Abstract
To test the feasibility of altering polyamine levels by influencing their catabolic pathway, we obtained transgenic tobacco (Nicotiana tabacum) plants constitutively expressing either maize (Zea mays) polyamine oxidase (MPAO) or pea (Pisum sativum) copper amine oxidase (PCuAO), two extracellular and H2O2-producing enzymes. Despite the high expression levels of the transgenes in the extracellular space, the amount of free polyamines in the homozygous transgenic plants was similar to that in the wild-type ones, suggesting either a tight regulation of polyamine levels or a different compartmentalization of the two recombinant proteins and the bulk amount of endogenous polyamines. Furthermore, no change in lignification levels and plant morphology was observed in the transgenic plants compared to untransformed plants, while a small but significant change in reactive oxygen species-scavenging capacity was verified. Both the MPAO and the PCuAO tobacco transgenic plants produced high amounts of H2O2 only in the presence of exogenously added enzyme substrates. These observations provided evidence for the limiting amount of freely available polyamines in the extracellular space in tobacco plants under physiological conditions, which was further confirmed for untransformed maize and pea plants. The amount of H2O2 produced by exogenously added polyamines in cell suspensions from the MPAO transgenic plants was sufficient to induce programmed cell death, which was sensitive to catalase treatment and required gene expression and caspase-like activity. The MPAO and PCuAO transgenic plants represent excellent tools to study polyamine secretion and conjugation in the extracellular space, as well as to determine when and how polyamine catabolism actually intervenes both in cell wall development and in response to stress.
The polyamines putrescine (Put), spermidine (Spd), and spermine (Spm) are low Mr metabolites naturally found in eukaryotic and prokaryotic cells (Cohen, 1998). Because of their polycationic nature at physiological pH, polyamines can bind strongly to negative charges in cellular components such as nucleic acids, various types of proteins, and acidic phospholipids (Cohen, 1998). In plant cells, polyamines can also be conjugated via an amide bond to hydroxycinnamic acids (Martin-Tanguy, 1997) and serve as precursors for secondary metabolites like nicotine. The function of the conjugated forms of polyamines is not known. However, they have long been associated with flowering (Martin-Tanguy, 1997) and plant-microbe interactions (Torrigiani et al., 1997; Mackintosh et al., 2001; Cowley and Walters, 2002).
Polyamines play important roles in DNA stabilization, RNA and protein synthesis, membrane stabilization, modulation of ion channels, and protection against oxygen radicals, and they are essential for cell homeostasis, cell growth, and tumorigenesis (Wallace et al., 2003). In particular, plant polyamines have been implicated in a variety of plant growth and developmental processes involving cell proliferation and differentiation, morphogenesis, seed dormancy and germination, tuberization, flower induction and development, fruit growth and ripening, embryogenesis, and senescence (for review, see Kumar et al., 1997; Tiburcio et al., 1997; Walden et al., 1997; Malmberg et al., 1998; Bouchereau et al., 1999). Polyamine involvement in defense mechanisms during biotic and abiotic stress (such as mineral nutrient deficiency, osmotic, salt, heat, chilling, wounding, oxidative stress, and pathogen infection) has also been demonstrated (Torrigiani et al., 1997; Yamakawa et al., 1998; Bouchereau et al., 1999; Mackintosh et al., 2001; Perez-Amador et al., 2002; Walters, 2003).
Polyamines are catabolized by the action of amine oxidases. Amine oxidases include the copper-containing amine oxidases (CuAO; EC 1.4.3.6), oxidizing the diamines Put and cadaverine at the primary amino groups, and the flavin-containing polyamine oxidases (PAO), which oxidize Spd and Spm at their secondary amino groups (Federico and Angelini, 1991). In plants, these enzymes are preferentially associated with the primary and secondary cell walls of tissues undergoing lignification, suberization, and wall stiffening (such as xylem, xylem parenchyma, endodermis, and epidermis), although their association to cortical parenchyma cell walls during specific developmental stages has also been reported (Federico and Angelini, 1991; Slocum and Furey, 1991; Liu et al., 1995; Laurenzi et al., 2001). CuAO reaction products from Put are Δ1-pyrroline, H2O2, and ammonia, while PAO yields Δ1-pyrroline and 1,5-diazabicyclononane from Spd and Spm respectively, along with 1,3-diaminopropane (Dap) and H2O2. Dap can be converted into β-Ala, whereas Δ1-pyrroline can be further catabolized to γ-aminobutyric acid (GABA) in a reaction catalyzed by pyrroline dehydrogenase (Flores and Filner, 1985). GABA is subsequently transaminated and oxidized to succinic acid, which is incorporated into the Kreb's cycle, thus ensuring the recycling of carbon and nitrogen from Put.
Far from being only a means of eliminating cellular polyamines, the enzymes involved in polyamine catabolism and the products deriving from their action contribute to important physiological processes (Martin-Tanguy, 1997; Šebela et al., 2001). The production of H2O2 through polyamine oxidation has been correlated with the oxidative burst, cell death, lignification, and suberization processes occurring during development and defense responses (Allan and Fluhr, 1997; Møller and McPherson, 1998; Rea et al., 1998, 2002; Cona et al., 2003; Walters, 2003). PAO are also involved in the production of uncommon polyamines, such as norspermidine, norspermine, cardopentamine, caldohexamine, homocaldopentamine, and homocaldohecamine (Phillips and Kuehn, 1991). Uncommon polyamines are of interest because they may be involved in mediating growth response of various organisms under extreme environmental conditions (Phillips and Kuehn, 1991).
In the last few years, mutant plants with aberrant polyamine biosynthesis and transgenic plants exhibiting overexpression or down-regulation of enzymes involved in polyamine metabolic pathways have been used for the study of polyamine function (DeScenzo and Minocha, 1993; Kumar et al., 1996; Burtin and Michael, 1997; Watson et al., 1998; Rafart-Pedros et al., 1999; Capell et al., 2000; Noury et al., 2000; Wisniewski and Brewin, 2000; Mehta et al., 2002; Thu-Hang et al., 2002). Most of these studies have focused on the polyamine biosynthetic pathways and only a few on the catabolic pathways (Wisniewski and Brewin, 2000). Furthermore, in several of these studies, only relatively weak variations in polyamine levels have been observed, indicating the occurrence of compensatory mechanisms to maintain polyamine homeostasis.
In order to test the feasibility of altering polyamine levels by influencing their catabolic pathway and to gain some insight into the physiological processes in which both polyamines and polyamine catabolism are involved, transgenic tobacco (Nicotiana tabacum) plants constitutively expressing either maize (Zea mays) polyamine oxidase (MPAO) or pea (Pisum sativum) copper amine oxidase (PCuAO) were obtained. The transgenic plants appeared morphologically normal, and their polyamine and lignin content was comparable to that of wild-type plants. Though exhibiting a small but significant increase in reactive oxygen species (ROS)-scavenging ability compared with wild-type plants, MPAO and PCuAO transgenic plants and cell suspensions derived from them produced high levels of H2O2 only in the presence of exogenously supplied enzyme substrate, indicating that polyamines in the extracellular space are limiting. Such an oxidative stress was sufficient to induce a programmed cell death (PCD)-like cell death in cell suspensions obtained from MPAO transgenic plants.
RESULTS
Molecular Characterization of MPAO and PCuAO Transgenic Tobacco Plants
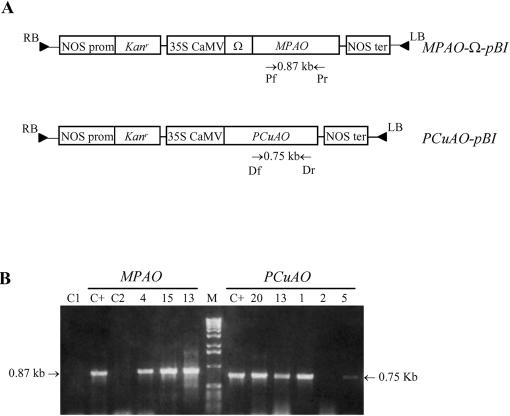
Tobacco plants were transformed with MPAO-Ω-pBI or PCuAO-pBI constructs (Fig. 1A) containing the cDNA encoding for MPAO (Tavladoraki et al., 1998) or PCuAO (Tipping and McPherson, 1995), respectively, under the control of the 35S cauliflower mosaic virus promoter. In the MPAO-Ω-pBI construct, the Ω element from tobacco mosaic virus (TMV) was also inserted to obtain elevated expression levels.
Figure 1.
Generation and molecular characterization of transgenic tobacco plants expressing MPAO or PCuAO. A, Map of the MPAO-Ω-pBI and PCuAO-pBI constructs used for Agrobacterium-mediated transformation of tobacco plants. Primers (Pr/Pf and Dr/Df) used for PCR analysis and lengths of amplified fragments are indicated. B, PCR analysis of alkali-treated leaf pieces from tobacco plants transformed with the MPAO-Ω-pBI and PCuAO-pBI constructs. Numbers represent independent MPAO and PCuAO transgenic lines. C+, positive control using purified MPAO or PCuAO cDNA; C1 and C2, negative controls using leaf pieces from untransformed plants and MPAO- or PCuAO-specific primers, respectively; M, Mr marker (1-kb DNA ladder; Life Technologies/Gibco-BRL, Cleveland).
Transgenic plants were selected in the presence of kanamycin, and several independent transgenic lines were obtained from two distinct transformation experiments. Primary MPAO or PCuAO transformants (T0 generation) were screened for the presence of the transgenes by PCR after alkali treatment to amplify a 0.87-kb or a 0.75-kb fragment of the MPAO or the PCuAO cDNA, respectively (Fig. 1B). No amplification product was obtained from the untransformed plants either with the MPAO- or the PCuAO-specific oligonucleotides (Fig. 1B).
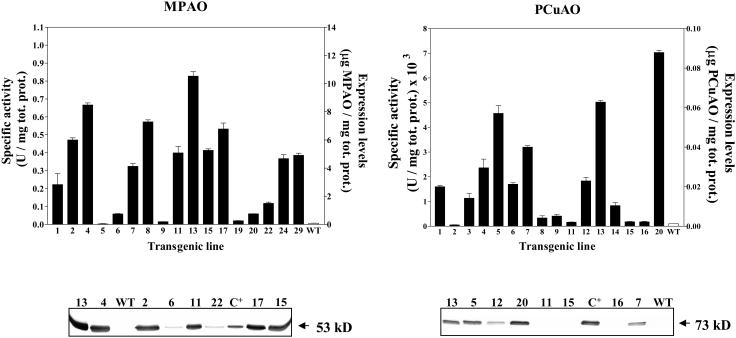
MPAO and PCuAO expression was determined in the leaves of primary transformants by western-blot analysis and enzyme activity assays (Fig. 2). The two methods gave comparable results and revealed different amounts of recombinant protein among the various transgenic lines. The maximum expression level observed for recombinant MPAO (transgenic line MPAO-13) was 0.01 mg of MPAO per milligram of total soluble proteins, which is about 10-fold higher than that of the native enzyme in maize leaves. For recombinant PCuAO, the maximum expression level observed (transgenic line PCuAO-20) was 0.088 μg of PCuAO per milligram of total soluble proteins, which is about 10-fold less than that of the native enzyme in the pea seedlings. No protein similar to MPAO and PCuAO was detectable in untransformed plants by both enzyme activity assays and western-blot analysis utilizing anti-MPAO and anti-PCuAO polyclonal antibodies (Fig. 2).
Figure 2.
Expression levels of MPAO and PCuAO in different transgenic tobacco lines. Expression levels were determined by enzyme activity assays (top) and western-blot analysis (bottom) in crude leaf extracts. Values are mean ± se from three replicates. Numbers represent independent MPAO and PCuAO transgenic lines. WT, untransformed tobacco plants; C+, purified MPAO or PCuAO protein. For western-blot analysis, extracts were normalized for the amount of total soluble proteins.
The inheritance of the MPAO or PCuAO transgenes was studied in three transgenic lines showing different expression levels of the transgenes. Primary transformants were selfed and the seeds harvested after maturation. Upon germination on medium containing kanamycin (T1 generation), we observed for all six lines a segregation ratio of 3:1, indicating the presence of a single integration locus.
None of the transgenic plants of the T1 and T2 generations exhibited an altered morphology during normal growth. Furthermore, they exhibited similar accumulation levels of the recombinant proteins to those of the corresponding primary transformants in all tissues tested (leaves, roots, and stems). Homozygous transgenic lines of the T2 generation from primary transformants having the highest transgene expression levels (transgenic lines MPAO-13H and PCuAO-20H) were selected for further analysis.
Localization of Transgene Expression
Native MPAO and PCuAO enzymes are prevalently apoplastic proteins associated with plant cell walls. Apoplastic localization is also expected for the recombinant proteins in the transgenic plants since the whole cDNAs, including the sequences encoding for the signal peptides that guide protein entry into the endoplasmic reticulum, were inserted in the transformation constructs.
To confirm the extracellular localization of the recombinant proteins in the transgenic plants, intercellular fluids from the MPAO and PCuAO transgenic plants were tested for transgene accumulation both by western-blot analysis (data not shown) and enzyme activity assays (Table I). As a control, intercellular fluids were also tested for levels of the cytosolic marker Glc-6-phosphate dehydrogenase (data not shown) and extracellular peroxidases (Table I). Data demonstrated that the MPAO- and PCuAO-specific activities are at least 10-fold higher in the intercellular fluids (fractions F1 and F2; Table I) than in the crude extracts (fraction T; Table I), confirming a prevalently extracellular localization for the two recombinant enzymes. On the contrary, PAO and CuAO activity could not be detected in intercellular fluids obtained from wild-type plants under our experimental conditions. Extracellular localization of the two recombinant enzymes in the transgenic plants was also confirmed by the lack of MPAO and PCuAO enzyme activity in protoplasts obtained from these plants. Transgene enzyme activity could be detected only upon cell wall formation (data not shown).
Table I.
Analysis of intercellular fluids from MPAO or PCuAO tobacco plants for transgene expression levels
| MPAO Specific Activity | PCuAO Specific Activity | POD Specific Activity | |
|---|---|---|---|
| units/mg tot. prot. | units/mg tot. prot. | units/mg tot. prot. | |
| F1 | 3.0 | 7.6 × 10−1 | 2.5 × 10−2 |
| F2 | 3.3 | 7.0 × 10−2 | 0.4 × 10−2 |
| I | 0.5 | 5.0 × 10−3 | 1.0 × 10−3 |
| T | 0.8 | 7.0 × 10−3 | 2.0 × 10−3 |
Two successive apoplastic extracts (fractions F1 and F2), intracellular extracts (fraction I), and total soluble extracts (fraction T) were tested for MPAO, PCuAO, and peroxidase (POD) enzymatic activity. The experiment was repeated twice, and data are from a single representative experiment.
H2O2 Levels in Transgenic Plants Overexpressing MPAO or PCuAO
The homozygous transgenic lines MPAO-13H and PCuAO-20H were analyzed for H2O2 production in different tissues (leaf discs, stem sections, and roots) by placing them onto agar plates containing KI and starch (Olson and Varner, 1993). H2O2 diffusing from plant tissues is indicated by the purple-blue color in surrounding areas of the medium. Using such an assay, H2O2 production could not be detected in any of the tissues analyzed, either from transgenic or wild-type plants up to 24 h of incubation. Elevated levels of H2O2 production were observed in all tissues tested of both MPAO and PCuAO transgenic plants only upon supply of enzyme substrate (1 mm Spd and 1 mm Put, respectively; Fig. 3). On the contrary, addition of Spd or Put to the various tissues of wild-type plants did not result in detectable H2O2 production (Fig. 3). The level of H2O2 production was higher in all tissues of MPAO transgenic plants than in those of PCuAO ones. This difference reflects the expression levels of recombinant MPAO and PCuAO in the transgenic plants. Furthermore, the above results also indicate that the limiting factor for H2O2 production in the transgenic tobacco plants is the polyamine content of the apoplast, where the recombinant proteins are localized.
Figure 3.
Detection of H2O2 production in plant tissues of tobacco, maize, and pea plants on KI- and starch-containing medium. A, Leaf discs, stem sections, and roots of untransformed plants (WT) and MPAO- or PCuAO-expressing tobacco transgenic plants were placed in the medium in the absence (−Spd/Put) or in the presence of 1 mm Spd (+Spd) or Put (+Put). B, Leaf discs from maize and pea plants were placed in the medium in the absence (−Spd; −Put) and in the presence of Spd (+Spd) or Put (+Put) at a final concentration of 1 mm. Color development was allowed for 24 h.
Using the same method, leaf discs from maize and pea seedlings expressing MPAO and PCuAO, respectively, were also tested for H2O2 production (Fig. 3). Similar to the transgenic plants, H2O2 was produced from maize and pea leaf discs only upon addition of Spd or Put, respectively, suggesting that polyamine transport to the apoplast is limited also in these plants.
In Situ Detection of H2O2 in Transgenic Plants Overexpressing MPAO or PCuAO
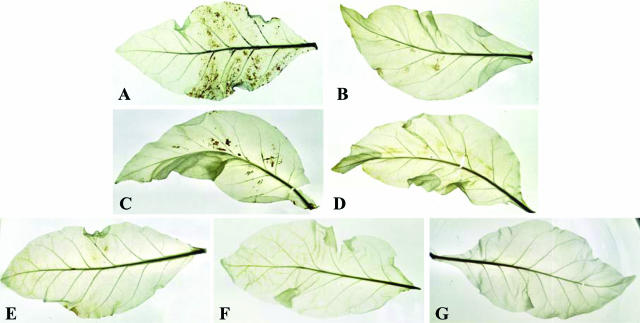
To exclude the possibility that the H2O2 detected in the transgenic segments by the KI/starch assay is a product of the recombinant enzymes liberated from the damaged cells at the tissue cutting site, H2O2 production in the MPAO and PCuAO transgenic plants also was evaluated in situ by allowing leaves to take up 3,3-diaminobenzidine (DAB), which in the presence of peroxidases polymerizes as soon as it comes into contact with H2O2, forming a brown precipitate. DAB polymerization was only observed in the transgenic plants, which had absorbed DAB in the presence of 1 mm exogenous polyamines (Fig. 4, A and C). As observed using the KI/starch assay, staining intensity was proportional to enzyme expression, and no precipitate was detected in polyamine-treated (Fig. 4, B and D) or untreated (Fig. 4G) wild-type plants. Furthermore, H2O2 production was not detected in transgenic leaves, which had not been treated with enzyme substrate (Fig. 4, E and F), confirming that the limiting factor for H2O2 production in the transgenic plants is polyamine levels in the extracellular space. These results also suggest that MPAO and PCuAO proteins are functionally expressed in situ in tobacco transgenic plants.
Figure 4.
In situ detection of H2O2 production by the DAB-uptake method in the MPAO and PCuAO transgenic tobacco plants. Leaves from wild-type (B, D, and G) and MPAO (A and E) or PCuAO (C and F) transgenic plants were supplied with DAB for 18 h in the absence (E–G) and in the presence of 1 mm Spd (A and B) or 1 mm Put (C and D).
Polyamine and Lignin Content in MPAO and PCuAO Transgenic Plants
The leaves of the MPAO-13 and PCuAO-20 transgenic lines were analyzed for polyamine levels. Despite the high expression levels of transgenes, only a slight, statistically not significant reduction in the levels of total free polyamines was observed in the MPAO and PCuAO transgenic plants compared with untransformed plants (Table II). To determine whether the two extracellularly localized recombinant proteins interfere with polyamine levels in the apoplast, intercellular fluids were examined for polyamine content. However, polyamines could not be detected in the apoplast either of transgenic or wild-type plants, as reported previously by Yamakawa et al. (1998) and Yoda et al. (2003).
Table II.
Free polyamine content in young leaves of wild-type and MPAO- or PCuAO-expressing tobacco plants
| Put | Spd | Spm | |
|---|---|---|---|
| nmol/g fresh weight | |||
| WT | 275.5 ± 79.0 | 130.3 ± 50.0 | 12.8 ± 5.8 |
| MPAO | 217.5 ± 56.4 | 102.5 ± 35.9 | 8.3 ± 3.5 |
| PCuAO | 242.4 ± 59.9 | 126.2 ± 17.5 | 11.2 ± 2.6 |
Polyamine levels were determined in leaves of 30-d-old wild-type and transgenic plants. The values represent the mean ± sd between independent plants (n = 10).
It has been suggested that H2O2 produced by polyamine oxidation in the cell wall may be involved in peroxidase-mediated lignification, suberization, and cell wall polymer cross-linking occurring during ontogenesis and defense responses (Møller and McPherson, 1998; Rea et al., 1998, 2002; Wisniewski et al., 2000). To investigate whether MPAO or PCuAO expression in the tobacco transgenic plants interferes with cell wall development, the transgenic plants were also histochemically analyzed for lignin content. No significant changes in lignification levels were observed in both transgenic lines compared to untransformed plants (data not shown).
The Cellular Redox State in MPAO and PCuAO Transgenic Plants
In order to verify whether overexpression of MPAO or PCuAO in the transgenic tobacco plants affected the ROS-scavenging activity, levels of ascorbate peroxidase (APX), catalase, dehydroascorbate reductase (DHAR), ascorbate (ASC), and dehydroascorbate (DHA) were also determined in transgenic and wild-type plants.
As shown in Table III, APX level was significantly increased in both transgenic lines as compared to the wild-type plants, while catalase activity did not vary. Furthermore, ASC content and redox state, i.e. the ratio between the reduced form of ASC and the total ASC pool (ASC + DHA), were increased only in the PCuAO transgenic plants (Table III). Interestingly, DHAR was significantly enhanced only in these plants. This could explain the shift toward the reduced form of ASC pool in the PCuAO transgenic plants. Moreover, since DHA is an unstable molecule, which is quickly degraded when it is not promptly reduced to ASC, the rise in DHAR activity could also explain the increased amount of the ASC pool present in the PCuAO transgenic plants.
Table III.
ROS-scavenging activity in MPAO or PCuAO transgenic tobacco plants
| APX (nmol ASCoxidized min−1 mg−1 prot.) | Catalase (nmol H2O2 min−1 mg−1prot.) | DHAR (nmol DHAreducedmin−1 mg−1 prot.) | Ascorbate Pool ([ASC + DHA]) (nmol g−1 fresh wt) | Ascorbate Redox State ([ASC] / [ASC+DHA]) | |
|---|---|---|---|---|---|
| WT | 326 ± 27 | 287 ± 7 | 191 ± 17 | 1,470 ± 30 | 0.87 |
| MPAO | 383 ± 17* | 295 ± 15 | 202 ± 22 | 1,300 ± 110 | 0.86 |
| PCuAO | 401 ± 23* | 258 ± 36 | 269 ± 27* | 1,550 ± 80* | 0.91* |
Ascorbate content and redox enzymes were determined in leaves of 30-d-old wild-type and transgenic plants. The values are the means of four experiments ± sd.
Differences from the wild type were significant at P < 0.05 (Student's t paired test).
Characterization of Cell Suspensions Obtained from MPAO Transgenic Plants
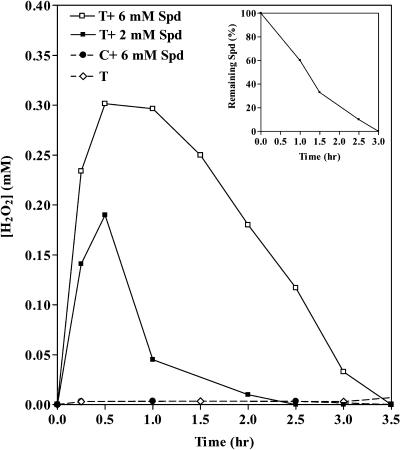
Cell suspensions were obtained from the leaves of wild-type and MPAO-13H transgenic plants and tested for H2O2 accumulation. The transgenic cell suspension maintained high expression levels of the recombinant MPAO, i.e. 20 μg of MPAO per milliliter packed cell volume. H2O2 could not be detected in the medium of either the MPAO or the wild-type cell suspension in the absence of exogenous Spd. Addition of Spd at a final concentration of 2 mm or 6 mm resulted in a steady-state accumulation of H2O2 in the culture medium of only the MPAO cell suspension (reaching a maximum concentration of about 0.18 mm with 2 mm Spd and 0.3 mm with 6 mm Spd after 0.5 h) and not in that of the wild-type one (Fig. 5). The H2O2 produced by the MPAO cell suspensions was quickly eliminated upon complete oxidation of the exogenously added Spd (Fig. 5), probably due to the presence of an efficient ROS-scavenging system (Fig. 5). Indeed, exogenously supplied H2O2 (0.35 mm) was immediately eliminated (Fig. 6). The wild-type and transgenic cell suspensions demonstrated a similar rate of H2O2 degradation (Fig. 6), which was not influenced by the presence of Spd (Fig. 6).
Figure 5.
Steady-state accumulation of H2O2 in cell suspensions from MPAO transgenic plants. Kinetics of H2O2 accumulation in the culture medium of cell suspensions obtained from wild-type (C) or MPAO transgenic (T) plants was determined spectrophotometrically after addition or not of 2 mm or 6 mm Spd. Data are from a single representative experiment, which was repeated in triplicate. The inset shows the remaining amount of Spd in the medium of MPAO cell suspensions at various time intervals after addition of 6 mm Spd.
Figure 6.
H2O2 scavenging in tobacco cell suspensions. Degradation of exogenously added H2O2 (0.35 mm) was determined in wild-type (W) and MPAO-expressing (T) cell suspensions in the absence (W; T) and in the presence (W + Spd) of 6 mm Spd. Data are from a single representative experiment, which was repeated in triplicate.
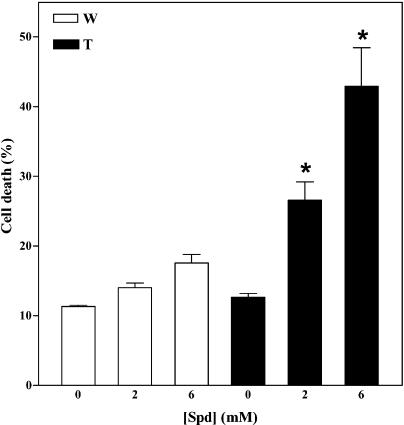
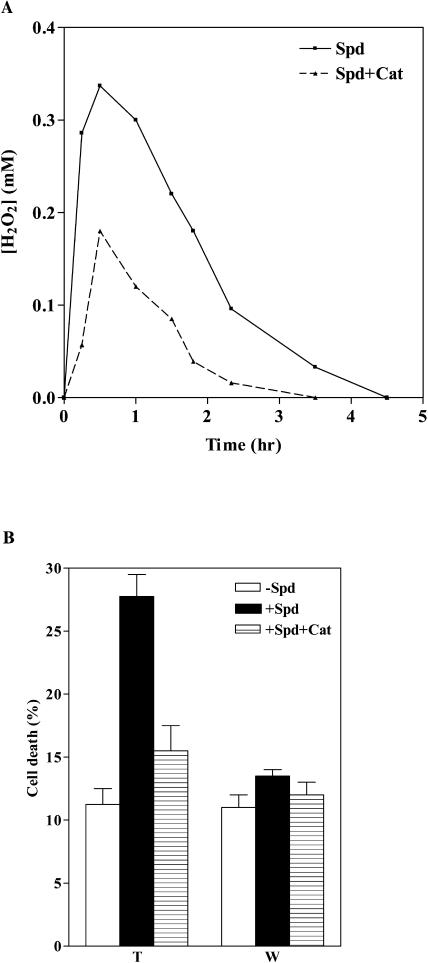
Addition of 2 mm or 6 mm Spd significantly enhanced cell death in the MPAO cell suspension, whereas very little cell death was observed in the wild-type cell suspension treated with the same amount of Spd or in the untreated MPAO cells (Fig. 7). To test whether the increased cell death observed in Spd-treated MPAO cells was due to a rise in H2O2 levels, cultures were preincubated with catalase before addition of Spd. Catalase treatment reduced both the steady-state levels of H2O2 generated by the oxidation of 6 mm Spd (Fig. 8A) and the cell-death response (Fig. 8B) in the MPAO cell suspension, indicating that the increased cell death observed was mainly H2O2 dependent.
Figure 7.
Effect of H2O2 on cell viability of tobacco cell suspensions. Cell death was measured by Evans blue staining 24 h after addition of various amounts of Spd (0, 2 mm, and 6 mm) in wild-type (W) and MPAO-expressing (T) cell suspensions. Values are mean ± se (n = 5). Asterisks indicate values significantly different from those of cells mock treated with H2O by one-way ANOVA test (P < 0.05).
Figure 8.
Induction of cell death by oxidative stress in tobacco cell suspensions. A, Kinetics of H2O2 accumulation induced by 6 mm Spd in MPAO-expressing cell suspensions. Cells were pretreated (Spd + Cat) or not (Spd) with catalase for 30 min before addition of Spd. Data are from a single representative experiment, which was repeated in triplicate. B, Cell death was determined by Evans blue staining in wild-type (W) and MPAO-expressing (T) cell suspensions 24 h after addition (+Spd) or not (−Spd) of 6 mm Spd. Cell death was also determined in cell suspensions pretreated with catalase for 30 min before addition of 6 mm Spd (+Spd+Cat). Each point represents mean value of three independent experiments.
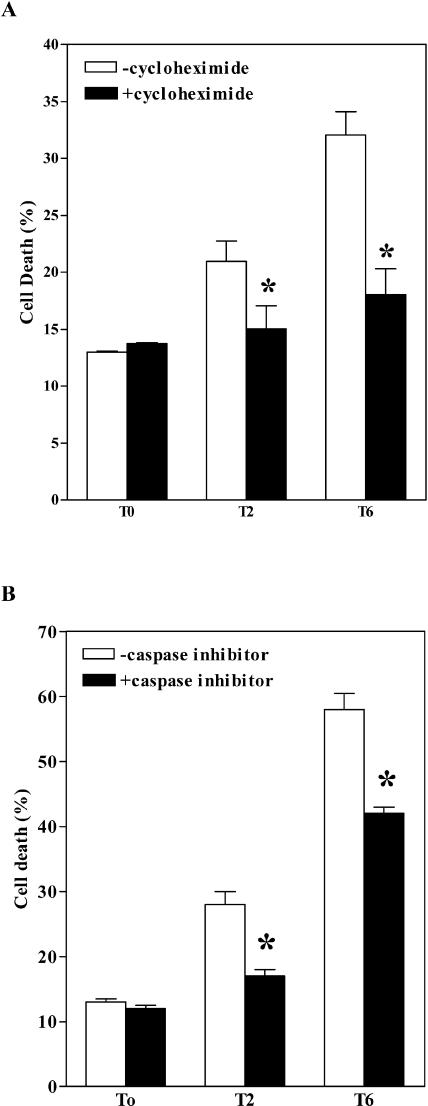
Recently, it has been demonstrated that H2O2-induced cell death is a programmed event requiring de novo transcription and translation (Desikan et al., 1998; Solomon et al., 1999; Clarke et al., 2000). To determine whether the Spd-induced oxidative stress in the MPAO-expressing tobacco cell suspensions requires gene expression, cells were preincubated with cycloheximide, an inhibitor of translation, for 30 min before the induction of oxidative stress. Cycloheximide reduced cell death induced by 2 mm or 6 mm Spd by 29% and 45%, respectively (Fig. 9A), suggesting that the cell death resulting from the oxidative stress in the MPAO-expressing tobacco cell suspensions is an active process that requires the expression of new proteins. Furthermore, to verify whether the oxidative stress generated by Spd in the MPAO cell suspension triggered a signaling mechanism similar to that described for H2O2- and NO-dependent cell death in soybean (Glycine max) and Arabidopsis cultures (Solomon et al., 1999; Clarke et al., 2000; Tiwari et al., 2002), such suspensions were preincubated with Z-YVAD, a competitive and irreversible inhibitor of caspase-1 and -4, for 30 min before addition of Spd. Addition of the caspase inhibitor reduced the cell death induced by 2 mm Spd by about 40% and that induced by 6 mm Spd by about 28% (Fig. 9B), suggesting that the oxidative stress induced an active PCD-like pathway. In these experiments, the levels of cell death induced by Spd appeared higher than in the previous ones, probably because of the presence of dimethylsulfoxide at a final concentration of 0.2% (v/v), used as a solvent for the caspase inhibitor.
Figure 9.
Induction of PCD by Spd-induced production of H2O2 in MPAO-expressing cell suspensions. Cell death was estimated by Evans blue staining in MPAO-expressing cell suspensions (T) 15 h after addition of 0, 2 mm, or 6 mm Spd. Where indicated, 40 μm cycloheximide (A) or 40 μm caspase inhibitor (B) was added to the culture medium 30 min prior to Spd administration. Values are mean ± se (n = 3). Asterisks indicate values significantly different from those of cells treated with the same amount of Spd and not with cycloheximide or caspase inhibitor P < 0.05 (by one-way ANOVA test).
DISCUSSION
There is increasing evidence that PAO and CuAO may be implicated in plant development and defense responses (Rea et al., 1998; Cowley and Walters, 2002; Cona et al., 2003; Yoda et al., 2003). Considering their extracellular localization, these H2O2-generating enzymes may act together with peroxidases to mediate protein cross-linking and lignin deposition in plant cell walls under mechanical or physiological stress. It also has been demonstrated that H2O2 produced by polyamine oxidation is correlated with oxidative stress and cell death during ontogenesis and in response to biotic and abiotic stress (Allan and Fluhr, 1997; Torrigiani et al., 1997; Møller and McPherson, 1998; Cowley and Walters, 2002; Rea et al., 2002; Yoda et al., 2003). In addition, CuAO and PAO may serve to modify polyamine levels and hence influence polyamine metabolism.
To gain a further insight into the physiological role of the two amine oxidases in plants and to verify the possibility of interfering with polyamine homeostasis, we obtained transgenic tobacco plants overexpressing MPAO or PCuAO. Although CuAO and PAO activity has recently been detected in wild-type plants of N. tabacum cv Samsun at very low amounts and after enzyme enrichment or using sensitive enzymatic assays (Biondi et al., 2001; Yoda et al., 2003), enzymes with similar molecular and catalytic properties to those of MPAO or PCuAO were not detectable under our experimental conditions in the wild-type tobacco plants used in this study (N. tabacum cv SR1). Thus, N. tabacum cv SR1 plants provide a suitable system for transgenic studies on the MPAO and PCuAO genes.
In this study, we show that the MPAO and PCuAO transgenic plants and MPAO cell suspensions produce high amounts of H2O2, compared to the untransformed ones, only in the presence of exogenously added substrate, leading to the conclusion that the two recombinant proteins are functionally expressed in the tobacco plants and that the limiting factor for their activity in the tobacco plants is the amount of polyamines available in the extracellular space. Polyamine transport to the extracellular space seems to be the limiting factor for H2O2 production by MPAO and PCuAO not only in tobacco plants but also in maize and pea plants (Fig. 3), indicating similar regulatory mechanisms of polyamine metabolism among the various plant species.
Despite the high expression level of the transgenes in the MPAO and PCuAO transgenic plants (1% of total soluble proteins for MPAO and 0.01% for PCuAO; Fig. 2), levels of free polyamines were only slightly reduced (Table II). This is probably due to a fine regulation of polyamine levels, which is achieved via a balance of their biosynthesis, degradation, uptake, transport, and conjugation. It may also be due to inaccessibility of MPAO and PCuAO in the transgenic plants by the bulk amount of polyamines owing to a different compartmentalization. Indeed, our results demonstrated a prevalently extracellular localization of the recombinant MPAO and PCuAO proteins in the transgenic tobacco plants, similar to the native enzymes in maize and pea plants, respectively (Federico and Angelini, 1991; Laurenzi et al., 2001). On the other hand, under our experimental conditions free polyamines could not be detected in the intercellular fluids of either wild-type or transgenic tobacco plants. Furthermore, vacuoles have been proposed as temporary storage sites of polyamines (Pistocchi et al., 1988). The question thus arises as to why maize and pea plants produce high amounts of amine oxidases in the apoplast in the absence of comparably high levels of enzyme substrate. It is possible that controlled polyamine transport to the apoplast may occur during certain physiological or stress conditions. Little is known about transport of diamines and polyamines around plants or about their distribution and their roles in the extracellular environment. In TMV-infected tobacco plants, it has been demonstrated that Put and Spm accumulate in the intercellular space during hypersensitive response (HR; Yamakawa et al., 1998; Yoda et al., 2003). Furthermore, enzyme activity of Orn decarboxylase and Arg decarboxylase increases during HR in tobacco plants infected with TMV, resulting in elevated concentrations of their products and their conjugates, mainly in necrotic regions (Torrigiani et al., 1997). In several cases, conjugated polyamines are also produced in response to abiotic injury and mineral nutrient deficiency. In their conjugated forms, polyamines are often associated with a variety of phenolic cell wall components, such as hydroxycinnamic acids. It is not known whether the conjugated polyamines in the plant cell wall have a structural role or serve as polyamine storage forms or whether they are biologically active molecules. Small amounts of pectin-associated polyamines are also present in the cell wall, which have been proposed to be part of an intrinsic signaling network of the extracellular matrix by modulating the transduction of the pectic signal (Messiaen and Van Cutsem, 1999).
Lignification levels in young MPAO- and PCuAO-expressing transgenic plants appeared quite similar to those of the untransformed plants, despite the high expression levels of the transgenes. This may reflect the lack of polyamine accumulation in the apoplast and suggests that polyamine catabolism is not involved in early stages of tobacco plant development under physiological conditions. However, this may not be true for all plants, as in the case of maize and pea. It is also possible that polyamine catabolism is specifically involved in plant development either during maturation and senescence or under stress conditions.
In this study, a small but significant increase in the ROS-scavenging capability of the MPAO and PCuAO transgenic plants has been demonstrated relative to the wild-type ones. In particular, higher APX levels have been demonstrated in both transgenic lines compared to the wild-type tobacco plants. These data suggest that a small amount of H2O2, not detectable under our experimental conditions, may be generated by transgene expression, inducing an increase in the ROS-scavenging capacity of the transgenic plants. However, this increase was not high enough to interfere with the high rate of H2O2 degradation in the MPAO cell suspensions when this ROS was exogenously added to the culture medium. In contrast to APX levels, significant variations in catalase activity between transgenic and wild-type plants were not observed. The different behavior of catalase and APX is in accordance with only a slight increase of H2O2 production in transformed plants. Indeed, APX, having a much higher affinity for H2O2 than catalase (Mittler, 2002), is much more sensitive even to low increase in H2O2 level. The different behavior of APX and catalase could also be a consequence of their different localization within cells. Indeed, catalase, which is localized within microbodies and mitochondria, may be less sensitive to H2O2 production in the plant cell wall by MPAO and PCuAO than APX, which is present in almost all cellular compartments, apoplastic space included. It is intriguing that although transgene expression levels in the PCuAO transgenic plants are lower than those in the MPAO plants, ASC content and ASC redox state were enhanced relative to the untransformed plants, only in the former and not in the latter. This rise in ASC content and ASC redox state is probably due to an increased capacity of the PCuAO transgenic plants to reduce DHA, the oxidized form of ASC. Indeed, DHAR activity is also increased in these transgenic plants but not in the MPAO ones. It is possible that Put levels in tobacco plant cell walls are higher than those of Spd or Spm, and this may produce stronger oxidative stress, triggering a cellular homeostatic rebalance of the ASC recycling capability and redox state.
There is evidence that ROS play key roles in the initiation of PCD in both animals and plants, and recent work suggested that H2O2 might determine the HR response in plants (Levine et al., 1994). It has been also demonstrated that H2O2 can induce an apoptosis-like cell-death program in suspension cultures of soybean and Arabidopsis (Desikan et al., 1998; Solomon et al., 1999; Tiwari et al., 2002). Here, we report that despite the presence of an efficient system of ROS-scavenging in the tobacco cell suspensions, the level of oxidative stress generated by exogenously supplied substrate in MPAO-expressing cell suspensions is sufficient to induce cell death, the latter being higher in the presence of higher concentrations of enzyme substrate. The levels of H2O2 accumulated in the MPAO-expressing cell suspensions (a maximum of about 0.3 mm in 1 h after addition of 6 mm Spd) was similar to that produced in Arabidopsis cell suspensions using Glc oxidase/Glc as an H2O2-generating system (Tiwari et al., 2002). Furthermore, the oxidative stress generated in the MPAO tobacco cell suspensions triggered a cell-death program requiring gene expression and caspase-like activity, similar to that observed in Arabidopsis and soybean cell suspensions (Desikan et al., 1998; Solomon et al., 1999; Tiwari et al., 2002). However, using the caspase inhibitor Z-YVAD, the reduction of cell death induced by 6 mm Spd appeared lower than that induced by 2 mm Spd. It is possible that the high oxidative stress induced by 6 mm Spd triggered cell death also via an uncontrolled necrotic pathway or via a caspase-independent pathway.
In conclusion, present data indicate that under physiological conditions the amount of freely available polyamines in the apoplast and cell wall is the limiting factor that regulates the catabolic pathway linked to polyamines. The fact that MPAO or PCuAO transgenic plants are normal both in their developmental pattern and metabolism and that an increase in cell wall polyamine content can be evidenced via H2O2 production renders them an excellent tool for the study of polyamine secretion and conjugation. In addition, they may be used to determine how and when these amine oxidases actually intervene both in cell wall development and in response to stress.
MATERIAL AND METHODS
Plant Material and Growth Conditions
Plants of tobacco (Nicotiana tabacum cv Petit Havana SR1), maize (Zea mays L. cv DK 300; Monsanto, Lodi, Italy), and pea (Pisum sativum) were used throughout. Plants and cell cultures were kept in a growth chamber with an irradiance of approximately 150 μE m−2 s−1, a mean temperature of 24°C, and a 16-h daylength.
Vector Construction and Plant Transformation
The cDNAs encoding for MPAO (Tavladoraki et al., 1998) and for PCuAO (Tipping and McPherson, 1995) were inserted in the pBI121 vector (Jefferson et al., 1987) between restriction sites SmaI and SacI, after excision of the gene encoding for β-glucuronidase, to form the MPAO-Ω-pBI and PCuAO-pBI constructs, respectively. In the MPAO-Ω-pBI construct, the Ω translational enhancer from TMV (Gallie, 1993) was also inserted downstream from 35S cauliflower mosaic virus promoter. Subsequently, the two constructs were inserted into Agrobacterium tumefaciens strain LBA4404 through electroporation and mobilized into tobacco plants via A. tumefaciens-mediated leaf-disc transformation (Horsch et al., 1985). Shoots were regenerated from leaf discs on Murashige and Skoog medium containing 3% (w/v) Suc, 1 mg L−1 indole-3-butyric acid, 1 mg L−1 6-benzilaminopurine, 100 mg L−1 kanamycin sulfate, 200 mg L−1 vancomycin, and 200 mg L−1 cefotaxim. Regenerated plants were transferred to rooting medium containing 1% (w/v) Suc, 100 mg L−1 kanamycin sulfate, 200 mg L−1 vancomycin, 200 mg L−1 cefotaxim, and 0.1 mg L−1 indole-3-butyric acid. Rooted plants (T0 generation) were either propagated in hormone-free medium containing 100 mg L−1 kanamycin sulfate or transferred to soil for seed preparation (T1 generation). Homozygous transgenic lines were selected by germination of a large amount of seeds obtained from individual plants of T1 generation on medium containing kanamycin.
Screening of Transgenic Plants by PCR Analysis
Genomic PCR amplification to detect MPAO and PCuAO cDNAs in transgenic plants was performed from alkali-treated leaf pieces obtained as described by Klimyuk et al. (1993). The PCR reaction was performed in a volume of 50 μL in the presence of 67 mm Tris-HCl, pH 8.8, 16 mm (NH4)2SO4, 0.01% (v/v) Tween 20, 0.5 μm gene-specific primers, 250 μm deoxynucleotide triphosphates, and 0.5 units of Taq DNA polymerase (PolyTaq; Polymed, Firenze, Italy). After an initial denaturation step for 5 min at 94°C, 35 amplification steps were carried out (each comprising denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min), followed by a 10 min extension at 72°C. The primers used for MPAO amplification were Pf (5′-GCCCATCGTCAACTCCACCCTCAAG-3′) and Pr (5′-GTAGCTCGTCGTCAGCCTGCTCGTC-3′), while for PCuAO amplification were Df (5′-GTACCTTATCAAGACCCTACAGAAG-3′) and Dr (5′-CGTATTTGTGGATAATCATCCTCTG-3′). Amplification fragments were analyzed on 1% (w/v) agarose gel.
Preparation of Intercellular Fluids and Crude Leaf Extracts
Leaves of tobacco plants were immersed in 10 mm sodium phosphate, pH 6.5 (for MPAO) or pH 7.0 (for PCuAO), and subjected to three consecutive rounds of vacuum for 2 min, followed by release of vacuum. Infiltrated leaves were gently dried and placed in a centrifuge tube on a grid separated from the tube bottom. Intercellular fluid was collected in the bottom of the tube after centrifugation for 15 min at 1,800g (fraction F1). Vacuum infiltration was repeated as below to obtain residual intercellular fluid (fraction F2). The rest of the leaves were homogenized in 0.2 m sodium phosphate, pH 6.5 (for MPAO) or pH 7.5 (for PCuAO), and the extract clarified by centrifugation for 10 min at 10,000g. The resulting supernatant is referred to as the intracellular extract (fraction I).
To prepare a crude protein extract, leaves were homogenized first with liquid nitrogen and then with 0.2 m sodium phosphate, pH 6.5 (for MPAO) or pH 7.0 (for PCuAO). The homogenate was centrifuged for 10 min at 10,000g, and the supernatant is referred to as the crude leaf extract (fraction T).
All fractions obtained (intercellular fluids, intracellular extracts, and crude extracts) were tested for extracellular peroxidase levels according to Smith and Barker (1988) and for the cytosolic marker Glc-6-phosphate dehydrogenase according to Simcox et al. (1977).
Determination of MPAO and PCuAO Enzyme Activity
MPAO and PCuAO enzymatic activities were determined from protein extracts by following the formation of a pink adduct (ɛ515 = 2.6 × 104 m−1 cm−1), resulting from the oxidation of 0.1 mm 4-aminoantipyrine and of 1.0 mm 3,5-dichloro-2-hydroxybenzenesulfonic acid (DCHBS) catalyzed by 0.08 mg/mL of horseradish peroxidase in 0.2 m sodium phosphate buffer, pH 6.5 (for MPAO) or pH 7.0 (for PCuAO), at 25°C (Smith and Barker, 1988). One unit of enzyme represents the amount of enzyme that catalyzes the oxidation of 1 μmol of substrate per min. Expression levels were calculated from enzyme activity in the plant protein extracts considering the specific activity of the purified enzymes (80 units mg−1 of protein). Quantification of total soluble proteins was performed using a protein assay kit (Bio-Rad, Hercules, CA) and bovine serum albumin as a standard.
Western-Blot Analysis
Western-blot analysis was performed according to Cona et al. (2003), utilizing rabbit anti-MPAO or anti-lentil CuAO polyclonal antibodies purified by affinity chromatography through a Sepharose 4B column (Amersham-Pharmacia Biotech, Uppsala) coupled to bromelain (Sigma, St. Louis).
Polyamine Analysis
Total free polyamine levels were determined in both whole tobacco leaves and intercellular spaces. For polyamine extraction from whole leaves, fresh leaf tissues were homogenized initially with liquid nitrogen and then with cold 5% (v/v) perchloric acid (200 mg fresh weight mL−1). Crude extracts were incubated at 4°C for 18 h and were clarified by centrifugation. The supernatant was used to analyze total free polyamines. For polyamine extraction from the intercellular fluids, 15 leaf discs (15 mm in diameter) were cut out, weighed, and washed with distilled water. Subsequently, the leaf discs were submerged in water in vacuo, and the intercellular fluid, recovered by centrifugation, was immediately mixed with perchloric acid to a final concentration of 5% (v/v).
Free polyamines were quantified after derivatization with dansyl chloride according to Smith and Davies (1985) with minor modifications. Dansylated polyamines were separated by HPLC (PU-980; Jasco, Tokyo) on a reverse-phase C18 column (Spherisorb S5 ODS2, 5 μm particle diameter, 4.6 × 250 mm) using an acetonitrile to water gradient (60% to 70% acetonitrile in 5.5 min, 70% to 80% in 1.5 min, 80% to 100% in 2 min, 100% for 2 min, 100% to 70% in 2 min, and 70% to 60% in 2 min at a flow rate of 1.5 mL min−1). Eluted peaks were detected by a spectrofluorometer (Jasco 821-FP, excitation 365 nm, emission 510 nm), recorded, and integrated by an attached computer using the JCL6000 software (Jones Chromatography, Hengoed, UK).
H2O2 Detection by KI/Starch Assay in Plant Tissue
H2O2 produced by tissues of transgenic plants was detected essentially as described by Olson and Varner (1993). Leaf discs, roots, and sections of stem were soaked in water and placed onto agar plates containing 50 mm KI and 4% (w/v) potato (Solanum tuberosum) starch. Samples were left at room temperature to allow color development. Color development was also examined after addition of 1 mm Spd or Put solutions on the plant tissue.
H2O2 Detection by 3,3-Diaminobenzidine Uptake Method in Plant Leaves
In situ H2O2 production was detected by an endogenous peroxidase-dependent staining procedure using DAB (Thordal-Christensen et al., 1997). Leaves were detached and placed in a solution of 1 mg mL−1 DAB, pH 3.8, for 18 h under light. Subsequently, the leaves were immersed in boiling 96% (v/v) ethanol for 10 min and then stored in 96% (v/v) ethanol. H2O2 production was visualized as a reddish-brown coloration. H2O2 production was also examined in the presence of 1 mm Spd or Put in the DAB solution.
Lignin Histochemical Analysis
Fresh transversal sections (200 μm) were obtained from the two youngest internodes of 4- to 5-week-old transgenic and wild-type tobacco plants. Lignin deposition was detected by the phloroglucinol/HCl method according to Rea et al. (1998) using a Zeiss Axiophot optical microscope (Jena, Germany).
Ascorbate System and H2O2-Scavenging Enzymes
Leaves were collected from three different 30-d-old wild-type or transformed plants. Attention was paid in order to select leaves in the same stage of development (about 3–4 cm in length, at the same internode level). For determination of ASC content and redox state, leaves were homogenized with 4 volumes of cold 5% (w/v) metaphosphoric acid at 4°C in a mortar. The homogenate was centrifuged at 20,000g for 15 min at 4°C, and the supernatant was collected for analysis of ASC according to Zhang and Kirkham (1996).
For the assays of ASC redox enzymes and catalase, leaves were ground in liquid nitrogen and homogenized at 4°C in 2 volumes (w/v) of extraction buffer containing 50 mm Tris-HCl, pH 7.8, 0.05% (w/v) Cys, and 0.1% (w/v) bovine serum albumin. The homogenate was centrifuged at 20,000g for 15 min. The supernatant was used for enzymatic assays. Activity of l-ASC:hydrogen peroxide oxidoreductase (APX; EC 1.11.1.11) and of glutathione:dehydroascorbate oxidoreductase (DHA reductase; EC 1.8.5.1) was determined according to de Pinto et al. (2000). Catalase activity was evaluated according to De Gara et al. (2003).
Cell Culture Conditions and Treatments
Young leaves, collected from 3- to 4-week-old in vitro grown wild-type and homozygous MPAO transgenic tobacco plants, were induced to form callus on an agar-solidified Murashige and Skoog medium supplemented with 3% (w/v) Suc, 1 mg L−1 naphthaleneacetic acid, and 0.2 mg L−1 kinetin, pH 5.7. Callus were grown in darkness at 26°C and subcultured on fresh medium every 4 weeks. After three subcultures, fast-growing callus were placed into liquid medium of the same composition. Suspensions were grown for 15 d, and large cell aggregates were eliminated by filtering through a 500-μm mesh filter. Cells settling on a 125-μm mesh filter were resuspended in fresh medium at a 1:10 dilution and grown in the same conditions giving rise to a fast-growing suspension culture. Subculturing was performed every 2 weeks, and cell suspensions were used 4 d after subculture. Before each experiment, the cells were washed with fresh culture medium and resuspended at a 1:4 dilution. For inhibitor experiments, aliquots of cells were pretreated with catalase (2,000 units mL–1), with Z-YVAD (40 μm), or with cycloheximide (40 μm) for 30 min prior to Spd addition. Controls were mock treated with sterile distilled water or dimethylsulfoxide, as appropriate.
To determine H2O2 steady-state levels in cell suspensions, 40 μL of culture medium were added to a cuvette containing 760 μL of 0.08 mg mL−1 horseradish peroxidase in 0.2 m sodium phosphate buffer, pH 9.0, 100 μL of 1 mm 4-aminoantipyrine, and 100 μL of 10 mm DCHBS. Formation of the colored adduct was measured at 515 nm, after incubation for 5 min at room temperature.
Cell death was quantified by Evans blue staining, as described by Levine et al. (1994). Briefly, samples were incubated for 15 min with 0.05% (w/v) Evans blue and then washed extensively to remove unbound dye. Cells treated with Spd were washed with culture medium before staining to avoid dye precipitation by polyamines. Dye bound to dead cells was solubilized in 50% (v/v) methanol and 1% (w/v) SDS at 60°C for 30 min, and quantified by absorbance at 600 nm. For 100% cell death, the culture was heated at 100°C for 5 min.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AJ002204 (MPAO) and L39931 (PCuAO).
Acknowledgments
We thank Prof. M.J. McPherson (Centre for Plant Sciences, University of Leeds, Leeds, UK) for the gift of PCuAO cDNA clone, Dr. C. Faso (Biology Department, Università degli Studi Roma Tre) for critical reading of the manuscript, and Dr. D. Pashkoulov (Floramiata SpA) for useful discussions and growth of the plants.
This work was supported by the Italian Ministry for University and Scientific Research (project PRIN to R.A. and L.D.G.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.036764.
References
- Allan AC, Fluhr R (1997) Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9: 1559–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi S, Scaramagli S, Capitani F, Altamura MM, Torrigiani P (2001) Methyl jasmonate upregulates biosynthetic gene expression, oxidation and conjugation of polyamines, and inhibits shoot formation in tobacco thin layers. J Exp Bot 52: 231–242 [PubMed] [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin–Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140: 103–125 [Google Scholar]
- Burtin D, Michael T (1997) Over-expression of arginine decarboxylase in transgenic plants. Biochem J 325: 331–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell T, Bassie L, Topsom L, Hitchin E, Christou P (2000) Simultaneous reduction of the activity of the two related enzymes involved in early steps of the polyamine biosynthetic pathway by a single antisense cDNA in transgenic rice. Mol Gen Genet 264: 470–476 [DOI] [PubMed] [Google Scholar]
- Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ (2000) NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J 24: 667–677 [DOI] [PubMed] [Google Scholar]
- Cohen SS (1998) A Guide to the Polyamines. Oxford University Press, Oxford
- Cona A, Cenci F, Cervelli M, Federico R, Mariottini P, Moreno S, Angelini R (2003) Polyamine oxidase, a hydrogen peroxide-producing enzyme, is up-regulated by light and down-regulated by auxin in the outer tissues of the maize mesocotyl. Plant Physiol 131: 803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley T, Walters DR (2002) Polyamine metabolism in barley reacting hypersensitively to the powdery mildew fungus Blumeria graminis f. sp. hordei. Plant Cell Environ 25: 461–468 [Google Scholar]
- De Gara L, de Pinto MC, Moliterni VM, D'Egidio MG (2003) Redox regulation and storage processes during maturation in kernels of Triticum durum. J Exp Bot 54: 249–258 [DOI] [PubMed] [Google Scholar]
- DeScenzo RA, Minocha SC (1993) Modulation of cellular polyamines in tobacco by transfer and expression of mouse ornithine decarboxylase cDNA. Plant Mol Biol 22: 113–127 [DOI] [PubMed] [Google Scholar]
- Desikan R, Reynolds A, Hancock JT, Neill SJ (1998) Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem J 330: 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pinto MC, Tommasi F, De Gara L (2000) Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tobacco Bright Yellow 2. Plant Physiol Biochem 38: 541–550 [Google Scholar]
- Federico R, Angelini R (1991) Polyamine catabolism in plants. In RD Slocum, HE Flores, eds, Biochemistry and Physiology of Polyamines in Plants. CRC Press, Boca Raton, FL, pp 41–56
- Flores HE, Filner P (1985) Polyamine catabolism in higher plants: characterization of pyrroline dehydrogenase. Plant Growth Regul 3: 277–291 [Google Scholar]
- Gallie DR (1993) Posttranscriptional regulation of gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 44: 77–105 [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 228: 1229–1231 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimyuk V, Carroll BJ, Thomas CM, Jones JDG (1993) Alkali treatment for rapid preparation of plant material for reliable PCR analysis. Plant J 3: 493–494 [DOI] [PubMed] [Google Scholar]
- Kumar A, Altabella T, Taylor MA, Tiburcio AF (1997) Recent advances in polyamine research. Trends Plant Sci 2: 124–130 [Google Scholar]
- Kumar A, Taylor MA, Mad-Arif SA, Davies H (1996) Potato plants expressing antisense and sense S-adenosylmethionine decarboxylase (SAMDC) transgenes show altered levels of polyamine and ethylene: antisense plants display abnormal phenotypes. Plant J 9: 147–158 [Google Scholar]
- Laurenzi M, Tipping AJ, Marcus SE, Knox JP, Federico R, Angelini R, McPherson MJ (2001) Analysis of the distribution of copper amine oxidase in cell walls of legume seedlings. Planta 214: 37–45 [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Liu L, Eriksson KEL, Dean JFD (1995) Localization of hydrogen peroxide production in Pisum sativum L. using epi-polarization microscopy to follow cerium perhydroxide deposition. Plant Physiol 107: 501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh CA, Slater LA, Walters DR, Robins DJ (2001) Synthesis of six novel N,N-dialkyl derivatives of spermidine and effects on growth of the fungal plant pathogen Pyrenophora avenae. FEMS Microbiol Lett 202: 221–225 [DOI] [PubMed] [Google Scholar]
- Malmberg RL, Watson MB, Galloway GL, Yu W (1998) Molecular genetic analysis of plant polyamines. Crit Rev Plant Sci 17: 199–224 [Google Scholar]
- Martin-Tanguy J (1997) Conjugated polyamines and reproductive development: biochemical, molecular and physiological approaches. Physiol Plant 100: 675–688 [Google Scholar]
- Mehta RA, Cassol T, Li N, Ali N, Handa AK, Mattoo AK (2002) Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat Biotechnol 20: 613–618 [DOI] [PubMed] [Google Scholar]
- Messiaen J, Van Cutsem P (1999) Polyamines and pectins. II. Modulation of pectic-signal transduction. Planta 208: 247–256 [DOI] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Møller SG, McPherson MJ (1998) Developmental expression and biochemical analysis of the Arabidopsis atao1 gene encoding an H2O2-generating diamine oxidase. Plant J 13: 781–791 [DOI] [PubMed] [Google Scholar]
- Noury M, Bassie L, Lepri O, Kurek I, Christou P, Capell T (2000) A transgenic rice cell lineage expressing the oat arginine decarboxylase (adc) cDNA constitutively accumulates putrescine in callus and seeds but not in vegetative tissues. Plant Mol Biol 43: 537–544 [DOI] [PubMed] [Google Scholar]
- Olson PD, Varner JE (1993) Hydrogen peroxide and lignification. Plant J 4: 887–892 [Google Scholar]
- Perez-Amador MA, Leon J, Green PJ, Carbonell J (2002) Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol 130: 1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GC, Kuehn GD (1991) Uncommon polyamines in plants and other organisms. In RD Slocum, HE Flores, eds, Biochemistry and Physiology of the Polyamines in Plants. CRC Press, Boca Raton, FL, pp 121–136
- Pistocchi R, Keller F, Bagni N, Matile P (1988) Transport and subcellular localization of polyamines in carrot protoplasts and vacuoles. Plant Physiol 87: 514–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafart-Pedros A, MacLeod MR, Ross HA, McRae D, Tiburcio AF, Davies HV, Taylor MA (1999) Manipulation of S-adenosylmethionine decarboxylase activity in potato tubers. Planta 209: 153–160 [DOI] [PubMed] [Google Scholar]
- Rea G, Laurenzi M, Tranquilli E, D'Ovidio R, Federico R, Angelini R (1998) Developmentally and wound regulated expression of the gene encoding a cell wall copper amino oxidase in chickpea seedlings. FEBS Lett 437: 177–182 [DOI] [PubMed] [Google Scholar]
- Rea G, Metoui O, Infantino A, Federico R, Angelini R (2002) Copper amine oxidase exoression in defense responses to wounding and Ascochyta rabiei invasion. Plant Physiol 128: 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šebela M, Radovà A, Angelini R, Tavladoraki P, Frébort I, Pêc P (2001) FAD-containing polyamine oxidases: a timely challenge for researchers in biochemistry and physiology of plants. Plant Sci 160: 197–207 [DOI] [PubMed] [Google Scholar]
- Simcox PD, Reid EE, Canvin DT, Dennis DT (1977) Enzymes of the glycolytic and pentose phosphate pathways in proplastids from the developing endosperm of Ricinus communis L. Plant Physiol 59: 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum RD, Furey MJ (1991) Electron-microscopic cytochemical localization of diamine and polyamine oxidases in pea and maize tissues. Planta 183: 443–450 [DOI] [PubMed] [Google Scholar]
- Smith TA, Barker JHA (1988) The di-and polyamine oxidase in plants. In V Zappia, AE Pegg, eds, Progress in Polyamine Research. Novel Biochemical, Pharmacological and Clinical aspects. Plenum Press, New York, pp 573–587
- Smith TA, Davies PJ (1985) Separation and quantitation of polyamines in plant tissue by high performance liquid chromatography of their dansyl derivatives. Plant Physiol 78: 89–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A (1999) The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11: 431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavladoraki P, Schininà ME, Cecconi F, Di Agostino S, Manera F, Rea G, Federico R, Mariottini P, Angelini R (1998) Maize polyamine oxidase primary structure from protein and cDNA sequencing. FEBS Lett 426: 62–66 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Thu-Hang P, Bassie L, Safwat G, Trung-Nghia P, Christou P, Capell T (2002) Expression of a heterologous S-adenosylmethionine decarboxylase cDNA in plants demonstrates that changes in S-adenosyl-L-methionine decarboxylase activity determine levels of the higher polyamines Spermidine and Spermine. Plant Physiol 129: 1744–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio AF, Altabella T, Borrell A, Masgrau C (1997) Polyamine metabolism and its regulation. Physiol Plant 100: 664–674 [Google Scholar]
- Tipping AJ, McPherson MJ (1995) Cloning and molecular analysis of the pea seeding copper amine oxidase. J Biol Chem 270: 16939–16946 [DOI] [PubMed] [Google Scholar]
- Tiwari BS, Belenghi B, Levine A (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128: 1271–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrigiani P, Rabiti AL, Bortolotti C, Betti L, Marani F, Canova A, Bagni N (1997) Polyamine synthesis and accumulation in the hypersensitive response to TMV in Nicotiana tabacum. New Phytol 135: 467–473 [Google Scholar]
- Walden R, Cordeiro A, Tiburcio AF (1997) Polyamines: small molecules triggering pathways in plant growth and development. Plant Physiol 113: 1009–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace HM, Fraser AV, Hughes A (2003) A perspective of polyamine metabolism. Biochem J 376: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters D (2003) Resistance to plant pathogens: possible roles for free polyamines and polyamine catabolism. New Phytol 159: 109–115 [DOI] [PubMed] [Google Scholar]
- Watson MB, Emory KK, Piatak RM, Malmberg RL (1998) Arginine decarboxylase (polyamine synthesis) mutants of Arabidopsis thaliana with altered root growth. Plant J 13: 231–239 [DOI] [PubMed] [Google Scholar]
- Wisniewski JP, Brewin NJ (2000) Construction of transgenic pea lines with modified expression of diamine oxidase and modified nodulation responses with exogenous putrescine. Mol Plant Microbe Interact 13: 922–928 [DOI] [PubMed] [Google Scholar]
- Wisniewski JP, Rathbun EA, Knox JP, Brewin NJ (2000) Involvement of diamine oxidase and peroxidase in insolubilization of the extracellular matrix: implications for pea nodule initiation by Rhizobium leguminasarum. Mol Plant Microbe Interact 13: 413–420 [DOI] [PubMed] [Google Scholar]
- Yamakawa H, Kamada H, Satoh M, Ohashi Y (1998) Spermine is a salicylate-independent endogenous inducer for both tobacco acidic pathogenesis-related proteins and resistance against tobacco mosaic virus infection. Plant Physiol 118: 1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda H, Yamaguchi Y, Sano H (2003) Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation in tobacco plants. Plant Physiol 132: 1973–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JX, Kirkham MB (1996) Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol 132: 361–373 [DOI] [PubMed] [Google Scholar]