Abstract
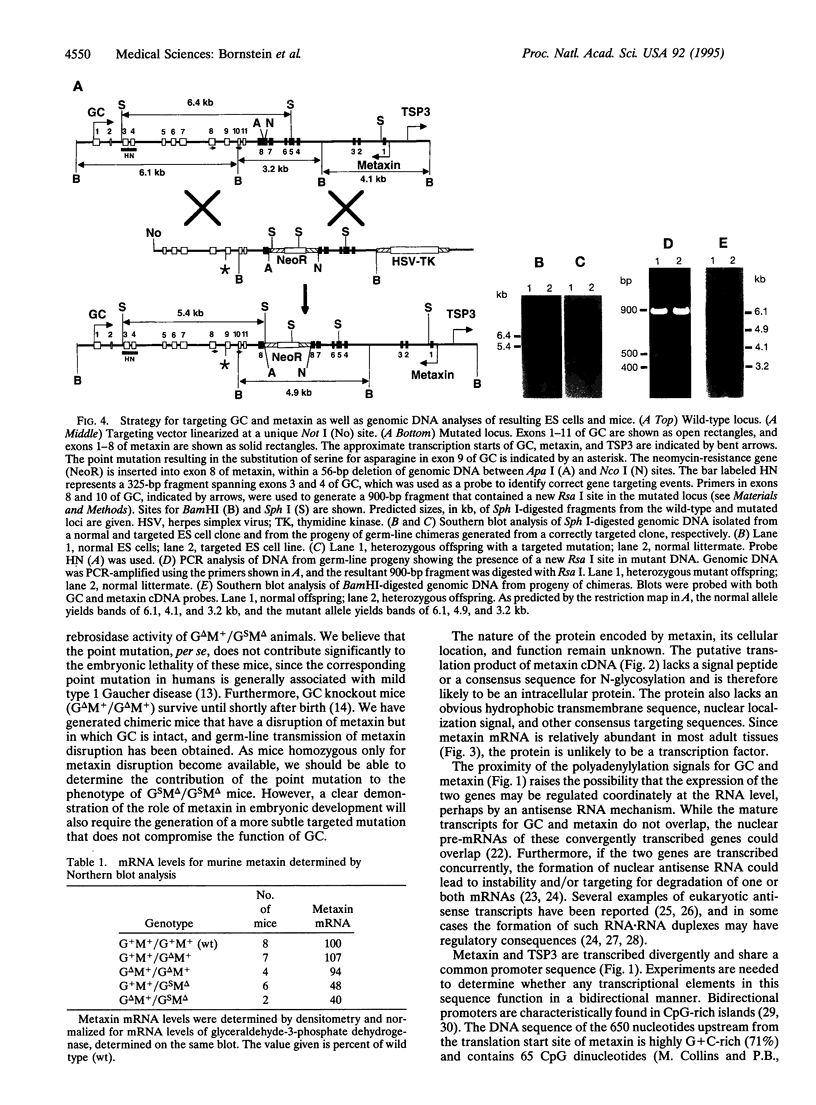
We have identified a murine gene, metaxin, that spans the 6-kb interval separating the glucocerebrosidase gene (GC) from the thrombospondin 3 gene on chromosome 3E3-F1. Metaxin and GC are transcribed convergently; their major polyadenylylation sites are only 431 bp apart. On the other hand, metaxin and the thrombospondin 3 gene are transcribed divergently and share a common promoter sequence. The cDNA for metaxin encodes a 317-aa protein, without either a signal sequence or consensus for N-linked glycosylation. Metaxin protein is expressed ubiquitously in tissues of the young adult mouse, but no close homologues have been found in the DNA or protein data bases. A targeted mutation (A-->G in exon 9) was introduced into GC by homologous recombination in embryonic stem cells to establish a mouse model for a mild form of Gaucher disease. A phosphoglycerate kinase-neomycin gene cassette was also inserted into the 3'-flanking region of GC as a selectable marker, at a site later identified as the terminal exon of metaxin. Mice homozygous for the combined mutations die early in gestation. Since the same amino acid mutation in humans is associated with mild type 1 Gaucher disease, we suggest that metaxin protein is likely to be essential for embryonic development in mice. Clearly, the contiguous gene organization at this locus limits targeting strategies for the production of murine models of Gaucher disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J., Lawler J. Extracellular matrix: the thrombospondin family. Curr Biol. 1993 Mar;3(3):188–190. doi: 10.1016/0960-9822(93)90270-x. [DOI] [PubMed] [Google Scholar]
- Beutler E. Gaucher disease as a paradigm of current issues regarding single gene mutations of humans. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5384–5390. doi: 10.1073/pnas.90.12.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Devarayalu S., Edelhoff S., Disteche C. M. Isolation and characterization of the mouse thrombospondin 3 (Thbs3) gene. Genomics. 1993 Mar;15(3):607–613. doi: 10.1006/geno.1993.1114. [DOI] [PubMed] [Google Scholar]
- Bornstein P., McKay J. The first intron of the alpha 1(I) collagen gene contains several transcriptional regulatory elements. J Biol Chem. 1988 Feb 5;263(4):1603–1606. [PubMed] [Google Scholar]
- Bornstein P. Thrombospondins: structure and regulation of expression. FASEB J. 1992 Nov;6(14):3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- Carstea E. D., Murray G. J., O'Neill R. R. Molecular and functional characterization of the murine glucocerebrosidase gene. Biochem Biophys Res Commun. 1992 May 15;184(3):1477–1483. doi: 10.1016/s0006-291x(05)80049-x. [DOI] [PubMed] [Google Scholar]
- Cross S. H., Charlton J. A., Nan X., Bird A. P. Purification of CpG islands using a methylated DNA binding column. Nat Genet. 1994 Mar;6(3):236–244. doi: 10.1038/ng0394-236. [DOI] [PubMed] [Google Scholar]
- Dolnick B. J. Cloning and characterization of a naturally occurring antisense RNA to human thymidylate synthase mRNA. Nucleic Acids Res. 1993 Apr 25;21(8):1747–1752. doi: 10.1093/nar/21.8.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavalas A., Dixon J. E., Brayton K. A., Zalkin H. Coexpression of two closely linked avian genes for purine nucleotide synthesis from a bidirectional promoter. Mol Cell Biol. 1993 Aug;13(8):4784–4792. doi: 10.1128/mcb.13.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä P., Soininen R., Tryggvason K. Directional regulatory activity of cis-acting elements in the bidirectional alpha 1(IV) and alpha 2(IV) collagen gene promoter. J Biol Chem. 1993 Nov 25;268(33):24677–24682. [PubMed] [Google Scholar]
- Hildebrandt M., Nellen W. Differential antisense transcription from the Dictyostelium EB4 gene locus: implications on antisense-mediated regulation of mRNA stability. Cell. 1992 Apr 3;69(1):197–204. doi: 10.1016/0092-8674(92)90130-5. [DOI] [PubMed] [Google Scholar]
- Horowitz M., Zimran A. Mutations causing Gaucher disease. Hum Mutat. 1994;3(1):1–11. doi: 10.1002/humu.1380030102. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Liska D. J., Sage E. H., Bornstein P. Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn. 1993 May;197(1):40–56. doi: 10.1002/aja.1001970105. [DOI] [PubMed] [Google Scholar]
- Kambouris M., Triarhou L. C., Dlouhy S. R., Sangameswaran L., Luo F., Ghetti B., Hodes M. E. Novel cDNA clones obtained by antibody screening of a mouse cerebellar cDNA expression library. Brain Res Mol Brain Res. 1994 Sep;25(3-4):183–191. doi: 10.1016/0169-328x(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. W. An antisense mRNA directs the covalent modification of the transcript encoding fibroblast growth factor in Xenopus oocytes. Cell. 1989 Nov 17;59(4):687–696. doi: 10.1016/0092-8674(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal G. W., Armstrong B. C., Battey J. F. N-myc mRNA forms an RNA-RNA duplex with endogenous antisense transcripts. Mol Cell Biol. 1990 Aug;10(8):4180–4191. doi: 10.1128/mcb.10.8.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavia P., Macleod D., Bird A. Coincident start sites for divergent transcripts at a randomly selected CpG-rich island of mouse. EMBO J. 1987 Sep;6(9):2773–2779. doi: 10.1002/j.1460-2075.1987.tb02572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A., D'Adamio L., Diener A. C., Clayton L. K., Reinherz E. L. CD3 zeta/eta/theta locus is colinear with and transcribed antisense to the gene encoding the transcription factor Oct-1. J Immunol. 1993 Sep 15;151(6):3152–3162. [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Miyajima N., Horiuchi R., Shibuya Y., Fukushige S., Matsubara K., Toyoshima K., Yamamoto T. Two erbA homologs encoding proteins with different T3 binding capacities are transcribed from opposite DNA strands of the same genetic locus. Cell. 1989 Apr 7;57(1):31–39. doi: 10.1016/0092-8674(89)90169-4. [DOI] [PubMed] [Google Scholar]
- O'Neill R. R., Tokoro T., Kozak C. A., Brady R. O. Comparison of the chromosomal localization of murine and human glucocerebrosidase genes and of the deduced amino acid sequences. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5049–5053. doi: 10.1073/pnas.86.13.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer A. P., Parry G., Patton S., Gendler S. J. Molecular cloning and analysis of the mouse homologue of the tumor-associated mucin, MUC1, reveals conservation of potential O-glycosylation sites, transmembrane, and cytoplasmic domains and a loss of minisatellite-like polymorphism. J Biol Chem. 1991 Aug 15;266(23):15099–15109. [PubMed] [Google Scholar]
- Tsuji S., Martin B. M., Barranger J. A., Stubblefield B. K., LaMarca M. E., Ginns E. I. Genetic heterogeneity in type 1 Gaucher disease: multiple genotypes in Ashkenazic and non-Ashkenazic individuals. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2349–2352. doi: 10.1073/pnas.85.7.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Tremblay M. L., LaMarca M. E., Willemsen R., Stubblefield B. K., Winfield S., Zablocka B., Sidransky E., Martin B. M., Huang S. P. Animal model of Gaucher's disease from targeted disruption of the mouse glucocerebrosidase gene. Nature. 1992 Jun 4;357(6377):407–410. doi: 10.1038/357407a0. [DOI] [PubMed] [Google Scholar]
- Vos H. L., Devarayalu S., de Vries Y., Bornstein P. Thrombospondin 3 (Thbs3), a new member of the thrombospondin gene family. J Biol Chem. 1992 Jun 15;267(17):12192–12196. [PubMed] [Google Scholar]
- Vos H. L., de Vries Y., Hilkens J. The mouse episialin (Muc1) gene and its promoter: rapid evolution of the repetitive domain in the protein. Biochem Biophys Res Commun. 1991 Nov 27;181(1):121–130. doi: 10.1016/s0006-291x(05)81390-7. [DOI] [PubMed] [Google Scholar]