Abstract
Inhibition of net photosynthesis (Pn) by moderate heat stress has been attributed to an inability of Rubisco activase to maintain Rubisco in an active form. To examine this proposal, the temperature response of Pn, Rubisco activation, chlorophyll fluorescence, and the activities of Rubisco and Rubisco activase were examined in species from contrasting environments. The temperature optimum of Rubisco activation was 10°C higher in the creosote bush (Larrea tridentata) compared with the Antarctic hairgrass (Deschampsia antarctica), resembling the temperature response of Pn. Pn increased markedly with increasing internal CO2 concentration in Antarctic hairgrass and creosote bush plants subjected to moderate heat stress even under nonphotorespiratory conditions. Nonphotochemical quenching of chlorophyll fluorescence, the effective quantum yield of photochemical energy conversion (ΔF/Fm′) and the maximum yield of PSII (Fv/Fm) were more sensitive to temperature in Antarctic hairgrass and two other species endemic to cold regions (i.e. Lysipomia pumila and spinach [Spinacea oleracea]) compared with creosote bush and three species (i.e. jojoba [Simmondsia chinensis], tobacco [Nicotiana tabacum], and cotton [Gossypium hirsutum]) from warm regions. The temperature response of activity and the rate of catalytic inactivation of Rubisco from creosote bush and Antarctic hairgrass were similar, whereas the optimum for ATP hydrolysis and Rubisco activation by recombinant creosote bush, cotton, and tobacco activase was 8°C to 10°C higher than for Antarctic hairgrass and spinach activase. These results support a role for activase in limiting photosynthesis at high temperature.
The temperature optimum for higher plant photosynthesis is usually rather broad and generally matches the average daytime temperature encountered in the natural environment (Berry and Björkman, 1980; Larcher, 1995). Significant inhibition of photosynthesis occurs at temperatures above the optimum, resulting in considerable loss of potential productivity. Inhibition of photosynthesis by heat stress has long been attributed to an impairment of electron transport activity, caused in part by changes in membrane fluidity (Raison et al., 1982; Havaux, 1993; Murakami et al., 2000). However, others (Bilger et al., 1987; Jones et al., 1998; Bukhov et al., 1999; Bukhov and Dzhibladze, 2002) support the idea that the initial site of inhibition is associated with a Calvin cycle reaction, specifically the inactivation of Rubisco (Weis, 1981a, 1981b; Kobza and Edwards, 1987). This idea has been revitalized recently by newer data from our laboratory (Feller et al., 1998; Law and Crafts-Brandner, 1999; Crafts-Brandner and Law, 2000; Crafts-Brandner and Salvucci, 2000) coupled with a more thorough understanding of the biochemistry of the activation process (Andrews, 1996; Spreitzer and Salvucci, 2002; Portis, 2003).
The activation state of Rubisco in leaves reflects a balance between sequestration of Rubisco active sites in a closed, inactive conformation and the reactivation of these sites by conformational changes induced by Rubisco activase (Andrews, 1996; Spreitzer and Salvucci, 2002; Portis, 2003). Previous studies provided a biochemical basis for inactivation of Rubisco under heat stress by showing that this balance shifts to a lower activation state at higher temperature because of faster rates of Rubisco inactivation and slower rates of activase activity (Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner, 2004). In cotton (Gossypium hirsutum), wheat (Triticum aestivum), tobacco (Nicotiana tabacum), and maize (Zea mays), a decrease in Rubisco activation under moderate heat stress correlated with reduced rates of net photosynthesis (Pn) and was accompanied by increased levels of RuBP and decreased levels of 3-phosphoglycerate (Kobza and Edwards, 1987; Law and Crafts-Brandner, 1999; Crafts-Brandner and Law, 2000; Sharkey et al., 2001; Crafts-Brandner and Salvucci, 2002). Analysis of the response of Pn to temperature and CO2 concentration in cotton under both photorespiratory and nonphotorespiratory conditions showed that the temperature response of Pn could be predicted from the kinetic properties of Rubisco if the measured changes in Rubisco activation were included in the calculation (Crafts-Brandner and Salvucci, 2000).
When taken together, the accumulated data from gas exchange and biochemical analyses suggest that deactivation of Rubisco, caused at least in part by thermal inactivation of activase (Salvucci et al., 2001), is the primary cause of inhibition of photosynthesis under moderate heat stress (Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner, 2004). To determine if this concept is broadly applicable and to further document the role of activase, we compared the temperature response of Pn, Rubisco activation, chlorophyll fluorescence, and Rubisco and activase activities in plants from contrasting thermal environments. The results suggest that the upper temperature limit of photosynthesis is determined by the thermal properties of activase.
RESULTS
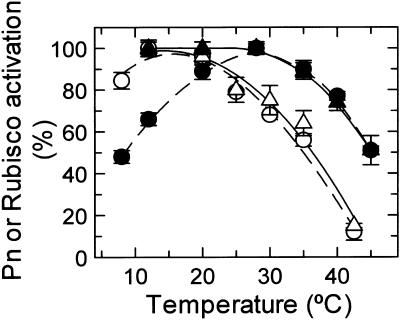
Pn and Rubisco activation were measured in two C3 plant species from contrasting thermal environments (Fig. 1). The Antarctic hairgrass (Deschampsia antarctica) is one of two angiosperms endemic to maritime Antarctica (Xiong et al., 1999; Alberdi et al., 2002). Creosote bush (Larrea tridentate) is a desert shrub adapted to hot, arid regions of the U.S. southwest (Mooney et al., 1978; Hamerlynck et al, 2000). Gas exchange measurements showed that the temperature optimum of Pn for Antarctic hairgrass and creosote bush differed by about 10°C. For Antarctic hairgrass, Pn was inhibited by temperatures higher than about 20°C, whereas inhibition of creosote bush photosynthesis occurred at temperatures higher than 30°C. Rubisco activation (i.e. the fraction of active Rubisco in the leaf) decreased at precisely the same temperatures that inhibited Pn, and the extent of inhibition of Pn and Rubisco activation was similar at a given high temperature. A similar close relationship between the responses of Pn and Rubisco activation to elevated temperature has been reported in isolated spinach (Spinacea oleracea) chloroplasts (Weis, 1981b) and in intact leaves of maize (Crafts-Brandner and Salvucci, 2002), wheat, and cotton (Law and Crafts-Brandner, 1999; Crafts-Brandner and Salvucci, 2000).
Figure 1.
Effect of temperature on Pn and Rubisco activation in Antarctic hairgrass and creosote bush. Pn (•, ○, dashed lines) was measured in attached leaves of Antarctic hairgrass (○) and creosote bush (•) in air at the indicated leaf temperatures. Rubisco activation (▵, ▴, solid lines) was determined by rapid extraction and assay of Antarctic hairgrass (▵) and creosote bush (▴) leaves, sampled by freeze-clamping the same leaves used for gas exchange immediately following the measurement. The data for Pn are expressed as a percentage of the rate at the temperature optimum; 29 ± 2 and 19.4 ± 1 μmol m−2 s−1 for creosote bush and Antarctic hairgrass, respectively. The data for Rubisco activation are expressed as a percentage of the activity of the fully light-activated enzyme measured at the temperature optimum; 87.5 ± 1 and 81.1 ± 3.9 μmol m−2 s−1 for creosote bush and Antarctic hairgrass, respectively. These activities were similar to the activities of the enzymes after carbamylation in vitro with CO2 and Mg2+.
The responsiveness of Pn to increasing internal CO2 concentration (Ci) was determined for Antarctic hairgrass and creosote bush at leaf temperatures that caused moderate heat stress. These measurements were conducted in low O2 (i.e. 10 mbar) to eliminate the complications associated with photorespiration (Monson et al., 1982; Kobza and Edwards, 1987). At air levels of CO2 under both atmospheric and low O2, Pn in creosote bush was inhibited by approximately 28% at 42.5°C compared with 28°C (Table I). When Ci was increased progressively at a leaf temperature of 42.5°C, the rate of Pn at 10 mbar O2 increased markedly. Similar results were observed with Antarctic hairgrass, even though the temperature required for inhibition of Pn was lower (Table II). For example, Pn at air levels of CO2 in Antarctic hairgrass was inhibited by approximately 38% at 36°C compared with 16°C under both atmospheric and low O2, and the rate of Pn at 36°C increased markedly when Ci was increased in low O2.
Table I.
Effect of temperature, Ci, and O2 on Pn and Rubisco activation in intact creosote bush leaves
| Temperature | O2 | Ci | Pn | Rubisco activation |
|---|---|---|---|---|
| °C | mbar | μbar | μmol m−2 s−1 | % |
| 28 | 210 | 261 | 29.0 ± 0.6 | 100 |
| 28 | 10 | 243 | 43.9 ± 1.1 | 79.4 |
| 40 | 210 | 258 | 23.3 ± 1.6 | 84.7 |
| 42.5 | 210 | 262 | 19.3 ± 1.6 | 60 |
| 42.5 | 10 | 241 | 32.8 ± 1.1 | NDa |
| 42.5 | 10 | 335 | 39.8 ± 1.4 | ND |
| 42.5 | 10 | 489 | 49.5 ± 0.7 | ND |
| 42.5 | 10 | 687 | 56.4 ± 0.7 | ND |
| 42.5 | 10 | 844 | 59.0 ± 0.3 | 39.1 |
The Pn of each of three sets of leaves was measured under all of the conditions listed below by continuously changing the O2, temperature, and Ci after first achieving a steady-state rate at 28°C, 210 mbar O2, and 261 μbar Ci. Rubisco activation was measured by rapid extraction and assay of freeze-clamped leaves. By necessity, separate sets of leaves were used for determining Rubisco activation.
ND, Not determined.
Table II.
Effect of temperature, Ci, and O2 on Pn and Rubisco activation in intact Antarctic hairgrass leaves
| Temperature | O2 | Ci | Pn | Rubisco activation |
|---|---|---|---|---|
| °C | mbar | μbar | μmol m−2 s−1 | % |
| 16 | 210 | 290 | 19.4 ± 0.5 | 100 |
| 16 | 10 | 270 | 25.6 ± 2.3 | 77.8 |
| 32 | 210 | 234 | 13.4 ± 0.4 | NDa |
| 36 | 210 | 241 | 11.4 ± 0.7 | 66.4 |
| 36 | 10 | 213 | 16.8 ± 1.0 | 50.3 |
| 36 | 10 | 284 | 22.1 ± 1.3 | ND |
| 36 | 10 | 395 | 28.9 ± 2.3 | ND |
| 36 | 10 | 549 | 36.1 ± 2.4 | ND |
| 36 | 10 | 702 | 40.8 ± 2.5 | 37.1 |
The Pn of each of three sets of leaves was measured under all of the conditions listed below by continuously changing the O2, temperature, and Ci after first achieving a steady-state rate at 16°C, 210 mbar O2, and 290 μbar Ci. Rubisco activation was measured by rapid extraction and assay of freeze-clamped leaves. By necessity, separate sets of leaves were used for determining Rubisco activation.
ND, Not determined.
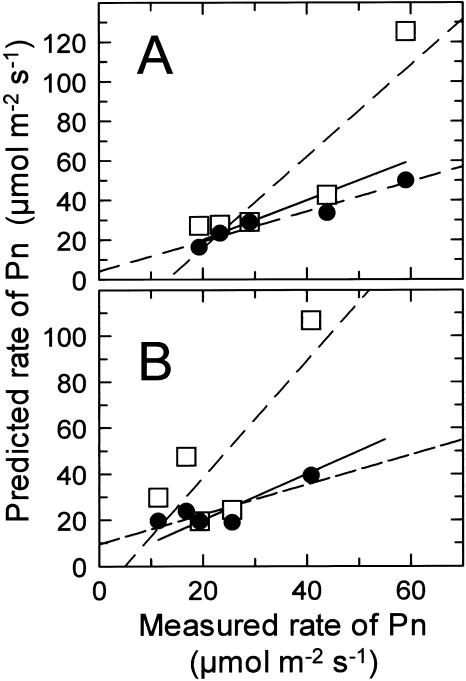
Measurements of the activation state of Rubisco in Antarctic hairgrass and creosote bush under the conditions described above revealed that Rubisco deactivated when leaf temperature and Ci were increased or when the O2 concentration was decreased (Tables I and II), consistent with previous observations with other plant species (Schnyder et al., 1984; Sharkey et al., 1986; Kobza and Edwards, 1987; Sage et al., 1989; Crafts-Brandner and Salvucci, 2000). These data were used to determine if the kinetic properties of Rubisco could account for the measured rates of Pn after adjustment for changes in Rubisco activation. For both creosote bush and Antarctic hairgrass, rates of Pn predicted from Rubisco kinetics without adjustment for changes in Rubisco activation generally deviated from the measured rates, with the most drastic differences occurring at high temperatures and Ci, i.e. conditions that promote considerable deactivation of Rubisco (Tables I and II). By contrast, the rates of Pn predicted from the kinetic properties of Rubisco were similar to the measured rate under all conditions when adjusted for changes in Rubisco activation (Fig. 2). The slight deviation between the measured rates of Pn and the rates predicted after adjustment for changes in Rubisco activation was probably caused by mitochondrial respiration that was not considered in the calculations because of uncertainty about its magnitude in the light.
Figure 2.
Relationship between the measured and predicted rates of Pn in nonstressed and heat stressed leaves of creosote bush and Antarctic hairgrass at ambient and elevated Ci. The predicted rates of Pn for creosote bush (A) and Antarctic hairgrass (B) were calculated from the kinetic properties of Rubisco with (•) and without (□) adjustment for the measured changes in Rubisco activation. The two dashed lines show the linear regression of the relationship between measured and predicted rates of Pn with (•) and without (□) adjustment for changes in Rubisco activation. Values for Pn and Rubisco activation, as well as the conditions of temperature, Ci, and O2, are from Tables I (A) and II (B). The solid line denotes a 1:1 relationship between measured and predicted rates.
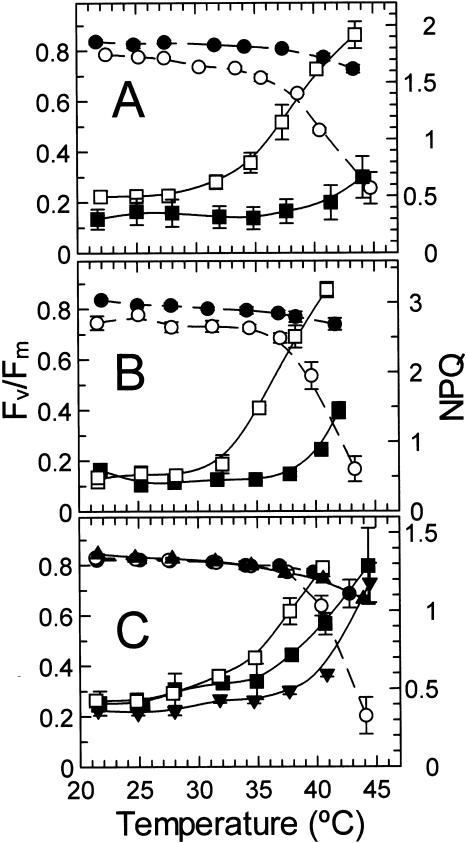
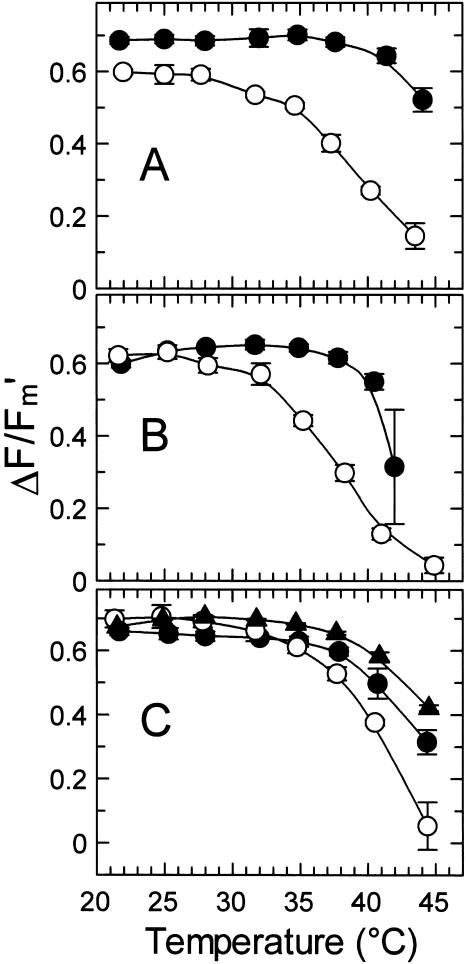
The effect of temperature on chlorophyll fluorescence was determined for attached leaves of Antarctic hairgrass and creosote bush (Fig. 3A). The values of Fv/Fm for the two plant species were relatively constant with temperature below about 35°C. At temperatures higher the 35°C, Fv/Fm decreased much more abruptly with temperature for Antarctic hairgrass compared with creosote bush. The temperature response of nonphotochemical quenching (NPQ) and ΔF/Fm′, the effective quantum yield of photochemical energy conversion (Genty et al., 1989), were markedly different for Antarctic hairgrass and creosote bush (Figs. 3A and 4A). NPQ increased and ΔF/Fm′ decreased when leaf temperatures exceeded about 28°C for Antarctic hairgrass and 38°C for creosote bush.
Figure 3.
Effect of temperature on NPQ of chlorophyll fluorescence and the maximum yield of PSII (Fv/Fm) in plants native to warm and cold regions. The Fv/Fm (○, •, ▴) and NPQ (□, ▪, ▾) were determined at the indicated temperatures for attached leaves of the following plant species: A, the Antarctic hairgrass (○, □) and the creosote bush, (•, ▪); B, the Andean monocot L. pumila (○, □) and the desert shrub jojoba (•, ▪); and C, spinach (○, □), cotton (•, ▪), and tobacco (▴, ▾).
Figure 4.
Effect of temperature on the effective quantum yield of photochemical energy conversion (ΔF/Fm′) in plants native to warm and cold regions. The ΔF/Fm′ were determined at the indicated temperatures for attached leaves of the following plant species: A, the Antarctic hairgrass (○) and the creosote bush (•); B, the Andean monocot L. pumila (○) and the desert shrub jojoba (•); and C, spinach (○), cotton (•), and tobacco (▴).
To determine if other cold and warm climate-adapted species also exhibited a differential effect of temperature on Fv/Fm, NPQ, and ΔF/Fm′, these parameters were measured in jojoba (Simmondsia chinensis), another shrub from the arid deserts of the U.S. southwest, and Lysipomia pumila, an Andean monocot adapted to the cool wet climate of the Altiplano region of South America (Figs. 3B and 4B), as well as in cultivated plants of temperate (i.e. spinach) and subtropical (i.e. cotton and tobacco) origin (Figs. 3C and 4C). The values of Fv/Fm in jojoba, L. pumila, and the three cultivated species were similar and relatively constant at temperatures lower than 37°C. At temperatures higher than about 37°C, Fv/Fm decreased more abruptly with temperature in L. pumila and spinach compared with jojoba, tobacco, and cotton. For all species, a marked increase in NPQ and a decrease in ΔF/Fm′ with temperature preceded the decrease in Fv/Fm. These changes in NPQ and ΔF/Fm′ occurred when temperatures exceeded 28°C to 35°C for L. pumila and spinach and 35°C to 38°C for tobacco, cotton, and jojoba.
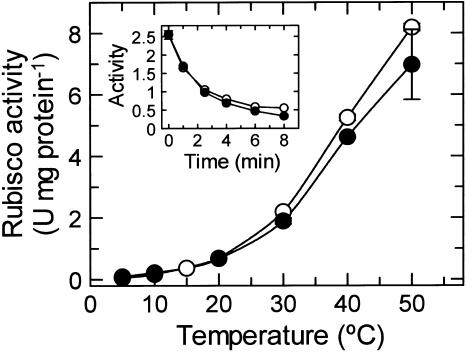
Since the activation state of Rubisco is determined by the balance between inactivation of Rubisco and its reactivation by activase (Spreitzer and Salvucci, 2002; Portis, 2003), we examined the thermal properties of both Rubisco and activase from Antarctic hairgrass and creosote bush. The temperature response of the carboxylase activities of fully activated (i.e. carbamylated) Rubisco isolated from Antarctic hairgrass and creosote bush were nearly identical; activity increased with increasing temperature to at least 50°C (Fig. 5). The rates of carboxylation and the response of activity to temperature were similar to the results reported previously for purified Rubisco from tobacco and cotton (Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner, 2004). Rubisco from Antarctic hairgrass and creosote bush also showed similar rates of inactivation when incubated at 40°C under catalytic conditions (Fig. 5, inset).
Figure 5.
Effect of temperature on the carboxylase activity of Rubisco isolated from Antarctic hairgrass and creosote bush. The carboxylase activity of Rubisco from Antarctic hairgrass (○) and creosote bush (•) was determined at the indicated temperatures. The enzyme was incubated with 30 mm NaHCO3 and 10 mm MgCl2 to fully carbamylated the enzyme prior to assay. Inset, Time course of inactivation of Rubisco under catalytic conditions. Fully carbamylated Rubisco from Antarctic hairgrass (○) and creosote bush (•) was incubated at 40°C in the presence of RuBP, and residual activity was determined at 30°C at the indicated times.
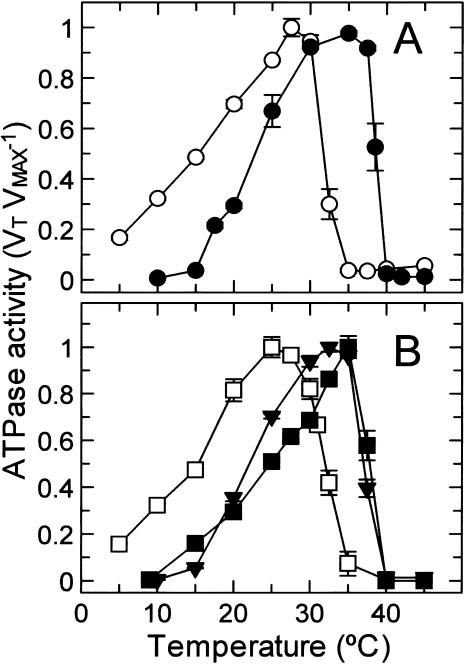
To facilitate purification and to obviate problems with proteolysis, activase cDNAs from creosote bush and Antarctic hairgrass were cloned, and the recombinant enzyme was analyzed after expression in Escherichia coli. Immunoblots of creosote bush and Antarctic hairgrass leaves showed that the α and β forms of activase (Salvucci et al., 2003) were present in approximately equal abundance in both of these species (data not shown). Preliminary experiments revealed that the ATPase activity of the α and β forms of activase within a species exhibited a similar response to temperature (data not shown). Consequently, a mixture of equal amounts of both forms of the enzyme was used for subsequent analysis. Activase from creosote bush had a broad temperature optimum for ATP hydrolysis centered at 35°C, whereas the enzyme from Antarctic hairgrass exhibited maximal rates at 27.5°C (Fig. 6A). At the optimum for creosote bush activase, activase from Antarctic hairgrass was inactive.
Figure 6.
Effect of temperature on the activity of recombinant activase from plants native to warm and cold regions. A, The ATPase activity of recombinant Antarctic hairgrass (○) and creosote bush (•) activase was measured at the indicated temperatures. B, The ATPase activity of recombinant spinach (□), tobacco (▪), and cotton (▾) activase was measured at the indicated temperatures. Results are expressed as VT VMAX−1, the ratio of the activities at the indicated temperature (VT) to the activity at the temperature optimum (VMAX). Maximum rates of ATP hydrolysis were 0.9, 0.56, 0.75, 0.86, and 0.59 units mg protein−1 for activase from Antarctic hairgrass, creosote bush, spinach, tobacco, and cotton, respectively.
Differences in the temperature response of ATPase activity between creosote bush and Antarctic hairgrass activases were also striking at temperatures between 5°C and 20°C. For example, at 15°C the rate of ATP hydrolysis by creosote bush activase was less than 5% of maximum compared to about 50% for Antarctic hairgrass activase. Despite the relatively poor performance of creosote bush activase at low temperatures, the activation state of Rubisco in creosote bush leaves was nearly 100% when leaf temperatures were less than 20°C (Fig. 1).
The temperature response of activity was also examined for recombinant activase from the three cultivated species used above for the chlorophyll fluorescence analysis. Since spinach and cotton express both forms of activase, equal amounts of the α and β forms were used for the experiments and compared to the results with the β form of tobacco activase, the only form expressed by this species (Portis, 2003). The temperature optimum for ATP hydrolysis by cotton and tobacco activase was almost 10°C higher than the optimum for the spinach enzyme (Fig. 6B). For example, a temperature of 35°C was nearly optimal for cotton and tobacco activase yet almost completely inhibited the enzyme from spinach. Differences in the temperature response of ATPase activity were apparent at temperatures between 5°C and 20°C. For example, at 15°C the rate of ATP hydrolysis by cotton activase was less than 5% of maximum compared to about 50% for spinach activase.
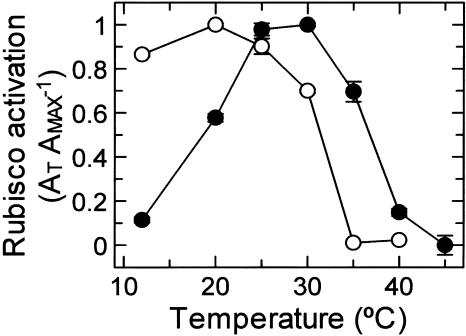
The effect of temperature on activation of Rubisco by activase was compared in an in vitro assay using purified activase and Rubisco (Fig. 7). Activation of inactive Antarctic hairgrass Rubisco by recombinant Antarctic hairgrass activase was optimum at 20°C and decreased progressively with increasing temperature. Activation was completely inhibited at temperatures of 35°C and higher. By contrast, the temperature optimum for activation of creosote bush Rubisco by recombinant creosote bush activase was 30°C, and considerable (i.e. approximately 70%) activation activity was evident at 35°C. At 12°C, Antarctic hairgrass activase promoted a high level of activation, whereas activase from creosote bush was ineffective in activating Rubisco at this temperature. Together, these differences in the temperature response of Rubisco activation between recombinant Antarctic hairgrass and creosote bush activases were consistent with the differences observed for ATPase activity.
Figure 7.
Effect of temperature on in vitro activation of Rubisco by recombinant activase from Antarctic hairgrass and creosote bush. Activation of Antarctic hairgrass Rubisco by recombinant Antarctic hairgrass activase (○) and creosote bush Rubisco by recombinant creosote bush activase (•) was measured at the indicated temperatures. The fraction of active sites converted from an inactive to an active form was determined by comparing the activity of Rubisco obtained after incubating the decarbamylated enzyme complexed with RuBP with activase to the activity of the fully carbamylated control. At each temperature, the values were adjusted for the fraction of sites that activated spontaneously, i.e. in the absence of activase. Results are expressed as AT AMAX−1, the ratio of the sites activated by activase at the indicated temperature (AT) to the sites activated at the temperature optimum (AMAX). The maximum extent of activation after correction for spontaneous activation was 79% and 28% of the sites for Antarctic hairgrass and creosote bush, respectively. The specific activity of the Antarctic hairgrass and creosote bush Rubisco used in these experiments was 1.2 and 1.4 units mg protein−1, respectively, at 30°C.
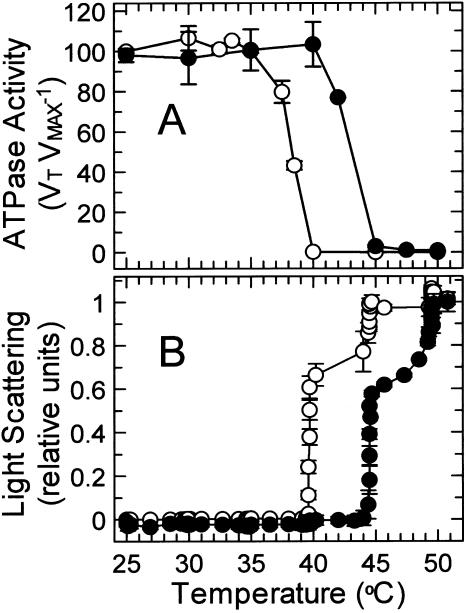
Loss of activase activity above the temperature optimum is probably caused by a structural breakdown of the protein. Consequently, the thermal stabilities of Antarctic hairgrass and creosote bush activase were compared by incubating the two enzymes at various temperatures in the presence of the substrate analog, ATPγS, and then measuring residual ATPase activity at a constant assay temperature (Fig. 8A). Since ATPγS is known to protect the enzyme against denaturation (Crafts-Brandner et al., 1997), the activase measured in these experiments was in its most stable form. Under these conditions, the temperature required to inactivate ATPase activity by 50% (i.e. T50) was 38.5°C and 43°C for Antarctic hairgrass and creosote bush activase, respectively. Similarly, measurements of light scattering during continuous heating in the presence of ATPγS showed that thermal denaturation and aggregation occurred at 39°C and 44°C for Antarctic hairgrass and creosote bush activase, respectively, (Fig. 8B). These temperatures were identical to the temperatures that completely inhibited enzyme activity (Fig. 8A), an indication that differences in the temperature response of activity between Antarctic hairgrass and creosote bush activase reflect differences in the inherent thermal stability of the protein.
Figure 8.
Effect of temperature on the stability of recombinant activase from Antarctic hairgrass and creosote bush. A, Residual ATPase activity of recombinant activase from Antarctic hairgrass (○) and creosote bush (•) was measured at 30°C after incubation for 10 min at the indicated temperatures in the presence of 0.75 mm ATPγS. Results are expressed as VT VMAX−1, the ratio of the activities after incubation at the indicated temperature (VT) to the control activity (VMAX) determined for enzyme maintained at 4°C. The rates of ATP hydrolysis by the controls were 0.85 and 0.51 units mg protein−1 for activase from Antarctic hairgrass and creosote bush, respectively. B, Thermal aggregation of recombinant activase from Antarctic hairgrass (○) and creosote bush (•) was determined by measuring light scattering at the indicated temperatures during a time course of increasing temperature. Recombinant activase (40 μg) was incubated in 400 μL in a thermostatted cuvette in the presence of 0.75 mm ATPγS. After 5 min at 25°C, the temperature of the cuvette was increased by increasing the temperature of circulating water bath by 5°C every 10 min.
DISCUSSION
Relationship between Pn and Rubisco Activation under Moderate Heat Stress
The temperature response of Pn is complex, reflecting the temperature dependencies of several interacting physical and biochemical processes. That the rate of Pn does not continue to increase with temperature has been attributed, in part, to reduced CO2 uptake and increased CO2 evolution caused by higher photorespiratory and respiratory activities and lower gas solubility at higher temperatures (Berry and Björkman, 1980; Monson et al., 1982; Jordan and Ogren, 1984). These factors are partially offset by increased carboxylation from the faster rates of Rubisco turnover at higher temperatures (Salvucci and Crafts-Brandner, 2004; Fig. 5). As a result, the response of Pn to temperature predicted from the kinetics of Rubisco is relatively flat above the optimum (Crafts-Brandner and Salvucci, 2000), indicating that another factor(s) must be responsible for the pronounced decrease in Pn at supraoptimal temperatures.
Recent studies with cotton, wheat, tobacco, and maize have confirmed earlier observations (Weis, 1981a, 1981b; Kobza and Edwards, 1987) that Rubisco deactivates markedly in response to moderate heat stress (Law and Crafts-Brandner, 1999; Crafts-Brandner and Law, 2000; Crafts-Brandner and Salvucci, 2000, 2002). Significant decreases in the activation state of Rubisco under moderate heat stress, when superimposed on the relatively flat temperature response predicted for Pn, exert a major inhibitory effect on Pn. As a result, we found that the progressive decrease in Rubisco activation that accompanies increasing leaf temperatures closely correlates with the extent of photosynthetic inhibition both here (Fig. 1) and in previous studies (Law and Crafts-Brandner, 1999; Crafts-Brandner and Salvucci, 2000, 2002).
Differential Effects of Temperature on Pn, Rubisco Activation, and Chlorophyll Fluorescence in Plants from Contrasting Thermal Environments
Photosynthetic rates are generally optimal at temperatures that are prevalent during the day in a species' native environment (Berry and Björkman, 1980). Consequently, the response of Rubisco activation to temperature should differ in plants from different thermal environments if there is a general involvement of activase in the inhibition of Pn by moderate heat stress. To test this idea, Rubisco activation and Pn were characterized in creosote bush and Antarctic hairgrass, two plant species from contrasting thermal environments. Both of these species are C3 plants, but creosote bush is frequently subjected to temperatures in excess of 45°C in its desert habitat (Hamerlynck et al., 2000), whereas Antarctic hairgrass from maritime Antarctica usually experiences daytime temperatures between 0°C and 6°C and seldom encounters temperatures higher than 20°C (Alberdi et al., 2002).
Pn and Rubisco activation exhibited a similar response to increasing temperature in both creosote bush and Antarctic hairgrass, despite a 10°C difference in the temperature optimum between these species (Fig. 1). The relationship between Pn and Rubisco activation was not maintained when temperatures were decreased from optimal to suboptimal for Pn, since the activation state of Rubisco in both creosote bush and Antarctic hairgrass was nearly 100% at suboptimal temperatures. Thus, unlike at high temperatures, Rubisco activation does not decrease at low temperatures under steady-state conditions of saturating irradiance, at least not when temperature is decreased from the optimum for Pn and Rubisco activation.
Measurements of chlorophyll fluorescence have consistently shown that NPQ increases markedly in heat-stressed leaves (Schreiber and Bilger, 1987; Jones et al., 1998; Lu and Zhang, 2000; Leakey et al., 2003). Since saturating irradiance produces a high degree of NPQ even in nonstressed leaves, measurements are often conducted at a low actinic irradiance, i.e. conditions that make it possible to observe large increases in energy quenching (Schreiber and Bilger, 1987; Law and Crafts-Brandner, 1999; Lu and Zhang, 2000). Under these conditions, marked differences in the temperature response of NPQ and the operating yield of PSII, ΔF/Fm′, were observed between creosote bush and Antarctic hairgrass. Although not directly comparable because of the different irradiance levels used for measurements, nevertheless differences in the response of NPQ and ΔF/Fm′ to temperature between creosote bush and Antarctic hairgrass resembled the differential responses of Pn and Rubisco activation (Fig. 1), suggesting that the underlying cause of photosynthetic inhibition by heat stress was similar under both high and low irradiance. In fact, measurements of Rubisco activation show that the activation state does indeed decrease under moderate heat stress when irradiance levels are low (S. Crafts-Brandner, unpublished data).
Similar results to those described above were observed when the temperature response of NPQ and ΔF/Fm′ in warm climate species (i.e. jojoba, tobacco, and cotton) was compared with the response in species like L. pumila and spinach that are native to cold environments. Interestingly, differential effects of temperature on Fv/Fm were also observed among species endemic to colder compared with warmer regions. Decreases in Fv/Fm are regarded as an indicator of inactivation of PSII reaction centers, caused by damage to the thylakoid membrane (Roháček, 2002). Regardless of the species, the temperatures that caused Fv/Fm to decrease were higher than those that promoted an increase in NPQ (see also Bilger et al., 1987; Schreiber and Bilger, 1987; Law and Crafts-Brandner, 1999; Lu and Zhang, 2000; Leakey et al., 2003) and inhibited Pn (Law and Crafts-Brandner, 1999; Xiong et al., 1999; Georgieva et al., 2000; Hamerlynck et al., 2000).
The Biochemical Basis for Inactivation of Rubisco under Heat Stress
The biochemical basis for inactivation of Rubisco under heat stress is an imbalance between the rates of Rubisco inactivation and reactivation by activase (Crafts-Brandner and Salvucci, 2000). As temperature increases, mechanism-based inactivation of Rubisco proceeds more rapidly, mainly because of a faster rate of substrate misprotonation, which increases the production of the closed, inactive form of the enzyme (Salvucci and Crafts-Brandner, 2004). Since conversion to the open conformation is extremely slow without activase (Andrews, 1996; Duff et al., 2000), maintenance of Rubisco in an active state at high temperatures requires faster rates of activase activity to offset the faster rates of Rubisco inactivation. However, in vitro assays using purified activase and Rubisco and saturating levels of ATP and RuBP have shown that activase activity, which is sufficient for Rubisco activation at optimal temperatures, is insufficient to keep pace with the faster rates of Rubisco inactivation at high temperatures (Crafts-Brandner and Salvucci, 2000). Thus, the activation state of Rubisco decreases under heat stress because activase activity cannot overcome the faster rates of Rubisco inactivation. The poor performance of activase at high temperature has been attributed to its relatively low temperature optimum for catalysis (Salvucci and Crafts Brandner, 2004), caused in part by thermal instability (Fig. 8; Salvucci et al., 2001; Rokka et al., 2001), as well as to other unspecified causes (Sharkey, 2000).
It is well known that the thermal properties of homologous enzymes from different species often match the temperature of the environments to which the organisms are adapted (Somero, 1995). This axiom did not hold true for Rubisco since the temperature response of Rubisco activity from creosote bush and Antarctic hairgrass was similar and nearly identical to the responses reported for cotton and tobacco Rubisco. Similar measurements by Sage (2002) showed that Rubisco activity was actually higher at elevated temperatures in species adapted to cool environments compared with species from warm environments.
In contrast to Rubisco, the thermal properties of activase in plant species from contrasting thermal environments differed, corresponding to the differential response of Pn and Rubisco activation to temperature. Specifically, the temperature response and thermal stability of activase from the desert shrub was skewed toward higher temperatures, while the activase from the Antarctic hairgrass performed optimally at much lower temperature. Similarly, activase from cotton and tobacco, two cultivated species of subtropical origin, was more thermotolerant than activase from the temperate species, spinach.
Limitations to Pn Imposed by the Thermal Properties of Activase
Since the activation state of Rubisco is determined by the balance between inactivation and subsequent reactivation by activase, and the rate of inactivation was similar in Rubisco from creosote bush and Antarctic hairgrass (Fig. 5, inset), then it follows that the temperature limits for Rubisco activation and probably photosynthesis are determined to a large extent by the thermal properties of activase. The stimulation of Pn by increasing Ci at low O2 for both creosote bush and Antarctic hairgrass (Tables I and II) supports this view by establishing that the supply of ATP for RuBP regeneration is not limiting at air levels of CO2 when Pn is inhibited by moderate heat stress (see also Monson et al., 1982; Crafts-Brandner and Law, 2000).
The increase in NPQ that accompanies moderate heat stress, while not a direct measure of ΔpH, nevertheless suggests that the proton gradient is increased or at least maintained under moderate heat stress to provide ample ATP for RuBP regeneration. A similar conclusion about the status of the transthylakoid pH gradient under moderate heat stress has been drawn from measurements of 9-aminoacridine fluorescence and light scattering (Bukhov et al., 1999). These and other measurements suggest that the transthylakoid pH gradient proton is maintained under heat stress by increased electron flow through PSI (Bukhov et al., 1999, 2000; Bukhov and Dzhibladze, 2002).
CONCLUSIONS
The ability of activase to maintain Rubisco in an active conformation appears to place a limit on the temperatures at which higher plants can photosynthesize. Heat shock proteins, changes in the chloroplast milieu, and probably other factors affect the properties of activase and other components of the photosynthetic apparatus. In turn, these factors undoubtedly modulate the precise response of photosynthesis and Rubisco activation to temperature, including the degree to which a given plant species can acclimate to temperatures outside its normal range (Law and Crafts-Brandner, 1999). While it will be interesting in the future to determine if the properties of activase change when plants acclimate to temperature, the focus of this study was to compare plants grown at temperatures similar to those encountered in their natural environments. The results showed that under natural growth temperatures the temperature response of Pn and Rubisco activation was consistent with the thermal properties of activase. Thus, the thermal stability of activase represents a major biochemical factor limiting the ability of plants to photosynthesize at high temperature. By limiting photosynthetic activity, activase may ultimately affect the geographic distribution of higher plants, their productivity in a particular thermal environment, and their ability to respond to changes in climate.
MATERIALS AND METHODS
Plant Material
Antarctic hairgrass (Deschampsia antarctica) plants were collected from the Stepping Stone Islands along the west coast of the Antarctic Peninsula by Dr. T.A Day (Arizona State University, Tempe, AZ; Xiong et al., 1999). Lysipomia pumila plants were collected near Achachi, Bolivia (16°3′S, 68°30′W) at an elevation of 4,268 m by Dr. T. Ayers (Northern Arizona University, Flagstaff, AZ). Plants were propagated in a growth chamber under a 14 h and 12°C light (300 μmol photons m−2 s−1)/10 h and 8°C dark regime. Creosote bush (Larrea tridentata) and jojoba (Simmondsia chinensis) plants were obtained from a local nursery and maintained under natural light conditions in an air-conditioned greenhouse under a 28°C and 12 h day/24°C and 12 h night regime. Maximum PAR on most days was 1,800 to 2,000 μmol photons m−2 s−1. Spinach (Spinacea oleracea) plants were grown from seed in a growth chamber under a 16 h and 21°C light (300 μmol photons m−2 s−1)/8 h and 18°C night. Tobacco (Nicotiana tabacum) and cotton (Gossypium hirsutum) plants were grown from seed in an air-conditioned greenhouse as described above.
Gene Cloning, Protein Expression, and Purification
Activase cDNAs were cloned by reverse transcription-PCR from mRNA isolated from leaves of creosote bush and Antarctic hairgrass (Salvucci et al., 2003). The clones were engineered for expression of the mature α and β forms of the protein in Escherichia coli by replacing nucleotides that encode for the transit peptide with an ATG start codon using PCR. The cDNAs for creosote bush (accession no. AY312575, α form; accession no. AY312576, β form) and Antarctic hairgrass (accession no. AY312573, α form; accession no. AY312574, β form) activases were inserted into the pET23d and 23a vectors, respectively, for expression in E. coli strain BL21(DE3) pLysS after induction with isopropyl-β-d-thiogalactopyranoside (van de Loo and Salvucci, 1996). Clones of the spinach, cotton, and tobacco activase have been described previously (van de Loo and Salvucci, 1996; Crafts-Brandner et al., 1997; Salvucci et al., 2003). Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining permission will be the responsibility of the requestor.
Activase protein was purified from E. coli cells as described previously (Salvucci and Klein, 1994; van de Loo and Salvucci, 1996), with the following changes. After precipitation with 37% (w/v) ammonium sulfate, the suspended protein was centrifuged at 237,000 g for 30 min in a Beckman SW55 rotor. The clarified supernatant was fractionated at 1 mL min−1 on a 2.6 × 60-cm Toyopearl HW-55S column in 50 mm HEPES-KOH, pH. 7.2, 10 mm MgCl2, and 2 mm dithiothreitol. This chromatography step replaced separation by rate zonal centrifugation on Suc gradients (Salvucci and Klein, 1994). Fractions containing ATPase activity were further fractionated by anion-exchange chromatography (Salvucci and Klein, 1994) on a 15-mL Q-Sepharose HiTrap column (Amersham Biosciences, Piscataway, NJ).
Rubisco was purified from leaves of creosote bush and Antarctic hairgrass as described previously (Crafts-Brandner and Salvucci, 2000) with the following changes. For isolation of creosote bush Rubisco, protein was precipitated with 70% (w/v) ammonium sulfate. For both creosote bush and Antarctic hairgrass, rate zonal centrifugation was replaced by gel-filtration chromatography through Toyopearl HW-55S as described above in 50 mm Tris-HCl, pH 7.6, 10 mm MgCl2, 10 mm NaHCO3, and 10 mm 2-mercaptoethanol. Fractions containing Rubisco activity were further fractionated by anion-exchange chromatography (Salvucci and Klein, 1994) on a 1 × 10-cm Mono-Q column (Amersham Biosciences).
Net Photosynthesis and Rubisco Activation
Pn of intact leaves was determined in an atmosphere of 350 μbar CO2 or as indicated, 210 or 10 mbar O2, and a saturating irradiance of 1,800 μmol photons m−2 s−1 using a LI-COR 6400 portable photosynthesis system (Law and Crafts-Brandner, 1999; Crafts-Brandner and Salvucci, 2000). Entire plants were placed inside a humidified plant growth chamber, and leaf temperature was increased or decreased after reaching steady-state photosynthesis at the optimal temperature by changing the temperature of both the leaf cuvette and the growth chamber (Law and Crafts-Brandner, 1999). Gas exchange measurements were taken after 45 min at each temperature. The mean internal CO2 concentrations (i.e. Ci) ±se over the entire temperature range for measurements conducted in air were 286 ± 9 and 262 ± 15 μbar for Antarctic hairgrass and creosote bush, respectively. The highest Ci occurred at the three highest temperatures for Antarctic hairgrass (i.e. 298 μbar) and the lowest temperatures for creosote bush (i.e. 278 μbar). The values presented are the means ± se of the steady-state rates measured for three different plants.
Rubisco activation was determined by rapid extraction and assay of leaf tissue sampled by freeze-clamping immediately following measurement of gas exchange (Law and Crafts-Brandner, 1999; Crafts-Brandner and Salvucci, 2000). The activation state was determined by comparing the activity of Rubisco immediately following extraction with the activity after incubation with 10 mm MgCl2 and NaHCO3. The values presented are the means ± se of the three different samples. The predicted rates of Pn were calculated from the gas solubilities and the kinetic properties of Rubisco without adjustment for dark respiration and assuming that RuBP was saturating (Crafts-Brandner and Salvucci, 2000). Predicted rates were normalized to the measured rate of Pn at the temperature where Rubisco activation was 100%, i.e. 28°C and 16°C for creosote bush or Antarctic hairgrass, respectively. The activation energy for Rubisco was determined experimentally using purified creosote bush or Antarctic hairgrass Rubisco (Fig. 5).
Enzyme Assays
ATPase activity was measured spectrophotometrically as described previously (Crafts-Brandner and Salvucci, 2000; Salvucci et al., 2001). The temperature response of activity was determined by incubating 50 μg of activase in assays without ATP for 5 min at the indicated temperatures and then initiating the reaction with ATP. The pH of the assay varied from 8.11 at 4°C to 7.84 at 50°C, a range that coincides with the broad pH optimum of activase (Robinson and Portis, 1989). Preliminary experiments showed that the activities of the linking enzymes were not limiting at any of the temperatures used in the experiments (data not shown). For measurement of thermal stability, activase was incubated for 10 min at the indicated temperatures in the presence of 0.75 mm ATPγS and then assayed for activity at 30°C as described previously (Crafts-Brandner et al., 1997).
Activation of Rubisco in vitro by activase was determined in a two-stage assay as described previously (Crafts-Brandner and Salvucci, 2000). To determine the temperature response of activation, 0.2 to 0.4 mg mL−1 activase was incubated at the indicated temperatures in reactions without ATP. The amount of activase used in the first stage of the assay affected the level of activation attained but not the temperature response profile (data not shown). After 5 min, ATP was added to the reactions followed 30 s later by 0.5 mg mL−1 decarbamylated Rubisco complexed with RuBP. The extent of activation was determined after 5 min by measuring Rubisco activity at 30°C in assays containing 100 mm Tricine-NaOH, pH 8, 10 mm MgCl2, 10 mm NaH14CO3 (1 mCi mmol−1), and 0.4 mm RuBP. Time course experiments showed that 5 min was sufficient time to reach full activation (see also van de Loo and Salvucci, 1996).
Rubisco activity and deactivation under catalytic conditions were determined for the purified enzyme in the absence of activase, as described previously (Crafts-Brandner and Salvucci, 2000). The pH of the assay varied from 8.10 at 4°C to 7.84 at 50°C, a range that coincides with the broad pH optimum of Rubisco (Andrews et al., 1975). Preliminary experiments established that the Mg2+ concentration was saturating for activity throughout the temperature range. Light-scattering measurements were conducted in the presence of 0.75 mm ATPγS as described previously (Salvucci et al., 2001). Assays of the isolated enzymes were conducted in at least duplicate and the values presented are the means ± se.
Chlorophyll Fluorescence
Chlorophyll fluorescence was measured in air using a PAM 2000 fluorometer (Heinz Walz GmbH, Effeltrich, Germany). Measurements were conducted with intact plants placed inside a humidified plant growth chamber (Law and Crafts-Brandner, 1999). The fiber optic probe was positioned above the leaf at a constant distance using an open leaf clip holder with a built-in thermocouple that was used to monitor leaf temperature. A preprogrammed protocol was used that determines Fo with weak modulated light, followed by Fm after a 0.8-s pulse of strong white light (>4,000 μmol photons m−2 s−1). After a 20-s lag, a 5 min quenching analysis was performed using continuous actinic light and 0.8-s pulses of saturating light every 20 s. The intensity of the actinic light was 125 μmol photons m−2 s−1at 665 nm, which produced a consistent NPQ value of between 0.2 and 0.4 at the starting temperature. After completion of each run, the leaf temperature was increased by 3°C to 5°C by increasing the temperature of the chamber, and a new analysis was started after dark-adapting the leaf material for 10 min. The values presented are the means ± se for measurements of three to six leaves each on separate plants. NPQ, and ΔF/Fm′ are defined by the fluorescence terms (Fm−Fm′)/Fm′ and (Fm−Fs)/Fm′, respectively, (Roháček, 2002) and were determined after 4.5 min of continuous actinic light.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AY312573–AY312576.
Acknowledgments
We thank Drs. T.A. Day (Arizona State University) and T.J. Ayers (Northern Arizona University) for providing Antarctic hairgrass and L. pumila plants, respectively. We acknowledge the expert technical assistance of Nancy Parks, Laura Salywon, and Donald Brummett.
Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that may also be suitable.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.038323.
References
- Alberdi M, Bravo LA, Gutiérrez A, Gidekel M, Corcuera LJ (2002) Ecophysiology of Antarctic vascular plants. Physiol Plant 115: 479–486 [DOI] [PubMed] [Google Scholar]
- Andrews TJ (1996) The bait in the Rubisco mousetrap. Nat Struct Biol 3: 3–7 [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Badger MR, Lorimer GH (1975) Factors affecting interconversion between kinetic forms of ribulose diphosphate carboxylase-oxygenase from spinach. Arch Biochem Biophys 171: 93–103 [DOI] [PubMed] [Google Scholar]
- Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31: 491–543 [Google Scholar]
- Bilger W, Schreiber U, Lange OL (1987) Chlorophyll fluorescence as an indicator of heat induced limitation of photosynthesis in Arbutus unedo. In JD Tenhumen, FM Catarino, OL Lange, WC Oechel, eds, Plant Response to Stress. Springer-Verlag, Berlin, pp 391–399
- Bukhov NG, Dzhibladze TG (2002) The effect of high temperature on the photosynthetic activity of intact barley leaves at low and high irradiance. Russ J Plant Physiol 49: 371–375 [Google Scholar]
- Bukhov NG, Samson G, Carpentier R (2000) Nonphotosynthetic reduction of the intersystem electron transport chain of chloroplasts following heat stress. Steady-state rate. Photochem Photobiol 72: 351–357 [PubMed] [Google Scholar]
- Bukhov NG, Wiese C, Neimanis S, Heber U (1999) Heat sensitivity of chloroplasts and leaves: Leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth Res 59: 81–93 [Google Scholar]
- Crafts-Brandner SJ, Law RD (2000) Effect of heat stress on the inhibition and recovery of ribulose-1,5-bisphosphate carboxylase/oxygenase activation state. Planta 212: 67–74 [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci USA 97: 13430–13435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME (2002) Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol 129: 1773–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, van de Loo FJ, Salvucci ME (1997) The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiol 114: 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff AP, Andrews TJ, Curmi PM (2000) The transition between the open and closed states of rubisco is triggered by the inter-phosphate distance of the bound bisphosphate. J Mol Biol 298: 903–916 [DOI] [PubMed] [Google Scholar]
- Feller U, Crafts-Brandner SJ, Salvucci ME (1998) Moderately high temperatures inhibit ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol 116: 539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Georgieva K, Tsonev T, Velikova V, Yordanov I (2000) Photosynthetic activity during high temperature treatment of pea plants. J Plant Physiol 157: 169–176 [Google Scholar]
- Hamerlynck EP, Huxman TE, Loik ME, Smith SD (2000) Effects of extreme high temperature, drought and elevated CO2 on photosynthesis of the Mojave Desert evergreen shrub, Larrea tridentata. Plant Ecol 148: 183–193 [Google Scholar]
- Havaux M (1993) Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ 16: 461–467 [Google Scholar]
- Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U (1998) Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooanthellae. Plant Cell Environ 21: 1219–1230 [Google Scholar]
- Jordan DB, Ogren WL (1984) The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase. Dependence on ribulosebisphosphate concentration, pH and temperature. Planta 161: 308–313 [DOI] [PubMed] [Google Scholar]
- Kobza J, Edwards GE (1987) Influences of leaf temperature on photosynthetic carbon metabolism in wheat. Plant Physiol 83: 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher W (1995) Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, Ed 3. Springer-Verlag, Berlin
- Law RD, Crafts-Brandner SJ (1999) Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol 120: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey ADB, Press MC, Scholes JD (2003) High-temperature inhibition of photosynthesis is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling. Plant Cell Environ 26: 1681–1690 [Google Scholar]
- Lu CM, Zhang JH (2000) Heat-induced multiple effects on PSII in wheat plants. J Plant Physiol 156: 259–265 [Google Scholar]
- Monson RK, Stidham MA, Williams GJ, Edwards GE, Uribe EG (1982) Temperature dependence of photosynthesis in Agropyron smithii Rydb. I. Factors affecting net CO2 uptake in intact leaves and contribution from ribulose-1,5-bisphosphate carboxylase measured in vivo and in vitro. Plant Physiol 69: 921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney HA, Björkman O, Collatz GJ (1978) Photosynthetic acclimation to temperature in the desert shrub, Larra divaricata. Plant Physiol 61: 406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Tsuyama M, Kobayashi Y, Kodama H, Iba K (2000) Trienoic fatty acids and plant tolerance of high temperature. Science 287: 476–479 [DOI] [PubMed] [Google Scholar]
- Portis AR, Jr (2003) Rubisco activase: Rubisco's catalytic chaperone. Photosynth Res 75: 11–27 [DOI] [PubMed] [Google Scholar]
- Raison JK, Roberts JKM, Berry JA (1982) Correlations between the thermal stability of chloroplast (thylakoid) membranes and the composition and fluidity of their polar lipids upon acclimation of the higher plant, Nerium oleander, to growth temperature. Biochim Biophys Acta 688: 218–228 [Google Scholar]
- Robinson SP, Portis AR, Jr. (1989) Adenosine triphosphate hydrolysis by purified Rubisco activase. Arch Biochem Biophys 268: 93–99 [DOI] [PubMed] [Google Scholar]
- Roháček K (2002) Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 40: 13–29 [Google Scholar]
- Rokka A, Zhang L, Aro E-M (2001) Rubisco activase: an enzyme with a temperature-dependent dual function? Plant J 25: 463–471 [DOI] [PubMed] [Google Scholar]
- Sage RF (2002) Variation in the kcat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. J Exp Bot 53: 609–620 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR (1989) Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant Physiol 89: 590–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120: 179–186 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Klein RR (1994) Directed mutagenesis of a reactive lysyl residue (K247) of Rubisco activase. Arch Biochem Biophys 314: 178–185 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Osteryoung KW, Crafts-Brandner SJ, Vierling E (2001) Exceptional sensitivity of Rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiol 127: 1053–1064 [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, van de Loo FJ, Stecher DS (2003) Two isoforms of Rubisco activase in cotton, the products of separate genes not alternative splicing. Planta 216: 736–744 [DOI] [PubMed] [Google Scholar]
- Schnyder H, Mächler F, Nösberger J (1984) Influence of temperature and O2 concentration on photosynthesis and light activation of ribulosebisphosphate carboxylase oxygenase in intact leaves of white clover (Trifolium repens L). J Exp Bot 35: 147–156 [Google Scholar]
- Schreiber U, Bilger W (1987) Rapid assessment of stress effects on plant leaves by chlorophyll fluorescence measurements. In JD Tenhumen, FM Catarino, OL Lange, WC Oechel, eds, Plant Response to Stress. Springer-Verlag, Berlin, pp 27–53
- Sharkey TD (2000) Some like it hot. Science 287: 435–437 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Badger MR, von Caemmerer S, Andrews TJ (2001) Increased heat sensitivity of photosynthesis in tobacco plants with reduced Rubisco activase. Photosynth Res 67: 147–156 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Seemann JR, Berry JA (1986) Regulation of ribulose-1,5-bisphosphate carboxylase activity in response to changing partial pressure of O2 and light in Phaseolus vulgaris. Plant Physiol 81: 788–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero GN (1995) Proteins and temperature. Annu Rev Physiol 57: 43–68 [DOI] [PubMed] [Google Scholar]
- Spreitzer RJ, Salvucci ME (2002) Rubisco:interactions, associations and the possibilities of a better enzyme. Annu Rev Plant Biol 53: 449–475 [DOI] [PubMed] [Google Scholar]
- van de Loo FJ, Salvucci ME (1996) Activation of ribulose-1,5-bisphosphate carboxylase/oxygenase involves Rubisco activase Trp16. Biochemistry 35: 8143–8148 [DOI] [PubMed] [Google Scholar]
- Weis E (1981. a) Reversible heat-inactivation of the Calvin Cycle: A possible mechanism of the temperature regulation of photosynthesis. Planta 151: 33–39 [DOI] [PubMed] [Google Scholar]
- Weis E (1981. b) The temperature-sensitivity of dark-inactivation and light-activation of the ribulose-1,5-bisphosphate carboxylase in spinach chloroplasts. FEBS Lett 129: 197–200 [Google Scholar]
- Xiong FS, Ruhland CT, Day TA (1999) Photosynthetic temperature response of the Antarctic vascular plants Colobanthus quitensis and Deschampsia antarctica. Physiol Plant 106: 276–286 [Google Scholar]