Abstract
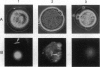
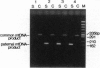
To examine whether mtDNA is uni- or biparentally transmitted in mice, we developed an assay that can detect sperm mtDNA in a single mouse embryo. In intraspecific hybrids of Mus musculus, paternal mtDNA was detected only through the early pronucleus stage, and its disappearance co-incided with loss of membrane potential in sperm-derived mitochondria. By contrast, in interspecific hybrids between M. musculus and Mus spretus, paternal mtDNA was detected throughout development from pronucleus stage to neonates. We propose that oocyte cytoplasm has a species-specific mechanism that recognizes and eliminates sperm mitochondria and mtDNA. This mechanism must recognize nuclearly encoded proteins in the sperm midpiece, and not the mtDNA or the proteins it encodes, because sperm mitochondria from the congenic strain B6.mtspr, which carries M. spretus mtDNA on background of M. musculus (B6) nuclear genes, were eliminated early by B6 oocytes as in intraspecific crosses. We conclude that cytoplasmic genomes are transmitted uniparentally in intraspecific crosses in mammals as in Chlamydomonas and that leakage of parental mtDNA is limited to interspecific crosses, which rarely occur in nature.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armbrust E. V., Ferris P. J., Goodenough U. W. A mating type-linked gene cluster expressed in Chlamydomonas zygotes participates in the uniparental inheritance of the chloroplast genome. Cell. 1993 Sep 10;74(5):801–811. doi: 10.1016/0092-8674(93)90460-8. [DOI] [PubMed] [Google Scholar]
- Ball E. H., Singer S. J. Mitochondria are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1982 Jan;79(1):123–126. doi: 10.1073/pnas.79.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham E., Lamb T., Avise J. C. Size polymorphism and heteroplasmy in the mitochondrial DNA of lower vertebrates. J Hered. 1986 Jul-Aug;77(4):249–252. doi: 10.1093/oxfordjournals.jhered.a110230. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Birky C. W., Jr Transmission genetics of mitochondria and chloroplasts. Annu Rev Genet. 1978;12:471–512. doi: 10.1146/annurev.ge.12.120178.002351. [DOI] [PubMed] [Google Scholar]
- Gyllensten U., Wharton D., Josefsson A., Wilson A. C. Paternal inheritance of mitochondrial DNA in mice. Nature. 1991 Jul 18;352(6332):255–257. doi: 10.1038/352255a0. [DOI] [PubMed] [Google Scholar]
- Gyllensten U., Wharton D., Wilson A. C. Maternal inheritance of mitochondrial DNA during backcrossing of two species of mice. J Hered. 1985 Sep-Oct;76(5):321–324. doi: 10.1093/oxfordjournals.jhered.a110103. [DOI] [PubMed] [Google Scholar]
- Hecht N. B., Liem H., Kleene K. C., Distel R. J., Ho S. M. Maternal inheritance of the mouse mitochondrial genome is not mediated by a loss or gross alteration of the paternal mitochondrial DNA or by methylation of the oocyte mitochondrial DNA. Dev Biol. 1984 Apr;102(2):452–461. doi: 10.1016/0012-1606(84)90210-0. [DOI] [PubMed] [Google Scholar]
- Hiraoka J., Hirao Y. Fate of sperm tail components after incorporation into the hamster egg. Gamete Res. 1988 Apr;19(4):369–380. doi: 10.1002/mrd.1120190408. [DOI] [PubMed] [Google Scholar]
- Hoeh W. R., Blakley K. H., Brown W. M. Heteroplasmy suggests limited biparental inheritance of Mytilus mitochondrial DNA. Science. 1991 Mar 22;251(5000):1488–1490. doi: 10.1126/science.1672472. [DOI] [PubMed] [Google Scholar]
- Hurst L. D., Hoekstra R. F. Evolutionary genetics. Shellfish genes kept in line. Nature. 1994 Apr 28;368(6474):811–812. doi: 10.1038/368811a0. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo R., Satta Y., Matsuura E. T., Ishiwa H., Takahata N., Chigusa S. I. Incomplete maternal transmission of mitochondrial DNA in Drosophila. Genetics. 1990 Nov;126(3):657–663. doi: 10.1093/genetics/126.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T., Avise J. C. Directional introgression of mitochondrial DNA in a hybrid population of tree frogs: The influence of mating behavior. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2526–2530. doi: 10.1073/pnas.83.8.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansman R. A., Avise J. C., Huettel M. D. Critical experimental test of the possibility of "paternal leakage" of mitochondrial DNA. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1969–1971. doi: 10.1073/pnas.80.7.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl K. F., Hausmann B., Robinson P. J., Guénet J. L., Wharton D. C., Winking H. Mta, the maternally transmitted antigen, is determined jointly by the chromosomal Hmt and the extrachromosomal Mtf genes. J Exp Med. 1986 Feb 1;163(2):334–346. doi: 10.1084/jem.163.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl K. F., Hausmann B., Robinson P. J., Guénet J. L., Wharton D. C., Winking H. Mta, the maternally transmitted antigen, is determined jointly by the chromosomal Hmt and the extrachromosomal Mtf genes. J Exp Med. 1986 Feb 1;163(2):334–346. doi: 10.1084/jem.163.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland B., Wang C. R., Yonekawa H., Hermel E., Lindahl K. F. Maternally transmitted histocompatibility antigen of mice: a hydrophobic peptide of a mitochondrially encoded protein. Cell. 1990 Mar 23;60(6):971–980. doi: 10.1016/0092-8674(90)90345-f. [DOI] [PubMed] [Google Scholar]
- Loveland B., Wang C. R., Yonekawa H., Hermel E., Lindahl K. F. Maternally transmitted histocompatibility antigen of mice: a hydrophobic peptide of a mitochondrially encoded protein. Cell. 1990 Mar 23;60(6):971–980. doi: 10.1016/0092-8674(90)90345-f. [DOI] [PubMed] [Google Scholar]
- Pikó L., Matsumoto L. Number of mitochondria and some properties of mitochondrial DNA in the mouse egg. Dev Biol. 1976 Mar;49(1):1–10. doi: 10.1016/0012-1606(76)90253-0. [DOI] [PubMed] [Google Scholar]
- Yonekawa H., Moriwaki K., Gotoh O., Miyashita N., Migita S., Bonhomme F., Hjorth J. P., Petras M. L., Tagashira Y. Origins of laboratory mice deduced from restriction patterns of mitochondrial DNA. Differentiation. 1982;22(3):222–226. doi: 10.1111/j.1432-0436.1982.tb01255.x. [DOI] [PubMed] [Google Scholar]
- Zouros E., Freeman K. R., Ball A. O., Pogson G. H. Direct evidence for extensive paternal mitochondrial DNA inheritance in the marine mussel Mytilus. Nature. 1992 Oct 1;359(6394):412–414. doi: 10.1038/359412a0. [DOI] [PubMed] [Google Scholar]
- Zouros E., Oberhauser Ball A., Saavedra C., Freeman K. R. An unusual type of mitochondrial DNA inheritance in the blue mussel Mytilus. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7463–7467. doi: 10.1073/pnas.91.16.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]