Abstract
Background
Clinical audits have emerged as a potential tool to summarize the clinical performance of healthcare over a specified period of time. However, the effectiveness of audit and feedback has shown inconsistent results and the impact of audit and feedback on clinical performance has not been evaluated for COPD exacerbations. In the present study, we analyzed the results of two consecutive nationwide clinical audits performed in Spain to evaluate both the in-hospital clinical care provided and the feedback strategy.
Methods
The present study is an analysis of two clinical audits performed in Spain that evaluated the clinical care provided to COPD patients who were admitted to the hospital for a COPD exacerbation. The first audit was performed from November–December 2008. The feedback strategy consisted of personalized reports for each participant center, the presentation and discussion of the results at regional, national and international meetings and the creation of health-care quality standards for COPD. The second audit was part of a European study during January and February 2011. The impact of the feedback strategy was evaluated in term of clinical care provided and in-hospital survival.
Results
A total of 94 centers participated in the two audits, recruiting 8,143 admissions (audit 1∶3,493 and audit 2∶4,650). The initially provided clinical care was reasonably acceptable even though there was considerable variability. Several diagnostic and therapeutic procedures improved in the second audit. Although the differences were significant, the degree of improvement was small to moderate. We found no impact on in-hospital mortality.
Conclusions
The present study describes COPD hospital care in Spanish hospitals and evaluates the impact of peer-benchmarked, individually written and group-oral feedback strategy on the clinical outcomes for treating COPD exacerbations. It describes small to moderate improvements in the clinical care provided to COPD patients with no impact on in-hospital mortality.
Introduction
The existence of a gap between the healthcare that patients actually receive and the guidelines for that healthcare is now well acknowledged [1]. The variations in clinical practice establish a complex interplay of different factors that impact the resulting outcomes in ways that cannot be explained solely by patient characteristics [2]. In this scenario, clinical audits have emerged as a potential tool to summarize the clinical performance of healthcare over a specified period of time. The aim of a clinical audit is to provide health professionals with information that they can use to assess and adjust their performance [3], improving the clinical care provided to patients and the clinical outcomes. Accordingly, the information obtained in an audit should be used to improve care, and several pathways for this aim have been described [4].
In this context, feedback from the audited information constitutes a key step to improve clinical practice [5]. A key question is how successful audit and feedback are in motivating health professionals to modify their clinical practice. To date, several systematic reviews have assessed the effectiveness of audit and feedback with inconsistent results [6], [7]. A recent Cochrane systematic review concluded that audit and feedback generally lead to small, but potentially important, improvements in health care that depend on both the baseline performance and how the feedback is provided [8]. Accordingly, the impact of audit and feedback should be monitored by auditing clinical practices after implementing an intervention [3]; however, it should be acknowledged that the potential effect may also be influenced by the characteristics of the studied disease.
Chronic obstructive pulmonary disease (COPD) is a major and growing health problem [9]. Patients with COPD often suffer episodes of exacerbation during the course of their disease that often require hospitalization; these exacerbations are associated with significant mortality and morbidity [10], [11] and are responsible for most of the social and economic burden of COPD [12]. Thus, the clinical care provided to patients who are admitted to the hospital for a COPD exacerbation should be carefully evaluated. However, the impact of audit and feedback on clinical performance has not been evaluated for COPD exacerbations.
In recent years, Spain had used an auditing process for COPD, named AUDIPOC, which has resulted in the completion of two major clinical audits in the country [13], [14]. In the present study, we evaluated the results of two consecutive clinical audits performed in Spain to assess the clinical care provided to patients who were admitted to the hospital with a physician discharge diagnosis of a COPD exacerbation. The analysis will compare the performances in both audits to evaluate the components of the feedback approach that enhance COPD clinical performance.
Methods
The present study is an analysis of two clinical audits performed in Spain that evaluated the clinical care provided to COPD patients who were admitted to the hospital for a COPD exacerbation. Both audits had a similar methodology that has been extensively reported in previous publications [13], [14]. Briefly, both studies were clinical audits with prospective case ascertainment of consecutive exacerbation hospital admissions and retrospective data gathering from medical records. All cases admitted to the hospital in any Department or Unit during a 2-month period with a discharge diagnosis of COPD exacerbation were included. The inclusion of a case in the audit was finally decided upon discharge if the diagnosis of COPD exacerbation was included in the discharge report as the cause of admission. The cases with a specific diagnosis that was different from a COPD exacerbation upon admission, including pulmonary edema, pneumonia, pulmonary embolism, pneumothorax, rib fractures, aspiration, pleural effusion or any other associated respiratory or non-respiratory condition, were excluded. The medical records of the included patients were reviewed, and the audit data were extracted. The survivals were followed-up for 90 days from hospitalization to evaluate their vital status and whether the patient had been readmitted. The participant investigators were asked to complete a resource and organization database recording the hospital and respiratory unit or department resources.
The first audit was conducted from November–December 2008, and 129 hospitals participated, recruiting 5,178 patients. The second audit was part of a European study in which 432 centers from 13 countries recruited 18,016 patients. In Spain, 94 hospitals participated and recruited a total of 5,271 cases during January–February 2011. For the purpose of the present study, we selected the centers that participated in both audits. Ninety-four centers were included in this analysis. Altogether, a total of 8,143 cases were included in both audits.
The recorded variables have been reported and included information on the patient characteristics (e.g., age and gender), disease characteristics (e.g., disease severity, clinical features and exacerbation), resources available (e.g., hospital structure, hospital materials and human resources) and clinical practice (e.g., the adopted diagnostic and therapeutic interventions and the adjustment to clinical guidelines). Adherence to the clinical practice guidelines was analyzed following the GOLD recommendations for exacerbation management, which were available at the time of each audit. An adequate use of mechanical ventilation or antibiotics was considered to be present when a patient with such prescriptions correctly received them or when a patient who did not have such prescriptions did not receive them accordingly.
After the first audit, feedback was planned in three different ways. First, a specific report was created for each participant center. In this report, the value of each recorded variable was presented for that center and benchmarked against the regional and national values. Second, the results of the audit were presented in the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) annual congress and in the European Respiratory Society (ERS) annual meeting, and all investigators were invited to attend. Additionally, several smaller local meetings were planned during the next two years following the first audit to communicate the results. Notably, investigators were encouraged to organize meetings at the regional level to discuss the audit results, and several meetings were organized. Third, after the first audit, the SEPAR organized a working committee to create COPD healthcare quality standards, which were made available to all respiratory physicians [15]. All these initiatives were performed during 2009 and 2010, and the second audit was conducted in 2011. After the first audit, participants were not informed of an upcoming second audit.
In Spain, both audits were approved by the institutional review board of Hospital Universitario Virgen del Rocío (approval acta 07/2008 and 16/2010) and confirmed by each participating hospital [13], [16]. Additionally, the hospital management director of each center authorized the audit and agreed not to inform the medical staff that the audit was being conducted so that their medical practice would not change. According to ethical regulations, all data were de-identified in the database by an audit number that was not related to the medical record number or to any personal data. There was no personal information in the database that could be used to identify the patient.
Statistical analysis
The statistical computations were performed with the Statistical Package for Social Sciences (SPSS, IBM Corporation, Somers, NY) version 18.0. Variables were characterized by the mean and standard deviation or the absolute and relative frequencies of their categories. For describing the centers, we calculated the inter-regional range (IRR), which indicates the region with the highest or lowest mean value for a particular variable. Inferential studies comparing both audits were analyzed with the unpaired Student’s t-test (evaluating the variance equivalence with the Levene’s test) or chi-squared test (with the Fisher’s exact test, if required). Kaplan-Meier curves were constructed that evaluated in-hospital survival using the log-rank test to compare the in-hospital global mortality in both audits. The alpha error was set at 0.05.
Results
Ninety-four Spanish hospitals participated in both clinical audits. The main characteristics of the participant hospitals are summarized in table 1. The participating hospitals were largely public hospitals, with a high proportion being university/teaching hospitals and having a respiratory ward or team. The distribution of the participating hospitals among the different administrative regions is given in table S1 of the online supplement. All the regions in the country participated in this study.
Table 1. General characteristics of the hospitals participating in both audits.
| Variables | Mean | IRR | p value* |
| General characteristics | |||
| Number of beds (n) | 555.3 | 273–1073 | 0.049 |
| Catchment population (inhabitants) | 324.344 | 149.799–705.727 | NS |
| University/teaching hospital (%) | 62 | 0–100 | NS |
| Public hospital (%) | 98.7 | 66.7–100 | NS |
| Hospital with intensive care unit (%) | 89.9 | 50–100 | 0.020 |
| Number of beds in intensive care unit (n) | 15 | 4–26 | NS |
| Spirometry available (%) | 100 | 100–100 | NS |
| Hospital with a respiratory ward (%) | 78.5 | 50–100 | NS |
| Hospital with a respiratory team (%) | 96.2 | 75–100 | NS |
| Material resources | |||
| Respiratory outpatient clinic (%) | 100 | 100–100 | NS |
| COPD outpatient clinic (%) | 59.5 | 0–100 | NS |
| Specialty triage system (%) | 8.9 | 0–100 | 0.039 |
| Emergency department (%) | 86.1 | 66.7–100 | NS |
| Intermediate care unit (%) | 30.4 | 0–100 | NS |
| Number of beds in intermediate care unit (n) | 7.8 | 4–30 | NS |
| Offer non-invasive ventilation for acidosis (%) | 96.2 | 66.7–100 | NS |
| Offer invasive ventilation for acidosis (%) | 81.0 | 50–100 | NS |
| Human resources | |||
| Chest physicians (n) | 8.7 | 5–16 | 0.071 |
| Chest physicians per 1000 beds (n) | 17.3 | 1.2–27.2 | 0.001 |
| Respiratory trainees (n) | 3.6 | 1.25–7.5 | 0.025 |
| Respiratory trainees per 100 beds (n) | 5.9 | 2.4–26 | <0.001 |
| Physiotherapists (n) | 1.4 | 0.25–3 | <0.001 |
| Physiotherapists per 1000 beds (n) | 3.1 | 0.3–15.4 | <0.001 |
| Specialist nurses (n) | 4.5 | 0–21.3 | <0.001 |
| Specialist nurses per 100 beds (n) | 7.1 | 0–20.4 | <0.001 |
| Lung function technicians (n) | 2.1 | 1.4–2.3 | 0.046 |
| Lung function technicians per 1000 beds (n) | 4.8 | 1.1–11.4 | 0.011 |
| Hospital performance | |||
| Admissions for any cause in the previous year (n) | 62.802.5 | 25.142–154.244 | 0.044 |
| Percentage of COPD admissions in the unit (%) | 57.9 | 29–83 | NS |
| Daily respiratory physician on call (%) | 25.3 | 0–100 | NS |
| Number of ward rounds (n) | 1.5 | 1–3.1 | NS |
| Percentage of patients seen by physiotherapist (%) | 21.9 | 0–90 | NS |
| Percentage of patients seen by respiratory physician (%) | 54.6 | 30–95 | NS |
| Capacity to perform NIMV on all eligible patients (%) | 56.6 | 0–100 | NS |
| Capacity to perform IMV on all eligible patients (%) | 76.6 | 50–100 | NS |
| Early discharge program (%) | 20.3 | 0–100 | <0.001 |
| Percentage of patients in the early discharge program (%) | 20.4 | 5–40 | NS |
| Ability to care for long-term oxygen therapy patients (%) | 97.5 | 66.7–100 | NS |
| Ability to care for home-ventilated patients (%) | 89.9 | 66.7–100 | NS |
| Percentage of patients with rehabilitation (%) | 29.7 | 10–100 | 0.021 |
Data are expressed as the mean or relative frequency according to the nature of the variable. IRR: Inter-regional range. NS: not significant.
*p value for the differences between the Spanish regions calculated by the ANOVA or chi-square test.
A total of 8,143 admissions were analyzed from the two audits (audit 1: 3,493; audit 2: 4,650). The characteristics of the patients are summarized in table 2. The cases were mostly males in their eighth decade of life with a high proportion of active smokers. The number of patients with no information on their forced expiratory volume in one second (FEV1) was 3,273, including 2,874 patients without spirometry and 399 patients with some spirometric information but no FEV1. Altogether, the availability of the FEV1 significantly improved in the second audit. The majority of these patients were considered to have severe or very severe disease. More than 40% of the patients had not been previously admitted, and this percentage increased significantly in the second audit. Although there were some statistical differences, the clinical presentation according to the three Anthonisen criteria was not clinically different between the audits.
Table 2. Characteristics of the patients included in each audit.
| Audit 1 (n = 3,493) | Audit 2 (n = 4,650) | p value* | |
| Age (years) | 73.3 (10.05) | 72.7 (10.6) | 0.007 |
| Males (n) | 3,036 (86.9) | 4,001 (86.0) | NS |
| Tobacco | |||
| • Current smokers (n) | 772 (22.1) | 1,129 (24.3) | 0.023 |
| • Ex-smokers (n) | 2,069 (59.2) | 3,022 (65.0) | <0.001 |
| • Never smokers (n) | 151 (4.3) | 211 (4.5) | NS |
| • Missing (n) | 501 (14.3) | 288 (6.2) | <0.001 |
| Tobacco history (pack-years) | 55.4 (29.6) | 51.8 (32.6) | <0.001 |
| Comorbidities (Charlson) | 2.8 (1.8) | 1.7 (1.7) | <0.001 |
| Body mass index (kg/m2) | 27.6 (5.3) | 27.7 (5.4) | NS |
| Spirometry: FEV1 (%) | 44.8 (17.0) | 45.1 (16.4) | NS |
| GOLD spirometric assessment: | |||
| • No information available | 1,812 (51.9) | 1,461 (31.4) | <0.001 |
| • No obstruction | 157 (4.5) | 247 (5.3) | NS |
| • Mild (n) | 50 (1.4) | 74 (1.6) | NS |
| • Moderate (n) | 422 (12.1) | 887 (19.1) | <0.001 |
| • Severe (n) | 737 (21.1) | 1,424 (30.6) | <0.001 |
| • Very severe (n) | 315 (9.0) | 557 (12.0) | <0.001 |
| First admission (n) | 1,415 (40.5) | 2,093 (45.0) | <0.001 |
| Respiratory ward (n) | 1,921 (55.5) | 2,637 (56.7) | NS |
| Dyspnea increase (n) | 3,328 (95.3) | 4,431 (95.3) | NS |
| Sputum increase (n) | 2,258 (64.6) | 3,140 (67.5) | 0.004 |
| Sputum color change (n) | 1,654 (47.4) | 2,349 (50.5) | 0.002 |
Data are expressed as the mean (standard deviation) or absolute (relative) frequencies. NS: not significant. FEV1: forced expiratory volume in one second.
*Calculated using the unpaired Student’s t-test or chi-square test.
The diagnostic procedures improved by the second audit (table 3). The proportion of cases with blood gas analysis significantly increased from 88.5 to 90.9%, and radiographies were also slightly more frequently performed in the second audit than in the first (99.5% vs. 97.2%). Although the increases were significant, they were not extremely different. The severity of the blood gas alterations was similar between the audits. An unexpected finding was the high proportion of consolidations in the radiographies for the second audit.
Table 3. Diagnostic procedures performed during admission in each audit.
| Audit 1 (n = 3,493) | Audit 2 (n = 4,650) | p value* | |
| Blood gas analysis performed (n) | 3,091 (88.5) | 4,227 (90.9) | <0.001 |
| With oxygen (n) | 621 (17.8) | 1,181 (26.1) | <0.001 |
| pH | 7.39 (0.06) | 7.39 (0.07) | NS |
| PaO2 (mmHg) | 58.9 (15.0) | 60.1 (21.5) | 0.007 |
| PaCO2 (mmHg) | 48.5 (14.7) | 48.5 (15.8) | NS |
| Bicarbonate (mEq/l) | 28.7 (5.1) | 28.6 (5.2) | NS |
| Acidosis (n) | 549 (15.7) | 737 (15.8) | 0.002 |
| Radiography performed (n) | 3,396 (97.2) | 4,627 (99.5) | <0.001 |
| Consolidation (n) | 12 (0.3) | 384 (8.3) | <0.001 |
| Interstitial (n) | 20 (0.6) | 152 (3.3) | <0.001 |
| Neoplasm (n) | 53 (1.5) | 66 (1.4) | NS |
| Pleural effusion (n) | 119 (3.4) | 161 (3.5) | NS |
| Pneumothorax (n) | 2 (0.1) | 6 (0.1) | NS |
Data are expressed as the mean (standard deviation) or absolute (relative) frequencies. NS: not significant. PaO2: partial pressure of oxygen in arterial blood. PaCO2: partial pressure of carbon dioxide in arterial blood.
*Calculated using the unpaired Student’s t-test or chi-square test.
The therapeutic interventions before admission, during hospitalization and at discharge are summarized in table 4. Before admission, the number of administered treatments increased in the second audit, with a special emphasis on short-acting bronchodilators, long-acting muscarinic antagonists (LAMA) and systemic steroids. During admission, there were also some differences between the audits, but they were less striking. Notably, there was a significant decrease in systemic steroid use and a slight decrease in oxygen use in the second audit. The therapeutic recommendations at discharge were similar. Although the differences were significant, the percentages of each therapeutic option were not very dramatic.
Table 4. Therapeutic interventions performed in both audits.
| Previous admission | During admission | At discharge | |||||||
| Audit 1 (n = 3,493) | Audit 2 (n = 4,650) | p value* | Audit 1 (n = 3,493) | Audit 2 (n = 4,650) | p value* | Audit 1 (n = 3,493) | Audit 2 (n = 4,650) | p value* | |
| SABA (n) | 1,227 (35.1) | 2,183 (46.9) | <0.001 | 3,069 (87.9) | 4,184 (90.0) | 0.003 | 1,703 (48.8) | 1,894 (40.7) | <0.001 |
| SAMA (n) | 587 (16.8) | 1,022 (22.0) | <0.001 | 3,062 (87.7) | 4,333 (93.2) | <0.001 | 780 (22.3) | 834 (17.9) | <0.001 |
| LABA alone (n) | 186 (5.3) | 265 (5.7) | <0.001 | – | – | – | 229 (6.6) | 339 (7.3) | <0.001 |
| LAMA (n) | 1,286 (36.8) | 2,446 (52.6) | <0.001 | – | – | – | 2,243 (64.2) | 3,055 (65.7) | <0.001 |
| ICS alone (n) | 277 (7.9) | 301 (6.5) | <0.001 | 448 (12.8) | 844 (18.2) | <0.001 | 284 (8.1) | 278 (6.0) | <0.001 |
| ICS/LABA combination (n) | 1,529 (43.8) | 2,819 (60.6) | <0.001 | – | – | – | 2,584 (74.0) | 3,487 (75.0) | <0.001 |
| Methylxanthines (n) | 235 (6.7) | 412 (8.9) | <0.001 | 347 (9.9) | 113 (2.4) | <0.001 | 336 (9.6) | 417 (9.0) | <0.001 |
| Systemic steroids (n) | 415 (11.9) | 771 (16.6) | <0.001 | 3,185 (91.2) | 4,123 (88.7) | <0.001 | 2,407 (68.9) | 2,908 (62.5) | <0.001 |
| Antibiotics (n) | 524 (15.0) | 847 (18.2) | <0.001 | 3,129 (89.6) | 4,309 (92.7) | <0.001 | 1,808 (51.8) | 2,284 (49.1) | <0.001 |
| Oxygen (n) | – | – | – | 3,355 (97.4) | 4,422 (96.5) | 0.009 | 1,474 (42.2) | 1,892 (40.7) | <0.001 |
| Non-invasive ventilation (n) | – | – | – | 353 (10.1) | 555 (11.9) | <0.001 | 210 (6.0) | 318 (6.8) | <0.001 |
| Invasive ventilation (n) | – | – | – | 28 (0.8) | 80 (1.7) | <0.001 | – | – | – |
Data are expressed as the mean (standard deviation) or absolute (relative) frequencies. NS: not significant. SABA: Short-acting beta agonist, SAMA: short-acting muscarinic antagonist. LABA: long-acting beta agonist, LAMA: long-acting muscarinic antagonist, ICS: inhaled corticosteroid.
*Calculated using the unpaired Student’s t-test or chi-square test. The figures for non-invasive ventilation at discharge refer to home mechanical ventilation. –: not applicable or not recorded.
The 10 recommendations evaluated according to the GOLD guidelines are summarized in table 5. The majority of these recommendations improved in the second audit. However, although the differences were significant, they were not very striking.
Table 5. Adjustment to the GOLD guidelines.
| Audit 1 (n = 3,493) | Audit 2 (n = 4,650) | p value* | |
| Spirometry performed | 2,066 (59.1) | 3,202 (68.9) | <0.001 |
| Blood gas analysis performed | 3,091 (88.5) | 4,227 (90.9) | <0.001 |
| Radiography performed | 3,396 (97.2) | 4,627 (99.5) | <0.001 |
| Treatment with oxygen | 3,355 (97.4) | 4,422 (96.5) | 0.009 |
| Short-acting bronchodilator use | 3,337 (95.5) | 4,473 (96.2) | NS |
| No methylxanthine use | 3,146 (90.1) | 4,537 (97.6) | <0.001 |
| Systemic steroids | 3,185 (91.2) | 4,123 (88.7) | <0.001 |
| Adequate antibiotic use | 1,930 (61.5) | 2,782 (60.3) | NS |
| Adequate NIMV use | 2,625 (85.1) | 3,491 (85.6) | NS |
| Adequate IMV use | 2,994 (97.1) | 3,906 (95.8) | 0.005 |
Data are expressed as the mean (standard deviation) or absolute (relative) frequencies. NS: not significant. NIMV: non-invasive mechanical ventilation. IMV: invasive mechanical ventilation.
*Calculated using the unpaired Student’s t-test or chi-square test.
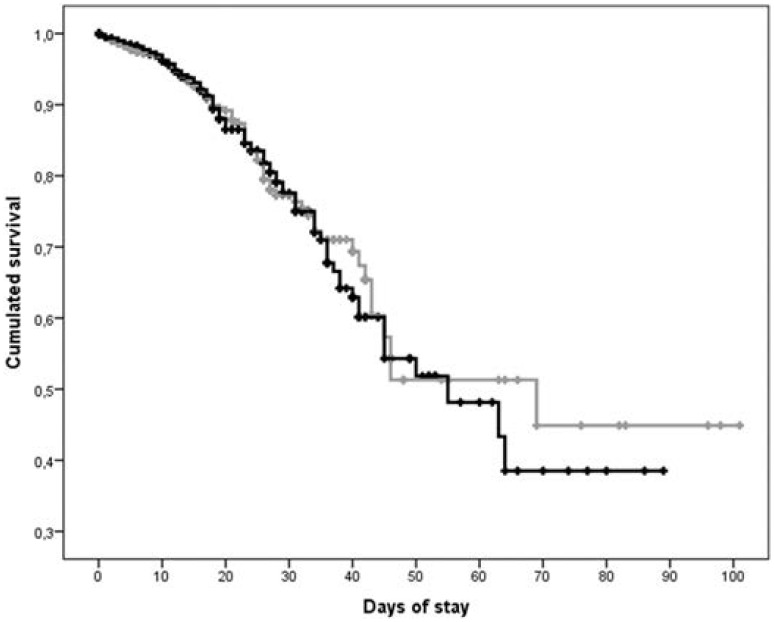
The survival curves for both audits are shown in figure 1. The in-hospital mortality was not different between the two audits. The first audit reported 164 (4.7%) deaths, and the second reported 202 (4.3%) deaths, and this difference was not significantly different. Accordingly, the median survival in the Kaplan-Meier analysis was not significantly prolonged in the second audit (56.7 days) compared with the first audit (64.6 days). The lengths of stay were very similar between the two audits, with 8.7 (7.8) days in audit 1 and 8.5 (7.3) days in audit 2 (not significantly different).
Figure 1. Kaplan-Meier curves comparing the in-hospital survival between the audits.
The grey line represents the first audit. The black line represents the second audit.
Discussion
The present study describes the state of COPD hospital care in Spanish hospitals and evaluates the impact of a feedback strategy on clinical care. After two consecutive audits, with feedback performed in between audits, we have observed some improvements in the clinical care provided to COPD patients. However, the majority of these improvements were small or moderate and did not impact the in-hospital mortality. This finding suggests that performing a clinical audit and providing the participant centers with the results according to our feedback strategy increased awareness and improved some aspects of COPD exacerbations, although there was no clear benefit on the outcomes.
Clinical audits have gained traction in healthcare systems as a way of obtaining information on the clinical care being provided. This information is of interest both for people funding healthcare, who want to ensure that the care they purchase is of the highest possible standard, and for patients who hope to receive safe and effective healthcare. However, despite evidence from many studies on audit and feedback, there is still limited information on how to use these data. A recent systematic review assessed the effectiveness of audit and feedback and reported an inconsistent picture; some evaluations obtained positive results, whereas others did not [8]. Interestingly, although previous studies suggest that audit and feedback may improve the performance of health care providers, the effects are generally moderate or small [17]. In our study, we also found moderate or small improvements in the clinical performance for COPD. Notably, audit and feedback measures are most likely to be beneficial when the existing practice is farthest away from what is desired and when the feedback is more intensive. However, the initial situation in our study was not far from the current guidelines; therefore, the feedback effect may be limited by a ceiling effect. The rationale for this limitation is most likely because clinical care for COPD exacerbations does not require complex interventions. Additionally, the recommendations for clinical care are closely followed in the medical community in our country, and the current guidelines are well known [18]. However, in this case, even small to moderate improvements in quality are worthwhile. COPD is a dramatic disease with a high prevalence [19], high in-hospital mortality and high readmission rate [16], and adequate clinical care can influence its outcome. Therefore, any effort to improve clinical care for these patients is worthwhile. Accordingly, some examples should be highlighted. The use of spirometry for diagnosis was improved in the second audit (table 2). Spirometry is a simple, non-invasive diagnostic procedure that is a necessary pillar for diagnosing COPD. In Spain, the use of spirometry has recently been evaluated, showing significant bottlenecks in primary and secondary care [20]. Thus, improvements in the use of spirometry will contribute to a better diagnosis and identification of these patients. In this regard, it is worth noting that 4.5% of patients in the first audit and 5.3% of those in the second audit did not show an obstructive pattern on spirometry. Therefore, such patients should not have been diagnosed with COPD and consequently COPD exacerbations. According to current guidelines, the diagnosis of COPD exacerbations should be solely based on clinical symptoms. However, such definition may be problematic and has been recently challenged [21]. Notably, in this study we were auditing the clinical care provided to patients deemed to have COPD exacerbations by the clinician in charge and thus treated accordingly. Arterial blood gas analysis was increased by 2.4%. The performance of a blood gas analysis is one of the key diagnostic measures for evaluating the severity of an exacerbation in hospitals. In those centers that do not offer this procedure, the alternative is to use pulse-oximetry to evaluate oxygen saturation. However, this method does not measure the pH or PaCO2, potentially leading to an incorrect assessment [22]. The use of chest radiography is another diagnostic intervention that is needed to eliminate other potential accompanying conditions that may mimic or worsen the exacerbation. In this regard, there is an ongoing debate on the significance of the consolidation frequently found in these COPD patients [23]. Additionally, from a therapeutic standpoint, several positive changes were observed after the feedback strategy was implemented. Short-acting bronchodilators were more frequently used, and the use of methylxanthines, which are no longer recommended, was decreased. Interestingly, the use of systemic steroids was also decreased, which is an unexplained finding that will need to be addressed in the future. The adequate use of invasive mechanical ventilation will also need to be further explored. However, all of these measures had a negligible impact on in-hospital mortality or length of stay and several factors are deemed to play a role in determining such clinically relevant endpoints. One potential explanation for such a finding is the presence of a ceiling effect with a good starting position for the majority of the items. Previous studies have demonstrated the influence of the baseline performance on the potential improvement gained [24]. Another explanation may be related to the feedback strategy used. Although it seems intuitive that health care professionals will modify their clinical practice if they receive feedback that their clinical practice is inconsistent with those of their peers or the accepted guidelines, this outcome has not been consistently demonstrated. Although there is some controversy that feedback with peer comparison is either more or less effective than other initiatives [25], [26], we provided feedback that was benchmarked against the peer average because providing this information may help participants understand the relative deviation of their own measures, which could improve patient outcomes.
Feedback can be delivered in different ways, and audit and feedback can be used as components of a multifaceted strategy to improve the quality of healthcare. In our study, we provided oral and written information at the individual level and oral information at the group level. Additionally, healthcare quality standards for COPD were constructed according to the experience with the first AUDIPOC audit [15]. Although this feedback strategy informs the participants of the audit results, alternative feedback strategies may result in different outcomes. For example, there are care bundles, including several evidence-based practices, that should be delivered to all patients to guarantee a set of minimum requirements for clinical care and several initiatives for COPD have been implemented [27], [28].
Some limitations must be considered when interpreting our results. Within each region, the participating hospitals volunteered. Therefore, although all regions were sampled, there was no attempt at representative sampling. As a consequence, the hospitals involved in the study were different in organizational terms and there was a certain degree of variability concerning the available resources (Table 1). However, the participating centers were identical for both audits. Additionally, the investigators were the same for the majority of the involved centers. We therefore believe that the potential impact of structural differences between the first and the second audits should be minimal. Another limitation is the considerably high number of investigators who recorded the information using different information sources, as is intrinsically associated with audits.
In summary, our study evaluated the impact of a peer-benchmarked, individually written and group-oral feedback strategy on the clinical outcomes for treating COPD exacerbations. The results of our study suggest that performing a clinical audit and providing the participant centers with the results according to our feedback strategy increased awareness and improved some aspects of COPD exacerbation treatment. Accordingly, other feedback strategies may yield different results. Although we did not observe a clear benefit in the clinical outcomes, several aspects of the diagnostic and therapeutic clinical care provided to the COPD patients admitted to hospitals seemed to improve, which, in turn, may reduce the gap between the healthcare that patients receive and the guidelines for that care.
Supporting Information
Percentual distribution of the cases included in both audits according to the region within the country.
(DOCX)
Membership of the AUDIPOC and the European COPD Audit studies.
(DOCX)
Acknowledgments
The authors are thankful to Enzo Emanuele, MD, PhD (Living Research s.a.s., Robbio, Italy) for his expert editorial assistance.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
AUDIPOC study was funded by Fondo de Investigación Sanitaria, Spanish Ministry of Health, project numbers PI07/90129, PI07/90309, PI07/90486, PI07/90503, PI07/90516, PI07/90721, PI08/90129, PI08/90578, PI08/90251, PI08/90529, PI08/90129, PI07/90403, PI08/90447, PI08/90457, PI08/90486, and PI08/90550. The European COPD audit was funded by the European Respiratory Society. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Asch SM, Kerr EA, Keesey J, Adams JL, Setodji CM, et al. (2006) Who is at greatest risk for receiving poor-quality health care? N Engl J Med 354: 1147–1156. [DOI] [PubMed] [Google Scholar]
- 2. Agabiti N, Belleudi V, Davoli M, Forastiere F, Faustini A, et al. (2010) Profiling hospital performance to monitor the quality of care: the case of COPD. Eur Respir J 35: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 3.Flottorp SA (2010) Using audit and feedback to health professionals to improve the quality and safety of health care. Copenhagen European Observatory on Health Systems and Policies: WHO. regional office for Europe; IX, 42 p. [Google Scholar]
- 4. Berwick DM, James B, Coye MJ (2003) Connections between quality measurement and improvement. Med Care 41: I30–38. [DOI] [PubMed] [Google Scholar]
- 5. Mugford M, Banfield P, O'Hanlon M (1991) Effects of feedback of information on clinical practice: a review. Bmj 303: 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stone EG, Morton SC, Hulscher ME, Maglione MA, Roth EA, et al. (2002) Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med 136: 641–651. [DOI] [PubMed] [Google Scholar]
- 7. van der Veer SN, de Keizer NF, Ravelli AC, Tenkink S, Jager KJ (2010) Improving quality of care. A systematic review on how medical registries provide information feedback to health care providers. Int J Med Inform 79: 305–323. [DOI] [PubMed] [Google Scholar]
- 8. Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, et al. (2012) Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 6: Cd000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez-Campos JL, Ruiz-Ramos M, Soriano JB (2014) Mortality trends in chronic obstructive pulmonary disease in Europe, 1994–2010: a joinpoint regression analysis. Lancet Respir Med 2: 54–62. [DOI] [PubMed] [Google Scholar]
- 10. Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez-Sala JL, et al. (2004) Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 59: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, et al. (2005) Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 60: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dalal AA, Shah M, D'Souza AO, Rane P (2011) Costs of COPD exacerbations in the emergency department and inpatient setting. Respir Med 105: 454–460. [DOI] [PubMed] [Google Scholar]
- 13. Pozo-Rodriguez F, Alvarez CJ, Castro-Acosta A, Melero Moreno C, Capelastegui A, et al. (2010) [Clinical audit of patients admitted to hospital in Spain due to exacerbation of COPD (AUDIPOC study): method and organisation]. Arch Bronconeumol 46: 349–357. [DOI] [PubMed] [Google Scholar]
- 14. Lopez-Campos JL, Hartl S, Pozo-Rodriguez F, Roberts CM, European CAt (2013) European COPD Audit: design, organisation of work and methodology. Eur Respir J 41: 270–276. [DOI] [PubMed] [Google Scholar]
- 15. Soler-Cataluna JJ, Calle M, Cosio BG, Marin JM, Monso E, et al. (2009) [Health-care quality standards in chronic obstructive pulmonary disease]. Arch Bronconeumol 45: 196–203. [DOI] [PubMed] [Google Scholar]
- 16. Pozo-Rodriguez F, Lopez-Campos JL, Alvarez-Martinez CJ, Castro-Acosta A, Aguero R, et al. (2012) Clinical audit of COPD patients requiring hospital admissions in Spain: AUDIPOC study. PLoS One 7: e42156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lilford RJ, Brown CA, Nicholl J (2007) Use of process measures to monitor the quality of clinical practice. Bmj 335: 648–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miravitlles M, Soler-Cataluna JJ, Calle M, Molina J, Almagro P, et al. (2014) Spanish guideline for COPD (GesEPOC). Update 2014. Arch Bronconeumol 50 Suppl 1: 1–16. [DOI] [PubMed] [Google Scholar]
- 19. Miravitlles M, Soriano JB, Garcia-Rio F, Munoz L, Duran-Tauleria E, et al. (2009) Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 64: 863–868. [DOI] [PubMed] [Google Scholar]
- 20. Lopez-Campos JL, Soriano JB, Calle M, Encuesta de Espirometria en Espana P (2013) A comprehensive, national survey of spirometry in Spain: current bottlenecks and future directions in primary and secondary care. Chest 144: 601–609. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Campos JL, Calero C, Lopez-Ramirez C. Exacerbations or complications? Redefining the concepts in COPD. Int J Clin Pract 2014 (in press). [DOI] [PubMed]
- 22. Tsai CL, Ginde AA, Blanc PG, Camargo CA (2009) Improved care of acute exacerbation of chronic obstructive pulmonary disease in two academic emergency departments. Int J Emerg Med 2: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roberts CM, Lopez-Campos JL, Pozo-Rodriguez F, Hartl S, European CAt (2013) European hospital adherence to GOLD recommendations for chronic obstructive pulmonary disease (COPD) exacerbation admissions. Thorax 68: 1169–1171. [DOI] [PubMed] [Google Scholar]
- 24. Ivers NM, Grimshaw JM, Jamtvedt G, Flottorp S, O'Brien MA, et al. (2014) Growing Literature, Stagnant Science? Systematic Review, Meta-Regression and Cumulative Analysis of Audit and Feedback Interventions in Health Care. J Gen Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kiefe CI, Allison JJ, Williams OD, Person SD, Weaver MT, et al. (2001) Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. Jama 285: 2871–2879. [DOI] [PubMed] [Google Scholar]
- 26. Sondergaard J, Andersen M, Stovring H, Kragstrup J (2003) Mailed prescriber feedback in addition to a clinical guideline has no impact: a randomised, controlled trial. Scand J Prim Health Care 21: 47–51. [DOI] [PubMed] [Google Scholar]
- 27. Hopkinson NS, Englebretsen C, Cooley N, Kennie K, Lim M, et al. (2012) Designing and implementing a COPD discharge care bundle. Thorax 67: 90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCarthy C, Brennan JR, Brown L, Donaghy D, Jones P, et al. (2013) Use of a care bundle in the emergency department for acute exacerbations of chronic obstructive pulmonary disease: a feasibility study. Int J Chron Obstruct Pulmon Dis 8: 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percentual distribution of the cases included in both audits according to the region within the country.
(DOCX)
Membership of the AUDIPOC and the European COPD Audit studies.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.