Abstract
Plant mitochondria were previously shown to comprise respiratory supercomplexes containing cytochrome c reductase (complex III) and NADH dehydrogenase (complex I) of I1III2 and I2III4 composition. Here we report the discovery of additional supercomplexes in potato (Solanum tuberosum) mitochondria, which are of lower abundance and include cytochrome c oxidase (complex IV). Highly active mitochondria were isolated from potato tubers and stems, solubilized by digitonin, and subsequently analyzed by Blue-native (BN) polyacrylamide gel electrophoresis (PAGE). Visualization of supercomplexes by in-gel activity stains for complex IV revealed five novel supercomplexes of 850, 1,200, 1,850, 2,200, and 3,000 kD in potato tuber mitochondria. These supercomplexes have III2IV1, III2IV2, I1III2IV1, I1III2IV2, and I1III2IV4 compositions as shown by two-dimensional BN/sodium dodecyl sulfate (SDS)-PAGE and BN/BN-PAGE in combination with activity stains for cytochrome c oxidase. Potato stem mitochondria include similar supercomplexes, but complex IV is partially present in a smaller version that lacks the Cox6b protein and possibly other subunits. However, in mitochondria from potato tubers and stems, about 90% of complex IV was present in monomeric form. It was suggested that the I1III2IV4 supercomplex represents a basic unit for respiration in mammalian mitochondria termed respirasome. Respirasomes also occur in potato mitochondria but were of low concentrations under all conditions applied. We speculate that respirasomes are more abundant under in vivo conditions.
Prerequisite for oxidative phosphorylation (OXPHOS) in mitochondria are five protein complexes termed NADH dehydrogenase (complex I), succinate dehydrogenase (complex II), cytochrome c reductase (complex III), cytochrome c oxidase (complex IV), and ATP synthase (complex V). These protein complexes can be separated by biochemical procedures and are well characterized for several organisms. However, there is mounting evidence that in vivo these protein complexes specifically interact forming supermolecular structures called supercomplexes: (1) purification protocols for individual OXPHOS complexes sometimes lead to the isolation of stoichiometric assemblies of two or more complexes which are functionally active (Hatefi et al., 1961; Hatefi and Rieske, 1967); (2) stable and enzymatically active supercomplexes can be reconstituted upon mixture of complexes I and III (Fowler and Hatefi, 1961; Fowler and Richardson, 1963; Hatefi, 1978; Ragan and Heron, 1978); (3) respiratory protein complexes from several bacteria were found to form specific supermolecular structures (Berry and Trumpower, 1985; Sone et al., 1987; Iwasaki et al., 1995; Niebisch and Bott, 2003); (4) inhibitor titration experiments reveal that the respiratory chain of yeast (Saccharomyces cerevisiae) behaves like a single functional unit (Boumans et al., 1998); and (5) flux control experiments indicate specific interactions of respiratory protein complexes (Genova et al., 2003). Several physiological roles were proposed for these respiratory supercomplexes, like substrate channeling, catalytic enhancement, protection of reactive reaction intermediates, and stabilization of individual protein complexes (Schägger and Pfeiffer, 2000; Genova et al., 2003).
Recently, characterization of mitochondrial supercomplexes was very much facilitated by the introduction of a novel experimental strategy which is based on protein solubilizations using mild nonionic detergents and separation of the solubilized protein complexes by Blue-native (BN) gel electrophoresis or gel chromatography (Arnold et al., 1998, 1999; Cruciat et al., 2000; Schägger and Pfeiffer, 2000; Zhang et al., 2002; Pfeiffer et al., 2003). Using this approach, several distinct supercomplexes could be described for mitochondria from different organisms (for review, see Schägger, 2001a, 2002).
In yeast, dimeric complex III (this protein complex always is dimeric for functional reasons) forms supercomplexes with one or two copies of complex IV. Furthermore, complex V was shown to partially occur in a dimeric state, which includes some dimer-specific subunits. In contrast, complex II from yeast does not form part of supermolecular structures under all experimental conditions applied. In beef, the complexes III2 and I form a supercomplex. Additionally, this supercomplex can include one to four copies of complex IV. The resulting large structures are called respirasomes, because they can autonomously carry out respiration in the presence of cytochrome c and ubiquinone (Schägger and Pfeiffer, 2000). Like in yeast, ATP synthase partially forms dimers, and complex II does not form part of supercomplexes.
Meanwhile, protein solubilizations using nonionic detergents and separations of solubilized protein complexes by BN-PAGE were used to systematically investigate the structure of the OXPHOS system of plants (Eubel et al., 2003). Three different supercomplexes were found in digitonin-solubilized mitochondrial fractions of Arabidopsis, potato (Solanum tuberosum), barley (Hordeum vulgare), and bean (Phaseolus vulgaris): (1) a 1,500-kD I1III2 supercomplex; (2) a 3,000-kD I2III4 supercomplex; and (3) a 1,100-kD dimeric ATP synthase complex. Depending on the plant investigated, the percentage of complex I integrated into the I1III2 supercomplex varies between 50% and 90%. The I2III4 supercomplex is of lower abundance and only becomes visible upon prolonged staining of BN gels. While the I1III2 and I2III4 supercomplexes are stable at high detergent to protein ratios, dimeric ATP synthase proved to be only stable at very low detergent concentrations. In contrast to yeast and mammals, cytochrome c oxidase (complex IV) of plant mitochondria did not form part of supercomplexes under all conditions applied. Instead, two different forms of monomeric complex IV are visible on BN gels, which are termed complex IVa and IVb (about 300 and 220 kD in Arabidopsis). Complex IVa includes at least one additional subunit, which is homologous to the Cox6b protein from mammals and yeast (Eubel et al., 2003).
Here we report a continuation of our efforts to carefully characterize the supermolecular structure of the OXPHOS system of plant mitochondria. Using highly active mitochondria isolated from freshly harvested potato tubers, five additional supercomplexes of about 850, 1,150, 1,850, 2,200, and 3,000 kD are visible on BN gels. All five protein complexes include complex IVa as shown by one-dimensional (1D) BN-PAGE, two-dimensional (2D) BN/SDS-PAGE, and 2D BN/BN-PAGE in combination with in-gel activity measurements for cytochrome c oxidase. The novel supercomplexes are of comparatively low abundance and have III2IV1, III2IV2, I1III2IV1, I1III2IV2, and I1III2IV4 compositions. Slightly smaller versions of these protein complexes occur in potato stem mitochondria, which include complex IVb instead of complex IVa. Hence, the OXPHOS complexes of plant mitochondria partially form respirasomes, which most likely have important physiological and/or regulatory functions.
RESULTS
Identification of Novel Supercomplexes in Potato Mitochondrial Fractions
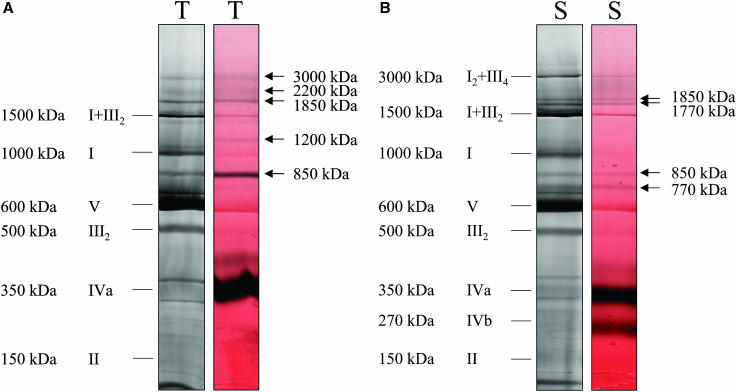
Previous investigations of digitonin-solubilized mitochondrial fractions from Arabidopsis, potato, bean, and barley by BN-PAGE led to the identification of I1III2 and I2III4 supercomplexes and dimeric ATP synthase (Eubel et al., 2003) but did not reveal hints on complex IV-containing supercomplexes which were described for yeast and mammalian mitochondria (Schägger and Pfeiffer, 2000). However, these findings were based on mitochondrial isolations from etiolated seedlings (bean and barley), aged storage organs (potato), and suspension cell cultures (Arabidopsis), and it so far cannot be ruled out that mitochondrial preparations from other tissues or organs might allow the discovery of further supercomplexes. In an attempt to re-examine our previous findings, freshly harvested potato tubers were used for mitochondrial isolations and subsequent characterizations of digitonin-solubilized protein extracts on 1D BN gels (Fig. 1A). In parallel, mitochondrial preparations from 20-d-old etiolated potato stems were analyzed by this procedure (Fig. 1B).
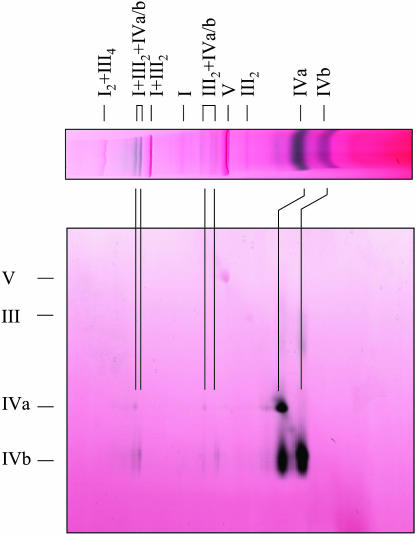
Figure 1.
Identification of complex IV-containing supercomplexes in potato tuber (T) and stem (S) mitochondria. Protein complexes were solubilized by 5 g digitonin per g protein, separated by 1D BN-PAGE and either visualized by Coomassie staining (left gel strips) or by in-gel activity staining for cytochrome c oxidase (right gel strips). Activity stains are given in false-color mode to increase color contrast (red, Coomassie; black, enzyme activity). Molecular masses and identities of known protein complexes are indicated on the left side of the gels in Roman numerals (I, NADH dehydrogenase; II, succinate dehydrogenase; III, cytochrome c reductase; IVa and IVb, large and small form of cytochrome c oxidase; V, ATP synthase; I + III2 and I2 + III4, supercomplexes of complexes I and III). Additional supercomplexes exhibiting cytochrome c oxidase activity are indicated by arrows.
All molecular masses of protein complexes given in this publication represent apparent molecular masses as deduced from separations on BN gels. These values should be considered with caution, because protein separations on BN gels do not exactly reflect calculated molecular masses. Some values for apparent molecular masses in this publication were corrected in comparison to the values given in Eubel et al. (2003): 600 kD for complex V (previously 550 kD), 350 kD for complex IVa (previously 300 kD), and 270 kD for complex IVb (previously 220 kD).
As expected, all known protein complexes of the OXPHOS system are visible on our gels (Fig. 1): complex I (approximately 1,000 kD), complex V (approximately 600 kD), and dimeric complex III (approximately 500 kD). Complex IVa (approximately 350 kD), complex IVb (approximately 270 kD), and complex II result in diffuse bands on the 1D gels but were clearly identified upon resolution of their subunits on second gel dimensions, which were carried out in the presence of SDS (data not shown). Finally, the I1III2 and I2III4 supercomplexes are visible. However, the occurrence of the I2III4 supercomplex and complex IVb was restricted to potato stem mitochondria. Dimeric ATP synthase could not be detected in both fractions, most likely because digitonin concentrations were too high.
Besides the known mitochondrial protein complexes and supercomplexes, additional complexes of low abundance showed up on our gels at approximately 850 kD and above 1,500 kD in both mitochondrial fractions (Fig. 1). To test if these protein supercomplexes include complex IV, in-gel activity measurements for cytochrome c oxidase were carried out. Indeed, five novel bands of approximately 850, 1,200, 1,850, 2,200, and 3,000 kD specifically were labeled in the potato tuber mitochondrial fraction (Fig. 1A). The 850- and 1,850-kD bands also are present in potato stem mitochondria and additionally two bands at approximately 770 and approximately 1,770 kD (Fig. 1). Identities of the newly discovered protein complexes were analyzed by 2D gel electrophoresis systems and are given below.
Physiological State of Mitochondrial Fractions Used for Supercomplex Characterizations
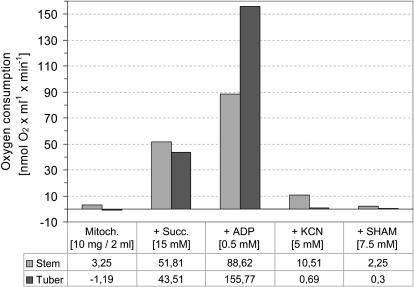
Oxygen uptake measurements were carried out using a Clark-type oxygen electrode to ensure that mitochondria used for the characterization of the novel supercomplexes are intact and physiologically active (Fig. 2). Organelles prepared from freshly harvested potato tubers exhibited high oxygen consumption rates (on average 155 nmol O2 min−1 mg−1 mitochondrial protein under state III conditions). In contrast, activity of potato stem mitochondria reproducibly was 40% to 50% lower under the same conditions. Mitochondria prepared from both organs had comparable state II respiration. Alternative respiration was low in mitochondrial isolations from potato stems and even lower in tuber mitochondria. We conclude that all mitochondrial fractions contained highly active organelles, but that mitochondria prepared from freshly harvested potato tubers exhibited highest state III respiration.
Figure 2.
Oxygen consumption of isolated mitochondria from potato tubers and stems. Values are based on three independent mitochondrial preparations.
Optimization of Protein Solubilizations for Supercomplex Characterizations
To allow optimal visualization of the novel mitochondrial supercomplexes, isolated mitochondria from potato tubers and stems were solubilized by varying concentrations of digitonin (Fig. 3). As previously reported (Eubel et al., 2003), 1 g digitonin per g mitochondrial protein only partially allowed solubilization of membrane-bound protein complexes as shown by resolutions on 1D BN gels. In contrast, solubilization of protein complexes and supercomplexes was very efficient between 2.5 and 10 g digitonin per g mitochondrial protein. Under these conditions, all known protein complexes and the newly discovered complexes of low abundance could be resolved. However, abundance of some supercomplexes decreased slightly in the presence of higher detergent to protein ratios. All further experiments were carried out with digitonin:protein ratios of 5 g/g.
Figure 3.
Resolution of mitochondrial protein complexes from potato mitochondria after solubilization using varying digitonin to protein ratios. Protein complexes were separated by 1D BN-PAGE and visualized by Coomassie staining. Detergent to protein ratios are given in g detergent per g mitochondrial protein. The OXPHOS complexes are designated by Roman numerals (see legend of Fig. 1). Unknown protein complexes are indicated by arrows.
Compositions of Newly Discovered Mitochondrial Supercomplexes
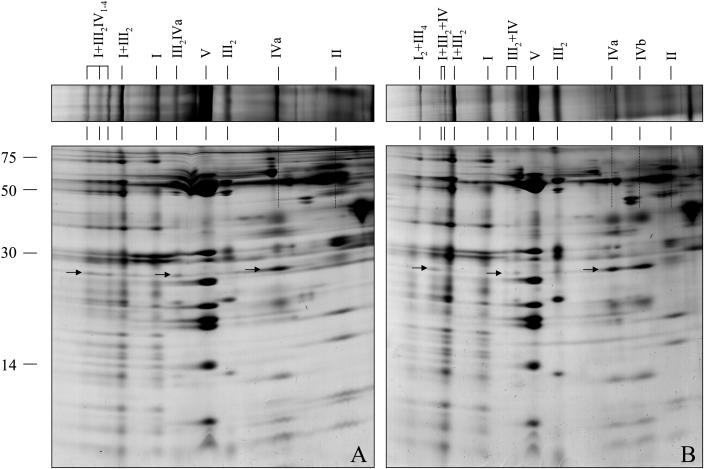
Two-dimensional BN/SDS-PAGE was carried out to characterize the subunit compositions of the novel mitochondrial protein supercomplexes (Fig. 4). High protein amounts had to be loaded onto the gels to overcome their low abundance and to obtain information on subunits of these supercomplexes. The 850-kD complex of potato tuber mitochondria contains subunits of complexes III and IV and most likely has III2IV composition (Fig. 4A). The 1,200-kD complex could not be detected on our 2D gels. The 1,850-, 2,200-, and 3,000-kD complexes of potato tuber mitochondria all contain the subunits of the I1III2 supercomplex and additionally the Cox2 protein, which is the most dominant subunit of complex IV on BN gels (Fig. 4A). Further subunits of complex IV probably are present but could not be detected because they overlap with subunits of the complexes I and III on our gels. Due to low abundance, densitometric measurements of individual protein spots did not allow resolution of the stoichiometry of the protein complexes within these supercomplexes. However, based on the apparent molecular masses on the BN gels, the 1,850-, 2,200-, and 3,000-kD supercomplexes probably have I1III2IV1, I1III2IV2, and I1III2IV4 compositions, which would be in accordance with findings on respiratory supercomplexes in mammalian mitochondria (Schägger and Pfeiffer, 2000). We conclude that complex IV forms part of supercomplexes in potato tuber mitochondria. However, about 90% of complex IV was in monomeric state under the conditions applied (Fig. 4A).
Figure 4.
Two-dimensional resolution of mitochondrial protein complexes from potato tubers (A) and potato stems (B) by BN/SDS-PAGE. Mitochondrial proteins were solubilized by 5 g digitonin per g protein. Gels were Coomassie stained. Strips of corresponding 1D BN gels and identities of protein complexes and supercomplexes are given above the 2D gels. The numbers on the left indicate the molecular masses of standard proteins. Subunit II of cytochrome c oxidase is marked by arrows.
Slightly different results were obtained upon resolution of mitochondrial protein complexes from potato stems by 2D BN/SDS-PAGE (Fig. 4B). First of all, about 50% of monomeric complex IV was not in the larger IVa (350 kD) but in the IVb form (270 kD), which could not be detected in the potato tuber mitochondrial fraction. Since the mitochondrial fractions from tubers and stems were treated equally, artificial generation of this smaller version of monomeric complex IV during mitochondrial isolations and/or BN-PAGE seems unlikely. As reported previously for Arabidopsis and bean, complex IVb lacks at least one 30-kD subunit, which was identified as being homologous to Cox6b proteins from yeast and mammals (Eubel et al., 2003). The 850- and 1,850-kD supercomplexes containing complex IV are also present in potato stem mitochondria and additionally two slightly smaller supercomplexes of 770 and 1,770 kD, which probably include complex IVb instead of complex IVa. The complex IV-containing 2,200- and 3,000-kD supercomplexes could not be found in mitochondrial isolations from potato stems. Instead, the 3,000-kD I2III4 supercomplex is present, which previously was described for Arabidopsis (Eubel et al., 2003).
Analysis of the Newly Discovered Supercomplexes by 2D BN/BN-PAGE
To further investigate the structure of the newly discovered complex IV-containing supercomplexes from potato, 2D gel electrophoreses were repeated using 2D BN/BN-PAGE (Schägger and Pfeiffer, 2000). This procedure is based on the separation of digitonin-solubilized protein complexes and supercomplexes on a first dimension BN-PAGE and subsequently a resolution of the separated supercomplexes on a second dimension BN-PAGE in the presence of dodecylmaltoside. Dodecylmaltoside is known to destabilize supercomplexes. Protein complexes and supercomplexes likewise stable in the presence of digitonin and dodecylmaltoside form a diagonal line on the resulting 2D gels, whereas supercomplexes destabilized by dodecylmaltoside dissociate into protein complexes of higher electrophoretic mobility.
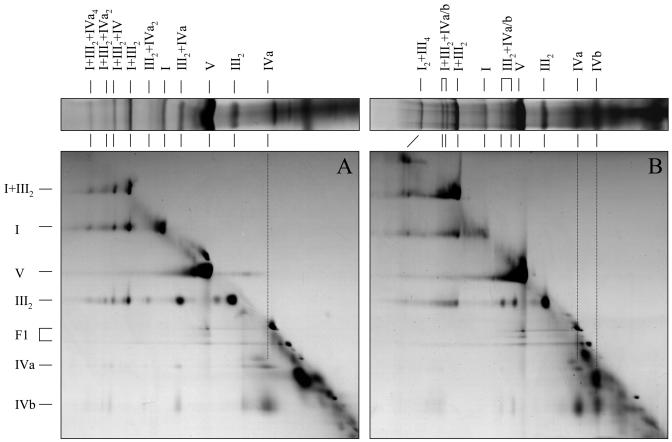
2D BN/BN-PAGE of mitochondrial fractions from potato tuber (Fig. 5A) confirmed all results obtained by 2D BN/SDS-PAGE: the 850-kD supercomplex consists of complexes III and IV and the 1,850-, 2,200-, and 3,000-kD supercomplexes of complexes I, III, and IV. Separation of all these supercomplexes not only revealed occurrence of complex IVa but also presence of the smaller complex IVb. However, since monomeric complex IVb is absent in potato tuber mitochondrial fractions after digitonin solubilizations (Figs. 1A and 4A) but present after additional dodecylmaltoside treatment (Fig. 5A), this version of complex IV most likely is artificially generated during BN/BN-PAGE under the conditions applied. In contrast to 2D BN/SDS-PAGE, 2D BN/BN-PAGE allowed the analysis of the 1,200-kD supercomplex present in potato tuber mitochondria. Like the 850-kD complex, this supercomplex only contains the complexes III and IV and probably has a III2IV2 composition.
Figure 5.
Two-dimensional resolution of mitochondrial protein complexes from potato tubers (A) and stems (B) by BN/BN-PAGE. Mitochondrial proteins were solubilized by 5 g digitonin per g protein. Corresponding strips of 1D BN gels are shown above the 2D gels. Identities of the resolved protein complexes and supercomplexes are given above and to the left of the gels in Roman numerals.
Analysis of mitochondrial fractions from potato stems by BN/BN-PAGE (Fig. 5B) also confirmed the findings obtained by 2D BN/SDS-PAGE: the 850- and 1,850-kD complexes include complexes III + IVa and I + III + IVa (complex IVa is partially converted into complex IVb as reported for mitochondria from potato tubers). The 770- and 1,770-kD complexes seem to have the same composition but most likely include complex IVb instead of complex IVa. The complex IV-containing 1,200-, 2,200-, and 3,000-kD supercomplexes of potato tuber mitochondria are absent, but a 3,000-kD I2III4 complex is present.
Interestingly, destabilization of the large complex IV-containing supercomplexes partially results in generation of the I1III2 but not of the III2IV1-2 supercomplexes (Fig. 5, A and B). We conclude that interactions between the complexes I and III are stronger than interactions between complexes III and IV.
In-Gel Activity Measurements for Cytochrome c Oxidase in 2D BN/BN Gels
To increase sensitivity, a 2D BN-BN gel for potato stem mitochondria was repeated and stained by in-gel activity measurements for cytochrome c oxidase. This measurement was not possible after polymerization of the 1D BN gel stripe into the sample gel of a second gel dimension, most likely because N,N,N′,N′-tetramethylethylenediamine (TEMED) and ammonium persulfate (APS) diffused into the gel stripe and destroyed enzymatic activities. However, fixation of the first gel dimension with agarose onto the second gel dimension proved to be compatible with this experimental approach. As shown in Figure 6, all previously made conclusions on complex IV-containing supercomplexes could be confirmed. Indeed, the 850- and 1,850-kD complexes include complex IVa, which partially dissociates into complex IVb in the presence of dodecylmaltoside. In contrast, the 770- and 1,770-kD complexes only contain the smaller IVb version of the cytochrome c oxidase complex (Fig. 6).
Figure 6.
Identification of cytochrome c oxidase-containing supercomplexes of potato stem mitochondria by in-gel activity staining on 2D BN/BN gels. Mitochondrial proteins were solubilized by 5 g digitonin per g protein. A corresponding stripe of an activity-stained 1D BN gel is shown above the 2D gel. Identities of protein complexes and supercomplexes of the OXPHOS system from potato are given in Roman numerals. The activity stain is given in false-color mode to increase color-contrast (red, Coomassie; black, enzyme activity).
DISCUSSION
Structure of Respiratory Supercomplexes in Plants, Animals, and Fungi
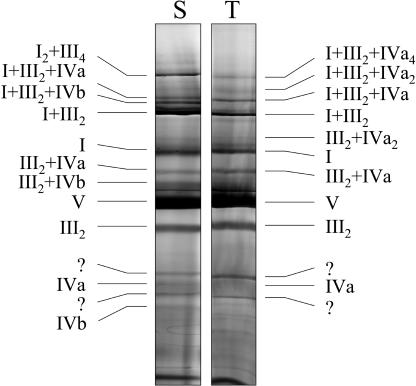
Besides the previously described I1III2 and I2III4 supercomplexes and dimeric ATP synthase, potato tuber mitochondria contain five additional respiratory supercomplexes of about 850-, 1,200-, 1,850-, 2,200-, and 3,000-kD, which include complex IV. The 850- and 1,200-kD complexes only contain complexes III and IV and probably have III2IV1 and III2IV2 compositions; the other three complex IV-containing supercomplexes additionally include complex I and most likely have I1III2IV1, I1III2IV2, and I1III2IV4 structures (Table I; Fig. 7). Similar supercomplexes were found in potato stem mitochondria. However, all newly described supercomplexes are of rather low abundance, because they only contain about 10% of total complex IV upon digitonin solubilizations and analysis on BN gels. Using comparable conditions, nearly 100% of yeast complex IV is associated with dimeric complex III (Cruciat et al., 2000; Schägger and Pfeiffer, 2000). In mammalian mitochondria—which include similar respiratory supercomplexes than potato (Schägger and Pfeiffer, 2000)—most complex IV also is present in the monomeric form. However, there are some striking differences between mammalian and plant mitochondria with respect to respiratory supercomplexes: most complex I of bovine mitochondria forms part of the I1III2IV1 complex, whereas in plants the I1III2 complex is of highest abundance. In fact the I1III2 complex seems to be of special stability in mitochondria from potato and other plants. Furthermore, a larger I2III4 supercomplex is present in plant mitochondria, which could not be described for mammalian mitochondria.
Table I.
Protein complexes and supercomplexes of the OXPHOS system in potato tuber and stem mitochondria
| Occurrence
|
||||
|---|---|---|---|---|
| Molecular Mass [kD] | Components | Proposed Composition | Tuber | Stem |
| 3,000 | I, III | I2 + III4 | — | x |
| 3,000 | I, III, IVa | I1 + III2 + IVa4 | x | — |
| 2,200 | I, III, IVa | I1 + III2 + IVa2 | x | — |
| 1,850 | I, III, IVa | I1 + III2 + IVa1 | x | x |
| 1,770 | I, III, IVb | I1 + III2 + IVb1 | — | x |
| 1,500 | I, III | I1 + III2 | x | x |
| 1,200 | III, IVa | III2 + IVa2 | x | — |
| 1,000 | I | I1 | x | x |
| 850 | III, IVa | III2 + IVa1 | x | x |
| 770 | III, IVb | III2 + IVb1 | — | x |
| 600 | V | V1 | x | x |
| 500 | III | III2 | x | x |
| 350 | IVa | IVa1 | x | x |
| 270 | IVb | IVb1 | — | x |
Figure 7.
Identities of protein complexes and supercomplexes of the OXPHOS system in potato tubers (T) and stems (S) after separation by 1D BN-PAGE. Proteins were solubilized by 5 g digitonin per g protein. The gels were Coomassie stained. Identities of the protein complexes and supercomplexes are given by Roman numerals.
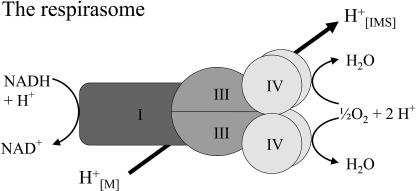
The I1III2IV4 supercomplex was suggested to represent a basic unit for respiration in mammalian mitochondria termed respirasome (Schägger and Pfeiffer, 2000). Respirasomes are also present in plant mitochondria (Fig. 8). However, only very minor amounts of complex IV form part of respirasomes in mammals and plants (<5%). On the other hand, these structures might be much more abundant in vivo and only destabilized under the experimental conditions used for their characterization. Indeed, low digitonin to protein ratios seem to allow solubilization of higher quantities of respirasomes in potato (Fig. 3). Possibly in vivo even larger structures than respirasomes are formed by oligomerization of supercomplexes. In fact, some very weak protein bands can be seen above 3,000 kD on the gels shown in Figure 7. The I2III4 supercomplex of plant mitochondria could be a building block of these proposed oligomeric structures.
Figure 8.
Structure and function of the respirasome in mitochondria. [M], Matrix; [IMS], mitochondrial intermembrane space.
Are Some Supercomplexes Artificially Formed during Protein Solubilizations?
So far, formation of specific respiratory supercomplexes by artificial aggregation cannot be completely excluded but is highly unlikely for several reasons: (1) all complex IV-containing supercomplexes proved to be active by in-gel activity measurements for cytochrome c oxidase; (2) higher abundance of complex IV-containing supercomplexes in potato tuber mitochondria in comparison to potato stem mitochondria correlated with higher state III respiration; (3) the five OXPHOS complexes could theoretically form 10 different heterodimeric supercomplexes (composed of two different monomeric complexes); however, only heterodimeric I-III and III-IV complexes were observed, which represent the only meaningful associations with respect to the physiology of the mitochondrial respiratory chain (besides II-III associations, which were not observed); and (4) several physiological data reviewed in the introduction section support specific supercomplex formations, like reconstitution, inhibitor titration, and flux control experiments (Hatefi and Rieske, 1967; Ragan and Heron, 1978; Boumans et al., 1998; Genova et al., 2003).
Assembly of Mitochondrial Supercomplexes
Currently the mechanisms for supercomplex formation in mitochondria are only poorly understood. In yeast cardiolipin proved to be essential for supercomplex stability. Based on studies with yeast mutants deficient in individual subunits of OXPHOS complexes, some proteins possibly forming part of supercomplex interphases could be defined (Pfeiffer et al., 2003). In potato the I1III2IV1-4 complexes partly dissociate into the I1III2 supercomplex and monomeric complex VI, indicating that the complex I-III association is much stronger than the interaction between these complexes and complex IV. This disassembly order might represent reverse assembly stages.
Experimental Conditions for Supercomplex Characterizations in Plants
Digitonin solubilization and BN-PAGE proved to be a powerful tool for the investigation of mitochondrial supercomplexes from plants. However, visualization of individual supercomplexes in mitochondrial fractions of plants very much depends on various factors:
The digitonin to protein ratio. Five grams detergent per g protein proved to be optimal for the quantitative solubilization of most supercomplexes (Fig. 3). However, lower detergent to protein ratios significantly increase the amounts of some supercomplexes on BN gels. In fact, solubilization using 1 g digitonin per g protein seems to mainly solubilize I1III2 and I1III2IV4 supercomplexes (lane 1 of the gels in Fig. 3).
The physiological state of the starting material for mitochondrial isolations. Freshly harvested potato tubers gave much better results concerning supercomplex visualization on BN gels than potato tubers stored for some weeks (data not shown). This most likely explains the absence of complex IV-containing supercomplexes of potato tubers in our previous investigations (however, some low amounts of the 850-kD III2IV1 supercomplex of potato mitochondria were overseen on the gel in Figure 4 in Eubel et al., 2003).
The plant organs selected for mitochondrial preparations. Potato tubers and stems slightly differ with respect to occurrence of individual supercomplexes. Overall, stem mitochondria contained less complex IV-containing respiratory supercomplexes. Furthermore, complex IV partially is present in the smaller IVb form in stem mitochondria. So far it cannot be distinguished whether these differences reflect tissue-specific variations or rather represent differences in physiological states of the organelles of these two tissues. Possibly etiolated seedlings or suspension cell cultures are not optimal as starting material for the characterization of labile interactions of mitochondrial protein complexes.
We speculate that complex IV-containing supercomplexes are present in other plants depending on the physiological state of the organs used for mitochondrial isolations but might be of low abundance. Indeed, mitochondria prepared from Arabidopsis leaves revealed some very small amounts of complex IV-containing supercomplexes (H. Eubel and H.-P. Braun, data not shown).
Functional Relevance of the Monomeric Cytochrome c Oxidase Complexes IVa and IVb of Plant Mitochondria
Monomeric complex IV is represented by two different forms in plants (Jänsch et al., 1996; Eubel et al., 2003; Sabar et al., 2003), the larger of which includes at least one additional protein subunit homologous to the Cox6b protein of fungi and mammals. The smaller complex IVb is generated by dissociation of the larger complex IVa in the presence of dodecylmaltoside. However, digitonin-solubilized mitochondrial fractions from potato tubers and stems differ considerably with respect to complex IVb, which is absent in digitonin extracts of potato tuber mitochondria (Fig. 4A) but represents about 50% of monomeric complex IV of stem mitochondria (Fig. 4B). Furthermore, supercomplexes of potato stem mitochondria seem to partially include the smaller IVb form of cytochrome c oxidase. Complex IVb is enzymatically active, but specific activity is significantly reduced in comparison to complex IVa (compare Figs. 1 and 4/5). At the same time, state III respiration of stem mitochondria is reduced as shown by oxygen consumption measurements of isolated mitochondria (Fig. 2). We therefore speculate that there might be distinct physiological roles of the two forms of cytochrome c oxidase in plants. Possibly plant mitochondria contain a pool of partially inactivated complex IV which rapidly can be activated upon association with the Cox6b protein.
An even larger probably monomeric form of complex IV can be seen by activity stainings of BN gels in the 400-kD range (Fig. 1). This version of complex IV is not visible on Coomassie-stained BN gels (Fig. 4), and its identity so far remains a mystery. Possibly this form of complex IV is a chaperone-bound assembly intermediate of cytochrome c oxidase. Similarly, a slightly larger form of complex III (550 instead of 500 kD) can be seen on the 2D BN/BN gel in Figure 5, which is invisible on the corresponding first gel dimension and might also represent a chaperone-bound form of this respiratory complex. Further experiments have to be carried out to explain these observations.
Outlook
Plant mitochondria exhibit several special features in comparison to mitochondria from heterotrophic eukaryotes. Due to the presence of numerous alternative oxidoreductases, the respiratory chain of plant mitochondria is very much branched (Vanlerberghe and McIntosh, 1997; Rasmusson et al., 1999; Moller, 2001; Moore et al., 2003). Furthermore, the protein complexes of the respiratory chain include plant-specific protein subunits (Braun and Schmitz, 1995; Eubel et al., 2003; Heazlewood et al., 2003a, 2003b; Millar et al., 2003). For instance, the two subunits of the mitochondrial processing peptidase form an integral part of complex III in plants (Braun et al., 1992b; Eriksson et al., 1994). As a consequence, respiratory supercomplexes most likely have special roles in plant mitochondria, e.g. in regulating access of alternative respiratory oxidoreductases to their substrates during respiration. Experiments to address these questions are under way in our laboratory.
MATERIALS AND METHODS
Isolation of Mitochondria from Potato Tubers and Stems
Freshly harvested potato (Solanum tuberosum var. cilena) tubers were purchased directly from a local farmer. Half of them were stored in the cold (4°C); the other half were planted into soil and grown in the dark at 20°C. Mitochondria were prepared from stored tubers and from etiolated potato stems after 20 d. Plant material (200 g) was homogenized at 4°C using a Waring blender for 3 × 5 s, filtrated through four layers of muslin, and subsequently organelles were purified by differential centrifugations and Percoll density gradient centrifugation as outlined previously (Braun et al., 1992a). Isolated mitochondria were either directly analyzed by gel electrophoresis or stored at −80°C.
Sample Preparation for Gel Electrophoresis
Mitochondrial samples of 500 μg (50 μg mitochondrial protein) were sedimented by centrifugation for 10 min at 14,000g, resuspended in 50 μL of digitonin solution (1%–10% digitonin/30 mm HEPES/150 mm potassium acetate/10% glycerol), and incubated for 20 min at 0°C. Afterwards samples were centrifuged for 10 min at 18,000g. Finally supernatants were supplemented with 5 μL of a Coomassie Blue solution (5% Coomassie Blue/750 mm aminocaproic acid) and directly loaded onto BN gels.
Gel Electrophoresis
BN-PAGE was carried out as described previously (Schägger, 2001b). Gels were destained by incubation in fixing solution (40% [v/v] methanol, 10% [v/v] acetic acid) overnight and subsequently stained with Coomassie colloidal (Neuhoff et al., 1985, 1990). Alternatively, strips of BN gels were transferred horizontally onto second gel dimensions. 2D BN/SDS-PAGE was carried out according to Schägger (2001b) and 2D BN/BN-PAGE according to Schägger and Pfeiffer et al. (2000). However, 1D gel strips for BN/BN-PAGE were fixed by 1.5% agarose onto the second gel dimension and not by direct polymerization into the stacking gel. This modification proved to be essential for subsequent in-gel activity measurements.
In-Gel Activity Stains for Cytochrome c Oxidase
In-gel activity of cytochrome c oxidase was measured according to Zerbetto et al. (1997) and Jung et al. (2000): 1D BN or 2D BN/BN gels were incubated in 20 mm phosphate buffer (pH 7.4), 1.0 mg/mL DAB (3,3′-diaminobenzidine), 24 units/mL catalase, 1 mg/mL cytochrome c, and 75 mg/mL sucrose. Reactions were carried out at room temperature for 1 h (1D gels) or overnight (2D gels). Staining was stopped by fixing the gels in 45% methanol/10% acetic acid. Finally, gels were scanned. To increase color contrast images were false-colored for Coomassie (red) and catalase activity (black) by Photoshop software (Adobe Systems, Mountain View, CA).
Oxygen Electrode Measurements
Oxidative phosphorylation of all mitochondrial preparations was analyzed using a Clark-type oxygen electrode with a reaction chamber of 2 mL (Oxygraph, Hansatech, Norfolk, England). Oxygen consumption of 10 mg mitochondria (1 mg mitochondrial protein) in reaction buffer (0.3 m mannitol, 10 mm K2HPO4 (pH 7.2), 10 mm KCl, 5 mm MgCl2) was measured after supplementation of succinate (15 mm), ADP (5 mm), KCN (5 mm), and salicylhydroxamic acid (SHAM; 7.5 mm). Mitochondrial oxygen consumption was calculated in nmol ΔO2 min−1 mg protein−1.
Acknowledgments
We thank Dagmar Lewejohann for expert technical assistance and Leila Matter, Dennis Kahlisch, and Prof. Dr. Udo Schmitz for critical reading of the manuscript.
This work was supported by the Fonds der Chemischen Industrie and the Deutsche Forschungsgemeinschaft (grant BR 1829–7/1.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.038018.
References
- Arnold I, Pfeiffer K, Neupert W, Stuart RA, Schägger H (1998) Yeast mitochondrial F1F0-ATP synthase exists as a dimer: identification of three dimer-specific subunits. EMBO J 17: 7170–7178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold I, Pfeiffer K, Neupert W, Stuart RA, Schägger H (1999) ATP synthase of yeast mitochondria: isolation of subunit j and disruption of the ATP18 gene. J Biol Chem 274: 36–40 [DOI] [PubMed] [Google Scholar]
- Berry EA, Trumpower BL (1985) Isolation of ubiquinol oxidase from Paracoccus denitrificans and resolution into cytochrome bc1 and cytochrome c-aa3 complexes. J Biol Chem 260: 2458–2467 [PubMed] [Google Scholar]
- Boumans H, Grivell LA, Berden JA (1998) The respiratory chain in yeast behaves as a single functional unit. J Biol Chem 273: 4872–4877 [DOI] [PubMed] [Google Scholar]
- Braun HP, Emmermann M, Kruft V, Schmitz UK (1992. a) Cytochrome c1 from potato: a protein with a presequence for targeting to the mitochondrial intermembrane space. Mol Gen Genet 231: 217–225 [DOI] [PubMed] [Google Scholar]
- Braun HP, Emmermann M, Kruft V, Schmitz UK (1992. b) The general mitochondrial processing peptidase from potato is an integral part of cytochrome c reductase of the respiratory chain. EMBO J 11: 3219–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun HP, Schmitz UK (1995) The bifunctional cytochrome c reductase/processing peptidase complex from plant mitochondria. J Bioenerg Biomembr 27: 423–436 [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Brunner S, Baumann F, Neupert W, Stuart RA (2000) The cytochrome bc1 and cytochrome c oxidase complexes associate to form a single supracomplex in yeast mitochondria. J Biol Chem 275: 18093–18098 [DOI] [PubMed] [Google Scholar]
- Eriksson AC, Sjöling S, Glaser E (1994) The ubiquinol cytochrome c oxidoreductase complex of spinach leaf mitochondria is involved in both respiration and protein processing. Biochim Biophys Acta 1186: 221–231 [Google Scholar]
- Eubel H, Jänsch L, Braun HP (2003) New insights into the respiratory chain of plant mitochondria. Supercomplexes and a unique composition of complex II. Plant Physiol 133: 274–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler LR, Hatefi Y (1961) Reconstitution of the electron transport system III. Reconstitution of DPNH oxidase, succinic oxidase, and DPNH succinic oxidase. Biochem Biophys Res Commun 5: 203–208 [DOI] [PubMed] [Google Scholar]
- Fowler LR, Richardson HS (1963) Studies on the electron transfer system. J Biol Chem 238: 456–463 [PubMed] [Google Scholar]
- Genova ML, Bianchi C, Lenaz G (2003) Structural organization of the mitochondrial respiratory chain. Ital J Biochem 52: 58–61 [PubMed] [Google Scholar]
- Hatefi Y (1978) Reconstitution of the electron-transport system of bovine heart mitochondria. Methods Enzymol 53: 48–54 [DOI] [PubMed] [Google Scholar]
- Hatefi Y, Haavik AG, Jurtshuk P (1961) Studies on the electron transport system. XXX. DPNH-cytochrome c reductase I. Biochim Biophys Acta 52: 106–118 [DOI] [PubMed] [Google Scholar]
- Hatefi Y, Rieske JS (1967) The preparation and properties of DPNH-cytochrome c reductase (complex I-III of the respiratory chain). Methods Enzymol 10: 225–231 [Google Scholar]
- Heazlewood JA, Howell KA, Millar AH (2003. b) Mitochondrial complex I from Arabidopsis and rice: orthologs of mammalian and yeast components coupled to plant-specific subunits. Biochim Biophys Acta 1604: 159–169 [DOI] [PubMed] [Google Scholar]
- Heazlewood JL, Whelan J, Millar AH (2003. a) The products of the mitochondrial ORF25 and ORFB genes are FO components of the plant F1FO ATP synthase. FEBS Lett 540: 201–205 [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Matsuura K, Oshima T (1995) Resolution of the aerobic respiratory system of the thermoacidophilic archaeon, Sulfolobus sp. Strain 7. I. The archael terminal oxidase supercomplex is a functional fusion of respiratory complexes III and IV with no c-type cytochromes. J Biol Chem 270: 30881–30892 [DOI] [PubMed] [Google Scholar]
- Jänsch L, Kruft V, Schmitz UK, Braun HP (1996) New insights into the composition, molecular mass and stoichiometry of the protein complexes of plant mitochondria. Plant J 9: 357–368 [DOI] [PubMed] [Google Scholar]
- Jung C, Higgins CMJ, Xu Z (2000) Measuring the quantity and activity of mitochondrial electron transport chain complexes in tissues of central nervous system using blue native polyacrylamide gel electrophoresis. Anal Biochem 286: 214–223 [DOI] [PubMed] [Google Scholar]
- Millar AH, Mittova V, Kiddle G, Heazlewood JL, Bartoli CG, Theodoulou FL, Foyer CH (2003) Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol 133: 443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52: 561–591 [DOI] [PubMed] [Google Scholar]
- Moore CS, Cook-Johnson RJ, Rudhe C, Whelan J, Day DA, Wiskich JT, Soole KL (2003) Identification of AtNDI1, an internal non-phosphorylating NAD(P)H dehydrogenase in Arabidopsis mitochondria. Plant Physiol 133: 1968–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff V, Stamm R, Eibl H (1985) Clear background and highly sensitive protein staining with Coomassie Blue dyes in polyacrylamide gels: a systematic analysis. Electrophoresis 6: 427–448 [Google Scholar]
- Neuhoff V, Stamm R, Pardowitz I, Arold N, Ehrhardt W, Taube D (1990) Essential problems in quantification of proteins following colloidal staining with Coomassie Brilliant Blue dyes in polyacrylamide gels, and their solution. Electrophoresis 11: 101–117 [DOI] [PubMed] [Google Scholar]
- Niebisch A, Bott M (2003) Purification of a cytochrome bc1-aa3 supercomplex with quinol oxidase activity from Corynebacterium glutamicum. Identification of a fourth subunit of cytochrome aa3 oxidase and mutational analysis of diheme cytochrome c1. J Biol Chem 278: 4339–4346 [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, Schägger H (2003) Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem 278: 52873–52880 [DOI] [PubMed] [Google Scholar]
- Ragan CI, Heron C (1978) The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Biochem J 174: 783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AG, Svensson AS, Knoop V, Grohmann L, Brennicke A (1999) Homologues of yeast and bacterial rotenone-insensitive NADH dehydrogenases in higher eukaryotes: two enzymes are present in potato mitochondria. Plant J 20: 79–87 [DOI] [PubMed] [Google Scholar]
- Sabar M, Gagliardi D, Balk J, Leaver CJ (2003) ORFB is a subunit of F(1)F(O)-ATP synthase: insight into the basis of cytoplasmic male sterility in sunflower. EMBO Rep 4: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H (2001. a) Respiratory chain supercomplexes. IUBMB Life 52: 119–128 [DOI] [PubMed] [Google Scholar]
- Schägger H (2001. b) Blue-native gels to isolate protein complexes from mitochondria. Methods Cell Biol 65: 231–244 [DOI] [PubMed] [Google Scholar]
- Schägger H (2002) Respiratory supercomplexes of mitochondria and bacteria. Biochim Biophys Acta 1555: 154–159 [DOI] [PubMed] [Google Scholar]
- Schägger H, Pfeiffer K (2000) Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J 19: 1777–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone N, Sekimachi M, Kutoh E (1987) Identification and properties of a quinol oxidase supercomplex composed of a bc1 complex and cytochrome oxidase in the thermophilic bacterium PS3. J Biol Chem 262: 15386–15391 [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L (1997) Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol 48: 703–734 [DOI] [PubMed] [Google Scholar]
- Zerbetto E, Vergani L, Dabbeni-Sala F (1997) Quantitation of muscle mitochondrial oxidative phosphorylation enzymes via histochemical staining of blue native polyacrylamide gels. Electrophoresis 18: 2059–2064 [DOI] [PubMed] [Google Scholar]
- Zhang M, Mileykovskaya E, Dowhan W (2002) Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem 277: 43553–43556 [DOI] [PubMed] [Google Scholar]