Abstract
Signaling events during abscisic acid (ABA) or methyl jasmonate (MJ)-induced stomatal closure were examined in Arabidopsis wild type, ABA-insensitive (ost1-2), and MJ-insensitive mutants (jar1-1) in order to examine a crosstalk between ABA and MJ signal transduction. Some of the experiments were performed on epidermal strips of Pisum sativum. Stomata of jar1-1 mutant plants are insensitive to MJ but are able to close in response to ABA. However, their sensitivity to ABA is less than that of wild-type plants. Reciprocally, the stomata of ost1-2 are insensitive to ABA but are able to close in response to MJ to a lesser extent compared to wild-type plants. Both MJ and ABA promote H2O2 production in wild-type guard cells, while exogenous application of diphenylene iodonium (DPI) chloride, an inhibitor of NAD(P)H oxidases, results in the suppression of ABA- and MJ-induced stomatal closure. ABA elevates H2O2 production in wild-type and jar1-1 guard cells but not in ost1-2, whereas MJ induces H2O2 production in both wild-type and ost1-2 guard cells, but not in jar1-1. MJ-induced stomatal closing is suppressed in the NAD(P)H oxidase double mutant atrbohD/F and in the outward potassium channel mutant gork1. Furthermore, MJ induces alkalization in guard cell cytosol, and MJ-induced stomatal closing is inhibited by butyrate. Analyses of the kinetics of cytosolic pH changes and reactive oxygen species (ROS) production show that the alkalization of cytoplasm precedes ROS production during the stomatal response to both ABA and MJ. Our results further indicate that JAR1, as OST1, functions upstream of ROS produced by NAD(P)H oxidases and that the cytoplasmic alkalization precedes ROS production during MJ or ABA signal transduction in guard cells.
Methyl jasmonate (MJ), a linolenic acid derivative, is involved in plant development and defense and is overproduced during wounding, fruit ripening, and drought stress (Creelman and Mullet, 1997). MJ affects plant transpiration (Lee et al., 1996; Wang, 1999) by promoting stomatal closure (Raghavendra and Reddy, 1987; Gehring et al., 1997; Suhita et al., 2003). MJ-induced stomatal closure is accompanied by an alkalization of the guard cell cytoplasm in Paphiopedilum spp. (Gehring et al., 1997). A recent study has shown that this response to MJ requires external calcium and involves a calmodulin-like domain, obviously of a protein kinase (Suhita et al., 2003). Interestingly, Evans (2003) demonstrated that MJ activates the outward potassium channel from guard cell protoplast of Vicia faba, the main conductance allowing K+ efflux and loss of turgor. These steps of cytoplasmic pH modification (Irving et al., 1992) and modulation of potassium channels at the guard cell plasma membrane (Armstrong et al., 1995) are also involved in abscisic acid (ABA)-induced stomatal closure.
In addition, cytoplasmic calcium waves (Allen et al., 2000), protein (de)phosphorylation (Leung et al., 1994, 1997; Meyer et al., 1994; Li et al., 2000; Merlot et al., 2001; Kwak et al., 2002; Mustilli et al., 2002) and reactive oxygen species (ROS) have all been identified to participate in ABA signaling (Gomez-Cadenas et al., 1999; Guan et al., 2000; Pei et al., 2000; Zhang et al., 2001a, 2001b; Klüsener et al., 2002). In guard cells, ABA induces ROS production, which in turn activates Ca2+ channels at the plasma membrane (Pei et al., 2000; Murata et al., 2001). Further, ABA-induced elevation in cytosolic Ca2+ leads to activation of slow anion channels and inactivation of inward rectifying K+ channels. The consequences are K+ efflux, guard cell turgor reduction, and stomatal closure. Interestingly, MJ together with various elicitors also induces an accumulation of H2O2 in leaves (Orozco-Cardenas and Ryan, 1999). Thus, it is likely that ABA and MJ transduction pathways leading to stomatal closure involve overlapping signaling elements. Such interaction has already been suggested by Herde et al. (1997) who observed that ABA deficient mutants were insensitive to jasmonic acid in reducing the transpiration stream. However, the jasmonate-insensitive mutant jar1-1 showed increased sensitivity to ABA inhibition of germination (Staswick et al., 1992). Although these observations suggest that the relationships between MJ and ABA signals affecting stomatal regulation and germination are different, they also indicate the existence of crosstalk between MJ and ABA signaling cascades. It remains largely unknown which molecular components are shared by ABA and MJ signal transduction.
In the study presented here, cytoplasmic pH changes and ROS production in response to ABA or MJ were studied in guard cells of Arabidopsis. Additionally, mutant plants affected in ABA signaling (ost1-2; Mustilli et al., 2002), MJ signaling (jar1-1; Staswick et al., 1992, 2002), plasma membrane catalytic subunits of the plasma membrane NAD(P)H oxidases (atrbohD/F; Kwak et al., 2003) or guard cell outward K+ channel (gork1; Hosy et al., 2003) were used to assess the respective roles of these genes in ABA or MJ signaling pathways leading to stomatal closure.
RESULTS
jar1-1 Mutants Are Insensitive to MJ But Not to ABA While ost1-2 Mutants Are Insensitive to ABA But Not to MJ
The jar1-1 MJ-insensitive mutant has been isolated on the basis of a diminished sensitivity of root growth to MJ (Staswick et al., 1992). The jar1-1 mutation affects the biochemical capability of JAR1 in the adenylation of jasmonic acid (Staswick et al., 2002). The ost1-2 mutant has been isolated using infrared thermography and characterized as ABA-insensitive at the stomatal level (Mustilli et al., 2002). OST1 is an ABA-activated protein kinase, an ortholog of the V. faba Ca2+-independent ABA-activated protein kinase (Li et al., 2000). The ost1-2 mutation (Gly-33 to Arg) affects an invariant residue required for ATP-binding and is thus predicted to abolish OST1 kinase activity (Mustilli et al., 2002).
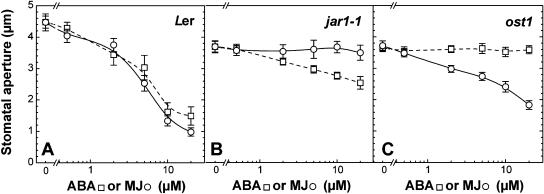
Figure 1 presents the stomatal sensitivity to ABA and MJ in wild-type plants, jar1-1, and ost1-2 mutant plants. Dose-response curves for MJ and ABA were quite similar in wild-type plants (Fig. 1A), with a 50% effect observed at around 5 μm. In the case of the jar1-1 mutant (Fig. 1B), stomata did not respond to MJ while a residual response to ABA was still observed, 28% of stomatal closure observed in wild-type plant at 20 μm ABA. As previously described (Mustilli et al., 2002), stomata from ost1-2 mutant plants were insensitive to ABA (Fig. 1C). However, they were still able to close in response to MJ, with a diminished sensitivity compared to wild-type plants, 60% of stomatal closure observed in wild-type plant at 20 μm MJ. These results demonstrate that JAR1 and OST1 are not absolutely required in a common ABA and MJ signaling pathway leading to stomatal closure. However the diminished response of ost1-2 to MJ and jar1-1 to ABA suggests a crosstalk between two signaling pathways through interacting elements.
Figure 1.
Dose-response curves of ABA- (squares) and MJ- (circles) induced stomatal closure in Landsberg erecta (A), jar1-1 mutant (B), and ost1-2 (C) mutant plants of Arabidopsis, respectively. The stomata of abaxial epidermis from leaves were allowed to open in light for 2 h, then ABA or MJ was applied for 2 h. Results are the averages ± se (n = 60) from at least 3 independent experiments.
Protein Kinases Are Essential Elements in Stomatal Closure by ABA and MJ
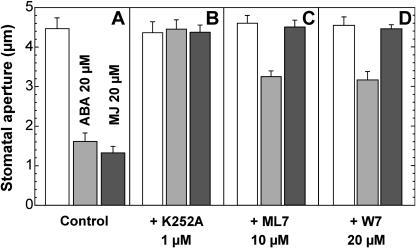
Protein (de)phosphorylation events play important roles in ABA signaling in guard cells (Li et al., 2000; Merlot et al., 2001; Kwak et al., 2002; Mustilli et al., 2002). Three compounds, K252a (broad range protein kinase inhibitor; Kase et al., 1987), ML7 (Ca2+-calmodulin protein kinase inhibitor; Saitoh et al., 1987; Hidaka and Kobayashi, 1999), and W7 (calmodulin inhibitor; Yorio et al., 1985), were compared in their abilities to inhibit ABA- or MJ-induced stomatal closure (Fig. 2). K252a was found to abolish the ABA- and MJ-induced stomatal closure (Fig. 2B). In contrast, ML7 and W7 were able to suppress the response to MJ but were only partly effective against ABA (Fig. 2).
Figure 2.
ABA- or MJ-induced stomatal closing (A) in the presence of the protein kinase inhibitors K252a (B), ML7 (C), and the calmodulin antagonist W7 (D). Stomata of leaf epidermis were allowed to open in light for 2 h, and then incubated for 2 h in ABA or MJ. K252a, ML7, and W7 were added 30 min before the addition of ABA or MJ. Results are the averages ± se (n = 60) from at least 3 independent experiments.
These results strongly suggest that at least one protein kinase, regulated by calcium and a calmodulin-like domain, is involved in stomatal response to MJ. The results also suggest that the ABA signaling cascade involved Ca2+-dependent and Ca2+-independent protein kinases as previously suggested (Allan et al., 1994; MacRobbie, 1998).
JAR1 and OST1 Are Located Upstream of ABA- and MJ-Induced H2O2 Production through an NAD(P)H Oxidase
ROS production in guard cells is induced by not only ABA (Pei et al., 2000; Murata et al., 2001) but also by chitosan, an elicitor of defense reactions (Lee et al., 1999), or in leaves by an exposure to MJ vapors (Orozco-Cardenas and Ryan, 1999). Analysis of ROS levels in guard cells using the fluorescent dye, 2′,7′-dichlorofluorescein diacetate (H2DCF-DA), confirmed that ROS production was significantly stimulated after a treatment with ABA and unraveled an even greater stimulation by MJ in wild-type plants (Table I). Interestingly, MJ did not increase ROS levels in jar1-1 guard cells, while ABA had no effect on ROS level in ost1-2 guard cells (Table I). Additionally, ROS production and stomatal closure triggered by ABA were slightly impaired in jar1-1, and identical results were obtained when ost1-2 mutants were submitted to MJ. However, externally applied H2O2 elicited a similar degree of stomatal closure in jar1-1 (55% decrease over control, without H2O2) and ost1-2 (60% decrease over control) as in wild-type plants (53% over control), suggesting that OST1 and JAR1 are placed upstream of ROS production in ABA or MJ signaling.
Table I.
ABA- and MJ-induced H2O2 production in guard cells and stomatal closure
| Ler
|
jar1-1
|
ost1-2
|
gork1
|
|||||
|---|---|---|---|---|---|---|---|---|
| H2O2 | SA | H2O2 | SA | H2O2 | SA | H2O2 | SA | |
| % | μm | % | μm | % | μm | % | μm | |
| Control | 100.0 ± 3.1 | 4.47 ± 0.27 | 94.4 ± 3.8 | 3.69 ± 0.17 | 98.6 ± 2.7 | 3.65 ± 0.12 | 101.1 ± 3.5 | 4.51 ± 0.21 |
| MJ | 128.4 ± 2.5 | 1.33 ± 0.16 | 97.5 ± 3.0 | 3.50 ± 0.26 | 112.8 ± 3.5 | 2.41 ± 0.17 | 127.4 ± 3.5 | 4.22 ± 0.15 |
| ABA | 121.5 ± 3.1 | 1.62 ± 0.29 | 116.0 ± 2.8 | 2.54 ± 0.20 | 99.3 ± 3.7 | 3.59 ± 0.13 | 120.5 ± 3.7 | 2.67 ± 0.18 |
Influence of jar1-1, ost1-2, and gork1 mutations on H2O2 production and stomatal closure in response to 20 μm MJ or ABA. Changes in ROS levels were analyzed by measuring H2DCF-DA fluorescence levels in guard cells in response to a 30-min treatment with ABA, MJ, or solvent control addition. To determine the consequence of mutations on stomatal closure, leaf epidermis were allowed to open in light for 2 h, then ABA or MJ was applied for 2 h. Results are the averages ± se (n = 60) of 3 to 4 independent experiments. The extents of H2O2 production in the guard cells of wild-type plants, without MJ or ABA, are taken as 100%. SA, stomatal apertures.
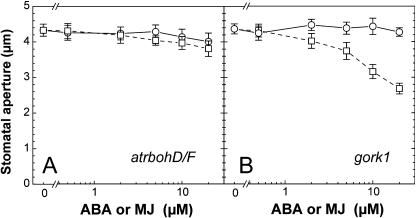
Pei et al. (2000) reported that diphenylene iodonium (DPI) partially prevented ABA-induced stomatal closure and proposed that DPI could limit H2O2 production in guard cells. To test that the fluorescence observed in guard cells was the result of the product of an NAD(P)H oxidase, DPI was supplied to the incubation medium for 30 min before treating the epidermal strips with ABA or MJ. In the presence of 12.5 μm DPI, ROS production was indeed restricted even after a treatment with 20 μm ABA or MJ (Table II). In parallel, stomatal closure by ABA and MJ was also prevented. These results indicate that ROS production by an NAD(P)H oxidase (Auh and Murphy, 1995) or another flavoenzyme (O'Donnell et al., 1993) is a key element in the MJ signaling pathway as already shown for ABA (Pei et al., 2000). A recent work has shown that a double mutant of two isoforms of catalytic subunits of plasma membrane NAD(P)H oxidases (atrbohD/F) led to a diminished ABA sensitivity at the stomatal level (Kwak et al., 2003). Interestingly, stomatal response to MJ was almost abolished in this double mutant (Fig. 3A), underlining the importance of NAD(P)H oxidases during not only ABA- but also MJ-induced stomatal closure.
Table II.
The effect of DPI on the patterns of ROS production in response to ABA or MJ in guard cells of Arabidopsis wild-type and mutant plants
| Plant | Hormone (20 μm)
|
|||||
|---|---|---|---|---|---|---|
| None
|
ABA
|
MJ
|
||||
| No DPI | DPI | No DPI | DPI | No DPI | DPI | |
| Wild type | 100 ± 1.9 | 103 ± 2.5 | 121 ± 2.2 | 104 ± 2.1 | 126 ± 3.5 | 102 ± 3.2 |
| jar1-1 | 96 ± 2.9 | 105 ± 3.2 | 112 ± 3.5 | 106 ± 3.2 | 97 ± 3.4 | 103 ± 3.1 |
| ost1-2 | 99 ± 2.8 | 101 ± 3.0 | 99 ± 3.1 | 98 ± 3.0 | 113 ± 2.8 | 97 ± 3.6 |
| gork1 | 101 ± 3.1 | 102 ± 2.9 | 120 ± 2.7 | 103 ± 3.3 | 127 ± 3.7 | 106 ± 2.9 |
The ROS production was monitored by using fluorescence dye, H2DCF-DA, as described in “Materials and Methods”. The extent of ROS production in guard cells, without 20 μm MJ or 20 μm ABA or 12.5 μm DPI in wild type, was taken as control and 100%. Results are averages (±se) from at least 3 experiments.
Figure 3.
Dose-response curves of ABA-induced (squares) and MJ-induced (circles) stomatal closure in atrbohD/F double mutant plants and gork1 mutant plants. Stomata of leaf epidermis were allowed to open for 2 h under light, then ABA or MJ was applied for 2 h. Results are the average ± se (n = 60) of 3 to 4 independent experiments.
Alkalization of pHCyt Is Necessary and Precedes ROS Production in Response to ABA and MJ
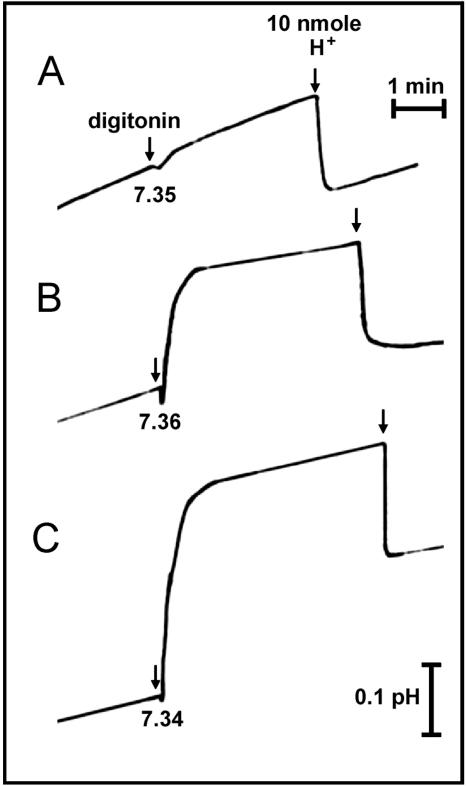
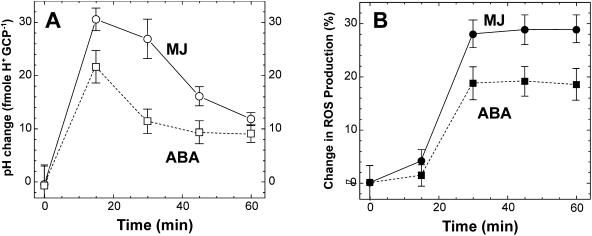
A modification of guard cell cytoplasmic pH is essential in ABA-induced stomatal closure (Irving et al., 1992; Blatt and Armstrong, 1993). We have therefore studied the response of the cytoplasmic pH to ABA and MJ. The null point method, first developed to measure cytoplasmic pH ([pH]Cyt) in animal cells, has been shown to be valuable in measuring [pH]Cyt changes induced by MJ in barley aleurone protoplast (Van der Veen et al., 1992). The basic principle of this method is that when extracellular solution pH ([pH]Ext) is equal to [pH]Cyt and buffering capacity is low, a selective disruption of the plasma membrane by digitonin will not affect [pH]Ext. We used this method to evaluate the [pH]Cyt change of guard cell protoplasts (GCPs) in response to MJ or ABA. In preliminary experiments, very little change of [pH]Ext was observed when GCPs were treated with digitonin at [pH]Ext 7.35 ± 0.03 (Fig. 4A). Then, two types of experiments were performed. In a first set [pH]Ext was fixed to 7.35 ± 0.03 using KOH, then changes in pH of external solution, after addition of digitonin to MJ- or ABA-treated GCPs, were recorded (Fig. 4).
Figure 4.
Change in the pH of the external solution after permeation of the guard cell plasma membrane by digitonin in control protoplasts (A), ABA-treated protoplasts (B), or MJ-treated protoplasts (C). Guard cell protoplasts were incubated in the presence of 20 μm ABA or MJ for 30 min before application of digitonin. Values indicate the external solution pH at time of application of digitonin; 10 nmol of HCl were injected into the medium at the end of each experiment for calibration.
In a second batch of experiments, the precise values of [pH]Cyt in presence of ABA or MJ were estimated by changing the external pH by 0.1 unit to determine the null-point value. Based on triplicate measurements, the estimated [pH]Cyt for untreated guard cell protoplasts was 7.33 ± 0.04 ([pH]Cyt ± se), and 7.47 ± 0.02 or 7.68 ± 0.02 after a 30 min treatment with 20 μm ABA or MJ, respectively. In the presence of MJ or ABA an alteration of the cytoplasmic pH took place in 15 min and then slowly decreased (Fig. 5). During the first 30 min of treatment ABA or MJ induced a ROS production increase in guard cells which then plateaued for at least 30 min (Fig. 5).
Figure 5.
Kinetics of pH change (A) and ROS production (B) in guard cells in response to 20 μm ABA or 20 μm MJ; pH changes (A) were determined using the null-point method and H2O2 production (B) using the fluorescent dye H2DCF-DA as described in “Materials and Methods”. Each data point is the mean ± se from at least 3 independent experiments.
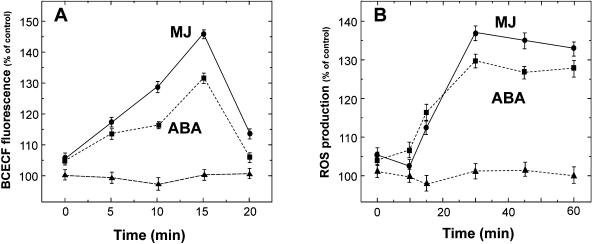
Technically it is difficult to use the null-point method for observing pH changes within 15 min. The pattern was therefore cross-checked with another species, Pisum sativum, using short durations of incubations and monitoring pH with a fluorescent dye, BCECF-AM. These results again indicated that the rise in pH of guard cells started within 5 min and peaked by 15 min, while the ROS production started only after 10 min and reaches maximum by 30 min (Fig. 6). Interestingly MJ triggered a stronger response than ABA despite kinetics that were similar. These results are in accordance with the results obtained by Gehring et al. (1997) using Paphiopedilum spp. guard cells and the fluorescent dye BCECF. The controls without ABA or MJ showed no significant change in pH (Fig. 6A).
Figure 6.
The pH changes and ROS production in guard cells of P. sativum. Changes in pH (A) or ROS (B) were monitored by using BCECF-AM or H2DCF-DA after the addition of ABA (squares) and MJ (circles). The pixel intensities of fluorescence at each given point were determined and the relative changes in pH or ROS production were expressed by considering solvent control at zero time as the standard (100%). Each data point is the mean ± se from at least 3 independent experiments. Note the different time scales in A and B.
Addition of the weak acid butyrate (0.5 mm), which causes an acidification of cytoplasm (Blatt and Thiel, 1994), limited stomatal closure caused by ABA or MJ in wild-type plants as well as in jar-1 and ost-1 mutants of Arabidopsis (Table III). Butyrate also diminished ABA- and MJ-induction of ROS production (Table IV; Fig. 7). Butyrate at 0.5 mm did not significantly affect the rates of either photosynthesis (122 μmol mg chl−1 h−1 in the absence and 120 μmol mg chl−1 h−1 in the presence of butyrate) or respiration (9 μmol mg chl−1 h−1 and 10 μmol mg chl−1 h−1, ±butyrate, respectively). Thus, butyrate at the concentration used in our experiments did not influence the metabolism of guard cells. These results together with the kinetics of ROS production and pH changes (Figs. 5 and 6), gave strong indications that pH changes preceded ROS production during stomatal closure induced by MJ and ABA. Moreover, preincubation of GCPs with 1 μm K252a also led to the suppression of the pH change (Table IV), suggesting that a protein kinase is involved upstream of pH change in ABA and MJ signaling cascades.
Table III.
Reversal by butyrate of stomatal closure by ABA or MJ in guard cells of Arabidopsis wild-type and mutant plants
| Plant
|
Control
|
ABA (20 μm)
|
MJ (20 μm)
|
|||
|---|---|---|---|---|---|---|
| No Butyrate | 0.5 mm Butyrate | No butyrate | 0.5 mm Butyrate | No butyrate | 0.5 mm Butyrate | |
| Stomatal aperture (μm) | ||||||
| Wild type | 4.12 ± 0.2 | 4.03 ± 0.5 | 1.09 ± 0.3 | 2.72 ± 0.2 | 0.98 ± 0.1 | 2.96 ± 0.2 |
| jar1-1 | 3.41 ± 0.2 | 3.24 ± 0.7 | 2.54 ± 0.2 | 2.75 ± 0.2 | 3.5 ± 0.3 | 3.29 ± 0.1 |
| ost1-2 | 3.87 ± 0.2 | 3.56 ± 0.4 | 3.59 ± 0.5 | 3.61 ± 0.1 | 1.83 ± 0.1 | 2.62 ± 0.3 |
| gork1 | 4.19 ± 0.3 | 4.07 ± 0.2 | 2.76 ± 0.2 | 2.89 ± 0.3 | 4.22 ± 0.2 | 4.01 ± 0.4 |
Modulation of pH change in the presence of butyrate on wild type, jar1-1, ost1-2, and gork1 with respect to stomatal closure in response to 20 μm ABA or MJ. The leaf epidermis were allowed to open in light for 2 h, then ABA or MJ or 0.5 mm butyrate was applied for 2 h. Results are the averages ±se (n = 60) of 3 to 4 independent experiments.
Table IV.
The effect of butyrate and K252a on alkalization and ROS production by guard cells of Arabidopsis in response to ABA, MJ
| Hormone
|
Rise in pH (fmol H+ GCP−1)
|
ROS Production (% of Control)
|
||||
|---|---|---|---|---|---|---|
| Control | + 0.5 mm Butyrate | + 1 μm K252a | Control | + 0.5 mm Butyrate | + 1 μm K252a | |
| 20 μm | ||||||
| None | 0.34 ± 0.01 | 0.26 ± 0.01 | 0.87 ± 1.2 | 100 ± 3.1 | 101 ± 3.5 | 101 ± 2.7 |
| ABA | 20.8 ± 1.21 | 8.6 ± 1.3 | 0.82 ± 2.1 | 122 ± 3.1 | 110 ± 3.5 | 105 ± 3.1 |
| MJ | 26.5 ± 1.15 | 10.1 ± 1.0 | 0.74 ± 1.4 | 128 ± 2.5 | 114 ± 3.7 | 103 ± 2.9 |
Changes in pH of external solution were recorded after permeation of guard cell plasma membrane by digitonin in control or hormone-treated protoplasts. The ROS production was monitored by using fluorescence dye, H2DCF-DA, as described in “Materials and Methods”. The extent of ROS production in guard cells, without MJ or ABA or butyrate or K252a (control), is taken as 100%. Results are averages (±se) from at least 3 experiments.
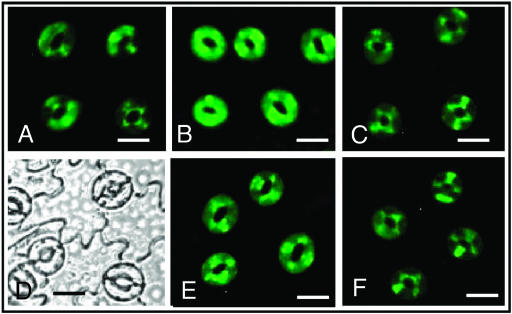
Figure 7.
MJ- or ABA-induced H2O2 production in guard cells is inhibited by 0.5 mm butyrate. Photographs were taken from a representative lot of guard cells from epidermal strips loaded with H2DCF-DA, untreated (A) or submitted to a 30-min pretreatment with 20 μm MJ (B), or 20 μm ABA (E). C and F, Effects of 0.5 mm butyrate on MJ- or ABA-induced H2O2 production, respectively. Photographs were taken using fluorescence (A–C, E, F) or light microscopy (D); bars represent 10 μm.
Role of Calcium or Calmodulin in pH or ROS Production
Although changes in calcium, pH, and ROS are observed in response to hormonal signals, their interrelationship and the exact sequence of these events have not been clear. It may be argued that changes in pH or ROS production in guard cells are brought out by external/internal calcium. The pattern of pH change and ROS production were therefore assessed after modulating calcium, by the addition of either external calcium or EGTA. Added external calcium or EGTA did not affect ROS production (Table V), confirming the involvement of calcium downstream of ROS production. We noticed that ML-7, a Ca2+-calmodulin (CaM) protein kinase inhibitor, was quite effective in reversing the stomatal closure caused by MJ but not that of ABA. Therefore, the effect of W7 (CaM antagonist) was checked. Again, W7 was effective in reversing the effect of MJ but not of ABA. Thus, a CaM-like domain appears to play a more active role in the case of MJ than that of ABA (Fig. 2, C and D).
Table V.
The effect of calcium or EGTA on stomatal closure, of alkalization, and ROS production in response to ABA or MJ, in guard cells of P. sativum
| Phenomenon and Calcium Modulator
|
Hormone (20 μm)
|
||
|---|---|---|---|
| None | ABA | MJ | |
| Stomatal opening (μm) | |||
| None | 4.0 ± 0.5 | 1.9 ± 1.0 | 1.8 ± 0.7 |
| 10 μm Ca(NO3)2 | 2.8 ± 0.5 | 2.5 ± 1.1 | 2.0 ± 0.9 |
| 2 mm EGTA | 4.8 ± 0.8 | 3.4 ± 1.2 | 4.4 ± 0.8 |
| Change in pH (% Control) | |||
| None | 100 ± 2.7 | 120 ± 2.1 | 125 ± 3.1 |
| 10 μm Ca(NO3)2 | 99 ± 3.2 | 121 ± 2.8 | 124 ± 2.9 |
| 2 mm EGTA | 100 ± 2.9 | 120 ± 3.2 | 124 ± 3.7 |
| Change in ROS (% Control) | |||
| None | 100 ± 3.3 | 122 ± 2.8 | 130 ± 3.2 |
| 10 μm Ca(NO3)2 | 105 ± 2.8 | 126 ± 3.1 | 135 ± 3.0 |
| 2 mm EGTA | 96 ± 3.4 | 118 ± 3.4 | 123 ± 3.7 |
Change in pH or ROS levels were analyzed by measuring BCECF-AM or H2DCF-DA fluorescence in guard cells in response to ABA or MJ or solvent control. Stomata were allowed to open in light for 2 h, then ABA or MJ was applied. The cellular pH and ROS production were examined after 15 and 30 min, respectively. The fluorescence intensity of the dye in the control sets (without ABA or MJ or calcium or EGTA) is taken as 100 and other values were expressed in relation to control. Results are the averages ±se of 3 to 4 independent experiments.
Gork1 Mutant Is Insensitive to MJ
The guard cell outward K+ channel GORK was for a long time suspected to be the main K+ conductance supporting ion efflux during stomatal closure. The molecular nature of this ion channel has been recently identified (Ache et al., 2000) and a GORK knockout mutant, gork1, characterized at the stomatal level (Hosy et al., 2003). In this mutant, the outward K+ currents, generally observed upon membrane depolarization, are absent in guard cell protoplasts, and the mutant displays a limited stomatal closure in response to the stress hormone ABA compared to wild-type plants.
We have therefore examined stomatal responses to ABA and MJ in gork1. As previously observed, the gork1 mutation led to a diminished response to ABA (Fig. 3B). Interestingly, stomatal closure in response to MJ was completely suppressed in the gork1 mutant. A recent work from Evans (2003) has already described a MJ dose-dependent modulation of inward and outward rectifier K+ channels at the guard cell plasma membrane. The results from our study suggest that GORK contributes to one of the conductances involved in K+ efflux during ABA-induced stomatal closure while it is an essential element in MJ-induced ion efflux and stomatal closure. MJ did not induce stomatal closure but caused significant ROS production in guard cells of gork1 (Table II).
DISCUSSION
ABA and MJ play a crucial role in plant adaptation to stress conditions. These two phytohormones inhibit root growth, limit transpiration, interfere with seed germination and cell cycle, and induce stomatal closure (Raghavendra and Reddy, 1987; Staswick et al., 1992; Wang, 1999; Swiatek et al., 2002). Considerable efforts have been devoted to identify signaling elements in the guard cell response to ABA, a fundamental process in drought resistance (Schroeder et al., 2001). In comparison, very few studies have focused on MJ signaling cascade leading to stomatal closure. It has been shown that MJ is produced during water stress (Creelman and Mullet, 1997) and that stomatal closure contributes to diminish the entry of certain pathogens in the leaf tissues (Agrios, 1997). Additionally, stomatal closure could limit plant growth and help redirect plant metabolism toward defense reactions. Previous studies have shown that some events in MJ- and ABA-signaling are similar, e.g. calcium requirement and protein (de)phosphorylation (Suhita et al., 2003), alkalization of the guard cell cytoplasm (Gehring et al., 1997), ROS production (Lee et al., 1999), and modulation of K+ channels at the guard cell plasma membrane (Evans, 2003).
In the study presented here, we observed that the response to MJ was more sensitive to Ca2+-calmodulin (CaM) protein kinase inhibitors than the stomatal response to ABA (Fig. 2). These inhibitors were able to reverse the response to MJ, while the response to ABA was only partially affected. These findings suggest that at least one protein kinase with a Ca2+-CaM like regulatory domain plays an essential role in MJ response, while such activity appears to participate to a limited extent in the ABA cascade. In contrast, a broad range inhibitor of protein kinase (K252a) was able to suppress both responses, suggesting that Ca2+-dependent and Ca2+-independent protein kinases are involved parallely during the ABA signaling. Interestingly, K252a was able to suppress MJ- or ABA-induced pH changes and ROS production (Table IV), suggesting that a protein phosphorylation event is essential and located upstream of these responses. While the kinetics of ROS production and pH change in response to MJ or ABA were almost similar, the amplitude of responses was always higher with MJ than that with ABA. These results confirm the previous observations from Gehring et al. (1997), who used different techniques and species. Additionally, the limited responses of jar1-1 and ost1-2 allow placing JAR1 and OST1 upstream of cytoplasmic alkalization in the MJ- and ABA-signaling pathways, respectively.
The outward-rectifying K+ channels appear to play an important role in stomatal closure. However, the reports on the regulation of these outward-rectifying K+ channels are ambiguous. These channels were found to be down-regulated by H2O2 (Köhler et al., 2003). However, a rise in cytoplasmic pH (which is expected to raise H2O2 levels) led to the up-regulation of these outward K+ channels (Miedema and Assmann, 1996). In a recent study, Evans (2003) found that MJ down-regulated the outward K+ channels. As per our observations, the gork1 mutant, whose outward K+ channels are impaired, was insensitive to MJ (Fig. 3B). Further, external addition of H2O2 caused marked stomatal closure in all the three mutants, including GORK. We suggest that GORK is one of the limiting elements during stomatal response to MJ and possible mechanisms could also be involved, for example modulation of influx of Suc or K+.
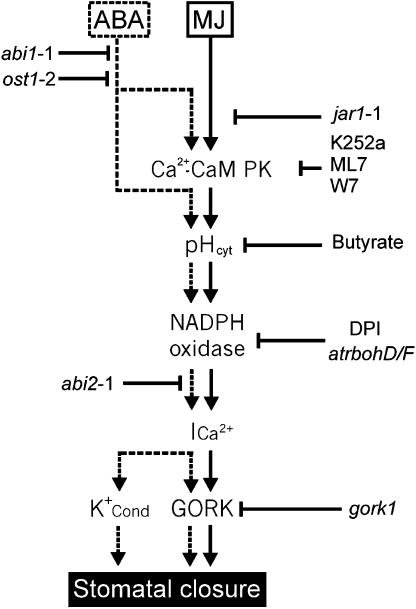
An important point from our study is that the sequence of events signaling stomatal closure can be traced, which appears broadly similar for MJ and ABA. At least one protein phosphorylation event is necessary for the cytoplasmic alkalization, which leads to ROS production by the NAD(P)H oxidase. In turn, ROS would activate hyperpolarization-activated Ca2+ channels (Pei et al., 2000; Murata et al., 2001) and the resulting elevation of free cytoplasmic Ca2+ triggers plasma membrane anion channels leading to cell depolarization. Hedrich et al. (1990) showed R type anion channel activation by extracellular CaCl2. Schroeder and Hagiwara (1989) showed S type anion channel activation by cytoplasmic Ca2+ elevation. ROS would also inhibit inward-rectifying K+ channels (Köhler et al., 2003; Torsethaugen et al., 1999). Among the last steps in the cascade leading to stomatal closure is the activation of the outward K+ rectifier from guard cells (Armstrong et al., 1995), allowing potassium efflux and loss of turgor. Thus, GORK appears to be an essential component in the MJ signaling cascade, leading to stomatal closure (Fig. 8).
Figure 8.
Model for the sequence of events in the MJ signaling cascade leading to stomatal closure. This linear model integrates our results from the different mutants and the use of inhibitors. Ca2+-CaM PK, calcium-calmodulin activated protein kinase; Ica, calcium influx at the plasma membrane. abi1 and abi2 have been placed according to Murata et al. (2001) and Mustilli et al. (2002).
CONCLUSION
The sequence of events occurring during stomatal closure is often debated. From the work presented here, using different mutants, a general scheme is proposed for stomatal response to MJ (Fig. 8). Comparative analysis of responses to MJ and ABA points out some specificity in the ABA cascade, which appears to involve parallel Ca2+-dependent and Ca2+-independent pathways and K+-conductance(s) other than GORK. There are still many open questions, e.g. the nature of the component leading to cytoplasmic alkalization, the elements linking pH change and H2O2 production in guard cells. The role of calcium also appears complex. From our study, a Ca2+-CaM protein kinase seems to be involved in a very early MJ signaling, whereas H2O2 production occurs downstream of phosphorylation events that activate Ca2+-channels at the plasma membrane leading to Ca2+ elevation in the cytoplasm. Thus, change in cytoplasmic free calcium could be an important signaling event involved in multiple steps of the signaling pathway leading to complex kinetics (Allen et al., 2000). Future studies will have to focus on these crucial points of stomatal physiology.
MATERIALS AND METHODS
Plant Material and Culture Conditions
The Arabidopsis Landsberg erecta, ost1-2 mutant plants (accession Landsberg, Mustilli et al., 2002), jar1-1 mutant plants (accession Columbia, Staswick et al., 1992), atrbohD/F mutant plants (accession Columbia, Torres et al., 2002), and gork1 mutant plants (accession Wassilewskija, Hosy et al., 2003) were grown on sand in hydroponic conditions in a growth chamber (8 h light, 300 μmol m−2 s−1, 70% relative humidity, 22°C; 16 h darkness, 75% relative humidity, 20°C) for 4 to 5 weeks. Plants were watered four times a day with a half-strength Hoagland solution. Most of the experiments were performed with Arabidopsis at CEA de Cadarache, DEVM/LEMS France.
Additional experiments were conducted with another species, Pisum sativum cv Arkel at Department of Plant Sciences, University of Hyderabad, India. Seedlings were grown in plastic trays filled with soil and farmyard manure (3:1, v/v). Plants were grown outdoors under natural photoperiod of approximately 12 h and average daily temperature of 30°C day/20°C night. The first and second fully expanded leaves were picked from 8- to 10-d-old plants.
Stomatal Aperture
Leaves from 4- to 5-week-old plants were harvested at the end of the night. Paradermal sections of abaxial epidermis were incubated in 30 mm KCl, 10 mm MES-KOH, pH 6.5, at 22°C. As indicated, ABA, MJ, K252a, ML7, W7, and other effectors were added to the solution. Stomatal apertures were measured with an optical microscope (Optiphot-2, Nikon, Tokyo) fitted with a camera lucida and a digitizing table (Houston Instrument TG 1017, Austin, TX) linked to a personal computer. For each treatment, at least 60 stomatal apertures were measured; each experiment was at least repeated thrice. It was ascertained that the three ecotypes of Arabidopsis used in this study have similar responses to ABA and MJ at the stomatal level (Table VI).
Table VI.
Responses of the three ecotypes of Arabidopsis to ABA or MJ
| Hormone (20 μm)
|
|||
|---|---|---|---|
| Ecotype | Control | ABA | MJ |
| Stomatal aperture (μm) | |||
| Landsberg | 3.9 ± 0.24 | 1.1 ± 0.21 | 1.2 ± 0.23 |
| Columbia | 3.8 ± 0.17 | 0.8 ± 0.25 | 1.0 ± 0.19 |
| Wassilewskija | 4.3 ± 0.21 | 1.2 ± 0.29 | 0.9 ± 0.13 |
The stomatal opening was measured as described in “Materials and Methods”, in the absence or presence of 20 μm ABA or MJ. Results are averages ±se of 3 to 4 independent experiments.
Guard Cell Protoplasts
GCPs were prepared essentially as reported by Pandey et al. (2002). They were then washed twice in 550 mm mannitol, 10 mm KCl, 1 mm CaCl2, 1 mm MgCl2, and 5 mm MES-NaOH, pH 7. For kinetic studies, GCPs were incubated in the same solution in the presence of ABA, MJ, or methanol solvent control for the indicated time at room temperature (around 22°C).
Fluorescent Dyes to Monitor ROS and pH
Hydrogen peroxide production in guard cells of Arabidopsis or P. sativum was monitored by using H2DCF-DA, as previously described (Murata et al., 2001). Epidermal leaf peels were mounted on a microscope slide with medical adhesive (Hollister, Libertyville, IL). Epidermal tissues were incubated for 3 h in 30 mm KCl and 10 mm MES-KOH, pH 6.5. The dye H2DCF-DA (30 μm) was added to the incubation medium. After 20 min, the excess of dye was removed by three washes with distilled water. When used, DPI (12.5 μm) was added 30 min before the dye to the epidermal strips. Epidermal tissues were then incubated for the indicated time with 20 μm ABA or MJ with an equal volume of methanol added to the control.
Changes in pH were examined in epidermis of P. sativum by incubation with 2′,7′-bis(2-carboxy-ethyl)-5(6)-carboxyfluorescein-acetoxymethylester (BCECF-AM) as described earlier by Irving et al. (1992). The epidermal tissues were incubated for 3 h with 50 mm KCl and 10 mm MES-KOH, pH 6.5 in light (350–450 μmol m−2 s−1). The strips were then treated with 20 μm BCECF-AM for 30 min in darkness. The strips were rinsed several times in incubation buffer so as to remove the excess dye. The epidermal tissues were then treated with 20 μm ABA or MJ (or methanol in the control) and examined under the fluorescent microscope.
Guard cells were then observed either with an epifluorescence microscope (Optiphot-2) fitted with a CCD camera (AxioCam, Zeiss, Gottingen, Germany) for the ROS fluorescence or with a fluorescence microscope fitted with camera (Eclipse TE 200, Niokon, Tokyo; Coolsnap CF, Photometrics, Roper Scientific, Tucson, AZ) for studies of pH change with BCECF-AM (20 μm) fluorescence. Images were captured and the relative fluorescence emission of guard cells was analyzed using the NIH software, as previously described in Murata et al. (2001).
Measurements of Cytoplasmic pH by the Null-Point Method
The null-point method used for barley aleurone protoplasts, as described in Van der Veen et al. (1992), was adapted for Arabidopsis guard cell protoplasts. Briefly, 106 GCPs were placed in a weakly buffered medium (0.5 mm MES, 10 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 500 mm mannitol, pH 7). The suspension was continuously stirred with a magnetic flea at low-speed magnetic stirring to avoid protoplast damage. GCPs were incubated with MJ or ABA 20 μm at room temperature. The [pH]Ext was adjusted to the required value with diluted KOH. Subsequently, digitonin (0.01%, w/v) was added to permeate the protoplast plasma membrane. The resulting pH changes in the external solution were recorded with a combined pH electrode (Ingold 104023522, Wilmington, MA) coupled to a pH-meter (pHM85, Radiometer Copenhagen, Copenhagen). Buffering capacity of the solution was determined by adding 10 nmol of HCl at the end of each experiment.
Chemicals
Chemicals were purchased from Sigma (St. Louis); Cellulase R10, Cellulase RS, and Pectolyase Y-23 from Sheishin Corporation (Tokyo); W7 and protein kinase inhibitors from BIOMOL (Plymouth, PA); and BCECF-AM from Molecular Probes (Juro, Switzerland).
Acknowledgments
We thank Drs. Elena Marin and Nathalie Leonhardt for their help in guard cell protoplast preparations and critical reading of the manuscript. The Arabidopsis mutants were generous gifts from Dr. Hervé Sentenac (gork1, ENSAM, Montpellier, France) and Dr. Jérôme Giraudat (ost1-2, ISV, Gif sur Yvette, France). We thank Julian Schroeder for communicating findings on AtrbohD and AtrbohF gene functions in ABA signaling prior to publication (supported by NIH grant no. GM60396 to J.S.) and for providing the atrbohD/F double mutant seeds.
This work was supported by grants from the Indo-French Centre for the Promotion of Advanced Research (grant no. 2203–1 to A.S.R. and A.V.) and the Council of Scientific and Industrial Research [grant no. 38(0949)/99/EMR–II to A.S.R.], both from New Delhi. J.M.K. was supported by a fellowship from the Human Frontier Science Program.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.032250.
References
- Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MR, Hedrich R (2000) GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett 486: 93–98 [DOI] [PubMed] [Google Scholar]
- Agrios GN (1997) Plant Pathology, Ed 4. Academic Press, San Diego, pp 46–52
- Allan AC, Fricker MD, Ward JL, Beale MH, Trewavas AJ (1994) Two transduction pathways mediate rapid effects of abscisic acid in Commelina guard cells. Plant Cell 6: 1319–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF (2000) Alternation of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 [DOI] [PubMed] [Google Scholar]
- Armstrong F, Leung J, Grabov A, Brearley J, Giraudat J, Blatt MR (1995) Sensitivity to abscisic acid of guard-cell K+ channels is suppressed by abi1-1, a mutant Arabidopsis gene encoding putative protein phosphatase. Proc Natl Acad Sci USA 92: 9250–9254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auh CK, Murphy TM (1995) Plasma membrane redox enzyme is involved in the synthesis of O2− and H2O2 by Phytophythora elicitor-stimulated rose cell. Plant Physiol 107: 1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR, Armstrong F (1993) K+ channels of stomatal guard cells: abscisic-acid-evoked control of the outward rectifier mediated by cytoplasmic pH. Planta 191: 330–341 [Google Scholar]
- Blatt MR, Thiel G (1994) K+ channels of stomatal guard cells: bimodal control of the K+ inward-rectifier evoked by auxin. Plant J 5: 55–56 [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol 48: 355–381 [DOI] [PubMed] [Google Scholar]
- Evans NH (2003) Modulation of guard cell plasma membrane potassium currents by methyl jasmonate. Plant Physiol 131: 8–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, McConchie R, Parish RW (1997) Jasmonates induce intracellular alkalization and closure of Paphiopedilum guard cell. Ann Bot 80: 485–489 [Google Scholar]
- Gomez-Cadenas A, Verhey SD, Holappa LD, Shen Q, Ho TH, Walker-Simmons MK (1999) An abscisic acid-induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. Proc Natl Acad Sci USA 96: 1767–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan LM, Zhao J, Scandalios JG (2000) Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J 22: 87–95 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Busch H, Raschke K (1990) Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J 9: 3889–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herde O, Peña-Cortés H, Willmitzer L, Fisahn J (1997) Stomatal responses to jasmonic acid, linolenic acid, and abscisic acid in wild type and ABA deficient tomato plants. Plant Cell Environ 20: 136–141 [Google Scholar]
- Hidaka H, Kobayashi R (1999) Use of protein (serine/threonine) kinase activators and inhibitors to study protein phosphorylation in intact cells. In DG Hardie, ed, Protein Phosphorylation: A Practical Approach. Oxford University Press, Oxford, pp 87–107
- Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, Poree F, Boucherez J, Lebaudy A, Bouchez D, Very AA, et al. (2003) The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements, plant transpiration. Proc Natl Acad Sci USA 100: 5549–5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving HR, Gehring CA, Parish RW (1992) Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proc Natl Acad Sci USA 89: 1790–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase H, Kazuyuki I, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M (1987) K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun 142: 436–440 [DOI] [PubMed] [Google Scholar]
- Klüsener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI (2002) Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol 130: 2152–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler B, Hills A, Blatt MR (2003) Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol 131: 385–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Moon JH, Murata Y, Kuchitsu K, Leonhardt N, DeLong A, Schroeder JI (2002) Disruption of a guard cell-expressed protein phosphatase 2A regulatory subunit, RCN1, confers abscisic acid insensitivity in Arabidopsis. Plant Cell 14: 2849–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori I, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom R, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi H, Suh S, Doo I-S, Oh K-Y, Choi EJ, Taylor SAT, Low PS, Lee Y (1999) Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reaction oxygen species from guard cells of tomato and Commelina communis. Plant Physiol 121: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, Lur H-S, Lin Y-H, Chu C (1996) Physiological and biochemical changes related to methyl jasmonate-induced chilling tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ 19: 65–74 [Google Scholar]
- Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chefdor F, Giraudat J (1994) Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science 264: 1448–1452 [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9: 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang XQ, Watson MB, Assmann SM (2000) Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 287: 300–303 [DOI] [PubMed] [Google Scholar]
- MacRobbie EAC (1998) Signal transduction and ion channels in guard cells. Philos Trans R Soc Lond B Biol Sci 353: 1475–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signaling pathway. Plant J 25: 295–303 [DOI] [PubMed] [Google Scholar]
- Meyer K, Leube MP, Grill E (1994) A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264: 1452–1455 [DOI] [PubMed] [Google Scholar]
- Miedema H, Assmann SMA (1996) Membrane-delimited effect of internal pH on the K+ outward rectifier of Vicia faba guard cells. J Membr Biol 154: 227–237 [DOI] [PubMed] [Google Scholar]
- Murata Y, Pei Z-M, Mori IC, Schroeder J (2001) Abscisic acid activation of plasma membrane Ca2+-channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell BV, Tew DG, Jones OT, England PJ (1993) Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 290: 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96: 6553–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Wang X-Q, Coursol SA, Assmann SM (2002) Preparation and application of Arabidopsis thaliana guard cell protoplasts. New Phytol 153: 517–526 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JL (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Reddy KB (1987) Action of proline on stomata differs from that of abscisic acid, G-substances or methyl jasmonate. Plant Physiol 144: 691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H (1987) Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem 262: 7796–7801 [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S (1989) Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338: 427–443 [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ (2001) Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature 410: 327–330 [DOI] [PubMed] [Google Scholar]
- Staswick PE, Su W, Howell SH (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89: 6837–6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I, Rowe ML (2002) Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 4: 1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D, Kolla VA, Vavasseur A, Raghavendra AS (2003) Different signaling pathways involved during the suppression of stomatal opening by methyl jasmonate or abscisic acid. Plant Sci 164: 481–488 [Google Scholar]
- Swiatek A, Lenjou M, Van Bockstaele D, Inze D, Van Onckelen H (2002) Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol 128: 201–211 [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsethaugen G, Pell EJ, Assmann SM (1999) Ozone inhibits guard cell K+ channels implicated in stomatal opening. Proc Natl Acad Sci USA 96: 13577–13582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Veen R, Heimovaara-Dijkstra S, Wang M (1992) Cytosolic alkalization mediated abscisic acid is necessary, but not sufficient, for abscisic acid-induced gene expression in barley aleurone protoplasts. Plant Physiol 100: 699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY (1999) Methyl jasmonate reduces water stress in strawberry. J Plant Growth Regul 18: 127–134 [DOI] [PubMed] [Google Scholar]
- Yorio T, Royce R, Mattern J, Oakford LX, Mia AJ, Tarapoom N (1985) Inhibition of the hydro-osmotic response to vasopressin and hypertonicity by phenothiazines and W7, calmodulin antagonists. Gen Pharmacol 16: 347–353 [DOI] [PubMed] [Google Scholar]
- Zhang X, Miao YC, An GY, Zhou Y, Shangguan ZP, Gao JF, Song CP (2001. a) K+ channels inhibited by hydrogen peroxide mediate abscisic acid signaling in Vicia guard cells. Cell Res 11: 195–202 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001. b) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126: 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]