Summary
Very little research on dispositional optimism (DO) has been carried out in the field of Parkinson’s disease (PD). The present cross-sectional study, focusing on this personality trait, was performed with two main aims: i) to compare DO between patients with PD and a control group (CG); ii) to perform, in the PD group, a regression analysis including health-related variables, such as depression, anxiety, quality of life (QoL) and activities of daily living.
Seventy PD participants and 70 healthy volunteers were enrolled in the study. The Mann-Whitney test was used to compare life orientation between the PD and CG groups. In the PD group, Pearson’s correlation analysis was used to investigate the relationship between the measures of DO and the other variables. Means of log-linear regression were also used. Mean ratios adjusted for sex, age, education, and severity of disease were estimated, with relative 95% confidence intervals and p-values. The main results were as follows: i) no significant difference in DO was found between the PD participants and the CG; ii) DO was positively associated with QoL and emotional distress and inversely correlated with the Unified Parkinson’s Disease Rating Scale; iii) DO was not correlated with disability. In conclusion, high DO predicts a satisfactory quality of life, low emotional distress and reduced disease severity in PD.
Keywords: disability, dispositional optimism, Parkinson’s disease
Introduction
Idiopathic Parkinson’s disease (PD) is a progressive, degenerative and disabling disease characterized by cardinal signs (resting tremor, rigidity, postural instability and general slowing) without atypical signs. PD also involves autonomic, cognitive and affective functions. Moreover, personality changes have been reported. PD patients are generally more suspicious and cautious than both healthy age-matched individuals and patients suffering from other chronic diseases (Poewe et al., 1983; Eatough et al., 1990; Menza et al., 1993; Hubble et al., 1995).
In spite of these changes, positive attitudes are reported. For example, as regards religious and spiritual beliefs there was no statistical difference on specific testing between PD participants and controls (Giaquinto et al., 2011). Dispositional optimism (DO) is a new focus of interest in PD. The concept of DO stretches back over centuries in philosophical fields. The word optimism comes from the Latin word “optimum” meaning “very good” and optimism can be considered an instrument of coping.
Optimism is also considered a dispositional construct. A few articles have dealt with DO in PD patients, but the difference with the general population was not evaluated. A longitudinal study on DO and disease severity in twelve PD patients found no consistent relationship (Shifren, 1996). By contrast, the Global Parkinson’s Disease Survey (2002) found a statistically significant impact of “current feelings of optimism” on quality of life (QoL) in a large sample of PD patients. More recently, low DO (or high pessimism) was associated with reduced QoL in PD (Gruber-Baldini et al., 2009).
These considerations raise the question of the extent to which PD patients may present DO. The present investigation, adopting a new perspective, compared PD patients with a control group of healthy volunteers (CG). The main objectives of this study were: i) to look for a statistical difference in DO between PD and CG subjects; ii) to construct a log-linear regression model to analyze the relationship between DO and health-related variables, e.g. QoL, emotional distress, activities of daily living (ADL), illness severity and disability, in PD patients.
Material and methods
Sample
The initial sample comprised ninety-seven consecutive patients with a diagnosis of idiopathic PD referred to our center by local health district general practitioners (GPs) in the period 2006–2012.
The inclusion criteria were: i) a confirmed diagnosis of idiopathic PD; ii) Hoehn and Yahr scale (HY) (1967) stage 1, 2 or 3; iii) more than five years of education. The exclusion criteria were: i) non-idiopathic PD; ii) mental impairment; iii) lack of informed consent; iv) high comorbidity.
The screening procedures, which are described further on, revealed that twenty-seven patients did not meet the inclusion criteria and these patients were not included. Thus, the study sample consisted of seventy participants (47 men and 23 women). Their mean age was 68.4 years (±SD 10.2) and their mean duration of education was 10.5 years (±SD 4.5). They were at HY stages 1, 2 or 3 (the HY scale, which rates the stage of disease, ranges from stage 1 to stage 5). Their mean disease duration was 6.5 years (±SD 2.1). The participants all lived at home, with a spouse or with family. Eight-one percent of them were retired. They had all been receiving optimized levodopa/dopa decarboxylase inhibitor therapy and/or dopamine agonists and were tested while on medications with optimal effects. Seventy healthy subjects were randomly selected from district GPs’ files. The mean age of the CG subjects was 60.9 years (±SD 10.3). The two groups had an equivalent duration of education.
The study was approved by the institution’s ethics committee and written consent was obtained from all the PD participants.
Measures
The subjects were evaluated using the following measures:
The Mini-Mental State Examination (MMSE) (Folstein et al., 1975). The MMSE consists of questions testing orientation, registration, attention and calculation, recall, language, and copying. It is considered a screening test for cognitive impairment. It has a maximum score of 30 points. Scores below 24 indicate mental deterioration. The Italian version was adopted (Magni et al., 1996).
The Unified Parkinson’s Disease Rating Scale (UPDRS) (Fahn et al., 1987). The UPDRS contains separate sections: I. Mentation, II. ADLs, III. Motor Examination, and IV. Complications of therapy, as well as a Total score (I + II + III). It comprises 42 items altogether. A rating of zero on an item indicates normality for that item while four is the worst result.
The Life Orientation Test – Revised version (LOT-R) (Scheier et al., 1994). DO was assessed using the Italian version of the LOT-R, which consists of three items in a positive direction and three items in a negative direction, plus four “neutral” items. The Italian version investigates optimism and pessimism together (Anolli, 2005). Each item is scored 1–5, and high values imply optimism. The total score is given by the sum of the six non-neutral items and it ranges from 6 to 30. The raw values are converted into percentiles. There are no cut-offs. The brevity of this test makes it suitable for use in projects evaluating several measures and involving old people.
The WHO-5 Well-being Index (WHO-5) (Bech, 2004). This scale is derived from a larger rating scale developed for a WHO project on QoL. The original items were reduced to five items covering positive mood (good spirits, relaxation), vitality (being active and waking up fresh and rested), and general interest (being interested in things). The short form is more suitable for people who demonstrate easy fatigability on testing. The scale has been successfully applied in a PD population, giving a Cronbach’s alpha value of 0.83 (Schneider et al., 2010). The Italian version (De Girolamo et al., 2000) was applied.
The Hospital Anxiety and Depression Scale (HADS) (Zigmond and Snaith, 1983). This is a self-assessment scale used to measure both anxiety and depression. Higher scores mean higher psychological distress. The cut-off scores are fixed at 5 for the partial scores and at 10 for the total score. Equivalent or higher scores classify subjects as psychologically distressed. The subscales are also valid measures of the severity of the emotional disorder. The Italian translation and validation of the scale was used (Costantini et al., 1999). In the present research the total score and corresponding sub-scales were included in the statistical analysis.
The Barthel Index (Shah et al., 1989). This instrument is widely used to evaluate ADL. The scale consists of 10 items that measure feeding, moving from wheelchair to bed and back, grooming, toilet use, bathing, walking on level surface, going up and down stairs, dressing, bowel continence and bladder continence. The highest score is 100, i.e. total independence. Since motor fluctuations are common in PD, the best score for each item in the last week was taken.
The Cumulative Illness Rating Scale (CIR) (Linn et al., 1968). The CIR identifies 14 items, corresponding to different systems. Each system is scored as follows: 1= none, no impairment of the specific organ/system; 2= mild, impairment does not interfere with normal activity; treatment may or may not be required; prognosis is excellent; 3= moderate, impairment interferes with normal activity, treatment is needed, prognosis is good; 4= severe, impairment is disabling, treatment is urgently needed, prognosis is guarded; 5= extremely severe, impairment is life threatening, treatment is urgent or of no avail, prognosis is not good. Patients could not be included in this study if they had a score of over 3 in any item.
Procedure
Since the subjects were GP referred, the diagnosis of idiopathic PD, based on the PD Brain Bank criteria (Hughes et al., 1992), first had to be confirmed by movement disorder specialists. All the patients had already undergone magnetic resonance imaging, giving scans that showed no changes due to other diseases. The case history was collected, focusing specifically on excluding previous traumatic brain injuries and use of dopamine receptor blockers. The case history and a clinical assessment also sought specifically to exclude major comorbidities, such as arthritis, cancer, arterial hypertension, small vessel and heart disease, and psychosis.
Once these screening steps had been completed, the participants were administered the above-listed instruments, which they were invited to answer at their own pace. The participants were always administered the tests by the examiner, who did not influence their choices among the multiple responses. The interview lasted about three hours in total. The order of presentation of the instruments was randomized across the participants. The UPDRS and the Barthel Index were compiled by the specialist with the assistance of a caregiver. Care was taken to avoid overtiring the patients. The healthy volunteers were required to compile only the LOT-R.
Data analysis
A general description of the study population was obtained by performing a univariate analysis, comparing socio-demographic variables (i.e., sex, age and education) and LOT-R scores between the PD patients and the CG; the PD group was also described by health-related and disease severity variables (i.e., WHO-5, HADS, UPDRS and Barthel Index scores). The Chi-square test was applied for categorical variables and the unpaired Student’s t-test was applied for continuous variables. The Mann-Whitney test was used to compare life orientation in the PD and CG groups. In the PD group, the Pearson’s correlation analysis was used to investigate the relationship between the LOT-R, UPDRS, WHO-5, HADS and Barthel Index scores. Life orientation scores were divided into three ranges: pessimistic attitude = LOT-R score <40; balanced attitude = LOT-R score ranging from 40 to 60; optimistic attitude = LOT-R score >60. The effect of life orientation on disability, emotional distress and well-being was investigated by means of log-linear regression. Mean ratios adjusted for sex, age, education, and UPDRS were estimated, with relative 95% confidence intervals and p-values. The threshold for statistical significance was set at p<0.05. The statistical software packages used for the statistical analyses were SPSS V13 (SPSS Inc. Released 2004. SPSS for Windows, Version 13.0. Chicago, SPSS Inc.) and STATA/SE V12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Results
Demographics
Table I shows the descriptive statistics of the sample. The LOT-R scores recorded in the CG and PD participants were not significantly different (Mann-Whitney test, p=0.10).
Table I.
Descriptive analysis of the sample.
| Controls | PD group | p-value | |
|---|---|---|---|
| Sex, n (%) | |||
| - male | 40 (57%) | 23 (33%) | 0.004a |
| - female | 30 (43%) | 47 (67%) | |
| Age, mean±SD | 63.96±7.46 | 68.41±10.15 | 0.004b |
| Years of education, mean±SD | 11.44±4.1 | 10.47±4.53 | 0.185b |
| LOT-R, n (%) | |||
| - score: <40 | 26 (37%) | 30 (43%) | 0.356a |
| - score: 40–60 | 16 (23%) | 20 (29%) | – |
| - score: >60 | 28 (40%) | 20 (29%) | – |
| WHO-5, median | – | 44 | – |
| HADS, median | – | 13 | – |
| UPDRS, median | – | 37.5 | – |
| Barthel Index, median | – | 37.5 | – |
Abbreviations and symbols: PD=Parkinson’s disease; LOT-R= Life Orientation Test-Revised version; WHO-5= WHO-5 Well-being Index; HADS=Hospital Anxiety and Depression Scale; UPDRS=Unified Parkinson’s Disease Rating Scale;
Chi-square test;
Unpaired t-test
Correlations
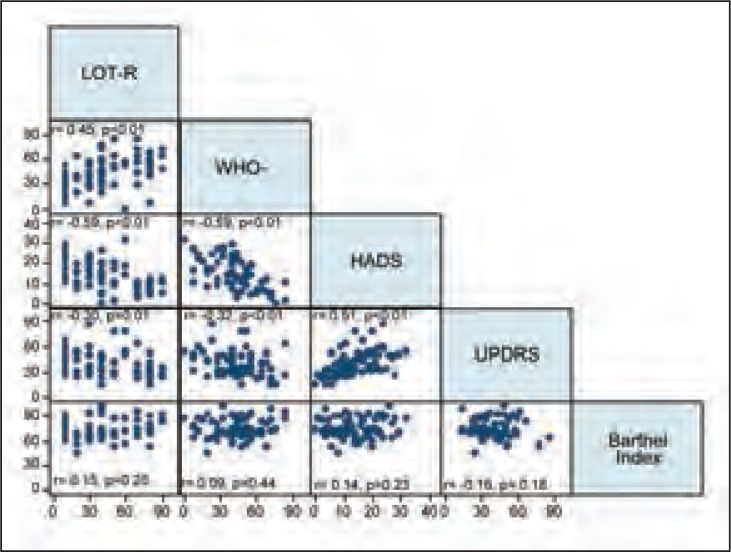
The LOT-R values were found to correlate with the WHO-5, the HADS, and the UPDRS scale scores. As shown in figure 1, the variables were all found to be significantly inter-correlated, except for the Barthel Index, which was not significantly correlated with any of the other variables (Fig. 1).
Figure 1.
Correlation of LOT-R values with the other study variables.
r=coefficient from Pearson’s correlation analysis; statistically significant correlation: p<0.05
LOT-R= Life Orientation Test-Revised version; WHO-5= WHO-5 Well-being Index; HADS=Hospital Anxiety and Depression Scale; UPDRS=Unified Parkinson’s Disease Rating Scale
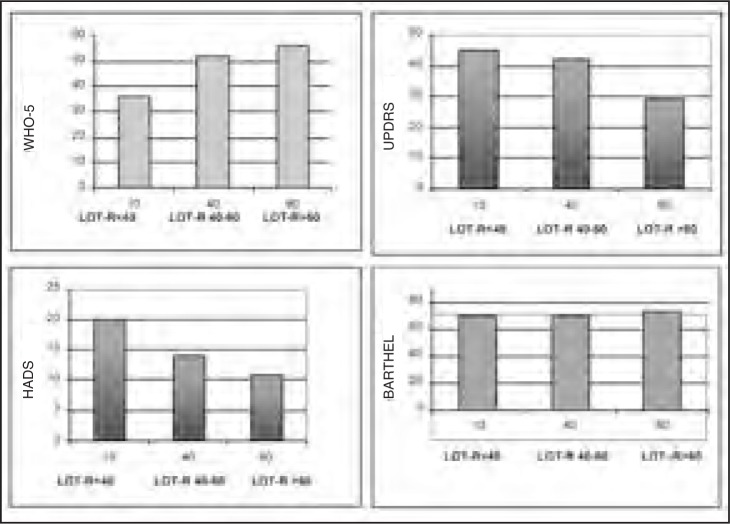
Figure 2 illustrates the relationship of life orientation, as evaluated by the LOT-R, with well-being (WHO-5), anxiety and depression (HADS), disability (Barthel Index) and PD severity (UPDRS).
Figure 2.
Relationships between LOT-R scores and well-being, anxiety and depression, disability and PD severity.
X and Y values are percentiles.
LOT-R= Life Orientation Test-Revised version; WHO-5= WHO-5 Well-being Index; HADS=Hospital Anxiety and Depression Scale; UPDRS=Unified Parkinson’s Disease Rating Scale; Barthel=Barthel Index (measuring disability)
The Barthel Index and UPDRS showed different relationships with the LOT-R scale. Indeed, high LOT-R scores were found to be correlated with high WHO-5 and low HADS and UPDRS scores. The Barthel Index values, on the other hand, were not correlated with any LOT-R score range.
Table II shows the results of a log-linear regression analysis adjusted for sex, education, age and PD severity (UPDRS score). As expected, life orientation, as evaluated by LOT-R, seemed to have an effect on anxiety and depression in people affected by PD. In particular, the mean ratio between the group with a balanced attitude (LOT-R score range: 40-60) and that with a pessimistic attitude (LOT-R score <40) was 0.63 (95%CI: [0.47; 0.84], p=0.002), and the mean ratio between the group with an optimistic attitude (LOT-R score >60) and that with a pessimistic attitude (LOT-R score <40) was 0.52 (95%CI: [0.39; 0.71], p<0.001). Life orientation was found to have no statistically significant effect either on independence in ADL, as measured by the Barthel Index, or on QoL. All estimates were adjusted for sex, education, age and UPDRS score.
Table II.
Effect of life orientation (LOT-R) on well-being (WHO-5), anxiety and depression (HADS) and disability (Barthel Index).
| Barthel Index | WHO-5* | HADS* | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean ratio | 95% CI | p-value | Mean ratio | 95% CI | p-value | Mean ratio | 95% CI | p-value | |
| Pessimistic attitude (LOT-R score: <40) | 1 | 1 | 1 | ||||||
| Balanced attitude (LOT-R score: 40–60) | 1.02 | [0.93 ; 1.12] | 0.657 | 1.24 | [0.84 ; 1.83] | 0.271 | 0.63 | [0.47 ; 0.84] | 0.002 |
| Optimistic attitude (LOT-R score: >60) | 1.05 | [0.95 ; 1.16] | 0.361 | 1.48 | [0.98 ; 2.23] | 0.059 | 0.52 | [0.39 ; 0.71] | <0.001 |
| Males | 1.03 | [0.95 ; 1.12] | 0.493 | 0.94 | [0.67 ; 1.33] | 0.730 | 0.76 | [0.58 ; 0.98] | 0.033 |
| Education | 1.00 | [1.00 ; 1.01] | 0.676 | 0.99 | [0.96 ; 1.03] | 0.680 | 1.01 | [0.99 ; 1.04] | 0.311 |
| Age | 1.00 | [0.99 ; 1.00] | 0.120 | 1.01 | [0.99 ; 1.02] | 0.392 | 1.01 | [0.99 ; 1.02] | 0.301 |
| UPDRS score | 1.00 | [1.00 ; 1.00] | 0.453 | 0.99 | [0.98 ; 1.00] | 0.078 | 1.02 | [1.01 ; 1.03] | <0.001 |
Abbreviations and symbols: LOT-R=Life Orientation Test – Revised; WHO-5=WHO-5 Well-being Index; HADS=Hospital Anxiety and Depression Scale; UPDRS=Unified Parkinson’s Disease Rating Scale; CI=confidence interval;
Log-transformation applied to original score + 1 [i.e.: ln(WHO5+1) and ln(HADS+1)].
Education, age and UPDRS were used as continuous covariates Statistical significance: p<0.05.
Discussion
Optimism is considered a dispositional construct. A few studies have dealt with DO in PD patients but they did not evaluate the difference versus the general population. In the present study the CG slightly outscored the PD participants but the difference was not significant on the Mann-Whitney test. This finding is perhaps surprising, given that the burden of motor impairment in PD and the prospect of a worsening of the condition might be expected to offset any optimistic expectancy. However, we suggest that two possible factors account for the lack of a striking difference between the two groups we studied. First of all, PD outpatients are different from PD patients who do not carry out rehabilitation. A rehabilitation center offers a pleasant setting, ideally characterized by up-to-date facilities staffed by skilled and courteous operators. Clients perceive the quality of the work done there and this climate may foster hope. The second factor concerns the so-called healthy volunteers. The global economic crisis of recent years has threatened people’s well-being. Worries over the cost of living, taxes, unemployment, being laid off, down-sizing and unsafe savings may have negatively influenced the interviews given by the control subjects, who are concerned for themselves and for their families. The PD patients were older than the controls (mean age 68.4 vs 60.9 years) but this difference is unlikely to have influenced the results.
We found a negative correlation between DO and UPDRS. This finding, like the lack of a clear statistical difference between CG and PD participants, was unexpected. Indeed, The UPDRS is a comprehensive instrument for evaluating disease severity, which has subscales investigating mentation, ADL, motor abilities, and complications of therapy. The UPDRS sub-scale Mentation includes Behavior and Mood. In this section the components Intellectual Impairment and Thought Disorder play no role, because mental deterioration was one of the exclusion criteria. By contrast, DO may have influenced the other components, namely Depression and Motivation/Initiative. A previous study has already found disability and DO to be uncorrelated in PD (Gruber-Baldini et al., 2009).
Our findings clearly indicate that DO is predictive of satisfactory QoL and low emotional distress in PD, at least in the early stages of the disease. QoL has been defined as “individuals’ perceptions of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns” (WHO, 1993). The concept encompasses physical health, psychological well-being, social aspects and environment. Living with PD is a stressful experience. However, the Global Parkinson’s Disease Survey (2002), on the basis of a single item, found that DO had a significant impact on health-related QoL. Gruber-Baldini et al. (2009) confirmed the importance of DO in predicting QoL. According to them, interventions that promote positive attitudes may be useful in PD. Our results clearly confirmed that view. Depression should always be carefully sought in PD patients, because it is associated with a longer disease duration and worse motor symptoms (Pålhagen et al., 2008) and a 40% decrease in QoL (Althaus et al., 2008). Depression occurs in approximately 45% of PD patients (Lemke, 2008). The prevalence of depressive symptoms in PD has been estimated to be 35.4% (Althaus et al., 2008) and a significant proportion of patients continue to experience depressive symptoms despite using anti-depressant medication. Our findings indicate a negative relationship between LOT-R and HADS, i.e. high DO indicates lower levels of both anxiety and depression. DO was not considered in previous studies on depression in PD, but this variable likely affected the results, allowing better adjustment to daily-life stressors.
The present research indicates that PD patients can retain a positive outlook despite being affected by a progressive disease. DO has a scientific basis. People who are dispositionally optimistic have generally positive expectancies for the future and experience less distress when coping with difficult situations (Andersson, 1996). DO is associated with better psychological adjustment to stressors. DO may stem from previously acquired experiences and hence it is to a certain extent prone to change (Carver et al., 2009; Vassar et al., 2010). Greater DO fosters better strategies and better adjustment to adverse events. In PD, DO can be more associated with approach coping and less with avoidance coping because expectancies of a positive outcome after rehabilitation lead participants to strive to overcome their PD-related problems. On the other hand, patients with low DO have expectancies of a negative outcome and may try to refuse treatment. In meta-analytic studies, approach coping strategies are positively associated with DO. As a consequence, stressors are controlled and minimized. By contrast, avoidance coping strategies are negatively associated and, in this case, stressors are avoided, ignored, or removed (Nes and Segerstrom, 2006). Nes and Segerstrom argue that, besides the approach/avoidance distinction, DO can be either problem-focused or emotion-focused, but this distinction appears less significant in our PD participants, because they had the support of a team, including a psychologist. Bucks et al. (2009), in 85 PD patients, investigated the predictive value of various coping processes for psychological and disease-specific aspects of health-related QoL in PD. Greater use of active, problem-solving coping was found to improve cognitive impairment, communication and bodily discomfort when PD participants felt themselves to be coping adequately in these areas. By contrast, greater use of the escape-avoidance coping strategy worsened emotional well-being, mood and social support. Thus, active coping is important for some aspects of health-related QoL in PD. Psychological interventions may be beneficial in PD, if they reduce the use of escape-avoidance strategies.
Dispositional optimism has positive influences in other fields. Kato et al. (2012) applied the LOT-R in centenarians and concluded that DO may contribute to successful aging. In a gerontological prospective cohort study with over 10 years of follow-up, a negative correlation between DO and feelings of loneliness was recorded at baseline. In this male population, lower levels of DO were strongly associated with greater feelings of loneliness. This inverse relationship was independent of potential socio-demographic and clinical age-related confounding variables. Thus, elderly subjects with low DO require attention for the prevention of loneliness (Rius-Ottenheim et al., 2012). Likewise, after moderate-to-severe traumatic brain injury higher levels of DO are related to better psychological functioning. As a consequence, DO could predict improved cognitive and functional outcome (Ramanathan et al., 2011).
In conclusion, a PD patient should not be regarded merely as a defective biomechanical device, but rather as a person who experiences illness, disability or restriction in the sphere of social participation. The Latin origin of the word “individual” means “indivisible”. Focusing on the concept of the person and paying attention to personality traits, coping strategies and self-regulation under stressful conditions should allow PD patients to receive more holistic and beneficial care. We acknowledge some limitations of the present study: i) it has a cross-sectional design in which the variables were measured at one time-point; ii) the sample included participants in the early stages of PD and cases with severely disabling PD were not included; iii) the Italian version of the DO scale treats optimism and pessimism as bipolar opposites. In spite of these limitations, this study suggests that it is wise to include evaluation of personality traits in current PD clinical models. Further research is warranted to discover whether interventions that promote personal control and positive attitudes may assist in reducing disability and improving quality of life in PD participants under rehabilitation.
References
- Althaus A, Becker OA, Spottke A, et al. Frequency and treatment of depressive symptoms in a Parkinson’s Disease registry. Parkinsonism Relat Disord. 2008;14:626–632. doi: 10.1016/j.parkreldis.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Andersson G. The benefits of optimism: a meta-analytic review of the Life Orientation Test. Personality and Individual Differences. 1996;21:719–725. [Google Scholar]
- Anolli L. Versione Italiana “L’ottimismo”. Bolgna: Il Mulino; 2005. Life Orientation Test-r. [Google Scholar]
- Bech P. Measuring the dimension of Psychological General Well-Being by the WHO-5. Quality of Life Newsletter. 2004;32:15–16. [Google Scholar]
- Bucks RS, Cruise KE, Skinner TC, et al. Coping processes and health-related quality of life in Parkinson’s disease. Int J Geriatr Psychiatry. 2011;26:247–255. doi: 10.1002/gps.2520. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, Miller CJ, et al. Optimism. In: Lopez SJ, Snyder CR, editors. Oxford Handbook of Positive Psychology. 2nd edn. New York: Oxford University Press; 2009. pp. 303–312. [Google Scholar]
- Costantini M, Musso M, Viterbori P, et al. Detecting psychological distress in cancer patients: validity of the Italian version of the Hospital Anxiety and Depression Scale. Support Care Cancer. 1999;7:121–127. doi: 10.1007/s005200050241. [DOI] [PubMed] [Google Scholar]
- De Girolamo G, Rucci P, Scocco P, et al. Quality of life assessment: validation of the Italian version of the WHOQOL-Brief. Epidemiol Psichiatr Soc. 2000;9:45–55. doi: 10.1017/s1121189x00007740. [DOI] [PubMed] [Google Scholar]
- Eatough VM, Kempster PA, Stern GM, Lees AJ. Premorbid personality and idiopathic Parkinson’s disease. Adv Neurol. 1990;53:335–337. [PubMed] [Google Scholar]
- Fahn S, Elton RL, Members of the UPDRS development Committee . Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease Hants. Macmillan Healthcare Information; 1987. pp. 153–1563. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Giaquinto S, Bruti L, Dall’Armi V, et al. Religious and spiritual beliefs in outpatients suffering from Parkinson Disease. Int J Geriatr Psychiatry. 2011;26:916–922. doi: 10.1002/gps.2624. [DOI] [PubMed] [Google Scholar]
- Global Parkinson’s Disease Survey GPDS Steering Committee Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov Disord. 2002;17:60–67. doi: 10.1002/mds.10010. [DOI] [PubMed] [Google Scholar]
- Gruber-Baldini AL, Ye J, Anderson KE, et al. Effects of optimism/pessimism and locus of control on disability and quality of life in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:665–669. doi: 10.1016/j.parkreldis.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Hoehn M, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hubble JP, Koller WC. The parkinsonian personality. Adv Neurol. 1995;65:43–48. [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Zweig R, Barzilai N, et al. Positive attitude towards life and emotional expression as personality phenotypes for centenarians. Aging (Albany NY) 2012;4:359–367. doi: 10.18632/aging.100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke MR. Depressive symptoms in Parkinson’s disease. Eur J Neurol. 2008;15(Suppl 1):21–25. doi: 10.1111/j.1468-1331.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- Linn BS, Linn MW, Gurel L. Cumulative Illness Rating Scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Magni E, Binetti G, Bianchetti A, et al. Mini-Mental State Examination: a normative study in Italian elderly population. Eur J Neurol. 1996;3:198–202. doi: 10.1111/j.1468-1331.1996.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Menza MA, Golbe LI, Cody RA, et al. Dopamine-related personality traits in Parkinson’s disease. Neurology. 1993;43:505–508. doi: 10.1212/wnl.43.3_part_1.505. [DOI] [PubMed] [Google Scholar]
- Nes LS, Segerstrom SC. Dispositional Optimism and coping: a meta-analytic review. Pers Soc Psychol Rev. 2006;10:235–251. doi: 10.1207/s15327957pspr1003_3. [DOI] [PubMed] [Google Scholar]
- Pålhagen SE, Carlsson M, Curman E, et al. Depressive illness in Parkinson’s disease – indication of a more advanced and widespread neurodegenerative process? Acta Neurol Scand. 2008;117:295–304. doi: 10.1111/j.1600-0404.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- Poewe W, Gerstenbrand F, Ransmayr G, et al. Premorbid personality of Parkinson patients. J Neural Transm Suppl. 1983;19:215–224. [PubMed] [Google Scholar]
- Ramanathan DM, Wardecker BM, Slocomb JE, et al. Dispositional optimism and outcome following traumatic brain injury. Brain Inj. 2011;25:328–337. doi: 10.3109/02699052.2011.554336. [DOI] [PubMed] [Google Scholar]
- Rius-Ottenheim N, Kromhout D, van der Mast RC, et al. Dispositional optimism and loneliness in older men. Int J Geriatr Psychiatry. 2012;27:151–159. doi: 10.1002/gps.2701. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Schneider CB, Pilhatsch M, Rifati M, et al. Utility of the WHO-Five Well-being Index as a screening tool for depression in Parkinson’s disease. Mov Disord. 2010;25:777–783. doi: 10.1002/mds.22985. [DOI] [PubMed] [Google Scholar]
- Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- Shifren K. Individual differences in the perception of optimism and disease severity: a study among individuals with Parkinson’s disease. J Behav Med. 1996;19:241–271. doi: 10.1007/BF01857768. [DOI] [PubMed] [Google Scholar]
- Vassar M, Bradley G. A reliability generalization study of coefficient alpha for the life orientation test. J Pers Assess. 2010;92:362–370. doi: 10.1080/00223891.2010.482016. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- WHO Study protocol for the World Health Organisation project to develop a Quality of Life assessment instrument (WHOQOL) Quality of Life Research. 1993;2:153–159. [PubMed] [Google Scholar]