Abstract
Pan is a poly(A)-specific 3′ exoribonuclease that, together with the CCR4-NOT complex, is responsible for initiating and controlling mRNA decay by degradation of the poly(A) tail. Now, more than twenty years after the enzyme's discovery, a surge of recent papers, including one in this issue of The EMBO Journal (Wolf et al, 2014) has revealed details of its unusual asymmetric structure and aspects of its mode of substrate binding.
See also: J Wolf et al (July 2014), S Jonas et al and B Schafer >et al
With few exceptions, eukaryotic mRNAs are distinguished from all other types of RNA by the unique combination of an m7G cap structure at the 5′ end and a poly(A) tail at the 3′ end. Cap and poly(A) tail share roles in two important cellular processes, the initiation of translation and the control of mRNA half-life. Unlike the cap, the poly(A) tail is not an all-or-nothing modification; it can be extended by poly(A) polymerases or shortened by poly(A)-specific 3′ exonucleases. Tail elongation can be used to promote, and tail shortening to inhibit, translation of specific messages, although recent evidence suggests that this regulatory mechanism may be restricted to certain cell types (Chang et al, 2014; Subtelny et al, 2014).
In most cells, poly(A) tail shortening—or deadenylation—serves mainly as a prelude to mRNA decay. Deadenylation begins more or less immediately after birth of a message; progress beyond a certain threshold (e.g. below a length of ˜12 nucleotides in yeast) signals mRNA ‘death’, either by decapitation (i.e. cap hydrolyzis) or by 3′-to-5′ degradation of the mRNA body. Continuous shortening from a relatively uniform initial length is the reason for the heterogeneous poly(A) tail length distribution seen in the steady-state mRNA population. The shortening rate is not the same for all mRNAs, and indeed, mRNA half-life is controlled by deadenylation rate differences. To this end, cells use either sequence-specific RNA-binding proteins or miRNAs to recruit poly(A) degrading exonucleases—hence the intense interest in such enzymes (Wahle & Winkler, 2013).
The Poly(A) nuclease (Pan), discovered more than twenty years ago as the first deadenylase in S. cerevisiae, has since been found to be almost universally conserved, although the CCR4-NOT complex is now considered the more important poly(A)-degrading enzyme. Classical purification of Pan revealed two subunits, Pan2 and Pan3 (Boeck et al, 1996; Brown et al, 1996). Pan2 contains an N-terminal WD40 domain followed by a low complexity ‘linker’ sequence, a ubiquitin-specific protease (USP)-like domain lacking catalytic residues, and a C-terminal 3′ exonuclease domain of the DEDD type (Fig1A). DEDD enzymes hydrolyze phosphodiester bonds by means of a two-metal-ion mechanism, and it has been confirmed that Pan's enzymatic activity resides in the DEDD domain (Uchida et al, 2004; Jonas et al, 2014; Schäfer et al, 2014; Wolf et al, 2014).
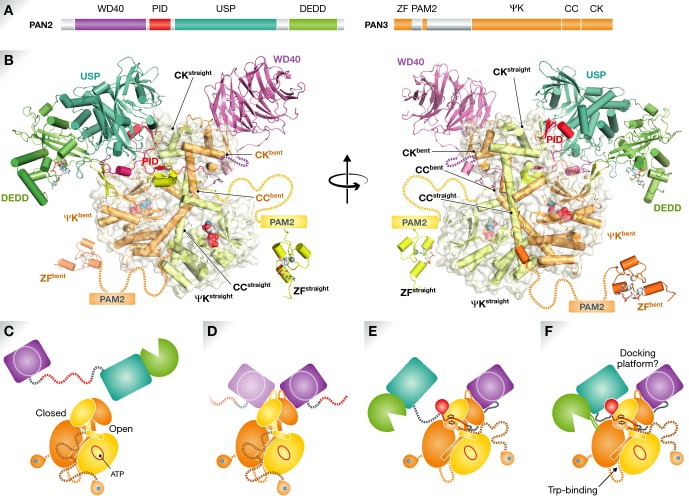
Figure 1. Organization of the Poly(A) nuclease Pan.
(A) Schematic of Pan2 and Pan3 domain (USP, ubiquitin-specific protease; DEDD, exonuclease; PID, Pan3-interacting domain; ZF, zinc finger; PAM2, PABP-interacting motif-2; ψK, pseudokinase; CC, coiled coil; CK, C-terminal knob). (B) Composite model of the Pan2–Pan3 complex derived from the available structural data (Jonas et al, 2014; Schäfer et al, 2014; Wolf et al, 2014) in two orientations. Asymmetry within the homodimeric Pan3 ψK–CK domain is most obvious in the coiled-coil helices (straight versus bent) of each protomer. The Pan2 linker connecting the WD40 and USP domains wraps around the waist of the Pan3 ψK–CK dimer, with the PID folding into the narrow groove between the ψKbent and CKstraight domains. PID and USP binding precludes recruitment of a second WD40 domain to CKstraight, ensuring 1:2 stoichiometry. The DEDD active site (marked by a docked A3 trinucleotide, sticks) is brought into vicinity of the two ψK ATP binding sites (spheres). Pan3 N-terminal zinc finger domains are presumably flexibly linked to the complex; the linker region includes PAM2. Note that it is not certain whether all interactions depicted in this composite model occur simultaneously. (C–F) Hypothetical Pan (sub)assemblies; an ordered assembly of the complex should not be inferred. (C) Both Pan2 and Pan3 possess globular N- and C-terminal domains that are thought to be separated by flexible linker peptides in the isolated proteins. (D) The asymmetric Pan3 C-terminal ψK–CK dimer is able to bind to two Pan2 WD40 domains in the absence of PID (binding), causing ordering of a conserved CK loop. (E) Engagement and folding of PID in the ψK-CK closed groove is mutually incompatible with the presence of a second Pan2 molecule. PID binding results in a restructuring of the Pan3 N-terminal segment with formation of a short antiparallel β-sheet. (F) Docking of the Pan2 USP and DEDD domains positions the nuclease active site close to the ATP binding sites of ψK, which binds to and structures an otherwise disordered DEDD domain loop. See text for further details.
From Pan's very first characterization, one of its defining characteristics was the dependence of its nuclease activity on the cytoplasmic poly(A)-binding protein (Pab1 in yeast; PABPC in humans), PABPC dependence being mediated by Pan3 (Mangus et al, 2004; Uchida et al, 2004). A short PABP-interacting motif 2 (PAM2) close to the N-terminus of Pan3 (Fig1A) binds the C-terminal domain of PABPC, as observed for other PAM2-containing proteins (Siddiqui et al, 2007), and point mutations affecting this interaction result in elongated poly(A) tails in yeast (Mangus et al, 2004; Siddiqui et al, 2007). While these data suggest that PABPC is required for Pan activity in vivo, all three new papers reveal that the PABPC dependence of Pan is less pronounced than once thought, with only a fourfold stimulation in vitro (Wolf et al, 2014). Even in the absence of PABPC, Pan remains poly(A)-specific, that is, the enzyme has an intrinsic substrate specificity.
Wolf et al (2014) report that not only PABPC interaction, but also direct RNA recognition is a function of Pan3: a CCCH-type zinc finger at the very N-terminus of Pan3 specifically binds poly(A), and its deletion modestly reduces Pan activity. The C-terminal part of Pan3 also binds RNA, albeit without sequence specificity. This portion of Pan3 contains a pseudokinase domain (ψK), which retains ATP binding properties but no kinase activity due to structural rearrangements and loss of active site residues (Christie et al, 2013). A long α-helix links the ψK domain to a globular C-terminal domain (C-terminal knob, CK) (Fig1B). The Pan3 ψK–CK region homodimerizes through the long central helices of the protomers, which form an intermolecular coiled-coil. Surprisingly, however, the Pan3 dimer is markedly asymmetric; whereas the central α-helix of one protomer is straight, that of the second protomer exhibits a pronounced kink. This in turn results in a close approach of the ψKbent and CKstraight domains and an accompanying separation of the opposing ψKstraight and CKbent domains (Fig1B).
So, how might Pan3 bring Pan2 and the poly(A) substrate together? In order to answer this question, three groups have adopted a ‘divide and conquer’ approach (Fig1B). Focussing on the Pan2 N-terminal region, the Izaurralde group demonstrates that the WD40 propeller binds exclusively to the Pan3 CK domain (Jonas et al, 2014), leading to an ordering of an otherwise disordered conserved loop between the central α-helix and CK in Pan3. Unusual for WD40 interactions, it is the edge of the propeller that contacts CK, leaving the usual ‘top’ binding surface free. As Pan3 is a dimer, two WD40 domains can bind in a symmetric arrangement. Extension of the Pan2 sequence beyond the WD40 domain results in an unexpected phenomenon, however: inclusion of more than ca. 100 additional Pan2 linker residues switches Pan2:Pan3 stoichiometry of 2:2 to 1:2.
A structural explanation for this is provided by the Passmore group (Wolf et al, 2014). They show that the Pan3 ψK–CK dimer binds with high affinity (Kd ˜ 10 nM) to a peptide from the Pan2 linker that they term the ‘Pan3-interacting domain’ (PID). The structure of the peptide complex reveals two major interaction sites: the PID N-terminal region binds in a narrow groove between the ψKbent and CKstraight domains, whereas the C-terminal region folds into a mini-domain that extends the two-stranded β-sheet of the CKstraight domain by a further two strands. As this latter interaction site overlaps with the WD40 binding site on the CKstraight domain, only one WD40–PID-containing protein can bind to the Pan3 dimer (at the CKbent domain). Insertion of the N-terminal PID region leads to at least one structural rearrangement in Pan3: residues immediately N-terminal to the ψK–CKstraight domain reorder to form a short antiparallel β-sheet with the PID peptide, potentially reorienting the PAM2 sequence and the N-terminal Zn-finger domain.
The direct observation of a 1:2 core complex by the Conti group (Schäfer et al, 2014) provides the icing on the cake, achieved with the help of constructs devoid of the Pan2 WD40 domain and of the Pan3 Zn-finger domains—although it proved crucial to include Pan3 residues N-terminal to the ψK–CK domain. Despite a resolution of 3.8 Å, the structure brings to light a number of intricate interactions: (i) Pan2 residues N-terminal to the PID peptide wrap around the ψK–CK domain, occupying the open groove between the ψKstraight and CKbent domains; (ii) residues of the Pan2 USP domain contact the Pan3 CKstraight domain (and probably also the folded PID C-terminal mini-domain) in a position that is also incompatible with binding of a second WD40 domain; and (iii) the DEDD domain active site juxtaposes the ψKbent domain. Comparison of bound and free Pan2 C-terminal domains (Jonas et al, 2014; Schäfer et al, 2014) also reveals that a disordered surface loop of the DEDD domain enters into an interaction with the ψKbent domain in a manner reminiscent of the activation loops of bona fide protein kinases.
How the Pan complex interacts with RNA is less clear. In addition to the zinc finger interaction, Wolf et al (2014) note a positively charged surface on Pan3 that might interact with the phosphate backbone of RNA. The residual ATP binding site in the ψK domain is required for mRNA deadenylation in vivo (Christie et al, 2013), and Schäfer et al (2014) postulate that this may represent a recognition site for poly(A) substrate bases. Although these interactions are each of low affinity, with KDs in the micromolar or even high micromolar range (discussed in Wolf et al, 2014), their multiplicity could well result in a high overall avidity of Pan for RNA, especially since each interaction can in principle occur twice in the homodimer. It should also be remembered that the complex can be recruited to RNA by other proteins. As an example, GW182 proteins are effector proteins that associate with miRNAs via interaction with argonaute proteins and recruit both Pan and the CCR4-NOT complex to miRNA targets (Braun et al, 2011; Chekulaeva et al, 2011; Fabian et al, 2011). GW182 proteins possess unstructured regions containing tryptophan residues known to be required for Pan3 binding. Serendipitously, the Pan3 homodimer crystal structure revealed a binding pocket for tryptophan at the base of the ψK–CK dimer near the dimer axis, and pull-down assays with mutant proteins confirmed that this pocket is indeed important for the Pan3–GW182 interaction (Christie et al, 2013). Exposure of the ‘usual’ WD40 propeller binding surface as well as of the vestigial ‘ubiquitin binding site’ in the USP-like domain (Jonas et al, 2014; Schäfer et al, 2014) also raises the possibility of a recruitment module at the ‘top’ of the Pan complex.
Summing up, all known interactions of Pan with its RNA substrate, be they direct (zinc finger, C-terminal domain) or indirect (via PABPC or GW182), depend on Pan3. Consistent with Pan2 contributing nothing but its active site to the RNA interactions, the isolated polypeptide has low nuclease activity and no detectable RNA-binding activity (Wolf et al, 2014). The inherent poly(A) specificity of Pan remains to be explained; the Pan3 zinc finger is unlikely to be solely responsible. It will therefore be of interest to know whether the Pan2 active site itself contributes to substrate specificity.
The recent structures provide tantalizing insights into the intricate organization of Pan, yet they also raise a wealth of new questions. Can all the Pan2–Pan3 interactions observed in the crystal structures (of protein constructs derived from different organisms) be satisfied simultaneously? Do both Pan3 protomers contribute to substrate binding and feeding to the Pan2 nuclease domain? Might PABPC1 and/or RNA substrate binding alter the Pan complex formation, organization and/or dynamics? And can the asymmetry of Pan3 be switched, allowing temporary engagement of a second Pan2 molecule? Deciphering Pan's secrets continues to be a rewarding task.
Acknowledgments
The authors are grateful to E. Conti, E. Izaurralde, and L. Passmore for providing coordinates prior to publication.
References
- Boeck R, Tarun S, Rieger M, Deardorff JA, Müller-Auer S, Sachs AB. The yeast Pan2 protein is required for poly(A)-binding protein-stimulated poly(A)-nuclease activity. J Biol Chem. 1996;271:432–438. doi: 10.1074/jbc.271.1.432. [DOI] [PubMed] [Google Scholar]
- Braun JE, Huntzinger E, Fauser M, Izaurralde E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol Cell. 2011;44:120–133. doi: 10.1016/j.molcel.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Brown CE, Tarun SZ, Boeck R, Sachs AB. PAN3 encodes a subunit of the Pab1p-dependent poly(A) nuclease in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:5744–5753. doi: 10.1128/mcb.16.10.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Lim J, Kim VN. TAIL-seq: genome-wide determination of poly(A) tail length and 3′ end modifications. Mol Cell. 2014;53:1044–1052. doi: 10.1016/j.molcel.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Chekulaeva M, Mathys H, Zipprich JT, Attig J, Colic M, Parker R, Filipowicz W. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat Struct Mol Biol. 2011;18:1218–1226. doi: 10.1038/nsmb.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M, Boland A, Huntzinger E, Weichenrieder O, Izaurralde E. Structure of the Pan3 pseudokinase reveals the basis for interactions with the Pan2 deadenylase and the GW182 proteins. Mol Cell. 2013;51:360–373. doi: 10.1016/j.molcel.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Fabian MR, Cieplak MK, Frank F, Morita M, Green J, Srikumar T, Nagar B, Yamamoto T, Raught B, Duchaine TF, Sonenberg N. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat Struct Mol Biol. 2011;18:1211–1217. doi: 10.1038/nsmb.2149. [DOI] [PubMed] [Google Scholar]
- Jonas S, Christie M, Peter D, Bhandari D, Loh B, Huntzinger E, Weichenrieder O, Izaurralde E. An asymmetric PAN3 dimer recruits a single PAN2 exonuclease to mediate mRNA deadenylation and decay. Nat Struct Mol Biol. 2014 doi: 10.1038/nsmb.2837. DOI: 10.1038/nsmb.2837. [DOI] [PubMed] [Google Scholar]
- Mangus DA, Evans MC, Agrin NS, Smith M, Gongidi P, Jacobson A. Positive and negative regulation of poly(A) nuclease. Mol Cell Biol. 2004;24:5521–5533. doi: 10.1128/MCB.24.12.5521-5533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer IB, Rode M, Bonneau F, Schüssler S, Conti E. The structure of the Pan2-Pan3 core complex reveals crosstalk between deadenylase and pseudokinase. Nat Struct Mol Biol. 2014 doi: 10.1038/nsmb.2834. DOI: 10.1038/nsmb.2834. [DOI] [PubMed] [Google Scholar]
- Siddiqui N, Mangus DA, Chang T-C, Palermino J-M, Shyu A-B, Gehring K. Poly(A) nuclease interacts with the C-terminal domain of polyadenylate-binding protein domain from poly(A) -binding protein. J Biol Chem. 2007;282:25067–25075. doi: 10.1074/jbc.M701256200. [DOI] [PubMed] [Google Scholar]
- Subtelny AO, Eichhorn SW, Chen GR, Sive H, Bartel DP. Poly(A) tail profiling reveals an embryonic switch in translational control. Nature. 2014;508:66–71. doi: 10.1038/nature13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Hoshino S, Katada T. Identification of a human cytoplasmic poly(A) nuclease complex stimulated by poly(A)-binding protein. J Biol Chem. 2004;279:1383–1391. doi: 10.1074/jbc.M309125200. [DOI] [PubMed] [Google Scholar]
- Wahle E, Winkler GS. RNA decay machines: deadenylation by the Ccr4-Not and Pan2-Pan3 complexes. Biochim Biophys Acta. 2013;1829:561–570. doi: 10.1016/j.bbagrm.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Wolf J, Valkov E, Allen MD, Meineke B, Gordiyenko Y, McLaughlin SH, Olsen TM, Robinson CV, Bycroft M, Stewart M, Passmore LA. Structural basis for Pan3 binding to Pan2 and its function in mRNA recruitment and deadenylation. EMBO J. 2014;33:1514–1526. doi: 10.15252/embj.201488373. [DOI] [PMC free article] [PubMed] [Google Scholar]