Abstract
All chlorophyll (Chl)-binding proteins involved in photosynthesis of higher plants are hydrophobic membrane proteins integrated into the thylakoids. However, a different category of Chl-binding proteins, the so-called water-soluble Chl proteins (WSCPs), was found in members of the Brassicaceae, Polygonaceae, Chenopodiaceae, and Amaranthaceae families. WSCPs from different plant species bind Chl a and Chl b in different ratios. Some members of the WSCP family are induced after drought and heat stress as well as leaf detachment. It has been proposed that this group of proteins might have a physiological function in the Chl degradation pathway. We demonstrate here that a protein that shared sequence homology to WSCPs accumulated in etiolated barley (Hordeum vulgare) seedlings exposed to light for 2 h. The novel 22-kD protein was attached to the outer envelope of barley etiochloroplasts, and import of the 27-kD precursor was light dependent and induced after feeding the isolated plastids the tetrapyrrole precursor 5-aminolevulinic acid. HPLC analyses and spectroscopic pigment measurements of acetone-extracted pigments showed that the 22-kD protein is complexed with chlorophyllide. We propose a novel role of WSCPs as pigment carriers operating during light-induced chloroplast development.
In light-grown seedlings and mature green plants, chlorophyll (Chl) a and Chl b as well as carotenoids are bound to various proteins within PSI and PSII (Ort and Yocum, 1996). These proteins differ in their Chl-binding properties: one group binds only Chl a, whereas the other can bind both Chl a and Chl b (for reviews, see von Wettstein et al., 1995; Green and Durnford, 1996). The first group is represented by the plastid-encoded pigment-binding proteins of the actual reaction centers (PSI-A/B and D1/D2 in PSII) and the photosynthetic cores of PSII (CP43 and CP47).
The second group comprises the light-harvesting Chl a/b-binding proteins LHCI and LHCII of PSI and PSII, respectively (Dreyfuss and Thornber, 1994; Kühlbrandt et al., 1994; Green and Durnford, 1996). These nuclear gene products form the outer antenna complexes of the two photosystems. The Chl content of LHCII accounts for approximately 50% of all Chls in the thylakoid membranes (Kühlbrandt et al., 1994). LHCII operates as a trimer (Dreyfuss and Thornber, 1994), and each monomer contains Chl a plus Chl b in a 7:6 stoichiometry (Kühlbrandt et al., 1994). Monomeric LHCII is embedded into the thylakoids via three α-helices (Kühlbrandt et al., 1994). Similar, structurally related α-helices also occur in other Chl-binding proteins, such as the minor light-harvesting Chl a/b-binding proteins CP29, CP26, CP24, and CP14 (summarized in Green et al., 1991; Jansson, 1994; Paulsen, 1995), and the early light-inducible proteins (ELIPs; Kloppstech et al., 1984; Grimm and Kloppstech, 1987; Grimm et al., 1989; for review, see Adamska, 1997). The S-subunit of PSII (PsbS) is a related but four-helix Chl-binding protein, which contains an additional fourth helix similar to helix 2 present in LHCII and the other Chl-binding proteins (Li et al., 2000). Previous work has shown that Chl-binding proteins such as LHCII and CP29 cross-react with anti-LHCI antibodies because they share structurally similar transmembrane helices (White and Green, 1987).
In dark-grown (etiolated) seedlings, protochlorophyllide (Pchlide) accumulates instead of Chl. This compound is the immediate precursor of chlorophyllide (Chlide) and differs from it by a double bond in ring D of the tetrapyrrole ring system (for details, see von Wettstein et al., 1995). Pchlide is bound to two NADPH:Pchlide oxidoreductases (EC 1.3.33.1), called PORA and PORB (Holtorf et al., 1995), which are supposed to be part of a larger complex (also known as the holochrome) in the prolamellar body of etioplasts (Reinbothe et al., 1999). Upon illumination, it catalyzes the conversion of Pchlide to Chlide (Boardman, 1962) and in turn rapidly disintegrates (Kahn, 1968). In vivo and in vitro analyses showed that after this reaction has been performed, either POR protein are degraded or Chlide released (C. Reinbothe et al., 1995; S. Reinbothe et al., 1995c). We hypothesized that Chlide released during the degradation of the PORA and PORB may bind to other pigment-binding proteins, such as LHCI and LHCII and their minor species, ELIPS and PsbS (Reinbothe and Reinbothe, 1996).
In this study, a biochemical approach was taken to identify putative Chlide carriers. We discovered a novel pigment-binding protein, which transiently accumulated as a soluble protein in the stroma of barley (Hordeum vulgare) etiochloroplasts, present in dark-grown plants that had been exposed to light for 2 h. The pigment-binding properties, chloroplast import characteristics, and the temporal expression pattern of this protein were compatible with its role as a Chlide carrier. This protein, named FCBP (free Chl[ide]-binding protein), was found to be a novel member of the family of water-soluble Chl proteins (WSCPs) of the Brassicaceae family, which thus far have only been implicated in pigment binding during photosynthetic acclimation and leaf senescence. Our results show for the first time, to our knowledge, that WSCPs, including FCBP, also operate in the shuttling of Chl intermediates during seedling de-etiolation.
RESULTS
Previous work had shown that etiochloroplasts isolated from etiolated barley plants, which had been exposed to light for a few hours, are experimentally depleted of endogenous PORA and PORB (Reinbothe et al., 1996). Due to their light-induced destabilization (C. Reinbothe et al., 1995; S. Reinbothe et al., 1995b, 1995c), PORA and PORB are degraded during plastid isolation in the light (Reinbothe et al., 1996). However, etiochloroplasts contain substantial amounts of the cytosolic PORA precursor (pPORA) at their outer envelope (Reinbothe et al., 1996). Because of the light-induced decline in the level of Pchlide, which is likely to be synthesized in the plastid envelope (Pineau et al., 1986; Joyard et al., 1990), import of the pPORA was blocked (Reinbothe et al., 1996). Import could be restored upon feeding isolated etiochloroplasts the Pchlide precursor 5-aminolevulinic acid (5-ALA; Reinbothe et al., 1996). Then, the envelope-bound precursor was chased into the plastids and processed to mature size (Reinbothe et al., 1996).
We exploited these previous observations in all subsequent experiments. Briefly, 5-d-old dark-grown barley seedlings were exposed to white light for 2 h, and plastids were isolated by Percoll density gradient centrifugation and purified. The whole plastid isolation and purification procedure was performed in white light and led to etiochloroplasts that were devoid of endogenous PORA and PORB (Fig. 1, Etiochloroplasts, lane 0). These PORA/B-depleted plastids were subsequently incubated either in darkness or white light with 5-ALA in the presence of 5 mM Mg-ATP (see “Materials and Methods”).
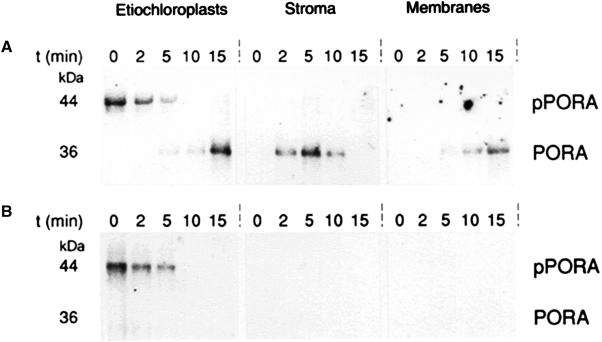
Figure 1.
Light-induced degradation of imported PORA. Barley etiochloroplasts bearing pPORA at their outer envelope were incubated with 5-ALA either in darkness (A) or white light (B). At the indicated time points, aliquots of the plastid suspension were withdrawn. Etiochloroplasts to be further fractionated were diluted into import buffer containing thermolysin (100 μg per 50-μL assay; Stroma and Membranes), whereas all other plastids were diluted into buffer lacking thermolysin (Etiochloroplasts). After a step of centrifugation, the protease-treated etiochloroplasts were lysed and fractionated into stroma and membranes. Nonprotease-treated etiochloroplasts, by contrast, were immediately extracted with TCA. Stromal and membrane proteins recovered from the thermolysin-treated etiochloroplasts were then subjected to immunoprecipitation using an antiserum against pPORA. The resulting immunocomplexes in turn were run electrophoretically, blotted onto nitrocellulose filters, and reprobed with the same antiserum, using the goat anti-rabbit IgG, rabbit anti-goat IgG alkaline phosphatase system. The blot shows POR-related proteins in the dark- and light-incubated, 5-ALA-fed etiochloroplasts and the indicated plastid subfractions.
Stromal proteins were recovered from lysed etiochloroplasts after various stages of import and subjected to immunoprecipitation (Wiedmann et al., 1987), using an antiserum against pPORA (Schulz et al., 1989). We assumed that nascent PORA-Pchlide-NADPH complexes formed upon import may reduce their Pchlide to Chlide and in turn would be degraded if the samples were illuminated. As shown previously, there is a light-induced, ATP- and metal ion-dependent, multicomponent stromal protease that degrades freshly formed POR-product complexes (C. Reinbothe et al., 1995; S. Reinbothe et al., 1995a). This so-called POR-degrading protease is unable to attack POR-Pchlide-NADPH complexes in darkness (C. Reinbothe et al., 1995; S. Reinbothe et al., 1995a).
Figure 1 shows the results of the immunoprecipitations. They unveiled the transient accumulation of mature PORA in the stroma of the 5-ALA-fed, dark-incubated etiochloroplasts and its subsequent targeting to the thylakoids (Fig. 1A). By contrast, no mature PORA could be seen in the 5-ALA-fed, illuminated samples (Fig. 1B). This finding supports our assumption that the imported and processed pPORA was degraded. We reasoned that Chlide (a possible phototoxin) released during the degradation of imported PORA would not exist free in the stroma but would be bound by other carrier proteins, such as members of the LHC superfamily (see introduction).
To detect such putative Chlide-binding proteins, immunoprecipitations were performed using a polyclonal antiserum against LHCII (Apel and Kloppstech, 1980). We reasoned that because of the shared structural features of known Chl-binding proteins (see introduction), this antiserum might recognize not only minor LHC proteins, such as CP29, CP26, CP24, and CP14, but also ELIPs and PsbS.
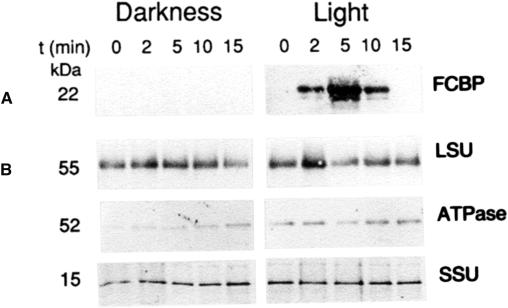
Figure 2A shows that a 22-kD protein could be immunoprecipitated from the stroma of 5-ALA-fed, illuminated etiochloroplasts. In addition to the main, prominent band, a slightly smaller band and a slightly larger band were observed (Fig. 2A). All three transiently accumulated as soluble proteins in the stroma. Their banding pattern was reminiscent of that observed for LHCII in the thylakoids (Apel and Kloppstech, 1980). Control experiments with antisera against the 52-kD subunit of the coupling factor O (CFO)/coupling factor 1 (CF1)-ATPase and the large and small subunits of Rubisco showed that the appearance of soluble Chl-binding proteins was most likely not due to photooxidative membrane destruction induced by excess Pchlide produced in the 5-ALA-fed, illuminated samples. Both in illuminated and dark-incubated etiochloroplasts, similar levels of the control proteins were seen (Fig. 2B).
Figure 2.
Detection of FCBP. Etiochloroplast-bound pPORA was imported either in darkness or white light, as described in Figure 1. At the indicated time intervals, stromal polypeptides were prepared from lysed plastids, subjected to immunoprecipitation using an antiserum against barley LHCII, and the resulting immunocomplexes were separated electrophoretically and blotted onto nitrocellulose filters. The blots were reprobed with the same antiserum. Parallel samples were precipitated and reprobed with antisera against barley large (LSU) and small subunits (SSU) of Rubisco and the 52-kD subunit of the CFO/CF1 ATPase. The blot highlights the detection of a group of related proteins, collectively referred to as FCBP, which accumulate only in the illuminated, not in the dark-incubated, 5-ALA-fed etiochloroplasts.
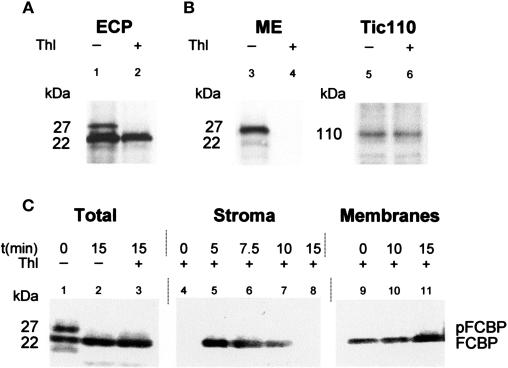
The appearance of the group of 22-kD proteins, which we collectively named FCBP, was investigated further. When total etiochloroplast proteins were probed with the anti-LHCII antiserum, a larger, 27-kD protein was observed in addition to the main 22-kD protein (Fig. 3A, lane 1). Pretreatment of isolated etiochloroplasts with thermolysin prior to protein extraction led to the disappearance of the 27-kD band (Fig. 3A, lane 2). A protein of similar electrophoretic mobility could be detected when isolated mixed envelopes, which had been prepared from purified barley etiochloroplasts, were probed with the anti-LHCII antiserum (Fig. 3B, lane 3). This protein disappeared upon thermolysin treatment prior to plastid fractionation (Fig. 3B, lane 4). The inner plastid envelope membrane protein Tic110 remained protease resistant, however (Fig. 3D, lanes 5 and 6). These results demonstrated that the isolated etiochloroplasts were largely intact. Upon feeding such etiochloroplasts 5-ALA in white light, the level of 27-kD protein dropped, and increasing amounts of 22-kD FCBP were detectable in the total etiochloroplast samples (Fig. 3C, Total, lanes 1–3). Fractionation experiments with etiochloroplasts, which had been treated with thermolysin after import, and subsequent immunoprecipitations with the anti-LHCII antiserum revealed that the 27-kD protein was imported and processed to mature size. The resulting 22-kD protein accumulated transiently in the stroma and subsequently was targeted to the inner membranes of chloroplasts (Fig. 3, Stroma versus Membranes).
Figure 3.
FCBP accumulates as a higher molecular mass precursor (pFCBP) in vivo. Etiochloroplasts were prepared as described in Figure 1. The plastids were then fed 5-ALA in white light. After the indicated time periods, aliquots were taken and intact and broken plastids separated on Percoll. Intact plastids were fractionated into mixed outer and inner plastid envelope membranes, stroma, and total membranes. Membrane fractions were solubilized with detergent. All fractions in turn were subjected to immunoprecipitation with the anti-LHCII antiserum described in Figure 2. A, Protease sensitivity of the 27-kD pFCBP, but not mature 22-kD FCBP, toward thermolysin (Thl) in intact etiochloroplasts (ECP; lanes 1 and 2). B, Detection of protease-sensitive pFCBP in mixed outer and inner plastid envelope membranes (ME) and its degradation by thermolysin prior to plastid fractionation (lanes 3 and 4). For comparison, lanes 5 and 6 show the level of the translocon of the inner envelope protein Tic110 before and after thermolysin treatment of isolated intact plastids. C, Import and routing of pFCBP studied with 5-ALA-fed, illuminated etiochloroplasts. The blot shows pFCBP and FCBP levels in total plastids (Total) as well as stromal (Stroma) and membrane (Membranes) fractions at the indicated times of import. Molecular masses are indicated.
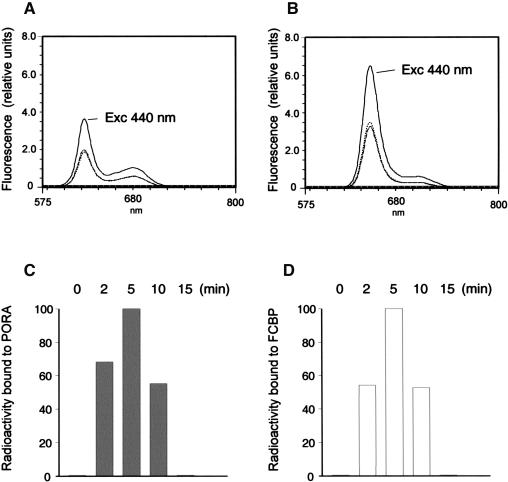
We next analyzed whether the freshly imported, processed FCBP may be complexed with Chlide. When we assayed immunocomplexes of stromal protein recovered after different stages of import with the anti-LHCII antiserum, unexpectedly no Chlide fluorescence could be seen using low temperature spectroscopy at 77 K (data not shown). Room temperature analyses of the same samples showed that there was acetone-extractable pigment in the illuminated etiochloroplasts, however (Fig. 4B). Its emission maximum at 652 nm was consistent with that of Chlide b. Additional fluorescence emission and excitation analyses at different excitation and emission wavelengths proved the identity of FCBP-extracted pigment as Chlide b (data not shown). When immunocomplexes were recovered from stromal extracts of 5-ALA-fed but dark-incubated etiochloroplasts using the PORA antiserum, Pchlide was the only acetone-extractable pigment (Fig. 4A). Because Pchlide a and Pchlide b display very similar fluorescence spectra at the chosen excitation wavelength of 440 nm, no definite answer on the identity of the detected pigment could be given at this stage. When import reactions were performed in the presence of 3H-5-ALA, 3H-Chlide b and 3H-Pchlide were found (Fig. 4, C and D). This result showed that de novo synthesized pigments bound to the FCBP and PORA, respectively.
Figure 4.
Time course of 5-ALA-derived pigment accumulation in dark- and light-incubated etiochloroplasts, respectively. Etiochloroplasts were prepared as described and treated with 5-ALA (A and B) or 3H-5-ALA (C and D) in darkness (A and C) or white light (B and D). Stromal proteins recovered after different stages of import by immunoprecipitation with the pPORA and LHCII antisera, respectively (see Figs. 1 and 2), were extracted with 100% acetone and analyzed by room temperature fluorescence measurements, at an excitation wavelength of 440 nm (A and B), or by liquid scintillation counting (C and D). The different curves show PORA-extracted (A) and FCBP-extracted (B) pigments accumulating at time zero (dotted line) and after 2 min (hatched line), 5 min (solid line), 10 min (stippled line), and 15 min (long dashes) of import. Bars in C and D show 3H-pigments extracted from PORA and FCBP at the indicated stages of import, expressed as percentages of maximum levels.
Fluorescence spectroscopy of acetone-extracted pigments does not allow distinguishing of whether porphyrins and chlorins, such as Pchlide and Chlide, may be present in their esterified or nonesterified forms. As shown previously, the phytol chain has no influence on the spectral properties of the dissolved compounds (for example, see Helfrich et al., 1994). We consequently sought for alternative methods permitting the identification of FCBP-extracted and PORA-extracted pigments.
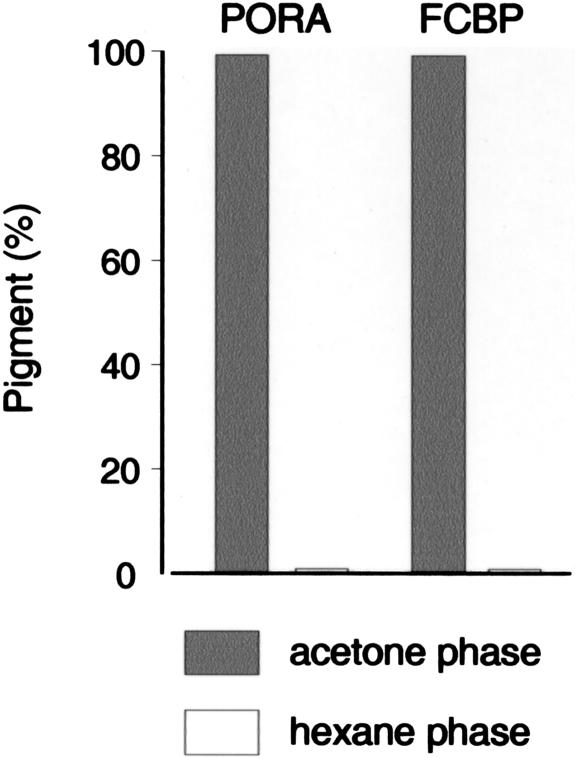
In the first experiment, differential solvent extractability of FCBP-bound and PORA-bound pigments with mixtures of acetone and hexane (see “Materials and Methods”) was tested. This method had previously been introduced by Helfrich et al. (1994) in order to separate esterified and nonesterified pigments. Figure 5 demonstrates that both FCBP-bound and PORA-bound pigments were almost quantitatively retained in the aqueous phase obtained after extraction of the samples, whereas only traces (less than 1%) of the total pigment were present in the hexane phase. Thus, both pigments were largely present in a nonesterified form.
Figure 5.
Differential solvent extractability of PORA- and FCBP-bound pigments and to distinguish esterified and nonesterified pigments. Stromal PORA and FCBP were prepared as described in Figure 4 and extracted with acetone and hexane. After phase separation, nonesterified pigments present in the acetone/buffer phase (gray bars) and esterified pigments present in the hexane phase (white bars) were quantified.
As a second approach, we analyzed acetone-extracted pigments by HPLC. As shown previously, Pchlide a and Pchlide b can well be resolved on C18 reverse phase columns (Scheumann et al., 1999; C. Reinbothe et al., 2003), whereas Chlide a and Chlide b as well as their esterified products are separable on C30 reversed phase materials (S. Reinbothe et al., 2003). During HPLC, pigment detection and identification was made by absorbance measurements, using a photodiode array detector (see “Materials and Methods”). For comparison, synthetic standards were prepared. These have been characterized (C. Reinbothe, S. Pollmann, C. Desvignes, M. Weigele, E. Beck, and S. Reinbothe, unpublished data).
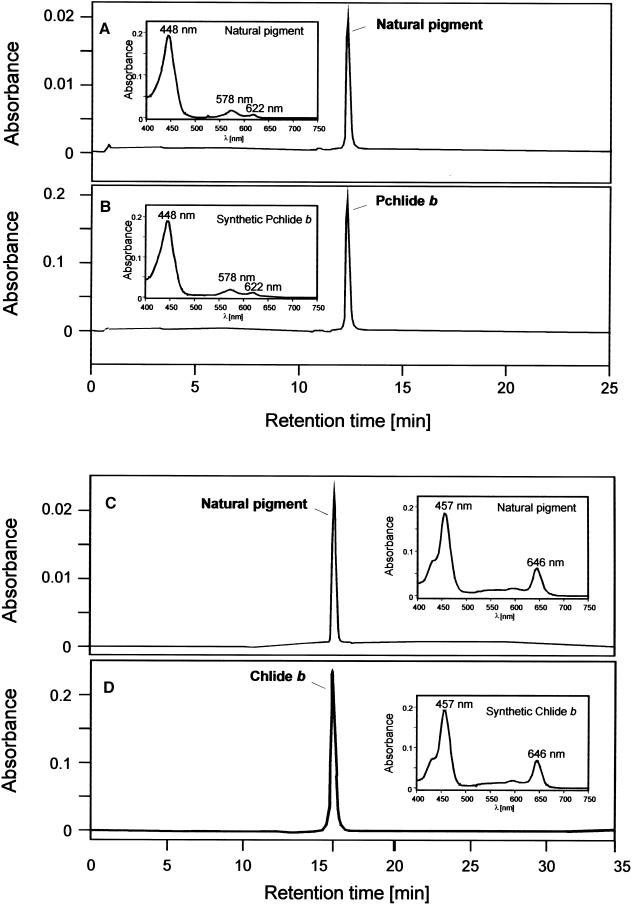
Figure 6A shows a representative HPLC chromatogram of PORA-extracted pigments. The inset depicts an absorbance spectrum of resolved pigment. Both the retention time of 12.5 min and the absorbance profile (Fig. 6A) are consistent with values previously reported for Pchlide b (Scheumann et al., 1999; C. Reinbothe et al., 2003). Pchlide b has a main maximum at 448 nm and two lower maxima at 578 nm and 622 nm, respectively (Scheumann et al., 1999). These correspond to the so-called Soret band (448 nm), the Qx band (578 nm), and the Qy band (622 nm; Scheumann et al., 1999). The Qx band had a higher absorbance than the Qy band, which is a typical feature of all investigated pigments of the proto b series (Schoch et al., 1995; Scheumann et al., 1999; C. Reinbothe et al., 2003).
Figure 6.
HPLC separation of PORA- and FCBP-extracted pigments. Isolated etiochloroplasts were fed 5-ALA either in darkness (A and B) or white light (C and D) and stromal polypeptides subjected to immunoprecipitation as described in Figure 4. Pigments were extracted with 100% acetone containing 0.1% diethyl pyrocarbonate. A, HPLC of PORA-extracted pigments on a C18 reverse phase column. B, as A, but showing the same sample diluted with a 10-fold molar excess of synthetic Pchlide b prior to separation. C, HPLC of FCBP-extracted pigments on a C30 reverse phase column. D, as C, but showing the same sample diluted with a 10-fold molar excess of synthetic Chlide b prior to separation. Absorbance spectra were recorded at either 455 nm (A and B) or 435 nm (C and D). Insets show absorbance profiles of natural (A and C) and synthetic (B and D) compounds.
Coinjection experiments were performed in order to prove the identity of PORA-extracted Pchlide b. Ten-fold molar excess of synthetic Pchlide b was added to the sample prior to separation. Figure 6B shows an absorption spectrum of synthetic Pchlide b, which was identical to that reported before for pigments extracted from the PORA (Fig. 6A, inset). The retention time of the synthetic standard was also indistinguishable from that of the PORA-extracted pigment, strongly suggesting that both compounds were identical.
Figure 6C shows a representative HPLC chromatogram of FCBP-extracted pigments after separation on a C30 column. The inset highlights the absorption properties of resolved pigment. The shape of the curve and the main absorption maxima were consistent with those reported for Chl(ide) b (e.g. Ito et al., 1996; Scheumann et al., 1998). Chl(ide) b has two main maxima at 457 nm and 646 nm, respectively, and a shoulder at 435 nm. Identical values were obtained for the synthetic standard (Fig. 6D, inset). Coelution of the natural pigment and synthetic standard (Fig. 6D) confirmed that both compounds were identical.
We next purified and sequenced the 22-kD FCBP. Import reactions were scaled up 103-fold and immunoprecipitates recovered with the anti-LHCII antiserum separated electrophoretically. Then, the 22-kD band was eluted, treated with either Staphylococcus aureus protease V8 or endoproteinase Lys C, and the mixtures subjected to HPLC. Several peptides were obtained that were subsequently sequenced.
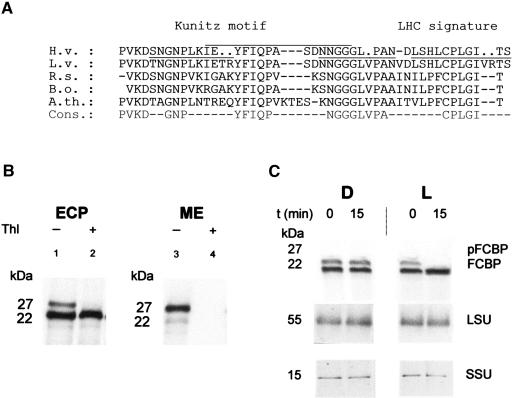
Figure 7A shows a sequence of 29 amino acids that could be constructed from three overlapping peptides. In this sequence, three positions could not be determined with certainty (Fig. 7A, dots). In addition, a fourth short peptide sequence was obtained. Upon aligning the resulting overall sequence with protein sequences deduced from the data banks, a relationship of the 22-kD protein to a group of previously identified seed storage proteins possessing the so-called Kunitz trypsin proteinase inhibitor motif became apparent (Fig. 7A). Interestingly, this Kunitz motif was also found in a distantly related member of the Chl-binding protein family, the so-called WSCPs of the Brassicaceae family, including Lepidium virginicum (Murata and Ishikawa, 1981; Itoh et al., 1982), cauliflower (Brassica oleracea var. botrys; Murata et al., 1971; Nishio and Satoh, 1997), brussels sprouts (B. oleracea var. gemmifera; Fig. 7A; Kamimura et al., 1997), wild mustard (B. nigra; Murata and Murata, 1971), and rapeseed (B. napus var. oleifera; Nishio and Satoh, 1997). WSCP relatives could also be found in Arabidopsis (Satoh et al., 1998) and Raphanus sativus (Fig. 7A; Lopez et al., 1994).
Figure 7.
Identification of FCBP as a WSCP. A, Partial amino acid sequence data. FCBP was recovered from the stroma of 5-ALA-fed, illuminated etiochloroplasts as described (see Fig. 2), purified, and treated with two different proteases. The resulting peptide mixtures were subjected to HPLC and individual peptides sequenced. The obtained partial sequence of the barley FCBP (H. vulgare, H.v.) was aligned with published WSCP amino acid sequences of L. virginicum (L.v.), R. sativus (R.s.), and B. oleracea (B.o.), as well as a drought-induced protein from Arabidopsis (A.th.). S. aureus V8 protease-derived and endoproteinase Lys C-derived sequences are overlined and underlined, respectively. Dots indicate amino acids, the identity of which could not be determined unambiguously. In the given consensus amino acid sequence (Cons.), nonidentical residues are marked by dashes. B, Cross-reactivity of FCBP with the L. virginicum WSCP. Intact barley etiochloroplasts (ECP) were prepared as described and treated without (−) or with (+) thermolysin (Thl; lanes 1 and 2, respectively) and extracted with TCA or fractionated into mixed outer and inner envelope membranes (ME; lanes 3 and 4), stroma, and membranes (data not shown) prior to protein extraction. Protein was separated electrophoretically and subjected to western blotting, using an antiserum against the WSCP of L. virginicum. C, Light-dependent import of pFCBP into 5-ALA-fed barley etiochloroplasts monitored with the anti-WSCP antiserum of L. virginicum. Import reactions were performed as described in Figure 1 and protein detected by western blotting. As a control, antisera against the large (LSU) and small subunits (SSU) of Rubisco were used. Molecular masses are indicated. D, darkness; L, light.
All WSCPs characterized thus far are able to bind Chl a and Chl b (for summary, see Satoh et al., 1998). Despite their similarity in terms of hydrophilicity, molecular mass, and subunit composition, two different subgroups of WSCPs have been discerned (Satoh et al., 1998). Members of the first group, comprising WSCPs from cauliflower, wild mustard, and brussels sprouts, show a Chl a content that is approximately 6- to 10-fold higher than that of Chl b (Murata and Murata, 1971; Murata et al., 1971; Kamimura et al., 1997; Satoh et al., 1998). Members of the second group are characterized by approximately equimolar contents of Chl a and Chl b (Murata and Ishikawa, 1981; Itoh et al., 1982).
The obtained partial sequence information shown in Figure 7A suggested that the barley FCBP may belong to group II WSCPs. We therefore reprobed western blots containing total etiochloroplast samples with a heterologous antiserum against the WSCP of L. virginicum. Figure 7B demonstrates that the antiserum cross-reacted with the previously identified 22-kD and 27-kD proteins (Fig. 7B, lane 1). Pretreatment of isolated etiochloroplasts with thermolysin degraded the 27-kD protein but did not affect the level of the 22-kD protein (Fig. 7B, lane 2). A similar protease-sensitive 27-kD band was detectable in mixed outer and inner plastid envelope membranes (Fig. 7B, lanes 3 and 4). Upon feeding isolated intact etiochloroplasts 5-ALA in white light, the 27-kD protein was imported and processed, as judged from the increased amounts of 22-kD protein on the blots (Fig. 7C, L). In dark controls, no import occurred (Fig. 7C, D).
DISCUSSION
In this study, a novel water-soluble pigment-binding protein was discovered in barley etiochloroplasts. This protein is related to a family of WSCPs studied in the Brassicaceae family. In contrast to previously described pigment-binding proteins, such as LHCII, its minor species CP29, CP26, CP24, and CP14, as well as ELIPs and PsbS, the barley WSCP, which we henceforth refer to as hvWSCP, was found to contain Chl(ide) (Fig. 4). The aqueous solubulity of this pigment combined with an HPLC retention time identical to that of Chlide b (Fig. 6) indicates that the pigment bound to hvWSCP is nonesterified. Moreover, hvWSCP precursor molecules (hvpWSCP) accumulated at the outer envelope of developing barley etiochloroplasts in vivo and were imported into the plastids only under conditions where Chlide b was produced (Figs. 1, 3, and 7).
The time course experiments shown in Figures 1 and 3 suggest that Chlide b may originate during the degradation of imported pPORA. Previous in vitro reconstitution experiments showing high affinity binding of Pchlide b to PORA and its conversion to Chlide b (C. Reinbothe et al., 1999; S. Reinbothe et al., 2003) seem well compatible with the determined pigment-binding properties of hvWSCP. Results (C. Reinbothe, S. Pollmann, C. Desvignes, M. Weigele, E. Beck, and S. Reinbothe, unpublished data) additionally demonstrate that hvWSCP is also able to bind Chlide a. hvWSCP thus appears to represent the first identified Chlide carrier from barley that functions during plant de-etiolation.
hvWSCP is remarkable in at least three more aspects. First, it could not be detected by in situ fluorescence measurements at −196°C (77 K). This finding suggests that Chlide b may be present in a nonphotoexcitable form if bound to hvWSCP or that it may dissipate its excitation energy in a radiation-less manner, for example, by interacting with carotenoids present in etioplasts. Interestingly, recombinant WSCP of cauliflower has recently been demonstrated to lack carotenoids but to quench triplet excited Chl and singlet oxygen in an as yet unknown manner (Schmidt et al., 2003). Alternatively, hvWSCP may interact with other proteins, such as stromal targeting factors, chaperones, or other, yet to be identified factors, which affect its bound pigments. As shown previously, some WSCPs display Chl fluorescence only if present in tetrameric or other higher, homo-oligomeric forms containing both Chl a and Chl b (Satoh et al., 1998; Schmidt et al., 2003). Their resistance against extraction with 80% acetone (Satoh et al., 1998) points to a highly ordered organization of protein and pigment. This property underscores the need for Chl intermediates, which are potent phototoxins, not to remain unbound in the stroma during de-etiolation and chloroplast dedifferentiation (senescence).
The second remarkable point is the apparent absence of structural similarity between hvWSCP and related WSCPs to other known Chl-binding proteins. The partial amino acid sequence obtained for hvWSCP indicated a relationship to the Kunitz trypsin inhibitor motif present in certain seed storage proteins. But it also unveiled that the Kunitz motif is a signature of WSCPs. As shown in Figure 7A, the Kunitz trypsin inhibitor motif is located adjacent to the (F/Y)DPLGL motif found in the hinge region between helix 2 and helix 3 of LHCII (Kühlbrandt et al., 1994; Satoh et al., 1998). The latter region is known from x-ray structures of pea (Pisum sativum) LHCII to bind Chl a1 and a head group of lutein (Kühlbrandt et al., 1994). The presence of the (F/Y)DPLGL motif in hvWSCP thus may partly explain pigment binding. However, there may be other, structurally distinctive high affinity pigment-binding sites in hvWSCP and other WSCPs that remain to be discovered.
The function of the Kunitz trypsin inhibitor motif is not yet understood. A role in inhibiting stromal proteases is conceivable but has not been demonstrated. This lack of information may be due to some specific cofactor requirements not met in previous in vitro tests using trypsin (Kamimura et al., 1997; Nishio and Satoh, 1997). On the other hand, the respective target protease in the stroma that may be inhibited by the Kunitz trypsin inhibitor motif has not yet been identified. It is tempting to speculate that it may be specifically inhibiting the POR-degrading protease.
As a third point, it is noteworthy that WSCPs thus far are believed to lack typical chloroplast transit peptides (Satoh et al., 1998). Instead, WSCPs were proposed to contain signal sequences reminiscent of those of secretory proteins (Satoh et al., 1998). Our results show that hvWSCP is synthesized as a larger precursor, which is imported into the chloroplast only in the presence of Chlide b. Interestingly, Eggink et al. (2001) proposed that import and membrane insertion of the nucleus-encoded Chl-binding proteins may be regulated by retention signals in the mature polypeptides that anchor the proteins to the inner plastid envelope membranes. Only in the presence of nascent Chl, import would occur (Eggink et al., 2001).
Satoh et al. (1998) advanced the hypothesis that WSCP may, under certain conditions, be able to enter the secretory pathway and speculated that WSCPs could funnel Chl or Chl catabolites for degradation in the vacuole. However, it seemed for a long time mysterious how plastid-bound WSCPs could be targeted to the vacuole. With the detection of a pool of envelope-bound 27-kD WSCP, this question may now find an explanation. We hypothesize that pigment-driven, bidirectional movement could give rise to both plastid and vacuolar forms of WSCPs. If so, this mechanism could allow envelope-localized porphyrins and chlorins (Pineau et al., 1986; Joyard et al., 1990) to be imported from the cytosol into the chloroplast or to be exported from the plastids to their surroundings. It is tempting to speculate that such bidirectional transport could have interesting implications for the regulation of chloroplast development and aging (senescence), which is known to be under the control of both nucleus- and plastid-derived signals (Reinbothe and Reinbothe, 1996). Work is in progress to clone and characterize the barley WSCP and to study its role during plant development.
MATERIALS AND METHODS
Plastid Isolation
Seeds of barley (Hordeum vulgare) L. cv Carina were grown on moist vermiculite for 5 d in the dark and exposed to white light (30 W m−2, provided by fluorescent bulbs) for 2 h (Reinbothe et al., 1996). Etiochloroplasts were isolated from such illuminated seedlings by Percoll density gradient centrifugation (S. Reinbothe et al., 1995c). After a subsequent purification step on Percoll (S. Reinbothe et al., 1995c), the plastids were resuspended in import buffer lacking ATP, to a final number of 5 × 107 per mL (S. Reinbothe et al., 1995c).
Import Assay
Fifty-microliter import assays consisted of 25 μL of a doubly concentrated import buffer lacking ATP (see above), 2 μL of Mg-ATP (5 mM final concentration), and 10 μL of the isolated, resuspended plastids. The import reaction was initiated by adding 2.5 μL of 5-ALA (0.5 mM final concentration; S. Reinbothe et al., 1995c). After various time intervals of incubation either in darkness or white light, the assay mixtures were diluted, if not stated otherwise, into import buffer containing 100 mg mL−1 thermolysin (modified after Cline et al., 1984) and centrifuged. Intact and broken plastids were separated on Percoll, and the intact plastids were fractionated into envelopes, stroma, and thylakoids (Cline et al., 1984).
Analysis of Stromal Polypeptides
Stromal polypeptides were subjected to gel filtration on Sephadex G15 (S. Reinbothe et al., 1995c), equilibrated in import buffer lacking ATP (see above), and incubated either with an anti-pPORA antiserum (Schulz et al., 1989) or anti-LHCII antiserum (Apel and Kloppstech, 1980). Immunoprecipitations were performed according to Wiedmann et al. (1987). Aliquots of the resulting immunoprecipitates were extracted with 100% acetone and analyzed either electrophoretically or spectroscopically, as indicated below.
Protein Extraction, Electrophoresis, Western Blotting, and Microsequencing of Proteins
Total protein was extracted from nonfractionated etiochloroplasts, which had been recovered from the import mixtures by centrifugation, as described previously (S. Reinbothe et al., 1995c). After differential centrifugation of the plastid homogenates at 2,652g and 18,272g, respectively, in a Sorvall RC5B centrifuge (DuPont, Wilmington, DE), protein was precipitated with trichloroacetic acid (TCA; 5% final concentration), washed with acetone, ethanol, and diethyl ether, and finally dissolved in sample buffer (Laemmli, 1970). By the same procedure, protein was recovered from stromal and solubilized membrane fractions, respectively. Total plastid protein as well as stromal and membrane polypeptides were run in denaturing 10% to 20% polyacrylamide gradients containing SDS (Scharf and Nover, 1982), blotted onto nitrocellulose filters, and subjected to western blot analyses (Towbin et al., 1979), using the indicated anti-pPORA and anti-LHCII antisera. As a control, western blots were probed with antisera against the small and large subunits of Rubisco (Lehmann et al., 1995) and the 52-kD subunit of the chloroplast CFO/CF1 ATPase. Stromal polypeptides that had been subjected to immunoprecipitation prior to electrophoretic analysis were probed identically. For microsequencing, protein was recovered by immunoprecipitations, separated by SDS-PAGE, and the relevant bands were eluted, pooled, and concentrated on preparative gels. The concentrated band was subjected to two-dimensional SDS-PAGE, including isoelectric focusing in the first dimension and SDS-gradient fractionation in the second dimension (O'Farrell, 1975). Individual spots were pooled and treated with endoproteinase Lys C and Staphylococcus aureus protease V8, respectively. The resulting mixtures were subjected to HPLC and individual peptides sequences determined as described by Chang et al. (1978) and Chang (1983). The partial amino acid sequences were aligned with protein sequences deduced from the data banks, using standard computer programs.
Pigment Analyses
Pigments were extracted from stromal fractions of lysed barley etiochloroplasts with 100% acetone containing 0.1% (v/v) diethyl pyrocarbonate. Separation by HPLC was performed on a C18 reverse phase silica gel column (250 × 4.6 mm, Nucleosil ODS 5 μm; Macherey-Nagel, Duren, Germany) as described by Scheumann et al. (1999). Either a step gradient was used, starting with 34% 25 mM aqueous ammonium acetate, 15% acetone, and 51% methanol (buffer A), increasing to 16% H2O, 60% acetone, and 24% methanol within 20 min (buffer B), and finally to 100% acetone another 4 min later, or linear gradients from buffer A to buffer B. Absorbance measurements were made at 455 nm to detect and quantify Pchlide. At this wavelength, which corresponds to the Soret band of Pchlide b, both Pchlide a and Pchlide b can be detected, although the extinction coefficients are 5-fold different (Scheumann et al., 1999). As internal standards, we used synthetic Pchlides a and b, which had been prepared from Chlides a and b, respectively, using an excess of 2,3-dichloro-5,6-dicyanobenzoquinone (Schoch et al., 1995; Scheumann et al., 1999). At a flow rate of 1 mL/min, Pchlide a has a retention time of 15 min and Pchlide b of 12.5 min (C. Reinbothe et al., 2003).
For separation of Chlides a and b and their esterified products, a C30 reverse phase column (250 × 4.6 mm, 5 μm; YMC, Wilmington, NC; Fraser et al., 2000; S. Reinbothe et al., 2003) was used. Absorbance measurements were made at 435 nm. The following retention times were obtained: Chlide b, 16.25 min; Chl b, 17.5 min; Chlide a, 19.5 min; and Chl a, 21 min. All HPLCs were performed in a Varian ProStar model 410 apparatus (Palo Alto, CA), including a ProStar model 240 pump and a ProStar 330 photodiode array detector.
Differential pigment extraction with acetone and hexane was performed according to Helfrich et al. (1994), with the following modifications. Stromal immunoprecipitates were recovered as described and extracted with a solution of 100% (v/v) acetone containing 0.5% (v/v) diethyl pyrocarbonate. The extracts were centrifuged and protein sedimented. Following re-extraction of pigments, the pooled acetone phases were supplemented with one-third volume of a reaction buffer containing 10 mM MgCl2 and 3 mM MnCl2 (Helfrich et al., 1994). To the resulting mixture, 50 mg DEAE-cellulose (Whatman, Clifton, NJ) and one-half volume of hexane were added. After phase separation, the amount of esterified and nonesterified pigments was determined in the upper and lower solvent phases, respectively (Helfrich et al., 1994). Esterified pigments are almost quantitatively retained in the upper, hexane phase, whereas nonesterified pigments partition into the lower, aqueous acetone phase (Helfrich et al., 1994).
Fluorescence spectroscopy of acetone-extracted pigments was performed in a Perkin Elmer LS50B spectrometer (Foster City, CA) at room temperature using the indicated excitation and emission wavelengths (Reinbothe et al., 1999).
Acknowledgments
This work was inaugurated in the Department of Prof. Dr. E. W. Weiler at the Institute for Plant Physiology, Ruhr-Universität Bochum, Bochum, Germany. We thank E.W. for his stimulating interest and continuous support of the work. We thank Dr. S. Pollmann, Bochum, for expert help with HPLC. Critical reading of the manuscript by Dr. J. Gray, University of Toledo, is gratefully acknowledged.
This work was supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany (grant no. RE1465/1–1,1–2 to C.R.).
This article is dedicated to Régis Mache on the occasion of his 70th birthday.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.033613.
References
- Adamska I (1997) ELIPs—Light-induced stress proteins. Physiol Plant 100: 75–85 [Google Scholar]
- Apel K, Kloppstech K (1980) The effect of light on the biosynthesis of the light-harvesting chlorophyll a/b protein. Evidence for the requirement of chlorophyll a for the stabilization of the apoprotein. Planta 150: 426–430 [DOI] [PubMed] [Google Scholar]
- Boardman NK (1962) Studies on a protochlorophyll-protein complex. I. Purification and molecular weight determination. Biochim Biophys Acta 62: 63–79 [DOI] [PubMed] [Google Scholar]
- Chang J-Y (1983) Manual micro-sequence analysis of peptides and proteins using 4-N,N-dimethylaminoazobenzene 4′-isothio-cyanate/phenylisothiocyanate double coupling method. FEBS Lett 93: 205–214 [Google Scholar]
- Chang J-Y, Brauer D, Wittmann-Liebold B (1978) Micro-sequence analysis of polypeptides using dimethyl-aminoazobenzene isothiocyanate. Methods Enzymol 91: 455–466 [DOI] [PubMed] [Google Scholar]
- Cline K, Werner-Washburne M, Andrews J, Keegstra K (1984) Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol 75: 675–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss BW, Thornber JP (1994) Assembly of the light-harvesting complexes (LHCs) of photosystem II. Monomeric LHCIIb complexes are intermediates in the formation of oligomeric LHC IIb complexes. Plant Physiol 106: 829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggink L, Park H, Hoober JK (2001) The role of chlorophyll b in photosynthesis: hypothesis. BMC Plant Biol. http://www.biomedcentral.com/1471-2229/1/2 (October 17) [DOI] [PMC free article] [PubMed]
- Fraser PD, Pinto EMS, Holloway DE, Bramley PM (2000) Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J 24: 551–555 [DOI] [PubMed] [Google Scholar]
- Green BR, Durnford DG (1996) The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 47: 685–714 [DOI] [PubMed] [Google Scholar]
- Green BR, Pichersky E, Kloppstech K (1991) The chlorophyll a/b-binding light-harvesting antennas of green plants: the story of an extended gene family. Trends Biochem Sci 16: 181–186 [DOI] [PubMed] [Google Scholar]
- Grimm B, Kloppstech K (1987) The early light-inducible proteins of barley. Characterization of two families of 2-h-specific nuclear coded chloroplast proteins. Eur J Biochem 167: 493–499 [DOI] [PubMed] [Google Scholar]
- Grimm B, Kruse E, Kloppstech K (1989) Transiently expressed early light-inducible thylakoid proteins share trans-membrane domains with light-harvesting chlorophyll binding proteins. Plant Mol Biol 13: 583–593 [DOI] [PubMed] [Google Scholar]
- Helfrich M, Schoch S, Lempert U, Rüdiger W (1994) Chlorophyll synthetase cannot synthesize chlorophyll a′. Eur J Biochem 219: 267–275 [DOI] [PubMed] [Google Scholar]
- Holtorf H, Reinbothe S, Reinbothe C, Bereza B, Apel K (1995) Two routes of chlorophyllide synthesis that are differentially regulated by light in barley. Proc Natl Acad Sci USA 92: 3254–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Ohtsuka T, Tanaka A (1996) Conversion of chlorophyll b to chlorophyll a via 7-hydroxymethyl chlorophyll. J Biol Chem 271: 1475–1479 [DOI] [PubMed] [Google Scholar]
- Itoh R, Itoh S, Sugawa M, Oishi O, Tabata K, Okada M, Nishimura M, Yakushiji E (1982) Isolation of crystalline water-soluble chlorophyll proteins with different chlorophyll a and b contents from stems and leaves of Lepidium virginicum. Plant Cell Physiol 23: 557–560 [Google Scholar]
- Jansson S (1994) The light-harvesting chlorophyll a/b binding proteins. Biochim Biophys Acta 1184: 1–19 [DOI] [PubMed] [Google Scholar]
- Joyard J, Block M, Pineau B, Albrieux C, Douce R (1990) Envelope membranes from mature spinach chloroplasts contain a NADPH:protochlorophyllide reductase on the cytosolic side of the outer membrane. J Biol Chem 265: 21820–21827 [PubMed] [Google Scholar]
- Kahn A (1968) Developmental physiology of bean leaf plastids. Tube transformation and protochlorophyll(ide) photo-conversion by a flash irradiation. Plant Physiol 43: 1781–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura Y, Mori T, Yamasaki T, Katoh S (1997) Isolation, properties and a possible function of a water-soluble chlorophyll a/b-protein from Brussels sprouts. Plant Cell Physiol 38: 133–138 [DOI] [PubMed] [Google Scholar]
- Kloppstech K, Meyer G, Bartsch K, Hundrieser J, Link G (1984) Control of gene expression during the early stages of chloroplast development. In W Wiessner, D Robinson, and R Starr, eds, Compartments in Algal Cells and Their Interaction. Springer-Verlag, Berlin, pp 36–46
- Kühlbrandt W, Wang DN, Fujiyoshi Y (1994) Atomic resolution of plant light-harvesting complex by electron crystallo-graphy. Nature 367: 614–621 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lehmann J, Atzorn R, Brückner C, Reinbothe S, Leopold J, Wasternack C, Parthier B (1995) Accumulation of jasmonate, abscisic acid, specific transcripts and proteins in osmotically stressed barley leaf segments. Planta 197: 156–163 [Google Scholar]
- Li X-P, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi K (2000) A pigment-binding protein essential for regulation of photosynthetic light-harvesting. Nature 403: 391–395 [DOI] [PubMed] [Google Scholar]
- Lopez F, Vansuyt G, Fourcroy P, Casse-Delbart F (1994) Accumulation of a 22 kDa protein and its mRNA in the leaves of Raphanus sativus in response to salt stress or water deficit. Physiol Plant 91: 605–614 [Google Scholar]
- Murata T, Ishikawa C (1981) Chemical, physicochemical and spectrophotometric properties of crystalline chlorophyll-protein complex from Lepidium virginicum. Biochim Biophys Acta 635: 341–347 [DOI] [PubMed] [Google Scholar]
- Murata T, Murata N (1971) Water-soluble chlorophyll-proteins from Brassica nigra and Lepidium virginicum L. Carnegie Inst Wash Year Book 70: 504–507 [Google Scholar]
- Murata T, Toda F, Uchino K, Yakushi E (1971) Water-soluble chlorophyll protein of Brassica oleracea var. botrys (cauliflower). Biochim Biophys Acta 245: 208–215 [DOI] [PubMed] [Google Scholar]
- Nishio N, Satoh H (1997) A water-soluble chlorophyll protein in cauliflower may be identical to BnD22, a drought-induced, 22-kilodalton protein in rapeseed. Plant Physiol 115: 841–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell PH (1975) High-resolution two-dimensional electrophoresis of proteins. J Biol Chem 250: 4007–4021 [PMC free article] [PubMed] [Google Scholar]
- Ort DT, Yocum CF (1996) Oxygenic Photosynthesis: The Light Reactions. Kluwer Academic Publishers, Dordrecht, The Netherlands
- Paulsen H (1995) Chlorophyll a/b binding proteins. Photochem Photobiol 62: 367–382 [Google Scholar]
- Pineau B, Dubertret G, Joyard J, Douce R (1986) Fluorescence properties of the envelope membranes from spinach chloroplasts. J Biol Chem 261: 9210–9215 [PubMed] [Google Scholar]
- Reinbothe C, Apel K, Reinbothe S (1995) A light-induced protease from barley plastids degrades NADPH:protochlorophyllide oxidoreductase complexed with chloro-phyllide. Mol Cell Biol 15: 6206–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe C, Buhr F, Pollmann S, Reinbothe S (2003) In vitro-reconstitution of LHPP with protochlorophyllides a and b. J Biol Chem 278: 807–815 [DOI] [PubMed] [Google Scholar]
- Reinbothe C, Lebedev N, Reinbothe S (1999) A proto-chlorophyllide light-harvesting complex involved in de-etiolation of higher plants. Nature 397: 80–84 [Google Scholar]
- Reinbothe S, Pollmann S, Reinbothe C (2003) In-situ-conversion of protochlorophyllide b to protochloro-phyllide a in barley: evidence for a novel role of 7-formyl reductase in the prolamellar body of etioplasts. J Biol Chem 278: 800–806 [DOI] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Holtorf H, Apel K (1995. a) Two NADPH:protochlorophyllide oxidoreductases in barley: evidence for the selective disappearance of PORA during the light-induced greening of etiolated seedlings. Plant Cell 7: 1933–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C (1996) The regulation of enzymes involved in chlorophyll biosynthesis. Eur J Biochem 237: 323–343 [DOI] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Neumann D, Apel K (1996) A plastid enzyme arrested in the step of precursor translocation in vivo. Proc Natl Acad Sci USA 93: 12026–12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Runge S, Apel K (1995. b) Enzymatic product formation impairs both the chloroplast receptor binding function as well as translocation competence of the NADPH:protochlorophyllide oxidoreductase, a nuclear-encoded plastid protein. J Cell Biol 129: 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe S, Runge S, Reinbothe C, van Cleve B, Apel K (1995. c) Substrate-dependent transport of the NADPH:proto-chlorophyllide oxidoreductase into isolated plastids. Plant Cell 7: 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Nakayama K, Okada M (1998) Molecular cloning and functional expression of a water-soluble chlorophyll protein, a putative carrier of chlorophyll molecules in cauliflower. J Biol Chem 273: 30568–30575 [DOI] [PubMed] [Google Scholar]
- Scharf K-D, Nover L (1982) Heat-shock-induced alterations of ribosomal protein phosphorylation in plant cell cultures. Cell 30: 427–437 [DOI] [PubMed] [Google Scholar]
- Scheumann V, Schoch S, Rüdiger W (1998) Chlorophyll a formation in the chlorophyll b reductase reaction requires reduced ferredoxin. J Biol Chem 273: 35102–35106 [DOI] [PubMed] [Google Scholar]
- Scheumann V, Klement H, Helfrich M, Oster U, Schoch S, Rüdiger W (1999) Protochlorophyllide b does not occur in barley etioplasts. FEBS Lett 445: 445–448 [DOI] [PubMed] [Google Scholar]
- Schmidt K, Fifezan C, Krieger-Liszkay A, Satoh H, Paulsen H (2003) Recombinant water-soluble chlorophyll protein from Brassica oleracea var. Botrys binds various chlorophyll derivatives. Biochemistry 42: 7427–7433 [DOI] [PubMed] [Google Scholar]
- Schoch S, Helfrich M, Wiktorsson B, Sundqvist C, Rüdiger W, Ryberg M (1995) Photoreduction of protopheophorbide with NADPH-protochlorophyllide oxido-reductase from etiolated wheat (Triticum aestivum). Eur J Biochem 229: 291–298 [DOI] [PubMed] [Google Scholar]
- Schulz R, Steinmüller K, Klaas M, Forreiter C, Rasmussen S, Hiller C, Apel K (1989) Nucleotide sequence of a cDNA coding for the NADPH-protochlorophyllide oxidoreductase (PCR) of barley (Hordeum vulgare L.) and expression in Escherichia coli. Mol Gen Genet 217: 355–361 [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wettstein D, Gough S, Kannangara CG (1995) Chlorophyll biosynthesis. Plant Cell 7: 1039–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White WJ, Green BR (1987) Antibodies to the photosystem I chlorophyll a + b antenna cross-react with polypeptides of CP29 and LHCII. Eur J Biochem 163: 545–551 [DOI] [PubMed] [Google Scholar]
- Wiedmann M, Kurzchalia T, Bielka H, Rapoport TA (1987) Direct probing of the interaction between the signal sequence of nascent preprolactin and the signal recognition particle by specific crosslinking. J Cell Biol 104: 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]