Abstract
Our earlier studies with the pgsA mutant of Synechocystis PCC6803 demonstrated the important role of phosphatidylglycerol (PG) in PSII dimer formation and in electron transport between the primary and secondary electron-accepting plastoquinones of PSII. Using a long-term depletion of PG from pgsA mutant cells, we could induce a decrease not only in PSII but also in PSI activity. Simultaneously with the decrease in PSI activity, dramatic structural changes of the PSI complex were detected. A 21-d PG depletion resulted in the degradation of PSI trimers and concomitant accumulation of monomer PSI. The analyses of PSI particles isolated by MonoQ chromatography showed that, following the 21-d depletion, PSI trimers were no longer detectable in the thylakoid membranes. Immunoblot analyses revealed that the PSI monomers accumulating in the PG-depleted mutant cells do not contain PsaL, the protein subunit thought to be responsible for the trimer formation. Nevertheless, the trimeric structure of PSI reaction center could be restored by readdition of PG, even in the presence of the protein synthesis inhibitor lincomycin, indicating that free PsaL was present in thylakoid membranes following the 21-d PG depletion. Our data suggest an indispensable role for PG in the PsaL-mediated assembly of the PSI reaction center.
Phosphatidylglycerol (PG) is an integral component of photosynthetic membranes. PG molecules are important for both the formation and functioning of photosynthetic apparatus. In cyanobacterial cells, PG is the only representative of the phospholipid family (Wada and Murata, 1998), and the majority of PG molecules are localized in the thylakoid membranes that are the site of oxygenic electron transport. Photosynthesis is the basic energy source of cyanobacteria and generally for photosynthetic organisms. The availability of the complete genomic sequence of Synechocystis PCC6803 (Kaneko et al., 1996) opened the way for studying the structural and functional roles of PG via molecular genetic approaches. The pgsA gene encoding PG phosphate synthase was inactivated in Synechocystis PCC6803 cells by inserting a kanamycin resistance gene cassette (Hagio et al., 2000). Maintenance of the recently generated pgsA mutant strain requires exogenously supplied PG. A 40% decrease in photosynthetic oxygen-evolving activity could be detected following a 3-d depletion of PG, which resulted in an approximately 50% decrease in the amount of PG molecules in the cellular membranes. The photosynthetic processes, as measured by fluorescence induction in the PG-depleted cells, slowed down, and a perturbation of the surroundings of the secondary quinone acceptor (QB) was observed (Gombos et al., 2002). This perturbation was similar to the effect of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), which can block electron transport between primary quinone acceptor (QA) and QB. Additionally, Fragata et al. (1991) demonstrated that in isolated barley (Hordeum vulgare) PSII particles, oxygen-evolving activity strongly depends on the presence of PG and Mg2+ in the system (Fragata et al., 1991). These results indicate that PG molecules are essential constituents of photosynthetic apparatus in both cyanobacteria and in higher plants. However, PSI activity was not affected by short-term PG deprivation. This observation seemed to contradict x-ray crystallographic data, which showed the presence of PG in PSI and the absence in PSII reaction centers (RCs; Jordan et al., 2001; Zouni et al., 2001). Nevertheless, the 3.8 Å resolution of these measurements did not allow the detection of lipid molecules in PSII (Zouni et al., 2001). Although x-ray crystallographic measurements indicated the presence of three PG molecules in PSI RCs (Jordan et al., 2001), PSI activity was not significantly affected, even after 90% depletion of the cellular PG content. This finding suggests that the strength of PG binding by PSII might be much weaker than those of the PSI RCs. Accordingly, following a 90% decrease in PG content, the cells still may contain enough PG molecules to maintain PSI activity, but looser binding of PG to PSII RC could lead to loss of PSII activity at an earlier stage. It was also demonstrated by using isolated PSII particles treated with phospholipase that PG molecules assist in PSII dimer formation (Kruse et al., 2000). Recent reports confirmed the deleterious effect of PG deprivation not only on the photosynthetic function but also the photosynthetic structures in cyanobacteria. Using a mutant defective in PG synthesis, it was evidenced that PG molecules in photosynthetic membranes are required for the efficient dimer formation of the PSII complex (Sakurai et al., 2003). Earlier studies indicated that in higher plants PG contribute to the development of chloroplasts (Hagio et al., 2002; Xu et al., 2002).
In contrast to higher plants, in which only the monomer form of PSI RC is present (Golbeck, 1992; Chitnis, 1996), in cyanobacteria both monomeric and trimeric forms of PSI RCs exist (Rögner et al., 1990a; Shubin et al., 1993; Boekema et al., 1994). On the other hand, dimerization of PSI could not be observed. In photosynthetic membranes of Synechocystis PCC6803, a dynamic equilibrium exists between the monomeric and trimeric forms of PSI. The physiological importance of the PSI trimers is unknown. Some authors suggested that such oligomers can protect the PSI RCs against light stress by permitting radiationless dissipation of excess energy into heat (Mukerji and Sauer, 1989; Karapetyan et al., 1999), that red chlorophylls (Chls) can funnel light energy to P700 (van Grondelle et al., 1994; Shubin et al., 1995) as well as increase the cross section of light absorption (Trissl, 1993). Earlier the effect of lipids on the formation of PSI RCs was suggested by measuring the effect of lipids on the PSI trimer formation (Kruip et al., 1999). The in vitro reconstitution of PSI trimers from monomers by phospholipid liposomes demonstrated that this specific oligomerization process requires specific lipids. Nevertheless, in vivo the effective involvement of lipids in PSI trimer formation has not yet been demonstrated.
PSI RCs contain several protein subunits (Sun et al., 1997; Fromme et al., 2001). PsaA and PsaB subunits form the heterodimeric core of the RC, and, in addition to the core proteins, the cyanobacterial PSI complex contains nine small subunits (PsaC to PsaM; Fromme, 1996). One protein subunit of the PSI RCs, PsaL, was suggested to be a key component of the trimer-forming domain in the structure of PSI (Chitnis and Chitnis, 1993; Schluchter et al., 1996). The importance of PsaL in the formation of PSI trimer was demonstrated by showing that a PsaL-deficient mutant is devoid of trimeric PSI (Fromme, 1996). The subunits PsaC, D, E, F, and J were found dispensable for PSI trimer formation (Kruip et al., 1997).
Using long-term PG depletion, we studied the effect of PG on the PSI oligomer formation in the PG-deficient pgsA. Following 21 d of depletion, there was no detectable amount of PG in the cells. Presumably, despite of the strong binding of PG molecules to the PSI RCs, by this stage (most of) the trimeric complexes lost their PG content. Using anion-exchange chromatography of solubilized thylakoid fractions, we observed a dramatic decrease in the level of PSI trimers. A concomitant increase of monomeric PSI content suggested that monomers were formed at the expense of trimers. To our knowledge, this is the first in vivo evidence of the essential role of PG in PSI trimer formation.
RESULTS
Lipid Analyses
Using thin-layer chromatography and gas chromatography, there was no detectable amount of PG in isolated thylakoid membranes of the pgsA mutant cells following a 21-d PG depletion (data not shown).
Effect of PG Deprivation on the Pigment Content and the Growth Rate
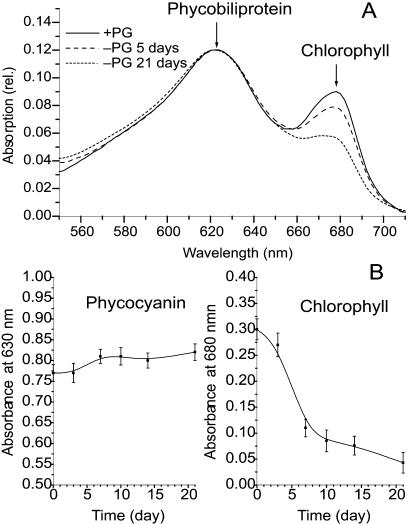
The absorption spectra of intact cells (Fig. 1A) show that the Chl content of the pgsA mutant decreases dramatically following 5 d of PG deprivation; however, the phycocyanin content remained constant. The phycocyanin content, as measured by the optical density at 630 nm (OD630; Fig. 1B), did not change appreciably, even after 21 d of PG depletion. By contrast, the relative Chl content monitored by OD680 showed a rapid decrease between days 2 and 7 of PG depletion, decreasing to about 30% of the initial value. Following day 7 of depletion, the decrease slowed down, and at the end of day 21 the Chl content still remained about 20% of the initial value. For the first 3 d following the transfer of mutant cells to PG-free medium, the growth rate was similar to that of the mutant cultures grown in PG-supplemented medium. Later on, the growth rate of PG-deprived cultures decreased significantly compared to those cultivated in the presence of PG. Following about 14 d of PG depletion, division of the cells was stopped, but it could be completely recovered by readdition of PG to the culture. This reversible stage of depletion lasted to about day 21 of PG deprivation (data not shown).
Figure 1.
The effect of PG depletion on the pigment content of pgsA cells. A, Absorption spectra of cell suspensions of the pgsA mutant of Synechocystis PCC6803; solid line, cells grown in the presence of PG (20 μm); broken line, cells after 5 d of PG depletion; dotted line, cells after 21 d of PG depletion. The spectra were normalized at 625 nm. B, Changes in phycocyanin (left) and Chl (right) contents in the pgsA mutant cells during PG depletion. Each value was calculated from three independent experiments.
Effect of Long-Term PG Depletion on the PSI Activity
PSI activity was monitored by measuring changes in the OD705 values in intact cells. These measurements can detect change in the redox state of PSI that is proportional to PSI activity. The results are summarized in Table I. All these data were calculated on the basis of Chl concentration. PSI activity did not show any significant change in the first week of PG depletion, but during the second week it decreased to about 80% of the initial activity. At the end of the 21-d PG depletion, only 40% of initial PSI activity was observed. Interestingly, if 20 μm of PG was added to the cells on day 21 of PG depletion, the PSI activity returned to initial level within 3 d of cultivation. Low temperature fluorescence emission dramatically decreased at longer wavelength characteristic for PSI emission following 14 d of PG depletion (data not shown). This observation, together with the decrease in the absorption change at 705 nm, clearly indicates the effect of long-term PG depletion on PSI activity.
Table I.
PSI activity in the pgsA mutant cells upon PG deprivation
| PG depletion (days) | 0 | 3 | 7 | 10 | 14 | 21 | Recov.a |
|---|---|---|---|---|---|---|---|
| ΔA705/μg Chl | 3.25 | 3.07 | 3.20 | 2.96 | 2.51 | 1.34 | 3.11 |
Effect of PG depletion on PSI activity measured by the change in absorbance at 705 nm on the basis of Chl content.
Recov., Following 21 d of PG deprivation and 3 d of readdition of 20 μM PG. Each OD value was calculated from three independent experiments; each sd is less than or equal to ±0.05.
PG Depletion Effect on the Oligomerization of PSI Complexes
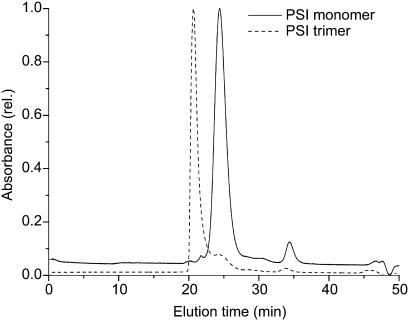
To investigate the oligomerization of RCs, pigment-protein complexes were isolated from thylakoid membranes. The separation of monomeric and trimeric forms of PSI was performed by anion-exchange chromatography combined with fluorescence spectroscopy. Fractions eluted with MgSO4 gradient were analyzed by fluorescence emission at 77 K. Separation of the thylakoid fraction isolated from pgsA mutant grown in the presence of PG resulted in four peaks of pigment-protein complexes (Fig. 2A). According to the fluorescence emission bands, peaks 1 and 4 contained monomeric and trimeric forms of PSI, respectively. The 77 K fluorescence emission spectra of these fractions were identical to those of purified PSI monomers and trimers described by other authors (Van der Lee et al., 1993). In peak 3, monomeric and dimeric forms of PSII were identified (Fig. 3), while peak 2 contained a mixture of PSI and PSII monomers (data not shown). Subsequent gel filtration chromatography on a TSK 3000 SW column revealed that peak 1 contains a pigment-protein complex with an estimated molecular mass of 300 kD, whereas the molecular mass of the pigment-protein complex in peak 4 exceeded 600 kD, as it eluted in the void volume of the column (Fig. 4).
Figure 2.
Elution profile of β-dodecylmaltoside-solubilized thylakoid fractions from Synechocystis pgsA mutant cells on an anion-exchange column (MonoQ HR 5/5). Absorbance was recorded at 437 nm. Peak 1, PSI monomer; peak 2, a mixture of PSI and PSII monomers; peak 3, PSII oligomer; and peak 4, PSI trimer. A, Thylakoids from cells grown in the presence of PG (20 μm). B, Thylakoids from cells following 7 d of PG depletion. C, Thylakoids from cells following 14 d of PG depletion. D, Thylakoids from cells following 21 d of PG depletion. E, Thylakoids from cells following 21 d of PG depletion and 3 d of PG readdition. F, Thylakoids from cells following 21 d of PG depletion and 20 h of PG readdition in the presence of lincomycin (0.4 mg mL−1). For details, see the text.
Figure 3.
77 K fluorescence emission spectra of fractions from the peak 1 (PSI monomer), peak 3 (PSII), and peak 4 (PSI trimer). The excitation wavelength was 437 nm.
Figure 4.
Elution profiles of PSI oligomers on TSK 3000 SW gel filtration column. Solid line indicates PSI monomer (from peak 1 of MonoQ profile) with a molecular mass of 300 kD, and the broken line represents PSI trimer (from peak 4 of MonoQ profile) with an estimated molecular mass above 600 kD.
By contrast, the same separation of thylakoids isolated from cells after 21 d of PG depletion revealed only three peaks of pigment-protein complexes, with an additional shoulder at peak 2 (Fig. 2D). No trimeric form of PSI could be detected by 77 K fluorescence analysis and/or gel filtration chromatography. Additionally, the relative ratio of free pigments to pigment-protein complexes was much higher in comparison to PG supplied cells. Interestingly, in thylakoid fractions isolated from 7-d PG-depleted cultures (Fig. 2B), the ratio of PSI trimer (peak 4) to monomer (peak 1) was smaller than that in cells cultured in the presence of PG (Fig. 2B). The relative amount of PSI RC trimer further decreased in 14-d PG-depleted cells (Fig. 2C); however, there still was a detectable level of PSI RC trimer content. Following a 21-d PG depletion, no PSI RC trimer could be detected (Fig. 2D).
In the thylakoid fraction isolated from PG-depleted cells 3 d after the readdition of PG to the culture medium, the trimeric form of PSI (peak 4) was restored (Fig. 2E). Following a 20-h recovery period, trimerization of PSI RCs was observed even in the presence of lincomycin, a protein synthesis inhibitor, at the concentration of 0.4 mg mL−1 (Fig. 2F).
Analysis of the Protein Composition of PSI Monomers and Trimers
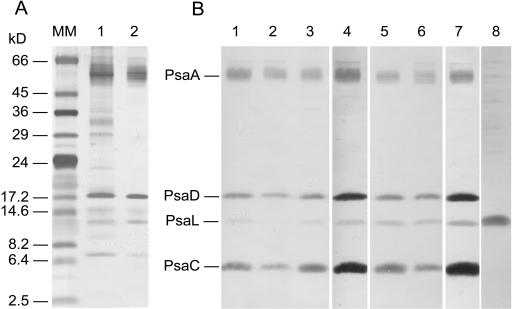
The protein composition of fractions from anion-exchange chromatography containing PSI monomers (peak 1) and trimers (peak 4) was analyzed by SDS-PAGE (Fig. 5A) and immunoblotting (Fig. 5B). In all PSI monomer- and trimer-containing fractions isolated from the pgsA mutant cells grown in the presence or absence of PG and following 3 d of PG readdition, the proteins reacting specifically with antibodies raised against individual subunits of cyanobacterial PSI (PsaA, PsaC, PsaD, and PsaL) were identified. Immunoblot analyses revealed a striking difference between the amounts of PsaL in peaks 1 and 4. The trimer fractions both in the PG-supplemented cells and in the 21-d PG-depleted cells following 3 d of PG readdition contained high amounts of PsaL (Fig. 5B, lanes 5 and 6). Compared to the trimer fractions, all the monomer fractions contained much lower amounts of PsaL. The PsaL content in the monomer fraction of 21-d PG-depleted cells was under the detectable level (Fig. 5B, lane 2). Despite of the remarkable decrease of PsaL in the 21-d PG-depleted PSI RC monomers, we could detect a high level of this protein in the isolated thylakoid fraction of the same cell sample (Fig. 5B, lane 8). This observation can explain the PG-induced recovery of the PSI trimers in the PG-depleted cells even in the presence of lincomycin.
Figure 5.
Analysis of protein content of PSI oligomers. A, Silver-stained SDS-PAGE (12%) of the monomer (lane 1) and trimeric (lane 2) fractions of PSI, from cells grown in the presence of PG, after anion-exchange chromatography. B, Immunoblot of PSI complexes after MonoQ anion-exchange chromatography. Filters were probed with antibodies specific for the cyanobacterial PsaA, PsaC, PsaD, and PsaL subunits (lanes 1–7). Lane 1, PSI monomers from cells grown in the presence of PG (20 μm); lane 2, monomers after 21 d of PG depletion; lane 3, monomers after 21 d of PG depletion followed by 3 d of PG readdition; lane 4, monomers from 21-d PG-depleted cells followed by 20 h of PG readdition in the presence of lincomycin (0.4 mg mL−1); lane 5, PSI trimers from cells grown in the presence of PG (20 μm); lane 6, trimers from cells of 21-d PG depletion followed by 3 d of PG readdition; and lane 7, trimers from cells of 21-d PG depletion followed by 20 h of PG readdition in the presence of lincomycin (0.4 mg mL−1). Lane 8, thylakoid membrane isolated from cells after 21 d of PG depletion probed with anti-PsaL antibody.
DISCUSSION
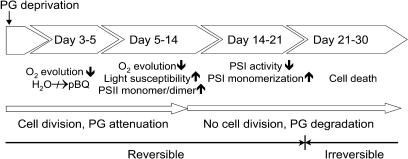
In this article, we describe the effects of prolonged PG deprivation on the photosynthetic RCs of the cyanobacterium Synechocystis PCC6803. During our work, we took advantage of pgsA mutant, which, due to a lesion in the PG phosphate synthase gene, is incapable of PG synthesis. Cultivation for 4 weeks in PG-deficient medium was found to be lethal for this mutant. Figure 6 shows a sequence of physiological and biochemical changes of the photosynthetic apparatus taking place upon long-term PG depletion. The first 3 to 5 d of PG deprivation caused mainly a disturbance in the photosynthetic electron transport. A 40% decrease in PG content could modify the functioning PSII RC by perturbing the membrane environment. This moderate PG depletion could affect the environment of QB site, which may result in a slowdown of electron transport between QA and QB (Gombos et al., 2002). This could indicate that PG molecules may be loosely bound to PSII RC and easily detached from the quinone site. Simultaneously, with the change in its function, the structure of PSII RC was also affected. In the second week of PG deprivation, the light-induced monomerization of PSII dimers was significantly enhanced (Sakurai et al., 2003). A severe PG depletion occurred in the third week of culturing. Following day 21, no PG could be detected by thin-layer chromatography in the lipid extract. This loss of PG was not compensated by an increase in the level of sulfoquinovosyldiacylglycerol, another anionic lipid, as has been observed in other photosynthetic organisms (Essigmann et al., 1998). A considerable inactivation of PSI activity was observed between days 14 and 21 of PG deprivation, as measured by OD705 changes. The PSI RC catalyzes the oxidation of reduced plastocyanin or cytochrome c6 and the reduction of soluble ferredoxin or flavodoxin. It plays an important role as a central part of electron transport, while also regulating the maintenance of physiological homeostasis of photosynthetic organisms. In cyanobacteria, a unique trimeric organization of PSI complexes has been observed (Fromme et al., 2001). However, the regulation of the transition and balance between PSI trimers and monomers has not yet been elucidated. Under our experimental conditions, the decrease of PSI activity was correlated with monomerization of the trimeric form. Earlier in vitro studies indicated that trimer formation was enhanced by the lipid environment (Kruip et al., 1999). The depletion of PG, a specific anionic lipid and the only phospholipid of cyanobacterial photosynthetic membranes, resulted in an unbalanced oligomer formation. This anionic lipid contains one negatively charged phosphate group bound to glycerol backbone, which can accommodate Ca2+ that has been suggested to be required for trimer formation (Fromme et al., 2001). In vitro it was demonstrated that Ca2+ can induce the aggregation of negatively charged lipids (Hincha, 2003). Under PG depletion, monomer PSI complexes became dominant at the expense of the trimers. Disappearance of PG molecules and concomitant complete monomerization of PSI RCs strongly suggest a requirement for PG in maintenance of the trimeric form. Although, except in PsaL, all of the protein subunits of the PSI RC are present in the monomer separated from the cells after 21 d of PG depletion, trimer formation could not be observed. This observation clearly demonstrates the importance of PsaL in trimer formation, while also suggesting that PG is required for the binding of PsaL to the PSI RC. PG-induced reconstitution of PSI trimers in the presence of lincomycin reveals that de novo protein synthesis is not required for the restoration of PSI oligomers. The high level of PsaL observed in the thylakoids of 21-d PG-depleted cells can provide the source of this protein for the restoration of PSI RC trimers, while readded PG molecules can stabilize the reformed PSI RC trimers. Our data confirm the indispensable role of PG molecules in the formation of the structures required for the functioning of the photosynthetic RCs.
Figure 6.
Chronology of PG depletion effects on cellular functions in Synechocystis PCC6803 cells. The arrows indicate increase or decrease in the physiological activity or amount of pigments and oligomers.
On the basis of our current knowledge, it can be asserted that (1) PG is needed for the formation of both PSII (Sakurai et al., 2003) and PSI oligomers, (2) PG acts by loosely binding to PSII RC for the maintenance of the quinone environment and supporting its function (Gombos et al., 2002), (3) PG molecules might regulate the optimal light capturing by balancing the PSI monomer to trimer ratio, and (4) PG could be important in protection against the excess of light exposure by supporting the formation of PSI trimers that might facilitate radiationless energy dissipation. Our pgsA mutant can be instrumental in the localization of PG-specific binding sites in the RCs by exploring the specificity of PG binding and interactions with RC protein components. Our studies can also lead to understanding the physiological relevance of PSI trimers.
MATERIALS AND METHODS
Organism and Growth Conditions
The pgsA mutant cells of Synechocystis PCC6803 were grown photoautotrophically in BG 11 medium (Allen, 1968) supplemented with 5 mm HEPES-NaOH (pH 7.5), 20 μg mL−1 kanamycin, and 20 μm dioleoyl-PG (18:1/18:1 PG; P-9664; Sigma, St Louis) at 30°C under continuous illumination at the intensity of 30 μmol photons m−2 s−1. PG depletion was achieved by washing the cells twice with PG-free medium, followed by cultivation in PG-free medium. Cultures were aerated on a gyratory shaker at 100 rpm. The concentration of cells in cultures was calculated on the basis of OD750.
Absorption Spectra and Pigment Concentration
Absorption spectra of cell suspensions were recorded on Shimadzu UV-3000 spectrophotometer (Columbia, MD). Chl and phycocyanin contents were estimated on the basis of the OD680 and OD630 values, respectively. Both pigment values were normalized on the basis of cell number. The Chl concentration was determined by the method of Arnon et al. (1974).
Lipid Analysis
Lipids were extracted from intact cells according to Bligh and Dyer (1959). Lipid and fatty acid analyses were carried out as described by Wada and Murata (1989).
Measurement of PSI Activity
Transient absorption changes at selected wavelengths in the microsecond to millisecond range were measured with a split-beam spectrophotometer, with the probing light obtained by a combination of two monochromators, a mechanical shutter and a 100-W tungsten-iodine lamp, according to Iwaki et al. (1999). Sample were excited by a 532-nm, 10-ns (full-width at half-maximum) pulse light from a Nd-YAG laser (Quanta Ray DCR-2-10) at a 0.05-Hz repetition rate. The sample in a cuvette with a 10-mm light path was placed in a temperature-controlled cuvette holder at 283 K during the measurements.
Isolation of Thylakoid Membranes
Thylakoid fraction was isolated from Synechocystis PCC6803 according to Komenda and Barber (1995). All subsequent steps of the isolation procedure were carried out at 4°C. Briefly, Synechocystis PCC6803 cells were collected by centrifugation at 2,500g for 10 min and resuspended in buffer A containing 50 mm MES (pH 6.5), 2 mm ɛ-amino-caproic acid, 5 mm EDTA, 1 mm phenylmethylsulfonyl fluoride (PMSF), and 1 mm benzamidine. The suspension was mixed with an equal volume of 0.1-mm glass beads and placed into a Bead Beater homogenizer (BioSpec Products, Bartlesville, OK). Cell disruption was performed 15 times for 1 min. Intact cells were removed by centrifugation at 2,000g for 5 min, and the supernatant was centrifuged again at 20,000g for 30 min. Pelleted thylakoids were resuspended either in buffer B (50 mm Tris, pH 7.5, 1 m Suc, 1 mm PMSF, and 1 mm benzamidine) or in buffer C (20 mm MES, pH 6.5, 10 mm CaCl2, 10 mm MgCl2, 0.5 m mannitol, 20% (v/v) glycerol, 1 mm PMSF, and 1 mm benzamidine) and stored at −80°C.
Isolation of Pigment-Protein Complexes from Thylakoids
Monomer and trimer PSI pigment-protein complexes were separated according to Rögner et al. (1990b), with slight modifications. All experiments were carried out at 4°C. Thylakoid membranes were resuspended in buffer C at 1 mg mL−1 Chl concentration. Following the addition of n-dodecyl-β-d-maltoside (DM) to 0.1% (w/v), the samples were shaken on an orbital shaker for 10 min in darkness. Then the membranes were pelleted by centrifugation for 30 min at 145,000g in a Beckman Ti 50 rotor (Fullerton, CA). The phycobilisome-containing supernatant was discarded, and the membrane pellet was resuspended in buffer C containing 1% (w/v) DM at 1 mg mL−1 Chl concentration. The suspension was shaken for 30 min and recentrifuged at 145,000g for 90 min. The supernatant, containing extracted pigment-protein complexes, was collected for further experiments.
HPLC separations were performed at 10°C with Varian Prostar (Varian, Palo Alto, CA) liquid chromatograph equipped with UV/Vis absorption and conductivity gradient detectors. Extracted pigment-protein complexes (100–300 μL) were loaded on MonoQ HR 5/5 column (Amersham-Pharmacia Biotech, Uppsala), equilibrated with buffer D containing 20 mm MES (pH 6.5), 10 mm CaCl2, 10 mm MgCl2, 0.5 m mannitol, 5 mm MgSO4, and 0.03% (w/v) DM, and eluted with a nonlinear gradient of 5 to 200 mm MgSO4, with a flow rate of 0.4 mL min−1, as described by Rögner et al. (1990b). The peaks were detected at 437 nm. One-milliliter fractions containing particular pigment-protein complexes, as detected by 77 K emission fluorescence, were pooled, dialyzed overnight against buffer E (20 mm MES, pH 6.5, supplemented with 1 mm PMSF and 1 mm benzamidine), and concentrated to approximately 100 μL by solid Sephadex G 150 (Amersham-Pharmacia Biotech), or the pigment-protein complexes were concentrated directly from the fractions using by StrataClean resin (Stratagene, La Jolla, CA).
Gel filtration chromatography was performed on a TSK 3000 SW (7.5- × 600-mm) column (Tosoh Bioscience, Tokyo) equilibrated with buffer D at a flow rate of 0.5 mL min−1. The column was calibrated using protein Mr standards (Bio-Rad, Hercules, CA).
Gel Electrophoresis and Immunoblotting
SDS-PAGE was performed according to the standard procedure, as described by Schagger and von Jagow (1987), using 12% gels. Equal amounts of proteins, as measured by Bradford assay, were loaded to each lane. Proteins separated by SDS-PAGE were silver stained as described by Ausubel et al. (1995) or transferred to nitrocellulose membranes (Protran BA 85; Schleicher & Schuell, Keene, NH) according to Towbin et al. (1979). Blots were probed with rabbit polyclonal antibodies raised against PSI subunits of Synechocystis PCC6803 (anti-PsaA and anti-PsaL) and of Synechococcus PCC7002 (anti-PsaC and anti-PsaD). Blots were developed by using goat anti-rabbit secondary antibodies conjugated with alkaline phosphatase using standard nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate staining protocol (Ausubel et al., 1995).
Fluorescence Spectroscopy
For 77 K measurements, the fractions obtained from MonoQ separation were cooled in liquid nitrogen. Steady-state fluorescence emission spectra (600–780 nm) were recorded, using 437-nm excitation wavelength, with Perkin-Elmer LS 50 spectrofluorimeter (Foster City, CA) equipped with liquid nitrogen attachment. The emission spectra were corrected for the wavelength dependence of the detection system and normalized at maximum.
Acknowledgments
We thank Dr. Jochen Kruip and Mrs. Tatjana Schwabe (Plant Biochemistry, Faculty of Biology, Ruhr-University Bochum, Bochum, Germany) for the gift of PsaL antibody. We also thank Erika Zukic for her excellent technical assistance. We thank Dr. Miklós Szekeres (Institute of Plant Biology, Biological Research Center of the Hungarian Academy of Sciences, Szeged, Hungary) for reading and correcting the manuscript.
This work was supported by grants from the Hungarian Science Foundation (OTKA; grant nos. T 34174 and T 38408), by the Dr. Rollin D. Hotchkiss Foundation (I.D.), and cofinanced by the European Union (Center of Excellence grant; contract no. BIER ICA1–CT–2000–70012) and the Polish Committee for Scientific Research (grant no. 158/E–338–/SPUB–M/5 PR UE/DZ 9/2001–2003).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.037754.
References
- Allen MM (1968) Simple conditions for growth of unicellular blue-green algae on plates. J Phycol 4: 1–4 [DOI] [PubMed] [Google Scholar]
- Arnon DI, McSwain BD, Tsujimoto HY, Wada K (1974) Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta 357: 231–245 [DOI] [PubMed] [Google Scholar]
- Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1995) Short Protocols in Molecular Biology, Chapter 10. John Wiley, New York
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Boekema EJ, Boonstra AF, Dekker JP, Rögner M (1994) Electron microscopic structural analysis of Photosystem I, Photosystem II, and the cytochrome b6/f complex from green plants and cyanobacteria. J Bioenerg Biomembr 26: 17–29 [DOI] [PubMed] [Google Scholar]
- Chitnis PR (1996) Photosystem I. Plant Physiol 111: 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis VP, Chitnis PR (1993) PsaL subunit is required for the formation of photosystem I trimers in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett 336: 330–334 [DOI] [PubMed] [Google Scholar]
- Essigmann B, Guler S, Narang RA, Linke D, Benning C (1998) Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 95: 1950–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragata M, Strzalka K, Nenonene EK (1991) MgCl2-induced reversal of oxygen evolution decay in photosystem II particles incubated with phosphatidylglycerol vesicles at high lipid/photosystem II ratio. J Photochem Photobiol B Biol 11: 329–342 [Google Scholar]
- Fromme P (1996) Structure and function of photosystem I. Curr Opin Struct Biol 6: 473–484 [DOI] [PubMed] [Google Scholar]
- Fromme P, Jordan P, Krauss N (2001) Structure of photosystem I. Biochim Biophys Acta 1507: 5–31 [DOI] [PubMed] [Google Scholar]
- Golbeck JH (1992) Structure and function of photosystem I. Annu Rev Plant Physiol Plant Mol Biol 43: 293–324 [Google Scholar]
- Gombos Z, Várkonyi Z, Hagio M, Iwaki M, Kovács L, Masamoto K, Itoh S, Wada H (2002) Phosphatidylglycerol requirement for the function of electron acceptor plastoquinone QB in the photosystem II reaction center. Biochemistry 41: 3796–3802 [DOI] [PubMed] [Google Scholar]
- Hagio M, Gombos Z, Várkonyi Z, Masamoto K, Sato N, Tsuzuki M, Wada H (2000) Direct evidence for requirement of phosphatidylglycerol in photosystem II of photosynthesis. Plant Physiol 124: 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagio M, Sakurai I, Sato S, Kato T, Tabata S, Wada H (2002) Phosphatidylglycerol is essential for the development of thylakoid membranes in Arabidopsis thaliana. Plant Cell Physiol 43: 1456–1464 [DOI] [PubMed] [Google Scholar]
- Hincha DK (2003) Effects of calcium-induced aggregation on the physical stability of liposomes containing plant glycolipids. Biochim Biophys Acta 1611: 180–186 [DOI] [PubMed] [Google Scholar]
- Iwaki M, Itoh S, Kamei S, Matsubara H, Oh-oka H (1999) Time-resolved spectroscopy of chlorophyll-a like electron acceptor in the reaction center complex of the green sulfur bacterium Chlorobium tepidum. Plant Cell Physiol 40: 1021–1028 [Google Scholar]
- Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature 411: 909–917 [DOI] [PubMed] [Google Scholar]
- Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, et al (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res 3: 109–136 [DOI] [PubMed] [Google Scholar]
- Karapetyan NV, Holzwarth AR, Rögner M (1999) The photosystem I trimer of cyanobacteria: molecular organization, excitation dynamics and physiological significance. FEBS Lett 460: 395–400 [DOI] [PubMed] [Google Scholar]
- Komenda J, Barber J (1995) Comparison of psbO and psbH deletion mutants of Synechocystis PCC 6803 indicates that degradation of D1 protein is regulated by the QB site and dependent on protein synthesis. Biochemistry 34: 9625–9631 [DOI] [PubMed] [Google Scholar]
- Kruip J, Chitnis PR, Lagoutte B, Rögner M, Boekema EJ (1997) Structural organization of the major subunits in cyanobacterial photosystem 1. Localization of subunits PsaC, -D, -E, -F, and -J. J Biol Chem 272: 17061–17069 [DOI] [PubMed] [Google Scholar]
- Kruip J, Karapetyan NV, Terekhova IV, Rögner M (1999) In vitro oligomerization of a membrane protein complex. liposome-based reconstitution of trimeric photosystem I from isolated monomers. J Biol Chem 274: 18181–18188 [DOI] [PubMed] [Google Scholar]
- Kruse O, Hankamer B, Konczak C, Gerle C, Morris E, Radunz A, Schmid GH, Barber J (2000) Phosphatidylglycerol is involved in the dimerization of photosystem II. J Biol Chem 275: 6509–6514 [DOI] [PubMed] [Google Scholar]
- Mukerji I, Sauer K (1989) Temperature dependent steady-state and picosecond kinetic fluorescence measurements of a Photosystem I preparation from spinach. In W Briggs, ed, Progress in Photosynthesis, Vol 8. Alan R Liss, New York, pp 105–122
- Rögner M, Mühlenhoff U, Boekema EJ, Witt HT (1990. a) Mono-, di- and trimeric PSI reaction center complexes isolated from the thermophilic cyanobacterium Synechococcus sp.: size, shape and activity. Biochim Biophys Acta 1015: 415–424 [Google Scholar]
- Rögner M, Nixon PJ, Diner BA (1990. b) Purification and characterization of photosystem I and photosystem II core complexes from wild-type and phycocyanin-deficient strains of the cyanobacterium Synechocystis PCC 6803. J Biol Chem 265: 6189–6196 [PubMed] [Google Scholar]
- Sakurai I, Hagio M, Gombos Z, Tyystjärvi T, Paakkarinen V, Aro E-M, Wada H (2003) Requirement of phosphatidylglycerol for maintenance of photosynthetic machinery. Plant Physiol 133: 1376–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166: 368–379 [DOI] [PubMed] [Google Scholar]
- Schluchter WM, Shen G, Zhao J, Bryant DA (1996) Characterization of psaI and psaL mutants of Synechococcus sp. strain PCC 7002: a new model for state transitions in cyanobacteria. Photochem Photobiol 64: 53–66 [DOI] [PubMed] [Google Scholar]
- Shubin V, Bezsmertnaya I, Karapetyan NV (1995) Efficient energy transfer from the long-wavelength antenna chlorophylls to P700 in photosystem I complexes from Spirulina platensis. J Photochem Photobiol B Biol 30: 153–160. [Google Scholar]
- Shubin V, Tsuprun VL, Bezsmertnaya IN, Karapetyan NV (1993) Trimeric forms of the photosystem I reaction center complex pre-exist in the membranes of the cyanobacterium Spirulina platensis. FEBS Lett 334: 79–82 [DOI] [PubMed] [Google Scholar]
- Sun J, Xu Q, Chitnis VP, Jin P, Chitnis PR (1997) Topography of the photosystem I core proteins of the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem 272: 21793–21802 [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trissl H-W (1993) Long-wavelength absorbing antenna pigments and heterogeneous absorption bands concentrate excitons and increase absorption cross section. Photosynth Res 35: 247–263 [DOI] [PubMed] [Google Scholar]
- Van der Lee J, Bald D, Kwa SLS, van Grondelle R, Rögner M, Dekker JP (1993) Steady-state polarized light spectroscopy of isolated Photosystem I complexes. Photosynth Res 35: 311–321 [DOI] [PubMed] [Google Scholar]
- van Grondelle R, Dekker JP, Gilbro T, Sundstrom V (1994) Energy transfer and trapping in photosynthesis. Biochim Biophys Acta 1187: 1–65 [Google Scholar]
- Wada H, Murata N (1989) Synechocystis PCC6803 mutants defective in desaturation of fatty acids. Plant Cell Physiol 30: 971–978 [Google Scholar]
- Wada H, Murata N (1998) Membrane lipids in Cyanobacteria. In P-A Siegenthaler, N Murata, eds, Lipids in Photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 65–81
- Xu C, Hartel H, Wada H, Hagio M, Yu B, Eakin C, Benning C (2002) The pgp1 mutant locus of Arabidopsis encodes a phosphatidylglycerolphosphate synthase with impaired activity. Plant Physiol 129: 594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouni A, Witt HT, Kern J, Fromme P, Krauss N, Saenger W, Orth P (2001) Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature 409: 739–743 [DOI] [PubMed] [Google Scholar]