with hMSCs remaining highly viable within BaCaps; naked hMSCs were poorly retained. Follow-up imaging 1 week after x-ray/MR imaging–guided intrapericardial delivery showed no evidence of pericardial adhesion and/or effusion or adverse effect on cardiac function. In contradistinction, BaCaps delivery with x-ray fluoroscopy without x-ray/MR imaging (n = 3) resulted in pericardial adhesions and poor hMSC viability after 1 week.
Conclusion
Intrapericardial delivery of BaCaps with hMSCs leads to high cell retention and survival. With x-ray/MR imaging guidance, intrapericardial delivery can be performed safely in the absence of preexisting pericardial effusion to provide a novel route for cardiac cellular regenerative therapy.
© RSNA, 2014
Introduction
Despite recent advances in pharmacotherapy and interventional surgical techniques, coronary heart disease remains the number one cause of heart failure in the Western world (1). Owing to limited regeneration capacity of the heart, therapeutic angiogenesis with exogenous agents, such as growth factors, gene therapy, or cellular therapeutics (2,3), may offer promise to patients with ischemic heart disease. In fact, stem and/or progenitor cell therapy has been shown to reduce infarct size and lessen adverse ventricular remodeling after myocardial infarction in preclinical (4) and clinical (5,6) settings. However, the long-term, sustained improvements are often not realized in clinical trials (7,8), which may be due in part to the poor survival and/or lack of sustained engraftment of the transplanted cells. The substantial cell loss that occurs shortly after stem cell administration has been attributed to the hypoxic environment of infarct tissue, lack of cell survival signals, or immunorejection (9,10). Indeed, retention and survival of stem cells delivered to the heart are poor, regardless of the administration route (11,12). Therefore, large numbers of cells are often administered to achieve observable benefit.
Current clinical trials have focused on local delivery of stem cells to the heart by using direct intramyocardial and/or transendocardial injections or intracoronary infusion (5,6,13,14). Whereas preclinical intracoronary cell administration effectiveness data are scarce, the transmyocardial administration effectiveness can be markedly altered, depending on whether cells are delivered to viable myocardium or hypoperfused and/or infarcted myocardium (15). The optimal cell delivery route remains under investigation.
The pericardial space, a potentially fluid-filled compartment between the epicardium and pericardial sac, may offer a less invasive approach for localized delivery of stem cell therapy to the heart (16). In fact, pericardial administration of angiogenic growth factors and other cardioactive agents have shown beneficial effects in preclinical studies (17,18). In a porcine chronic myocardial ischemia model, the deposition of fibroblast growth factor in the pericardial sac induced functionally significant epicardial angiogenesis (18). Indeed, application of a tissue-engineered cell layer over the infarcted epicardial surface appears to produce enhanced outcomes compared with transmyocardial cell delivery (19).
Alginate microencapsulation of allogeneic and xenogeneic islet cells has been developed as a method to prevent host immunorejection (20). The alginate microcapsule provides a porous layer that allows the diffusion of small molecules, such as cytokines, oxygen, nutrients, and waste products, but restricts large molecules, such as immunoglobulins and immune cells. Recently, stem cell encapsulation with radiopaque substances has been developed to enable cell tracking by using standard x-ray fluoroscopy (21). In a rabbit model of critical limb ischemia, arteriogenesis was enhanced by the administration of microencapsulated bone marrow–derived, allogeneic mesenchymal stem cells (MSCs) compared with unencapsulated MSCs (22).
We hypothesized that microencapsulated xenogeneic stem cells visible with x-rays could be delivered safely into the pericardial space with fused x-ray and magnetic resonance (MR) imaging (x-ray/MR imaging) guidance to improve cell retention and survival. X-ray/MR imaging guidance can exploit the strengths of each imaging modality—namely, the high temporal resolution and availability of delivery devices for x-ray imaging and the excellent soft-tissue contrast of MR imaging allow direct delineation of the myocardium and epicardial vasculature—thereby enabling safe delivery of cellular therapeutics to the pericardial space. In addition, through the use of stem cell microencapsulation visible with x-rays, retention of the cellular therapeutics and effects on cardiovascular function can subsequently be assessed with noninvasive imaging. The purpose of this study was to assess intrapericardial delivery of microencapsulated, xenogeneic human MSCs (hMSCs) by using x-ray/MR imaging guidance as a potential treatment for ischemic cardiovascular disease in an immunocompetent swine model.
Materials and Methods
This study was a collaboration between Siemens Corporate Research and The Johns Hopkins University. Siemens provided technical support and works in progress for image fusion. The authors who were not employees of Siemens had full control of the inclusion of all the data and information submitted for publication.
hMSC Encapsulation
The hMSCs (Lonza, Gaithersburg, Md) were expanded four to eight passages in MSC growth medium (MSCG SingleQuots; Lonza) according to the manufacturer’s instruction. Microencapsulation of hMSCs was performed as described previously (23) by using 10% (wt/vol) barium sulfate (Sigma, St Louis, Mo) in 1.5% (wt/vol) alginate (Pronoval UP LVG; FMC Polymer, Sandvika, Norway) (Appendix E1 [online]). In vitro encapsulated hMSC viability was examined by using cell staining that showed live and dead cells (2 μmol/L of calcein AM or 5 μmol/L of propidium iodide; Trevigen, Gaithersburg, Md).
Thrombogenicity
Thombogenicity of approximately 150 empty barium-alginate microcapsules (BaCaps) and 150 BaCaps with hMSCs was assessed in triplicate by means of activated clotting time (Appendix E1 [online]).
Animals
All animal studies were approved by The Johns Hopkins University Institutional Animal Care and Use Committee. Female Yorkshire pigs (approximately 25 kg) were sedated with a combination of ketamine (10 mg/mL), xylazine (100 mg/mL), and telazol (100 mg/mL) at a dose of 1 mL per 25 kg intramuscularly and induced with propofol to effect intravenously (approximately 4 mg/kg). Animals were intubated and maintained with general anesthesia with 1%–2% isoflurane by using mechanical ventilation. Complete blood counts and chemistry were acquired prior to intrapericardial access and at day 7.
X-ray/MR Imaging Protocol
Scout images were acquired with a 1.5-T MR unit (Espree; Siemens Healthcare, Erlangen, Germany) by using a surface coil (six-element body matrix and spinal array coil) in anesthetized pigs placed supine to determine the location and extent of the left ventricle. Cine short-axis MR images were acquired with contiguous multisection, breath-hold, electrocardiography-gated steady-state free precession by using the following imaging parameters: repetition time (msec)/echo time (msec), 25.48/1.59; 280 × 245-mm field of view; 192 × 192 image matrix; 6-mm section thickness; and 80° flip angle. Subsequently, navigator-gated, three-dimensional (3D), whole-heart steady-state free precession MR imaging was performed by using the following parameters: 290/1.67, 320 × 240-mm field of view, 256 × 173 image matrix, acceleration factor of two, 64 sections acquired, and 2-mm section thickness. Endocardial and epicardial borders were segmented on the short-axis and 3D whole-heart MR images by using vendor software (Argus; Siemens) on the basis of a combination of signal intensity–based segmentation and manual editing.
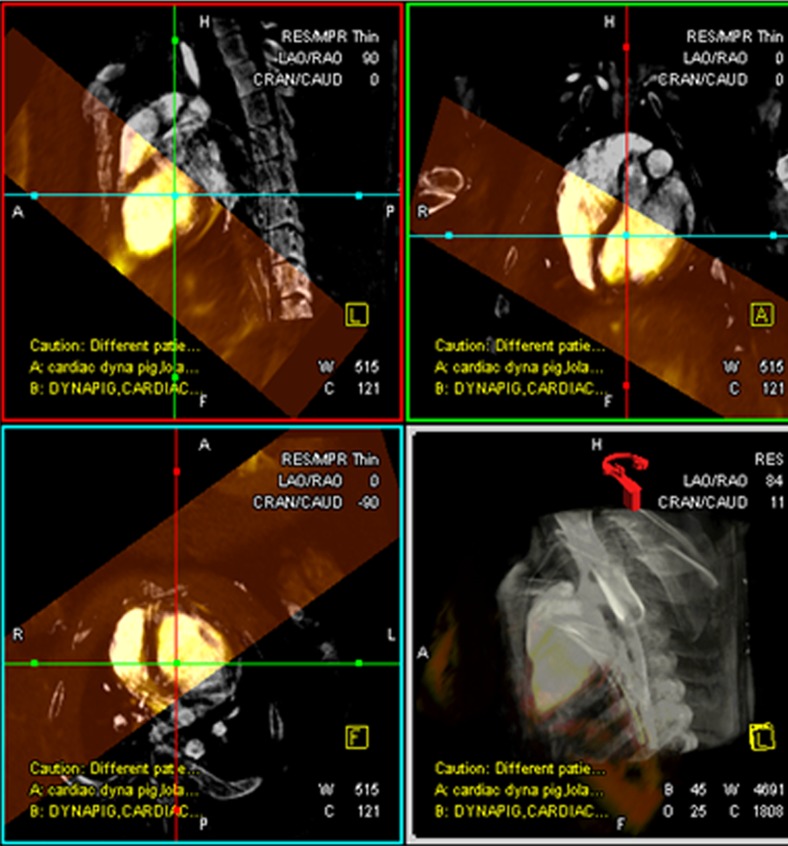
After MR imaging, the pigs were transferred to an x-ray angiographic system (Axiom Artis dFA; Siemens, Forchheim, Germany) with the use of a mechanized transport table that allows bidirectional transfer between the MR imaging and x-ray suites (Miyabi transfer system; Siemens). Cardiac-gated C-arm computed tomography (CT) (dynaCT; Siemens) with 190° rotation, 0.5° flip angle, 20-second acquisition, four rotations, 48-cm field of view, and 90-kV tube potential was performed during a breath hold and with 50:50 dilution of iohexol (Omnipaque; GE Healthcare, Princeton, NJ) injected in the aortic root or left main coronary artery. The cardiac-gated C-arm CT images were reconstructed by using the electrocardiography trigger to match the end-diastolic 3D whole-heart MR images (Movie 1 [online]). After 3D-3D registration of MR images and C-arm CT images (Appendix E1 [online]), a surface rendering of the ventricles from MR imaging and volume-rendered C-arm CT was projected onto live x-ray fluoroscopic images (syngo InSpace 3D/3D fusion; Siemens) (Fig 1, Movie 2 [online]).
Figure 1a:
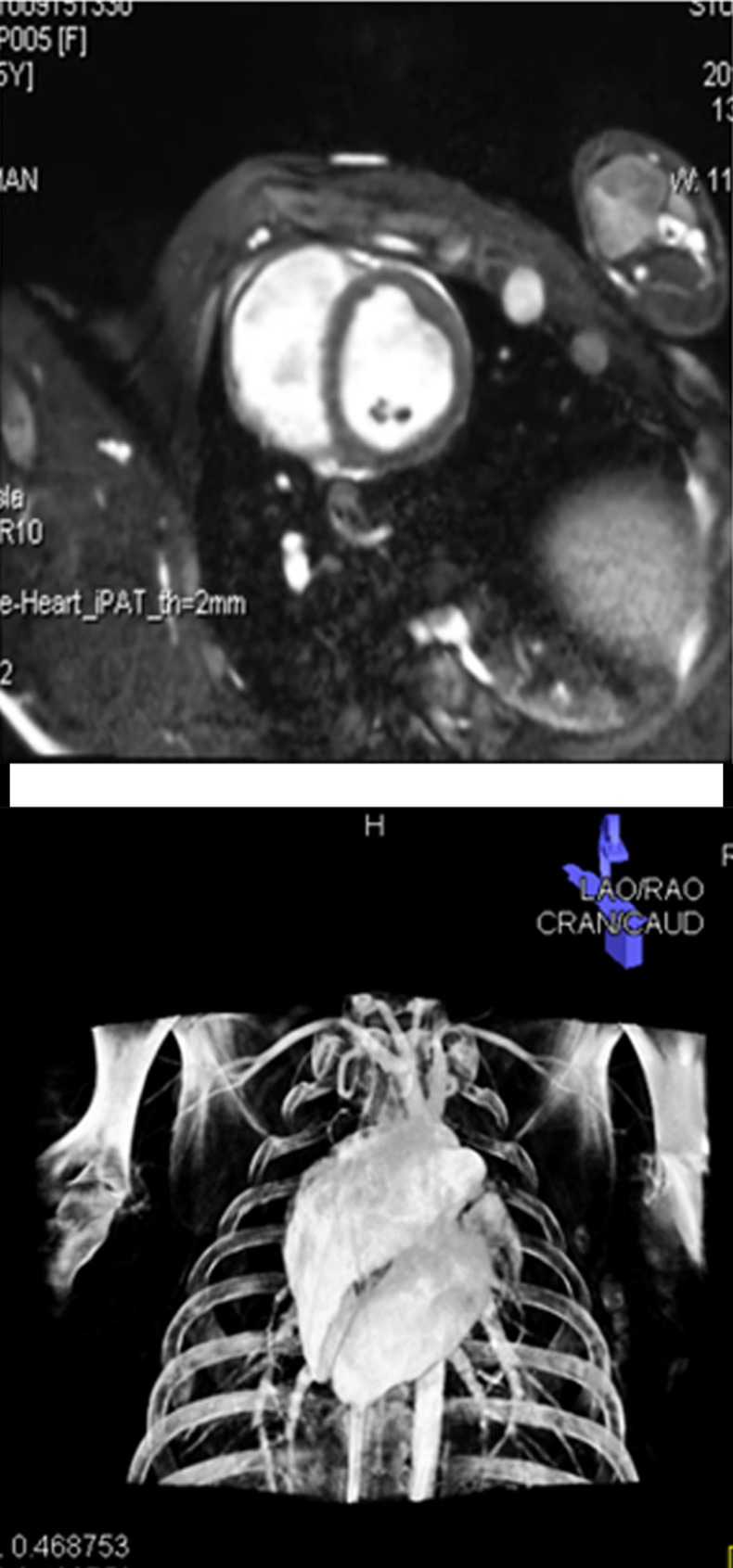
Imaging workflow of x-ray/MR imaging for intrapericardial delivery. (a) Navigator-gated 3D whole-heart MR image (top) was obtained to define ventricular borders for intrapericardial injection, followed by cardiac-gated C-arm CT image (bottom). (b) 3D registration of whole-heart MR imaging and C-arm CT was performed, followed by (c) surface rendering of the ventricles from MR imaging (top) and volume-rendered C-arm CT (bottom) overlaid on (d) a live x-ray fluoroscopic image (top) and fused image (bottom) to guide percutaneous access to the pericardial space.
Figure 1b:
Imaging workflow of x-ray/MR imaging for intrapericardial delivery. (a) Navigator-gated 3D whole-heart MR image (top) was obtained to define ventricular borders for intrapericardial injection, followed by cardiac-gated C-arm CT image (bottom). (b) 3D registration of whole-heart MR imaging and C-arm CT was performed, followed by (c) surface rendering of the ventricles from MR imaging (top) and volume-rendered C-arm CT (bottom) overlaid on (d) a live x-ray fluoroscopic image (top) and fused image (bottom) to guide percutaneous access to the pericardial space.
Figure 1c:
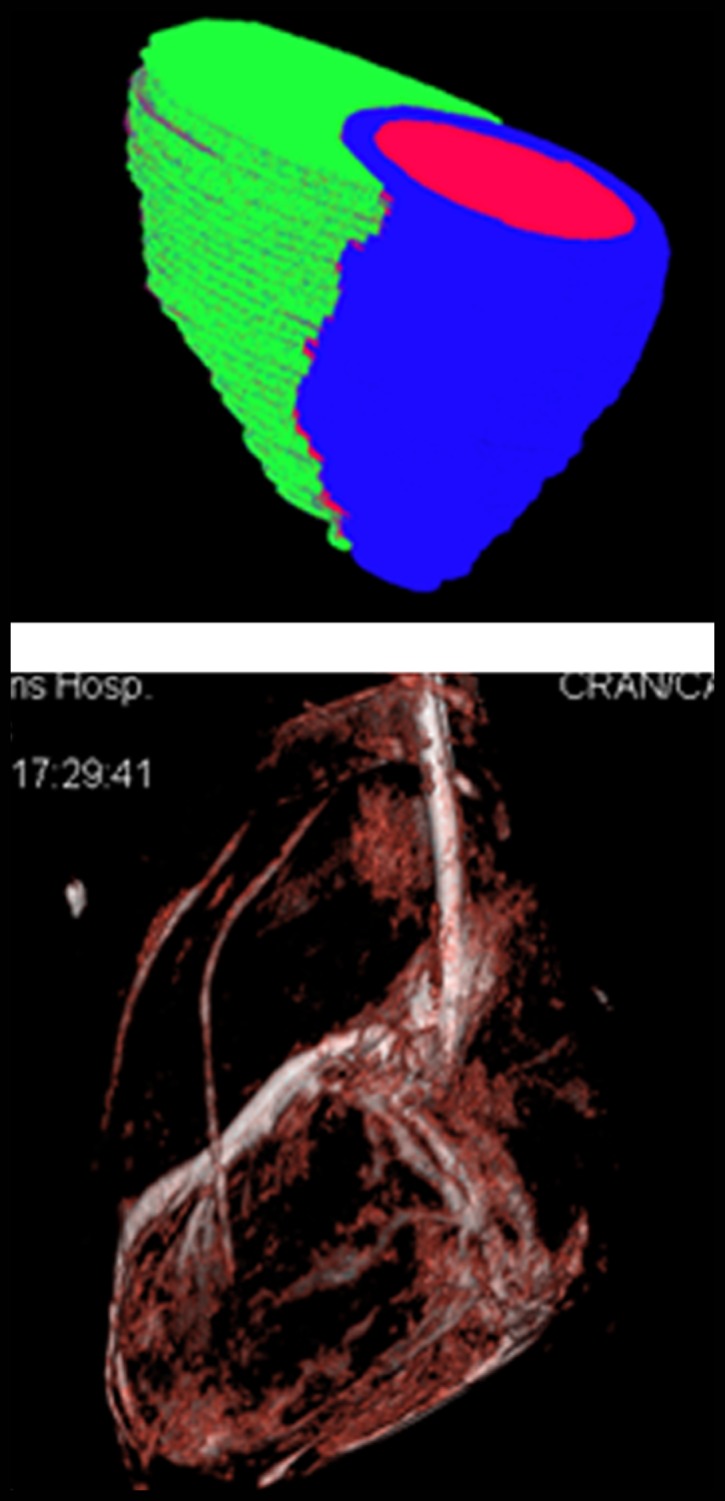
Imaging workflow of x-ray/MR imaging for intrapericardial delivery. (a) Navigator-gated 3D whole-heart MR image (top) was obtained to define ventricular borders for intrapericardial injection, followed by cardiac-gated C-arm CT image (bottom). (b) 3D registration of whole-heart MR imaging and C-arm CT was performed, followed by (c) surface rendering of the ventricles from MR imaging (top) and volume-rendered C-arm CT (bottom) overlaid on (d) a live x-ray fluoroscopic image (top) and fused image (bottom) to guide percutaneous access to the pericardial space.
Figure 1d:
Imaging workflow of x-ray/MR imaging for intrapericardial delivery. (a) Navigator-gated 3D whole-heart MR image (top) was obtained to define ventricular borders for intrapericardial injection, followed by cardiac-gated C-arm CT image (bottom). (b) 3D registration of whole-heart MR imaging and C-arm CT was performed, followed by (c) surface rendering of the ventricles from MR imaging (top) and volume-rendered C-arm CT (bottom) overlaid on (d) a live x-ray fluoroscopic image (top) and fused image (bottom) to guide percutaneous access to the pericardial space.
Intrapericardial Delivery of BaCaps
Animals were randomized to receive the same volume of empty BaCaps (approximately 10 mL), naked hMSCs (1 × 108 cells), BaCaps with hMSCs (8 × 107 cells), or saline intrapericardially with x-ray/MR imaging guidance (n = 8, Table E1 [online]). In addition, three subacute studies (one with empty BaCaps and two with BaCaps containing hMSCs) were performed without x-ray/MR imaging guidance, subsequent to the acute and several subacute studies in which x-ray/MR imaging was used. The injections were performed by an experienced cardiologist (P.V.J., with 13 years of experience in interventional cardiology) by using a subxiphoid percutaneous approach. Briefly, a 17-gauge Touhy needle was advanced into the pericardial space, taking care to avoid the epicardial coronary vasculature on the basis of the InSpace fusion display. A 0.035-inch guidewire was advanced into the pericardial space, and the Touhy needle was exchanged for a 4-F vascular sheath. Intrapericardial injections were then performed during digital subtraction angiography (90 kV, 520 mA, 15 frames per second). The sheath was then removed, and breath-hold, cardiac-gated, C-arm CT was repeated without iodinated contrast material administration. In one animal, C-arm CT was repeated after the animal was placed in the prone position.
Follow-up Examination
Follow-up MR imaging and C-arm CT were performed at 1 week after delivery with general anesthesia to assess pericardial integrity, cardiac function, and BaCaps persistence in eight pigs. After imaging, the animals were euthanized humanely. The heart and pericardium were exposed through a median sternotomy for gross anatomic evaluation, including the presence or absence of pericardial effusion. The heart was then removed, sectioned, and paraffin embedded.
Left Ventricular Function Assessment
Left ventricular ejection fraction at baseline and 1 week after delivery was determined from tracing the endocardial borders at end-diastole and end-systole on the cine MR images by using the vendor software (Argus; Siemens). Left ventricular ejection fraction was compared between baseline and 1-week follow-up.
Immunohistopathologic Analysis
Hematoxylin-eosin (H-E), trichrome, and periodic acid Schiff staining was performed in consecutive heart tissue sections (5 μm) to evaluate the inflammatory response, collagen deposition, and persistence of the BaCaps, respectively. For animals that received hMSCs, digital photomicrographs of the epicardial region adjacent to the hMSC injections stained with human nuclear antigen (HuNA; Millipore, Billerica, Mass) were evaluated for the presence of hMSCs.
Statistical Analysis
All data are presented as means ± standard deviations. Ventricular function between baseline and at 1 week after delivery was evaluated by using a paired Student t test. Comparison of thrombogenicity between empty BaCaps, BaCaps containing hMSCs, and porcine blood was performed by using one-way analysis of variance (SPSS statistical software, version 21.0; SPSS, Armonk, NY). A value of P < .05 was considered to indicate a significant difference.
Results
hMSC Encapsulation and in Vitro Characterization
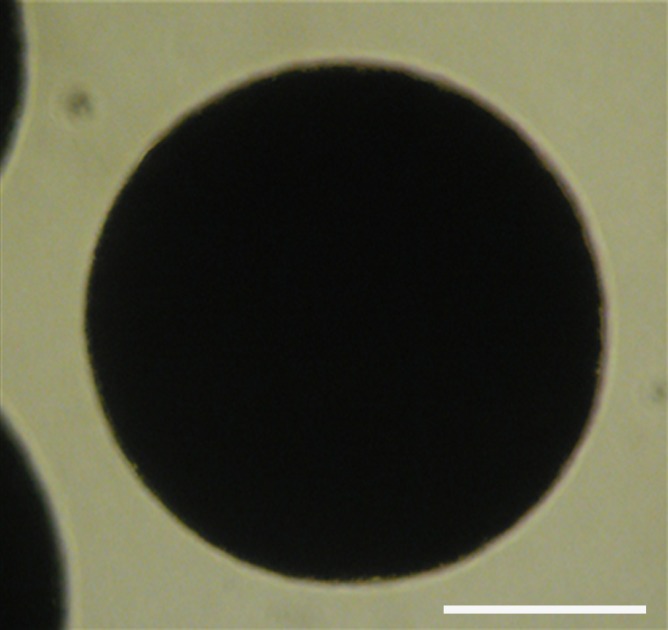
BaCaps were uniform in size, with a mean diameter ± standard deviation of 257 μm ± 9. Compared with alginate microcapsules, which are transparent, BaCaps were homogeneously opaque (Fig 2a, 2b). In vitro viability assessment of BaCaps with hMSCs yielded high cell survival (92% ± 5) immediately after encapsulation. Prior to delivery, cell viability was maintained (94.8% ± 6; P = 0.78) at 2 days afer encapsulation (Fig 2c).
Figure 2a:
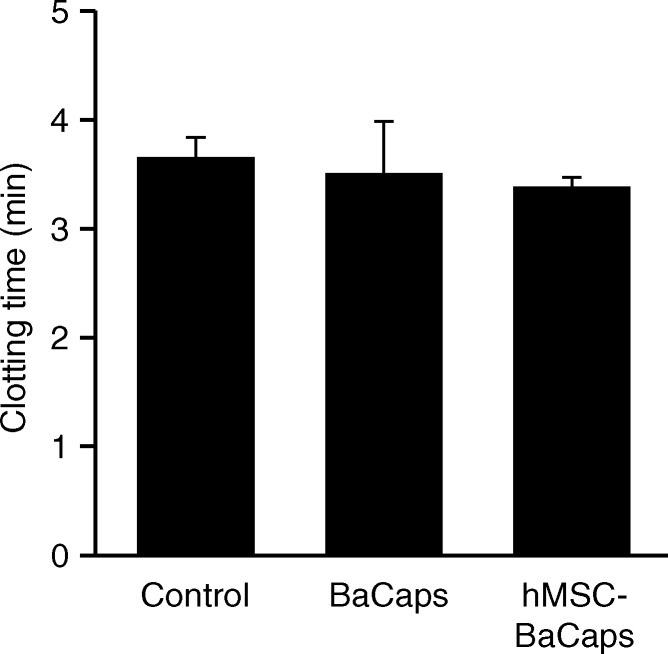
hMSC microencapsulation. (a) Macroscopic image of BaCaps. (b) Microscopic image of BaCaps (original magnification, ×10). (c) Viability staining (original magnification, ×10) of encapsulated hMSCs was performed with calcein (green, live cells) and propidium iodide (red, dead cells). Bars = 100 μm. (d) The time to clot formation shows no significant difference among porcine whole blood, empty BaCaps, and BaCaps that contained hMSCs.
Figure 2b:
hMSC microencapsulation. (a) Macroscopic image of BaCaps. (b) Microscopic image of BaCaps (original magnification, ×10). (c) Viability staining (original magnification, ×10) of encapsulated hMSCs was performed with calcein (green, live cells) and propidium iodide (red, dead cells). Bars = 100 μm. (d) The time to clot formation shows no significant difference among porcine whole blood, empty BaCaps, and BaCaps that contained hMSCs.
Figure 2c:
hMSC microencapsulation. (a) Macroscopic image of BaCaps. (b) Microscopic image of BaCaps (original magnification, ×10). (c) Viability staining (original magnification, ×10) of encapsulated hMSCs was performed with calcein (green, live cells) and propidium iodide (red, dead cells). Bars = 100 μm. (d) The time to clot formation shows no significant difference among porcine whole blood, empty BaCaps, and BaCaps that contained hMSCs.
For thrombogenicity testing, no significant difference in clotting time was observed between whole porcine blood (3.7 minutes ± 0.2), empty BaCaps (3.5 minutes ± 0.5), and BaCaps with hMSCs (3.4 minutes ± 0.1) (Fig 2d).
Figure 2d:
hMSC microencapsulation. (a) Macroscopic image of BaCaps. (b) Microscopic image of BaCaps (original magnification, ×10). (c) Viability staining (original magnification, ×10) of encapsulated hMSCs was performed with calcein (green, live cells) and propidium iodide (red, dead cells). Bars = 100 μm. (d) The time to clot formation shows no significant difference among porcine whole blood, empty BaCaps, and BaCaps that contained hMSCs.
X-ray/MR Imaging Guidance of hMSC Delivery into the Pericardial Space
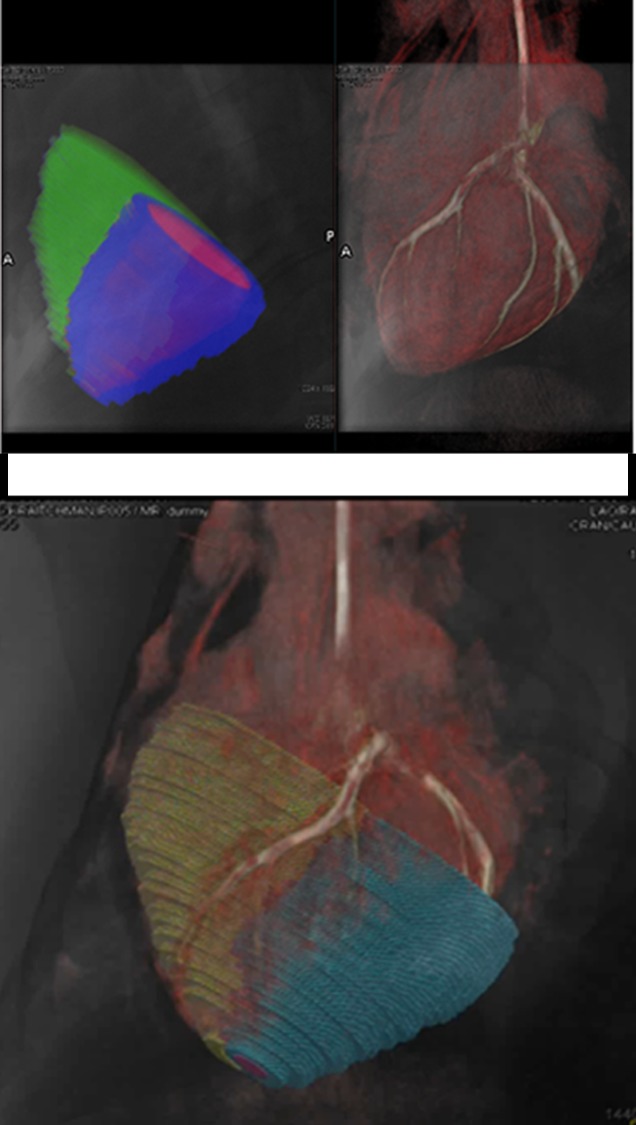
Intrapericardial access was obtained successfully in all 11 animals. With x-ray/MR imaging guidance, the increased soft-tissue visibility from MR imaging and C-arm CT enabled precise positioning of the needle in the pericardial space while avoiding the coronary vasculature, as compared with standard x-ray fluoroscopy (Fig 3a, 3b; Movie 3 [online]). C-arm CT images showed the presence of BaCaps in the pericardial space immediately after injection (Fig 3c). Immediately after injection, the capsules were free floating, as noted by the tendency to accumulate into the dependent portion of the heart—for example, the heart base when the pig was supine and the apex when the pig was prone (Movie 4 [online]). At the 1-week follow-up examination, BaCaps visibility was preserved on x-ray fluoroscopic images and C-arm CT images (Fig 3d). At 1 week after administration, BaCaps formed a tissuelike patch that adhered to the epicardium but not the pericardium (Fig 4a, 4b). BaCaps were not grossly visible in other organs. Careful visual inspection of the hearts of animals that were implanted with empty BaCaps or hMSC-containing BaCaps showed white opaque clumps of microcapsules that were still intact (Fig 4a, 4b). Importantly, no apparent pericardial effusion and adhesion were observed at follow-up MR imaging for the animals that underwent x-ray/MR image–guided delivery (Fig 5a, 5b; Fig E2A, E2B [online]).
Figure 3a:
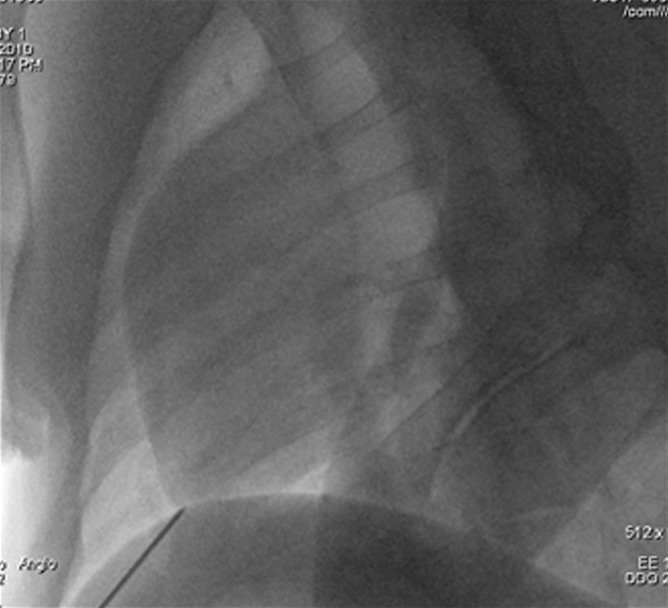
Images show intrapericardial delivery via x-ray/MR imaging or x-ray–only guidance. (a) Fluoroscopically guided pericardial puncture shows the lack of visualization of coronary vasculature and myocardial borders. (b) Image obtained with x-ray/MR imaging (gray scale indicates the x-ray portion of the image, and color indicates MR imaging) of the pig heart shows the coronary vasculature and ventricular boundaries. (c) C-arm CT image of the heart obtained immediately after BaCaps delivery demonstrates the presence of free-floating opaque BaCaps (arrow) in the pericardial space. (d) C-arm CT image in the same animal as in c shows the distribution of the BaCaps (arrows) 1 week after transplantation.
Figure 3b:
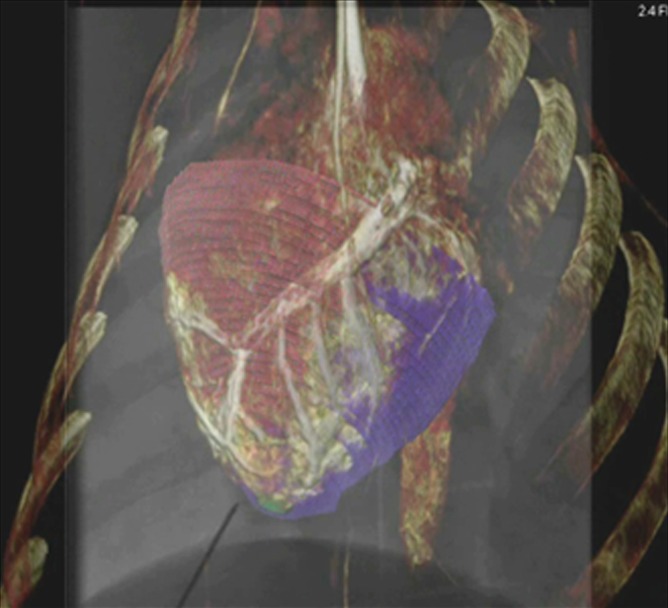
Images show intrapericardial delivery via x-ray/MR imaging or x-ray–only guidance. (a) Fluoroscopically guided pericardial puncture shows the lack of visualization of coronary vasculature and myocardial borders. (b) Image obtained with x-ray/MR imaging (gray scale indicates the x-ray portion of the image, and color indicates MR imaging) of the pig heart shows the coronary vasculature and ventricular boundaries. (c) C-arm CT image of the heart obtained immediately after BaCaps delivery demonstrates the presence of free-floating opaque BaCaps (arrow) in the pericardial space. (d) C-arm CT image in the same animal as in c shows the distribution of the BaCaps (arrows) 1 week after transplantation.
Figure 3c:
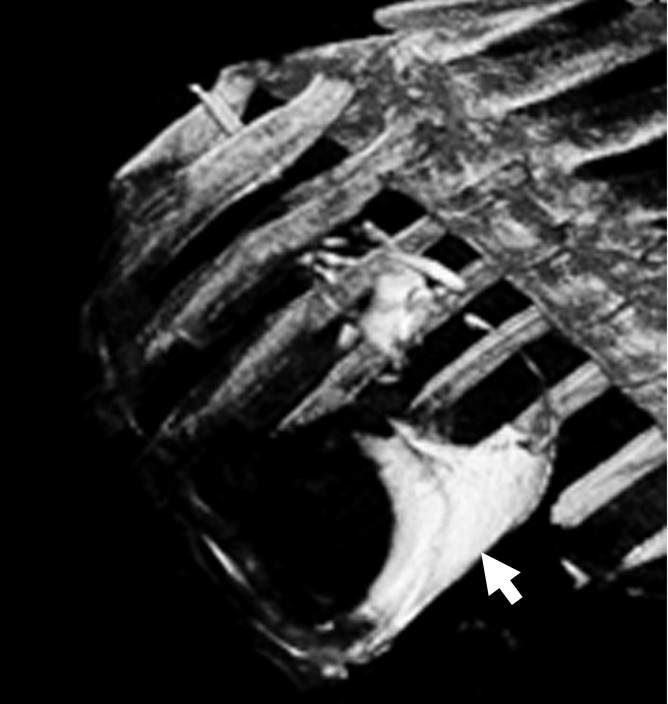
Images show intrapericardial delivery via x-ray/MR imaging or x-ray–only guidance. (a) Fluoroscopically guided pericardial puncture shows the lack of visualization of coronary vasculature and myocardial borders. (b) Image obtained with x-ray/MR imaging (gray scale indicates the x-ray portion of the image, and color indicates MR imaging) of the pig heart shows the coronary vasculature and ventricular boundaries. (c) C-arm CT image of the heart obtained immediately after BaCaps delivery demonstrates the presence of free-floating opaque BaCaps (arrow) in the pericardial space. (d) C-arm CT image in the same animal as in c shows the distribution of the BaCaps (arrows) 1 week after transplantation.
Figure 3d:
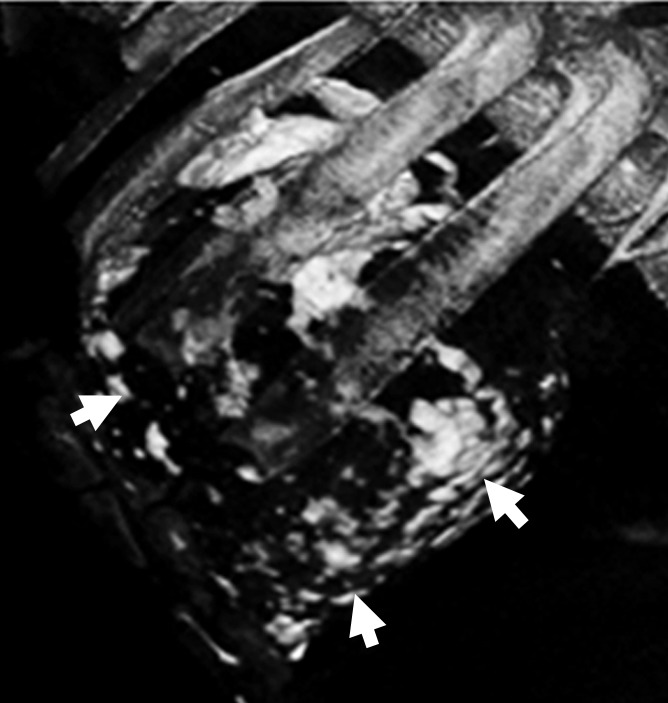
Images show intrapericardial delivery via x-ray/MR imaging or x-ray–only guidance. (a) Fluoroscopically guided pericardial puncture shows the lack of visualization of coronary vasculature and myocardial borders. (b) Image obtained with x-ray/MR imaging (gray scale indicates the x-ray portion of the image, and color indicates MR imaging) of the pig heart shows the coronary vasculature and ventricular boundaries. (c) C-arm CT image of the heart obtained immediately after BaCaps delivery demonstrates the presence of free-floating opaque BaCaps (arrow) in the pericardial space. (d) C-arm CT image in the same animal as in c shows the distribution of the BaCaps (arrows) 1 week after transplantation.
Figure 4a:
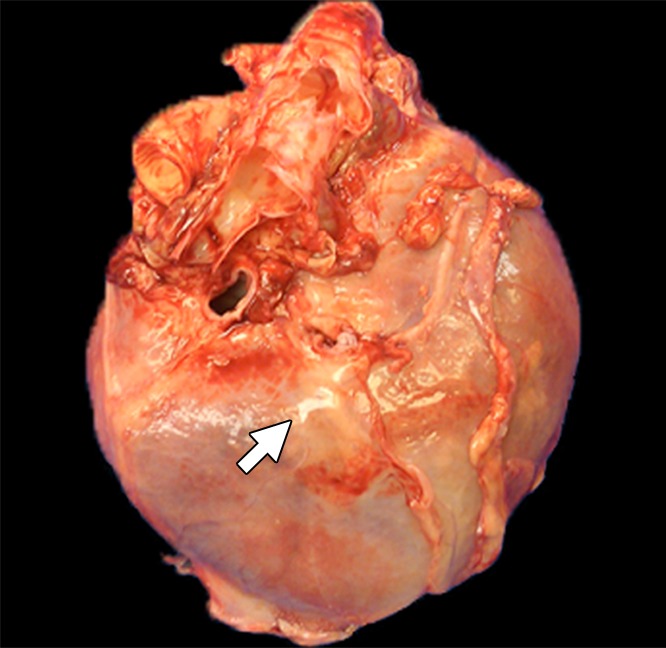
Photographs depict the postmortem analysis of the hearts that underwent x-ray/MR imaging or x-ray–guided intrapericardial delivery. (a, b) Ex vivo gross digital images of the heart with (a) and without (b) the pericardial sac 1 week after x-ray/MR imaging–guided delivery show the pseudotissue patch created by the microcapsules on the epicardial surface (arrows). (c) Digital image of the heart ex vivo 1 week after x-ray–guided intrapericardial delivery demonstrates hemorrhage secondary to pericardial delivery (arrow). (d) A short-axis heart slice obtained after formalin fixation demonstrates marked epicardial thickening 1 week after delivery and the presence of BaCaps (white arrow) and hemorrhage (black arrow) in an animal that underwent delivery without x-ray/MR imaging guidance.
Figure 4b:
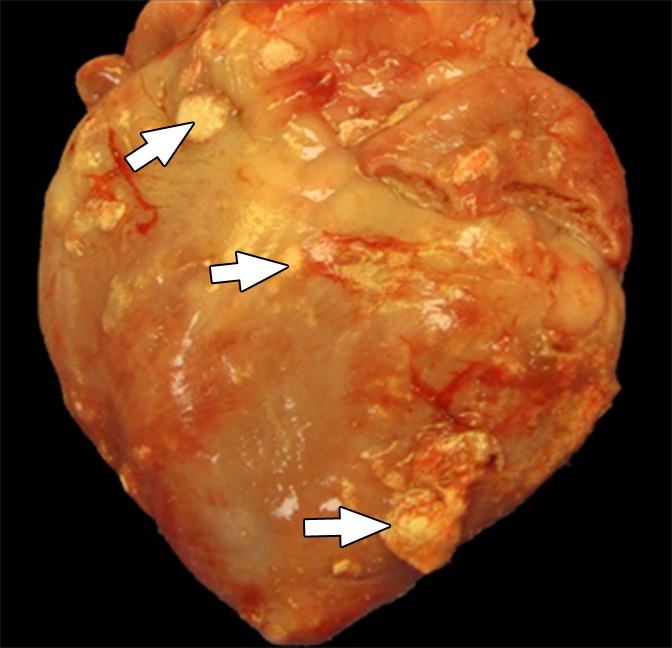
Photographs depict the postmortem analysis of the hearts that underwent x-ray/MR imaging or x-ray–guided intrapericardial delivery. (a, b) Ex vivo gross digital images of the heart with (a) and without (b) the pericardial sac 1 week after x-ray/MR imaging–guided delivery show the pseudotissue patch created by the microcapsules on the epicardial surface (arrows). (c) Digital image of the heart ex vivo 1 week after x-ray–guided intrapericardial delivery demonstrates hemorrhage secondary to pericardial delivery (arrow). (d) A short-axis heart slice obtained after formalin fixation demonstrates marked epicardial thickening 1 week after delivery and the presence of BaCaps (white arrow) and hemorrhage (black arrow) in an animal that underwent delivery without x-ray/MR imaging guidance.
Figure 5a:

MR images of the hearts that underwent x-ray/MR imaging or x-ray–guided intrapericardial delivery. (a, b) Short-axis MR images of the heart at baseline (a) and 1 week (b) after delivery of BaCaps containing hMSCs with x-ray/MR imaging guidance show no pericardial effusion or adhesion. (c, d) Short-axis cardiac MR images show pericardial enhancement (arrows), indicative of pericardial thickening 1 week after delivery in an animal that received BaCaps with an injection guided with x-ray only (d) as compared with baseline (c).
Figure 5b:
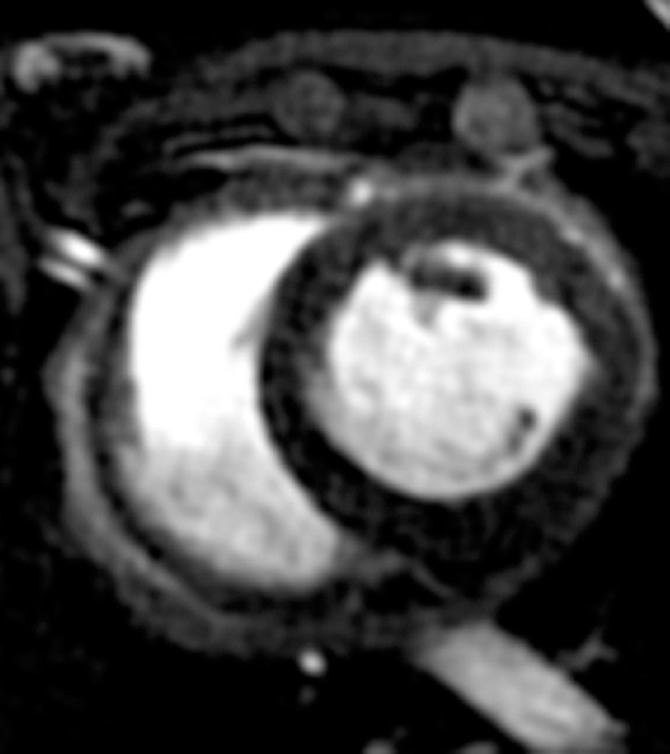
MR images of the hearts that underwent x-ray/MR imaging or x-ray–guided intrapericardial delivery. (a, b) Short-axis MR images of the heart at baseline (a) and 1 week (b) after delivery of BaCaps containing hMSCs with x-ray/MR imaging guidance show no pericardial effusion or adhesion. (c, d) Short-axis cardiac MR images show pericardial enhancement (arrows), indicative of pericardial thickening 1 week after delivery in an animal that received BaCaps with an injection guided with x-ray only (d) as compared with baseline (c).
Intrapericardial Delivery of hMSC without X-ray/MR Imaging
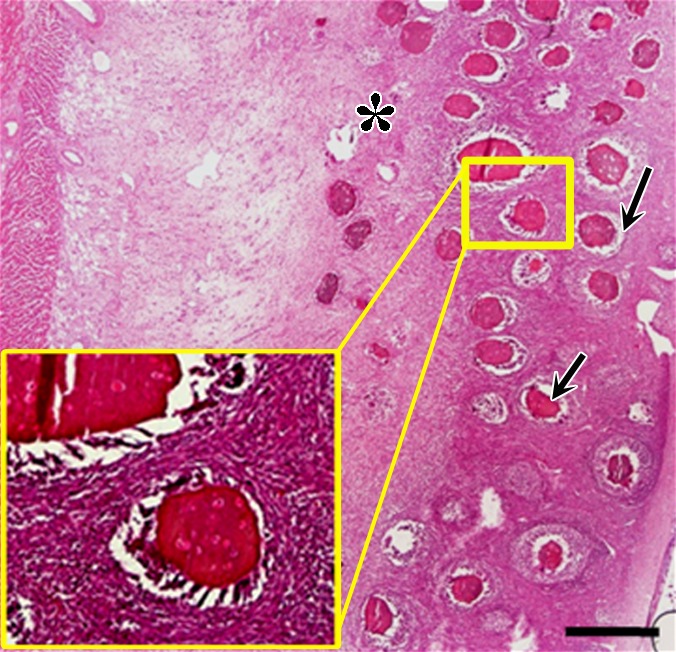
In contradistinction, x-ray–guided intrapericardial delivery without x-ray/MR imaging was challenging because of the lack of visibility of coronary vasculature and myocardial borders (Fig 3a). In two-thirds of these studies, a skilled cardiac interventionalist (P.V.J.) sensed that accidental ventricular puncture had occurred without frank development of hemopericardium during delivery. Gross postmortem examination of the hearts 1 week after delivery showed organized hematomas (Fig 4c) and severe epicardial thickening (Fig 4d), while the microcapsule integrity appeared to be preserved (Fig E1 [online]). These findings were consistent with the presence of signal hyperintensity—that is, pericardial fibrosis and/or effusion—on MR images at 1-week follow-up (Fig 5c, 5d; Fig E2C, E2D [online]). Nonetheless, no animals showed clinical signs of pericardial compromise 1 week after delivery.
Figure 4c:

Photographs depict the postmortem analysis of the hearts that underwent x-ray/MR imaging or x-ray–guided intrapericardial delivery. (a, b) Ex vivo gross digital images of the heart with (a) and without (b) the pericardial sac 1 week after x-ray/MR imaging–guided delivery show the pseudotissue patch created by the microcapsules on the epicardial surface (arrows). (c) Digital image of the heart ex vivo 1 week after x-ray–guided intrapericardial delivery demonstrates hemorrhage secondary to pericardial delivery (arrow). (d) A short-axis heart slice obtained after formalin fixation demonstrates marked epicardial thickening 1 week after delivery and the presence of BaCaps (white arrow) and hemorrhage (black arrow) in an animal that underwent delivery without x-ray/MR imaging guidance.
Figure 4d:
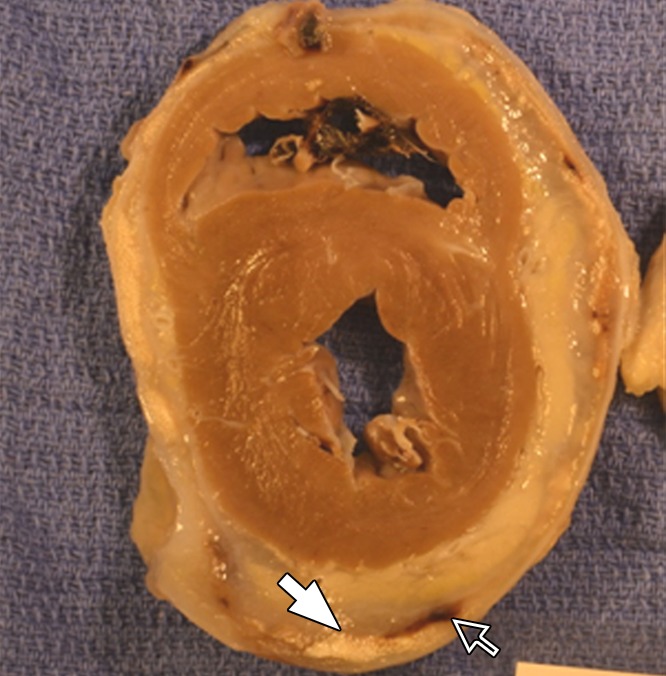
Photographs depict the postmortem analysis of the hearts that underwent x-ray/MR imaging or x-ray–guided intrapericardial delivery. (a, b) Ex vivo gross digital images of the heart with (a) and without (b) the pericardial sac 1 week after x-ray/MR imaging–guided delivery show the pseudotissue patch created by the microcapsules on the epicardial surface (arrows). (c) Digital image of the heart ex vivo 1 week after x-ray–guided intrapericardial delivery demonstrates hemorrhage secondary to pericardial delivery (arrow). (d) A short-axis heart slice obtained after formalin fixation demonstrates marked epicardial thickening 1 week after delivery and the presence of BaCaps (white arrow) and hemorrhage (black arrow) in an animal that underwent delivery without x-ray/MR imaging guidance.
Figure 5c:
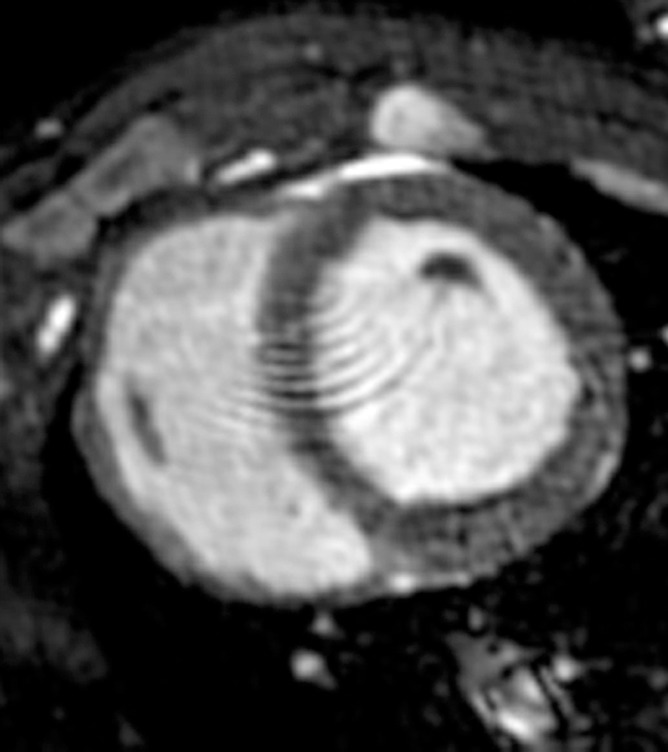
MR images of the hearts that underwent x-ray/MR imaging or x-ray–guided intrapericardial delivery. (a, b) Short-axis MR images of the heart at baseline (a) and 1 week (b) after delivery of BaCaps containing hMSCs with x-ray/MR imaging guidance show no pericardial effusion or adhesion. (c, d) Short-axis cardiac MR images show pericardial enhancement (arrows), indicative of pericardial thickening 1 week after delivery in an animal that received BaCaps with an injection guided with x-ray only (d) as compared with baseline (c).
Figure 5d:
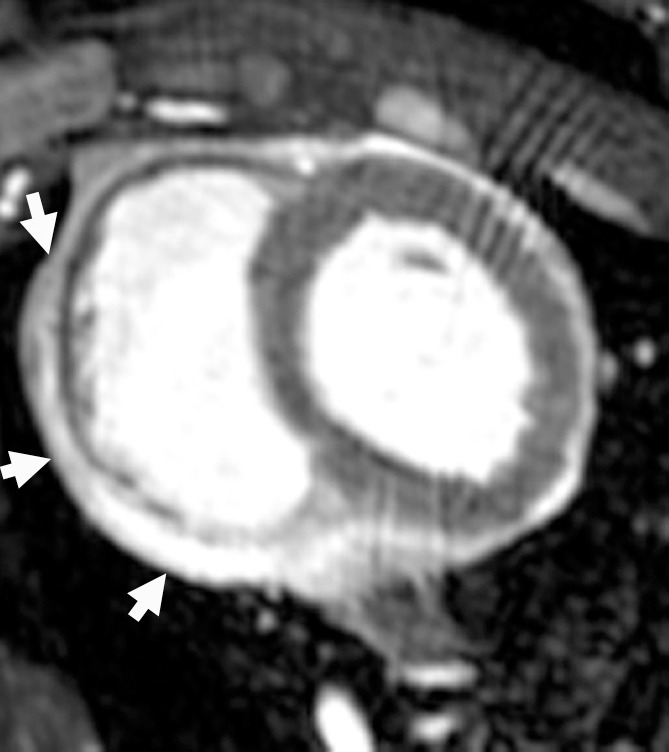
MR images of the hearts that underwent x-ray/MR imaging or x-ray–guided intrapericardial delivery. (a, b) Short-axis MR images of the heart at baseline (a) and 1 week (b) after delivery of BaCaps containing hMSCs with x-ray/MR imaging guidance show no pericardial effusion or adhesion. (c, d) Short-axis cardiac MR images show pericardial enhancement (arrows), indicative of pericardial thickening 1 week after delivery in an animal that received BaCaps with an injection guided with x-ray only (d) as compared with baseline (c).
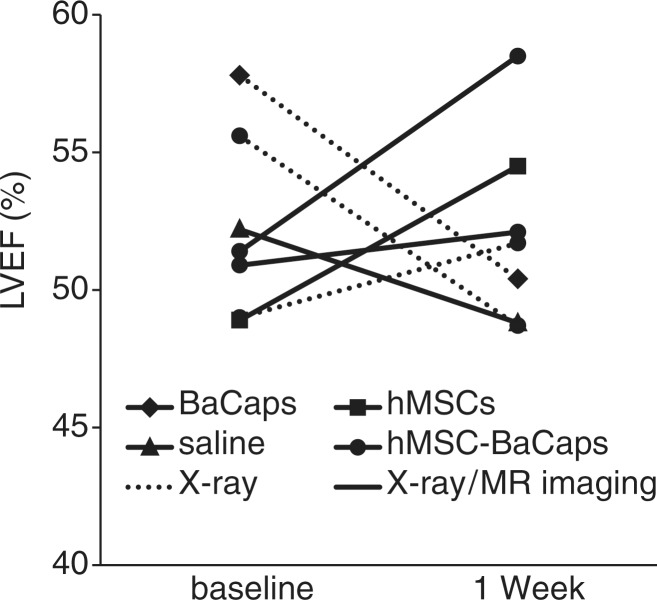
In all animals, cardiac function was preserved 1 week after delivery (left ventricular ejection fraction: 50.8% ± 4 at baseline vs 53.1% ± 3 at 1 week; n = 7; P = .932; Fig 6). However, a decline in ejection fraction was noted in two of the three subacute animals without x-ray/MR imaging–guided delivery. Blood counts were within normal limits for all animals.
Figure 6:
Graph shows the effect of intrapericardial delivery cardiac function. Left ventricular ejection fraction (LVEF) of the heart measured with MR imaging was maintained 1 week after delivery (n = 7, P = .932). Solid lines = x-ray/MR imaging–guided delivery, dotted lines = x-ray–guided delivery without x-ray/MR imaging.
Histologic Studies
Histologic analysis of H-E–stained heart sections demonstrated moderate fibroplasia 1 week after x-ray/MR imaging–guided delivery of BaCaps with hMSCs (Fig 7a). No foreign-body reaction was observed. Trichrome staining (Fig E3A [online]) of an adjacent section showed a slight deposition of collagen on the epicardial surface adjacent to where BaCaps containing hMSCs were, while periodic acid Schiff staining (Fig E3B [online]) showed the integrity of the microcapsules and the lack of glycogen formation. Similarly, minimal fibrosis was observed for animals that received empty BaCaps (Fig 7b) as compared with those that received naked hMSCs (Fig E3C [online]) or saline (Fig E3D [online]) with x-ray/MR imaging guidance.
Figure 7a:
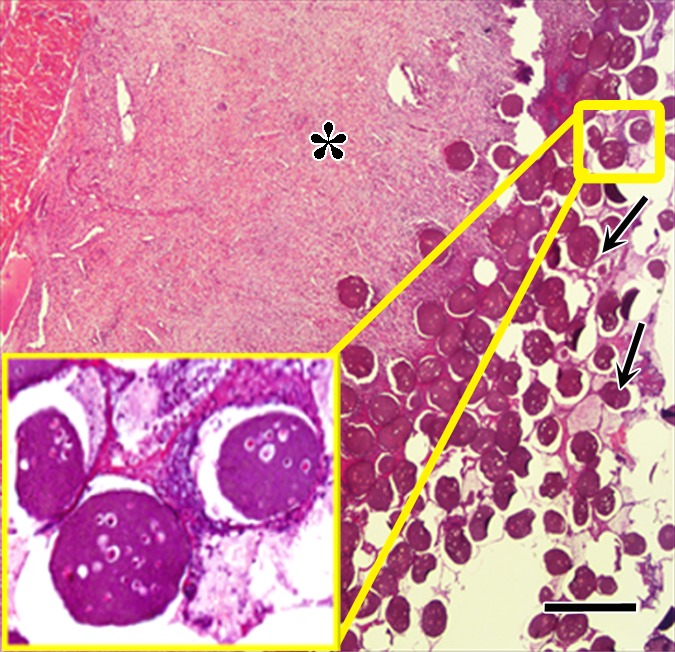
Photomicrographs demonstrate histopathologic analysis and hMSC retention in the heart. (a) H-E staining of the heart (original magnification, ×2) 1 week after x-ray/MR imaging–guided delivery of BaCaps with hMSCs (arrows). Insert shows viable hMSCs, with clear nuclear morphology and absence of a foreign-body reaction. Subjacent to the BaCaps is a moderate bed of fibroplasia and capillaries. (b) H-E staining of the heart (original magnification, ×2) after receiving empty BaCaps (arrows) with x-ray/MR imaging guidance. The higher-magnification insert shows representative empty BaCaps and absence of a foreign-body reaction. (c) H-E staining of the heart (original magnification, ×2) after receiving x-ray–guided delivery of BaCaps with hMSCs without x-ray/MR imaging. Note the formation of severe fibrosis and inflammatory infiltrates around the microcapsules 1 week after delivery. (d) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows the presence of viable hMSCs (green) within BaCaps that adhered on the epicardial surface 1 week after delivery. Blue represents cell nuclei stained with 4’,6-diamidino-2-phenylindole, or DAPI. (e) Human nuclear antigen staining of the heart (original magnification, ×40) after receiving naked hMSCs shows no detectable hMSCs (ie, green fluorescence) 1 week after delivery. Only autofluorescence (yellow areas [arrows]) in the myocardium is observed. (f) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows no detectable viable hMSCs inside the microcapsules. Bars in a–c = 1 mm, and bars in d–f = 200 μm. * = epicardium.
Figure 7b:
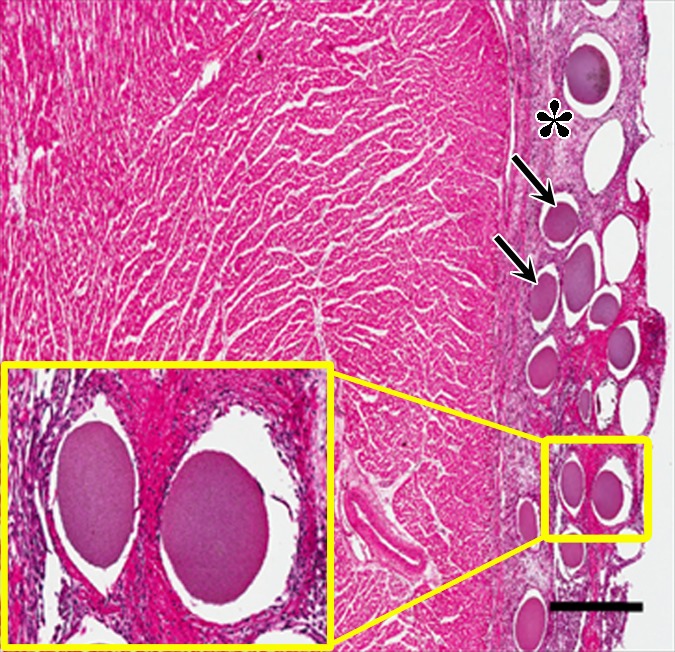
Photomicrographs demonstrate histopathologic analysis and hMSC retention in the heart. (a) H-E staining of the heart (original magnification, ×2) 1 week after x-ray/MR imaging–guided delivery of BaCaps with hMSCs (arrows). Insert shows viable hMSCs, with clear nuclear morphology and absence of a foreign-body reaction. Subjacent to the BaCaps is a moderate bed of fibroplasia and capillaries. (b) H-E staining of the heart (original magnification, ×2) after receiving empty BaCaps (arrows) with x-ray/MR imaging guidance. The higher-magnification insert shows representative empty BaCaps and absence of a foreign-body reaction. (c) H-E staining of the heart (original magnification, ×2) after receiving x-ray–guided delivery of BaCaps with hMSCs without x-ray/MR imaging. Note the formation of severe fibrosis and inflammatory infiltrates around the microcapsules 1 week after delivery. (d) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows the presence of viable hMSCs (green) within BaCaps that adhered on the epicardial surface 1 week after delivery. Blue represents cell nuclei stained with 4’,6-diamidino-2-phenylindole, or DAPI. (e) Human nuclear antigen staining of the heart (original magnification, ×40) after receiving naked hMSCs shows no detectable hMSCs (ie, green fluorescence) 1 week after delivery. Only autofluorescence (yellow areas [arrows]) in the myocardium is observed. (f) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows no detectable viable hMSCs inside the microcapsules. Bars in a–c = 1 mm, and bars in d–f = 200 μm. * = epicardium.
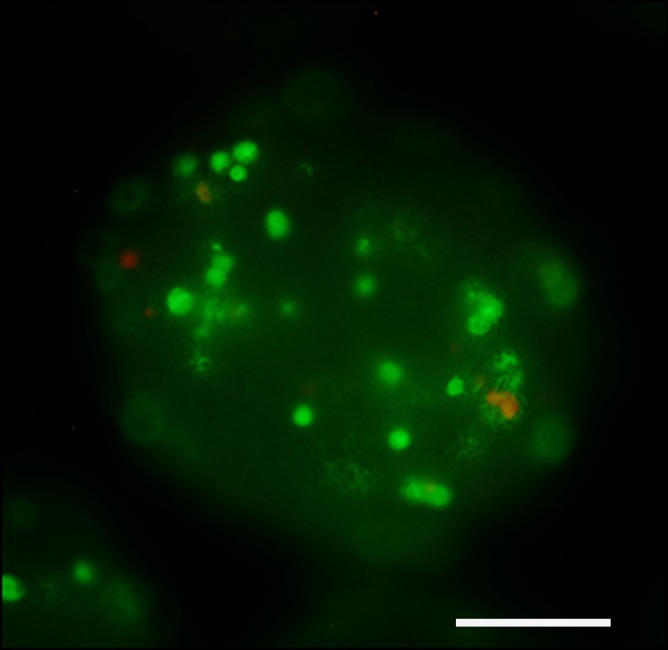
One week after delivery of BaCaps-containing hMSCs, immunohistochemical staining for human nuclear antigen yielded positive results, demonstrating the presence and retention of hMSCs within the microcapsules (Fig 7d), whereas naked hMSCs were not detected (Fig 7e).
Figure 7d:
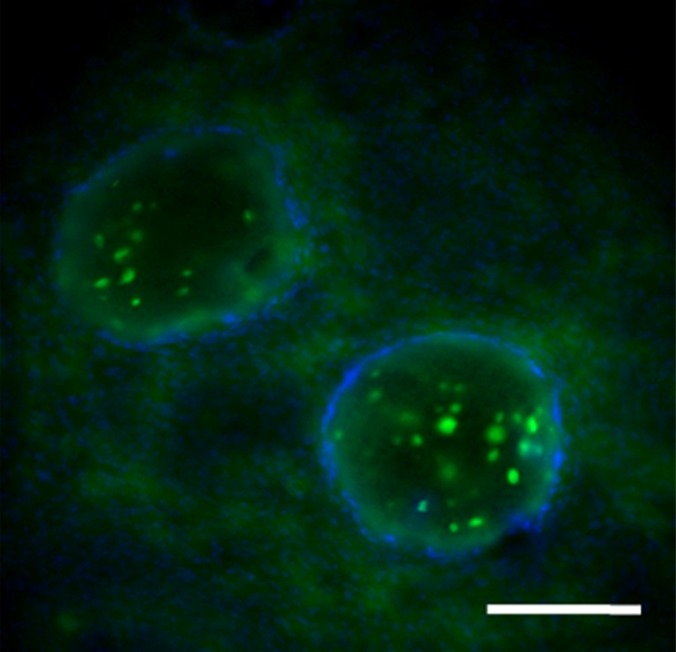
Photomicrographs demonstrate histopathologic analysis and hMSC retention in the heart. (a) H-E staining of the heart (original magnification, ×2) 1 week after x-ray/MR imaging–guided delivery of BaCaps with hMSCs (arrows). Insert shows viable hMSCs, with clear nuclear morphology and absence of a foreign-body reaction. Subjacent to the BaCaps is a moderate bed of fibroplasia and capillaries. (b) H-E staining of the heart (original magnification, ×2) after receiving empty BaCaps (arrows) with x-ray/MR imaging guidance. The higher-magnification insert shows representative empty BaCaps and absence of a foreign-body reaction. (c) H-E staining of the heart (original magnification, ×2) after receiving x-ray–guided delivery of BaCaps with hMSCs without x-ray/MR imaging. Note the formation of severe fibrosis and inflammatory infiltrates around the microcapsules 1 week after delivery. (d) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows the presence of viable hMSCs (green) within BaCaps that adhered on the epicardial surface 1 week after delivery. Blue represents cell nuclei stained with 4’,6-diamidino-2-phenylindole, or DAPI. (e) Human nuclear antigen staining of the heart (original magnification, ×40) after receiving naked hMSCs shows no detectable hMSCs (ie, green fluorescence) 1 week after delivery. Only autofluorescence (yellow areas [arrows]) in the myocardium is observed. (f) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows no detectable viable hMSCs inside the microcapsules. Bars in a–c = 1 mm, and bars in d–f = 200 μm. * = epicardium.
Figure 7e:
Photomicrographs demonstrate histopathologic analysis and hMSC retention in the heart. (a) H-E staining of the heart (original magnification, ×2) 1 week after x-ray/MR imaging–guided delivery of BaCaps with hMSCs (arrows). Insert shows viable hMSCs, with clear nuclear morphology and absence of a foreign-body reaction. Subjacent to the BaCaps is a moderate bed of fibroplasia and capillaries. (b) H-E staining of the heart (original magnification, ×2) after receiving empty BaCaps (arrows) with x-ray/MR imaging guidance. The higher-magnification insert shows representative empty BaCaps and absence of a foreign-body reaction. (c) H-E staining of the heart (original magnification, ×2) after receiving x-ray–guided delivery of BaCaps with hMSCs without x-ray/MR imaging. Note the formation of severe fibrosis and inflammatory infiltrates around the microcapsules 1 week after delivery. (d) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows the presence of viable hMSCs (green) within BaCaps that adhered on the epicardial surface 1 week after delivery. Blue represents cell nuclei stained with 4’,6-diamidino-2-phenylindole, or DAPI. (e) Human nuclear antigen staining of the heart (original magnification, ×40) after receiving naked hMSCs shows no detectable hMSCs (ie, green fluorescence) 1 week after delivery. Only autofluorescence (yellow areas [arrows]) in the myocardium is observed. (f) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows no detectable viable hMSCs inside the microcapsules. Bars in a–c = 1 mm, and bars in d–f = 200 μm. * = epicardium.
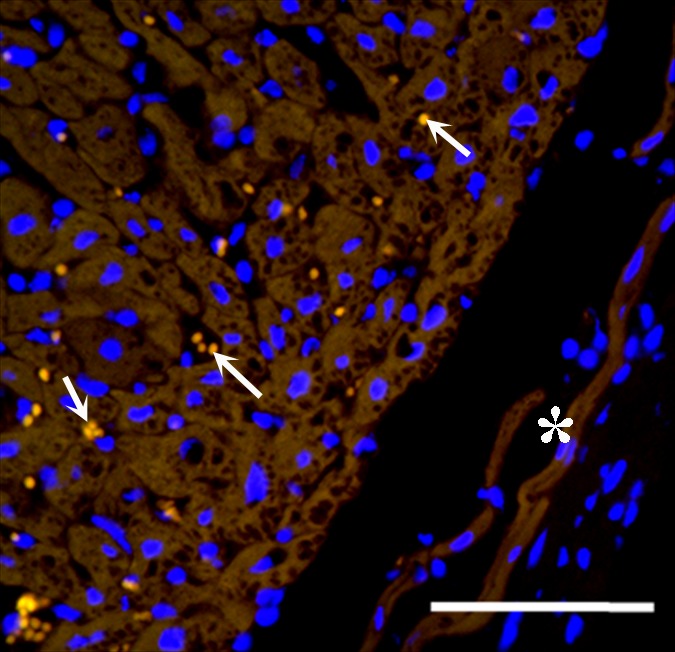
In contrast, animals that received BaCaps with hMSCs without x-ray/MR imaging guidance had severe fibrosis and showed inflammatory infiltrates with H-E staining of the heart 1 week after delivery (Fig 7c). In addition, human nuclear antigen staining showed that most encapsulated hMSCs were no longer alive (Fig 7f).
Figure 7c:
Photomicrographs demonstrate histopathologic analysis and hMSC retention in the heart. (a) H-E staining of the heart (original magnification, ×2) 1 week after x-ray/MR imaging–guided delivery of BaCaps with hMSCs (arrows). Insert shows viable hMSCs, with clear nuclear morphology and absence of a foreign-body reaction. Subjacent to the BaCaps is a moderate bed of fibroplasia and capillaries. (b) H-E staining of the heart (original magnification, ×2) after receiving empty BaCaps (arrows) with x-ray/MR imaging guidance. The higher-magnification insert shows representative empty BaCaps and absence of a foreign-body reaction. (c) H-E staining of the heart (original magnification, ×2) after receiving x-ray–guided delivery of BaCaps with hMSCs without x-ray/MR imaging. Note the formation of severe fibrosis and inflammatory infiltrates around the microcapsules 1 week after delivery. (d) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows the presence of viable hMSCs (green) within BaCaps that adhered on the epicardial surface 1 week after delivery. Blue represents cell nuclei stained with 4’,6-diamidino-2-phenylindole, or DAPI. (e) Human nuclear antigen staining of the heart (original magnification, ×40) after receiving naked hMSCs shows no detectable hMSCs (ie, green fluorescence) 1 week after delivery. Only autofluorescence (yellow areas [arrows]) in the myocardium is observed. (f) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows no detectable viable hMSCs inside the microcapsules. Bars in a–c = 1 mm, and bars in d–f = 200 μm. * = epicardium.
Figure 7f:
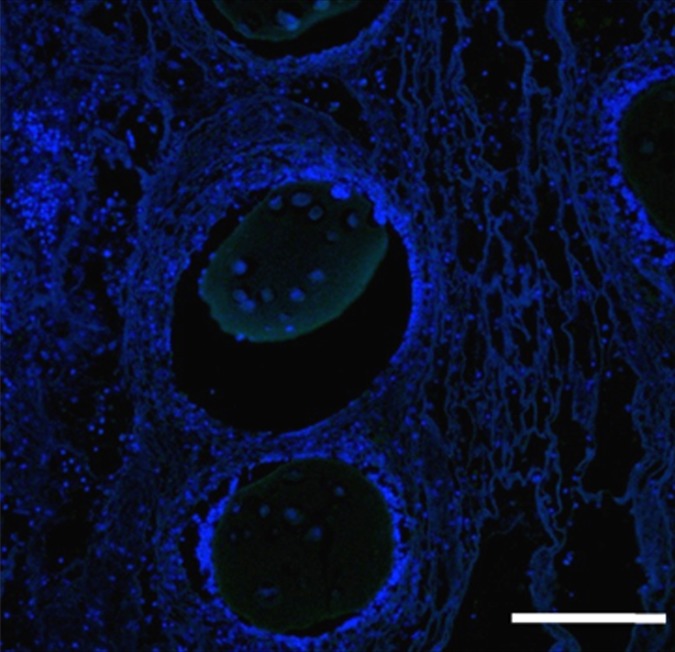
Photomicrographs demonstrate histopathologic analysis and hMSC retention in the heart. (a) H-E staining of the heart (original magnification, ×2) 1 week after x-ray/MR imaging–guided delivery of BaCaps with hMSCs (arrows). Insert shows viable hMSCs, with clear nuclear morphology and absence of a foreign-body reaction. Subjacent to the BaCaps is a moderate bed of fibroplasia and capillaries. (b) H-E staining of the heart (original magnification, ×2) after receiving empty BaCaps (arrows) with x-ray/MR imaging guidance. The higher-magnification insert shows representative empty BaCaps and absence of a foreign-body reaction. (c) H-E staining of the heart (original magnification, ×2) after receiving x-ray–guided delivery of BaCaps with hMSCs without x-ray/MR imaging. Note the formation of severe fibrosis and inflammatory infiltrates around the microcapsules 1 week after delivery. (d) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows the presence of viable hMSCs (green) within BaCaps that adhered on the epicardial surface 1 week after delivery. Blue represents cell nuclei stained with 4’,6-diamidino-2-phenylindole, or DAPI. (e) Human nuclear antigen staining of the heart (original magnification, ×40) after receiving naked hMSCs shows no detectable hMSCs (ie, green fluorescence) 1 week after delivery. Only autofluorescence (yellow areas [arrows]) in the myocardium is observed. (f) Human nuclear antigen staining of the heart (original magnification, ×10) after receiving BaCaps with hMSCs with x-ray/MR imaging guidance shows no detectable viable hMSCs inside the microcapsules. Bars in a–c = 1 mm, and bars in d–f = 200 μm. * = epicardium.
Discussion
In this study, we developed and evaluated an integrated approach for minimally invasive, localized stem cell delivery to the heart with improved cell retention. By using a large animal model, the feasibility and reliability of x-ray/MR imaging–guided intrapericardial delivery of microencapsulated hMSCs visible with x-rays was shown.
Compared with other local delivery techniques (ie, intramyocardial, intracoronary, or intravenous), intrapericardial delivery has several potential advantages in terms of safety and dose tolerance. Unlike transvascular infusions in which delivery efficiency can be affected by coronary patency, intrapericardial delivery can expose the entire epicardial surface to the delivered therapeutic material, regardless of vasculature status, while still limiting dispersion to other organs. Indeed, the pericardial sac has been used as a drug depot for treating pericardial diseases (24,25). Thus, the deposition of stem cells or other novel therapeutics in the pericardial space may offer a method to promote a longer dwell time of stem cells in close proximity to the myocardium for optimal induction of angiogenesis and myocardial regeneration via paracrine effects. There is growing evidence that direct regeneration of cardiomyocytes and vasculature from stem cells transplanted to the heart are limited, and that, instead, the beneficial effects of such cells are mediated through paracrine mechanisms by the secretion of cytoprotective factors and cytokines that induce resident stem cells homing to injured sites (26,27). Whether the diffusion of these factors from the pericardial space to the myocardium will be sufficient to induce a beneficial effect will need to be tested in future studies.
In clinical practice, pericardiocentesis in the presence of pathologic pericardial effusion, which significantly increases the pericardial space and makes cardiac perforation less likely, is routinely performed with ultrasonographic (US), fluoroscopic, or electrocardiographic guidance, with the latter primarily performed alone in emergency situations (28). Here, without pericardial effusion, the pericardium is immediately adjacent to the epicardium, leaving little room for error. While not tested rigorously, cardiac perforation and subsequent hemopericardium in all three non–x-ray/MR imaging studies resulted in a marked inflammatory reaction to the delivered microcapsules with ensuing pericardial adhesions. In contradistinction, there was no evidence of cardiac or coronary perforation in the x-ray/MR imaging–guided studies in normal pigs. In addition, in subacute studies, no pericardial effusion or adhesions were created by x-ray/MR imaging–guided pericardial delivery, and myocardial function was preserved. While used here to facilitate pericardial access, x-ray/MR imaging guidance could readily be adapted to other delivery paradigms in the heart and other organs for precise deposition of cellular therapeutics to the targeted regions, such as intramyocardial delivery to the infarct or its border zone or biopsy of cardiac masses.
Although stem cell therapy has shown promise in several clinical studies to improve cardiac function after myocardial infarction, cell retention and survival, even for autologous cells, is known to be low (29), which may significantly limit the therapeutic effects. Recently, 3D scaffolds—such as gelfoam, agarose gel (30), and collage matrix (31)—and alginate microcapsules (32) have been investigated for stem cell deposition into the myocardium, onto the heart surface, or into the pericardial sac to improve cell retention. In one study, Ladage et al demonstrated the successful transfer of autologous MSCs suspended in an absorbable sponge material to the pericardial space in a porcine myocardial infarction model (16) to enhance cell retention. However, the bioavailability of these cells may continue to decrease in response to host immune attack. Encapsulation of stem cells may help to increase cell retention while immunoprotecting cells within the heart. In fact, intramyocardial delivery of microencapsulated allogeneic MSCs showed the persistence of magnetically labeled alginate microcapsules (32). However, transmyocardial delivery of microcapsules resulted in a large inflammatory response with an abundance of phagocytic multinucleated giant cells. In the current study, the lack of foreign-body reaction bodes well for x-ray/MR image–guided intrapericardial delivery of BaCaps. In addition, impregnation of the microcapsules with barium sulfate allowed the evaluation of microcapsule persistence with x-ray imaging over time and demonstrated the change from free-floating capsules to engraftment of the hMSCs on the epicardial surface. To our knowledge, this is the first demonstration of xenogeneic cell survival in an immunocompetent large animal model after pericardial stem cell delivery by using microcapsules that are visible with imaging. Unlike other scaffolds, microcapsules can protect nonautologous cells from immunorejection. Compared with naked cells or scaffold materials, another potential advantage of microencapsulated cells is the prevention of cell migration or uptake by lymphatic drainage and clearance from the pericardial space. Our previous work has demonstrated the stability of barium sulfate–impregnated alginate microcapsules in vivo for at least 1 month (21) and the effectiveness in a rabbit peripheral arterial disease model (22). In barium-based oral contrast material–enhanced studies of the gastrointestinal tract, leaks of a small amount of barium outside the gastrointestinal tract have not resulted in severe long-term consequences, in part because of the poor water solubility of barium sulfate (33,34). In the current study, the total load of barium sulfate in the microcapsules was approximately 570 mg, which is a small fraction of the amount given for contrast-enhanced gastrointestinal studies. Nevertheless, given the short-term follow-up of 1 week in the current study, long-term stability and biocompatibility of these microcapsules, especially due to the presence of the heavy metal barium, would be warranted. An alternative biocompatible imaging contrast agent, such as perfluorooctylbromide (35), which can be cleared from the body by exhalation, may offer a similar ability to follow capsule persistence with CT or US. However, radiopacity during real-time delivery would be reduced.
The consolidation of the microcapsules on the epicardial surface in a pseudotissue patch without microscopic evidence of microcapsule rupture suggests that direct participation of the stem cells in cardiac repair would be limited in the first week. Previous work with a similar formulation of microencapsulated stem cells injected directly into skeletal muscle in an animal model of peripheral artery disease resulted in substantial local angiogenesis, which was attributed to paracrine effects, without fibrosis around the capsules at 2 weeks (22). We anticipate that a similar paracrine mechanism would be the primary contributing therapeutic process with intrapericardial delivery. These prior results, taken in concert with lack of inflammatory infiltrate or substantial collagen deposition at 1 week with x-ray/MR imaging delivery in the current study, would suggest that encapsulated MSCs may have a similar survival profile when delivered to the pericardial space. On the other hand, accidental puncture of the ventricle with the subsequent introduction of red blood and inflammatory cells into the pericardial space is associated with substantial fibrosis and poor stem cell survival. Further studies are necessary to determine the time frame over which encapsulated MSCs can survive and exert a beneficial effect on the heart when delivered to the pericardial space, and, furthermore, how these effects compare with naked cells administered via the pericardium or by using other delivery techniques.
The current x-ray/MR imaging technique does require the acquisition of both MR imaging and C-arm CT images for 3D-3D registration. For visualization, the segmentation of the MR imaging data set was the least automated and most time-consuming process. However, numerous software algorithms are available that could accelerate this process. In the current implementation, the volume overlays were only acquired in a single cardiac phase at end expiration. Thus, breathing and cardiac motion would result in misregistration with the live fluoroscopic images. During critical needle punctures, suspension of respiration in the appropriate phase, such as breath holds, can be used to minimize these registration errors. Despite these limitations of x-ray/MR imaging implementation, we have demonstrated for the first time the safety and feasibility of intrapericardial delivery with x-ray/MR imaging guidance of microencapsulated xenogeneic MSCs for the treatment of cardiac disease in nonimmunosuppressed animals to monitor and track cell retention. Furthermore, barium-alginate encapsulation of hMSCs improves cell retention compared with naked cells. Thus, this approach shows significant promise as a new, minimally invasive method for stem cell delivery by using standard clinical imaging equipment.
In summary, by using clinically available drug formulation and clinical angiographic and MR imaging units, x-ray/MR image–guided intrapericardial stem cell transplantation could be readily translatable and provide a safe, minimally invasive route to deliver cellular therapeutics in patients with ischemic cardiovascular disease.
Advance in Knowledge
■ X-ray fused with MR imaging (x-ray/MR imaging) for guiding delivery of microencapsulated stem cells to the pericardial space can be performed safely, with high cell retention and viability.
Implication for Patient Care
■ In patients with ischemic heart disease, x-ray/MR imaging–guided intrapericardial delivery of encapsulated stem cells may provide a novel route for cardiac regenerative therapy and enable tracking of engrafted encapsulated cells with conventional imaging systems.
APPENDIX
SUPPLEMENTAL FIGURES
SUPPLEMENTAL TABLE
SUPPLEMENTAL MOVIES
Acknowledgments
Acknowledgments
The authors thank Judith Cook and Jeremy Maurer for help with the animals and immunohistopathologic study.
Received July 17, 2013; revision requested September 17; revision received January 6, 2014; accepted January 21; final version accepted February 7.
Supported by Siemens Healthcare.
Funding: This research was supported by the National Institutes of Health (grant R21/R33-HL89029) and the Maryland Stem Cell Research Fund (grant MD-SCRFII-0399).
Disclosures of Conflicts of Interest: Y.F. No relevant conflicts of interest to disclose. N.A. No relevant conflicts of interest to disclose. T.E. Financial activities related to the present article: author is an employee of Siemens Healthcare. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. A.F. Financial activities related to the present article: author is an employee of Siemens Healthcare. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. W.D.G. Financial activities related to the present article: author is an employee of Siemens. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. K.G. No relevant conflicts of interest to disclose. C.R.W. No relevant conflicts of interest to disclose. J.W.M.B. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: author has a patent pending. M.S. No relevant conflicts of interest to disclose. P.V.J. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: author received money for a consultancy with Biocardia. Other relationships: none to disclose. D.L.K. Financial activities related to the present article: institution received a grant from Siemens. Financial activities not related to the present article: author received money for a consultancy with Surefire; author and institution received royalties from Samsung; and institution received grants or has grants pending with Boston Scientific and Siemens. Other relationships: none to disclose.
Abbreviations:
- BaCaps
- barium-alginate microcapsules
- H-E
- hematoxylin-eosin
- hMSC
- human MSC
- MSC
- mesenchymal stem cell
- 3D
- three-dimensional
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012;125(1):e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cittadini A, Monti MG, Petrillo V, et al. Complementary therapeutic effects of dual delivery of insulin-like growth factor-1 and vascular endothelial growth factor by gelatin microspheres in experimental heart failure. Eur J Heart Fail 2011;13(12):1264–1274. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki H, Kawamoto A, Tjwa M, et al. PlGF repairs myocardial ischemia through mechanisms of angiogenesis, cardioprotection and recruitment of myo-angiogenic competent marrow progenitors. PLoS ONE 2011;6(9):e24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee ST, White AJ, Matsushita S, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol 2011;57(4):455–465. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011;378(9806):1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012;308(22):2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 2006;113(10):1287–1294. [DOI] [PubMed] [Google Scholar]
- 8.Leistner DM, Fischer-Rasokat U, Honold J, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): final 5-year results suggest long-term safety and efficacy. Clin Res Cardiol 2011;100(10):925–934. [DOI] [PubMed] [Google Scholar]
- 9.Haider HKh, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol 2008;45(4):554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol 2001;33(5):907–921. [DOI] [PubMed] [Google Scholar]
- 11.Doyle B, Kemp BJ, Chareonthaitawee P, et al. Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med 2007;48(10):1708–1714. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation 2005;111(17):2198–2202. [DOI] [PubMed] [Google Scholar]
- 13.Perin EC, Silva GV, Henry TD, et al. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF). Am Heart J 2011;161(6):1078–1087, e3. [DOI] [PubMed] [Google Scholar]
- 14.Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA 2011;306(19):2110–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin P, Wang E, Wang YH, et al. Central zone of myocardial infarction: a neglected target area for heart cell therapy. J Cell Mol Med 2012;16(3):637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladage D, Turnbull IC, Ishikawa K, et al. Delivery of gelfoam-enabled cells and vectors into the pericardial space using a percutaneous approach in a porcine model. Gene Ther 2011;18(10):979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews KG, Devlin GP, Stuart SP, et al. Intrapericardial IGF-I improves cardiac function in an ovine model of chronic heart failure. Heart Lung Circ 2005;14(2):98–103. [DOI] [PubMed] [Google Scholar]
- 18.Laham RJ, Rezaee M, Post M, et al. Intrapericardial delivery of fibroblast growth factor-2 induces neovascularization in a porcine model of chronic myocardial ischemia. J Pharmacol Exp Ther 2000;292(2):795–802. [PubMed] [Google Scholar]
- 19.Hamdi H, Planat-Benard V, Bel A, et al. Epicardial adipose stem cell sheets results in greater post-infarction survival than intramyocardial injections. Cardiovasc Res 2011;91(3):483–491. [DOI] [PubMed] [Google Scholar]
- 20.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980;210(4472):908–910. [DOI] [PubMed] [Google Scholar]
- 21.Barnett BP, Kraitchman DL, Lauzon C, et al. Radiopaque alginate microcapsules for X-ray visualization and immunoprotection of cellular therapeutics. Mol Pharm 2006;3(5):531–538. [DOI] [PubMed] [Google Scholar]
- 22.Kedziorek DA, Hofmann LV, Fu Y, et al. X-ray-visible microcapsules containing mesenchymal stem cells improve hind limb perfusion in a rabbit model of peripheral arterial disease. Stem Cells 2012;30(6):1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnett BP, Arepally A, Stuber M, Arifin DR, Kraitchman DL, Bulte JW. Synthesis of magnetic resonance-, x-ray- and ultrasound-visible alginate microcapsules for immunoisolation and noninvasive imaging of cellular therapeutics. Nat Protoc 2011;6(8):1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolderman RW, Hermans JJ, Rademakers LM, et al. Intrapericardial delivery of amiodarone and sotalol: atrial transmural drug distribution and electrophysiological effects. J Cardiovasc Pharmacol 2009;54(4):355–363. [DOI] [PubMed] [Google Scholar]
- 25.Xiao YF, Sigg DC, Ujhelyi MR, Wilhelm JJ, Richardson ES, Iaizzo PA. Pericardial delivery of omega-3 fatty acid: a novel approach to reducing myocardial infarct sizes and arrhythmias. Am J Physiol Heart Circ Physiol 2008;294(5):H2212–H2218. [DOI] [PubMed] [Google Scholar]
- 26.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005;11(4):367–368. [DOI] [PubMed] [Google Scholar]
- 27.Li TS, Cheng K, Malliaras K, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol 2012;59(10):942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bishop LH, Jr, Estes EH, Jr, McIntosh HD. The electrocardiogram as a safeguard in pericardiocentesis. J Am Med Assoc 1956;162(4):264–265. [DOI] [PubMed] [Google Scholar]
- 29.Fukushima S, Varela-Carver A, Coppen SR, et al. Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation 2007;115(17):2254–2261. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Dreessen de Gervai P, Sun J, Glogowski M, Gussakovsky E, Kupriyanov V. MRI studies of cryoinjury infarction in pig hearts: ii. Effects of intrapericardial delivery of adipose-derived stem cells (ADSC) embedded in agarose gel. NMR Biomed 2012;25(2):227–235. [DOI] [PubMed] [Google Scholar]
- 31.Kutschka I, Chen IY, Kofidis T, et al. In vivo optical bioluminescence imaging of collagen-supported cardiac cell grafts. J Heart Lung Transplant 2007;26(3):273–280. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Mauricio RG, Acarregui A, Sánchez-Margallo FM, et al. A preliminary approach to the repair of myocardial infarction using adipose tissue-derived stem cells encapsulated in magnetic resonance-labelled alginate microspheres in a porcine model. Eur J Pharm Biopharm 2013;84(1):29–39. [DOI] [PubMed] [Google Scholar]
- 33.Gollub MJ, Bains MS. Barium sulfate: a new (old) contrast agent for diagnosis of postoperative esophageal leaks. Radiology 1997;202(2):360–362. [DOI] [PubMed] [Google Scholar]
- 34.Tanomkiat W, Galassi W. Barium sulfate as contrast medium for evaluation of postoperative anastomotic leaks. Acta Radiol 2000;41(5):482–485. [DOI] [PubMed] [Google Scholar]
- 35.Mattrey RF. Perfluorooctylbromide: a new contrast agent for CT, sonography, and MR imaging. AJR Am J Roentgenol 1989;152(2):247–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.