This work describes additional characterization of the localization of AtCYP20-2: this single domain cyclophilin was found to specifically associate with membrane regions enriched in photosystem II supercomplexes. In addition to the FK506 binding proteins (FKBPs), cyclophilins are defined as immunophilins, a ubiquitous protein family originally identified as the intracellular receptors of immunosuppressant drugs. Immunophilins are known to be localized in all plant subcellular compartments, and proteomic analysis has revealed the presence of 15 isoforms that are targeted to the chloroplast: one in the stroma (Lippuner et al., 1994), and up to 14 in the thylakoid lumen (Peltier et al., 2002; Schubert et al., 2002; Friso et al., 2004). Immunophilins exist as both single and multidomain isoforms, which have been shown to function both as active protein foldases and as regulatory components of thylakoid membrane complexes. TLP20, the spinach (Spinacia oleracca) homolog of AtCYP20-2, has been shown to be responsible for the major component of peptidyl-prolyl cis-trans isomerase activity within the thylakoid lumen (Edvardsson et al., 2003). In vitro studies have shown that the multidomain spinach cyclophilin TLP40 associates with and regulates the activity of a PSII-specific protein phosphatase within the thylakoid membrane, an essential process in the coordination of proteolysis and integration of newly synthesized D1 subunits during PSII protein turnover (Fulgosi et al., 1998; Vener et al., 1999). As an example of the specificity of single domain chloroplast immunophilin function, the precursor of the lumenal AtFKBP13 has been shown to interact with the Rieske Fe-S protein, a component of the cytochrome b6f complex, suggesting association occurs along the import pathway. Antisense AtFKBP13 lines have increased Rieske levels, suggesting it may act as a suppressor of Rieske accumulation (Gupta et al., 2002).
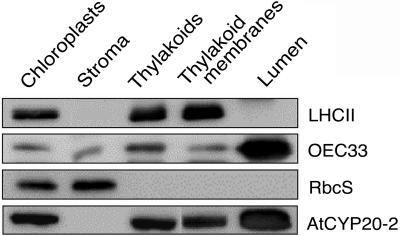
Given the abundance of immunophilin-like proteins in the thylakoid membrane system, it is likely that PPIase activity may be an integral component of the photosynthetic acclimation response; in particular, these proteins may play an important role in catalyzing correct folding and integration of proteins in and around the thylakoid membrane system. Using chromatographic and immunohistochemical approaches, we sought to determine the specific membrane location of AtCYP20-2. A specific antibody was obtained by using the C-terminal motif CGQLPMSEA as an antigen. The antibody reacted specifically with recombinant AtCYP20-2 and did not react with any proteins extracted from knockout plants lacking the AtCYP20-2 (data not shown). Thus it was concluded the antibody is highly specific for the AtCYP20-2 protein. To determine the precise localization of AtCYP20-2, chloroplast fractionation was carried out in conjunction with western blotting using the specific AtCYP20-2 antibody (Fig. 1). Control blots carried out using the integral thylakoid membrane protein LHCII, the 33-kD subunit of the oxygen-evolving complex (OEC33), and the small subunit of Rubisco (RbcS) allowed verification of the purity and composition of the fractions. As expected, LHCII was found to be exclusively located within the thylakoid membrane, whereas the RbcS was present only in the chloroplast stroma. AtCYP20-2 was localized to the thylakoid lumen, however, a significant portion of this cyclophilin was also bound to the thylakoid membrane fraction (Fig. 1). This finding was unexpected because AtCYP20-2 is a hydrophilic and soluble protein and, in contrast to the multidomain lumenal cyclophilin TLP40 (Fulgosi et al., 1998; Vener et al., 1999), has no specific membrane-binding domains. The membrane association of TLP20, the spinach homolog of AtCYP20-2, has not been reported (Edvardsson et al., 2003). On the other hand, AtCYP20-2 was identified among the proteins copurifying with thylakoid membranes in a proteomic study (Peltier et al., 2002).
Figure 1.
Localization of AtCYP20-2. Western analysis of AtCYP20-2 protein levels in chloroplast fractions (40 μg of protein loaded for AtCYP20-2, 10 μg for OEC33, LHCII, and RbcS). A total of 20 g of Arabidopsis leaf material was ground 7 × 1 s in a blender in 1× grinding buffer (330 mm sorbitol, 20 mm Tricine-KOH, pH 8.4, 5 mm EGTA, 5 mm EDTA, 10 mm NaCO3, 0.1% bovine serum albumin [BSA], and 330 mg/L isoascorbate). The suspension was then filtered through four layers of muslin and centrifuged for 5 min at 1,000g to pellet the crude chloroplast fraction. After resuspension of the chloroplasts in 1× grinding buffer the suspension was layered on top of two 35 mL continuous Percoll gradients. The gradients were generated by 40 min centrifugation at 48,000g of 50% (v/v) Percoll/2× grinding buffer (660 mm sorbitol, 40 mm tricine-KOH, pH 8.4, 10 mm EGTA, 10 mm EDTA, 20 mm NaCO3, 0.2% BSA, and 660 mg/L isoascorbate). The gradient was also supplemented with 132 mg/L (final concentration) of gluthathione. The loaded gradients were centrifuged for 15 min at 3,000g in a swing-out rotor with no brake. The lower band containing intact chloroplasts was collected, washed once in 1× grinding buffer, and diluted to 0.5 mg mL−1 chlorophyll. The chlorophyll concentration during the remainder of the preparation was kept at 0.5 mg mL−1. Intact chloroplasts were then lysed by resuspension in 25 mm NH4HCO3 and incubation on ice for 20 min. The lysed chloroplasts were then centrifuged for 90 s at 16,000g, and the supernatant, consisting of the stromal fraction, was collected and the pelleted thylakoid fraction washed once in 25 mm NH4HCO3 before resuspension in 1× grinding buffer. Intact thylakoids were then lysed by passing them twice through a Yeda press (10 MPa), and the thylakoid membrane and lumen fractions separated by 30 min ultracentrifugation at 200,000g. Thylakoid membranes were finally resuspended in 1× grinding buffer. Protein samples were separated by SDS-PAGE using the buffer system of Davis (1964), and the protocol of Sambrook et al. (1989). A 9% stacking gel and a 15% separating gel were used in all cases. Proteins separated by SDS-PAGE were transferred onto nitrocellulose membrane in transfer buffer (25 mm Tris/HCl, pH 8.3; 192 mm Gly, 20% methanol) with a Bio-Rad blotting kit at 30 mA overnight. The membrane was then blocked in TBS (50 mm Tris-buffered saline (20 mm Tris, 0.5 m NaCl), 5% milk powder, 0.5% Tween 20) for 1 h, followed by 1 h in TBS + primary antibody (dilutions varied according to antibody). Blots were washed for 15 min and 3 × 5 min with Tris-buffered saline followed by incubation in a 1:5000 dilution of the secondary antibody (alkaline phosphatase-conjugated goat anti-rabbit IgG, Amersham Biosciences) for 1 h. The blots were washed again as above and immunodetection was carried out with an enhanced chemiluminescence kit (Amersham Biosciences).
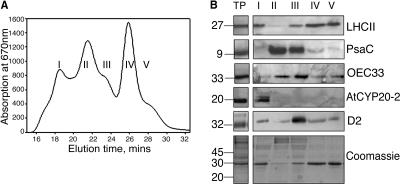
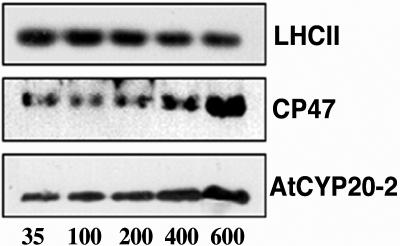
To refine the specific localization of membrane associated AtCYP20-2, intact thylakoid membranes were subjected to mild solubilization with n-dodecyl α-d-maltoside. This technique involves the size-based separation and isolation of distinct thylakoid membrane particles, allowing excellent preservation of three-dimensional structure and separation of PSI and PSII rich membrane regions (Boekema et al., 2000). Figure 2, A and B, show absorption at 670 nm and western analysis of digested fractions collected by gel filtration chromatography. The content of each fraction was verified by western-blot analysis using specific antibodies. Fraction I consists largely of membrane fragments enriched in PSII supercomplexes: these are PSII complexes composed of a dimeric core (D1, D2, CP43, and CP47), surrounded by two symmetrically arranged structures consisting of the minor light harvesting proteins CP26 and CP29 and one LHCII trimer. This basic supercomplex can bind up to two additional LHCII trimers and one additional monomeric complex (Boekema et al., 1999a, 1999b; Yakushevska et al., 2001). PsaC is a 9-kD protein which is associated with the core of PSI. Western analysis using a PsaC specific antibody shows that fraction II is enriched in stromal lamellae composed primarily of PSI core complexes, although a small amount of PSII core is also present. As shown by the abundance of D2 and OEC33 protein, fraction III contains the largest complement of PSII core complexes, as well as a small proportion of tightly bound LHCII. LHCII trimers separated from the PSII core complexes of fraction III are eluted in fraction IV, with monomeric LHCII being collected last in fraction V. Elution of the ATP synthase and cytochrome b6f complexes is known to occur in fractions II and III, respectively (van Roon et al., 2000). Western-blot analysis of each of these five fractions revealed maximal enrichment of AtCYP20-2 in fractions containing PSII supercomplexes. Interestingly however, virtually no AtCYP20-2 was found to be associated with PSII core complexes, indicating that the majority of AtCYP20-2 may be peripherally associated with the PSII complex. Having determined the location of the AtCYP20-2 protein to PSII supercomplexes, we sought to verify its response to changes in growth light conditions. The photosynthetic apparatus is highly sensitive to growth light: under high light conditions (400 μmol m−2 s−1), the thylakoid membrane is geared toward electron transport resulting in increased levels of PSII complexes and reduced levels of LHCII antennae. Under these conditions, increased levels of Rubisco accelerate carbon fixation resulting in high photosynthetic rates. Conversely, low light-grown plants maximize antenna size while minimizing reaction center content. Under light-limiting conditions (200 μmol m−2 s−1), photon capture is maximized by an increased concentration of LHCII trimers while relatively lower PSII and Rubisco levels result in lowered photosynthetic rates (Leong and Anderson, 1984; Wild et al., 1986; Anderson et al., 1988; Bailey et al., 2001). Arabidopsis plants were grown at light intensities from 35 to 600 μmol m−2 s−1 and the amount of AtCYP20-2 protein determined by western analysis of thylakoid protein preparations (Fig. 3). As previously documented (Bailey et al., 2001), increased growth irradiance resulted in decreased levels of chlorophyll a/b-binding protein LHCII and a concomitant increase in PSII reaction centers, as shown by increased levels of the PSII core protein CP47. AtCYP20-2 levels increased under increasing growth irradiances with maximal levels detectable at 600 μmol m−2 s−1. As samples were loaded on an equal chlorophyll basis, the increase in AtCYP20-2 levels may be attributed to its association and stoichiometric relation with photosystem II, corroborating the localization evidence suggesting PSII association described above (Fig. 2B).
Figure 2.
A, Absorbance at 670 nm of fractions obtained from thylakoid membranes purified from LL-grown Columbia-0 plants. B, Western analysis of fractions enriched for (1) PSII supercomplexes, (2) PSI, (3) PSI + PSII core, (4) trimeric LHCII, and (5) monomeric LHCII (protein equivalent of 2 μg of chlorophyll loaded in each well). TP, total thylakoid preparation. Thylakoids were prepared by homogenizing fresh tissue in ice-cold grinding medium (330 mm sorbitol, 5 mm MgCl2, 10 mm Na4P2O7 (pH 6.5), 2 mm ascorbate) with a polytron. The homogenate was then filtered through four layers of muslin followed by two layers of muslin and one layer of cotton wool. The filtrate was centrifuged for 10 min at 5,000g, and the chloroplast enriched pellet resuspended in wash buffer (330 mm sorbitol, 10 mm MES, pH 6.5), followed by a further 10 min centrifugation at 5,000g. The pellet was then resuspended in 5 mm MgCl2 for 30 s to lyse any remaining intact chloroplasts, followed by an equal volume of osmoticum (660 mm sorbitol, 20 mm KCl, 2 mm EDTA and 100 mm HEPES, pH 6.5) to break open the stacked grana. Thylakoid membranes were freshly prepared from 4- to 5-week old Arabidopsis plants as described above with a final resuspension in 20 mm Bis-Tris (pH 6.5), 5 mm MgCl2. Thylakoid samples were diluted to a final chlorophyll concentration of 1.4 mg mL−1 chlorophyll and solubilized by addition of n-dodecyl α-d-maltoside to a final concentration of 0.7% to 0.85%. Samples were vortexed thoroughly for 1 min and centrifuged for 1 min at 16,000g. The supernatant was then filtered through a 0.45-μm nylon filter and subjected to gel filtration chromatography using a Superdex 200 HR 10/30 column in an Amersham-Pharmacia ÄKTApurifier system using the same buffer and similar conditions as before (Yakushevska et al., 2001; Ruban et al., 2003).
Figure 3.
Thylakoid protein extracts from plants grown in steady-state light environments ranging from 35 to 600 μmol m−2 s−1 (inset, representative of 4 experiments) (protein equivalent of 2 μg of chlorophyll/well). Each blot is representative of four separate experiments. Plants were grown under short days (8 h light, 20°C; 16 h dark, 15°C); growth light was provided by color 84 fluorescent tubes (Philips TLD 35 W, or Philips PL-l 55 W heat-filtered through 2 mm polyacetate). Photon fluence rates were measured at the leaf surface using a Skye Instruments (Llandrindod Wells, UK) SKP200 PAR detector.
DISCUSSION
The finding that a significant amount of AtCYP20-2 protein is detected in the thylakoid membrane fraction is surprising given AtCYP20-2 is a hydrophilic protein without any predicted membrane-binding domains. Chromatographic separation and analysis of the thylakoid membrane complexes (Fig. 2A) showed that the membrane-associated AtCYP20-2 coeluted with the fraction enriched in PSII supercomplexes (Fig. 2B). Such a localization of AtCYP20-2 differs completely from that found for the complex lumenal cyclophilin TLP40, which has additional protein-protein interaction and phosphatase binding modules besides the cyclophilin-like catalytic domain (Fulgosi et al., 1998; Vener et al., 1999). It has been demonstrated that TLP40 binds to the protein phosphatase in the PSI-enriched stroma-exposed regions of thylakoid membrane (Fulgosi et al., 1998). The finding that, unlike OEC33, AtCYP20-2 was not detected in PSII core complexes suggests that it may be associated with the peripheral regions of the PSII supercomplex, such as those encompassing the moderately bound trimers of LHCII previously described (Yakushevska et al., 2001; Ruban et al., 2003) or the PsbS subunit (Funk et al., 1995). Western analysis of thylakoid membrane proteins obtained from Arabidopsis plants grown in light conditions spanning 35 to 600 μmol m−2 s−1 revealed an increase in the amount of AtCYP20-2 under high irradiances (Fig. 3). Like other proteins involved in photosynthesis, AtCYP20-2 levels are carefully balanced to meet the requirements of prevailing environmental conditions. The finding that, like photosystem II, AtCYP20-2 levels increase with increasing growth irradiance, adds support to our suggestion that it may be specifically associated with, and stoichiometrically related to this photosynthetic complex. While, based on the above findings, it is not possible to assign any substrates to AtCYP20-2, it may be surmised that specific interactions may occur with PSII proteins. Insertion of chlorophyll binding proteins such as LHCII into the thylakoid membrane is known to be a complex, dynamic process (Kutkatt et al., 1995). All chlorophyll a/b-binding proteins possess a conserved Pro residue within the stromal loop domain; reconstitution experiments have shown that substitution of this residue in recombinant LHCII renders it unable to assemble with pigments in vitro (Heinemann and Paulsen, 1999), suggesting that the presence and correct conformation of proline containing domains is crucial for its integration.
This work was supported by the Natural Environment Research Council, the Biotechnology and Biological Sciences Research Council (grant no. 50/P16446), the Swedish Research Council, the Swedish Research Council for Environment, Agriculture, and Spatial Planning (Formas), and Nordic Joint Committee for Agricultural Research (NKJ).
References
- Anderson JM, Chow WS, Goodchild DJ (1988) Thylakoid membrane organisation in sun/shade acclimation. Aust J Plant Physiol 15: 11–26 [Google Scholar]
- Bailey S, Walters RG, Jansson S, Horton P (2001) Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta 213: 794–801 [DOI] [PubMed] [Google Scholar]
- Boekema EJ, van Roon H, Calkoen F, Bass R, Dekker JP (1999. a) Multiple types of association of photosystem II and its light-harvesting antenna in partially solubilized photosystem II membranes. Biochemistry 38: 2233–2239 [DOI] [PubMed] [Google Scholar]
- Boekema EJ, van Roon H, Van Breemen JFL, Dekker JP (1999. b) Supramolecular organization of photosystem II and its light-harvesting antenna in partially solubilized photosystem II membranes. Eur J Biochem 266: 444–452 [DOI] [PubMed] [Google Scholar]
- Boekema EJ, Van Breemen JFL, Van Roon H, Dekker JP (2000) Arrangement of photosystem II supercomplexes in crystalline macrodomains within the thylakoid membrane of green plant chloroplasts. J Mol Biol 301: 1123–1133 [DOI] [PubMed] [Google Scholar]
- Davis BJ (1964) Disc electrophoresis-II: method and application to human serum proteins. Ann NY Acad Sci 121: 404–427 [DOI] [PubMed] [Google Scholar]
- Edvardsson A, Eshaghi S, Vener AS, Andersson B (2003) The major peptidyl-prolyl isomerase activity in thylakoid lumen of plant chloroplasts belongs to a novel cyclophilin TLP20. FEBS Lett 542: 137–141 [DOI] [PubMed] [Google Scholar]
- Friso G, Giacomelli L, Ytterberg AJ, Peltier J-B, Rudella A, Sun Q, van Wijk KJ (2004) In-depth analysis of the thylakoid membrane proteome of Arabidopsis thaliana chloroplasts: new proteins, new functions and a plastid proteome database. Plant Cell 16: 478–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulgosi H, Vener AV, Altschmied L, Herrmann RG, Andersson B (1998) A novel multi-functional chloroplast protein: identification of a 40 kDa immunophilin-like protein located in the thylakoid lumen. EMBO J 17: 1577–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C, Schröder WP, Napiwotzki A, Tjus SE, Renger G, Andersson B (1995) The PSII-S protein of higher plants: a new type of pigment-binding protein. Biochemistry 34: 11133–11141 [DOI] [PubMed] [Google Scholar]
- Gupta R, Mould RM, He ZY, Luan S (2002) A chloroplast FKBP interacts with and affects the accumulation of Rieske subunit of cytochrome bf complex. Proc Natl Acad Sci USA 99: 15806–15811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann B, Paulsen H (1999) Random mutations directed to transmembrane and loop domains of the light-harvesting chlorophyll a/b protein: impact on pigment binding. Biochemistry 38: 14088–14093 [DOI] [PubMed] [Google Scholar]
- Kutkatt R, Grimm R, Paulsen H (1995) Light-harvesting chlorophyll a/b-binding protein inserted into isolated thylakoids binds pigments and is assembled into trimeric light-harvesting complex. Plant Physiol 109: 1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong TY, Anderson JM (1984) Adaptations of the thylakoid membranes of pea chloroplasts to light intensities. I. Study on the distribution of chlorophyll-protein complexes. Photosynth Res 5: 105–115 [DOI] [PubMed] [Google Scholar]
- Lippuner V, Chou IT, Scott SV, Ettinger WF, Theg SM, Gasser CS (1994) Cloning and characterisation of chloroplast and cytosolic forms of cyclophilin from Arabidopsis thaliana. J Biol Chem 269: 7863–7868 [PubMed] [Google Scholar]
- Peltier JB, Emanuelsson O, Kalume DE, Ytterberg J, Friso G, Rudella A, Liberles DA, Söderberg L, Roepstorff P, von Heijne G, Van Wijk KJ (2002) Central functions of the lumenal and peripheral thylakoid proteome of Arabidopsis determined by experimentation and genome-wide prediction. Plant Cell 14: 211–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Wentworth M, Yakushevska AE, Andersson J, Lee PJ, Keegstra W, Dekker JP, Boekema EJ, Jansson S, Horton P (2003) Plants lacking the main light harvesting complex retain photosystem II macro-organization. Nature 421: 648–652 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor Press, Cold Spring Harbor, NY.
- Schubert M, Petersson UA, Haas BJ, Funk C, Schröder WP, Kieselbach T (2002) Proteome map of the chloroplast lumen of Arabidopsis thaliana. J Biol Chem 277: 8354–8365 [DOI] [PubMed] [Google Scholar]
- van Roon H, van Breemen JFL, deWeerd FL, Dekker JP, Boekema EJ (2000) Solubilization of green plant thylakoid membranes with n-dodecyl-α,D-maltoside. Implications for the structural organization of Photosystem II, Photosystem I, ATP synthase and cytochrome b6f complexes. Photosynth Res 64: 155–166 [DOI] [PubMed] [Google Scholar]
- Vener AV, Rokka A, Fulgosi H, Andersson B, Herrmann RG (1999) A cyclophilin-regulated PP2A-like protein phosphatase in thylakoid membranes of plant chloroplasts. Biochemistry 38: 14955–14965 [DOI] [PubMed] [Google Scholar]
- Wild A, Hopfner M, Ruhle W, Richter M (1986) Changes in the stoichiometry of photosystem II components as an adaptive response to high light conditions during growth. Z Naturforsch Teil C Biochem Biophys Biol Virol 41: 597–603 [Google Scholar]
- Yakushevska A, Jensen PE, Keegstra W, Van Rooon H, Scheller HV, Boekema EJ, Dekker JP (2001) Supermolecular organization of photosystem II and its associated light-harvesting antenna in Arabidopsis thaliana. Eur J Biochem 268: 6020–6028 [DOI] [PubMed] [Google Scholar]