Abstract
There is a growing body of evidence that excess lipids, hypoxic stress and other inflammatory signals can stimulate endoplasmic reticulum (ER) stress in metabolic diseases. However, the pathophysiological importance and the underlying mechanisms of this phenomenon remain unknown. The current study investigated that 50 ng/ml oxidized LDL promoted unfolded protein response (UPR) and ER stress in J774A1 murine macrophages, which was blocked by extracts (PPE) of purple Perilla frutescens, a plant of the mint family Lamiaceae. The ER stressor tunicamycin was employed as a positive control. Treating 1–10 µg/ml oxidized LDL for 24 h elicited lipotoxic apoptosis in macrophages with obvious nuclear condensation and DNA fragmentation, which was inhibited by PPE. Tunicamycin and oxidized LDL activated and induced the UPR components of activating transcription factor 6 and ER resident chaperone BiP/Grp78 in temporal manners and such effects were blocked by ≥5 µg/ml PPE. In addition, PPE suppressed the enhanced mRNA transcription and splicing of X-box binding protein 1 (XBP1) by tunicamycin and oxidized LDL. The protein induction and nuclear translocation of XBP1 were deterred in PPE-treated macrophages under ER stress. The induction of ATP-binding cassette transporter A1 (ABCA1), scavenger receptor-B1 (SR-B1) and intracellular adhesion molecule-1 (ICAM-1) was abolished by the ER stressor in activated macrophages. The protein induction of ABCA1 and ICAM1 but not SR-B1 was retrieved by adding 10 µg/ml PPE to cells. These results demonstrate that PPE inhibited lipotoxic apoptosis and demoted the induction and activation of UPR components in macrophages. PPE restored normal proteostasis in activated macrophages oxidized LDL. Therefore, PPE was a potent agent antagonizing macrophage ER stress due to lipotoxic signals associated with atherosclerosis.
Introduction
An accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER) lumen causes a cellular ER stress initiating unfolded protein response (UPR) [1]. The UPR functions in order to primarily restore normal proteostasis in the ER by halting protein translation and activating the signaling pathways leading to the production of molecular chaperones involved in protein folding [2]. In the UPR scenario, transmembrane ER resident kinases including inositol-requiring enzyme 1α (IRE1α) are activated, and membrane-anchored activating transcription factor 6 (ATF6) is monomerized, followed by activation of versatile transcription factors of X-box binding protein 1 (XBP1) [1], [2]. The UPR instigates cell survival by improving protein folding capacity of ER, or promotes cell death following chronic ER stress [3]. Excessive activation of sustained UPR results in cellular dysfunction and cell death as major contributors to cancer and neurodegenerative diseases [4], [5]. Unresolved ER stress is involved in a variety of metabolic disorders, such as obesity and type 2 diabetes mellitus [6].
Emerging preclinical and clinical evidence supports the notion that pharmacological modulators of ER stress have therapeutic potential as novel treatments of metabolic disorders, including obesity, fatty liver disease, and atherosclerosis [7], [8]. The molecular mechanisms of UPR in the pathogenesis of metabolic diseases emphasize the roles of various UPR components in animal disease models and human pathologies [9], [10]. The emergence of compounds that target specific UPR signaling components, such as ATF6, IRE1, spliced XBP1 and the proteasomes, would provide promising therapeutics for the treatment of human metabolic diseases [11], [12]. Chemical chaperones such as sodium phenylbutyrate, reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes [13]. The ER modification by chemical chaperones in macrophages and adipocytes has therapeutic efficacy against atherosclerosis in mouse models [14].
Some clinically approved pharmacological agents used in clinical settings may affect the UPR pathways. The lipid-lowering compound pravastatin ameliorates ER stress in cultured neonatal rat cardiomyocytes and in pressure-overloaded hearts, and the anti-diabetic agent pioglitazone represses hepatic ER stress in reducing insulin resistance [15], [16]. Recent evidence suggests potential benefits from phytochemicals and polyphenols in reducing the elevated oxidative and lipid-mediated ER stress [17], [18]. Vaticanol B, a resveratrol derivative, inhibits inflammation and improves the ER environment by reducing the ER protein load and by maintaining the ER membrane integrity [19]. Additionally, grape seed proanthocyanidin extracts alleviate oxidative stress and ER stress in the skeletal muscle in the pathogenesis of type 2 diabetes mellitus [20]. The phytoalexins glyceollins enhances insulinotropic actions in enteroendocrine cells and normalizes glucose homeostasis through diminishing ER stress [21]. Furthermore, quercetin protects macrophages from oxidized low-density lipoprotein (LDL)-induced apoptosis by inhibiting the ER stress-CHOP signaling pathway [22].
Purple perilla (Perilla frutescens) has been used in traditional medicine for respiratory afflictions and seasonal allergies due to its anti-allergic and anti-inflammatory activity [23]. In our previous study [24], purple perilla frutescens leaf extract (PPE) inhibited aldose reductase activity. The present study attempted to investigate that PPE inhibited the induction of UPR signaling components in the ER stressor tunicamycin-exposed murine macrophages. This study examined ER stress components and PPE cytoprotection from lipotoxicity in lipid-laden macrophages. Furthermore, it was explored that PPE inhibited ER stress-triggered proteostasis of atherogenesis-associated biomarkers in activated macrophages.
Materials and Methods
Materials
Dulbecco’s Modified Eagle Medium (DMEM) chemicals, fatty acid-BSA, tunicamycin, and β-actin antibody were purchased from Sigma Aldrich Chemical (St. Louis, MO), as were all other reagents, unless specifically stated elsewhere. Fetal bovine serum (FBS) and penicillin-streptomycin were obtained from Lonza (Basel, Switzerland). Antibodies of ATF6, BiP/GRP78 and XBP1 were obtained from Novus Biologicals (Littleton, CO). Antibodies of ABCA1, scavenger receptor B1 (SR-B1) and intracellular adhesion molecule-1 (ICAM-1) were provided from Abcam (Cambridge, UK). The liver X receptor (LXR) agonist T091317 was purchased by Cayman Chemical Company (Ann Arbor, MI). HRP-conjugated goat anti-rabbit IgG was supplied from Jackson ImmunoResearch Laboratory (West Grove, PA). 4′,6-Diamidino-2-phenylindole (DAPI) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Preparation of purple perilla extracts
Purple perilla was purchased from a local market in Chuncheon, Korea. A voucher sample (RIC-2012-5) has been deposited at the Center for Efficacy Assessment and Development of Functional Foods and Drugs, Hallym University, Chuncheon, Korea. The specimens were authenticated by Emeritus Professor Hyung-Joon Chi, Seoul National University, Korea. Dried leaves of purple perilla frutescens (2 kg) were extracted 3 times with 99.5% methanol for 5 h. The solvent was evaporated under reduced pressure below 45°C to give a methanol extract (11.68% yields). The extract was suspended in distilled water and partitioned with n-hexane (n-Hex), methylene chloride (CH2Cl3), ethyl acetate (EtOAc), n-butanol (n-BuOH), and H2O to yield 40.83 g n-Hex (purple perilla extract, PPE), 25.20 g EtOAc, 22.24 g CH2Cl3, 116.88 g n-BuOH, and 27.42 g H2O fractions [24].
Preparation and oxidation of human plasma LDL
Human plasma LDL was prepared by a discontinuous density gradient ultra-centrifugation as previously described [25], [26]. The plasma LDL isolation was approved by the Hallym University Institutional Review Board (HIRB-2011-007-2). The written informed consent was waived by the HIRB for use of blood samples. A pooled human normolipidemic plasma LDL fraction was dialyzed and used within 4 weeks. The concentration of total cholesterol was measured by using commercial diagnostic kits (Asan Pharmaceuticals, Seoul, Korea). Oxidized LDL was prepared by incubating with 10 µM CuSO4 in F-10 medium at 37°C for 24 h. The extent of LDL oxidative modification was routinely determined using TBARS measurements and eletrophoretic mobility assay.
Macrophage culture and viability
Mouse (BALB/cN, ascites) macrophage-like cell line J774A1 (American Type Culture Collection, Manassas, VA) were grown in DMEM supplemented with 10% FBS at 37°C in a humidified atmosphere of 5% CO2 in air [26]. Macrophages were pre-treated with 1–10 µg/ml PPE and exposed to 50 µg/ml cholesterol-oxidized LDL or 1 µM tunicamycin for various times. J774A1 macrophages were incubated in DMEM supplemented with 0.4% fatty acid-free BSA and treated with CuSO4-oxidized LDL.
The cytotoxicity of 1 µM tunicamycin was determined using MTT assay after culture of J774A1 macrophages in the presence of PPE. J774A1 cells were incubated in a fresh medium containing 1 mg/ml MTT for 3 h at 37°C. The purple formazan product was dissolved in 0.5 ml isopropanol with gentle shaking. Absorbance of formed formazan was measured at λ = 570 nm using a microplate reader (Bio-Rad Model 550, Hercules, CA). In the current study PPE per se did not induce toxicity of cells within the concentration range of 1–10 µg/ml (data not shown).
DNA laddering
The DNA laddering assay was conducted with a commercial apoptotic DNA ladder detection kit (invitrogen, Camarillo, CA), according to the manufacture’s instruction. Cells were treated with 1 µM tunicamycin or 10 µg/ml PPE and extracted in a Tris-EDTA lysis buffer. Ammonium acetate solution and 100% ethanol were added for the precipitation of DNA debris at –20°C. After centrifugation at 12,000 rpm for 10 min, DNA pellets were washed with 70% ethanol and air-dried for 10 min. Dried DNA pellets were re-suspended by adding DNA suspension buffer containing Tris, glycerol, and orange G and then electrophoresed on 1% agarose gel with 0.5 µg/ml ethidium bromide. For the visualization of DNA ladders, gel images were taken by TFX-20 M model-UV transilluminator (Vilber-Lourmat, Marne-la-Vallée, France).
Hoechst 33258 staining
After treating 10 µg/ml PPE to macrophages exposed to 1 µM tunicamycin on a glass-chamber slide, a brief washing with PBS-0.2% Tween 20 was conducted. Cells were fixed with 4% formaldehyde for 15 min. After blocking cells with a 4% FBS for 1 h, cells were stained with 1 mg/ml of the nuclear dye Hoechst 33258 (Promega Co., Madison, WI) to visualize nuclear condensation and fragmentation under fluorescence microscopy. Images of each slide were taken using an optical microscope system (Axiomager, Zeiss, Oberkochen, Germany).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
For the measurement of cellular apoptosis and DNA fragmentation, TUNEL assay was conducted by using commercial Florometric TUNEL kits (Promega Co.). J774A1 murine macrophages were incubated with 1 µM tunicamycin for 24 h in the absence and presence of 10 µg/ml PPE and fixed with 4% formaldehyde 4°C for 25 min. Fixed cells were permeabilized with 0.2% Triton X-100 and labeled fragmented DNA with fluorescein-dUTP at 37°C for 1 h. DAPI was used for counter-staining nuclei that were visualized and quantified with using an Axiomager optical microscope system.
Western blot analysis
Following culture protocols, cells were lysed in a lysis buffer. Equal protein amounts of cell lysates were electrophoresed on 6–8% SDS-PAGE and transferred onto a nitrocellulose membrane. After blocking with either 5% non-fat dry skim milk or 3% fatty acid-free bovine serum albumin or for 3 h, membrane was incubated overnight at 4°C with polyclonal rabbit antibodies of ATF6, BiP/GRP78, XBP1, ABCA1, SR-B1and ICAM-1. After triple washes, membrane was incubated for 1 h with a goat anti-rabbit IgG conjugated to horseradish peroxidase. The individual protein level was determined using Immobilon Western Chemiluminescnet horseradish peroxidase substrate (Millipore, Billerica, MA). Incubation with mouse β-actin antibody was also performed for comparative controls. After performing immunoblotting, the blot bands were visualized on Agfa X-ray film (Agfa-Gevaert, Belgium).
RT-PCR analysis
Total RNA was obtained from J774A1 cells using a commercial Trizol reagent kit (Invitrogen, Carlsbad, CA). The cDNA was synthesized using 5 µg of total RNA with 200 units of reverse transcriptase and 0.5 mg/ml oligo-(dT)15 primer (Bioneer, Daejeon, Korea). The PCR was accomplished using mRNA transcripts of mouse spliced XBP1 (forward primer: 5′-TACGGGAGAAAACTCACGGC-3′, reverse primer: 5′-TTCCAGCTTGGCTGATGAGG-3′, 421 bp) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, forward primer: 5′-AACTTTGGCATTGTGGAAGGG-3′, reverse primer: 5′-GACACATTG GGGGTAGGAACAC-3′, 224 bp) with an addition of 25 µl of 10 mM Tris-HCl (pH 9.0) containing 25 mM MgCl2, 10 mM dNTP and 5 units of Taq DNA polymerase. XBP1 was conducted 27 cycles and each cycle consisted of 30 s at 94°C, 30 s at 60°C and 45 s at 72°C, and the final extension was for 10 min at 72°C. After thermocycling and electrophoresis of the PCR products (10 µl) on 3% agarose gel containing 0.5 mg/ml ethidium bromide, the bands were visualized using a Vilber-Lourmat UV transilluminator and gel photographs were obtained. The absence of contaminants was routinely checked by the RT-PCR assay of negative control samples without a primer addition.
Immunocytochemical analysis
Immunofluorescent cytochemical staining for monolayer cells of J774A1 cells grown on glass coverslips was performed using mouse spliced XBP1 antibody and Cy3-conjugated anti-rabbit IgG. Cells were fixed with 4% formaldehyde for 15 min and permeated with 0.1% Triton X-100 for 1 min on ice. Cells were blocked using a 20% FBS for 1 h, and anti-mouse XBP1 was applied to cells. Triple washing was followed and incubation with Cy3-conjugated goat anti-rabbit IgG was achieved for 1 h. Nuclear staining was carried out with 4 mg/ml DAPI. Each slide was mounted in a mounting medium (Sigma Aldrich Chemical). Images of each slide were taken using an optical microscope system. The spliced XBP1 levels were quantified by an image analysis program from the microscope system.
Data analysis
The results are presented as means ± SEM. Statistical analyses were conducted using the SAS software package version 6.12 (SAS Institute, Cary, NC). One-way ANOVA was used to determine inhibitory effects of PPE on cellular effects of oxidized LDL in macrophages. Differences among treatment groups were analyzed with Duncan’s multiple-range test and considered significant at P<0.05.
Results
Suppressive effect of PPE on ER stress-induced apoptosis
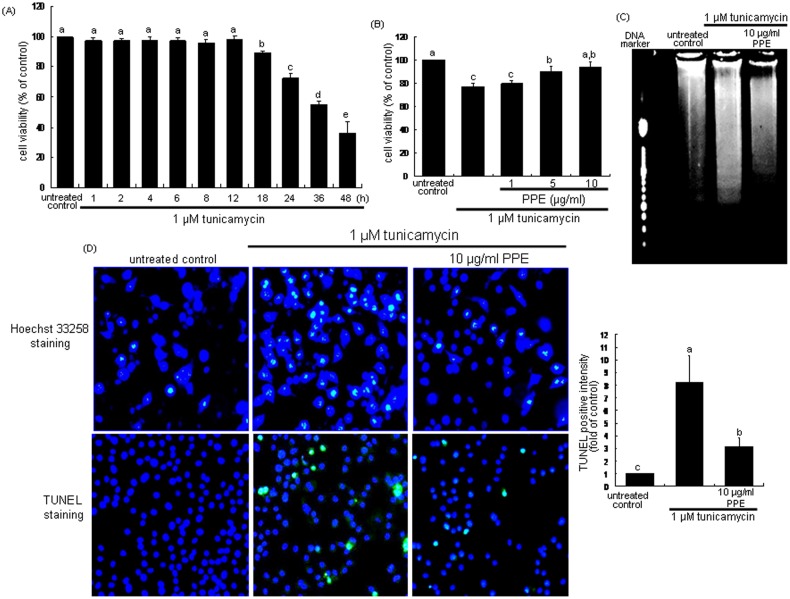
To examine the macrophage death by the ER stressor tunicamycin, MTT analysis was performed. When J774A1 macrophages were incubated with 1 µM tunicamycin for 48 h, ≈30% of macrophages were dead for 24 h (Figure 1A). In contrast, the decreased cell viability was near-completely restored by treating 10 µg/ml PPE (Figure 1B). The DNA laddering was obvious in tunicamycin-exposed macrophages, which was blunted by PPE (Figure 1C). In addition, tunicamycin-induced ER stress caused nuclear condensation and fragmentation of macrophages, while such condensation and fragmentation disappeared in 10 µg/ml PPE-treated cells, evidenced by Hoechst 33258 staining and TUNEL (Figure 1D). Accordingly, PPE alleviated macrophage apoptosis induced by ER stress.
Figure 1. Time course response of cytotoxicity by tunicamycin (A), elevation of cell viability by PPE (B), and DNA laddering (C) and inhibition of nuclear condensation and DNA fragmentation (D) and by PPE in tunicamycin-treated macrophages.
MTT assay was performed for the measurement of cell survival of 1 µM tunicamycin-exposed J774A1 murine macrophages by 1–10 µg/ml PPE (A and B). The bar graph data represent mean ± SEM from 4 independent experiments with multiple estimations. Values are expressed as percent cell survival relative to untreated control cells (cell viability = 100%). For the measurement of cellular apoptosis, DNA laddering, Hoechst33258 staining and TUNEL assay (C and D) were conducted with light microscopy and fluorescent microscopy, respectively. Histograms showing the relative fluorescent staining intensity of TUNEL-positive cells (D). Magnification: 200-fold. Means without a common letter differ, P<0.05.
Blockade of ATF6 activation and BiP/GRP78 induction by PPE
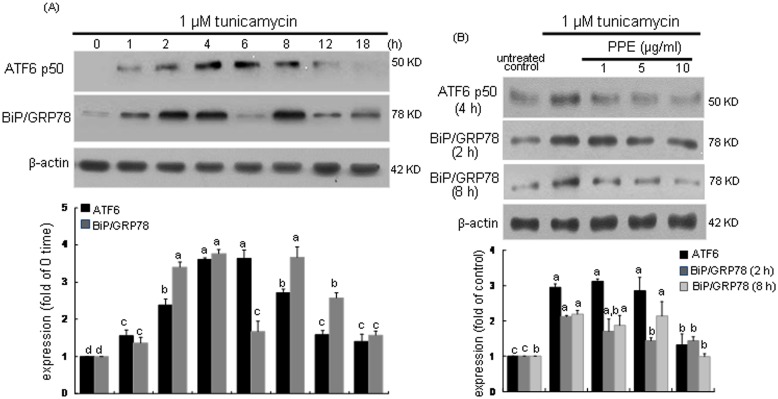
This study investigated whether PPE was able to inhibit the induction of UPR components and then alleviate macrophages ER stress. When macrophages were exposed to 1 µM tunicamycin, the ER transmembrane transcription factor ATF6 and the molecular chaperone BiP/GRP78 were temporally induced. As shown in Figure 2A, tunicamycin initiated ATF6 induction from 2 h after its exposure, and the induction was sustained up to 8 h. The ATF6 induction by treating tunicamycin for 6 h was dose-dependently suppressed by ≥5 µg/ml PPE (Figure 2B). On the other hand, tunicamycin also promoted the BiP/GRP78 induction in a dual fashion, in which early induction of BiP/GRP78 occurred from 1 h after the addition of tunicamycin (Figure 2A). In addition, its late induction ensued from 8 h-post exposure. PPE at the doses of ≥5 µg/ml suppressed both early (2 h elapsed) and late (8 h elapsed) inductions of BiP/GRP78 by tunicamycin (Figure 2B).
Figure 2. Time course responses of ATF6 and BiP/GRP78 induction (A) and their inhibition by 1–10 µg/ml PPE (B) in 1 µM tunicamycin-treated macrophages.
For the measurement of induction of ATF6 and BiP/GRP78 (B), total cell lysates were subject to Western blot analysis with a primary antibody against ATF6 or BiP/GRP78. β-Actin was used as an internal control. The bar graphs (mean ± SEM, n = 3) represent quantitative densitometric results of upper bands. Means without a common letter differ, P<0.05.
Inhibition of XBP1 induction by PPE
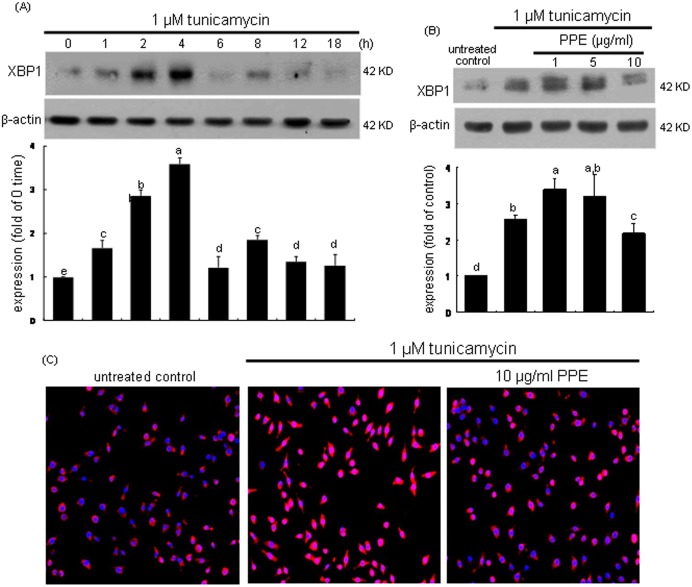
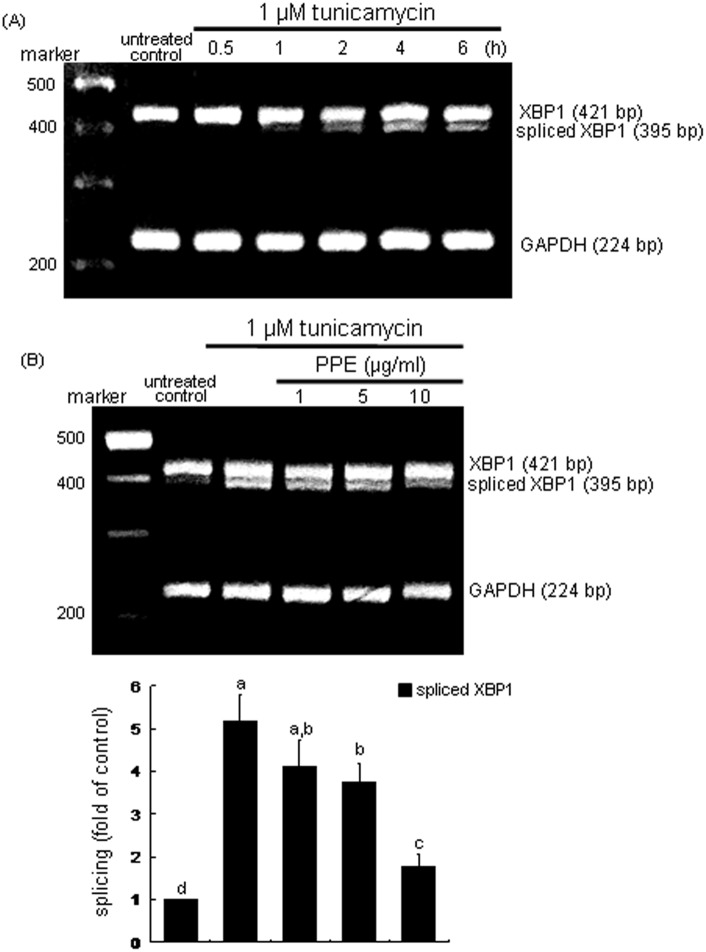
The transcription factor XBP-1 of macrophages was induced in an early stage within 2 h by tunicamycin (Figure 3A). The XBP1 induction was demoted by treating macrophages with 10 µg/ml PPE (Figure 3B). The nuclear translocation of XBP1 (pinkish staining) was enhanced in tunicamycin-exposed cells, which was deterred by administrating 10 µg/ml PPE (Figure 3C). These results indicates that PPE halted the formation of activated XBP1 leading to promoted transcriptional activation of UPR components. When the ER stress occurs, XBP1 mRNA is spliced by the endoRNase IRE1 and translocated into nucleus [1], [2]. As shown in Figure 4A, the XBP1 mRNA splicing was induced from 2 h after tunicamycin insult and such induction was sustained for another 2 h. In PPE-treated cells, the XBP-1 mRNA remained unspliced (Figure 4B). Accordingly, tunicamycin-triggered activation and nuclear translocation of the transcription factor XBP1, which was disturbed by adding PPE to macrophages.
Figure 3. Time course response of XBP1 induction (A) and its inhibition by 1–10 µg/ml PPE (B) and nuclear translocation (C) in 1 µM tunicamycin-exposed macrophages.
For the measurement of XBP1 induction, total cell lysates were subject to Western blot analysis with a primary antibody against XBP1. β-Actin was used as an internal control. The bar graphs (mean ± SEM, n = 3) represent quantitative densitometric results of upper bands. Means without a common letter differ, P<0.05. Nuclear translocation of XBP1 was detected by immunofluorocytochemical staining with Cy3-conjugated XBP1 antibody and nuclear counter-staining was carried out with DAPI (C). Microscopic observation was done by fluorescent microscopy. Magnification: 200-fold.
Figure 4. Temporal induction of XBP1 transcription (A) and inhibition of XBP1 transcription (B) by PPE.
J774A.1 macrophages were treated with 1 µM tunicamycin for 6 h in the absence and presence of 1–10 µg/ml PPE. XBP1 mRNA levels were measured by quantitative RT-PCR assay (A and B). GAPDH was used for the internal control (n = 3). The bar graphs (mean ± SEM, n = 3) represent quantitative densitometric results of upper bands. Means without a common letter differ, P<0.05.
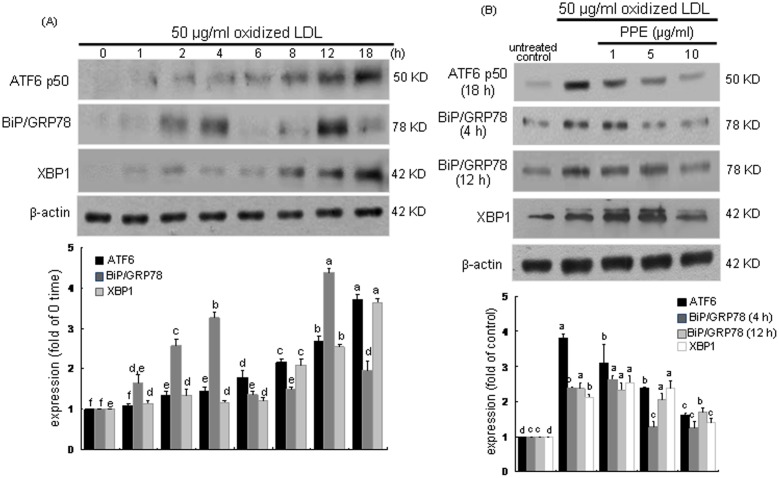
Repressive effect of PPE on oxidized LDL Induction of ER stress
Oxidized LDL is a critical factor in atherogenesis by triggering activation of inflammatory signaling pathway that may contribute to endothelial activation and dysfunction [27]. This study examined whether oxidized LDL elicited ER stress like tunicamycin and PPE inhibited ER stress in lipid-laden macrophages. Temporal induction of the ER stress markers of ATF6, BiP/GRP78 and XBP1 by oxidized LDL was observed (Figure 5A). Western blot analysis revealed that ATF6 activation and XBP1 expression were enhanced from 8 h after the exposure to 50 µg/ml oxidized LDL. The BiP/GRP78 induction was also enhanced in a dual manner, where oxidized LDL induced the BiP/GRP78 expression from 2 h to 4 h and at 12 h after its exposure (Figure 5A). It should be noted that oxidized LDL induced the respective UPR components later than tunicamycin did. When 10 µg/ml PPE was added to oxidized LDL-experienced macrophages, the enhanced ATF6 activation and XBP1 induction were significantly attenuated (Figure 5B). In addition, PPE abolished the dual induction of BiP/GRP78. Therefore, PPE blocked oxidized LDL-elicited ER stress via up-regulating the activation and induction of the UPR components of ATF6, BiP/GRP78 and XBP1.
Figure 5. Time course responses of ATF6, BiP/GRP78 and XBP1 (A) and their inhibition by 1–10 µg/ml PPE (B) in 1 µM tunicamycin-treated macrophages.
For the measurement of induction of ATF6, BiP/GRP78 and XBP1 (B), total cell lysates were subject to Western blot analysis with a primary antibody against ATF6, BiP/GRP78 or XBP1. β-Actin was used as an internal control. The bar graphs (mean ± SEM, n = 3) represent quantitative densitometric results of upper bands. Means without a common letter differ, P<0.05.
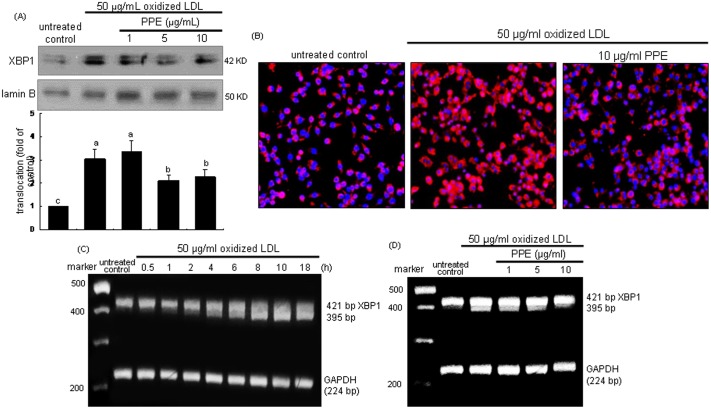
As shown in Figure 6A, nuclear XBP1 protein level was enhanced by treating cells with 50 µg/ml oxidized LDL, which was repressed by ≥5 µg/ml PPE. Consistently, the oxidized LDL-triggered nuclear translocation of XBP1 was disturbed in 10 µg/ml PPE-administrated macrophages, evidenced by Cy3-immunofluorocytochemical staining (Figure 6B). The XBP1 mRNA splicing was noticeably observed from 4 h after exposure to oxidized LDL (Figure 6C). In contrast, the spliced XBP-1 mRNA level was diminished in 1–10 µg/ml PPE-treated cells (Figure 6D). Thus, PPE was effective in inhibiting the transcriptional activity of XBP1 in lipid-laden macrophages.
Figure 6. Inhibition of nuclear translocation (A and B), and temporal response of XBP1 transcription and its inhibition by PPE (C and D).
J774A.1 macrophages were treated with 50 µg/ml oxidized LDL for 18 h in the absence and presence of 1–10 µg/ml PPE. Nuclear extracts were isolated by nuclear fraction assay and Western blot analysis was conducted by using primary anti-XBP1 (A). Lamin B was used as a nuclear control. Values (mean ± SEM, n = 3) not sharing a common letter are different at P<0.05. Nuclear translocation of XBP1 was also detected by immunofluorocytochemical staining with Cy3-conjugated XBP1 antibody and nuclear counter-staining was carried out with DAPI (B). Microscopic observation was done by fluorescent microscopy. Magnification: 200-fold. XBP1 mRNA levels were measured by quantitative RT-PCR assay (C and D). GAPDH was used for the internal control (n = 3).
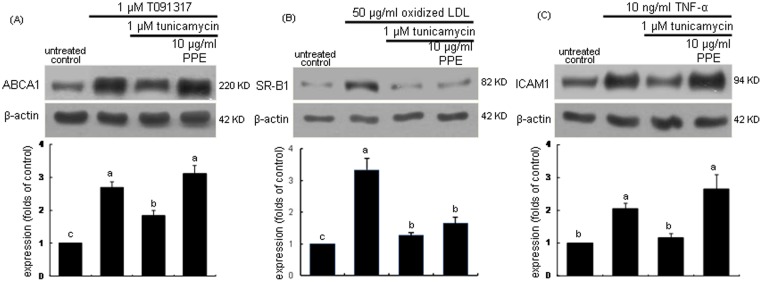
Restoration of protein folding activity by PPE
This study examined whether PPE retrieved the protein folding activity impaired by ER stress in macrophages. The LXR agonist T091317 at 1 µM, 50 µg/ml oxidized LDL and 10 ng/ml tumor necrosis factor (TNF)-α were employed for the macrophage induction of ABCA1, SR-B1, and ICAM1, respectively. These proteins were significantly stimulated by the respective stimulators (Figure 7). When the stimulated cells were exposed to 1 µM tunicamycin, the induction of ABCA1, SR-B1, and ICAM1 was abolished to their basal levels. In contrast, the treatment with 10 µg/ml PPE regained the induction of ABCA1 and ICAM1. These results demonstrate that PPE recovered the protein folding capacity of macrophage ER for the induction of ABCA1 and ICAM1. In contrast, the protein induction of SR-B1 by oxidized LDL was still impaired in 10 µg/ml PPE-treated macrophages, indicating that PPE did not affect the SR-B1 folding activity inhibited by the ER stressor.
Figure 7. PPE restoration of induction of ABCA1(A), SR-B1 (B) and ICAM-1 (C) demoted in tunicamycin-treated J774A1 murine macrophages.
Cells were stimulated with 1 µM T091317 for 12 h, 50 µg/ml Cu2+-oxidized LDL for 6 h, and 10 ng/ml TNF-α for 6 h in the absence and presence of 1 µM tunicamycin and 10 µg/ml PPE. For the measurement of expression of ABCA1, SR-B1 and ICAM-1, total cell lysates were subject to Western blot analysis with a primary antibody against ABCA1, SR-B1 and ICAM-1. β-Actin was used as an internal control. The bar graphs (mean ± SEM, n = 3) represent quantitative densitometric results of upper bands. Means without a common letter differ, P<0.05. Values (mean ± SEM, n = 3) not sharing a common letter are different at P<0.05.
Discussion
Seven major findings were observed from this study. 1) J774A.1 macrophages incubated with 1 µM tunicamycin for 24 h were dead by ≈30%, which was reversed by treating ≥5 µg/ml PPE. 2) PPE alleviated the ER stressor-induced apoptosis of macrophages with the concomitant inhibition of nuclear condensation and DNA laddering. 3) The PPE treatment ameliorated ER stress by reducing the activation and induction of UPR components of ATF6 and BiP/GRP78. 4) Tunicamycin induced XBP1 splicing and activation in macrophages, which was disturbed by administrating PPE to cells. 5) Oxidized LDL caused macrophage ER stress slowly with temporal activation and induction of ATF6, BiP/GRP78 and XBP1. 6) PPE ameliorated the ER stress in lipid-laden macrophages by demoting the activation and induction of these components. 7) Tunicamycin suppressed the protein induction of ABCA1 and ICAM1 in activated macrophages, whereas PPE retrieved the induction of these proteins. These observations demonstrate that PPE encumbered apoptosis and lipotoxicity, and restored normal proteostasis in ER stress-experienced macrophages. Therefore, PPE would be a promising candidate alleviating ER stress in macrophages involved in atherogenesis.
Cellular stimuli such as oxidative insult and calcium disturbance lead to the accumulation of unfolded proteins, a condition referred to as ER stress [1], [4]. In this study oxidized LDL containing oxysterols resulted in ER stress as the ER stress enhancer tunicamycin. Oxidized LDL highly activated the transmembrane transcription factor ATF6 and the transcription factor XBP1 in macrophages. The UPR increases the production of ER-resident chaperones triggering protein folding signaling pathways [1]. Oxidized LDL like tunicamycin induced the molecular chaperone BiP/GRP78 in a dual fashion. If excessive UPR in the ER of macrophages is sustained, cells will experience apoptotic cell death. Consistently, the nuclear condensation and DNA fragmentation appeared in macrophages treated with tunicamycin for 24 h. Since ER stress is involved in a variety of metabolic disorders [7], the therapeutic interventions targeting against UPR components and reducing ER stress would be promising strategies to treat metabolic diseases [11], [12]. Several studies demonstrate that phytochemicals and polyphenols reduce oxidative and lipid-mediated ER stress, hence inhibiting inflammation, obesity, and atherosclerosis [17]–[20]. Grape seed proanthocyanidins alleviate oxidative stress and skeletal muscle ER stress in the pathogenesis of type 2 diabetes mellitus [20]. In the present study PPE suppressed ER stress-induced macrophage apoptosis by tunicamycin through disturbing the induction and activation of UPR components. ER stress pertaining to the induction of small molecular chemical chaperones is a highly promising therapeutic target for metabolic diseases.
The balance between protein load and protein folding capacity of ER influences cell survival [3]. There is a growing body of evidence that a key cellular event in vulnerable atherosclerotic plaques is ER stress-induced macrophage apoptosis [12], [14], [28]. The mechanisms for the transition between adaptation to ER stress and ER stress-induced cell death are still being understood. The current study revealed that tunicamycin suppressed the protein synthesis of ABCA1, SR-B1 and ICAM-1 in activated macrophages, all involved in HDL biogenesis or atherogenesis. The protein ABCA1 is known as the cholesterol efflux regulatory protein and is a major regulator of cellular cholesterol and phospholipid homeostasis [29]. PPE restored the ABCA1 folding activity demoted by ER stress, suggesting that PPE may improve HDL biogenesis. Although the ICAM-1 induction in activated macrophages declined under ER stress, PPE also retrieved its protein expression and restored normal proteostasis. However, PPE did not improve SR-B1 expression deterred by ER stress in lipid-laden macrophages. The protein SR-B1 regulates the uptake of HDL-derived cholesterol and cholesteryl ester in steroidogenic tissues and facilitates macrophage cholesterol flux that may partly account for its effects on atherogenesis [30], [31]. It cannot be ruled out that PPE per se may attenuate SR-B1 induction by oxidized LDL despite its alleviation of ER stress.
The activation of these UPR signaling pathways plays a pro-survival role in the early stage of atherosclerosis [32]. With the progression of atherosclerosis, the increased intensity of ER stress in atherosclerotic lesions lead to prolonged and enhanced UPR signaling. UPR pathways induce expression of death effectors and activate apoptosis signaling pathways, leading to apoptosis of macrophages and smooth muscle cells in advanced lesions [32]. Prolonged oxidized LDL induced macrophage ER stress and apoptosis with the up-regulation of UPR components. Macrophages in atherosclerotic plaques produce abundant secretory proteins via UPR signaling pathways, which potentially induce ER stress in these cells [33]. Therapeutic approaches of PPE targeting the UPR may have promise in the prevention and/or regression of atherosclerosis. Similarly, quercetin protects macrophages from oxidized LDL-induced apoptosis by inhibiting the ER stress-CHOP signaling pathway [22].
Macrophage apoptosis can be caused by the accumulation of free cholesterol in the ER, leading to activation of the UPR [34], [35]. It is deemed that oxysterols present in oxidized LDL were the predominant regulator of oxidized LDL-induced macrophage ER stress. PPE promoted cholesterol efflux from macrophages through restoring the ABCA1 induction, possibly inhibiting lipotoxic apoptosis. In addition, PPE can encumber the uptake of oxidized lipids, which may render macrophages resistant to oxidized LDL-induced ER stress. The SR-B1 induction was not enhanced in PPE-administrated macrophages under ER stress.
In summary, the current study demonstrated that PPE reduced ER stress-induced apoptosis and alleviated UPR in J774A1 murine macrophages. Oxidized LDL promoted the induction of ER transcription factors and molecular chaperones, which was suppressed by PPE. In addition, PPE enhanced the induction of ABCA1 and ICAM1 demoted due to ER stress in activated macrophages. Accordingly, PPE encumbered lipotoxic apoptosis and restored normal proteostasis under ER stress of macrophages. Although PPE may serve as a beneficial in vitro modulator targeting against ER stress, the in vivo role of PPE remains unclear.
Supporting Information
Hallym University Institutional Review Board (HIRB-2011-007-2) for the plasma LDL isolation.
(PDF)
Certification for the exemption of written consent for use of blood samples.
(PDF)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This study was supported by High Value-added Food Technology Development Program, Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (112085-03-1-SB010) and by National Research Foundation of Korea through the Human Resource Training Project for Regional Innovation (2012-01-A-05-003-12-010100). The funders had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
References
- 1. Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, et al. (2013) Endoplasmic Reticulum and the Unfolded Protein Response: Dynamics and Metabolic Integration. Int Rev Cell Mol Biol 301: 215–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vandewynckel YP, Laukens D, Geerts A, Bogaerts E, Paridaens A, et al. (2013) The paradox of the unfolded protein response in cancer. Anticancer Res 33: 4683–4694. [PubMed] [Google Scholar]
- 3. Sano R, Reed JC (2013) ER stress-induced cell death mechanisms. Biochim Biophys Acta 1833: 3460–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernales S, Soto MM, McCullagh E (2012) Unfolded protein stress in the endoplasmic reticulum and mitochondria: a role in neurodegeneration. Front Aging Neurosci 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Endres K, Reinhardt S (2013) ER-stress in Alzheimer’s disease: turning the scale? Am J Neurodegener Dis 2: 247–265. [PMC free article] [PubMed] [Google Scholar]
- 6. Lee J, Ozcan U (2014) Unfolded protein response signaling and metabolic diseases. J Biol Chem 289: 1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cao SS, Kaufman RJ (2013) Targeting endoplasmic reticulum stress in metabolic disease. Expert Opin Ther Targets 17: 437–448. [DOI] [PubMed] [Google Scholar]
- 8. Pagliassotti MJ (2012) Endoplasmic reticulum stress in nonalcoholic fatty liver disease. Annu Rev Nutr 32: 17–33. [DOI] [PubMed] [Google Scholar]
- 9. Ryno LM, Wiseman RL, Kelly JW (2013) Targeting unfolded protein response signaling pathways to ameliorate protein misfolding diseases. Curr Opin Chem Biol 17: 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iwawaki T, Oikawa D (2013) The role of the unfolded protein response in diabetes mellitus. Semin Immunopathol 35: 333–350. [DOI] [PubMed] [Google Scholar]
- 11. Engin F, Hotamisligil GS (2010) Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab 12 (Suppl 2)108–115. [DOI] [PubMed] [Google Scholar]
- 12. Minamino T, Komuro I, Kitakaze M (2010) Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res 107: 1071–1082. [DOI] [PubMed] [Google Scholar]
- 13. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, et al. (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, et al. (2009) Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 15: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao H, Liao Y, Minamino T, Asano Y, Asakura M, et al. (2008) Inhibition of cardiac remodeling by pravastatin is associated with amelioration of endoplasmic reticulum stress. Hypertens Res 31: 1977–1987. [DOI] [PubMed] [Google Scholar]
- 16. Yoshiuchi K, Kaneto H, Matsuoka TA, Kasami R, Kohno K, et al. (2009) Pioglitazone reduces ER stress in the liver: direct monitoring of in vivo ER stress using ER stress-activated indicator transgenic mice. Endocr J 56: 1103–1111. [DOI] [PubMed] [Google Scholar]
- 17. Zingg JM, Hasan ST, Meydani M (2013) Molecular mechanisms of hypolipidemic effects of curcumin. Biofactors 39: 101–121. [DOI] [PubMed] [Google Scholar]
- 18. Chuang CC, McIntosh MK (2011) Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu Rev Nutr 31: 155–176. [DOI] [PubMed] [Google Scholar]
- 19. Tabata Y, Takano K, Ito T, Iinuma M, Yoshimoto T, et al. (2007) Vaticanol B, a resveratrol tetramer, regulates endoplasmic reticulum stress and inflammation. Am J Physiol Cell Physiol 293: C411–C418. [DOI] [PubMed] [Google Scholar]
- 20. Ding Y, Dai X, Jiang Y, Zhang Z, Bao L, et al. (2013) Grape seed proanthocyanidin extracts alleviate oxidative stress and ER stress in skeletal muscle of low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Mol Nutr Food Res 57: 365–369. [DOI] [PubMed] [Google Scholar]
- 21. Park S, Ahn IS, Kim JH, Lee MR, Kim JS, et al. (2010) Glyceollins, one of the phytoalexins derived from soybeans under fungal stress, enhance insulin sensitivity and exert insulinotropic actions. J Agric Food Chem 58: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 22. Yao S, Sang H, Song G, Yang N, Liu Q, et al. (2012) Quercetin protects macrophages from oxidized low-density lipoprotein-induced apoptosis by inhibiting the endoplasmic reticulum stress-C/EBP homologous protein pathway. Exp Biol Med (Maywood) 237: 822–831. [DOI] [PubMed] [Google Scholar]
- 23. Liu JY, Chen YC, Lin CH, Kao SH (2013) Perilla frutescens leaf extract inhibits mite major allergen Der p2-induced gene expression of pro-allergic and pro-inflammatory cytokines in human bronchial epithelial cell BEAS-2B. PLoS One 8: e77458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paek JH, Shin KH, Kang YH, Lee JY, Lim SS (2013) Rapid identification of aldose reductase inhibitory compounds from Perilla frutescens. Biomed Res Int 2013: 679463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jeong YJ, Choi YJ, Kwon HM, Kang SW, Park HS, et al. (2005) Differential inhibition of oxidized LDL-induced apoptosis in human endothelial cells treated with different flavonoids. Br J Nutr 93: 581–591. [DOI] [PubMed] [Google Scholar]
- 26. Park SH, Kim JL, Lee ES, Han SY, Gong JH, et al. (2011) Dietary ellagic acid attenuates oxidized LDL uptake and stimulates cholesterol efflux in murine macrophages. J Nutr 141: 1931–1937. [DOI] [PubMed] [Google Scholar]
- 27. Li D, Mehta JL (2005) Oxidized LDL, a critical factor in atherogenesis. Cardiovasc Res 68: 353–354. [DOI] [PubMed] [Google Scholar]
- 28. Tabas I, Seimon T, Timmins J, Li G, Lim W (2009) Macrophage apoptosis in advanced atherosclerosis. Ann N Y Acad Sci 1173 (Suppl 1)E40–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yokoyama S (2006) ABCA1 and biogenesis of HDL. J Atheroscler Thromb 13: 1–15. [DOI] [PubMed] [Google Scholar]
- 30. Valacchi G, Sticozzi C, Lim Y, Pecorelli A (2011) Scavenger receptor class B type I: a multifunctional receptor. Ann N Y Acad Sci 1229: E1–E7. [DOI] [PubMed] [Google Scholar]
- 31. Ji A, Meyer JM, Cai L, Akinmusire A, de Beer MC, Webb NR, et al. (2011) Scavenger receptor SR-BI in macrophage lipid metabolism. Atherosclerosis 217: 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou AX, Tabas I (2013) The UPR in atherosclerosis. Semin Immunopathol 35: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352: 1685–1695. [DOI] [PubMed] [Google Scholar]
- 34. Feng B, Yao PM, Li Y, Devlin CM, Zhang D, et al. (2003) The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5: 781–792. [DOI] [PubMed] [Google Scholar]
- 35. DeVries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, et al. (2005) Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol 171: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hallym University Institutional Review Board (HIRB-2011-007-2) for the plasma LDL isolation.
(PDF)
Certification for the exemption of written consent for use of blood samples.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.