Abstract
Purpose of review
Recent clinical trials using T-cell engaging immunotherapies such as bispecific antibodies which target T cells and tumor cells, as well as engineered T cells that express targeting and activation molecules known as chimeric antigen receptors, have demonstrated powerful proof of concept. These therapies result in a significant degree of immune activation in the patient, which has correlated with greatly increased efficacy but also with notable toxicity. These therapies produce nonphysiologic T-cell activation, which is the hallmark of these new, highly active treatments.
Recent findings
We and others have noted cytokine activation profiles that correlate with both toxicity and efficacy in patients receiving T-cell engaging therapies. Effector cytokines such as interferon-γ are elevated, but so are cytokines that are associated with macrophage activation syndrome/hemophagocytic lymphohistiocytosis, such as interleukin (IL)-10 and IL-6. Although corticosteroids can control some of these toxicities, a targeted approach may produce superior toxicity control without interfering with efficacy. One approach we have developed targets IL-6, a key cytokine in the toxicity response, using the IL-6 receptor antagonist tocilizumab.
Summary
Detailed studies of the T-cell activation produced by these novel therapies has led to more targeted approaches that have the potential to control toxicity while maintaining efficacy.
Keywords: Acute lymphoblastic leukemia, blinatumomab, chimeric antigen receptor, cytokine release syndrome
INTRODUCTION
The recent development of a number of novel T-cell engaging therapies has come with considerable promise to treat patients with refractory malignancies; however, as active forms of these therapies have become available, we have also seen unique toxicities. Two of these, chimeric antigen receptor-modified T cells (CART) with specificity against CD19 and blinatumomab, a novel bispecific T-cell engaging (BiTE) single-chain antibody construct that links CD3+ T lymphocytes with CD19+ B cells, have demonstrated remarkable efficacy against lymphoid malignancies in early phase clinical trials [1,2▪▪,3,4]. Porter et al. [1] demonstrated that CART against CD19 (CART-19) is highly effective in adults with relapsed/refractory chronic lymphocytic leukemia. Our group then showed that CART-19 is very effective in children with relapsed/refractory acute lymphoblastic leukemia (ALL), results later confirmed by other groups in adults with ALL [2▪▪,5▪]. Although it is effective, patients treated with CART often develop cytokine release syndrome (CRS, also referred to as ‘cytokine storm’) that can be mild to very severe. Similarly, blinatumomab was shown to be highly active in adults and children with relapsed/refractory ALL and in adults with relapsed/refractory non-Hodgkin’s lymphoma, and patients treated with blinatumomab also commonly develop CRS [4,6▪▪,7,8▪].
Interferon-γ (IFN-γ) is one principal effector cytokine that is markedly elevated in patients treated with CART-19 and blinatumomab who develop CRS [1,2▪▪,6▪▪,9]. Less predictably, the cytokines interleukin-6 (IL-6) and IL-10 are elevated after such therapies, with IL-6 showing very marked elevation in some patients. Interestingly, these cytokines are also elevated in patients who develop macrophage activation syndrome/hemophagocytic lymphohistiocytosis (MAS/HLH), and we hypothesized and subsequently demonstrated that some patients treated with CART and blinatumomab develop a clinical picture that mirrors HLH, raising the question of whether abnormal activation of macrophages is driving the cytokine storm after these therapies [2▪▪,10▪▪]. We also showed that cytokine-directed therapy using the IL-6 receptor (IL-6R) inhibitor tocilizumab could reverse clinically significant CRS without appearing to compromise the efficacy of the T-cell engaging therapy [2▪▪,10▪▪]. This review is dedicated to describing the toxicities of these novel T-cell engaging therapies, with particular focus on the biology and management of CRS.
BLINATUMOMAB: CLINICAL ACTIVITY AND TOXICITY PROFILE
Blinatumomab belongs to a new class of bispecific T cell-engagers (BiTE) [11]. BiTEs direct T-effector memory cells toward target cells and trigger target cell-specific cytotoxicity, leading to cell lysis. Blinatumomab targets CD19. In humans, CD19 is only expressed on B cells, and it is developmentally expressed from very early in the B cell lineage (early pro-B) through mature B cells [12]. Blinatumomab was shown to be very active in preclinical models of B cell malignancies, leading to clinical trials using the drug [13]. Blinatumomab was first studied in adults with lymphoma, demonstrating a greater than 35% objective response rate in patients with refractory disease [3].
Blinatumomab was studied in a phase 2 study in adults with minimal residual disease (MRD)+ ALL [4]. On this study, adults were treated at 15µg/m2/day continuous intravenous infusion over 4-week cycles. The primary efficacy endpoint of this trial was conversion from MRD-positive to MRD-negative, and 16 of 21 individuals met this endpoint. Many of the individuals underwent allogeneic hematopoietic stem cell transplant (HSCT). A subset of patients did not have a HSCT after blinatumomab, and some of them remain in remission (six of 11 individuals) with a median follow up of almost 3 years [8▪]. Based on these data, a phase 2 dose escalation trial of blinatumomab in adults with refractory/relapsed ALL was initiated [7]. The majority of individuals on this trial had marked replacement of bone marrow with leukemic blasts at the initiation of treatment. Early data demonstrate an impressive 75% morphologic complete response (CR) rate. Blinatumomab is currently under investigation in a phase 1 trial for children with relapsed/refractory B-cell acute lymphoblastic leukemia (NCT01471782). Early results of the trial are promising, with a 47% morphologic and MRD negative (<10−4 by PCR) CR rate [14]. A dose has been defined and the phase 2 portion of the study opened to accrual in October 2013.
In all of these studies, a number of patients developed significant toxicities, including CRS, disseminated intravascular coagulation, and central nervous system events (seizure or encephalopathy). Serious adverse events appear to be more common in patients treated with gross morphologic disease. Lowering the initial dose of blinatumomab to 5µg/m2/day and the addition of dexamethasone has been shown to reduce the toxicity of the agent [4,6▪▪]. Despite these efforts, some individuals develop more severe CRS that can progress to multisystem organ failure, and which, rarely, has been fatal. CRS has only been found during the first cycle of treatment. Presence or absence of CRS does correlate with treatment response; however, the severity of CRS varies between patients and does not appear to be associated with degree of response [6▪▪].
CART AGAINST CD19: CLINICAL ACTIVITY AND TOXICITY DATA
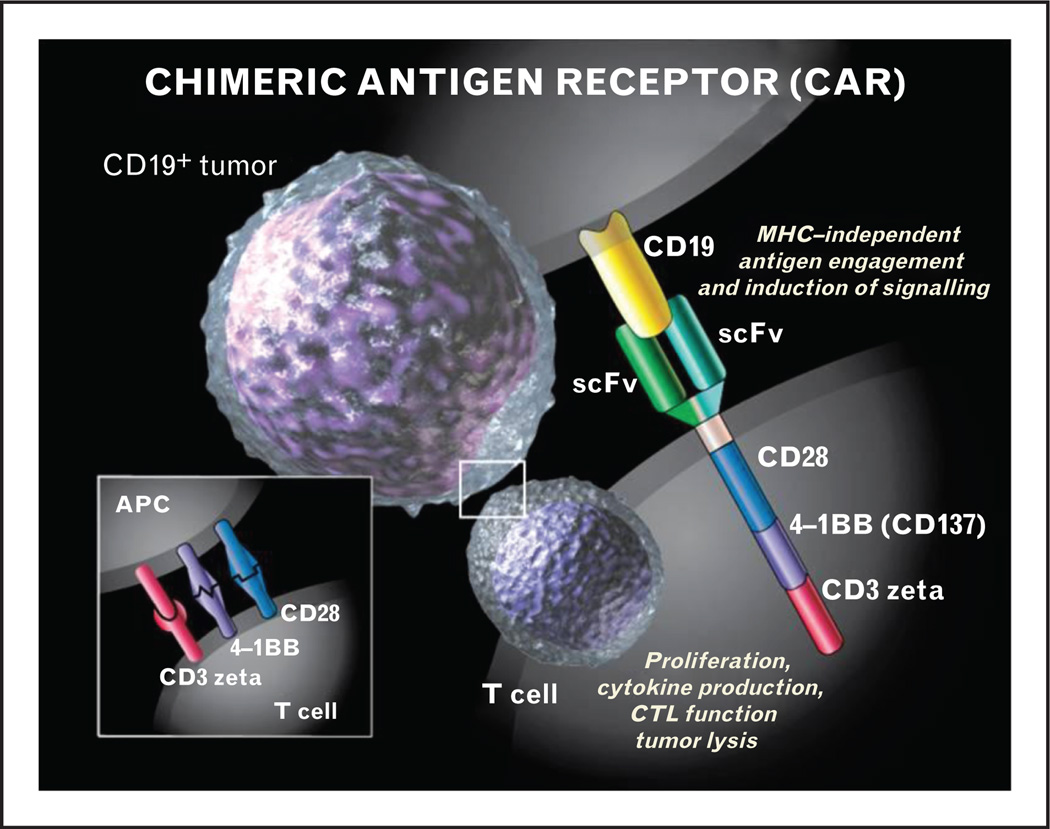
A second strategy to target CD19 is to use adoptive transfer of lymphocytes armed with chimeric antigen receptors (CAR) (Fig. 1) [15▪]. Although the concept of the CAR was described by Eshhar et al. [16] more than 20 years ago, limited expansion and persistence of the engineered T cells led to minimal toxicities but also to disappointing clinical efficacy. This changed with the reports of highly active CAR therapy against CD19 (CART19) in both adults with chronic lymphocytic leukemia (CLL) and children with relapsed ALL [1,2▪▪]. In the adult report, three patients with refractory and poor-prognosis CLL were given infusions of CART19, with two of three experiencing sustained complete remissions of disease and the third a partial response [1]. This was accompanied by some adverse events, including a delayed tumor lysis syndrome managed with alkalinization and supportive care. The pediatric report also described stunning efficacy in the setting of primary refractory ALL [2▪▪]. The first patient had relapsed twice with ALL despite intensive chemotherapy and had a significant disease burden at the time of treatment with CART19. The patient experienced a rapid elimination of disease that is sustained out to 18 months. Updates on subsequently treated patients indicate excellent response rates, including safely harvesting lymphocytes from patients who have previously undergone allogeneic transplant and returning them to the patient after CART19 modification without induction of graft-versus-host disease, despite the fact that the median donor chimerism in the infused T cells was 100% [17]. As of October 2013, 16 pediatric patients with relapsed or refractory ALL have been treated with CART19, yielding a CR rate of more than 80% (13/16) at Day 28. Eleven of the 13 patients in a CR were also MRD negative, with 2/13 remaining MRD positive at 0.1% or less MRD by flow cytometry. Levels of CRS vary with treated patients, ranging from mild (low-grade temperatures) to severe (hypotension requiring multiple vasoactive agents and ICU care). Four patients have been treated with tocilizumab (see below), with prompt resolution of fevers and stabilization of clinical status. Longer-term follow up and analysis of serum cytokine levels are needed before any associations between disease burden, level of CRS and disease response can be made.
FIGURE 1.
The chimeric antigen receptor consists of the single chain variable fragment of an antibody (scFv) that recognizes the CD19 protein on B cells and leukemia cells coupled to the CD3 zeta activation domain and costimulatory domains from CD28 and/or 4-1BB. This combines the major histocompatibility complex (MHC)-recognition of a tumor antigen with the activating potential of the T-cell receptor, allowing redirection of T cells to leukemia. Reproduced with permission from Sue Seif.
CYTOKINE RELEASE SYNDROME AND HEMOPHAGOCYTIC LYMPHOHISTIOCYTOSIS AFTER T-CELL ENGAGING THERAPIES
A subset of patients treated with blinatumomab and CART19 develop clinically significant CRS. In many patients, the CRS is mild and patients present with flu-like symptoms, including fever, myalgia, fatigue, and headache. In contrast, other patients develop more fulminant CRS with multisystem organ failure. Thus far, there are no reported fatalities from CRS after CART-19; however, a CRS-related fatality was recently described after blinatumomab treatment [14]. Some patients treated with both therapies develop laboratory evidence of CRS without clinical symptomatology. As the number of patients treated with either therapy is small, correlations between presence or absence of laboratory or clinical evidence of CRS and disease response are difficult to make. Moreover, some patients treated with blinatumomab have been shown to develop flu-like symptoms without marked hypercytokinemia [6▪▪].
Recent data demonstrate that IL-10, IL-6, and IFN-γ are the most highly elevated cytokines in patients who develop CRS after blinatumomab or CART-19 treatment [6▪▪]. These are the same cytokines commonly elevated in HLH/MAS [18, 19].HLH is a rare disorder with inappropriate immune system activation and cytokine release. HLH patients typically present with fever and splenomegaly in association with a number of abnormal laboratory values, including hyperferritinemia, hypertriglyceridemia, coagulopathy, and pancytopenia [20]. HLH is classified as primary or secondary. Primary HLH is caused by germline mutations in one of several genes involved in cytolytic granule exocytosis. Secondary HLH, also known as MAS, was formerly considered the ‘nonhereditary’ form, triggered by infection, malignancy, or autoimmune disease. New data suggest patients with secondary HLH may in fact have heterozygous mutations in the same genes, leading to mild dysfunctions in cytolytic granule exocytosis [20–23]. As patients with primary HLH have a complete or near complete deficiency in cytolytic granule exocytosis, no trigger or a very mild trigger initiates the HLH disease process. In contrast, patients with secondary HLH appear to only have partial defects and need a significant cytokine trigger.
Blinatumomab and CART-19 lead to transient cytokine activation, which could be a sufficient trigger for HLH/MAS in predisposed patients. We hypothesize a subset of patients with cancer treated with T-cell engaging therapies may develop HLH/MAS as a consequence of cytokine release in the setting of impaired cytolytic granule exocytosis. We believe that patients treated with BiTE antibody products and CAR T-cell therapies should be monitored prospectively for HLH/MAS. With further study, we may find that we can predict which patients may be more at risk to develop HLH/MAS from these types of therapies by screening for heterozygous mutations in HLH-causative genes, including PRF1, STX11, and MUNC13-4. Given that blinatumomab engages T cells through the T-cell receptor (TCR) and that CART19 bypasses the TCR, producing full T-cell activation from a chimeric set of intracellular activation domains (including CD3zeta and CD137), this indicates that CRS can result regardless of the mechanism of T-cell activation. This may have implications for other cellular therapies, including the use of high-affinity TCR-based adoptive cellular therapies [24].
IL-10, IL-6, and IFN-γ are not the only cytokines elevated in HLH or after CART19 or blinatumomab. Patients treated with CART-19 and blinatumomab have also been demonstrated to have elevated IL-2R, monocyte chemoattractant protein-1, and macrophage inflammatory protein-1-beta and normal levels of IL-1B, IL-4, IL-5, IL-7, IL-12, IL-13, IL-17, tumor necrosis factor (TNF)-a, and granulocyte macrophage colony-stimulating factor [6▪▪,9,10▪▪]. This cytokine profile is identical to the profile found in studies investigating cytokine levels in children with HLH [18, 19, 25, 26]. The relative degree in elevation of a particular cytokine can vary between patients; however, the reproducibility and consistency of the overall profile, with the same cytokines being elevated and the same cytokines being normal in patients with primary HLH and experiencing CRS after CART-19 or blinatumomab, is quite striking, and strongly suggests similar pathophysiologic processes are involved in the CRS. Cytokine profiles of T-cells engaged in vitro with BiTes or CART19 have demonstrated high levels of IFN-γ, IL-2, and TNF-α; and this cytokine release profile has been used in vitro as a marker of a potentially effective therapy [27,28].
IL-10 is an immunoregulatory cytokine whose primary function is to control innate and cell-mediated immunity by inhibiting activated macrophages [29]. It is primarily produced by monocytes/macrophages, but can also be produced by mast cells, B cells, regulatory T cells, and T helper cells (TH2). It is not commonly produced by cytotoxic T lymphocytes. IL-6 is an inflammatory cytokine involved in a large number of cellular functions, including neutrophil trafficking, acute phase response, angiogenesis, bone and lipid metabolism, B-cell differentiation, and autoantibody production [30▪]. It is produced by a number of cells, including monocytes/macrophages, dendritic cells, T cells, fibroblasts, keratinocytes, endothelial cells, adipocytes, myocytes, mesangial cells, and osteoblasts. IFN-γ is an inflammatory cytokine produced by cytotoxic T cells, T-helper cells (TH1), and natural killer cells. The principal functions of IFN-γ are macrophage activation, TH1 differentiation, induction of major histocompatibility complex (MHC) Class I, and B-cell isotype switching [31].
The high levels of IFN-γ may be expected after blinatumomab or CART19 treatment, because it is an effector cytokine released by activated cytotoxic T cells after engagement. In contrast, the high levels of IL-6 and IL-10 would not be expected with a cytotoxic T-cell response alone and are better explained by HLH/MAS. In thinking about targeting cytokines involved in CRS, as IL-10 is a negative regulator of macrophage function, targeting it may not be prudent in HLH/MAS. The high IFN-γ may be needed for blinatumomab to induce T cells to kill leukemia, and targeting it could inhibit the activity of the drug. IFN-γ may also be locally acting to increase tumor antigenicity by upregulating MHC Class I, enabling nonblinatumomab or CART19-based immune recognition. IL-6 is most likely released by the macrophages, either causing or being a secondary result of the inflammatory response. In theory, targeting IL-6 could relieve the toxic effects of HLH/MAS, yet not inhibit the activity of the T-cell-directed therapy. Conversely, the IL-6 may represent a part of a cytokine feedback loop that enhances T-cell proliferation, and targeting too early in the response could inhibit the clinical efficacy.
Tocilizumab is a recombinant humanized monoclonal antibody against the IL-6R that prevents IL-6 from binding to membrane-bound and soluble IL-6R [32, 33]. Tocilizumab has been studied in phase 1–3 trials in children and adults with juvenile idiopathic arthritis (JIA) and adult rheumatoid arthritis (RA), respectively. In adults, there have been eight randomized controlled trials treating over 2000 patients with tocilizumab [32, 33]. In most of these studies, tocilizumab is combined with additional immune suppressants and given on an every 4-week schedule. The most common adverse events reported are elevation of liver enzymes and cytopenias. Liver enzyme elevation occurred in 34–41% of patients in phase 3 trials, compared with 17% receiving placebo; however, most of these patients were receiving concomitant hepatotoxic medications, including methotrexate. Severe elevations in liver enzymes (>5× upper limit of normal) are rare (<1%) and elevations usually normalize after cessation of the drug. Myelosuppression (neutropenia and thrombocytopenia) is also observed with repeat dosing; however, this is typically mild, with fewer than 1% of patients developing significant neutropenia (ANC<500/µl) or thrombocytopenia (platelet count <50 000/µL). Tocilizumab has also been studied in randomized controlled trials in children with adverse effects mirroring the adult trials [34, 35, 36▪]. It is FDA and European Medicines Agency approved for RA and for JIA in patients above 2 years old and is also approved for Castleman’s disease in Japan. As indicated above, our experience in patients receiving CART19, as well as a patient receiving blinatumomab, is that IL-6 is highly elevated in these patients and temporally correlates with maximum T-cell activation/proliferation. Most importantly, we have seen rapid, dramatic and complete resolution of life-threatening CRS resulting from both therapies after a single dose of the IL-6 receptor antagonist tocilizumab [2▪▪,10▪▪]. As the CRS in these patients lasts for less than 2 weeks, later redosing of tocilizumab at monthly intervals is not required.
A few case reports have suggested that tocilizumab may temporarily mask or control clinical symptoms of systemic inflammation as a result of MAS in patients receiving tocilizumab for JIA [37▪▪]. In five patients receiving tocilizumab monotherapy for JIA, clinical evidence of MAS developed in the form of cytopenias, transaminase elevations, and increased levels of IL-6 and IL-18; however, several typical markers of MAS (such as fever, organomegaly, lymphadenopathy, hyperferritinemia, and elevated C-reactive protein) were absent. In these five patients, tocilizumab ameliorated the CRS but did not treat the underlying cause of the HLH/MAS. Thus, the concern in JIA patients experiencing MAS is that tocilizumab would only provide temporary control of the syndrome, which would then reflare as the IL-6 blockade wore off. In a circumstance in which the process driving the T-cell activation/ MAS feedback loop is temporary (as in T-cell engaging therapies), this may be less of a concern.
Other approaches that could be considered include the use of corticosteroids or inhibitors of IL-2R (CD25), IL-1R, or TNF-α. sIL-2R was markedly elevated in patients treated with blinatumomab and CART-19. It is also markedly elevated in patients with HLH and is used as a diagnostic criterion. Based on the high levels of sIL-2R in HLH, a number of case reports have investigated the efficacy of targeting CD25 with the monoclonal antibody daclizumab, demonstrating potential efficacy [38,39]. Unfortunately, daclizumab is no longer commercially available. Other CD25 inhibitors are in clinical use; however, they have not been studied in CRS or HLH. CD25 is present on activated T cells, making it less attractive for CART-19 or blinatumomab, as it has the potential to impact efficacy.
The recombinant anti-IL1R inhibitor anakinra has been has used for HLH/MAS that develops as a consequence of JIA [40]. Patients with JIA develop high levels of IL-1β. IL-1R is a naturally occurring IL-1 inhibitor produced by a number of cells, including macrophages, in response to elevated IL-1. Accordingly, biologic rationale supports the use of recombinant anti-IL1R in IL-1β-mediated disease. The CRS that develops after blinatumomab or CART-19 does not include marked elevation of IL-1β. A subset of patients do have a modest increase in IL-1α after treatment with blinatumomab or CART-19, raising the possibility that anakinra could be useful for CRS after T-cell engaging therapies. Similarly, TNF-α is elevated in inflammatory syndromes and serves as a target for blockade with etanercept in rheumatologic disorders [41]. Etanercept was given to the first pediatric ALL patient during severe CRS without apparent clinical benefit, and the level of TNF-α is not routinely elevated in CART19 therapy, making this a less promising target [2▪▪].
Lastly, corticosteroids have a long and well-studied benefit when treating inflammatory syndromes and are the mainstay of treating activated T-cell-based disorders such as graft-versus-host disease [42]. The only patient in the report by Porter et al. [1] to have a less than CR was the patient receiving steroids during the proliferative phase. Conversely, we have reported that an ALL patient had proliferation and efficacy despite steroids early after CART19, a finding also observed by Brentjens et al. [2▪▪,5▪]. Although corticosteroids will likely have a prominent role in managing CRS, their potential to block the therapeutic aspect of cellular therapies will remain a strong consideration. In contrast to CART19, corticosteroids are used commonly to help manage CRS after blinatumomab. In-vitro studies evaluating the effects of corticosteroids on BiTE molecules demonstrated a reduction in cytokine production without decreased T-cell activation or proliferation [43].
CONCLUSION
As we enter the era of highly active T-cell engaging therapies, as exemplified by the bispecific antibody blinatumomab and engineered CAR+ T cells such as CART19, we are seeing that the high degrees of T-cell activation that result in dramatic clinical responses are accompanied by toxicities such as CRS. In the case of CAR+ T cells, it may well be the case that significant clinical responses require a high degree of in-vivo proliferation, and it is the ability to achieve this proliferation that has resulted in such recent improvement in clinical trial results. In addition, we have now demonstrated that nonphysiological T-cell activation, which is the hallmark of these T-cell engaging therapies, creates a positive cytokine feedback loop that results in MAS, a syndrome which may contribute significantly to these toxicities. The challenge is to control the toxicity without interfering with efficacy. Minimizing the need for steroids to control CRS may help maintain efficacy. Our data suggest tocilizumab is effective at reversing CRS and controlling MAS without inhibiting the efficacy of CART19 or blinatumomab. Further studies are needed.
KEY POINTS.
CRS is a potentially serious complication of the nonphysiological T-cell activation produced by powerful T-cell engaging therapies such as bispecific T-cell engaging antibodies and chimeric antigen receptor-engineered T cells.
CRS caused by T-cell engaging therapies may be caused by abnormal activation of macrophages (MAS or secondary hematophagocytic lymphohistiocytosis).
Cytokine-directed therapy with the anti-IL6 receptor inhibitor tocilizumab may be effective in reversing CRS without hampering the efficacy of the T-cell engaging therapies.
Acknowledgements
The study is supported in part by grants from the National Institutes of Health (R01CA116660), the Leukemia and Lymphoma Society, Pennsylvania Department of Health, Cookies for Kids Cancer, Solving Kids Cancer, the WW Smith Charitable Trust and the Weinberg Funds.
Footnotes
Conflicts of interest
S.G.: Novartis research and clinical trial support.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. This article described the first pediatric use of CART19 cells in ALL, defines MAS/ HLH as a toxicity of T-cell activation, and describes the first use of tocilizumab to control MAS/HLH.
- 3.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 4.Topp MS, Kufer P, Gokbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 5. Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. This article demonstrated the use of an anti-CD19 CAR that uses CD28 as the costimulatory domain, with excellent activity in adult ALL
- 6. Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119:6226–6233. doi: 10.1182/blood-2012-01-400515. Description of the use of blinatumomab with high response rates in patients with ALL.
- 7.Topp MS, Goekbuget N, Zugmaier G, et al. Anti-CD19 BiTE blinatumomab induces high complete remission rates in adult patients with relapsed B-precursor ALL: updated results of an on-going phase II trial. Blood. 2011;118:252. [Google Scholar]
- 8. Topp MS, Gokbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120:5185–5187. doi: 10.1182/blood-2012-07-441030. This article highlights the activity of blinatumomab in adults with ALL with more extended follow up.
- 9.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. This article extends the concept of MAS/HLH resulting from nonphysiological T-cell activation to blinatumomab and describes rapid response of severe CRS to a single dose of tocilizumab.
- 11.Wolf E, Hofmeister R, Kufer P, et al. BiTEs: bispecific antibody constructs with unique antitumor activity. Drug Discov Today. 2005;10:1237–1244. doi: 10.1016/S1359-6446(05)03554-3. [DOI] [PubMed] [Google Scholar]
- 12.Portell CA, Advani AS. Antibody therapy for acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2012;7:153–159. doi: 10.1007/s11899-012-0120-7. [DOI] [PubMed] [Google Scholar]
- 13.Dreier T, Lorenczewski G, Brandl C, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100:690–697. doi: 10.1002/ijc.10557. [DOI] [PubMed] [Google Scholar]
- 14.Gore L, Zugmaier G, Handgretinger R, et al. Cytological and molecular remissions with blinatumomab treatment in second or later bone marrow relapse in pediatric acute lymphoblastic leukemia (ALL) J Clin Oncol. 2013;31(15 Suppl: 10007) [Google Scholar]
- 15. Lee DW, Barrett DM, Mackall C, et al. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin Cancer Res. 2012;18:2780–2790. doi: 10.1158/1078-0432.CCR-11-1920. Recent review of the CAR field with an emphasis on pediatrics.
- 16.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grupp SA, Kalos M, Barrett D, et al. Use of CD19-targeted Chimeric Antigen Receptor-modified T (CART19) cells in ALL and CLL produce transient cytokine release syndrome (CRS), macrophage activation syndrome and durable response. AACR 2013. 2013;16:CD008794. [Google Scholar]
- 18.Tang Y, Xu X, Song H, et al. Early diagnostic and prognostic significance of a specific Th1/Th2 cytokine pattern in children with haemophagocytic syndrome. Br J Haematol. 2008;143:84–91. doi: 10.1111/j.1365-2141.2008.07298.x. [DOI] [PubMed] [Google Scholar]
- 19.Xu XJ, Tang YM, Song H, et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr. 2012;160:984–990. doi: 10.1016/j.jpeds.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 20.Risma K, Jordan MB. Hemophagocytic lymphohistiocytosis: updates and evolving concepts. Curr Opin Pediatr. 2012;24:9–15. doi: 10.1097/MOP.0b013e32834ec9c1. [DOI] [PubMed] [Google Scholar]
- 21.Zhizhuo H, Junmei X, Yuelin S, et al. Screening the PRF1, UNC13D, STX11, SH2D1A, XIAP, and ITK gene mutations in Chinese children with Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2012;58:410–414. doi: 10.1002/pbc.23216. [DOI] [PubMed] [Google Scholar]
- 22.Zhang K, Jordan MB, Marsh RA, et al. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood. 2011;118:5794–5798. doi: 10.1182/blood-2011-07-370148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trizzino A, zur Stadt U, Ueda I, et al. Genotype-phenotype study of familial haemophagocytic lymphohistiocytosis due to perforin mutations. J Med Genet. 2008;45:15–21. doi: 10.1136/jmg.2007.052670. [DOI] [PubMed] [Google Scholar]
- 24.Robbins PF, Li YF, El-Gamil M, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olin RL, Nichols KE, Naghashpour M, et al. Successful use of the anti-CD25 antibody daclizumab in an adult patient with hemophagocytic lymphohistiocytosis. Am J Hematol. 2008;83:747–749. doi: 10.1002/ajh.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura K, Kanazawa T, Tsukada S, et al. Increased serum monocyte chemoattractant protein-1, macrophage inflammatory protein-1beta, and interleukin-8 concentrations in hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;51:662–668. doi: 10.1002/pbc.21660. [DOI] [PubMed] [Google Scholar]
- 27.Brischwein K, Parr L, Pflanz S, et al. Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J Immunother. 2007;30:798–807. doi: 10.1097/CJI.0b013e318156750c. [DOI] [PubMed] [Google Scholar]
- 28.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mihara M, Hashizume M, Yoshida H, et al. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci. 2012;122:143–159. doi: 10.1042/CS20110340. Highly relevant review of IL-6 in disease.
- 31.Saha B, Jyothi Prasanna S, Chandrasekar B, Nandi D. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine. 2010;50:1–14. doi: 10.1016/j.cyto.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2009;4:CD008794. doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh JA, Beg S, Lopez-Olivo MA. Tocilizumab for rheumatoid arthritis: a Cochrane systematic review. J Rheumatol. 2011;38:10–20. doi: 10.3899/jrheum.100717. [DOI] [PubMed] [Google Scholar]
- 34.Woo P, Wilkinson N, Prieur AM, et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther. 2005;7:R1281–R1288. doi: 10.1186/ar1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokota S, Miyamae T, Imagawa T, et al. Therapeutic efficacy of humanized recombinant antiinterleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:818–825. doi: 10.1002/art.20944. [DOI] [PubMed] [Google Scholar]
- 36. De Benedetti F, Brunner HI, Ruperto N, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2385–2395. doi: 10.1056/NEJMoa1112802. Pediatric data on the use of tocilizumab.
- 37. Shimizu M, Nakagishi Y, Kasai K, et al. Tocilizumab masks the clinical symptoms of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome: the diagnostic significance of interleukin-18 and interleukin-6. Cytokine. 2012;58:287–294. doi: 10.1016/j.cyto.2012.02.006. This article describes temporary responses (’masking’) of MAS to tociluzimab in JIA.
- 38.Tomaske M, Amon O, Bosk A, et al. Alpha-CD25 antibody treatment in a child with hemophagocytic lymphohistiocytosis. Med Pediatr Oncol. 2002;38:141–142. doi: 10.1002/mpo.1294. [DOI] [PubMed] [Google Scholar]
- 39.Lackner H, Urban C, Sovinz P, et al. Hemophagocytic lymphohistiocytosis as severe adverse event of antineoplastic treatment in children. Haematologica. 2008;93:291–294. doi: 10.3324/haematol.11704. [DOI] [PubMed] [Google Scholar]
- 40.Verbsky JW, White AJ. Effective use of the recombinant interleukin 1 receptor antagonist anakinra in therapy resistant systemic onset juvenile rheumatoid arthritis. J Rheumatol. 2004;31:2071–2075. [PubMed] [Google Scholar]
- 41.Horneff G, Schmeling H, Biedermann T, et al. The German etanercept registry for treatment of juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63:1638–1644. doi: 10.1136/ard.2003.014886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin PJ, Rizzo JD, Wingard JR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18:1150–1163. doi: 10.1016/j.bbmt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandl C, Haas C, d’Argouges S, et al. The effect of dexamethasone on polyclonal T cell activation and redirected target cell lysis as induced by a CD19/CD3-bispecific single-chain antibody construct. Cancer Immunol Immunother. 2007;56:1551–1563. doi: 10.1007/s00262-007-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]