Abstract
Sucrose synthase (SUS) is phosphorylated on a major, amino-terminal site located at Ser-15 (S15) in the maize (Zea mays) SUS1 protein. Site- and phospho-specific antibodies against a phosphorylated S15 (pS15) peptide allowed direct analysis of S15 phosphorylation in relation to membrane association. Immunoblots of the maize leaf elongation zone, divided into 4-cm segments, demonstrated that the abundance of soluble (s-SUS) and membrane (m-SUS) SUS protein showed distinct positional profiles. The content of m-SUS was maximal in the 4- to 8-cm segment where it represented 9% of total SUS and occurred as a peripheral membrane protein. In contrast, s-SUS was highest in the 12- to 16-cm segment. Relative to s-SUS, m-SUS was hypophosphorylated at S15 in the basal 4 cm but hyperphosphorylated in apical segments. Differing capabilities of the anti-pS15 and anti-S15 peptide antibodies to immunoprecipitate SUS suggested that phosphorylation of S15, or exposure of unphosphorylated SUS to slightly acidic pH, altered the structure of the amino terminus. These structural changes were generally coincident with the increased sucrose cleavage activity that occurs at pH values below 7.5. In vitro S15 phosphorylation of the S170A SUS protein by a maize calcium-dependent protein kinase (CDPK) significantly increased sucrose cleavage activity at low pH. Collectively, the results suggest that (1) SUS membrane binding is controlled in vivo; (2) relative pS15 content of m-SUS depends on the developmental state of the organ; and (3) phosphorylation of S15 affects amino-terminal conformation in a way that may stimulate the catalytic activity of SUS and influence membrane association.
In maize (Zea mays), the enzyme sucrose synthase (SUS) is usually a homo- or heterotetrameric protein complex (Su and Preiss, 1978; Chourey et al., 1986) composed of subunits encoded by the sh1, sus1, and sus3 genes (Carlson et al., 2002). The SUS protein is expressed in many organs including maize endosperm, roots, stems, and leaves (Chourey et al., 1986; Nguyen-Quoc et al., 1990; Carlson et al., 2002) and catalyzes a readily reversible reaction that interconverts Suc and UDP into UDP-Glc and Fru (Tsai, 1974). The pH optimum for the Suc cleavage activity of SUS occurs at pH 6.0 to 6.5, while the synthesis of Suc by SUS is maximal at alkaline pH (8.0–8.8; Tsai, 1974; Su and Preiss, 1978). Therefore, the Suc cleavage capability of SUS increases substantially as cytosolic pH is lowered over the physiological range of 7.6 to 6.8. Such decreases in intracellular pH are known to occur, for example, during anoxia (Gout et al., 2001). The activity of SUS increases under hypoxic and/or anoxic conditions, and tolerance to these stresses is dependent upon SUS activity (Ricard et al., 1998; Zeng et al., 1998; Subbaiah and Sachs, 2001). SUS is particularly enriched in heterotrophic organs, and the importance of its Suc cleavage activity is evidenced by SUS mutants that show reductions in cellulose, callose, and starch formation (Chourey et al., 1998; Sturm and Tang, 1999; Subbaiah and Sachs, 2001).
The soluble SUS (s-SUS) enzyme has traditionally been studied, although SUS may also occur in association with the actin cytoskeleton (Winter et al., 1998; Azama et al., 2003), plasma membrane (Amor et al., 1995; Carlson and Chourey, 1996; Winter et al., 1997; Sturm et al., 1999; Zhang et al., 1999; Haigler et al., 2001; Komina et al., 2002), Golgi (Buckeridge et al., 1999), symbiosome membrane (Zhang et al., 1999; Wienkoop and Saalbach, 2003), and vacuole (Etxeberria and Gonzalez, 2003). The plasma membrane-associated form is postulated to channel UDP-Glc derived from Suc cleavage toward the synthesis of cellulose and callose (Amor et al., 1995; Winter and Huber, 2000; Haigler et al., 2001). The affinity of SUS for membranes has been described as “tight,” requiring detergents or harsh chaotropes to dissociate it (Amor et al., 1995; Carlson and Chourey, 1996; Zhang et al., 1999; Komina et al., 2002). However, while generally accepted to be a non-trans-membrane protein, a clear demonstration that membrane SUS (m-SUS) is a peripheral membrane protein has not been reported.
SUS is a phosphoprotein in maize leaves (Huber et al., 1996), roots (Subbaiah and Sachs, 2001), stems (Winter et al., 1997), and suspension cultured cells (Shaw et al., 1994). Phosphorylation of SUS by calcium-dependent protein kinases (CDPKs) is known to occur on two conserved sites, located at Ser-15 (S15; Huber et al., 1996) and Ser-170 (S170; Hardin et al., 2003) in the SUS1 protein expressed in elongating maize leaves. The S170 phosphorylation site is minor, relative to S15, but has been implicated as part of the mechanism regulating proteolytic turnover of SUS (Hardin et al., 2003). The major SUS phosphorylation site targeted by CDPKs occurs at S15, and this modification has been implicated in regulating enzyme activity and/or localization (Huber et al., 1996; Winter et al., 1997; Winter and Huber, 2000). Changes in the intracellular distribution of SUS attributed to S15 phosphorylation and monitored by 32P-labeling have been noted during the exposure of maize root tips to anaerobic conditions (Subbaiah and Sachs, 2001) and in graviresponding maize pulvini (Winter et al., 1997). These results suggested that phosphorylation promotes a soluble phase localization of SUS (Winter et al., 1997; Winter and Huber, 2000) and is consistent with the decreased surface hydrophobicity that occurs in response to phosphorylation (Winter et al., 1997; Zhang et al., 1999). The m-SUS protein in mature soybean (Glycine max) nodules was also found to be specifically hypophosphorylated on S11 (the residue homologous to S15 in maize SUS1) relative to the soluble form (Komina et al., 2002), although no difference in the level of 32P-labeling of SUS was noted in relation to intracellular localization in cotton (Gossypium hirsutum) fibers (Haigler et al., 2001).
Phosphorylation of S15 has also been implicated in altering the kinetic properties of SUS. Several studies have noted that the Suc cleavage, but not synthetic, activity of SUS is specifically affected by phosphorylation, often as a result of an increased affinity for Suc and/or UDP (Huber et al., 1996; Nakai et al., 1998, 1999; Anguenot et al., 1999; Tanase et al., 2002). However, effects of phosphorylation on enzymatic activity have not been universally observed or the detected changes were minor and therefore interpreted as inconsequential (Winter et al., 1997; Zhang et al., 1999; Chikano et al., 2001).
Previous studies addressing the role(s) of SUS phosphorylation have been disadvantaged by several inherent experimental limitations. First, the partially purified native SUS enzyme possesses a variable stoichiometry of phosphorylation at S15 (or its homolog) that may be difficult to assess and then manipulate in vitro (Huber et al., 1996; Winter et al., 1997; Anguenot et al., 1999; Zhang et al., 1999). Second, the use of 32P-labeling in vivo as a means to assess phosphorylation status (Huber et al., 1996; Anguenot et al., 1999; Haigler et al., 2001; Subbaiah and Sachs, 2001) may be biased by the differential incorporation and/or turnover rates that are likely to occur at distinct intracellular locations. Therefore, in vivo 32P-labeling may be different for s-SUS versus m-SUS. Third, many of the previous studies describing the membrane localization of SUS and its affinity for membranes were potentially influenced by the use of noninverted membrane vesicles (Carlson and Chourey, 1996; Zhang et al., 1999; Subbaiah and Sachs, 2001; Komina et al., 2002) that possess a cytoplasmic side-in orientation and likely contain entrapped soluble enzymes (Johansson et al., 1995). This latter possibility has been excluded in some cases (Zhang et al., 1999; Komina et al., 2002). Finally, the recent verification that SUS1 has at least two CDPK phosphorylation sites residing at S15 and S170 demonstrates that 32P-incorporation is not sufficient to specifically monitor S15 phosphorylation, especially in vitro. Phosphorylation state-specific antibodies against an S15 homologous residue have been utilized to partially overcome this limitation (Komina et al., 2002), although all previous studies utilizing in vitro kinase labeling of recombinant wild-type SUS proteins to assess effects of S15 phosphorylation (Nakai et al., 1998; Zhang et al., 1999) would have benefited from a site-directed mutant at the S170 site (e.g. the S170A mutant protein). Consequently, the recognition of these insufficiencies necessitates a reevaluation of the role(s) of S15 phosphorylation utilizing approaches that circumvent these previous limitations.
The major objective of this study was to discern the influence of S15 phosphorylation on the SUS protein, focusing on effects to the structural and functional properties of the enzyme as well as to its location within the cell. The current study implements approaches divergent from prior work in the use of phosphorylation-specific antibodies to pS15-SUS and an S170 site mutant, which jointly enabled the specific assessment of the effects of S15 phosphorylation on SUS. Unique conclusions related to the role of pS15 in membrane association and enzyme activity were also made possible by expanding the developmental context and pH range, respectively, beyond that investigated in previous studies. The results obtained demonstrate that S15 phosphorylation alters the structure of the amino terminus in a manner similar to that invoked by exposure of unphosphorylated SUS to low pH, and that these structural changes may have an impact on both the membrane association and enzymatic activity of SUS.
RESULTS
Both s-SUS and m-SUS Are Phosphorylated at the S15 Site in Elongating Maize Leaves
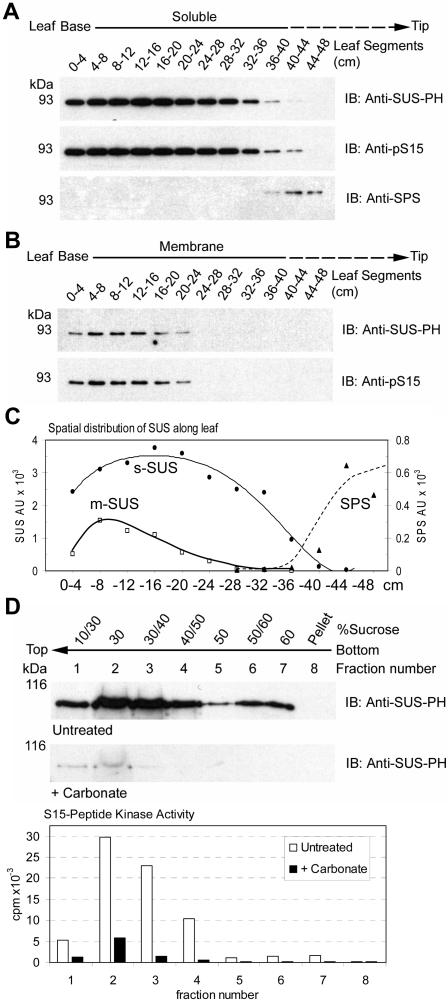
Protein extracts prepared from sequential 4-cm segments, covering the basal 48 cm of the maize leaf, were separated into soluble and membrane fractions under conditions that preserved the phosphorylation status of proteins and reduced soluble protein contamination in microsomes by vesicle entrapment. When immunoblots containing an equal amount of total protein per lane were probed with an antibody that detects SUS protein independent of its phosphorylation state (i.e. anti-SUS-PH), SUS was readily detected as both a soluble and membrane-associated protein (Fig. 1, A and B). When probed with the site- and phosphorylation-specific anti-pS15 antibodies, it was apparent that both s-SUS and m-SUS were phosphorylated at the S15 site (Fig. 1, A and B). Verification that anti-pS15 accurately and specifically detects S15 phosphorylation on SUS has been demonstrated (Hardin et al., 2003). Moreover, anti-pS15 reactivity in both the soluble and membrane fractions was completely blocked by preincubation with the pS15 peptide but not by the S15 peptide (data not shown). Densitometry of these blots (Fig. 1C) demonstrated that the amount of SUS found in the membrane fraction was highest in the 4- to 8-cm segment of the leaf and represented approximately 9% of total cellular SUS protein within this region. In contrast, s-SUS was most abundant within the 12- to 16-cm segment and represented 94% of total cellular SUS protein in this region. SUS protein levels in both the soluble and membrane phases were enriched in heterotrophic regions of the leaf and dramatically decreased during the transition to autotrophic growth, which was marked by accumulation of sucrose phosphate synthase (SPS) protein (Fig. 1, A and C).
Figure 1.
The distribution of SUS between the soluble and membrane phases is developmentally regulated in maize leaves. A, Equal amounts of total soluble protein extracts (1 μg) isolated from sequential 4-cm segments of elongating maize leaves were probed on immunoblots (IB) with the antibodies listed on the right of each panel. The positions of PAGE molecular mass markers are shown in kilodaltons on the left of each panel. B, Equal amounts of total membrane phase protein extracts (1 μg) isolated from sequential 4-cm segments of maize leaves were probed on immunoblots as in (A). C, Densitometry of immunoblots in A and B showing the distribution of anti-SUS-PH and anti-SPS antibody reactive proteins along the leaf. Arbitrary units (AU) are shown. D, Membrane preparations from maize leaves were untreated or treated with carbonate and subjected to flotation on discontinuous Suc gradients. Immunoblots (IB) were performed on the subsequent fractions with the antibodies listed to the right of each panel. S15-peptide kinase activities (cpm × 10−3) within the untreated (white bars) and carbonate-treated (black bars) fractions is also shown.
To clarify the nature of the association of SUS with membranes, microsomes were prepared from the basal 8 cm of the maize leaf and treated with buffer or sodium carbonate. The microsomes were then fractionated by flotation to the top of a Suc gradient by ultracentrifugation as a rigorous demonstration of membrane association that does not rely on sedimentation. Immunoblot analyses of fractions from the untreated sample (Fig. 1D) demonstrated that SUS was indeed membrane-associated as the majority of m-SUS floated out of the 60% Suc layer, were it was initially placed, to lower-density Suc fractions at the top of the gradient. As expected, soluble phase extracts containing s-SUS did not migrate from the bottom 60% Suc layer during centrifugation (data not shown). Immunoblot analyses of Suc gradient fractions containing membranes treated with carbonate (Fig. 1D) demonstrated that m-SUS was nearly completely dissociated from these cytoplasmic side-out vesicles by a treatment diagnostic for peripherally associated membrane proteins (Fujiki et al., 1982). As a control, we monitored membrane-associated CDPK activity using a synthetic peptide based on the S15 site. As expected (Ohto and Nakamura, 1995), carbonate treatment also released the majority of the calcium-dependent peptide kinase activity (Fig. 1D). It should be noted that the yield (data not shown) and flotation behavior of these membranes was not altered by carbonate treatment as evidenced by the residual m-SUS protein and kinase activity found in fractions 1 and 2 (Fig. 1D). The remainder of the m-SUS protein and CDPK activity, detected in the untreated samples, was removed with the soluble phase carbonate buffer wash and thus does not appear in the recovered membrane phase used for flotation.
Positional Profiles for S15 Phosphorylation and Intracellular SUS Localization within the Leaf Elongation Zone
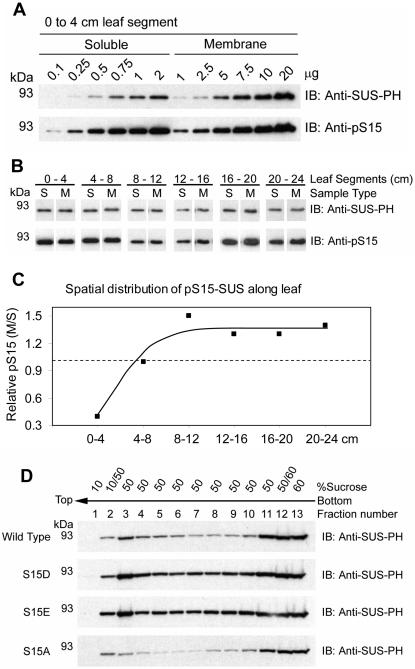
In order to assess the contribution that S15 phosphorylation may have on the membrane localization of SUS, increasing amounts of soluble and membrane extracts from the basal 4-cm segment of the maize leaf were probed on immunoblots for the corresponding amounts of total SUS (anti-SUS-PH) and pS15-SUS (anti-pS15) proteins (Fig. 2A). This analysis was repeated on extracts prepared from 4-cm segments covering the region between 4 and 24 cm. Representative portions of these latter immunoblots, determined to contain roughly equivalent amounts of total SUS (anti-SUS-PH) in both the soluble and membrane fractions, are presented in Figure 2B. It is readily apparent from the corresponding signals generated by the anti-pS15 antibodies that the relative phosphorylation state of S15 in m-SUS varied among these segments.
Figure 2.
Relative phosphorylation of S15 is variable along elongating maize leaves. A, Increasing amounts of total soluble (0.1–2 μg) and membrane phase (1–20 μg) protein extracts isolated from the basal 0- to 4-cm segment of maize leaves were probed on immunoblots (IB) with the antibodies listed on the right of each panel. The positions of PAGE molecular mass markers are shown in kilodaltons on the left of each panel. B, Representative data of single load comparisons extracted from 12 immunoblots performed as in A that included extracts isolated from sequential 4-cm segments of maize leaves covering the region from 0 to 24 cm. C, Relative pS15 content of m-SUS compared to s-SUS in different regions of the elongation zone. Densitometry of immunoblots, such as the dilution series shown in A, was used to determine the slopes of total protein versus antibody signal (AU). These values were used to determine the relative phosphorylation of S15 (per unit SUS protein) in the membrane and soluble phase for each 4-cm segment over the basal 24 cm of the leaf and are depicted as a ratio. Values greater than 1.0 (dashed line) indicate hyperphosphorylation of S15 in m-SUS relative to s-SUS while values less than 1.0 indicate hypophosphorylation of S15. D, Membrane preparations recovered from the SUS recombinants listed to the left of each panel were subjected to flotation on discontinuous Suc gradients. Immunoblots (IB) were performed on the subsequent fractions with the antibodies listed to the right of each panel.
To obtain quantitative differences in pS15-SUS in relation to intracellular localization, densitometry of immunoblots containing increasing amounts of soluble and membrane extracts (such as the one shown in Fig. 2A) was used to derive the empirical relationship between immunoblot signal intensity (AU) for both antibodies with increasing total protein (μg) in the soluble and membrane preparations (data not shown). The linear equations (mean R2 = 0.92 ± 0.07) derived from these graphs were used to generate ratios of the slope of pS15-SUS to total SUS in the soluble phase and in the membrane phase. Comparisons of these ratios allowed us to generate a numerical expression for the relative phosphorylation state of S15 in relation to intracellular localization and spatial distribution within the developing maize leaf (Fig. 2C). This analysis revealed that m-SUS was hypophosphorylated (approximately 40%) on S15 relative to s-SUS within the most basal segment (0–4 cm), and hyperphosphorylated (approximately 125%) in the region from 8 to 24 cm (Fig. 2C). These ratios do not reveal absolute S15 phosphorylation stoichiometry but are valuable in the comparative evaluation of different samples for relative phosphorylation of S15. An equivalent distribution of pS15-SUS between the soluble and membrane phases was observed within the 4- to 8-cm segment (Fig. 2C), which was the region that contained the highest amount of m-SUS protein (Fig. 1C). Decreases in m-SUS abundance were associated with both hypo- and hyperphosphorylated states (Figs. 1C and 2C). Therefore, phosphorylation of S15 may influence the steady-state intracellular distribution of SUS differently, dependent upon the particular region of the maize leaf under consideration.
During the purification of recombinant proteins from Escherichia coli we observed that the SUS protein possessed an inherent affinity for membranes. A significant portion of recombinant wild-type SUS1 co-sedimented with E. coli membranes and would float to the top of a Suc gradient with the bacterial membranes (Fig. 2D), similar to native m-SUS found on maize leaf vesicles (Fig. 1D). Under identical conditions, the recombinant SUS1 purified from the soluble phase did not float and remained at the bottom of these gradients as expected (data not shown). These observations are described in detail in a forthcoming manuscript (S.C. Hardin, K. Duncan, and S.C. Huber, unpublished data). This system was exploited as an approach to determine if S15 phosphorylation influenced the membrane affinity of SUS. Site-directed mutants of SUS1 were used that contained acidic residue substitutions at S15 (i.e. S15D and S15E) to mimic the negative charge imparted on the amino terminus by phosphorylation of S15. When membrane extracts from bacteria expressing the mutant proteins were subjected to Suc gradient flotation, these pS15 phospho-mimetics floated to an even greater extent than wild type (Fig. 2D). A control, neutral substitution mutant (i.e. S15A) did not promote enhanced flotation. These results suggested that the presence of negative charge in the amino terminus can increase binding of SUS to certain membranes, which may explain the presence of relatively hyperphosphorylated native pS15-SUS on membranes in certain regions of the maize leaf (Fig. 2C).
Phosphorylation of S15 and Acidic pH Affect the Conformation of the Amino Terminus
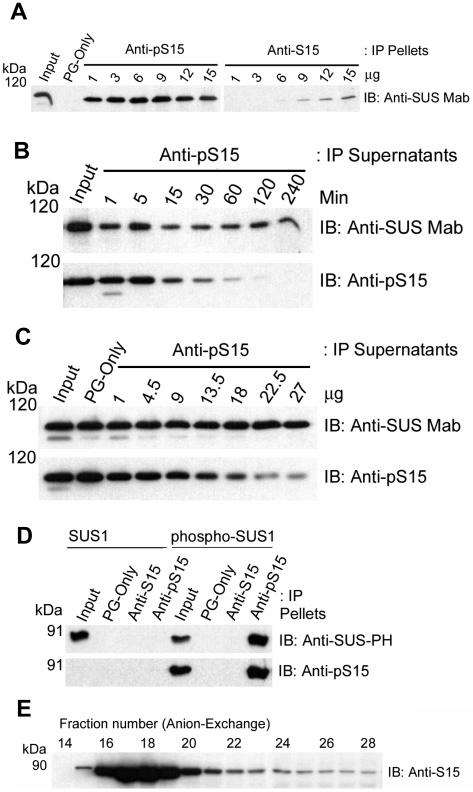
During the course of our studies, we made the interesting observation that the anti-pS15 antibodies could readily immunoprecipitate (IP) partially purified, native s-SUS whereas the anti-S15 antibodies could not. For example, when increasing amounts of each antibody were used to IP a constant amount of s-SUS and the resulting pellets probed on immunoblots for total SUS with an anti-SUS monoclonal antibody (i.e. anti-SUS Mab), 1 μg of the anti-pS15 antibody immunoprecipitated more s-SUS than 15 μg of the anti-S15 antibody (Fig. 3A). The difference in recovery of s-SUS was quantitated by densitometry to be approximately 30-fold (data not shown). These anti-peptide antibodies were generated using the same amino acid sequence, but will only IP the SUS protein if the corresponding region in the native conformation is solvent-exposed and in an unstructured loop or turn. The presence of secondary structure (e.g. helix) would be expected to eliminate their ability to IP SUS (Lu and Hodges, 2002). Therefore, these results suggested that phosphorylation at the S15 site produced a change in amino-terminal accessibility or conformation of SUS that was manifested as a large difference in the reactivity of these two antibodies for the native protein.
Figure 3.
Phosphorylation of S15 affects the conformation of the amino terminus of SUS. A, Immunoprecipitation (IP) of native s-SUS was performed with blank Protein-G beads (PG-only) or with the antibodies listed at the top of the figure. Immunoblots (IB) were performed on the IP pellets with the antibody listed to the right of the figure. The positions of PAGE molecular mass markers are shown in kilodaltons on the left of the figure. B, Immunoprecipitation (IP) of native s-SUS was performed for increasing amounts of time with 13.5 μg of the antibody listed at the top of the figure. Immunoblots (IB) were performed on the IP supernatants with the antibodies listed to the right of each panel. The positions of PAGE molecular mass markers are shown in kilodaltons (kD) on the left of each panel. C, Immunoprecipitation (IP) of native s-SUS was performed with blank Protein-G beads (PG-only) or with increasing amounts of the antibody listed at the top of the figure for 120 min. Immunoblots (IB) were performed on the IP supernatants with the antibodies listed to the right of each panel. D, Immunoprecipitation (IP) of recombinant wild-type SUS1 or CDPKII-phosphorylated SUS1 (phospho-SUS1 including pS15 and pS170) was performed with blank Protein-G beads (PG-only) or with the antibodies listed at the top of the figure. Immunoblots (IB) were performed on the IP pellets with the antibodies listed to the right of each panel. E, Native s-SUS protein was resolved by anion-exchange chromatography and immunoblot (IB) analysis performed on the SDS-denatured form with anti-S15 antibodies.
Two other possibilities that could explain the reduced ability of anti-S15 antibodies to IP SUS would be that (1) nearly all the s-SUS protein was phosphorylated on S15, and (2) that the S15 residue was modified in some other way (e.g. O-glycosylation). To test the first possibility, soluble phase extracts were incubated with a fixed amount of anti-pS15 antibody for increasing amounts of time, and the resulting supernatants were probed on immunoblots for total SUS and pS15-SUS (Fig. 3B). Under these conditions, immunodepletion was complete as pS15-SUS became nearly undetectable in the supernatants after 1 h and no further decreases in total SUS were observed up to 4 h (Fig. 3B). The SUS left in these supernatants apparently represents a discrete pool of tetrameric SUS completely devoid of S15 phosphorylation on any of its subunits. In complementary experiments, increasing amounts of anti-pS15 antibody were used to IP s-SUS for a fixed period of time. Immunoblots of these supernatants with the anti-SUS monoclonal and anti-pS15 antibodies yielded comparable results (Fig. 3C). Although somewhat variable in efficiency, the pertinent observation in both of these experiments is that a significant amount of unphosphorylated SUS protein remained in the supernatant after immunodepletion of essentially all the pS15-SUS (Fig. 3, B and C). Therefore, lack of unphosphorylated SUS in these maize extracts could not explain the inability of anti-S15 to IP native s-SUS. Moreover, we note that these totally unphosphorylated SUS tetramers existed in the soluble phase since the extracts used for IP were 100,000g supernatants. To further test these two possibilities, we performed immunoprecipitations with anti-S15 and anti-pS15 antibodies on bacterially expressed wild-type recombinant SUS1, that would not be glycosylated or phosphorylated, and on recombinant SUS1 phosphorylated by maize CDPKII (i.e. phospho-SUS1 including pS15 and pS170; Fig. 3D). Immunoblotting of the recombinant protein inputs established that wild-type SUS1 was unphosphorylated as expected and that CDPKII readily phosphorylated S15 (Fig. 3D). More important, the results also demonstrated that anti-S15 antibodies would not IP the completely unphosphorylated wild-type recombinant protein (Fig. 3D). These results suggest that neither the lack of unphosphorylated S15 nor modification of S15 by O-glycosylation were responsible for preventing anti-S15 antibodies from immunoprecipitating native SUS (Fig. 3A). As expected, anti-pS15 immunoprecipitated only the S15 phosphorylated SUS1 recombinant (Fig. 3D). As a means of further validation, we demonstrated that the anti-S15 antibodies recognized denatured SUS on immunoblots containing samples derived from an anion-exchange fractionated SUS peak (Fig. 3E). The anti-S15 antibodies recognized denatured SUS on these immunoblots at a 1,000-fold greater dilution than that used for IP of native s-SUS. Although not directly comparable, the noteworthy observation is that the anti-S15 antibodies perform much better against denatured SUS. The denaturation steps that precede gel electrophoresis destroy native SUS structure and recovered the anti-S15 epitope. Therefore, the inability of anti-S15 antibodies to IP native SUS was seemingly due to structural properties of SUS itself and not to properties of the antibody.
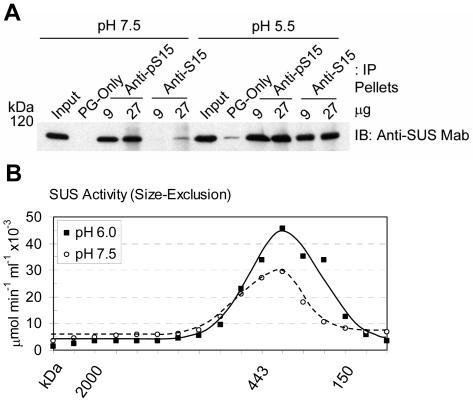
All of the IP experiments described so far (Fig. 3) were conducted at near neutral pH (i.e. pH 7.5). However, since the pH optimum for the Suc cleavage activity of SUS is 6.0 to 6.5 (Tsai, 1974; Su and Preiss, 1978), acidic conditions were tested to determine whether pH affected the ability of the anti-S15 and anti-pS15 antibodies to IP native SUS (Fig. 4A). Two different amounts of each antibody were used to IP native s-SUS that had been isolated at pH 5.5 or pH 7.5, and the resulting pellets were probed for total SUS (anti-SUS Mab). As expected, the anti-S15 antibodies were essentially ineffective at immunoprecipitating native SUS at pH 7.5 (Fig. 4A). However, when immunoprecipitations were performed against native s-SUS equilibrated to pH 5.5, a dramatic increase in the ability of anti-S15 to recognize SUS became apparent (Fig. 4A). Under these conditions, the anti-S15 and anti-pS15 antibodies performed almost identically. These results suggested that when exposed to low pH, the putative secondary structure of the amino terminus of unphosphorylated SUS assumed a more unstructured conformation that allowed recognition of the native protein by anti-S15 antibodies. This low pH-induced structural change may be similar to the phosphorylation-promoted amino-terminal conformation that occurred at pH 7.5 on pS15-SUS. To exclude the possibility that changes in the oligomeric structure of SUS were responsible for the observed effect of low pH on the ability of the anti-S15 antibodies to IP, size-exclusion chromatography was performed at pH 6.0 (Fig. 4B). Enzymatic analysis of these fractions demonstrated that SUS remained tetrameric at low pH. The peak of SUS cleavage activity occurred at an estimated molecular mass of 350 kD, as expected for the tetrameric SUS enzyme, whether chromatography was performed at pH 6.0 or pH 7.5 (Fig. 4B). Therefore, the increased ability of anti-S15 antibodies to IP SUS at low pH cannot be explained by aggregation or dissociation of SUS into dimers or monomers. The ability of anti-S15 antibodies to IP s-SUS at pH 6.0 was still much greater than at pH 7.5 (data not shown), and no significant differences in reactivity of the anti-S15 antibodies to denatured SUS were noted on immunoblots probed at acidic (6.0) or neutral (7.5) pH (data not shown). These results again implied that the effect of pH on the ability of the anti-S15 antibodies to IP SUS reflected changes in native s-SUS itself and not the antibody. Additionally, it is relevant to note that at pH 5.5 and pH 6.0 the native SUS protein remained completely soluble as the extracts for both IP and chromatography were 100,000g supernatants.
Figure 4.
Low pH affects the conformation of the amino terminus of SUS without affecting oligomerization state of the native protein. A, Immunoprecipitation (IP) of native s-SUS was performed at pH 7.5 or pH 5.5 with blank Protein-G beads (PG-only) or with the antibodies listed at the top of the figure. Immunoblots (IB) were performed on the IP pellets with the antibody listed to the right of the figure. The positions of PAGE molecular mass markers are shown in kilodaltons on the left of the figure. B, Sucrose cleavage activity (μmol UDP-Glc min−1 mL−1 × 10−3) of native s-SUS resolved by size-exclusion chromatography at pH 6.0 (▪, solid line) or pH 7.5 (○, dashed line). The elution positions of molecular mass standards are shown in kilodaltons (kD) under the graph.
Phosphorylation of S15 Stimulates the Sucrose Cleavage Activity of SUS
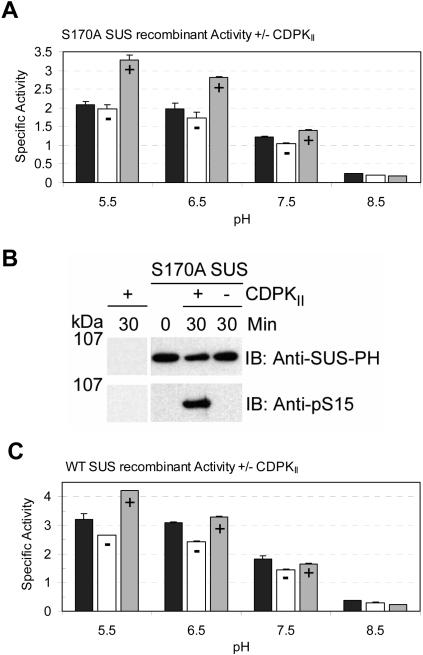
Since both the S15 and S170 residues of SUS1 can be phosphorylated by the maize leaf CDPKII enzyme (Hardin et al., 2003), a recombinant SUS1 protein containing a nonphosphorylatable amino acid substitution at S170 (i.e. S170A) was produced to determine whether S15-specific in vitro phosphorylation of SUS1 affected its Suc cleavage activity. Enzymatic analysis of the S170A SUS1 protein demonstrated that sucrolytic activity was highest at acidic pH (5.5, 6.5; Fig. 5A), similar to native maize SUS (Tsai, 1974; Su and Preiss, 1978). To test for an effect of S15 phosphorylation on this activity, the S170A protein was preincubated for 30 min at pH 7.5 with ATP and divalent cations (Mg+2 and Ca+2) in the presence or absence of CDPKII. When assayed after incubation with ATP but without CDPKII, the specific activity of the S170A SUS1 protein was essentially unchanged compared to the zero time controls at all four pH values (Fig. 5A). Therefore, SUS1 was not activated or losing significant amounts of activity during preincubation. However, when incubated with both ATP and CDPKII, the specific activity of the S170A SUS1 protein dramatically increased at acidic pH values compared to the zero time and minus CDPKII controls (Fig. 5A). The anion-exchange chromatography-purified CDPKII enzyme used for in vitro phosphorylation did not possess detectable SUS protein on immunoblots (Fig. 5B) or Suc cleavage activity (<0.1%; data not shown). The stimulation of activity by phosphorylation of S15 was most apparent at acidic pH and relative activation was 1.7-, 1.6-, 1.3-, and 0.9-fold at pH 5.5, 6.5, 7.5, and 8.5, respectively (Fig. 5A). Immunoblot analysis of the S170A proteins incubated with and without CDPKII confirmed that enzyme activation was indeed associated with the phosphorylation of S15 (Fig. 5B). These data strongly suggested that pS15 stimulated the Suc cleavage activity of SUS1 and that this effect was enhanced by decreased pH. In previous experiments similar to the one presented in Figure 5A, the stoichiometry of S15 phosphorylation by CDPKII in the S170A protein was only about 30% (Hardin et al., 2003); therefore, the maximum effect of pS15 on the Suc cleavage activity of SUS could actually be much higher when completely phosphorylated at the S15 site. In similar experiments, the wild-type SUS1 recombinant, containing both phosphorylatable residues (S15 and S170), was subjected to CDPKII phosphorylation and analysis of enzymatic activity (Fig. 5C). When incubated with ATP and CDPKII, the specific activity of wild-type SUS1 protein also increased compared to the zero time and minus CDPKII controls (Fig. 5C). The stimulation of activity in phospho-SUS1 (including both pS15 and pS170) was again most apparent at acidic pH, and relative activation by CDPKII phosphorylation was 1.6-, 1.4-, 1.1-, and 0.8-fold at pH 5.5, 6.5, 7.5, and 8.5, respectively (Fig. 5C). These observations demonstrated that the activity response of the S170A mutant protein to S15 phosphorylation was not conditioned by the mutation, as a similar response was observed in the wild-type protein. The magnitude of the effect of pS15 on activity was slightly reduced in the wild-type protein compared to the S170A protein (Fig. 5, A and C). This observation was not unexpected, as phosphorylation of S170 is without consequence on the activity of SUS (Hardin et al., 2003), and the reduced effect seen in the wild-type protein may simply reflect the reduction in phosphorylation of S15 in the presence of a second phosphorylatable residue. Moreover, it exemplifies the difficulty in assigning an activity effect to the phosphorylation of S15 previously encountered in all prior studies in which wild-type SUS substrates were phosphorylated in vitro and activity measured only at neutral pH.
Figure 5.
Phosphorylation affects the Suc cleavage activity of SUS. A, Specific Suc cleavage activities (μmol UDP-Glc min−1 mg−1) at pH 5.5, 6.5, 7.5, and 8.5 of S170A SUS1 recombinants at time zero (black bars) or after a 30-min incubation in vitro in the presence (+, gray bars) or absence (−, white bars) of CDPKII. B, Immunoblot (IB) analyses of CDPKII and S170A SUS1 recombinants at time zero or after a 30-min incubation in vitro in the presence (+) or absence (−) of CDPKII, with the antibodies listed to the right of each panel. The positions of PAGE molecular mass markers are shown in kilodaltons on the left of the figure. C, Specific Suc cleavage activities (μmol UDP-Glc min−1 mg−1) at pH 5.5, 6.5, 7.5, and 8.5 of wild-type SUS1 recombinants at time zero (black bars) or after a 30-min incubation in vitro in the presence (+, gray bars) or absence (−, white bars) of CDPKII.
DISCUSSION
In this study, m-SUS is clearly shown to be a peripheral membrane protein (Fig. 1D), as would be expected of a protein known to also exist as a soluble, globular protein. Interestingly, maize SUS1 has some inherent affinity for lipids (Fig. 2D) that may contribute to its membrane association in vivo. However, m-SUS is not simply in equilibrium with s-SUS in vivo (i.e. it does not reflect a constant proportion of the soluble phase enzyme; Fig. 1C), and thus membrane binding is controlled within the plant cell.
Phosphorylation of the primary S15 site has been suggested as one factor controlling several parameters including membrane association and enzyme activity, and was the specific focus of this work. The results obtained in this study suggest that phosphorylation of the S15 site (1) could either promote or inhibit membrane binding (as discussed below); (2) stimulates Suc cleavage activity at acidic pH; and (3) affects the apparent conformation of the amino terminus. Conceivably, the conformational change caused by phosphorylation may be responsible for changes in activity and membrane binding. However, the in vivo control of SUS distribution between the soluble and membrane phases is seemingly not solely due to changes in S15 phosphorylation state. Apparently, the influence of pS15 on membrane association may also depend on membrane lipid and/or protein composition, both of which are likely to vary among different organs or with the developmental status of the organ under study (Figs. 1 and 2). Furthermore, our results suggest that the presence of multiple phosphorylation sites in SUS, the indirect monitoring of pS15, the use of right side-out membrane vesicles, and the determination of SUS enzyme activity solely at neutral pH were important factors contributing to previous contradictory results on the roles of S15 phosphorylation on SUS activity and localization.
S15 Phosphorylation and Conformation of the Amino Terminus of SUS
Immunoprecipitation data demonstrating the differential ability of anti-S15 and anti-pS15 antibodies to IP native s-SUS suggested that phosphorylation of SUS at S15 or exposure to low pH changed the accessibility or conformation of the amino terminus (Figs. 3 and 4). Considering the proximity of this region to the terminus of the protein and the accessibility of the S15 site to protein kinases (Hardin et al., 2003), masking of the anti-S15 epitope by decreased accessibility is unlikely. The more likely interpretation is that the conformation of the amino terminus is the main factor involved in determining the efficiency with which the anti-S15 and anti-pS15 antibodies IP native s-SUS. Observation of pH-sensitive epitope masking has been documented previously (Bell-Parikh et al., 2001; Matsunaga et al., 2001). We suggest that the amino terminus of unphosphorylated SUS exists predominantly in a helical conformation at neutral pH that precludes recognition by the anti-peptide antibodies (Lu and Hodges, 2002). Indeed, in silico modeling of the SUS1 amino terminus using the Protein Sequence Analysis server (http://bmerc-www.bu.edu/psa) gave a probability higher than 90% that it exists in a helix. Unfortunately these algorithms cannot predict the effects of phosphorylation or pH on secondary structure. However, our data suggest that there is a reduction in the predicted helical content of the amino terminus upon phosphorylation, which promotes recognition by anti-pS15 antibodies at neutral pH, or upon exposure of unphosphorylated SUS to low pH, which promotes recognition by anti-S15 antibodies (Figs. 3 and 4). Since the presumed helical conformation of the amino terminus is likely to be in equilibrium with an unstructured loop conformation, the effect that phosphorylation and low pH have on helical structure need not be to entirely promote a loop conformation. The observed effects could also reflect a small shift toward an unstructured conformation in the transitional equilibrium between helix and loop. In an IP approach, where antibody binding is essentially irreversible and the retained form is sequestered into a nonequilibrating state, then a small displacement of the initial equilibrium toward an immunoprecipitable form could eventually generate large differences in product capture. In other words, the unphosphorylated neutral pH and low pH-exposed proteins, as well as the S15 phosphorylated protein, could all still exist with a predominantly helical amino terminus. Phosphorylation and low pH may only slightly shift the equilibrium toward an unstructured conformation. Helical wheel projections of the SUS1 amino terminus place S15 directly between an acidic (D7, E18, and D22) and a basic (R8, R12, and R19) face of the helix, indicating that if the amino terminus were still predominantly helical when phosphorylated, then the negative charge contributed to this region by pS15 may potentially disrupt the basic face or enhance the acidic face of the helix. It is also interesting to note that immediately adjacent to S15 is a conserved His residue (H14) whose pKa and protonation state could be differentially affected by pH in S15- versus pS15-SUS. These considerations may partially explain the seemingly interrelated effects that phosphorylation and pH have on amino-terminal structure.
S15 Phosphorylation and SUS Activity
In attempts to reconcile previous contradictory observations, we have taken into account the additional phosphorylation site in SUS (S170) and have assayed for cleavage activity across the pH range 5.5 to 8.5. Significantly, the utilization of these approaches allowed us to demonstrate that the specific phosphorylation of S15 (i.e. in the absence of additional phosphorylation sites) increased the sucrolytic activity of SUS at acidic pH (Fig. 5). This effect is probably related to an increased affinity for Suc and/or UDP as previously demonstrated (Huber et al., 1996; Nakai et al., 1998; Tanase et al., 2002). Curiously, the effect of phosphorylation was most pronounced under low pH conditions (5.5, 6.5) coincident with the pH optimum for Suc cleavage activity (Fig. 5; Tsai, 1974; Su and Preiss, 1978). At near neutral pH (7.5) the effect of phosphorylation on S170A SUS was still readily apparent (Fig. 5) but reduced. These observations may be particularly relevant toward explaining previous variability and negligible effects of phosphorylation on activity because all prior studies were performed at near neutral pH (Huber et al., 1996; Winter et al., 1997; Nakai et al., 1998, 1999; Anguenot et al., 1999; Zhang et al., 1999; Chikano et al., 2001; Tanase et al., 2002). In studies in which an effect of phosphorylation on enzymatic activity was noted, it was the Suc cleavage activity of SUS that was specifically affected (Huber et al., 1996; Nakai et al., 1998, 1999; Anguenot et al., 1999; Tanase et al., 2002). Our data suggest that the stimulation of cleavage activity by phosphorylation of S15 may be related to unstructuring of the amino terminus that also occurs in response to low pH (Figs. 3 and 4).
Physiological Implications of S15 Phosphorylation and Intracellular pH in SUS Regulation
The diversity of roles performed by SUS is probably dictated in part by its intracellular localization (Sturm and Tang, 1999; Winter and Huber, 2000; Haigler et al., 2001). At its peak of abundance, m-SUS represented 9% of the total cellular SUS protein within the 4- to 8-cm segment of maize leaves (Fig. 1C). This observation is consistent with the 9.9% found previously by 32P-incorporation to exist on the membrane in maize leaf (Winter et al., 1997) and similar to the 3% to 14% that occurred on the membrane in maize endosperm based on SUS activity measurements (Carlson and Chourey, 1996). However these values, and the 1.5% to 4% detected on mature nodule membranes (Zhang et al., 1999), are much less than the amounts of SUS detected on cotton fiber membranes or carrot (Daucus carota) protoplasts where it accounted for between 25% to 50% of total SUS (Amor et al., 1995; Sturm et al., 1999). Discovering the basis for these differences in membrane association among species and organs remains an important task for future studies. However, both s-SUS and m-SUS perform preferentially catabolic functions in heterotrophic organs and supply hexose sugars and sugar-nucleotides derived from Suc cleavage to metabolic and biosynthetic pathways, and are subject to reversible phosphorylation at S15 and exposure to decreases in cellular pH.
The effects invoked by changes in phosphorylation and pH probably control the catabolic activity of both s- and m-SUS in response to fluctuations in Suc supply and environmental stresses. However, these two events (i.e. S15 phosphorylation and fluctuating pH) are inherently different. Phosphorylation is a specific and directed modification that is responsive to cellular calcium levels and CDPK activity, whereas exposure to significant fluctuations in pH is an indirect occurrence attributed to a particular cellular location or physiological state. The relative influence exerted by each is therefore variable as conditions warrant, and they potentially may augment or counteract each other. For example, the phosphorylation state of m-SUS is sensitive to anoxia in maize root tips (Subbaiah and Sachs, 2001), nitrogen and salt stresses in soybean nodules (Komina et al., 2002), and gravity in maize pulvini (Winter et al., 1997). Furthermore, m-SUS could also experience fluctuating pH if localized, as some data suggest (Etxeberria and Gonzalez, 2003), in membrane microdomains next to sugar-proton cotransporters. The physiological effects of exposure to hypoxia or anoxia are particularly relevant to the regulation of SUS, as not only does phosphorylation state change (Subbaiah and Sachs, 2001), but also cytosolic pH (7.5) rapidly decreases to approximately 6.8 (Gout et al., 2001). Although this event is transient and the pH partially recovers to around 7.0 within about 25 min after exposure (Gout et al., 2001), this intracellular acidification would potentially stimulate the Suc cleavage activity of s- and m-SUS, and the S15 phosphorylated protein would potentially be relatively more activated (Fig. 5) in either location. Moreover, under these hypoxic conditions invertase activity decreases (Ricard et al., 1998) making SUS the predominant Suc-catabolizing enzyme. The activity of SUS increases dramatically over the first 3 h of hypoxic and/or anoxic conditions (Ricard et al., 1998; Zeng et al., 1998), perhaps reflecting the effects of reduced pH (Gout et al., 2001) and increased phosphorylation that occurs on both s-SUS and m-SUS (Subbaiah and Sachs, 2001). Reversible phosphorylation of S15 ensures the opportunity for rapid modulation of SUS activity in response to pH fluctuations, whereas prolonged anoxic conditions require the involvement of transcriptional regulatory mechanisms (Zeng et al., 1998). The efficient utilization of Suc under anoxic conditions has been identified as an important factor in the tolerance to these stresses (Ricard et al., 1998). The critical role played by SUS in this tolerance highlights the importance of this enzyme and the functional significance of phosphorylation of S15 (Ricard et al., 1998; Zeng et al., 1998; Subbaiah and Sachs, 2001).
MATERIALS AND METHODS
Upon written request, all novel materials and reagents described in this publication will be made available in limited quantities and in a timely manner for noncommercial purposes.
Plant Materials and Growth Conditions
Maize (Zea mays L. cv Pioneer 3183) plants were grown in a soil mixture in a greenhouse and fertilized three times weekly with a modified Hoagland solution. Temperature was controlled by a Grotron II system (ACME, Muskogee, OK) and supplemental lighting was provided by metal halide lamps during winter months. Elongating leaves were harvested from 4- to 8-week-old plants. The basal 8 cm or sequential 4-cm segments from the base (starting at the site of insertion) were removed, immediately frozen in liquid nitrogen, and stored at −80°C.
Protein Extraction and Ultracentrifugal Fractionation
Frozen maize leaf organ was ground in two volumes of protein extraction buffer (100 mm MOPS (pH 7.5), 10 mm dithiothreitol (DTT), 5 mm EDTA, 1 mm EGTA, 20 mm NaF, 10 mm Na2MoO4, 1 mm Na3VO4, 0.5 μm microcystin-LR, 1 mm phenylmethylsulfonyl fluoride (PMSF), 1 mm benzamidine-HCl, 5 mm caproic acid, 2 μm N-(trans-epoxysuccinyl)-Leu-4-guanidinobutylamide (E64), 5 μm leupeptin, 5 μg mL−1 soybean trypsin inhibitor, 10 μm carbobenzoxy-leucyl-leucyl-leucinal (MG132; CalBiochem, San Diego), 1% (w/v) polyvinylpolypyrrolidone (PVPP), 0.25 m Suc, and 2% (w/v) polyethylene glycol-8000. Clarified extracts were produced by filtration through Miracloth (CalBiochem) and two sequential centrifugations at 10,000g and 4°C for 15 min. This supernatant was clarified at 100,000g for 1 h at 4°C and retained as the soluble phase extract.
The 100,000g microsomal pellet was salt-washed and vesicles inverted (Johansson et al., 1995) to release entrapped protein by resuspension in 50 mm MOPS (pH 7.5), 5 mm DTT, 2 mm EDTA, 1 mm EGTA, 20 mm NaF, 10 mm Na2MoO4, 1 mm Na3VO4, 0.1 μm microcystin-LR, 1 mm PMSF, 1 mm benzamidine-HCl, 5 mm caproic acid, 2 μm E64, 5 μm leupeptin, 5 μg mL−1 soybean trypsin inhibitor, 10 μm MG132, 150 mm NaCl, and 0.1% (w/v) polyoxyethylene 20 cetyl ether (Brij 58). The membrane fraction was pelleted at 100,000g for 1 h at 4°C and resuspended on ice in 2% of the original volume of protein extraction buffer. Measurements of latent SUS and CDPK activity with increasing amounts of Triton X-100 indicated that at least 75% of these vesicles existed in a sealed, inside-out orientation. Following membrane solubilization in 1% (v/v) Triton X-100 and 0.25% (w/v) CHAPS, insoluble material (e.g. cytoskeletal complexes, etc.) was removed by centrifugation at 40,000g for 1 h at 4°C and the supernatant retained as the membrane phase extract. The soluble phase extracts were also subsequently adjusted to contain 1% (v/v) Triton X-100 and 0.25% (w/v) CHAPS to ensure equivalency.
Recombinant Protein Expression and Suc Gradient Flotation of Membranes
Soluble phase extracts from Escherichia coli BL21(DE3) transformants expressing wild-type and site-directed mutant (S170A, S15D, S15E) recombinant SUS1 proteins were purified by chromatography on Ni2+-charged iminodiacetic acid-agarose and SOURCE 15Q anion-exchange resin as described (Hardin et al., 2003). The final dialysis of peak fractions was performed against 10 mm MOPS (pH 7.0), 1 mm DTT, and 50 mm Suc at 4°C.
Discontinuous Suc gradient flotation of maize and E. coli membranes was done essentially as described (Ahola et al., 1999). Inverted membrane vesicles were prepared from maize leaf organ essentially as described above but omitting detergent solubilization. Duplicate aliquots were resuspended in MOPS buffer (50 mm MOPS (pH 7.5), 1 mm DTT, 0.1 mm EDTA, 5 mm NaF, 0.5 mm benzamidine-HCl, 1 μm E64, 250 mm Suc) or carbonate buffer (100 mm Na2CO3 (pH 11.5), 1 mm DTT, 0.1 mm EDTA, 5 mm NaF, 0.5 mm benzamidine-HCl, 1 μm E64, 250 mm Suc) for 30 min on ice (Fujiki et al., 1982). Membrane vesicles were prepared from equal fresh weights of BL21(DE3) transformants expressing wild-type and site-directed mutant (S15D, S15E, S15A) recombinant SUS1 proteins by resuspending the cells in 50 mm MOPS, pH 7.5, 50 mm Suc, 50 mm NaCl, 250 μg mL−1 lysozyme and incubating at room temperature for 30 min. Prior to sonication in an ice bath, 1 mm DTT, 1 mm EDTA, 10 μm leupeptin, 1 mm 4-(2-amino-ethyl) benzene sulfonyl fluoride, 0.5 mm benzamidine, and 1 μm E64 were added. Extracts were clarified of any potential intact cells or inclusion bodies by filtration through Miracloth (CalBiochem) and two sequential centrifugations at 10,000g and 4°C for 15 min. Membrane vesicles within these E. coli supernatants and the buffer-washed maize membrane suspension were recovered at 145,000g for 1.5 h at 4°C, and resuspended in a 60% (w/w) Suc solution made in 50 mm MOPS, pH 7.5, 50 mm NaCl, 1 mm DTT, 1 mm EDTA, 10 μm leupeptin, 1 mm AEBSF, 0.5 mm benzamidine, and 1 μm E64. These layers were sequentially overlaid with 50, 40, 30, and 10% (w/w) Suc solutions made in 50 mm MOPS, pH 7.5, 100 mm NaCl, 1 mm DTT, and 1 mm EDTA. Centrifugation was performed at 145,000g for 20 h at 4°C and fractions were collected from the top of the gradient.
Protein Extraction and Size-Exclusion Chromatography
Frozen maize leaf base organ was ground in four volumes of protein extraction buffer (100 mm MOPS (pH 7.5) or 100 mm MES (pH 6.0), 5 mm DTT, 5 mm EDTA, 20 mm NaF, 0.5 μm microcystin-LR, 1 mm PMSF, 1 mm benzamidine, 1 mm caproic acid, 2 μm E64, and 0.1 g/g fresh weight PVPP). Clarified extracts were produced by filtration through Miracloth (CalBiochem) and centrifugation at 10,000g and 4°C for 20 min. This supernatant was clarified at 100,000g for 1 h at 4°C and applied to a 130 mL Fractogel TSK HW55(S) 1.6- × 65-cm column equilibrated in 50 mm MOPS, pH 7.5, or 50 mm MES, pH 6.0, 10 mm MgCl2, 2 mm DTT, 50 mm Suc, and 50 mm NaCl. Separation was performed at 4°C by isocratic elution in the same buffer at a flow rate of 0.5 mL min−1. The column was calibrated using blue dextran (2,000 kD), apoferritin (443 kD), and alcohol dehydrogenase (150 kD).
Immunological Methods
The SUS1 peptides pS15 (CRVLSRLHpSVRERIGD) and PH (CHILRVPFRTENGIVRKWISR), or the SPS peptide (KAQVDVGNLKFDAIRRRKCI) were purified by HPLC, verified by mass spectrometry and used to generate immune serum in rabbits (Bethyl Laboratories, Montgomery, TX). Immunosorbent columns coupled with PH or SPS peptides were used to obtain the affinity-purified anti-SUS-PH and anti-SPS antibodies. Affinity purification of the peptide- and phosphopeptide-specific antibodies was achieved by sequentially processing the serum over immunosorbent columns coupled with the unphosphorylated S15 (CRVLSRLHSVRERIGD) and phosphorylated (pS15) versions of the peptides. The pS15:S15 reactivity ratio of the anti-pS15 antibodies against immobilized peptides was 99:1 by ELISA (testing performed by Bethyl). The mouse anti-SUS Mab were kindly provided by Dr. Prem Chourey (University of Florida, Gainesville). Proteins were denatured, separated on 7% or 12.5% polyacrylamide-0.1% SDS gels, transferred onto polyvinylidene fluoride membranes (Immobilon-P, Millipore, Bedford, MA) and immunoblotted as described (Hardin et al., 2003). Densitometry was performed on a Molecular Dynamics (Sunnyvale, CA) Personal Densitometer SI (model #375) of films scanned at 12-bit digital resolution and 100-micron pixel size. Volume quantitation was performed on ImageQuant 5.0 software. Protein concentrations were determined in duplicate by the dye-binding assay (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard.
Extracts for immunoprecipitations were prepared from frozen maize leaf base organ in four volumes of protein extraction buffer (100 mm MOPS (pH 7.5) or 100 mm MES (pH 5.5), 5 mm DTT, 5 mm EDTA, 20 mm NaF, 0.5 μm microcystin-LR, 1 mm PMSF, 1 mm benzamidine, 1 mm caproic acid, 2 μm E64, and 0.1 g/g fresh weight PVPP). Clarified extracts were produced by filtration through Miracloth (Calbiochem) and centrifugation at 10,000g and 4°C for 20 min. This supernatant was clarified at 100,000g for 1 h at 4°C and adjusted by a 5-fold dilution to contain equivalent total protein quantities (usually approximately 20 μg) and include 0.1% (v/v) Triton X-100. Various amounts of each immunoprecipitating antibody were prebound to Protein-G Sepharose (Sigma, St. Louis), washed in 20 mm immunoprecipitation buffer (MES pH 5.5 or MOPS pH 7.5) that included 1 mm DTT, 1 mm EDTA, 4 mm NaF, 0.2 mm PMSF, 0.2 mm benzamidine, 0.2 mm caproic acid, and 0.1% (v/v) Triton X-100, and mixed with equal volumes of the adjusted protein extracts at 4°C for various periods of time. The Protein-G beads were removed by brief centrifugation and portions of the supernatants denatured in SDS-PAGE sample buffer. The Protein-G bead pellets were washed twice in immunoprecipitation buffer, and twice in 5 mm NaH2PO4, pH 7.5, 150 mm NaCl prior to denaturation in SDS-PAGE sample buffer. Alternatively, recombinant wild-type SUS1 (1 μg) preincubated with maize CDPKII in the presence or absence of ATP was immunoprecipitated from 25 mm MOPS, pH 7.5, 1 mm DTT, 50 mm Suc, 150 mm NaCl, and 0.1% Triton X-100 as described above.
Enzyme Activity Assays
SUS activity was assayed in the catabolic (cleavage) direction essentially as described (Huber et al., 1996) using a fixed-time assay in 50 mm buffer (MES, pH 5.5 and 6.5, MOPS, pH 7.5, or Tricine, pH 8.5) containing 1 mm UDP and 100 mm Suc. Production of UDP-Glc was determined enzymatically using UDP-Glc-dehydrogenase (Calbiochem) coupled to the reduction of NAD. Peptide kinase assays were performed as described (Hardin et al., 2003) using an S15-containing peptide (VLARLHSVRERIKK).
In Vitro Phosphorylation
The maize CDPKII enzyme was partially purified from leaf base extracts by SOURCE 15Q anion-exchange chromatography as described (Hardin et al., 2003). For use in immunoprecipitation, purified wild-type recombinant SUS1 protein was incubated in 50 mm MOPS, pH 7.5, 50 mm Suc, 0.5 μm microcystin-LR, 10 mm MgCl2, 0.5 mm CaCl2, 20% (v/v) CDPKII (approximately 100 μg total protein) in the presence or absence of 2 mm ATP. For use in activity assays, purified wild-type and S170A recombinant SUS1 proteins were incubated in 5 mm MOPS, pH 7.5, 5 mm MgCl2, 0.5 mm CaCl2, 0.5 mm ATP in the presence or absence of 10% (v/v) CDPKII (approximately 60 μg total protein). Reactions preceded at room temperature for 30 min were diluted into 50 mm buffer (MES, pH 5.5 and 6.5, MOPS, pH 7.5, or Tricine, pH 8.5) and immediately used in SUS cleavage activity assays. Portions of the reactions were denatured in SDS-sample buffer to verify phosphorylation on immunoblots.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number L29418.
Acknowledgments
The authors gratefully acknowledge the contribution of anti-SUS monoclonal antibodies by Dr. Prem Chourey (University of Florida, Gainesville). Mention of a trademark or proprietary product does not constitute a guarantee or warranty by the U.S. Department of Agriculture (USDA)-Agricultural Research Service and does not imply its approval to the exclusion of other products that might also be suitable.
This research was supported in part by funds from the U.S. Department of Energy (grant no. DE-AI05-91ER20031 to S.C.H.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.036780.
References
- Ahola T, Lampio A, Auvinen P, Kaariainen L (1999) Semliki Forest virus mRNA capping enzyme requires association with anionic membrane phospholipids for activity. EMBO J 18: 3164–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP (1995) A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA 92: 9353–9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguenot R, Yelle S, Nguyen-Quoc B (1999) Purification of tomato sucrose synthase phosphorylated isoforms by Fe(III)-immobilized metal affinity chromatography. Arch Biochem Biophys 365: 163–169 [DOI] [PubMed] [Google Scholar]
- Azama K, Abe S, Sugimoto H, Davies E (2003) Lysine-containing proteins in maize endosperm: a major contribution from cytoskeleton-associated carbohydrate-metabolizing enzymes. Planta 217: 628–638 [DOI] [PubMed] [Google Scholar]
- Bell-Parikh LC, Eipper BA, Mains RE (2001) Response of an integral granule membrane protein to changes in pH. J Biol Chem 276: 29854–29863 [DOI] [PubMed] [Google Scholar]
- Buckeridge MS, Vergara CE, Carpita NC (1999) The mechanism of synthesis of a mixed-linkage (1-3),(1-4)β-d-Glucan in maize. Evidence for multiple sites of glucosyl transfer in the synthase complex. Plant Physiol 120: 1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SJ, Chourey PS (1996) Evidence for plasma membrane-associated forms of sucrose synthase in maize. Mol Gen Genet 252: 303–310 [DOI] [PubMed] [Google Scholar]
- Carlson SJ, Chourey PS, Helentjaris T, Datta R (2002) Gene expression studies on developing kernels of maize sucrose synthase (SUS) mutants show evidence for a third SUS gene. Plant Mol Biol 49: 15–29 [DOI] [PubMed] [Google Scholar]
- Chikano H, Ogawa M, Ikeda Y, Koizumi N, Kusano T, Sano H (2001) Two novel genes encoding SNF1-related protein kinases from Arabidopsis thaliana: differential accumulation of AtSR1 and AtSR2 transcripts in response to cytokinins and sugars, and phosphorylation of sucrose synthase by AtSR2. Mol Gen Genet 264: 674–681 [DOI] [PubMed] [Google Scholar]
- Chourey PS, Latham MD, Still PE (1986) Expression of two sucrose synthase genes in endosperm and seedling cells of maize: evidence of tissue specific polymerization of protomers. Mol Gen Genet 203: 251–255 [Google Scholar]
- Chourey PS, Taliercio EW, Carlson SJ, Ruan Y-L (1998) Genetic evidence that the two isoenzymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol Gen Genet 259: 88–96 [DOI] [PubMed] [Google Scholar]
- Etxeberria E, Gonzalez P (2003) Evidence for a tonoplast-associated form of sucrose synthase and its potential involvement in sucrose mobilization from the vacuole. J Exp Bot 54: 1407–1414 [DOI] [PubMed] [Google Scholar]
- Fujiki Y, Hubbard AL, Fowler S, Lazarow PB (1982) Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol 93: 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout E, Boisson A-M, Aubert S, Douce R, Bligny R (2001) Origin of the cytoplasmic pH changes during anaerobic stress in higher plant cells. Carbon-13 and phosphorous-31 nuclear magnetic resonance studies. Plant Physiol 125: 912–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler CH, Ivanova-Datcheva M, Hogan PS, Salnikov VV, Hwang S, Martin K, Delmer DP (2001) Carbon partitioning to cellulose synthesis. Plant Mol Biol 47: 29–51 [PubMed] [Google Scholar]
- Hardin SC, Tang G-Q, Scholz A, Holtgraewe D, Winter H, Huber SC (2003) Phosphorylation of sucrose synthase at serine 170: occurrence and possible role as a signal for proteolysis. Plant J 35: 588–603 [DOI] [PubMed] [Google Scholar]
- Huber SC, Huber JL, Liao P-C, Gage DA, McMichael RW, Chourey PS, Hannah CL, Koch K (1996) Phosphorylation of serine-15 of maize leaf sucrose synthase: occurrence in vivo and possible regulatory significance. Plant Physiol 112: 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson F, Olbe M, Sommarin M, Larsson C (1995) Brij 58, a polyoxyethylene acyl ether, creates membrane vesicles of uniform sidedness. A new tool to obtain inside-out (cytoplasmic side-out) plasma membrane vesicles. Plant J 7: 165–173 [DOI] [PubMed] [Google Scholar]
- Komina O, Zhou Y, Sarath G, Chollet R (2002) In vivo and in vitro phosphorylation of membrane and soluble forms of soybean nodule sucrose synthase. Plant Physiol 129: 1664–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SM, Hodges RS (2002) A de novo designed template for generating conformation-specific antibodies that recognize α-helices in proteins. J Biol Chem 277: 23515–23524 [DOI] [PubMed] [Google Scholar]
- Matsunaga Y, Peretz D, Williamson A, Burton D, Mehlhorn I, Groth D, Cohen FE, Prusiner SB, Baldwin MA (2001) Cryptic epitopes in N-terminally truncated prion protein are exposed in the full-length molecule: dependence of conformation on pH. Proteins 44: 110–118 [DOI] [PubMed] [Google Scholar]
- Nakai T, Konishi T, Zhang X-Q, Chollet R, Tonouchi N, Tsuchida T, Yoshinaga F, Mori H, Sakai F, Hayashi T (1998) An increase in apparent affinity for sucrose of mung bean sucrose synthase is caused by in vitro phosphorylation or directed mutagenesis of ser11. Plant Cell Physiol 39: 1337–1341 [DOI] [PubMed] [Google Scholar]
- Nakai T, Tonouchi N, Konishi T, Kojima Y, Tsuchida T, Yoshinaga F, Sakai F, Hayashi T (1999) Enhancement of cellulose production by expression of sucrose synthase in Acetobacter xylinum. Proc Natl Acad Sci USA 96: 14–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Quoc B, Krivitzky M, Huber SC, Lecharny A (1990) Sucrose synthase in developing maize leaves: regulation of activity by protein level during the import to export transition. Plant Physiol 94: 516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto M-a, Nakamura K (1995) Sugar-induced increase of calcium-dependent protein kinases associated with the plasma membrane in leaf tissues of tobacco. Plant Physiol 109: 973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard B, VanToai T, Chourey P, Saglio P (1998) Evidence for the critical role of sucrose synthase for anoxic tolerance of maize roots using a double mutant. Plant Physiol 116: 1323–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JR, Ferl RJ, Baier J, St Clair D, Carson C, McCarty DR, Hannah LC (1994) Structural features of the maize sus1 gene and protein. Plant Physiol 106: 1659–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Lienhard S, Schatt S, Hardegger M (1999) Tissue-specific expression of two genes for sucrose synthase in carrot (Daucus carota L.). Plant Mol Biol 39: 349–360 [DOI] [PubMed] [Google Scholar]
- Sturm A, Tang G-Q (1999) The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci 4: 401–407 [DOI] [PubMed] [Google Scholar]
- Su J-C, Preiss J (1978) Purification and properties of sucrose synthase from maize kernels. Plant Physiol 61: 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Sachs MM (2001) Altered patterns of sucrose synthase phosphorylation and localization precede callose induction and root tip death in anoxic maize seedlings. Plant Physiol 125: 585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanase K, Shiratake K, Mori H, Yamaki S (2002) Changes in the phosphorylation state of sucrose synthase during development of Japanese pear fruit. Physiol Plant 114: 21–26 [DOI] [PubMed] [Google Scholar]
- Tsai C-Y (1974) Sucrose-UDP glucosyltransferase of Zea mays endosperm. Phytochemistry 13: 885–891 [Google Scholar]
- Wienkoop S, Saalbach G (2003) Proteome analysis. Novel proteins identified at the peribacteroid membrane from Lotus japonicus root nodules. Plant Physiol 131: 1080–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Huber JL, Huber SC (1997) Membrane association of sucrose synthase: changes during the graviresponse and possible control by protein phosphorylation. FEBS Lett 420: 151–155 [DOI] [PubMed] [Google Scholar]
- Winter H, Huber JL, Huber SC (1998) Identification of sucrose synthase as an actin-binding protein. FEBS Lett 430: 205–208 [DOI] [PubMed] [Google Scholar]
- Winter H, Huber SC (2000) Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Plant Sci 19: 31–67 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE (1998) Differential regulation of sugar-sensitive sucrose synthases by hypoxia and anoxia indicate complementary transcriptional and posttranscriptional responses. Plant Physiol 116: 1573–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-Q, Lund AA, Sarath G, Cerny RL, Roberts DM, Chollet R (1999) Soybean nodule sucrose synthase (nodulin-100): further analysis of its phosphorylation using recombinant and authentic root-nodule enzymes. Arch Biochem Biophys 371: 70–82 [DOI] [PubMed] [Google Scholar]