Abstract
Background
Vitamin D may play a role in preserving cognitive function. However, there is a paucity of prospective studies on the relationship between vitamin D and cognition with aging. The aim of this study was to examine the association between plasma levels of vitamin D and subsequent cognitive function.
Methods
This is a prospective study including 1,185 women aged 60–70 years from the Nurses’ Health Study, who had plasma 25-hydroxy-vitamin D levels measured in 1989–1990 and completed an initial Telephone Interview of Cognitive Status approximately 9 years later. Subsequently, three follow-up cognitive assessments were conducted at 1.5–2.0 years intervals. We used multivariable-adjusted linear regression to model initial cognitive function, and mixed linear regression to model change in cognitive function over time.
Results
Lower vitamin D levels were associated with significantly worse cognitive function 9 years later. For example, the mean global composite score averaging all the cognitive tests was 0.20 lower (95% Confidence Interval (CI):−0.33,−0.08; p-trend=0.009) in women in the lowest quintile (median=14.1 ng/mL) compared with women in the highest quintile of vitamin D (median=38.4 ng/mL). The observed differences were equivalent to the effect estimates we found for women who were approximately 4–6 years apart in age. However, vitamin D levels were not significantly associated with subsequent cognitive decline during 6 years of follow-up.
Conclusions
Higher levels of plasma vitamin D in women aged 60–70 years were associated with better cognitive function about a decade later but were not associated with cognitive decline during 6 years of follow-up.
Keywords: Vitamin D, Cognitive Function, Women’s Health
INTRODUCTION
Understanding the neurobiological mechanisms underlying cognitive decline and the identification of potentially modifiable risk factors represents a public health priority (1). Emerging evidence suggests that vitamin D, which is critically important for bone, muscle health and the prevention of falls (2–4), may also play a role in brain function (5–7). Vitamin D receptors are located in a variety of areas, including the human cortex and hippocampus that are crucial for cognitive function (8), and polymorphisms in vitamin D receptor gene have been associated with cognitive decline and neurodegenerative disorders such as Alzheimer’s and Parkinson disease (9–12).
Vitamin D may exert neuroprotective effects through different pathways, including anti-inflammatory and antioxidant mechanisms (8, 13–14). Indeed, vitamin D deficiency has been associated with both a pro-inflammatory state (13) and increased oxidative stress (14), conditions that may alter the homeostasis among the biological systems involved in the maintenance of cognitive function. The majority of previous epidemiological studies have shown significant cross-sectional associations between both low plasma levels (6, 15–19) and inadequate dietary intake (5) of vitamin D and cognitive function. To date, however, the results have been inconsistent (20–22), with some studies showing adverse associations with low vitamin D levels (21), while others did not find significant associations (20,22). Furthermore, because the decline in cognitive function occurs over a long period of time, prospective studies of long duration are needed to examine the long-term relationships between vitamin D levels and cognitive function with aging. Therefore, we conducted a prospective study to examine the association between plasma levels of 25-hydroxy-vitamin D in women aged 60–70 years old and cognitive function assessed about a decade later in 1,185 women in the Nurses’ Health Study (NHS).
METHODS
Study population
The NHS began in 1976, when 121,700 female registered nurses aged 30–55 years, residing in 11 US states, returned a mailed questionnaire on their lifestyle and health status. Follow-up of the original cohort is ongoing via biennial mailed questionnaires. The response rate was >90%, with higher follow-up for those who donated blood samples (>95%). All women who were active participants at the inception of collection, completed the 1988 questionnaire, and responded positively to the question about blood drawing were invited to provide a blood sample. During 1989–1990, 32,826 NHS participants sent their blood samples overnight on ice and stored in liquid nitrogen. Previous work has documented the long-term stability of plasma samples collected and stored under this protocol (23). These samples have been previously used to study the relationship between many plasma biomarkers, including plasma vitamin D, and the development of chronic diseases (24). Health and lifestyle characteristics were similar between the whole Nurses’ Health Study cohort and those who returned blood samples (25). The Institutional Review Board of Brigham and Women’s Hospital, Harvard Medical School (Boston, MA) approved this study.
Cognitive Function Assessment
From 1995 through 2001, 22,715 NHS participants aged 70 years and older who were free of stroke were selected for a substudy on cognitive function. Of those who were eligible, 19,415 (93%) completed the initial cognitive interview. Three follow-up cognitive assessments were conducted at approximately two-year intervals, and participation rate remained high (>90%) over time. The interviews were administered by trained nurses and included the Telephone Interview of Cognitive Status (TICS) (26) and a telephone adaptation of the Mini-Mental State Examination (MMSE). Subsequently, the following tests were added: 1) immediate and delayed recalls of the East Boston Memory Test; 2) a test of category fluency during which women were asked to name as many animals as possible in one minute; 3) delayed recall of the TICS 10-word list; and 4) digit span backwards, in which women repeated backward an increasingly long series of digits. In a validation study, the performance on the telephone cognitive battery was strongly correlated (r=0.81) with performance on a comprehensive battery of neuropsychological tests in a detailed, in-person interview conducted in 61 highly educated women aged 70 years or older. Inter-interviewer reliability was also high across 10 interviewers (r>0.95 for each cognitive test).
Our primary outcome of interest was the global composite score (averaging all six tests in our cognitive battery). We also considered a verbal memory composite score (averaging immediate and delayed recalls of the TICS 10-word list and the East Boston Memory Test), the TICS score, category fluency task and the digit backwards. The composite scores were obtained by first converting the result from each cognitive test to z-scores then taking the average of the z-scores (27). We have tested the adequacy of the global composite score as a summary measurement by using principal component analyses as an alternative and found similar results.
Assessment of Vitamin D
Plasma 25-hydroxy-vitamin D was measured by the radioimmunoassay procedure (DiaSorin, Stillwater, MN). The method used to assay plasma 25-hydroxy-vitamin D has been described in detail previously (28). Across the 23 batches of blood samples used for this study, the mean CV in blinded quality control samples for 25-hydroxy-vitamin D was 10.7% (ranging from 4.9% to 18.5%), indicating an overall high reliability of measurements.
Population for Analysis
Of the 19,415 women enrolled in the cognitive cohort, 6,863 had provided a blood sample in 1989–1990, and 1,185 women were selected in the nested case-control studies from previous research in this cohort measuring vitamin D levels as part of nested case-control studies of: breast, colon, ovarian, pancreatic and endometrial cancer, Non-Hodgkin’s lymphoma, diabetes and multiple sclerosis. As indicated in Figure 1, we excluded all “cases” of these outcomes in the selected nested case-control studies and only utilized the control samples for the purpose of this study, resulting in a final analytical sample of 1,185 women (99.6% were Caucasian).
Figure 1.
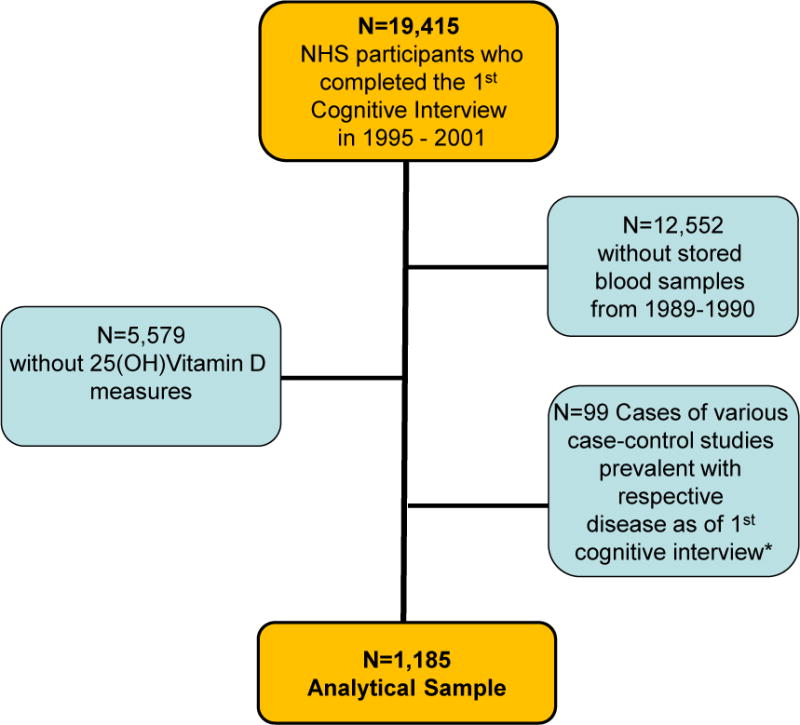
Derivation of the Population for Analysis
*Cases in the nested case-control studies with 25(OH)Vitamin D levels measured: cases of breast, colon, ovarian, pancreatic and endometrial cancer, Non-Hodgkin’s Lymphoma, diabetes and multiple sclerosis
Statistical Analysis
We used multiple linear regression models to estimate mean differences in cognitive function in later life according to quintiles of vitamin D in women aged 60–70 years old. For tests for trend, we modeled the median value of each quintile as a continuous variable in the multivariable regression model. Linear models were deemed appropriate for the analyses, especially given our sample size (29). Alternatively, we defined cut-points of vitamin D as used in previous studies (30–31): <15, 15–29.9 and >30 ng/mL. We also used polynomial and spline models to evaluate the presence of any non-linear/dose-response relationship, and to gain more insights into the possible etiology of low vitamin D levels ~ 10 years prior to cognitive testing, we also evaluated the relation with rate of cognitive decline (32). To test the association between plasma vitamin D and decline in cognitive function over four repeated assessments, we used multivariable-adjusted mixed linear regression models with random intercepts and slopes. This analytical approach assumes that a participant’s change in cognitive function follows that of the population mean except for random effects for initial level of cognitive function (i.e., random intercepts) and rates of change (i.e., random slopes). Main-effect terms for the exposure, covariates, and continuous time were included in the model to account for relations with initial level of cognitive function. We included interaction terms for the exposure and each covariate with continuous time to estimate associations with change in cognitive function. SAS version 9.1 was used for all analyses (SAS Institute, Inc, Cary, NC).
We used basic and fully-adjusted models to control for potential confounders. In basic models, we adjusted for age at interview (in years), highest attained education (Registered Nurse, Bachelor’s degree, Master’s and above), assay batch (1–23), time between blood draw and cognitive interview (years) and season at the time of blood draw (winter, summer, spring, autumn). In fully-adjusted models, we also included the following covariates as of blood-draw: husband’s education (less than high school, some high school, high school graduate, Bachelor’s, Master’s or above), cigarette smoking (current, past, never), aspirin use (<3/week, 3+/week), age at menopause (<50, 50–52, 53+ years), current use of vitamin E supplements (yes, no), antidepressant use (yes, no), the mental health index (0–52, 53–100) and the energy-fatigue index (0–49, 50–100) from the Short Form-36 Questionnaire (33–34), history of hypertension (yes, no), history of high cholesterol (yes, no), alcohol intake (0, <5, 5–14, 15+ g/day), postmenopausal hormone use (current, past, never), body mass index (<22, 22–24, 25–29, 30+ kg/m2), and physical activity (quintiles of metabolic-equivalent-hours/week).
We performed several secondary analyses. As an alternative to adjusting for batch in the model, we repeated the analyses after creating quintiles of vitamin D that were specific to each of the 23 batches. Also, because of the variability of the CVs for vitamin D in the different batches (ranging from 4.9 to 18.5), we repeated the analyses after excluding those with CVs >15%. In addition, we eliminated from the sample those with very low cognitive function performance at the first assessment, defined as the lowest 10% for each cognitive function score, to evaluate whether our results were driven by this specific segment of the population. We also examined effect modification by age at 1st cognitive assessment (median, ≤ 74 vs >74 years), highest attained education (Registered Nurse vs. Bachelor’s, Master’s or above), body mass index (≤25 vs >25 kg/m2), median level of physical activity (< 11.5 vs. ≥11.5 metabolic-equivalent-hours/week), season (May–October vs. November–April). In addition, because the seasons may not be comparable across the US, we examined whether the associations adjusted for season differed by US geographical region (Northern versus Middle or Southern).
RESULTS
The characteristics of participants by quintiles of plasma vitamin D levels as of blood draw in 1989–1990 are shown in Table 1. The average age at blood draw (63 years) or at first cognitive interview (74 years) was not substantially different across quintiles of vitamin D. Women with higher vitamin D levels in 1989–1990 had better cognitive performance on all tests in the initial assessment in 1995–2001. They were also more physically active, and had higher use of alcohol, vitamin E supplements and lower prevalence of obesity and cigarette smoking than women with lower plasma levels of vitamin D as of blood draw. These differences were adjusted for in multivariable analyses.
Table 1.
Age and age-standardized characteristics by plasma vitamin D at blood draw
| General Characteristics* | Quintiles of Vitamin D | P-value | ||
|---|---|---|---|---|
| 1s (Lowest) |
3th | 5th (Highest) |
||
|
|
||||
| Number of participants (N=1185) | 237 | 227 | 239 | |
| Age at cognitive interview (years); mean (SD) | 74.0 (2.2) | 74.1 (2.2) | 73.9 (2.0) | 0.72 |
| Age at blood draw (years); mean (SD) | 63.2 (2.5) | 63.0 (2.6) | 62.9 (2.4) | 0.44 |
| Plasma 25-hydroxy-vitamin D (ng/mL); mean (SD) | 13.6 (3.0) | 24.8 (1.2) | 40.7 (7.1) | <0.001 |
| Vitamin D from Supplements (IU/day); mean (SD) | 108 (269) | 145 (212) | 194 (215) | <0.001 |
| Vitamin D from Food (IU/day); mean (SD) | 198 (130) | 221 (108) | 235 (115) | <0.001 |
| TICS score; mean (SD)† | 33.9 (2.7) | 34.2 (2.6) | 34.3 (2.5) | 0.22 |
| Verbal score; mean (SD)† | −0.09 (0.74) | 0.02 (0.71) | 0.04 (0.72) | 0.20 |
| Category fluency score; mean (SD)† | 16.8 (4.6) | 17.3 (4.7) | 17.8 (4.6) | 0.03 |
| Digit backwards score; mean (SD)† | 6.6 (2.4) | 6.6 (2.4) | 7.2 (2.4) | 0.08 |
| Global score; mean (SD)† | −0.12 (0.63) | −0.01 (0.63) | 0.06 (0.58) | 0.02 |
| Masters/Doctorate degree; % | 6 | 7 | 7 | 0.81 |
| Husband with Masters/Doctorate degree; % | 22 | 21 | 23 | 0.58 |
| High Physical Activity*; % | 13 | 17 | 31 | <0.001 |
| Current smoking; % | 20 | 7 | 12 | 0.02 |
| Alcohol use (>15 g/day); % | 10 | 10 | 21 | <0.001 |
| Current postmenopausal hormone use; % | 25 | 26 | 35 | 0.02 |
| Age at menopause (≥53 years); % | 21 | 17 | 20 | 0.93 |
| Vitamin E use; % | 11 | 13 | 22 | <0.001 |
| Multivitamin use; % | 34 | 42 | 47 | 0.002 |
| Aspirin 3+/week; % | 16 | 21 | 22 | 0.14 |
| Current antidepressant use; % | 7 | 4 | 5 | 0.14 |
| Hypertension; % | 32 | 35 | 31 | 0.78 |
| High cholesterol; % | 36 | 36 | 33 | 0.73 |
| Obesity (BMI ≥ 30); % | 16 | 10 | 6 | <0.001 |
| Blood drawn in the winter; % | 37 | 29 | 20 | 0.04 |
Characteristics at blood draw (1989–1990); cognitive test performance at first assessment (1995–2001); TICS= Telephone interview of Cognitive Status; High physical activity=highest quintile of metabolic-equivalent-hours/week.
Global score was the average of all of the individual 6 tests converted into z scores (score – mean of the score / standard deviation of the score), with mean of 0. Verbal score was the average of 4 tests converted into z scores, with mean of 0. TICS score ranged from 0 to 41; category fluency score had a minimum value of 0 with no upper limit; the digit backwards score ranged from 0 to 12.
Cognitive Function at first assessment
Lower plasma levels of vitamin D in women aged 60–70 years old were significantly associated with worse cognitive performance assessed approximately 9 years later on the global and category fluency scores (Table 2). For example, compared to the highest quintile of vitamin D (median=38.4 ng/mL), those in the lowest quintile (median=14.1 ng/mL) had a mean difference of −0.20 (95% Confidence Interval (CI):−0.33, −0.08) for the global score and of −1.17 (95% CI: −2.06, −0.27) for category fluency, after adjustment for potential confounders. We did not find significant differences in mean cognitive scores across quintiles of vitamin D for verbal, TICS and digit backwards scores. When we used pre-defined cut-off points for vitamin D rather than quintiles, we found that women with vitamin D <15 ng/mL had a significantly lower mean global score (mean difference =−0.18, 95% CI: −0.31, −0.04) compared with women with levels of vitamin D ≥ 30 ng/mL.
Table 2.
Mean differences (CI) in initial levels of cognitive function (1995–2001) by quintiles of plasma levels of vitamin D (1989–1990)
| 1st | 2nd | 3rd | 4th | 5th | p-trend | |
|---|---|---|---|---|---|---|
| Median (range) | 14.1 (3.9–17.8) | 20.4 (17.9–22.7) | 24.7 (22.7–27.1) | 29.7 (27.1–33.1) | 38.4 (33.2–74.0) | |
| Global Score | ||||||
| Model 1* | −0.21 (−0.34, −0.09) | −0.06 (−0.18, 0.06) | −0.05 (−0.17, 0.07) | −0.10 (−0.22, 0.02) | REF | 0.005 |
| Model 2† | −0.20 (−0.33,−0.08) | −0.07 (−0.19, 0.05) | −0.03 (−0.15, 0.09) | −0.10 (−0.22, 0.02) | 0 | 0.009 |
| Verbal Score | ||||||
| Model 1 | −0.16 (−0.31, −0.01) | −0.05 (−0.19, 0.09) | 0.00 (−0.14, 0.15) | −0.09 (−0.23, 0.05) | 0 | 0.10 |
| Model 2 | −0.15 (−0.30, 0.00) | −0.06 (−0.20, 0.09) | 0.02 (−0.13, 0.17) | −0.09 (−0.24, 0.05) | 0 | 0.14 |
| TICS Score | ||||||
| Model 1 | −0.45 (−0.92, 0.03) | −0.32 (−0.79, 0.15) | −0.05 (−0.53, 0.43) | −0.50 (−0.97, −0.04) | 0 | 0.15 |
| Model 2 | −0.42 (−0.91, 0.08) | −0.32 (−0.80, 0.16) | −0.01 (−0.50, 0.48) | −0.58 (−1.05, −0.10) | 0 | 0.22 |
| Category Fluency Score | ||||||
| Model 1 | −1.19 (−2.05, −0.33) | −0.07 (−0.93, 0.79) | −0.38 (−1.26, 0.49) | 0.12 (−0.74, 0.98) | 0 | 0.01 |
| Model 2 | −1.17 (−2.06, −0.27) | −0.14 (−1.02, 0.74) | −0.36 (−1.25, 0.54) | 0.18 (−0.69, 1.06) | 0 | 0.01 |
| Digit Backward | ||||||
| Model 1 | −0.55 (−1.07, −0.03) | −0.42 (−0.91, 0.08) | −0.50 (−1.00, 0.00) | −0.53 (−1.03, −0.03) | 0 | 0.05 |
| Model 2 | −0.47 (−1.00, 0.06) | −0.34 (−0.85, 0.16) | −0.37 (−0.88, 0.14) | −0.55 (−1.06, −0.05) | 0 | 0.14 |
Abbreviations: TICS= Telephone Interview of Cognitive Status
Model 1= Multiple linear regression models adjusted for age at interview (years), highest attained education (Registered Nurse, Bachelor and Master’s or above), assay batch (categories of 1–23), time between blood draw and cognitive interview (years), and season at blood draw (winter, summer, spring, autumn).
Model 2= Model 1+ husband’s education (Bachelor, Master’s or above), cigarette smoking (current, past, never), aspirin use (<3/week, 3+/week), age at menopause (<50, 50–52, 53+ years), current use of vitamin E supplements, antidepressant use, mental health index (0–52, 53–100) and energy-fatigue index (0–49, 50–100) from the Short Form-36 Questionnaire, history of high blood pressure, history of high cholesterol, alcohol consumption (0,<5g/day, 5–14g/day, 15+ g/day), postmenopausal hormone use (current, past, never), body mass index (<22, 22–24, 25–29, 30+ kg/m2) and physical activity (quintiles of metabolic-equivalent-hours/week).
Secondary Analyses
The distribution of vitamin D was comparable across the 23 batches, suggesting that presence of possible errors related to batch is minimal. Furthermore, the results did not substantially change when we repeated the analyses using batch-specific quintiles of vitamin D (the mean difference in the lowest versus highest quintile: −0.16, 95% CI: −0.29,−0.03 for global score), and when we excluded those with scores in the worst 10 percent of cognitive function (the mean difference in the lowest versus the highest quintile: −0.19, 95% CI:−0.30, −0.09 for global score). When we repeated the analyses after excluding those in batches with CVs > 15% (n= 276; 23.3%), the results remained significant for the global score. Additional control for other potential confounders such as dietary intake of fish (which is the main source of omega-3 fatty acids) or supplemental intake of vitamins A and C did not meaningfully change our results. Finally, we found no significant interactions between plasma vitamin D levels and age, education, region, and body mass index in relation to cognitive outcomes (data not shown).
To evaluate whether there was a non-linear association between vitamin D and cognitive function, we used polynomial and spline models and found a weak non-linear relationship between vitamin D and the global and category fluency score (p=0.01 for vitamin D squared term for both outcomes; effect estimate of −0.0003 for global score and −0.002 for category fluency score), where the increase in scores with increasing plasma vitamin D levels tapered off at the 95th percentile (~44 ng/mL). There was no evidence of non-linear relationships with the other outcomes. Finally, we examined whether the associations adjusted for season differed by US geographical region (Northern versus Middle or Southern). Because the interaction terms by region were not significant, any potential biases would likely be minimal.
Decline in Cognitive Function
Over the 6 years of follow-up, we observed some overall modest declines. In particular, the mean difference between the last assessment and the baseline was −0.12 for the global composite score, −0.06 for the verbal score, −0.87 for the category fluency score and −0.46 for the digit span backwards test. When we converted these changes to annual rates of decline and analyzed these rates by plasma levels of vitamin D, we observed that plasma levels of vitamin D were not significantly associated with change in any of the cognitive function tests (global, verbal, TICS, category fluency, and digit backwards scores) during the 6 years of follow-up (Table 3). For example, the mean difference in annual rate of change in global score between women in the lowest versus the highest quintile of vitamin D was 0.01 (95% CI: −0.03, 0.01; p-trend=0.31).
Table 3.
Mean differences (CI)* in change in cognitive function (1995 – 2006) by quintiles of plasma levels of vitamin D (1989–1990)
| Quintiles (ng/mL) | 1st | 2nd | 3rd | 4th | 5th | p-trend |
|---|---|---|---|---|---|---|
| Median (range) | 14.1 (3.9–17.8) | 20.4 (17.9–22.7) | 24.7 (22.7–27.1) | 29.7 (27.1–33.1) | 38.4 (33.2–74.0) | |
|
|
||||||
| Global Score | 0.01 (−0.01, 0.03) | 0.01 (−0.01, 0.03) | 0.00 (−0.02, 0.02) | 0.01 (−0.01, 0.03) | REF | 0.31 |
| Verbal Score | 0.02 (−0.01, 0.04) | 0.01 (−0.02, 0.03) | −0.01 (−0.03, 0.02) | 0.01 (−0.01, 0.04) | 0 | 0.34 |
| TICS Score | 0.00 (−0.10, 0.09) | 0.02 (−0.07, 0.12) | −0.01 (−0.10, 0.09) | 0.06 (−0.03, 0.15) | 0 | 0.79 |
| Category Fluency | 0.12 (−0.04, 0.28) | 0.01 (−0.14, 0.17) | 0.04 (−0.12, 0.19) | −0.03 (−0.18, 0.12) | 0 | 0.13 |
| Digit Backward | −0.01 (−0.09, 0.06) | −0.02 (−0.09, 0.06) | 0.04 (−0.03, 0.11) | 0.03 (−0.04, 0.10) | 0 | 0.54 |
Abbreviations: TICS= Telephone Interview of Cognitive Status
Mixed linear regression models adjusted for age at interview (years), highest attained education (Registered Nurse, Bachelor, Master’s or above), assay batch (categories of 1–23), time between blood draw and cognitive interview (years), and season at blood draw (winter, summer, spring, autumn), husband’s education (Bachelor, Master’s or above), cigarette smoking (current, past, never), aspirin use (<3/week, 3+/week), age at menopause (<50, 50–52, 53+ years), current use of vitamin E supplements, antidepressant use, mental health index (0–52, 53–100) and energy-fatigue index (0–49, 50–100) from the Short Form-36 Questionnaire, history of high blood pressure, history of high cholesterol, alcohol consumption (0,<5g/day, 5–14g/day, 15+ g/day), postmenopausal hormone use (current, past, never), body mass index (<22, 22–24, 25–29, 30+ kg/m2) and physical activity (quintiles of metabolic-equivalent-hours/week).
DISCUSSION
In this prospective study, we found that lower plasma levels of 25-hydroxy-vitamin D in women aged 60–70 years were associated with worse cognitive function 9 years later, based on our global composite score. The differences in cognitive function that we found for those with low versus higher levels of vitamin D were clinically significant as they were equivalent to the differences in cognition that we observed between women who were 4 to 6 years apart in age in our cohort. Associations were also observed with category fluency score. Overall, we did not find significant associations between levels of vitamin D and change in cognitive function during 6 years of follow-up. Because our participants were highly educated and were young-old, it is likely that a longer time of follow-up is needed to observe a detectable change in cognitive function (17).
Our findings on the significant association between levels of vitamin D and cognitive performance are in line with previous cross-sectional studies showing a significant association between lower levels of vitamin D and measures of cognitive function (15, 16, 35). To our knowledge, only two prospective studies are currently available on the association between levels of vitamin D and cognitive decline in older persons (21, 22). In one study, Llewellyn et al. (21) found that lower levels of 25-hydroxy-vitamin D were associated with greater decline in the Mini Mental State Examination score during 6 years of follow-up in 858 Italians who were 65 years or older. Because greater variability in education of the participants may have led to greater variability in the trajectories of cognitive change with time, it is possible that this study may have had particularly high power to detect effects compared with our study population of healthy nurses with similar high education and socioeconomic levels. In the other study, Slinin et al.(21) found no association between levels of 25-hydroxy-vitamin D and baseline cognitive function (Modified Mini-Mental State Exam and Trails B test) and also found no significant associations with incident cognitive decline over 4.6 years of follow-up in 1,604 US men aged 65 years or older with overall high education. Thus, clearly more research from prospective studies on vitamin D and cognitive decline is needed.
Vitamin D may have indirect and direct effects on cognitive function. For example, low vitamin D has been associated with increased risk for cardiovascular disease, hypertension and diabetes mellitus (30, 36–38), conditions that are associated with cognitive function. Furthermore, experimental studies have shown that vitamin D reduces levels of pro-inflammatory markers in the blood (39) and hippocampus (40,41). It also enhances nerve conduction and brain detoxification by inhibiting the synthesis of nitric oxide synthase and by protecting neurons from reactive oxygen species (42, 43), and oxidative stress is considered to be one of the early initiators of the pathophysiologic mechanisms underlying cognitive decline and dementia (44). These indirect and direct mechanisms linking vitamin D with cognitive function, that likely occur at an early stage of cognitive decline, may explain, at least in part, our findings on the association between lower concentrations of vitamin D at ages 60–70 years and initial cognitive function scores 9 years later.
The long length of time (9 years) between the assessment of the exposure and the outcome of interest minimizes the possibility of reverse causation, where a low level of vitamin D may have been caused by overall poor cognitive ability and health status. On the other hand, because cognitive function was not assessed at the time of blood collection, we cannot rule out the possibility that differences in cognitive function observed at the first cognitive assessment 9 years later were already present at the time of blood collection. However, we repeated the analyses after excluding women with the lowest 10% of initial cognitive function scores, who are likely to represent a particularly vulnerable group with poor cognitive ability and health status at blood draw 9 years earlier, and the results did not meaningfully change. Therefore, it is unlikely that reverse causation entirely explains our results. Furthermore, residual confounding by unmeasured or unknown factors [such as depression,(45) low social activity,(46) physical function,(47) fractures,(48) osteoporosis,(49) parathyroid hormone (50), nutritional deficiencies (51, 52)] might have affected vitamin D levels during the 9-year interval. However, residual confounding, although possible, is unlikely to explain all of the results given the stability of the effect estimates from the basic to the fully-adjusted model, which includes multiple health-related factors such as the use of aspirin, history of high blood pressure and of high cholesterol. In addition, vitamin D levels may simply be a marker of another understudied risk factor for cognition. For example, a recent study found that sunlight exposure, which is a strong determinant of vitamin D levels, as well as other climactic factors, may be novel risk factors for cognitive function (53). Another potential limitation is that for examining change in cognitive function over time, we might have lacked power given the short follow-up time (6 years) in our population of generally well-educated women (15). It should also be acknowledged that there is an ongoing debate of the most accurate method to measure 25-hydroxy-vitamin D levels, and a lack of standardization in the measurements.(54, 55) Although this limitation might have affected our results, the effect of the additional errors and misclassifications likely dampened with the categorizations of quintiles, and the errors, if present, would have biased the results towards the null. Because our sample included a healthy subset (with no major cancers, diabetes or multiple sclerosis) of a highly educated cohort of mostly (99.6%) Caucasian women, our results may not generalize to populations with different characteristics. Interestingly, although our population was unique, the distribution of plasma vitamin D levels did not differ greatly with that observed for the general US female population of 60–69 year olds (e.g., in the NHANES III (1988–1992). For example, the percentage of women with very low levels of <10 ng/mL were about 2–3% (56) and the corresponding percentage was 2.84% in our population).
In conclusion, higher plasma vitamin D in women aged 60–70 years was associated with better cognitive function about a decade later, but it was not associated with change from the initial assessment in the subsequent 6 years of follow-up. Further prospective studies with larger sample sizes and longer follow-up are needed to confirm these results. To further explore the age at which low vitamin D levels may be most etiologic, prospective studies with repeated assessments of plasma vitamin D levels from middle (40–50 years) to old age in populations with high variability in plasma vitamin D levels may be most informative. Furthermore, future studies on genetic variation on the vitamin D receptors would provide insights into the underlying factors contributing to the link between vitamin D and decline in cognitive function. Finally, our study provided a unique opportunity to evaluate the relation between vitamin D and cognitive function approximately a decade later and supports the importance of maintaining optimal vitamin D levels in older persons. The identification of potentially modifiable risk factors for cognitive decline would help the development of cost-effective intervention strategies aimed at reducing its detrimental consequences and, ultimately, at improving quality of life in older persons.
Acknowledgments
This study was supported by grants P01 CA87969 and R01 CA49449 from the National Institutes of Health and, in part, by R01AG020727 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIA or NIH. The authors’ responsibilities were as follows—BB and JHK: designed this study, had full access to all of the data in the study and take responsibility for the accuracy of the data analysis; BB: wrote the manuscript; ED, FG and JHK: contributed to the data collection and to the final content of the manuscript.
Footnotes
All authors read and approved the final manuscript and none of them has or had any conflicts of interest/disclosures.
Author Contributions:
Bartali: study concept and design, analysis and interpretation of data, and preparation of manuscript.
Devore: interpretation of data.
Grodstein: acquisition of subjects and interpretation of data.
Kang: study concept and design, analysis and interpretation of data.
Sponsor’s Role:
None.
Contributor Information
Benedetta Bartali, New England Research Institutes, Epidemiology, Watertown, MA.
Elizabeth Devore, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital/Harvard Medical School, Boston, MA.
Francine Grodstein, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital/Harvard Medical School, Boston, MA.
Jae H. Kang, Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital/Harvard Medical School, Boston, MA.
References
- 1.Bennett DA. Diabetes and change in cognitive function. Arch Intern Med. 2000;160(2):141–3. doi: 10.1001/archinte.160.2.141. [DOI] [PubMed] [Google Scholar]
- 2.Christakos S, Hewison M, Gardner DG, Wagner CL, Sergeev IN, Rutten E, Pittas AG, Boland R, Ferrucci L, Bikle DD. Vitamin D: beyond bone. Ann N Y Acad Sci. 2013 May;1287:45–58. doi: 10.1111/nyas.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boersma D, Demontiero O, Mohtasham Amiri Z, Hassan S, Suarez H, Geisinger D, Suriyaarachchi P, Sharma A, Duque G. Vitamin D Status in Relation to Postural Stability in the Elderly. J Nutr Health Aging. 2012;3:270–275. doi: 10.1007/s12603-011-0345-5. [DOI] [PubMed] [Google Scholar]
- 4.Peterson A, Mattek N, Clemons A, Bowman GL, Buracchio T, Kaye J, Quinn J. Serum vitamin D concentrations are associated with falling and cognitive function in older adults. J Nutr Health Aging. 2012;10:898–901. doi: 10.1007/s12603-012-0378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annweiler C, Schott AM, Rolland Y, Blain H, Herrmann FR, Beauchet O. Dietary intake of vitamin D and cognition in older women: a large population-based study. Neurology. 2010;75(20):1810–6. doi: 10.1212/WNL.0b013e3181fd6352. [DOI] [PubMed] [Google Scholar]
- 6.Llewellyn DJ, Lang IA, Langa KM, Melzer D. Vitamin D and cognitive impairment in the elderly U.S. population. J Gerontol A Biol Sci Med Sci. 2011;66(1):59–65. doi: 10.1093/gerona/glq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med. 2008;29(6):415–22. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–5. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann DJ, Refsum H, Warden DR, Medway C, Wilcock GK, Smith AD. The vitamin D receptor gene is associated with Alzheimer’s disease. Neurosci Lett. 2011;504(2):79–82. doi: 10.1016/j.neulet.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 10.Butler MW, Burt A, Edwards TL, Zuchner S, Scott WK, Martin ER, et al. Vitamin D receptor gene as a candidate gene for Parkinson disease. Ann Hum Genet. 2011;75(2):201–10. doi: 10.1111/j.1469-1809.2010.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annweiler C, Llewellyn DJ, Beauchet O. Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2013;33(3):659–74. doi: 10.3233/JAD-2012-121432. [DOI] [PubMed] [Google Scholar]
- 12.Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79(13):1397–405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc AV, Yuksel M, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94(10):4023–30. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 15.Buell JS, Scott TM, Dawson-Hughes B, Dallal GE, Rosenberg IH, Folstein MF, et al. Vitamin D is associated with cognitive function in elders receiving home health services. J Gerontol A Biol Sci Med Sci. 2009;64(8):888–95. doi: 10.1093/gerona/glp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seamans KM, Hill TR, Scully L, Meunier N, Andrillo-Sanchez M, Polito A, et al. Vitamin D status and measures of cognitive function in healthy older European adults. Eur J Clin Nutr. 2010;64(10):1172–8. doi: 10.1038/ejcn.2010.117. [DOI] [PubMed] [Google Scholar]
- 17.Rubin EH, Storandt M, Miller JP, Kinscherf DA, Grant EA, Morris JC, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Arch Neurol. 1998;55(3):395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- 18.Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202–5. doi: 10.1016/j.abb.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Lee DM, Tajar A, Ulubaev A, Pendleton N, O’Neill TW, O’Connor DB, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722–9. doi: 10.1136/jnnp.2008.165720. [DOI] [PubMed] [Google Scholar]
- 20.Brouwer-Brolsma EM, Feskens EJ, Steegenga WT, de Groot LC. Associations of 25-hydroxyvitamin D with fasting glucose, fasting insulin, dementia and depression in European elderly: the SENECA study. Eur J Nutr. 2013;52(3):917–25. doi: 10.1007/s00394-012-0399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135–41. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slinin Y, Paudel ML, Taylor BC, Fink HA, Ishani A, Canales MT, et al. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology. 2010;74(1):33–41. doi: 10.1212/WNL.0b013e3181c7197b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hankinson SE, London SJ, Chute CG, Barbieri RL, Jones L, Kaplan LA, et al. Effect of transport conditions on the stability of biochemical markers in blood. Clin Chem. 1989;35:2313–6. [PubMed] [Google Scholar]
- 24.Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33(9):2021–3. doi: 10.2337/dc10-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okereke O, Hankinson SE, Hu FB, Grodstein F. Plasma C peptide level and cognitive function among older women without diabetes mellitus. Arch Intern Med. 2005;165(14):1651–6. doi: 10.1001/archinte.165.14.1651. [DOI] [PubMed] [Google Scholar]
- 26.Brandt J, Folstein MF. Telephone Interview for Cognitive Status: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 27.Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, et al. The apolipoprotein E e4 allele and decline in different cognitve systems during a 6-year period. Arch Neurol. 2002;59:1154–60. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 28.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39(3):529–33. [PubMed] [Google Scholar]
- 29.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–69. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 30.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 32.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75(12):1070–8. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ware J, Snow K, Kosinski M, Bandek B. SF-36 health survey: manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 34.McHorney CA, Ware JEJ, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Dhesi JK, Bearne LM, Moniz C, Hurley MV, Jackson SH, Swift CG, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17(5):891–7. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 36.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 37.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29(3):722–4. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- 39.Amer M, Qayyum R. Relation between serum 25-hydroxyvitamin D and C-reactive protein in asymptomatic adults (from the continuous National Health and Nutrition Examination Survey 2001 to 2006) Am J Cardiol. 2012;109(2):226–30. doi: 10.1016/j.amjcard.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 40.Moore ME, Piazza A, McCartney Y, Lynch MA. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem Soc Trans. 2005;33(Pt 4):573–7. doi: 10.1042/BST0330573. [DOI] [PubMed] [Google Scholar]
- 41.Moore M, Piazza A, Nolan Y, Lynch MA. Treatment with dexamethasone and vitamin D3 attenuates neuroinflammatory age-related changes in rat hippocampus. Synapse. 2007;61(10):851–61. doi: 10.1002/syn.20433. [DOI] [PubMed] [Google Scholar]
- 42.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? Faseb J. 2008;22(4):982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 43.Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, Abel K, et al. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2009;17(3):703–17. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- 44.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464(7288):529–35. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329–35. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.James BD, Wilson RS, Barnes LL, Bennett DA. Late-Life Social Activity and Cognitive Decline in Old Age. J Int Neuropsychol Soc. 2011;17(6):998–1005. doi: 10.1017/S1355617711000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clouston SAP, Brewster P, Kuh D, Richards M, Cooper R, Hardy R, et al. The Dynamic Relationship Between Physical Function and Cognition in Longitudinal Aging Cohorts. Epidemiol Rev. 2013;35:33–50. doi: 10.1093/epirev/mxs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seitz DP, Adunuri N, Gill SS, Rochon PA. Prevalence of Dementia and Cognitive Impairment Among Older Adults With Hip Fractures. Journal of the American Medical Directors Association. 2011;12(8):556–64. doi: 10.1016/j.jamda.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Luckhaus C, Mahabadi B, Grass-Kapanke B, Janner M, Willenberg H, Jager M, et al. Blood biomarkers of osteoporosis in mild cognitive impairment and Alzheimer’s disease. J Neural Transm. 2009;116(7):905–11. doi: 10.1007/s00702-009-0241-x. [DOI] [PubMed] [Google Scholar]
- 50.Bjorkman MP, Sorva AJ, Tilvis RS. Does elevated parathyroid hormone concentration predict cognitive decline in older people? Aging Clin Exp Res. 2010;22(2):164–9. doi: 10.1007/BF03324791. [DOI] [PubMed] [Google Scholar]
- 51.Gillette Guyonnet S, Abellan Van Kan G, Andrieu S, Barberger Gateau S, Berr C, Bonnefoy M, Dartigues JF, de Groot L, Ferry M, Galan P, Hercberg S, Jeandel C, Morris MC, Nourhashemi F, Payette H, Poulain JP, Portet F, Roussel AM, Ritz P, Rolland Y, Vellas B. IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging. 2007 Mar-Apr;11(2):132–52. [PubMed] [Google Scholar]
- 52.Kong HY, Cheng DM, Pang W, Sun SD, Liu J, Huang CY, Jiang YG. Homocysteine levels and cognitive function scores measured with MMSE and BCAT of middle-aged and elderly subjects in Tianjin City. J Nutr Health Aging. 2013;6:527–532. doi: 10.1007/s12603-013-0026-7. [DOI] [PubMed] [Google Scholar]
- 53.Kabagambe E, Wadley V, Kent S, Howard V, Crosson W, Al-Hamdan M, et al. The Relationship Between Long-Term Sunlight Radiation and Cognitive Decline in the REGARDS Cohort Study. Epidemiology. 2012;23(5S):S–474. doi: 10.1007/s00484-013-0631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: national surveys and the problem of standardization. Scandinavian journal of clinical and laboratory investigation. Supplementum. 2012;243:32–40. doi: 10.3109/00365513.2012.681935. [DOI] [PubMed] [Google Scholar]
- 55.Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004;50(11):2195–7. doi: 10.1373/clinchem.2004.040683. [DOI] [PubMed] [Google Scholar]
- 56.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]


