Abstract
The heterocyclic aldehyde 5-hydroxymethyl-2-furfural (5HMF) interacts allosterically with the abnormal form of haemoglobin (Hb), HbS, in red blood cells (RBCs) from patients with sickle cell disease (SCD), thereby increasing oxygen affinity and decreasing HbS polymerization and RBC sickling during hypoxia. We hypothesized that should 5HMF also inhibit the main cation pathways implicated in the dehydration of RBCs from SCD patients – the deoxygenation-induced cation pathway (Psickle), the Ca2+-activated K+ channel (the Gardos channel) and the K+–Cl− cotransporter (KCC) – it would have a synergistic effect in protection against sickling, directly through interacting with HbS, and indirectly through maintaining hydration and reducing [HbS]. This study was therefore designed to investigate the effects of 5HMF on RBC volume and K+ permeability in vitro. 5HMF markedly reduced the deoxygenation-induced dehydration of RBCs whether in response to maintained deoxygenation or to cyclical deoxygenation/re-oxygenation. 5HMF was found to inhibit Psickle, an effect which correlated with its effects on sickling. Deoxygenation-induced activation of the Gardos channel and exposure of phosphatidylserine were also inhibited, probably indirectly via reduced entry of Ca2+ through the Psickle pathway. Effects of 5HMF on KCC were more modest with a slight inhibition in N-ethylmaleimide (NEM, 1 mm)-treated RBCs and stimulation in RBCs untreated with NEM. These findings support the hypothesis that 5HMF may also be beneficial through effects on RBC ion and water homeostasis.
Key points
We addressed the hypothesis that the heterocyclic aldehyde 5-hydroxymethyl-2-furfural (5HMF) may act synergistically to ameliorate the complications of sickle cell disease through effects on red blood cell (RBC) membrane transport, in addition to its well-known action of increasing the oxygen affinity of the abnormal form of haemoglobin, HbS.
5HMF was found to reduce deoxygenation-induced dehydration of RBCs, whether in response to maintained deoxygenation or cyclical deoxygenation/re-oxygenation.
Acting at low millimolar concentrations, 5HMF reduced the activity of deoxygenation-induced cation conductance (sometimes termed Psickle), an effect which correlated with reduction in sickling. 5HMF similarly inhibited deoxygenation-induced activation of the Ca2+-activated K+ channel (or Gardos channel), an effect not seen following pharmacologically mediated increases in intracellular Ca2+ via the ionophore A23187. Deoxygenation-induced phosphatidylserine exposure, which is associated with Ca2+ entry via Psickle, was also inhibited by 5HMF.
By contrast, effects of 5HMF on the K+–Cl− cotransporter (KCC) were modest, with slight inhibition following treatment with N-ethylmaleimide (NEM) to abolish activity of its regulatory protein kinases, but stimulation in RBCs untreated with NEM.
It would therefore appear that an important beneficial action of 5HMF, in addition to effects on HbS oxygen affinity, is reduction in Psickle-mediated Ca2+ entry following RBC sickling, thereby inhibiting the deleterious sequelae of Gardos channel activation, RBC dehydration and also lipid scrambling.
Introduction
The complications of sickle cell disease (SCD) originate from the presence and polymerization of the abnormal form of haemoglobin (Hb), HbS, in the patient's red blood cells (RBCs). Compared with normal adult HbA, HbS shows a single amino acid substitution at the β6 position, at which glutamic acid is replaced by valine (Perutz & Mitchison, 1950; Bunn & Forget, 1986). Following the deoxygenation-induced conformational change of Hb, the loss of these negative charges allows neighbouring HbS molecules to aggregate and form long, rigid polymers which distort RBC shape. Other deleterious sequelae include altered rheology, RBC fragility, solute loss and dehydration and increased stickiness (Bunn & Forget, 1986). The consequent clinical complications fall into two groups: (i) a chronic anaemia consequent upon increased RBC destruction, and (ii) acute ischaemic signs following blockage of the microvasculature (Steinberg, 1999; Nagel & Platt, 2001; Rees et al. 2010). The precise clinical signs depend on the organ(s) involved but can be numerous. However, severity varies markedly among individuals, although the reason for this is not understood (Steinberg, 1999).
There is at present no specific treatment for SCD (Steinberg, 1999; Rees et al. 2010), although hydroxyurea has received considerable attention (Charache et al. 1987, 1992; Platt, 2008; Rees, 2011). The mechanisms of action of hydroxyurea remain uncertain but are probably mostly attributable to increased expression of fetal Hb (HbF). As HbF is not incorporated into HbS polymers and serves to dilute the intracellular concentration of HbS, its expression reduces the tendency for HbS to polymerize and for HbS-containing RBCs to sickle. Hydroxyurea, however, is not without risks: it is potentially teratogenic, especially in the long term; it achieves variable responses among individuals, and it raises issues of non-compliance (Platt, 2008). Such factors have restricted its use to more severely affected individuals (Rees, 2011).
Investigations continue into the development of other effective therapies. A promising alternative approach has involved the development of compounds which directly interpolate with HbS molecules and thereby reduce polymerization upon deoxygenation. The most encouraging of these have been the aromatic aldehydes such as vanillin (Zaugg et al. 1977; Abraham et al. 1991; Abdulmalik et al. 2005), and similar substituted benzaldehydes such as 12C79 (valerosol) and 589C80 (tucaresol) (Kneen & White, 1981; Beddell et al. 1984). These aldehydes form Schiff bases with HbS, allosterically increasing its oxygen affinity, and reducing polymerization and RBC sickling. Although they effectively reduce sickling and increase RBC hydration, none of these compounds has proved useful clinically (e.g. Fitzharris et al. 1985; Keidan et al. 1986; Arya et al. 1996). More recently, naturally occurring heterocyclic derivatives of furanic acid have been tested (Safo et al. 2004; Abdulmalik et al. 2005). These are active at lower concentrations and appear to be better tolerated. One of them, 5-hydroxymethyl-2-furfural (5HMF, also known as Aes-103), is currently in phase II clinical trials in SCD patients in the USA and UK (National Institutes of Health, 2013).
Several abnormal, or abnormally regulated, cation transporters participate in the pathogenesis of SCD (Joiner, 1993; Gibson & Ellory, 2002; Lew & Bookchin, 2005). These include the well-established pathways of the K+–Cl− cotransporter (or KCC) and the Ca2+-activated K+ channel (or Gardos channel), together with a third pathway, sometimes termed ‘Psickle’. Unlike the KCC and the Gardos channel, the molecular identity of Psickle is unknown but it appears to be a cation conductance pathway activated by deoxygenation, HbS polymerization and RBC shape change (Joiner et al. 1988; Joiner, 1993; Ma et al. 2012). Psickle mediates both entry of Ca2+ (Rhoda et al. 1990; Etzion et al. 1993) and exit of Mg2+ (Ortiz et al. 1990; Willcocks et al. 2002), with subsequent activation of the Gardos channel and also perhaps KCC. Deoxygenation-induced Ca2+ entry through Psickle is further implicated in the loss of phosphatidylserine (PS) asymmetry in RBCs from SCD patients (Lubin et al. 1981; Blumenfeld et al. 1991; Kuypers, 1998; de Jong et al. 2001; Weiss et al. 2012; Cytlak et al. 2013), subsequent to activation of a Ca2+-dependent scrambling process (Haest, 2003).
Together the three cation pathways interact to mediate solute loss (Gibson, 2001; Lew & Bookchin, 2005), thereby concentrating HbS, which greatly reduces the lag time for polymerization upon deoxygenation (Eaton & Hofrichter, 1987). PS exposure also increases RBC stickiness (Kuypers, 2008). These processes thereby increase the likelihood of sickling and vascular occlusion of the microvasculature.
Through its effects on HbS polymerization, it is possible that 5HMF also modulates the cation permeability of RBCs in SCD patients. Should 5HMF reduce solute loss and maintain better hydration of RBCs, HbS polymerization would be inhibited by two potentially synergistic mechanisms: a direct interaction of 5HMF with HbS stabilizing the oxygenated conformation, and a second mechanism through a reduction in [HbS], thus increasing the lag time to sickling on deoxygenation. This hypothesis was investigated in the present study. Findings show that RBC volume is better preserved in response to maintained deoxygenation and also to cyclical deoxygenation/re-oxygenation. Radioactive tracer methodologies were used to investigate the effects of 5HMF on K+ permeability, and specifically the activities of Psickle, the Gardos channel and KCC in RBCs from SCD patients. FITC-labelled lactadherin was also used to measure PS exposure. Results show that 5HMF modulates all three cation transport pathways and PS scrambling, and also affects HbS polymerization and sickling. These additional actions of 5HMF may be of significant consideration in assessments of its clinical use and also in the design of similar more efficacious compounds to ameliorate the complications of SCD.
Methods
Chemicals
Bumetanide, 5HMF, 3-[N-morpholino] propane sulphonic acid (MOPS), N-ethylmaleimide (NEM), ouabain and salts were purchased from Sigma Chemical Co. (Poole, UK). Clotrimazole and A23187 were purchased from Calbiochem (Nottingham, UK). 86Rb+ was supplied by Perkin Elmer (Beaconsfield, UK). Fluorescein isothiocyanate-conjugated lactadherin (LA-FITC) came from Haematologic Technologies, Inc. (Essex Junction, VT, USA) supplied via Cambridge Bioscience (Cambridge, UK), and phycoerythrin (PE)-conjugated anti-glycophorin A from Becton Dickinson Biosciences (San Jose, CA, USA).
Sample collection and handling
Blood samples were taken for routine tests according to clinical indications, from patients homozygous (HbSS) for SCD into the anticoagulant EDTA. Once routine testing had been completed, discarded and anonymized blood was analysed. The use of discarded blood was approved by the local ethics committee following guidelines set out in the Declaration of Helsinki. Samples were kept at 4°C until use within 48 h.
Solutions and tonometry
The standard saline (Cl-MBS) comprised 145 mm NaCl, 1.1 mm CaCl2, 5 mm glucose and 10 mm MOPS, (pH 7.4 at 37°C; 290 ± 5 mosmol (kg H2O)−1. For experiments in which Cl− dependence of K+ influx was examined, NO3− containing salts replaced those containing Cl− (N-MBS). To prevent the rapid RBC shrinkage that would otherwise occur following maximal stimulation of the Gardos channel in experiments in which intracellular Ca2+ was directly raised by incubation with the Ca2+ ionophore A23187, a high-K+ and low-Ca2+ containing saline was used with Ca2+ buffered with EGTA, comprising 80 mm KCl, 70 mm NaCl, 2 mm CaCl2, 0.15 mm MgCl2, 2 mm EGTA, 5 mm glucose and 10 mm MOPS (HK-MBS) with a free [Ca2+]o of 10 μm. The wash solution to remove unincorporated 86Rb+ comprised isotonic MgCl2 (107 mm), buffered with MOPS (10 mm), pH 7.4 at 4°C (Mg-MBS). Stock solutions of bumetanide (10 mm) were prepared in 100 mm Tris base and used at a final concentration of 10 μm. Stock solutions of ouabain (10 mm) were prepared in distilled water and used at a final concentration of 100 μm. Stocks of clotrimazole (CLT; 5 mm) were prepared in DMSO and used at a final concentration of 5 μm. In most experiments, whole blood was washed five times in N-MBS to remove Cl−, plasma and buffy coat. RBC suspensions were then pre-incubated at 15% haematocrit (Hct) in Eppendorf tubes with or without 5HMF (5 mm) for 30 min at 37°C and then placed in tonometers (Eschweiler GmbH & Co., Kiel, Germany) to equilibrate at the requisite oxygen tension before flux measurement (still in N-MBS). Tonometers were flushed with warm, humidified gas mixtures, supplied at the appropriate O2 tension using a Wösthoff gas mixing pump (Speake et al. 1997). For flux measurements, RBC suspensions were then diluted 10-fold into flux tubes, still equilibrated at the required oxygen tension. In instances in which its effects were investigated, tonometers and flux tubes also contained 0.1–5.0 mm 5HMF. For experiments involving A23187, the ionophore (6 μm final) was added to warm saline at 37°C in test tubes whilst vortexing. For experiments involving NEM, NEM (1 mm) was present during pre-incubation with 5HMF, after which aliquots were again diluted 10-fold into flux tubes. For CLT, dissolved in DMSO, appropriate controls were all treated with the same concentration of solvent (0.1% final).
Measurements of RBC sickling
To assess morphological sickling, RBCs were incubated in tonometers at 2% Hct for 20 min after which samples were fixed in solution identical to that used during incubation except that 0.3% glutaraldehyde was added. Control experiments showed that this protocol was sufficient to maintain RBC shape for several weeks. Sickling was assessed by light microscopy. Several hundred RBCs (typically 300–400) were counted using an Improved Neubauer haemocytometer. Control experiments examined the effect of 5HMF (5 mm) on sickling at an oxygen tension of 10 mmHg. These showed that the action of 5HMF was not immediate and that some pre-incubation was required, probably as a result of the time taken for aldehydes like 5HMF to enter RBCs and form adducts with Hb (Abraham et al. 1991; Abdulmalik et al. 2005). Thus, in a representative experiment at 10 mmHg O2, inhibition of sickling by 5HMF over 20 min was 20% without pre-incubation, but increased to 47%, 68%, 71% and 79% after 15, 30, 45 and 60 min, respectively, of pre-incubation. In subsequent experiments, 5HMF was therefore added to RBC suspensions at 37°C for 30 min prior to flux and volume assays, and for 60 min if PS exposure was to be measured.
Measurement of RBC water content
The effects of different oxygen tensions (with RBCs maintained under full oxygenation or deoxygenated at 0 mmHg or 15 mmHg O2, or during cyclical deoxygenation/re-oxygenation), all for 60 min at 37°C, on RBC volume were investigated. For oxygenation/deoxygenation cycles, gas delivered by the Wösthoff pump was switched between oxygen tensions of 0 mmHg and 100 mmHg every 10 min. RBC water content was then measured by the wet weight minus dry weight method (Borgese et al. 1991). In brief, after incubation for 60 min at 5% Hct, RBCs were pelleted by centrifugation at 12,000 g for 15 min at 4°C. The extruded pellet was weighed immediately (to an accuracy of 0.01 mg) and again after drying for 18 h at 95°C. Water content was expressed as ml water per g dry cell solids (ml g−1 d.c.s.).
K+ flux measurements
To determine the activity of the K+ transport pathways, K+ influx was measured at 37°C using 86Rb+ as a congener for K+ (Dunham & Ellory, 1981; Hannemann et al. 2011). RBCs washed in N-MBS were equilibrated in tonometers for 20 min then diluted 10-fold into saline, pre-equilibrated at the appropriate oxygen tension, at 260 mosmol kg−1 and pH 7. Hypotonic swelling and low pH were chosen in order to stimulate the KCC. 86Rb+ was added in 150 mm KNO3 to give a final [K+] of 7.5 mm in all experiments except those with HK saline and A23187-treated RBCs. Typically, three flux conditions were used: (i) Cl-MBS; (ii) Cl-MBS with clotrimazole, and (iii) N-MBS with clotrimazole. After incubation with radioisotope for 10 min, RBCs were washed five times in ice-cold Mg-MBS to remove extracellular 86Rb+. Ouabain (100 μm) and bumetanide (10 μm) were present in all experiments to obviate any K+ transport through the Na+/K+ pump and the Na+–K+–2Cl− cotransporter, respectively. KCC activity was assayed as Cl−-dependent K+ influx [using flux conditions (ii) and (iii)]; Gardos channel activity as the CLT-sensitive (5 μm) K+ influx [using conditions (i) and (ii)]; and Psickle as the deoxygenation-induced, clotrimazole-independent K+ influx measured in the absence of Cl− [condition (iii)]. When Gardos channel activity was measured in the presence of A23187, a K+ uptake measurement was carried out with serial samples of RBCs taken at 1, 3, 5, 7 and 10 min after addition of 86Rb+, with aliquots added directly into ice-cold Mg-MBS and then washed a further four times. Either microhaematocrit determination or the cyanohaemoglobin method was used to measure the Hct. Appropriate samples for this were taken before the start of each experiment.
Labelling of PS exposure
To investigate the effect of 5HMF on deoxygenation-induced PS exposure, RBC suspensions (0.5% Hct) were placed in a 24-well plate (Nunclon) in Cl-MBS with 1 mm vanadate, and continuously shaken at 2 g in a hypoxic Galaxy R incubator (Model 300, RS Biotech; C & M Scientific Ltd, Livingston, UK) for up to 4 h. Oxygen tension was set at 20 mmHg. To measure PS exposure, 5 μl aliquots (105 RBCs) of each sample were placed in 250 μl of LA-FITC binding buffer (16 nm LA-FITC in standard saline with 1 mm vanadate) and incubated in the dark at room temperature for 10 min. RBCs were then pelleted by centrifugation for 10 s at 16,100 g, washed once in saline to remove unbound LA-FITC and kept on ice until flow cytometry analysis. Control unlabelled RBCs treated with 5HMF (5 mm) established the absence of aldehyde-dependent self-fluorescence.
FACS acquisition and analysis
Externalized PS was measured with FITC using an excitation wavelength of 488 nm in the FL1 channel with an emission wavelength of 519 nm on a fluorescence-activated flow cytometer (FACSCalibur; Becton Dickinson Biosciences) and analysed with CellQuest Pro software (Becton Dickinson Biosciences) using a protocol published previously (Cytlak et al. 2013). Measurements were taken using a logarithmic gain. Forward scatter (FSC, size) and side scatter (SSC, granularity) gates for RBCs were identified in control experiments using anti-glycophorin A-PE labelled RBCs. The positive fluorescent gate was set using RBCs unlabelled with FITC-LA. For each measurement 10,000 events were gated. PS-positive cells were defined as all events falling within the preset FSC, SSC and positive fluorescent gates.
Statistics
Results are presented as the mean ± s.e.m. unless otherwise stated. Where appropriate, comparisons were made using paired Student's t tests. When transport activities were measured over a range of oxygen tensions, t tests followed by Holm–Šídák corrections for multiple comparisons were used (Graphpad Prism Version 6.0; GraphPad Software, Inc., La Jolla, CA, USA). Some correlations were made using the Pearson correlation test. The level of significance was set at P < 0.05.
Results
Effects of 5HMF on sickling in RBCs from SCD patients
In the first series of experiments, the effects of 5HMF on sickling were confirmed (Fig. 1). In the absence of 5HMF, sickling in fully deoxygenated RBCs approached about 80% of the total, compared with about 10% in fully oxygenated RBCs. The latter is probably indicative of the presence of irreversibly sickled cells, which sometimes accumulate in the circulation. 5HMF had no significant effect on percentage sickling at the two extremes of oxygen tension. At intermediate oxygen tensions (10–40 mmHg), however, 5HMF reduced sickling by about 50%, which is consistent with previous reports (Safo et al. 2004; Abdulmalik et al. 2005).
Figure 1. Effects of 5-hydroxymethyl-2-furfural (5HMF) on sickling of red blood cells (RBCs) from patients with sickle cell disease (SCD).
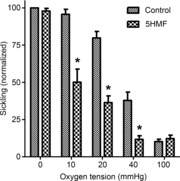
RBCs (15 % haematocrit, Hct) were pre-incubated with 5HMF (5 mm) for 30 min, after which they were equilibrated in Eschweiler tonometers at the indicated oxygen tension for 20 min. Aliquots were then fixed with glutaraldehyde and RBC shape assessed by light microscopy. The percentage of sickled RBCs at each oxygen tension was normalized to that at 0 mmHg O2 in the absence of 5HMF, which had a value of 84 ± 1.4% (range: 63–88%). Data are means ± s.e.m., n = 6 or 7. *P < 0.05 for control RBCs in the absence of 5HMF versus those incubated in its presence.
Effects of 5HMF on RBC volume in samples from SCD patients
The effects of 5HMF on RBC volume were then addressed (Fig. 2). In fully oxygenated RBCs, RBC volume was unaffected by the addition of 5HMF. Under low oxygen tension (≤15 mmHg) RBC volume declined significantly in control RBCs. By contrast, in RBCs also incubated with 5HMF, volume was unchanged compared with those held under fully oxygenated conditions and was significantly increased above control RBCs lacking 5HMF. The effect of cyclical deoxygenation/re-oxygenation was also tested. In this case, the presence of 5HMF again produced an increase in RBC volume compared with RBCs incubated in its absence. These effects of 5HMF are consistent with inhibition of the cation efflux pathways, which are upregulated in both oxygenated and deoxygenated RBCs from SCD patients (Lew & Bookchin, 2005). The mechanisms were therefore investigated further.
Figure 2. Effects of 5HMF on cell volume of red blood cells (RBCs) from patients with sickle cell disease (SCD).
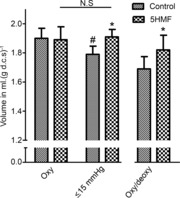
Cell volume was measured by wet weight minus dry weight and expressed as ml water per g dry cell solids [ml (g d.c.s.)−1] in RBCs maintained for 60 min under fully oxygenated conditions (Oxy), under continual deoxygenation (≤15 mmHg O2), or under cycles of deoxygenation/re-oxygenation (Oxy/deoxy), in which oxygen tension was changed from 100 mmHg to 0 mmHg every 10 min. Data are means ± s.d., n = 3–7, and in all cases were paired experiments carried out in the absence and presence of 5HMF. #P < 0.05 for control oxygenated RBCs versus control RBCs under the different deoxygenation protocols. *P < 0.05 for deoxygenated RBCs in the absence of 5HMF versus those incubated in its presence. NS, for oxygenated and deoxygenated RBCs in the presence of 5HMF.
Effects of 5HMF on the activity of Psickle in RBCs from SCD patients
The non-specific deoxygenation-induced conductance termed Psickle is thought to be activated upon reduction of oxygen tension, HbS polymerization and morphological sickling (Mohandas et al. 1986). Consistent with this postulate, 5HMF reduced Psickle activity (Fig. 3A), with inhibition of about 40% at 20 mmHg and 10 mmHg O2, and 15% at 0 mmHg O2. Sickling and Psickle activity correlated in both control RBCs (Pearson correlation, r = 0.885, P < 0.0001) and those treated with 5HMF (r = 0.789, P < 0.0001) (Fig. 3B).
Figure 3. Effects of 5HMF on the activity of Psickle in red blood cells (RBCs) from patients with sickle cell disease (SCD).
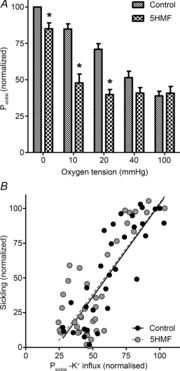
RBCs were treated as described in Fig. 1 except that after equilibration at different oxygen tensions in tonometers, aliquots were diluted 10-fold into test tubes for measurement of K+ influx [calculated as mmol (l cells h)−1 and measured at an extracellular [K+] of 7.5 mm], all in the absence or presence of 5HMF (5 mm). Psickle is defined as the K+ influx in Cl−-free saline in the presence of ouabain (100 μm), bumetanide (10 μm) and clotrimazole (5 μm). A, activity of Psickle at each oxygen tension was normalized to that at 0 mmHg O2 in the absence of 5HMF, which had a value of 1.51 ± 0.27 mmol (l cells h)−1 [range: 0.66–2.45 mmol (l cells h)−1]. Data are means ± s.e.m., n = 6 or 7. *P < 0.05 for control RBCs in the absence of 5HMF versus those incubated in its presence. B, correlation of sickling and activity of Psickle in RBCs from patients with SCD. Percentage sickling and activity of Psickle, from Figs 1 and 3A, both normalized to values in control RBCs at 0 mmHg, were correlated in RBCs pre-incubated with 5HMF (5 mm) or handled similarly but in the absence of this heterocyclic aldehyde. Pearson correlations were calculated as 0.885 in untreated RBCs (P < 0.0001) and 0.789 in the presence of 5HMF (P < 0.0001).
Effects of 5HMF on the activity of the Gardos channel in RBCs from SCD patients
The Gardos channel is also activated by deoxygenation, HbS polymerization and intracellular Ca2+ accumulation mediated probably via Psickle. Reducing sickling and Psickle activity might be expected to inhibit Gardos channel activity indirectly through reduction in Ca2+ entry. This pattern was observed (Fig. 4A). Inhibition of deoxygenation-induced activation of the Gardos channel was about 70% at 20 mmHg and 10 mmHg O2 and 30% at 0 mmHg O2, and thus larger than apparent with Psickle. A possible direct effect of 5HMF on the Gardos channel independent of any effects on Ca2+ entry was also tested. In these experiments, RBCs were treated with A23187 and a free extracellular Ca2+ of 10 μm (buffered with 2 mm EGTA) to produce near maximal activation of the Gardos channel. K+ flux approached about 300 mmol (l cells h)−1 and some evidence of saturation of 86Rb+ accumulation was observed by about 7 min (Fig. 4B). The similarity of the K+ uptake curves in the absence (filled symbols) and presence (open symbols) of 5HMF in ionophore-treated RBCs, however, argues against a direct effect of 5HMF on Gardos channel activity.
Figure 4. Effects of 5HMF on the activity of the Gardos channel in red blood cells (RBCs) from patients with sickle cell disease (SCD).
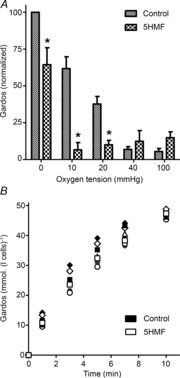
A, RBCs were treated as described in Fig. 3A, all in the absence or presence of 5HMF (5 mm). Gardos channel activity is defined as the K+ influx [calculated as mmol (l cells h)−1 and measured at an extracellular [K+] of 7.5 mm] in the absence or presence of clotrimazole (5 μm). Ouabain (100 μm) and bumetanide (10 μm) were included in all experiments. Activity of the Gardos channel at each oxygen tension was normalized to that at 0 mmHg O2 in the absence of 5HMF, which had a value of 3.1 ± 0.77 mmol (l cells h)−1 [range: 0.65–5.96 mmol (l cells h)−1]. Data are means ± s.e.m., n = 6 or 7. *P < 0.05 for control RBCs in the absence of 5HMF versus those incubated in its presence. B, effect of 5HMF on K+ uptake in Ca2+-loaded RBCs from patients with SCD. Total K+ uptake [given as mmol (l cells)−1 and measured at an extracellular [K+] of 80 mm] was measured over 10 min in RBCs treated with the ionophore A23187 (6 μm) in high K+-containing saline, at a free extracellular [Ca2+] of 10 μm (clamped with 2 mm EGTA), in the absence or presence of 5HMF (5 mm). Under these conditions, K+ uptake in the absence of ionophore was <0.5 mmol (l cells)−1 over 10 min. Ouabain (100 μm) and bumetanide (10 μm) were included in all experiments. Symbols represent paired data points from four different experiments.
Effects of 5HMF on deoxygenation-induced PS exposure in RBCs from SCD patients
Entry of Ca2+ into RBCs following HbS polymerization and activation of Psickle upon deoxygenation is also associated with increased externalization of PS mediated via the Ca2+-dependent scrambling process (Weiss et al. 2012; Cytlak et al. 2013). As well as inhibiting the Gardos channel, inhibition of Psickle would be expected to reduce deoxygenation-induced PS exposure. This was investigated by incubating RBCs from SCD patients in 24-well plates while they were shaken in a hypoxic incubator (20 mmHg O2) over 4 h. Results are shown in Fig. 5. Deoxygenation increased PS exposure and externalization was significantly reduced by treatment with 5HMF.
Figure 5. Effects of 5HMF on phosphatidylserine (PS) exposure in red blood cells (RBCs) from patients with sickle cell disease (SCD).
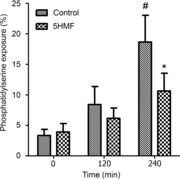
After a 60 min pre-incubation, RBC suspensions in Cl-MBS were deoxygenated (20 mmHg O2) in shaken 24-well plates in a low oxygen incubator for up to 4 h in the absence and presence of 5HMF (5 mm). Aliquots were removed at intervals and the percentage of RBCs positive for PS exposure measured using FACS. Data are means ± s.e.m., n = 4. #P < 0.05 for control RBCs at time 0 versus at 240 min. *P < 0.05 for control RBCs in the absence of 5HMF versus those incubated in its presence at 240 min.
Effects of 5HMF on activity of KCC in RBCs from SCD patients and normal individuals
Unlike in RBCs from normal individuals, in RBCs from SCD patients KCC activity is substantial and is observed in both oxygenated RBCs and following deoxygenation (Gibson et al. 1998), probably reflecting differences in the regulatory pathways in RBCs from these two genotypes (Merciris et al. 2001). KCC activity is shown in untreated RBCs from SCD patients and in the presence of 5HMF (Fig. 6). Under fully oxygenated conditions and also at oxygen tensions below the P50 for HbS, 5HMF produced a significant stimulation of KCC activity, although the magnitude of this effect declined with oxygen tension. In fully deoxygenated RBCs, 5HMF had no significant effect on KCC activity.
Figure 6. Effects of 5HMF on the activity of the K+–Cl− cotransporter (KCC) in red blood cells (RBCs) from patients with sickle cell disease (SCD).
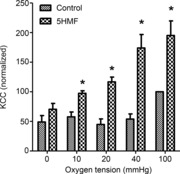
RBCs were treated as described in Fig. 3A, all in the presence or absence of 5HMF (5 mm). KCC activity is defined as the K+ influx [calculated as mmol (l cells h)−1 and measured at an extracellular [K+] of 7.5 mm] in the absence or presence of Cl− (substituted with NO3−). Ouabain (100 μm), bumetanide (10 μm) and clotrimazole (5 μm) were included in all experiments. Activity of KCC at each oxygen tension was normalized to that at 100 mmHg O2 in the absence of 5HMF, which had a value of 1.7 ± 0.31 mmol (l cells h)−1 [range: 0.56–2.54 mmol (l cells h)−1]. Data are means ± s.e.m., n = 5 or 6. *P < 0.05 for control RBCs in the absence of 5HMF versus those incubated in its presence.
KCC activity is regulated by a cascade of protein kinase and phosphatase enzymes with increased activity associated with the dephosphorylation of regulatory serine-threonine residues (Cossins et al. 1994; Gibson & Ellory, 2003). The effect of 5HMF could therefore be mediated either directly on the transporter or indirectly via these regulatory enzymes. These possibilities were investigated by pre-treating RBCs with NEM, an alkylating reagent which acts on –SH (sulphydryl) groups and which activates KCC and prevents inhibition by inhibitors of protein phosphatases, together with any effect by modalities such as volume and oxygen tension that require their activity (Lauf, 1983; Cossins et al. 1994). As expected, KCC activity was elevated by treatment with 1 mm NEM (Fig. 7). In the presence of NEM, the addition of 5HMF produced a modest inhibition of KCC activity, by contrast with the stimulation observed in the absence of NEM treatment.
Figure 7. Effects of N-ethylmaleimide (NEM) and 5HMF on the activity of KCC in red blood cells (RBCs) from patients with sickle cell disease (SCD).
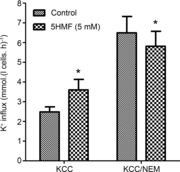
RBCs were pre-incubated for 30 min with or without NEM (1 mm) and/or 5HMF (5 mm) after which they were diluted 10-fold into test tubes for measurement of K+ influx, all in the absence or presence of 5HMF (5 mm). KCC activity is defined as the K+ influx [calculated as mmol (l cells h)−1 and measured at an extracellular [K+] of 7.5 mm] in the absence or presence of Cl− (substituted with NO3−). Ouabain (100 μm), bumetanide (10 μm) and clotrimazole (5 μm) were included in all experiments. Data are means ± s.e.m., n = 5. *P < 0.05 for RBCs in the absence versus presence of 5HMF.
KCC is also observed in oxygenated RBCs from normal individuals, albeit at lower levels of activity. 5HMF was therefore also tested in normal RBCs. KCC activity in control conditions increased from a mean ± s.e.m. of 0.32 ± 0.05 mmol (l cells h)−1 to 0.49 ± 0.07 mmol (l cells h)−1 (n = 3; P < 0.02). NEM also increased KCC activity in these RBCs, but in this case the addition of 5HMF had no significant effect.
Dose-dependence of 5HMF in RBCs from SCD patients
Previous studies on HbS solutions, whole blood or in sickle transgenic mice in vivo have used concentrations of 5HMF in the low millimolar range (Safo et al. 2004; Abdulmalik et al. 2005), similar to the concentration of Hb in RBCs. The dose-dependence of 5HMF for increased oxygen affinity and reduction of sickling in these previous studies indicate an IC50 of about 1 mm. It is also important to establish whether lower concentrations of the reagent are effective on the transport pathways studied here. This was investigated in the experiments shown in Fig. 8. Significant levels of inhibition of Psickle, the Gardos channel and PS exposure were apparent at 5HMF concentrations of 1 mm, 0.1 mm and 2 mm, respectively. In these deoxygenated RBCs, stimulation of KCC was significant by 5 mm.
Figure 8. Dose-dependence of 5HMF on the activities of Psickle, Gardos channel and KCC, and phosphatidylserine (PS) exposure in red blood cells (RBCs) from patients with sickle cell disease (SCD).
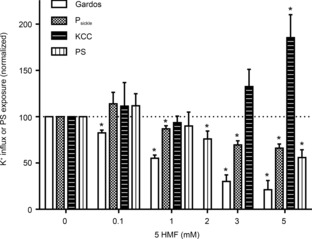
To measure transport activities, RBCs were treated as described in Figs 3A, 4A and 6 with transport activity measured as mmol (l cells h)−1 (at an extracellular [K+] of 7.5 mm) at 10 mmHg O2. PS exposure was measured as described in Fig. 5 at 20 mmHg O2. Transport activities and PS exposure were normalized to that in the absence of 5HMF. Data are means ± s.e.m., n = 4 for fluxes and n = 5 for PS exposure. *P < 0.05 in RBCs incubated in the presence of 5HMF versus in its absence.
Discussion
The present results demonstrate that 5HMF prevented dehydration of RBCs from SCD patients during maintained deoxygenation and during cyclical deoxygenation/re-oxygenation regimes. Further, 5HMF was found to inhibit two of the main cation pathways contributing to dehydration. Thus, as well as reducing morphological sickling in response to hypoxia, 5HMF inhibited both the deoxygenation-induced cation conductance (Psickle) and the Ca2+-activated K+ channel (the Gardos channel), probably by inhibiting Psickle-mediated Ca2+ entry. Consistent with the latter, deoxygenation-induced PS exposure, associated with Ca2+ entry through Psickle, was also reduced. The third pathway participating in solute loss, the KCl cotransporter (KCC), was stimulated, probably through an effect on its regulatory phosphorylation cascade.
5HMF is a naturally occurring, five-membered, heterocyclic aromatic aldehyde shown to interact allosterically with HbS to increase oxygen affinity and reduce sickling in vitro (Safo et al. 2004). 5HMF combines with HbS to form high-affinity Schiff bases, at least with the N-terminal α valine and probably at other sites (Safo et al. 2004). 5HMF is found in several food substances, such as honey, and along with other furanic compounds is present in traditional Chinese medicines given for SCD (Rehmanniae Radix) (Lin et al. 2008). Its low toxicity profile, which is better even than that of the aromatic aldehyde vanillin, makes it likely to be well tolerated in vivo. In vivo experiments with a transgenic mouse model of SCD showed that 5HMF was found to have good pharmacokinetic properties (Abdulmalik et al. 2005). As well as reducing RBC sickling, 5HMF prolonged the survival time of SCD mice under severe hypoxia. Similar findings were later observed in non-SCD mice under acute hypobaric hypoxia (Li et al. 2011). 5HMF appeared to be well tolerated in normal healthy volunteers, in whom it also produced significant increases in Hb oxygen affinity (Stern et al. 2012). It has now progressed to phase II clinical trials in patients with SCD (National Institutes of Health, 2013).
The current results also demonstrate powerful effects on volume and K+ permeability of RBCs from SCD patients. As its morphological effects on sickling led us to expect, 5HMF reduced activation of Psickle, a pathway thought to be activated by HbS polymerization and the sickling shape change (Mohandas et al. 1986). Psickle activity elevates intracellular Ca2+ and depletes Mg2+ (Ortiz et al. 1990; Rhoda et al. 1990; Willcocks et al. 2002). That the Ca2+-activated K+ channel or Gardos channel activity was also inhibited by 5HMF is consistent with these effects; its lack of effect following Ca2+ loading of RBCs with ionophore suggests the absence of a direct action of 5HMF on the channel. In a similar way, 5HMF also reduced deoxygenation-induced PS exposure, which is probably also mediated via Ca2+ entry and activation of the Ca2+-dependent scrambling process (Weiss et al. 2012; Cytlak et al. 2013). These findings and the correlation between percentage sickling and Psickle activity (Fig. 3) suggest that the main action of 5HMF on K+ permeability would appear to be linked to its effects on HbS polymerization. These observations are consistent with the mediation of the main effects of HbS on RBC cation permeability via its polymerization (Mohandas et al. 1986). However, the possibility of a direct inhibitory effect of 5HMF on Psickle cannot be excluded.
The effects of 5HMF on KCC are more complicated. In control RBCs, KCC activity was moderately increased and this effect was more evident at higher oxygen tensions. Following treatment with NEM, by contrast, 5HMF was found to modestly inhibit transporter activity. These findings are consistent with an action of 5HMF via the regulatory phosphorylation cascade that controls KCC activity (Cossins et al. 1994; Gibson & Ellory, 2003). It is possible that Mg2+ depletion, secondary to Psickle activity, may stimulate KCC through inhibition of protein phosphorylation (Delpire & Lauf, 1991; Muzyamba et al. 2006). If this was the case, however, a stimulatory effect of 5HMF would be expected to be more pronounced following deoxygenation, but this was not found. The exact mechanism by which 5HMF interacts with KCC requires further investigation. In this context, it is worth noting that 5HMF was previously reported to be without effect on Na+ transport pathways (Na+/K+ pump, Na+/H+ exchange and Na+–K+–2Cl− cotransporter) (Safo et al. 2004).
The present results demonstrate a protective action of 5HMF against RBC dehydration under continuous hypoxia and also during cyclical deoxygenation/re-oxygenation. By contrast, the insignificant effect of 5HMF on the volume of fully oxygenated RBCs suggests that although KCC activity was probably increased, the effect was negligible. It is worth pointing out that although KCC activity was increased by 5HMF, the absolute magnitudes of the fluxes were relatively small.
In conclusion, the hypothesis that 5HMF may reduce sickling through synergistic effects directly on HbS polymerization and indirectly through reduction in solute loss and increased hydration appear valid. The effect of 5HMF on K+ permeability and RBC volume would be useful measurements in clinical trials in SCD patients.
Acknowledgments
U.M.C. receives a Biotechnology and Biological Sciences Research Council studentship.
Glossary
- 5HMF
5-hydroxymethyl-2-furfural
- d.c.s.
dry cell solids
- Hb
haemoglobin
- KCC
K+–Cl− cotransporter
- NEM
N-ethylmaleimide
- PS
phosphatidylserine
- RBC
red blood cell
- SCD
sickle cell disease
Additional information
Competing interests
None declared.
Author contributions
The study was planned by J.S.G., A.H., D.C.R. and S.T. Experiments and data analyses were carried out by A.H. and U.M.C. The manuscript was written by J.S.G. and A.H., with additional input from D.C.R. and S.T. Samples were supplied by D.C.R. and S.T. All authors read and approved the final version of this manuscript.
Funding
Action Medical Research and the Medical Research Council are thanked for their financial support.
References
- Abdulmalik O, Safo MK, Chen Q, Yang J, Brugnara C, Ohene-Frempong K, Abraham DJ, Asakura T. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br J Haematol. 2005;128:552–561. doi: 10.1111/j.1365-2141.2004.05332.x. [DOI] [PubMed] [Google Scholar]
- Abraham DJ, Mehanna AS, Wireko FC, Whitney J, Thomas RP, Orringer EP. Vanillin, a potential agent for the treatment of sickle cell anemia. Blood. 1991;77:1334–1341. [PubMed] [Google Scholar]
- Arya R, Rolan PE, Wootton R, Posner J, Bellingham AJ. Tucaresol increases oxygen affinity and reduces haemolysis in subjects with sickle cell anaemia. Br J Haematol. 1996;93:817–821. doi: 10.1046/j.1365-2141.1996.d01-1744.x. [DOI] [PubMed] [Google Scholar]
- Beddell CR, Goodford PJ, Kneen G, White RD, Wilkinson S, Wootton R. Substituted benzaldehydes designed to increase the oxygen affinity of human haemoglobin and inhibit the sickling of sickle erythrocytes. Br J Pharmacol. 1984;82:397–407. doi: 10.1111/j.1476-5381.1984.tb10775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld N, Zachowski A, Galacteros F, Beuzard Y, Devaux PF. Transmembrane mobility of phospholipids in sickle erythrcoytes: effect of deoxygenation on diffusion and asymmetry. Blood. 1991;77:849–854. [PubMed] [Google Scholar]
- Borgese F, Motais R, Garcia-Romeu F. Regulation of Cl-dependent K transport by oxy-deoxyhemoglobin transitions in trout red cells. Biochim Biophys Acta. 1991;1066:252–256. doi: 10.1016/0005-2736(91)90194-d. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Forget BG. Hemoglobin: Molecular, Genetic and Clinical Aspects. Philadelphia, PA: Saunders; 1986. [Google Scholar]
- Charache S, Dover GJ, Moore RD, Eckert S, Ballas SK, Koshy M, Milner PFA, Orringer EP, Phillips G, Jr, Platt OS, Thomas GH. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992;79:2555–2565. [PubMed] [Google Scholar]
- Charache S, Dover GJ, Moyer MA, Moore JW. Hydroxyurea-induced augmentation of fetal hemoglobin production in patients with sickle cell anemia. Blood. 1987;69:109–116. [PubMed] [Google Scholar]
- Cossins AR, Weaver YR, Lykkeboe G, Nielsen OB. Role of protein phosphorylation in control of K flux pathways of trout red blood cells. Am J Physiol Cell Physiol. 1994;267:C1641–C1650. doi: 10.1152/ajpcell.1994.267.6.C1641. [DOI] [PubMed] [Google Scholar]
- Cytlak UM, Hannemann A, Rees DC, Gibson JS. Identification of the Ca2+ entry pathway involved in deoxygenation-induced phosphatidylserine exposure in red blood cells from patients with sickle cell disease. Pflugers Arch. 2013;465:1651–1660. doi: 10.1007/s00424-013-1308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpire E, Lauf PK. Magnesium and ATP dependence of K-Cl co-transport in low K+-sheep red blood cells. J Physiol. 1991;441:219–231. doi: 10.1113/jphysiol.1991.sp018747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham PB, Ellory JC. Passive potassium transport in low potassium sheep red cells: dependence upon cell volume and chloride. J Physiol. 1981;318:511–530. doi: 10.1113/jphysiol.1981.sp013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JW, Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987;70:1245–1266. [PubMed] [Google Scholar]
- Etzion Z, Tiffert T, Bookchin RM, Lew VL. Effects of deoxygenation on active and passive Ca2+ transport and on the cytoplasmic Ca2+ levels of sickle cell anemia red cells. J Clin Invest. 1993;92:2489–2498. doi: 10.1172/JCI116857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzharris P, McLean AEM, Sparks RG, Weatherley BC, White RD, Wootton R. The effects in volunteers of BW12C, a compound designed to left-shift the blood-oxygen saturation curve. Br J Clin Pharmacol. 1985;19:471–481. doi: 10.1111/j.1365-2125.1985.tb02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JS. Oxygen-sensitive cation transport in sickle cells. Blood Cells Mol Dis. 2001;27:112–120. doi: 10.1006/bcmd.2000.0361. [DOI] [PubMed] [Google Scholar]
- Gibson JS, Ellory JC. Membrane transport in sickle cell disease. Blood Cells Mol Dis. 2002;28:1–12. doi: 10.1006/bcmd.2002.0515. [DOI] [PubMed] [Google Scholar]
- Gibson JS. K+-Cl− cotransport in vertebrate red cells. In: Bernhardt I, Ellory JC, Ellory JC, editors. Red Cell Membrane Transport in Health and Disease. Berlin: Springer Verlag; 2003. pp. 197–220. [Google Scholar]
- Gibson JS, Speake PF, Ellory JC. Differential oxygen sensitivity of the K+-Cl− cotransporter in normal and sickle human red blood cells. J Physiol. 1998;511:225–234. doi: 10.1111/j.1469-7793.1998.225bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haest CWM. Distribution and movement of membrane lipids. In: Bernhardt I, Ellory JC, editors. Red Cell Membrane Transport in Health and Disease. Berlin: Springer Verlag; 2003. pp. 1–25. [Google Scholar]
- Hannemann A, Cytlak UM, Wilkins RJ, Ellory JC, Rees DC. The use of radioisotopes to characterise the abnormal permeability of red blood cells from sickle cell patients. In: Singh N, Gibson JS, editors. Radioisotopes: Applications in Bio-medical Science. Rijeka: InTech; 2011. pp. 151–172. [Google Scholar]
- Joiner CH. Cation transport and volume regulation in sickle red blood cells. Am J Physiol Cell Physiol. 1993;264:C251–C270. doi: 10.1152/ajpcell.1993.264.2.C251. [DOI] [PubMed] [Google Scholar]
- Joiner CH, Dew A, Ge DL. Deoxygenation-induced fluxes in sickle cells. I. Relationship between net potassium efflux and net sodium influx. Blood Cells. 1988;13:339–348. [PubMed] [Google Scholar]
- de Jong K, Larkin SK, Styles LA, Bookchin RM, Kuypers FA. Characterization of the phosphatidylserine-exposing subpopulations of sickle cells. Blood. 2001;98:860–867. doi: 10.1182/blood.v98.3.860. [DOI] [PubMed] [Google Scholar]
- Keidan AJ, White RD, Huehns ER, Franklin IM, Joy M, Stuart J. Effect of BW12C on oxygen affinity of haemoglobin in sickle-cell disease. Lancet. 1986;327:831–834. doi: 10.1016/s0140-6736(86)90941-4. [DOI] [PubMed] [Google Scholar]
- Kneen G, White RD. BW12C: a new anti-sickling agent. Br J Pharmacol. 1981;74:965–970. [Google Scholar]
- Kuypers FA. Phospholipid asymmetry in health and disease. Curr Opin Hematol. 1998;5:122–131. doi: 10.1097/00062752-199803000-00007. [DOI] [PubMed] [Google Scholar]
- Kuypers FA. Red cell membrane lipids in hemoglobinopathies. Curr Mol Med. 2008;8:633–638. doi: 10.2174/156652408786241429. [DOI] [PubMed] [Google Scholar]
- Lauf PK. Thiol-dependent passive K/Cl transport in sheep red cells. I. Dependence on chloride and external K+ (Rb+) ions. J Membr Biol. 1983;73:237–246. doi: 10.1007/BF01870538. [DOI] [PubMed] [Google Scholar]
- Lew VL, Bookchin RM. Ion transport pathology in the mechanism of sickle cell dehydration. Physiol Rev. 2005;85:179–200. doi: 10.1152/physrev.00052.2003. [DOI] [PubMed] [Google Scholar]
- Li M-M, Wu L-Y, Zhao T, Wu K-W, Xiong L, Zhu L-L, Fan M. The protective role of 5-hydroxymethyl-2-furfural (5-HMF) against acute hypobaric hypoxia. Cell Stress Chaperones. 2011;16:529–537. doi: 10.1007/s12192-011-0264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A-S, Qian K, Usami Y, Lin L, Itokawa H, Hsu C, Morris-Natshke SL, Lee K-H. 5-hydroxmethyl-2-furfural, a clinical trials agent for sickle cell anemia, and its mono/di-glucosides from classically processed steamed Rehmanniae Radix. J Nat Med. 2008;62:164–167. doi: 10.1007/s11418-007-0206-z. [DOI] [PubMed] [Google Scholar]
- Lubin B, Chiu D, Bastacky J, Roelofsen B, Van Deenen LLM. Abnormalities in membrane phospholipid organization in sickled erythrocytes. J Clin Invest. 1981;67:1643–1649. doi: 10.1172/JCI110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y-L, Rees DC, Gibson JS, Ellory JC. The conductance of red blood cells from sickle cell patients: ion selectivity and inhibitors. J Physiol. 2012;590:2095–2105. doi: 10.1113/jphysiol.2012.229609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merciris P, Hardy-Dessources MD, Giraud F. Deoxygenation of sickle cells stimulates Syk tyrosine kinase and inhibits a membrane tyrosine phosphatase. Blood. 2001;98:3121–3127. doi: 10.1182/blood.v98.10.3121. [DOI] [PubMed] [Google Scholar]
- Mohandas N, Rossi ME, Clark MR. Association between morphologic distortion of sickle cells and deoxygenation-induced cation permeability increases. Blood. 1986;68:450–454. [PubMed] [Google Scholar]
- Muzyamba MC, Campbell EH, Gibson JS. Effect of intracellular magnesium and oxygen tension on K+-Cl− cotransport in normal and sickle human red cells. Cell Physiol Biochem. 2006;17:121–128. doi: 10.1159/000092073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel RL. General pathophysiology of sickle cell anemia. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, Platt OS, editors. Disorders of Hemoglobin. Cambridge: Cambridge University Press; 2001. pp. 494–526. [Google Scholar]
- National Institutes of Health. 2013. Evaluation of different dose regimes of Aes-103 given for 28 days to subjects with stable sickle cell disease http://www.clinicaltrials.gov/ct2/show/NCT01987908/
- Ortiz OE, Lew VL, Bookchin RM. Deoxygenation permeabilizes sickle cell anaemia red cells to magnesium and reverses its gradient in the dense cells. J Physiol. 1990;427:211–226. doi: 10.1113/jphysiol.1990.sp018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz MF, Mitchison JM. The state of haemoglobin in sickle-cell anaemia. Nature. 1950;166:677–679. doi: 10.1038/166677a0. [DOI] [PubMed] [Google Scholar]
- Platt OS. Hydroxyurea for the treatment of sickle cell anemia. N Engl J Med. 2008;358:1362–1369. doi: 10.1056/NEJMct0708272. [DOI] [PubMed] [Google Scholar]
- Rees DC. The rationale for using hydroxycarbamide in the treatment of sickle cell disease. Haematologica. 2011;96:488–491. doi: 10.3324/haematol.2011.041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- Rhoda MD, Apovo M, Beuzard Y, Giraud F. Ca2+ permeability in deoxygenated sickle cells. Blood. 1990;75:2453–2458. [PubMed] [Google Scholar]
- Safo MK, Abdulmalik O, Danso-Danquah R, Burnett JC, Nokuri S, Joshi GS, Musayev FN, Asakura T, Abraham DJ. Structural basis for the potent antisickling effect of a novel class of five-membered heterocyclic aldehydic compounds. J Med Chem. 2004;47:4665–4676. doi: 10.1021/jm0498001. [DOI] [PubMed] [Google Scholar]
- Speake PF, Roberts CA, Gibson JS. Effect of changes in respiratory blood parameters on equine red blood cell K-Cl cotransporter. Am J Physiol Cell Physiol. 1997;273:C1811–C1818. doi: 10.1152/ajpcell.1997.273.6.C1811. [DOI] [PubMed] [Google Scholar]
- Steinberg MH. Management of sickle cell disease. New Engl J Med. 1999;340:1021–1030. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- Stern W, Matthews D, McKew JC, Shen J, Kato GJ. A phase 1, first-in-man, dose–response study of Aes-103 (5-HMF), an anti-sickling, allosteric modifier of hemoglobin oxygen affinity in healthy normal volunteers. 2012. p. 3210. American Society of Hematology 54th Annual Meeting, 8–11 December 2012, Atlanta, GA.
- Weiss E, Cytlak UM, Rees DC, Osei A, Gibson JS. Deoxygenation-induced and Ca2+-dependent phosphatidylserine externalisation in red blood cells from normal individuals and sickle cell patients. Cell Calcium. 2012;51:51–56. doi: 10.1016/j.ceca.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Willcocks JP, Mulquiney PJ, Ellory JC, Veech RL, Radda GK, Clarke K. Simultaneous determination of low free Mg2+ and pH in human sickle cells using 31P NMR spectroscopy. J Biol Chem. 2002;277:49911–49920. doi: 10.1074/jbc.M207551200. [DOI] [PubMed] [Google Scholar]
- Zaugg RH, Walder JA, Klotz IM. Schiff base adducts of hemoglobin. Modifications that inhibit erythrocyte sickling. J Biol Chem. 1977;252:8542–8548. [PubMed] [Google Scholar]


