Distinguishing patient cohorts that require ongoing mechanical ventilation has been made problematic by the use of various terms, which, in turn, leads to difficulty in comparative studies and uncertainty in the timing of clinical decision making. Consensus conferences have achieved little in terms of standardizing transition terms and information that can be broadly applied to include the many clinical specialities reflecting the continuum of care. Accordingly, this study aimed to identify the defining features of key transition points for individuals requiring ongoing ventilator assistance based on expert-derived consensus.
Keywords: Home ventilation, Long-term mechanical ventilation, Mechanical ventilation, Pediatric ventilation, Prolonged mechanical ventilation, Transition
Abstract
BACKGROUND:
Various terms, including ‘prolonged mechanical ventilation’ (PMV) and ‘long-term mechanical ventilation’ (LTMV), are used interchangeably to distinguish patient cohorts requiring ventilation, making comparisons and timing of clinical decision making problematic.
OBJECTIVE:
To develop expert, consensus-based criteria associated with care transitions to distinguish cohorts of ventilated patients.
METHODS:
A four-round (R), web-based Delphi study with consensus defined as >70% was performed. In R1, participants listed, using free text, criteria perceived to should and should not define seven transitions. Transitions comprised: T1 – acute ventilation to PMV; T2 – PMV to LTMV; T3 – PMV or LTMV to acute ventilation (reverse transition); T4 – institutional to community care; T5 – no ventilation to requiring LTMV; T6 – pediatric to adult LTMV; and T7 – active treatment to end-of-life care. Subsequent Rs sought consensus.
RESULTS:
Experts from intensive care (n=14), long-term care (n=14) and home ventilation (n=10), representing a variety of professional groups and geographical areas, completed all Rs. Consensus was reached on 14 of 20 statements defining T1 and 21 of 25 for T2. ‘Physiological stability’ had the highest consensus (97% and 100%, respectively). ‘Duration of ventilation’ did not achieve consensus. Consensus was achieved on 13 of 18 statements for T3 and 23 of 25 statements for T4. T4 statements reaching 100% consensus included: ‘informed choice’, ‘patient stability’, ‘informal caregiver support’, ‘caregiver knowledge’, ‘environment modification’, ‘supportive network’ and ‘access to interprofessional care’. Consensus was achieved for 15 of 17 T5, 16 of 20 T6 and 21 of 24 T7 items.
CONCLUSION:
Criteria to consider during key care transitions for ventilator-assisted individuals were identified. Such information will assist in furthering the consistency of clinical care plans, research trials and health care resource allocation.
Abstract
HISTORIQUE :
Divers termes, y compris prolonged mechanical ventilation (PMV) et long-term mechanical ventilation (LTMV), sont utilisés de manière interchangeable pour distinguer les cohortes de patients qui ont besoin de ventilation, ce qui complique les comparaisons et le moment choisi pour prendre des décisions cliniques.
OBJECTIF :
Élaborer des critères consensuels d’experts associés aux transitions des soins pour distinguer les cohortes de patients sous ventilation.
MÉTHODOLOGIE :
Les chercheurs ont effectué une analyse Delphi en ligne en quatre passages (P), dont le consensus était défini comme supérieur à 70 %. Au P1, les participants énuméraient en leurs mots les critères qui, selon eux, devraient et ne devraient pas définir sept transitions. Ces transitions s’établissaient comme suit : T1 – ventilation aiguë à PMV; T2 – PMV à LTMV; T3 – PMV ou LTMV à ventilation aiguë (transition inversée); T4 – soins hospitaliers à soins communautaires; T5 – aucune ventilation à LTMV; T6 – LTMV pédiatrique à adulte; et T7 – traitement actif à soins de fin de vie. Les P suivants visaient l’atteinte du consensus.
RÉSULTATS :
Des experts de la ventilation en soins intensifs (n=14), en soins de longue durée (n=14) et à domicile (n=10) au sein de divers groupes professionnels et de diverses régions géographiques ont fait tous les P. Ils sont parvenus à un consensus sur 14 des 20 énoncés définissant la T1 et sur 21 des 25 énoncés définissant la T2. La physiological stability obtenait le plus grand consensus (97 % et 100 %, respectivement). La ventilation duration n’obtenait pas de consensus. Toutefois, les experts sont parvenus à un consensus sur 13 des 18 énoncés définissant la T3 et sur 23 des 25 énoncés définissant la T4. Les énoncés définissant la T4 atteignant un consensus de 100 % incluaient informed choice, patient stability, informal caregiver support, caregiver knowledge, environment modification, supportive network et access to interprofessional care. Les experts sont parvenus à un consensus au sujet de 15 des 17 points de T5, 16 des 20 points de T6 et 21 des 24 points de T7.
CONCLUSION :
Les chercheurs ont déterminé les critères à envisager pendant les principales transitions des soins aux personnes sous ventilation. L’information contribuera à améliorer la cohérence des plans de soins cliniques, des essais de recherche et de l’affectation des ressources en santé.
Various clinical terms are used interchangeably to describe patient cohorts requiring mechanical ventilation (MV). This produces variability in reported patient prevalence, makes comparison of studies problematic and may contribute to decisional uncertainty regarding the most appropriate time to modify the goals of care. Many clinicians and researchers use duration of ventilation (consecutive days) and number of hours per day during which the patient receives ventilation as key descriptive terms. In 1998, a consensus conference recommended that long-term MV (LTMV) be defined as ≥30 days for ≥6 h (1) and, in 2005, another consensus conference recommended that prolonged MV (PMV) be defined as ≥21 consecutive days of MV for ≥6 h (2). More recently derived terms include ‘prolonged acute mechanical ventilation’ (≥96 consecutive hours) (3) and chronic critical illness defined as receiving MV in an intensive care unit for >14 days (4), although other definitions exist (5). Despite consensus conferences, transition terms are not standardized, with studies reporting PMV ranging from >6 h (6) to >29 days (7), and LTMV beginning in some instances at >3 days (8).
Other clinically defining features include tracheostomy placement and transfer to a lower intensity care location outside of the intensive care unit. In some jurisdictions, such definitions are linked to reimbursement plans. For example, in both the United States and Taiwan, PMV, defined as ≥21 consecutive days of MV, may trigger transfer to long-term acute care facilities (1) or respiratory care centres (9), respectively. Tracheostomy placement is usually guided by the anticipated need for PMV; however, timing is also highly variable (10).
While using duration of MV or other markers, such as tracheostomy placement or transfer of care to another centre, offer the advantages of simplicity, uniformity and relative ease of identification in the medical record or administrative databases (11), many clinicians will use other clinical markers to assist with indications for a change in clinical management, the overall plan of care or information provided to patients and their families. However, there is little information regarding the key transition points used by health care professionals that may reflect change across the spectrum of care. Moreover, such information needs to be broadly based to include many clinical specialties reflecting the continuum of care. Therefore, our aim was to identify defining features of seven key transition points across the care continuum for individuals requiring ongoing ventilator assistance based on expert-derived consensus. Our goal is that these definitional criteria will inform decision making by clinicians, administrators and policy makers.
METHODS
Study design
A two-stage approach was used to develop consensus for defining features of care transitions. First, a national workshop was held that invited 34 key stakeholders including clinicians and administrators providing services in three distinct areas – intensive care, long-term care and ventilation in the community – as well as international faculty from the United States and Europe, to identify clinical, research and policy priorities for ventilator-assisted individuals (VAIs) (12). An outcome of the workshop was identification of seven important transition points across the care continuum, and the need for greater clarity surrounding clinically defining features. These transitions comprised: T1 – ventilation in the acute phase of illness to PMV; T2 – PMV to LTMV; T3 – PMV or LTMV to acute critical care ventilation (reverse transition); T4 – institutional to community care; T5 – being ‘at-risk of but not currently receiving’ to ‘requiring’ LTMV; T6 – transition from pediatric to adult LTMV; and T7 – active treatment to end-of-life care. In stage 2, a four-round (R) Delphi approach was used to establish consensus for the defining features of each transition. The Delphi approach is advocated as an effective tool for establishing consensus in health care (13).
Delphi participants
Invited participants comprised clinicians and administrators considered to have expertise in service provision for VAIs in an institutional (acute or long-term) or community setting. Participants were identified by the authors’ national advisory group members. Additional participants required to meet purposeful diversity sampling targets were identified during development of a national inventory of service providers to VAIs (12). Invited participants also nominated potential participants either as a designate or as an additional participant. Purposeful diversity sampling was used to obtain professional (medicine, nursing, respiratory therapy and physiotherapy; adult and pediatric specialities) and geographical (across Canadian provinces) diversity representing the stakeholder groups. Although recommended minimum or maximum sample sizes for Delphi panels vary, key aspects include common sense and practical logistics (14). To achieve 10 participants representing each stakeholder group (acute, long-term institutional and community) the authors oversampled by 15 (45 participants in total) due to anticipated attrition. Only participants providing responses to the preceding R were invited to continue participation.
Delphi instrument and Rs
In R1, participants were provided with the title of the seven transitions listed above and requested to list, using free text, criteria they perceived should (Part A) and should not (Part B) define each. Participants were directed to consider potentially relevant timeframes, patient characteristics and physical locations. The R1 questionnaire weblink was provided by e-mail; three reminders were sent over a four-week period. Raw data generated in R1 were then subjected to inductive content analysis to identify categories and to generate an appropriate statement representing each category to be included in R2 (see data analysis section). In R2, participants rated agreement with each statement on a five-point Likert scale. Three reminders were sent over a six-week period.
For R3, the questionnaire was modified by removing Part B and adding statements not already represented in Part A, ensuring neutral language. This was performed for two reasons: first, in R1, many criteria suggested to define a transition were also suggested as criteria that should not define the transition producing duplication. Second, participants reported that difficulty rating agreement with suggested criteria should not be considered as definitional because most were worded in a negative direction such as ‘lack of expertise’ or ‘failure to consider patient wishes’.
In R3, participants re-assessed or confirmed R2 responses. The most common aggregate group response (mode) was provided in addition to the participant’s own response. Some statements had two R2 modes; both were presented. Participants also rated level of agreement with the ‘new’ neutrally restated statements derived from Part B. In R4, participants re-assessed or confirmed responses to these new statements (Figure 1).
Figure 1).
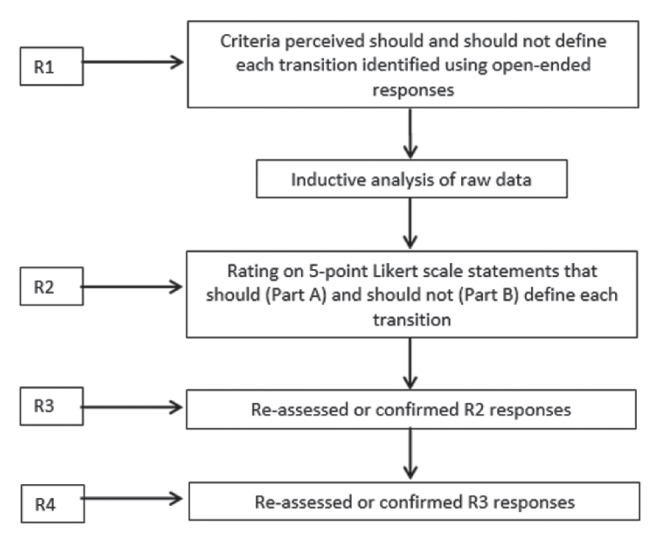
Delphi method. R Round
Ethics considerations
Research Ethics Boards of the University of Toronto (#26199) and St Michael’s Hospital (Toronto, Ontario) approved the study. Participation was voluntary and consent implied by questionnaire return.
Data analysis
Inductive content analysis of R1 data comprised, for each transition, independent reading and rereading, open coding, data grouping, category identification and sorting of items into categories (15) by three survey research unit members contracted to manage the Delphi process and five study investigators. Study investigators then identified an appropriate statement to represent each category for inclusion in R2.
Five-point Likert scale responses were grouped into three categories: agree, no opinion and disagree. Frequencies and percentages were calculated for each item on completion of R2, R3 and R4 to ascertain whether raw scores achieved >70% consensus. Although no standard threshold for consensus is recommended, >70% was selected because it has been recommended as a reasonable cut-off point (16). Failure to gain consensus was defined as ≤70% consensus in either agree or disagree groupings. Stability of individual opinion was measured using change scores (mean ± SD) for each transition, with a score of 0 reflecting no change in responses across rounds.
RESULTS
Of the 73 experts identified, 45 completed R1 and R2, 39 R3 and 38 R4 (84% retention). Fourteen participants represented intensive care, 14 long-term care and 10 home ventilation services; five specialized in pediatrics (Table 1). R1 generated 291 statements of criteria that should define the seven transitions and 221 of what should not. Inductive content analysis resulted in identification of 150 unique definitional criteria.
TABLE 1.
Participant diversity
| Intensive care | LTMV institutional | Home/community care | |
|---|---|---|---|
| Medical doctor | 10 | 2 | 3 |
| Registered nurse | 1 | 6 | 0 |
| RT/physiotherapist | 2 | 8 | 3 |
| Pediatrics | 1 | 1 | 4 |
| Total | 14 | 14 | 10 |
Data presented as n. LTMV Long-term mechanical ventilation; RT Respiratory therapist
Transition from acute to PMV
Thirteen of 20 (65%) statements achieved consensus that they should define transition from ventilation during acute illness to PMV; one achieved consensus that it should not be included (Table 2). Six statements did not achieve consensus. These were as follows: ‘possibility of future successful weaning’ (68%); ‘number of consecutive days on MV’ (64%); ‘patient transfer to an alternative care setting’ (64%); ‘tracheostomy in situ’ (62%); ‘number of consecutive hours of MV each day’ (59%); and ‘current care environment’ (28%). Although not achieving consensus of >70%, ≥21 consecutive days (13 of 25 [52%]) and ≥6 consecutive h (21 of 23 [91%]) of MV were the most frequent responses in terms of duration from participants that perceived they should define transition.
TABLE 2.
Transition from ventilation in the acute phase of illness to prolonged mechanical ventilation
| Criteria that should define transition | % |
|---|---|
| Patient stability from a physiological perspective | 97 |
| Repeated unsuccessful weaning attempts | 92 |
| The patient’s wishes | 92 |
| The patient’s prognosis | 90 |
| Quality of life | 90 |
| Availability of appropriately trained staff and resources to facilitate transition | 87 |
| The need for nocturnal noninvasive ventilation alone | 84 |
| An established routine for ventilation and weaning | 82 |
| The patient’s diagnosis | 77 |
| Patient motivation and agreement to transition | 77 |
| Availability of expertise in ventilator weaning | 76 |
| Patient characteristics | 74 |
| Family motivation and agreement to transition | 72 |
| Criterion that should NOT define transition | |
|
| |
| Ongoing requirement for controlled ventilation | 74 |
Transition from PMV to LTMV
Eighteen of 25 (72%) statements achieved consensus that they should define transition from PMV to LTMV; three statements achieved consensus that they should not (Table 3). Four statements did not achieve consensus: ‘minimum number of consecutive hours on MV’ (69%), ‘inadequate attempts at weaning’ (66%), ‘minimum number of consecutive days of MV’ (59%) and ‘family/informal caregiver capability/resources to assume care’ (14%). Although not achieving consensus, ≥30 days (14 of 23 [61%]) and ≥6 h (24 of 27 [89%]) were the most frequent responses in terms of duration from participants perceiving they should define transition.
TABLE 3.
Transition from prolonged mechanical ventilation to long-term mechanical ventilation (LTMV)
| Criteria that should define transition | % |
|---|---|
| Patient stability from a physiological perspective | 100 |
| Establishment of a transition plan | 95 |
| Option of withdrawal of care is discussed | 95 |
| Acceptance and motivation of the patient based on informed choice | 92 |
| Redefinition of the goals of care/care plan | 90 |
| Patient’s prognosis | 87 |
| Requirement for invasive mechanical ventilation on an indefinite basis | 87 |
| Patient care needs can be managed in a community or a long-term care facility | 85 |
| Capability of the healthcare team to manage LTMV | 85 |
| Acceptance/motivation of family/informal caregivers based on informed choice | 82 |
| Likelihood of acceptable quality of life | 82 |
| Availability of an appropriate interface (eg, facemask or tracheostomy) | 80 |
| Patient’s diagnosis | 77 |
| Requirement for noninvasive ventilation on an indefinite basis | 77 |
| Patient’s inability to participate in decisions (ie, if persistent vegetative state or similar, should not be classified as LTMV) | 76 |
| Availability of appropriate resources | 74 |
| Availability of a transition placement | 74 |
| Family desire to assume care | 71 |
| Criteria that should NOT define transition | |
|
| |
| Patient demographic characteristics | 87 |
| Family resources | 84 |
| Potential impact on hospital resources | 82 |
Reverse transition from PMV or LTMV to acute care
Ten of 18 (56%) statements achieved consensus that they should define reverse transition; three statements achieved consensus that they should not (Table 4). Five statements did not achieve consensus: ‘caregiver burnout’ (64%), ‘irreversible or untreatable condition’ (50%), ‘unavailability of caregivers (paid/unpaid)’ (26%), ‘patient’s mental health’ (16%) and ‘inadequate community resources’ (15%).
TABLE 4.
Transition from prolonged mechanical ventilation (MV) or long-term MV (LTMV) to acute critical care ventilation (reverse transition)
| Criteria that should define transition | % |
|---|---|
| Acute loss of physiological stability | 95 |
| The patient’s and family’s wishes for full intervention for reversible condition | 95 |
| Need for escalating medical/health care that cannot be provided in current environment | 95 |
| Patient’s wishes based on informed choice (ie, right to decline) | 92 |
| Availability of resources outside of intensive care unit to manage LTMV (ie, patients should not be admitted to an intensive care unit solely because ventilated | 92 |
| Existing plan of care/advanced directives agreed by patient, family and health care team | 87 |
| Nonacute/progressive deterioration of medical condition warranting acute critical care | 85 |
| Planned admission for redefining care plan | 80 |
| Withdrawal of life support when resources to do so are not available in the patient’s community | 77 |
| Community placement availability (ie, need to return to an acute care institution due to unavailability of community placement | 71 |
| Criteria that should NOT define transition | |
|
| |
| Patient demographic characteristics | 84 |
| Minimum number of consecutive days/hours on MV | 87 |
| Gradual, expected increase in ventilator demands/progression of underlying condition | 74 |
Transition from institutional care to care within the community (home/assisted living)
Twenty-one of 25 (84%) statements achieved consensus that they should define transition to community care; two statements achieved consensus that they should not (Table 5). Two statements did not achieve consensus: ‘patient’s diagnosis’ (62%) and ‘location of patient’s home’ (16%).
TABLE 5.
Transition from institutional care to care within the community (home/assisted living)
| Criteria that should define transition | % |
|---|---|
| Availability of ongoing access to continuing interprofessional care | 100 |
| Willingness and ability of supportive network comprising family/friends/caregivers to provide required care | 100 |
| Adequate modification of environment/physical space to accommodate patient and equipment | 100 |
| Informed choice on behalf of the patient to live in the community | 100 |
| Physiological stability of patient to live in the community | 100 |
| Availability of appropriate support for informal caregivers including respite | 100 |
| Client/caregivers ability to demonstrate required knowledge/skills to live in community safely | 100 |
| Availability of equipment that is adequately funded and resourced | 97 |
| Availability of a most responsible physician to lead care | 97 |
| Established plan of care for ongoing management | 97 |
| Realistic expectations of family caregivers | 97 |
| Availability of necessary community resources | 97 |
| Ability to secure required resources/supports in a timely manner | 97 |
| Availability of formal caregivers | 97 |
| Physiological stability for a minimum number of days | 95 |
| Availability of financial support for caregivers, equipment, supplies, medication, and health care professionals | 92 |
| An absence in delay in transition once community resources are ready | 90 |
| Stable and uncomplicated airway | 90 |
| Ability of patient to direct care | 74 |
| Patient’s prognosis | 73 |
| Patient’s ability to tolerate brief interruptions in ventilation | 71 |
| Criteria that should NOT define transition | |
|
| |
| Rules/regulations of involved institutions | 84 |
| Limitations imposed by health care funding | 73 |
Transition from ‘no ventilation’ to ‘requiring’ LTMV
Ten (59%) of 17 statements achieved consensus that they should define transition from absence of ventilation to requiring LTMV; five statements achieved consensus that they should not (Table 6). Two statements did not achieve consensus: ‘inadequate treatment of underlying disease’ (69%) and ‘willingness/ability of family/friends/caregivers to provide support’ (68%).
TABLE 6.
Transition from ‘no ventilation’ to ‘requiring’ long-term mechanical ventilation
| Criteria that should define transition | % |
|---|---|
| Recurrent/progressive ventilatory failure without complicating treatable acute illness/reversible factors | 100 |
| Degree of physiological impairment | 97 |
| Acceptance and motivation of the patient based on informed choice | 89 |
| Symptom profile | 81 |
| Likelihood of acceptable quality of life | 78 |
| Patient’s diagnosis | 78 |
| Medical prognosis | 78 |
| Time (ie, duration of symptoms/ventilatory failure; time from diagnosis) | 78 |
| Access to appropriate health care personnel and technology | 76 |
| Availability of resources | 73 |
| Criteria that should NOT define transition | |
|
| |
| Health care team coercion | 97 |
| Patient demographic characteristics | 97 |
| Patient’s physical location | 89 |
| Funding availability | 81 |
| Ventilator availability | 78 |
Transition from pediatric to adult LTMV services
Thirteen of 20 (65%) statements achieved consensus that they should define transition from paediatric to adult LTMV; three statements achieved consensus that they should not (Table 7). Four statements did not achieve consensus: ‘patient’s cognitive maturity’ (70%), ‘financial resource availability’ (58%), ‘physical maturity’ (55%) and ‘increased role of patient as opposed to parents in directing care’ (18%).
TABLE 7.
Transition from pediatric to adult long-term mechanical ventilation services
| Criteria that should define transition (n=34)* | % |
|---|---|
| Transfer of care from pediatric team/specialists to adult team/specialists | 100 |
| A plan that commenced in adolescence that views transition as a continuum | 100 |
| Chronological age | 97 |
| A transition plan being in place | 97 |
| Appropriate environment/equipment for developmental stage | 94 |
| Appropriate environment for physical size | 94 |
| Availability of trained/skilled health care workers | 88 |
| The likelihood of an acceptable quality of life | 82 |
| Adequate resources in the adult sector | 79 |
| Family readiness | 79 |
| Patient readiness | 74 |
| Persistent vegetative state (ie, withdrawal of care should be discussed with parents/guardians of these children as opposed to considering transition to adult services) | 73 |
| Patient’s underlying condition | 71 |
| Criteria that should NOT define transition | |
|
| |
| Bed availability | 97 |
| Physiological instability | 88 |
| No longer in school | 82 |
Individuals without experience dealing with pediatric to adult transition were advised they did not need to rate items in this section
Transition from active treatment to end-of-life care for PMV or LTMV
Seventeen (71%) of 24 statements achieved consensus that they should define transition to end-of-life care; four statements reached consensus that they should not (Table 8). Three statements did not achieve consensus: ‘resource availability for ongoing care’ (63%), ‘family/caregiver expectations in terms of prognosis’ (55%) and ‘loss of communication ability’ (13%).
TABLE 8.
Transition from active treatment to end-of-life care for prolonged mechanical ventilation or long-term mechanical ventilation
| Criteria that should define transition | % |
|---|---|
| Patient exhibiting prolonged suffering (physical, mental, emotional, spiritual) | 100 |
| Patient’s advance directives/previous expressed wishes based on informed choice | 100 |
| Informed consent to proceed from patient or appropriate designate | 100 |
| Treatment goal changed to palliation/comfort care | 97 |
| Family/surrogate decision maker wishes based on informed choice | 97 |
| Best anticipated functional outcome no longer being acceptable to the patient | 97 |
| Quality of life | 95 |
| Patient, family and health care team agreement on plan for end-of-life care | 95 |
| Patient values (cultural/religious/moral) | 92 |
| Current accepted professional and ethical standards | 92 |
| Illness that is irreversible and/or nontreatable | 87 |
| Ability to medically manage end-of-life process in resource-appropriate manner | 84 |
| Physiological end points | 82 |
| An appropriate environment | 82 |
| Multisystem organ failure | 82 |
| Availability of team with specialized palliative care support | 79 |
| Health care team agreement | 71 |
| Criteria that should NOT define transition | |
|
| |
| Patient’s physical location | 95 |
| Age | 87 |
| Time on ventilatory support | 84 |
| Level of physical dependency | 79 |
Stability of change scores
The mean (± SD) individual change scores demonstrated good stability for all seven transitions. For acute ventilation to PMV scores ranged from 0±0.30 (consecutive MV days) to 0.31±0.80 (patient charactersitic, sex) (81% of change scores = 0); PMV to LTMV: 0.05±0.41 (consecutive MV hours) to 0.69±1.00 (family capability/resources to assume care) (75% = 0); reverse transition: 0.03±0.87 (planned admission for redefining care plan) to 0.54±1.14 (caregiver burnout) (77% = 0); institutional to community care: 0±0.24 (financial support availability) to 0.47±0.74 (rules/regulations of involved institutions) (88% = 0); at risk to requiring LTMV: 0±0.94 (patient acceptance/motivation) to 0.60±0.95 (ventilator availability) (76% = 0); pediatric to adult: 0±0.73 (cognitive maturity) to 0.67±0.92 (bed availability) (81% = 0); and to end-of-life care: 0±0.73 (goal for palliation/comfort care) to 0.67±0.92 (physical dependency) (83% = 0).
DISCUSSION
The present study was the first to use an anonymized Delphi-derived expert consensus of national scope, with representation across professions, regions and differing health provision care sectors (acute, long-term and community) as well as adult and pediatric specialists, to identify defining features of important transitions across the care continuum for VAIs. Consistent across the seven transition points were: safety, including physiological stability; consideration of patient/family wishes and motivation based on informed choice; prognosis and anticipated quality of life; establishment of a transition plan that includes redefinition of care goals; and timely availability of adequate resources. These features represent a global set of issues for consideration during health care transition for VAIs.
Concern for patient safety and the need for physiological stability recognizes the potential reduction in care intensity due to different staffing models either in the same care location or in an alternative venue such as a long-term acute care hospital, specialized weaning centre, long-term care facility or assisted living unit (4,17). The need to consider patient and family wishes enabled through informed choice, including an understanding of prognosis and anticipated quality of life, was encouraging to note because it reflects acceptance of the importance of collaborative communication processes and shared decision making endorsed by professional societies (18,19).
Establishment of a transition plan, a care routine and redefinition of care goals were recognized as key in many transitions, consistent with an understanding that a health care transition is a process as opposed to an event (20). Transition is frequently required due to a change in health status, symptoms or functioning (21), regardless of whether it requires a change in physical location or service providers. Timely availability of adequate resources was highlighted as a defining feature for transition from institutional to community care, consistent with studies in both adult and pediatric populations that have identified issues associated with delays and gaps in service provision and potential out of pocket expenses of family caregivers (22–25).
Contrary to previous definitions (1,2), the number of consecutive days of ventilation did not exceed the threshold of 70% used to identify consensus in defining PMV or LTMV. Although participants did not regard these arbitrary time points to be pivotal in changing the overall plan of care in the present survey, in some jurisdictions, time-based changes in ventilation may trigger resource availability, which is a key determinant of level of care (4,5). Moreover, the lack of consensus should not infer discontinuing these time-based criteria at present because they remain important bedside clinical markers that may continue to play a role in clinical and research trials. Repeated unsuccessful weaning attempts and an indefinite need for invasive or noninvasive ventilation were considered to be strong indicators of transition.
There are several reasons why consistency of definitions that demarcate patient transitions is important. First, clinicians may benefit from being aware of factors that may alert them to the need for reconsidering their care plan, the information they provide to patients and their families as well as the care locations that may encourage a more rehabilitative focus. Second, policy decisions that determine resource allocations are simplified if descriptions of incidence and prevalence rely on consistent criteria (1). Third, such consistency promotes accurate benchmarking of outcomes such as survival, hospital length of stay, functional dependence or health care utilization, as well as various care models across different jurisdictions (26,27).
Limitations of the present study include the study being restricted to one country. Although we received advice regarding the selection of transitions from international faculty, the extent to which the identified criteria are consistent with perspectives from other countries with different professional outlooks and health care systems remains to be explored. Such an understanding would be an essential next step if our definitions would be of benefit to an international consensus panel interested in advancing guidelines in this area. Despite diversity in sampling, there is the potential that some defining features of transition were not identified. Those that were identified constitute a checklist that will require refinement and reduction before being of value for decision making at the bedside. Despite reaching consensus using a predefined threshold of >70% on several transition statements, few reached 100%, underscoring the subjectivity, range of opinion and potential differences in health care organization. Notwithstanding the above, our observations provide useful information for streamlining care plans and achieving increased clarity for the care of those who require ongoing ventilator support.
CONCLUSION
Using a Delphi-derived expert consensus approach we identified criteria to be considered during key health care transitions for individuals requiring ongoing ventilator assistance. Such information may be used to better integrate clinical decision making, policy development, resource utilization and informational material for patients and their families.
Footnotes
ON BEHALF OF THE CANuVENT GROUP: Reshma Amin, The Hospital for Sick Children; Monica Avendano, West Park Healthcare Centre; Sandra Dial, Montreal Chest Institute; Eddy Fan, Mount Sinai Hospital; Ian Fraser, Toronto East General Hospital; Robert Fowler, Sunnybrook Health Sciences Centre; Roger Goldstein, West Park Healthcare Centre; Sherri Katz, Children’s Hospital of Eastern Ontario; Judy King, University of Ottawa; David Leasa, London Health Sciences Centre; Cathy Mawdsley, London Health Sciences Centre; Douglas McKim, Ottawa Hospital; Mika Nonoyama, University of Toronto; Jeremy Road, Provincial Respiratory Outreach Program, Vancouver Coastal Health; Louise Rose, University of Toronto; Gordon Rubenfeld, Sunnybrook Health Sciences Centre.
INSTITUTION: This work was conducted at the CRICH Survey Research Unit at St Michael’s Hospital and the University of Toronto.
FUNDING/DISCLOSURES: The work was funded by a grant from the Partnerships for Health Systems Improvement competition of the Canadian Institutes of Health Research. The authors have no financial disclosures or conflicts of interest to declare.
AUTHOR CONTRIBUTIONS: All authors contributed to study design, conduct, analysis of R1 data, manuscript drafting and revision. LR and CP were responsible for data collection and management. LR takes responsibility for the integrity of the work as a whole.
REFERENCES
- 1.Make B, Hill N, Goldberg A, et al. Mechanical ventilation beyond the intensive care unit: Report of a consensus conference of the American College of Chest Physicians. Chest. 1998;113:289S–344S. doi: 10.1378/chest.113.5_supplement.289s. [DOI] [PubMed] [Google Scholar]
- 2.MacIntyre NR, Epstein SK, Carson S, Scheinhorn D, Christopher K, Muldoon S. Management of patients requiring prolonged mechanical ventilation: Report of a NAMDRC Consensus Conference. Chest. 2005;128:3937–54. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 3.Zilberberg MD, Luippold RS, Sulsky S, Shorr AF. Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit Care Med. 2008;36:724–30. doi: 10.1097/CCM.0B013E31816536F7. [DOI] [PubMed] [Google Scholar]
- 4.Kahn J, Werner R, David G, Ten Have T, Benson N, Asch D. Effectiveness of long-term acute care hospitalization in elderly patients with chronic critical illness. Med Care. 2013;51:4–10. doi: 10.1097/MLR.0b013e31826528a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiencek C, Winkelman C. Chronic critical illness: Prevalence, profile, and pathophysiology. AACN Adv Crit Care. 2010;21:44–61. doi: 10.1097/NCI.0b013e3181c6a162. [DOI] [PubMed] [Google Scholar]
- 6.Faenza S, Ravaglia MS, Cimatti M, Dante A, Spedicato S, Labate AM. Analysis of the causal factors of prolonged mechanical ventilation after orthotopic liver transplant. Transplant Proc. 2006;38:1131–4. doi: 10.1016/j.transproceed.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 7.Gracey DR, Naessens HM, Krishan I, Marsh HM. Hospital and posthospital survival in patients mechanically ventilated for more than 29 days. Chest. 1992;101:211–4. doi: 10.1378/chest.101.1.211. [DOI] [PubMed] [Google Scholar]
- 8.Wallace CJ, Oniki T, Bailey P, Clemmer TP. Implementation and testing of a respiratory failure care process model for patients with long-term mechanical ventilation. Proc AMIA Symp. 1998:1093. [Google Scholar]
- 9.Hung MC, Lu HM, Chen L, et al. Life expectancies and incidence rates of patients under prolonged mechanical ventilation: A population-based study during 1998 to 2007 in Taiwan. Crit Care. 2011;15:R107. doi: 10.1186/cc10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman B, Morris P. Tracheostomy practice in adults with acute respiratory failure. Crit Care Med. 2012;40:2890–6. doi: 10.1097/CCM.0b013e31825bc948. [DOI] [PubMed] [Google Scholar]
- 11.Carson S. Definitions and epidemiology of the chronically critically ill. Respir Care. 2012;57:848–56. doi: 10.4187/respcare.01736. [DOI] [PubMed] [Google Scholar]
- 12.Rose L. Understanding prolonged and long term mechanical ventilation in Canada. Toronto: Centre for Inner City Health St Michael’s Hospital; 2012. < www.stmichaelshospital.com/crich/sru/sru-ventilation/> (Accessed June 11, 2013). [Google Scholar]
- 13.Murphy M, Black N, Lamping D, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2:1–88. [PubMed] [Google Scholar]
- 14.Keeney S, Hasson F, McKenna H. Consulting the oracle: Ten lessons from using the Delphi technique in nursing research. J Adv Nurs. 2006;53:205–12. doi: 10.1111/j.1365-2648.2006.03716.x. [DOI] [PubMed] [Google Scholar]
- 15.Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62:107–15. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- 16.Sumison T. The Delphi technique: An adaptive research tool. Br J Occ Ther. 1998;61:153–6. [Google Scholar]
- 17.Rose L, Fraser I. Patient characteristics and outcomes of a provincial prolonged-ventilation weaning centre: A retrospective cohort study. Can Respir J. 2012;19:216–20. doi: 10.1155/2012/358265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson J, Powers K, Hedayat K, et al. Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004–2005. Crit Care Med. 2007;35:605–22. doi: 10.1097/01.CCM.0000254067.14607.EB. [DOI] [PubMed] [Google Scholar]
- 19.Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;2009:364–70. doi: 10.1002/jhm.510. [DOI] [PubMed] [Google Scholar]
- 20.Scal P. Transition for youth with chronic conditions: Primary care physicians’ approaches. Pediatrics. 2002;110:1315–21. [PubMed] [Google Scholar]
- 21.Thielke S, Diehr P. Transitions among health states using 12 measures of successful aging in men and women: Results from the Cardiovascular Health Study. J Aging Res. 2012;2012:243263. doi: 10.1155/2012/243263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kun S, Edwards J, Ward S, Keens T. Hospital readmissions for newly discharged pediatric home mechanical ventilation patients. Ped Pulmonol. 2012;47:409–14. doi: 10.1002/ppul.21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noyes J, Godfrey C, Beecham J. Resource use and service costs for ventilator-dependent children and young people in the UK. Health Soc Care Community. 2006;14:508–522. doi: 10.1111/j.1365-2524.2006.00639.x. [DOI] [PubMed] [Google Scholar]
- 24.Dybwik K, Tollali T, Nielsen E, Brinchmann B. “Fighting the system”: Families caring for ventilator-dependent children and adults with complex health care needs at home. BMC Health Serv Res. 2011;11:156. doi: 10.1186/1472-6963-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wise M, Hart N, Davidson C, et al. Home mechanical ventilation. BMJ. 2011;342:1687. doi: 10.1136/bmj.d1687. [DOI] [PubMed] [Google Scholar]
- 26.Cox CE, Carson SS, Govert JA, Chelluri L, Sanders GD. An economic evaluation of prolonged mechanical ventilation. Crit Care Med. 2007;35:1918–27. doi: 10.1097/01.CCM.0000275391.35834.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Resp J. 2007;29:1033–56. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]


