Summary
Weight bearing on the paretic lower extremity and transfer of weight from one lower extremity to the other are important goals of stroke rehabilitation. Improvements in these limb loading and weight transfer abilities have been shown to relate to improved performance of many functional activities. Unfortunately, valid and practical clinical measures of paretic lower extremity loading and weight transfer have not been identified.
The purpose of this study was to quantitatively assess, through center of foot pressure (CoP) analysis of quiet upright stance control, recovery of paretic limb loading as a measure of weight transfer in early stroke subjects, testing the effectiveness of a targeted rehabilitation intervention based on audio-visual biofeedback.
Thirty-seven adults with lower extremity motor impairment following unilateral, non-cerebellar stroke, were tested twice, at an interval of at least one month post stroke and following rehabilitation intervention aimed at correcting their asymmetrical weight bearing. The intervention was performed with (Study Group, SG) or without (Control Group, CG) a postural audio-visual biofeedback approach. Indices of postural stability and of balance control asymmetry were estimated by acquiring the movements of the CoP during quiet upright stance condition with or without visual input (eyes open, EO and eyes closed, EC). Clinical scales were also administered. Both the CG and the SG subjects showed improved control in upright stance posture as documented by significant improvements in the scale scores and indices of stability during both the EO and the EC condition. Only the SG showed a significantly reduced CoP index of asymmetry.
The CoP index of asymmetry, correlating with clinical motor scales, is a valid measure of paretic limb loading during stroke recovery. Postural audio-visual biofeedback represented the more effective approach for reducing weight loading asymmetry of the lower limbs in stroke.
Keywords: asymmetry index, biofeedback, neurorehabilitation, posturography, stroke, weight bearing
Introduction
In developed countries stroke is the leading cause of long-term disability in adults, with many stroke survivors experiencing a significant reduction in their quality of life (Paolucci et al., 2003; Mercer et al., 2009; Bersano et al., 2012).
Post-stroke patients often suffer from impaired postural and balance control with some never regaining the ability to stand. The balance of those who do prove able to resume standing is typically characterized by increased sway during quiet stance and by asymmetrical lower limb weight distribution (Verheyden et al., 2006; Hsieh et al., 2002; de Oliveira et al., 2008; Hendrickson et al., 2014).
A reduction in weight bearing on the paretic side is a common finding in stroke survivors and it has been negatively related to both motor function and recovery of independence in activities of daily living (Fong et al., 2001; Paolucci et al., 2003; Semprini et al., 2009; Hsieh et al., 2002; Dickstein et al., 1984; Winstein, 1991). Previous studies addressing balance deficits in stroke patients focused on different aspects of postural control including asymmetrical weight distribution, and they suggested that the asymmetrical stance of people with hemiparesis may be a compensatory strategy to overcome muscle weakness and perceptual deficits (Dickstein et al., 2004; de Haart et al., 2004; Januário et al., 2010; Lee et al., 1997). Moreover, these studies showed that the area of the center of foot pressure (CoP) sway pattern was much larger in stroke patients than in controls, with patients showing a greater tendency to sway during movement, particularly in the mediolateral direction (Lee et al., 1997).
As balance control can be considered a fundamental motor skill learned by the central nervous system, postural control strategies can become more efficient and effective with training and practice (French et al., 2010; Varoqui et al., 2011; de Haart et al., 2004). In recent years, evidence in support of postural rehabilitation has been increasing and a growing number of studies have shown that training performed by means of postural platforms is more effective than traditional physiotherapy approaches (Srivastava et al., 2009; Barclay-Goddard et al., 2004; Januário et al., 2010; Cheng et al., 2004; Pistarini and Molteni, 2009; Zelaschi, et al. 1995).
However, only a few studies have used instrumental postural analysis to evaluate the biomechanical parameters underlying improvements observed in postural control and weight-bearing distribution (Januário et al., 2010; de Haart et al., 2004) after rehabilitation training based on a dynamic balance platform with audio-visual biofeedback.
This study was performed with two main aims: i) to test, in subacute stroke patients, the effectiveness of a rehabilitation program based on the use of a dynamic balance platform with audio-visual feedback; ii) to quantitatively assess postural stability and weight-bearing distribution, through analysis of CoP indices.
Materials and methods
Participants
The study enrolled consecutive patients with a first-ever ischemic or hemorrhagic stroke, referred to the Neurorehabilitation Unit at IRCCS NEUROMED, Pozzilli, between January 2012 and June 2013, who were able to start a rehabilitation program. The inclusion criteria were: i) first-ever ischemic or hemorrhagic (not evacuated) stroke, diagnosed according to WHO criteria and confirmed by neuroimaging (computed tomography or magnetic resonance); ii) age between ≥ 40 and ≤ 60 years; iii) time since acute stroke ≤ 30 days; iv) standing balance score ≥ 2; v) Mini-Mental State Examination score ≥ 24. Exclusion criteria were: i) additional neurological or psychiatric disorders; ii) a personal history of diabetes mellitus; iii) a personal history of vestibular diseases; iv) peripheral neuropathies (confirmed by electroneurography/electromyography); v) aphasia; vi) neglect; vii) visual disturbances; viii) use of ortheses and/or prostheses.
All the participants gave their written informed consent to participate in the study. All the procedures were fully approved by the local ethics committee and the study was conducted in accordance with the revised version of the Declaration of Helsinki.
Study design
A stratified, single-center, single-blinded randomized controlled trial design was used to investigate the effects of a rehabilitation program based on the use of a dynamic balance platform with audio-visual feedback. Patients who met the inclusion criteria were considered eligible for the study and randomly assigned to the Study Group (SG) or the Control Group (CG) using a computerized random number generator.
A single experienced neurologist, blinded to treatment allocation, performed the clinical evaluations. All the instrumental evaluations were performed by the same bioengineer, also blinded to the patients’ treatment allocation.
Rehabilitation program
All the patients underwent a two-week rehabilitation program (one 60-minute session/day, six days/week, for a total of 12 sessions) involving patient-tailored one-to-one treatment (Van Peppen et al., 2004; Pollock et al., 2007; Levin and Panturin, 2011; Lennon and Ashburn, 2000) administered by experienced physical therapists. The rehabilitation program was based on traditional rehabilitation techniques supported by literature evidence (Borg, 1982; Pollock et al., 2000; Fletcher et al., 2001; Moreland et al., 2003; Gordon et al., 2004; Verheyden et al., 2009). More precisely, the physical therapy interventions consisted of: i) therapeutic exercises involving passive, active and active-assisted range of movement (RoM) training, ii) motor retraining activities such as static and dynamic standing, iii) gait training involving walking over ground, on a treadmill and up and down stairs, and iv) functional performance training, such as sit-to-stand maneuvers at various heights and bed mobility exercises.
In addition, all the patients both in the SG and the CG received 30 minutes of specific postural training based on: weight-shifting exercises (SG), or physiotherapy (CG).
The balance board training, undertaken by the SG, entailed upright stance posture exercises enriched by visual and auditory feedback, performed with a commercially available computerized balance platform (Balance System SD, Biodex Medical Systems, Shirley NY, USA). This training was performed under a physiotherapist’s supervision (verbal, without body contact) in order to direct the patient’s attention and to guarantee his/her safety. The balance platform focuses on the proprioceptive neuromuscular mechanisms that seem to affect dynamic joint and postural stability (Srivastava et al., 2009), with the advantage of allowing paretic limb muscle training in an upright position with the limb loaded.
The balance board exercises, performed in an upright standing position, were intended to enhance the ability to maintain postural stability during paced movements, i.e.: i) leg flexion and extension, ii) trunk flexion and extension on the left and right side alternately, and iii) pelvis abduction and adduction. All the activities were conducted while the patients monitored, in real time, their CoP movements, directly related to lower extremity weight bearing. CoP movements were represented as a moving dot in a patient-centered frame of reference and shown on a 12.1” color touchscreen display with adjustable height, so as to be at the level of the patient’s head.
The balance board training was performed using Biodex Balance System Training Mode software (Biodex Medical Systems, Shirley NY, USA), specifically the Weight Shift Training and Percent Weight-Bearing Training modes. In the first mode, patients stood on the balance platform, with each arm lying along the lateral aspect of the hip, and were required to move a dot, representing the current CoP position, in a target zone by shifting their own weight along the mediolateral direction. The target zone was set for each patient on the basis of previously defined individual motor performances. In the second mode, patients had to shift their own weight on the lower limbs paying attention to the real-time audio-visual weight-bearing feedback, trying to achieve perfectly symmetrical weight distribution along both the mediolateral and the anteroposterior directions.
The additional therapy in the CG was based on traditional rehabilitation techniques aimed at improving control of standing balance through postural maneuvers, focusing on increasing trunk and pelvic RoM, normalizing muscle tone, and stimulating appropriate balance responses involving weight shift, pelvic tilting, and trunk movements. All the exercises were manually and verbally facilitated/guided by a physiotherapist (Pollock et al., 2007).
Clinical evaluation
All eligible patients performed a clinical and functional evaluation before the randomization (T0) and at the end of treatment (T1).
The following validated clinical scales and scores were used:
Standing balance score (Bohannon and Leary, 1995; Bohannon, 1989a): an ordinal scale which has been shown to correlate significantly with transfer, walking and stair climbing performance in patients with stroke (Bohannon, 1989b; Bohannon et al., 1993; Bohannon, 1988,1995). The scores range from 0 to 6, with 0 = unable to stand without assistance, and 6 = able to stand independently for 30 seconds on either lower extremity alone.
Unified Balance Scale (UBS) (La Porta et al., 2011): a 27-item, activity-based “from bed to community” scale obtained by combining the scores of the Berg Balance Scale, Performance-Oriented Mobility Assessment scales, Tinetti Gait and Balance scale, and Fullerton Advanced Balance Scale with psychometric methods and Rasch analysis.
Functional Independence Measure (FIM) (Dodds et al., 1993; Platz et al., 1999; Cohen and Marino, 2000; Keith et al., 1987): a 13-item international validated scale used to assess disability in terms of independence or need of assistance in daily activities. To better disclose the motor recovery of the lower extremities, the following five items from the FIM motor domain were used (Leung et al., 2010): transfers (3 items = bed/chair/wheelchair, toilet, tub/shower) and locomotion (2 items = walk/wheelchair, stair).
A satisfaction score was also provided by each subject at the end of treatment (1 = poor; 2 = sufficient; 3 = good; 4 = excellent) (Lambercy et al., 2011).
Instrumental evaluation and CoP detection
Postural assessments were performed using the Balance System SD. The quiet upright posture assessment was performed in order to detect the instant position of the CoP. Briefly, the patients were asked to stand quietly on the force platform with their feet spaced 17 cm apart (distance measured between the heels) and with a 14° angle between the feet (McIlroy and Maki, 1997), with their arms at their sides, looking at a visual target positioned 40 cm in front of them at the height of their eyes. All the patients received the same instructions: to keep their gaze fixed on the visual target, remaining standing for at least 40 seconds. Foot positions were marked on the platform to ensure consistency across trials.
The eyes open (EO) and eyes closed (EC) conditions were recorded and six consecutive trials (Pinsault and Vuillerme, 2009) (3 EO, 3 EC, randomly assigned) were collected. In order to avoid fatigue, patients had a one-minute rest between trials.
The force platform signals were acquired at 20 Hz, in a time window of 40 seconds. The signals recorded during the first and last five seconds were discarded to avoid transient periods.
The evaluations were performed in a quiet room with very low background noise and diffuse light.
CoP analysis
Center of pressure displacement was analyzed off-line from the unfiltered platform signal by using two different parameterization techniques (Baratto et al., 2002): i) global parameterization, which numerically expresses the overall size of the sway patterns, in the time and frequency domains; and ii) structural parameterization, which identifies subunits of posturographic data, relating them to the underlying motor control process.
To compute structural parameters, a sway density plot (SDP) was calculated by counting the number of consecutive samples of the posturographic trajectory that, for each time instant, fell within a test circle (Ø 2.5 mm) (Baratto et al., 2002). The SDP describes a regular series of peaks and valleys. Peaks correspond to time instants where the ankle torque is relatively stable and associated with feed-forward control actions (motor commands); valleys are related to time instants where the ankle torque rapidly shifts between two consecutive stable points.
The following indices, subsequently referred to as “indices of postural stability”, were computed for each trial and condition (EO, EC):
the sway path (SP) of the CoP (integrating the instant velocity of the CoP over the total recording time);
the frequency bandwidth [including 80% of the area under the amplitude spectrum (FB1) (Baratto et al., 2002)], separately computed for the anteroposterior (A-P) and mediolateral (M-L) CoP movement directions;
the mean amplitude of the peaks (MP) of the SDP – MP is an a-dimensional value and it estimates the degree of postural stability;
the mean distance (MD) between two consecutive peaks of the SDP [for details see (Baratto et al. 2002)] – this represents the amplitude of the feed-forward control actions (motor commands);
the mean CoP position along the M-L direction (M-L mean CoP) - M-L mean CoP was calculated as an index of patients’ asymmetrical weight distribution on their feet. This index will subsequently also be referred to as the “CoP index of asymmetry”.
Statistical analysis
The mean values ± standard deviation of the clinical scale scores were calculated for each group, for both visual conditions (EO and EC) and both sessions (T0 and T1). The mean value ± standard error mean of the indices of postural stability and the CoP index of asymmetry were calculated for each group, for both visual conditions (EO and EC) and both sessions (T0 and T1). Unpaired Student’s t-test was performed to evaluate differences between groups at T0 and T1, while paired Student’s t-test was performed to evaluate the differences between the EO and EC conditions for each group at T0 and T1. To assess the usefulness of the indices of postural stability, Pearson’s correlation between these indices and the clinical scale scores was calculated. The statistical significance level was p<0.05.
Results
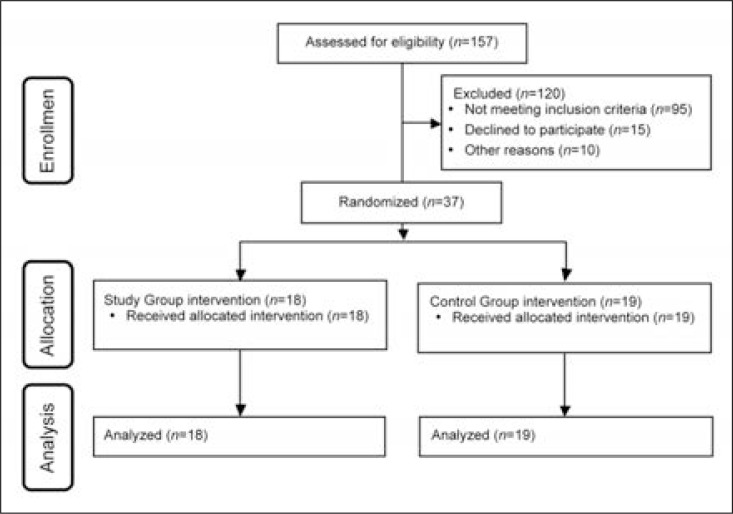
Of the total 157 patients enrolled, 37 (18 F, 19 M; mean age 57.9±11.3 years) met the inclusion criteria and were assigned to the CG or SG using a software-based randomization process (Fig. 1).
Figure 1.
CONSORT flow diagram.
The patients’ demographic and clinical data are shown in table I.
Table I.
Demographic data and clinical measures in first-ever ischemic or hemorrhagic stroke patients.
| Control Group (n=19) | T1 vs T0 (p) | Study Group (n=18) | T1 vs T0 (p) | |
|---|---|---|---|---|
| Age (years) | 61.3±11.8 | - | 55.7±10.4 | - |
| Sex, male/female | 9/10 | - | 10/8 | - |
| Lesion side, right/left | 12/7 | - | 8/10 | - |
| Stroke type, hemorrhagic/ischemic | 9/10 | - | 8/10 | - |
| UBS | <0.001 | <0.001 | ||
| T0 | 13.7±11.2 | 24.7±17.3 | ||
| T1 | 33.1±14.6 | 41.8±12.2 | ||
| FIM total | <0.001 | <0.001 | ||
| T0 | 50.8±14.5 | 54.0±11.4 | ||
| T1 | 97.6±18.2 | 101.3±15.7 | ||
| FIM motor | <0.001 | <0.001 | ||
| T0 | 8.0±2.4 | 8.5±3.4 | ||
| T1 | 23.1±7.3 | 24.8±6.5 | ||
| Standing balance score | <0.001 | <0.001 | ||
| T0 | 0.2±0.4 | 0.5±1.1 | ||
| T1 | 3.2±1.3 | 3.6±0.7 |
Abbreviations: UBS=Unified Balance Scale; FIM=Functional Independence Measure. Values are mean ±standard deviation or number.
No statistically significant differences were found between the two groups in demographics, clinical measures and indices in postural stability at T0 or T1.
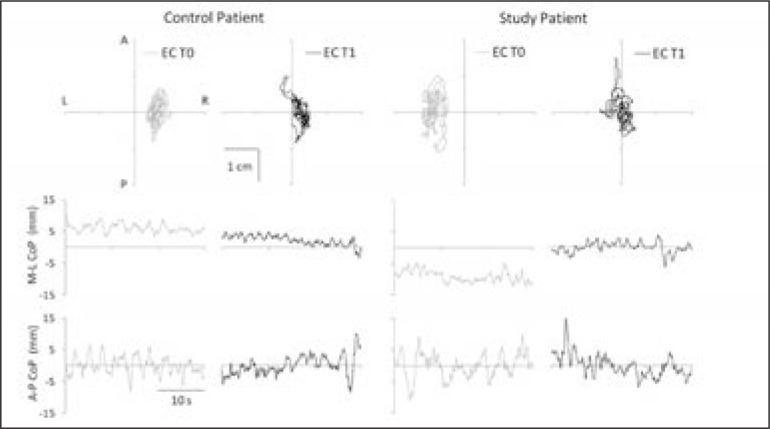
The within-group analysis revealed statistically significant improvements in all clinical scale scores (UBS, FIM total, FIM motor and standing balance) between admission and the end of treatment, in both groups (Table I). The CoP displacements along the A-P and M-L directions are shown in figure 2. The CoP oscillation obtained from two representative patients, one from the SG and one from the CG, was plotted and showed, in both cases, a consistent reduction in amplitude between T0 and T1.
Figure 2.
Statokinesigrams and stabilograms of a control patient and a study group patient. Top, statokinesigrams (2D plots of the planar oscillation of the CoP) and bottom, stabilograms (traces plotted against time) of a control patient (left half) and a study group patient (right half) plotted during eyes closed condition (EC) before (gray lines) and after (black lines) the rehabilitation intervention (T0 and T1, respectively). The stabilograms are the projections of the statokinesigrams along the mediolateral (M-L) and anteroposterior (A-P) direction.
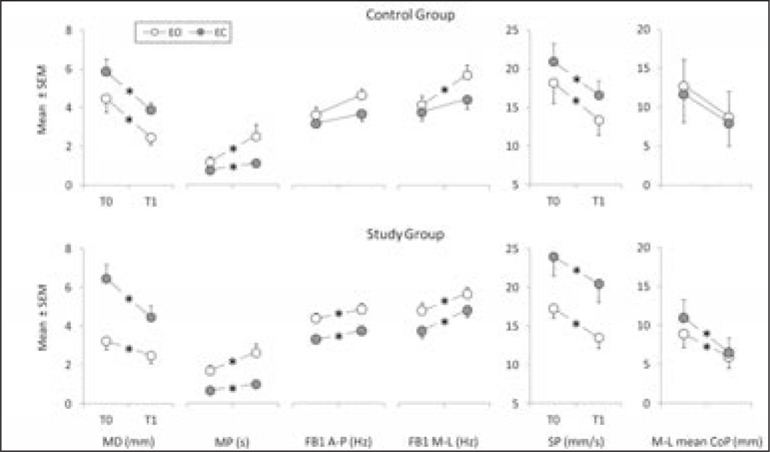
The indices of postural stability showed statistically significant differences between T0 and T1, for both the EO and EC conditions, in the SG; this pattern was also observed in the CG, with the exception of FB1 A-P, FB1 M-L and M-L mean CoP in the EC condition (Fig. 3). A significant medium correlation between the clinical scale scores and the indices of postural stability (r < −0.3, for MD, SP and M-L mean CoP; r > 0.3 for MP, FB1 A-P and FB1 M-L) was found in the SG patients, while in the CG, a significant medium correlation was found only for MD and SP (r < −0.46), MP and FB1 M-L (r > 0.37).
Figure 3.
Indices of postural stability recorded in the control group and study group during eyes open (EO) and eyes closed (EC) conditions.
MD and MP (structural parameters), FB1 A-P, FB1 ML, and SP (global parameters), and M-L mean CoP (CoP index of asymmetry) recorded at T0 and T1.Data are expressed as mean ± standard error mean. The asterisks represent significant differences (p<0.05).
No severe adverse events (such as fall, hypotension or heart problems) were reported during the rehabilitation program.
The satisfaction score was 3.5±0.7 (good level of satisfaction) in the SG and 2.4±0.9 (sufficient satisfaction) in the CG.
Discussion
Nowadays, posturographic analysis is widely performed to provide a guide for stroke rehabilitation, as it can be used to quantify postural instability and to analyze the course of a patient’s recovery of the standing posture (Nardone and Schieppati, 2010) and the level of asymmetry in weight-bearing distribution, thanks to its direct relationship with the mean CoP position along the M-L axis (Genthon et al., 2008).
Previous studies have demonstrated that the CoP signal correlates with the ankle torque, which is the combination of the descending motor commands modulating, contemporaneously, ankle torque and ankle stiffness, as well as the mechanical properties of the muscles acting around the ankle (Morasso and Sanguineti, 2002; Morasso and Schieppati, 1999; Baratto et al., 2002). Therefore, the clinical relevance of CoP analysis lies in the fact that the temporal structure of the CoP can be related to the postural control mechanism and its pathological modifications (Baratto et al., 2002).
This study, demonstrating reduction of asymmetrical weight bearing in stroke patients and, to a lesser extent, an improvement in postural stability, showed the effects of a rehabilitation program based on the use of a dynamic balance platform with audio-visual biofeedback.
After the rehabilitation program, in fact, both groups showed significantly improved postural control ability, as measured by the clinical scale scores, but only the SG showed a significant improvement of indices of postural stability, namely FB1 A-P and M-L mean CoP. As established by Pearson’s analysis, all the indices of postural stability were directly related to validated clinical scale scores, confirming their reliability in evaluating balance stability and asymmetry in weight-bearing distribution.
Time structure analysis of the CoP signal was performed through global (FB1 and SP) and structural (MD and MP) parameters, which express the size of sway patterns, in both the time and frequency domains, as well as identifying subunits in the posturographic data related to the postural control mechanism and its underlying motor processes (Baratto et al., 2002). On the other hand, the CoP index of asymmetry (M-L mean CoP) gives a direct appraisal of the difference in weight-bearing distribution between the lower extremities.
Since an excessive reliance on visual information after stroke has been reported (Bonan et al., 2004a,b), we tested the EO and EC conditions to evaluate the level of visual dependence in our sample (Bonan et al., 2004a,b; De Nunzio and Schieppati, 2007; Sozzi et al., 2011; De Nunzio et al., 2005).
The positive effect of postural rehabilitation, independently of the techniques, was confirmed by MD index decrease and MP increase, in both the SG and the CG, and under both visual conditions (EO and EC) (Fig. 3). The MD (mean distance between two consecutive peaks of the SDP) represents the mean distance between data clusters of the CoP signal (points in which the anticipatory muscle control actions or posturographic commands are stable) and its reduction indicates a decreased amplitude of posturographic commands, while a rise in the MP index is related to an increase in the degree of stabilization obtained with posturographic commands (Baratto et al., 2002).
The FB1 index calculated for both the A-P and the M-L CoP oscillations increased significantly, under the EO and EC conditions only in the SG, showing a meaningful increase in frequency bandwidth of the CoP signal and therefore in the presence of quick transients in the posturographic commands (Baratto et al., 2002).
Considering the FB1 and SP values together, a meaningful decrease in the amplitude of CoP oscillations as well as an enhancement of the effectiveness of the posturographic commands was revealed. At the end of treatment, the effectiveness of the posturographic commands was enhanced for the control of CoP oscillations in the A-P and M-L directions in the SG; in the CG, on the other hand, a significant improvement was found only in the M-L direction.
This result represents a further consideration supporting the usefulness and effectiveness of audio-visual biofeedback rehabilitation.
Even though the biofeedback exercises were meant to induce a symmetrical weight-bearing distribution focusing on M-L shifts, they had a “cross-over effect”, also inducing better control along the A-P direction. In our opinion, this “whole balancing effect” could be due to the real-time 2D representation of the CoP movements during the biofeedback training, which led the patients to control the CoP position in both the M-L and A-P directions.
The recovery of symmetrical weight-bearing distribution due to the audio-visual biofeedback approach was shown by the M-L mean CoP values that were reduced only in the SG, at the end of the rehabilitation program, under both visual conditions.
Data obtained from this study allow two main conclusions to be drawn: first, the indices of postural stability, derived from CoP signal analysis, provided a meaningful approach for studying postural control recovery, where clinical scales failed to do so. Second, the audio-visual biofeedback rehabilitation program seemed to induce a significant ameliorating effect on the asymmetrical weight-bearing distribution, as well as on the postural control recovery in these stroke patients.
Although these data are encouraging they are far from definitive and further studies should seek to better investigate the neurophysiological mechanisms involved in the recovery of sensory-motor integration, manipulating different sensory inputs, during upright quiet stance control.
References
- Baratto L, Morasso PG, Re C, et al. A new look at posturographic analysis in the clinical context: sway-density versus other parameterization techniques. Motor Control. 2002;6:246–270. doi: 10.1123/mcj.6.3.246. [DOI] [PubMed] [Google Scholar]
- Barclay-Goddard R, Stevenson T, Poluha W, et al. Force platform feedback for standing balance training after stroke. Cochrane Database Syst Rev. 2004;(4):CD004129. doi: 10.1002/14651858.CD004129.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersano A, Baron P, Lanfranconi S, et al. Lombardia GENS Group Lombardia GENS: a collaborative registry for monogenic diseases associated with stroke. Funct Neurol. 2012;27:107–117. [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW. Standing balance, lower extremity muscle strength, and walking performance of patients referred for physical therapy. Percept Mot Skills. 1995;80:379–385. doi: 10.2466/pms.1995.80.2.379. [DOI] [PubMed] [Google Scholar]
- Bohannon RW. Determinants of transfer capacity in patients with hemiplegia. Physiother Can. 1988;40:236–239. [Google Scholar]
- Bohannon RW. Correlation of lower limb strengths and other variables with standing performance in stroke patients. Physiother Can. 1989a;41:198–202. [Google Scholar]
- Bohannon RW. Selected determinants of ambulatory capacity in patients with hemiplegia. Clin Rehabil. 1989b;3:47–53. [Google Scholar]
- Bohannon RW, Leary KM. Standing balance and function over the course of acute rehabilitation. Arch Phys Med Rehabil. 1995;76:994–996. doi: 10.1016/s0003-9993(95)81035-8. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Walsh S, Joseph MC. Ordinal and timed balance measurements: reliability and validity in patients with stroke. Clin Rehabil. 1993;7:9–13. [Google Scholar]
- Bonan IV, Colle FM, Guichard JP, et al. Reliance on visual information after stroke. Part I: Balance on dynamic posturography. Arch Phys Med Rehabil. 2004;85:268–273. doi: 10.1016/j.apmr.2003.06.017. [DOI] [PubMed] [Google Scholar]
- Bonan IV, Yelnik AP, Colle FM, et al. Reliance on visual information after stroke. Part II: Effectiveness of a balance rehabilitation program with visual cue deprivation after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2004;85:274–278. doi: 10.1016/j.apmr.2003.06.016. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Cheng PT, Wang CM, Chung CY, et al. Effects of visual feedback rhythmic weight-shift training on hemiplegic stroke patients. Clin Rehabil. 2004;18:747–753. doi: 10.1191/0269215504cr778oa. [DOI] [PubMed] [Google Scholar]
- Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 Suppl 2):S21–29. doi: 10.1053/apmr.2000.20620. [DOI] [PubMed] [Google Scholar]
- de Haart M, Geurts AC, Huidekoper SC, et al. Recovery of standing balance in postacute stroke patients: a rehabilitation cohort study. Arch Phys Med Rehabil. 2004;85:886–895. doi: 10.1016/j.apmr.2003.05.012. [DOI] [PubMed] [Google Scholar]
- De Nunzio AM, Nardone A, Schieppati M. Head stabilization on a continuously oscillating platform: the effect of a proprioceptive disturbance on the balancing strategy. Exp Brain Res. 2005;165:261–272. doi: 10.1007/s00221-005-2297-7. [DOI] [PubMed] [Google Scholar]
- De Nunzio AM, Schieppati M. Time to reconfigure balancing behaviour in man: changing visual condition while riding a continuously moving platform. Exp Brain Res. 2007;178:18–36. doi: 10.1007/s00221-006-0708-z. [DOI] [PubMed] [Google Scholar]
- de Oliveira CB, de Medeiros IR, Frota NA, et al. Balance control in hemiparetic stroke patients: main tools for evaluation. J Rehabil Res Dev. 2008;45:1215–1226. [PubMed] [Google Scholar]
- Dickstein R, Nissan M, Pillar T, et al. Foot-ground pressure pattern of standing hemiplegic patients. Major characteristics and patterns of improvement. Phys Ther. 1984;64:19–23. doi: 10.1093/ptj/64.1.19. [DOI] [PubMed] [Google Scholar]
- Dickstein R, Shefi S, Marcovitz E, et al. Anticipatory postural adjustment in selected trunk muscles in post stroke hemiparetic patients. Arch Phys Med Rehabil. 2004;85:261–267. doi: 10.1016/j.apmr.2003.05.011. [DOI] [PubMed] [Google Scholar]
- Dodds TA, Martin DP, Stolov WC, et al. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil. 1993;74:531–536. doi: 10.1016/0003-9993(93)90119-u. [DOI] [PubMed] [Google Scholar]
- Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: a statement for health-care professionals from the American Heart Association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- Fong KN, Chan CC, Au DK. Relationship of motor and cognitive abilities to functional performance in stroke rehabilitation. Brain Inj. 2001;15:443–453. doi: 10.1080/02699050010005940. [DOI] [PubMed] [Google Scholar]
- French B, Thomas L, Leathley M, et al. Does repetitive task training improve functional activity after stroke? A Cochrane systematic review and meta-analysis. J Rehabil Med. 2010;42:9–14. doi: 10.2340/16501977-0473. [DOI] [PubMed] [Google Scholar]
- Genthon N, Gissot AS, Froger J, et al. Posturography in patients with stroke: estimating the percentage of body weight on each foot from a single force platform. Stroke. 2008;39:489. doi: 10.1161/STROKEAHA.107.493478. [DOI] [PubMed] [Google Scholar]
- Gordon NF, Gulanick M, Costa F, et al. Physical activity and exercise recommendations for stroke survivors: an American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Circulation. 2004;109:2031–2041. doi: 10.1161/01.CIR.0000126280.65777.A4. [DOI] [PubMed] [Google Scholar]
- Hendrickson J, Patterson KK, Inness EL, et al. Relationship between asymmetry of quiet standing balance control and walking post-stroke. Gait Posture. 2014;39:177–181. doi: 10.1016/j.gaitpost.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Sheu CF, Hsueh IP, et al. Trunk control as an early predictor of comprehensive activities of daily living function in stroke patients. Stroke. 2002;33:2626–2630. doi: 10.1161/01.str.0000033930.05931.93. [DOI] [PubMed] [Google Scholar]
- Januário F, Campos I, Amaral C. Rehabilitation of postural stability in ataxic/hemiplegic patients after stroke. Disabil Rehabil. 2010;32:1775–1779. doi: 10.3109/09638281003734433. [DOI] [PubMed] [Google Scholar]
- Keith RA, Granger CV, Hamilton BB, et al. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- Lambercy O, Dovat L, Yun H, et al. Effects of a robot-assisted training of grasp and pronation/supination in chronic stroke: a pilot study. J Neuroeng Rehabil. 2011;8:63. doi: 10.1186/1743-0003-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Porta F, Franceschini M, Caselli S, et al. Unified Balance Scale: an activity-based, bed to community, and aetiology-independent measure of balance calibrated with Rasch analysis. J Rehabil Med. 2011;43:435–444. doi: 10.2340/16501977-0797. [DOI] [PubMed] [Google Scholar]
- Lee MY, Wong MK, Tang FT, et al. Comparison of balance responses and motor patterns during sit-to-stand task with functional mobility in stroke patients. Am J Phys Med Rehabil. 1997;76:401–410. doi: 10.1097/00002060-199709000-00011. [DOI] [PubMed] [Google Scholar]
- Lennon S, Ashburn A. The Bobath concept in stroke rehabilitation: a focus group study of the experienced physiotherapists’ perspective. Disabil Rehabil. 2000;22:665–674. doi: 10.1080/096382800445461. [DOI] [PubMed] [Google Scholar]
- Leung AW, Cheng SK, Mak AK, et al. Functional gain in hemorrhagic stroke patients is predicted by functional level and cognitive abilities measured at hospital admission. NeuroRehabilitation. 2010;27:351–358. doi: 10.3233/NRE-2010-0619. [DOI] [PubMed] [Google Scholar]
- Levin MF, Panturin E. Sensorimotor integration for functional recovery and the Bobath approach. Motor Control. 2011;15:285–301. doi: 10.1123/mcj.15.2.285. [DOI] [PubMed] [Google Scholar]
- McIlroy WE, Maki BE. Preferred placement of the feet during quiet stance: development of a standardized foot placement for balance testing. Clin Biomech (Bristol, Avon) 1997;12:66–70. doi: 10.1016/s0268-0033(96)00040-x. [DOI] [PubMed] [Google Scholar]
- Mercer VS, Freburger JK, Chang SH, et al. Measurement of paretic-lower-extremity loading and weight transfer after stroke. Phys Ther. 2009;89:653–664. doi: 10.2522/ptj.20080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasso PG, Sanguineti V. Ankle muscle stiffness alone cannot stabilize balance during quiet standing. J Neurophysiol. 2002;88:2157–2162. doi: 10.1152/jn.2002.88.4.2157. [DOI] [PubMed] [Google Scholar]
- Morasso PG, Schieppati M. Can muscle stiffness alone stabilize upright standing? J Neurophysiol. 1999;82:1622–1626. doi: 10.1152/jn.1999.82.3.1622. [DOI] [PubMed] [Google Scholar]
- Moreland JD, Goldsmith CH, Huijbregts MP, et al. Progressive resistance strengthening exercises after stroke: a single-blind randomized controlled trial. Arch Phys Med Rehabil. 2003;84:1433–1440. doi: 10.1016/s0003-9993(03)00360-5. [DOI] [PubMed] [Google Scholar]
- Nardone A, Schieppati M. The role of instrumental assessment of balance in clinical decision making. Eur J Phys Rehabil Med. 2010;46:221–237. [PubMed] [Google Scholar]
- Paolucci S, Caltagirone C, Mastrilli F, et al. Planning availability in stroke rehabilitation units. Funct Neurol. 2003;18:191–194. [PubMed] [Google Scholar]
- Pinsault N, Vuillerme N. Test-retest reliability of centre of foot pressure measures to assess postural control during unperturbed stance. Med Eng Phys. 2009;31:276–286. doi: 10.1016/j.medengphy.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Pistarini C, Molteni F. New technologies and high specialization rehabilitation. Funct Neurol. 2009;24:169–171. [PubMed] [Google Scholar]
- Platz T, Hesse S, Mauritz KH. Motor rehabilitation after traumatic brain injury and stroke - Advances in assessment and therapy. Restor Neurol Neurosci. 1999;14:161–166. [PubMed] [Google Scholar]
- Pollock A, Baer G, Pomeroy V, et al. Physiotherapy treatment approaches for the recovery of postural control and lower limb function following stroke. Cochrane Database Syst Rev. 2007;(1):CD001920. doi: 10.1002/14651858.CD001920.pub2. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Franklin BA, Balady GJ, et al. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association Position paper endorsed by the American College of Sports Medicine. Circulation. 2000;101:828–833. doi: 10.1161/01.cir.101.7.828. [DOI] [PubMed] [Google Scholar]
- Semprini R, Sale P, Foti C, et al. Gait impairment in neurological disorders: a new technological approach. Funct Neurol. 2009;24:179–183. [PubMed] [Google Scholar]
- Sozzi S, Monti A, De Nunzio AM, et al. Sensori-motor integration during stance: time adaptation of control mechanisms on adding or removing vision. Hum Mov Sci. 2011;30:172–189. doi: 10.1016/j.humov.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Taly AB, Gupta A, et al. Post-stroke balance training: role of force platform with visual feedback technique. J Neurol Sci. 2009;287:89–93. doi: 10.1016/j.jns.2009.08.051. [DOI] [PubMed] [Google Scholar]
- Van Peppen RP, Kwakkel G, Wood-Dauphinee S, et al. The impact of physical therapy on functional outcomes after stroke: what’s the evidence? Clin Rehabil. 2004;18:833–862. doi: 10.1191/0269215504cr843oa. [DOI] [PubMed] [Google Scholar]
- Varoqui D, Froger J, Pélissier JY, et al. Effect of coordination biofeedback on (re)learning preferred postural patterns in post-stroke patients. Motor Control. 2011;15:187–205. doi: 10.1123/mcj.15.2.187. [DOI] [PubMed] [Google Scholar]
- Verheyden G, Vereeck L, Truijen S, et al. Additional exercises improve trunk performance after stroke: a pilot randomized controlled trial. Neurorehabil Neural Repair. 2009;23:281–286. doi: 10.1177/1545968308321776. [DOI] [PubMed] [Google Scholar]
- Verheyden G, Vereeck L, Truijen S, et al. Trunk performance after stroke and the relationship with balance, gait and functional ability. Clin Rehabil. 2006;20:451–458. doi: 10.1191/0269215505cr955oa. [DOI] [PubMed] [Google Scholar]
- Winstein CJ. Knowledge of results and motor learning -implications for physical therapy. Phys Ther. 1991;71:140–149. doi: 10.1093/ptj/71.2.140. [DOI] [PubMed] [Google Scholar]
- Zelaschi F, Felicetti G, Di Patrizi S. Motor rehabilitation: evolution of functional markers in trained hemiparetic patients and effectiveness of synchronous techniques. Funct Neurol. 1995;10:203–207. [PubMed] [Google Scholar]