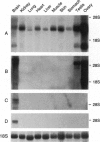
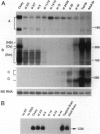
Abstract
A large family of genes encodes proteins with RNA recognition motifs that are presumed to bind RNA and to function in posttranscriptional regulation. Neural-specific members of this family include elav, a gene required for correct differentiation and maintenance of neurons in Drosophila melanogaster, and a related gene, HuD, which is expressed in human neuronal cells. I have identified genes related to elav and HuD in Xenopus laevis, zebrafish, and mouse that define a family of four closely related vertebrate elav-like genes (elrA, elrB, elrC, and elrD) in fish, frogs, and mammals. In addition to protein sequence conservation, a segment of the 3'-untranslated sequence of elrD is also conserved, implying a functional role in elrD expression. In adult frogs, elrC and elrD are exclusively expressed in the brain, whereas elrB is expressed in brain, testis, and ovary. During Xenopus development, elrC and elrD RNAs are detected by late gastrula and late neurula stages, respectively, whereas a nervous system-specific elrB RNA species is expressed by early tadpole stage. Additional elrB transcripts are detected in the ovary and early embryo, demonstrating a maternal supply of mRNA and possibly of protein. These expression patterns suggest a role for different elav-like genes in early development and neuronal differentiation. Surprisingly, elrA is expressed in all adult tissues tested and at all times during development. Thus, the widely expressed elrA is expected to have a related function in all cells.
Full text
PDF

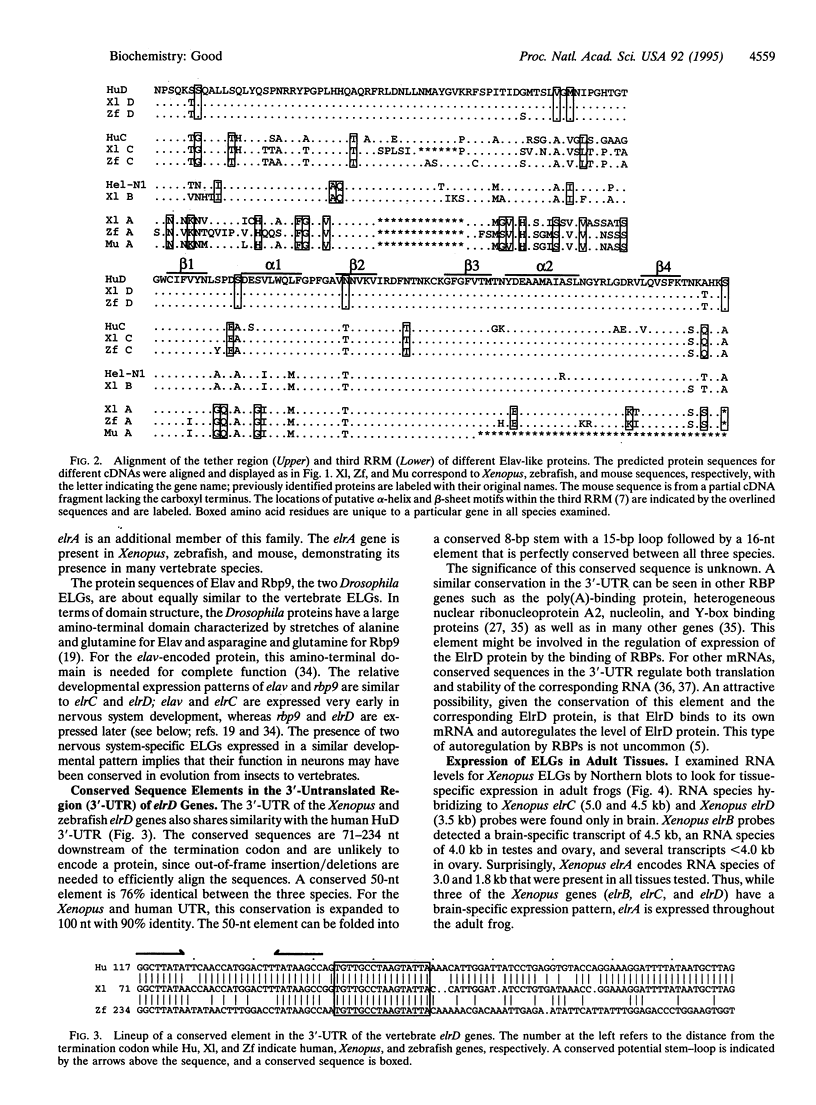
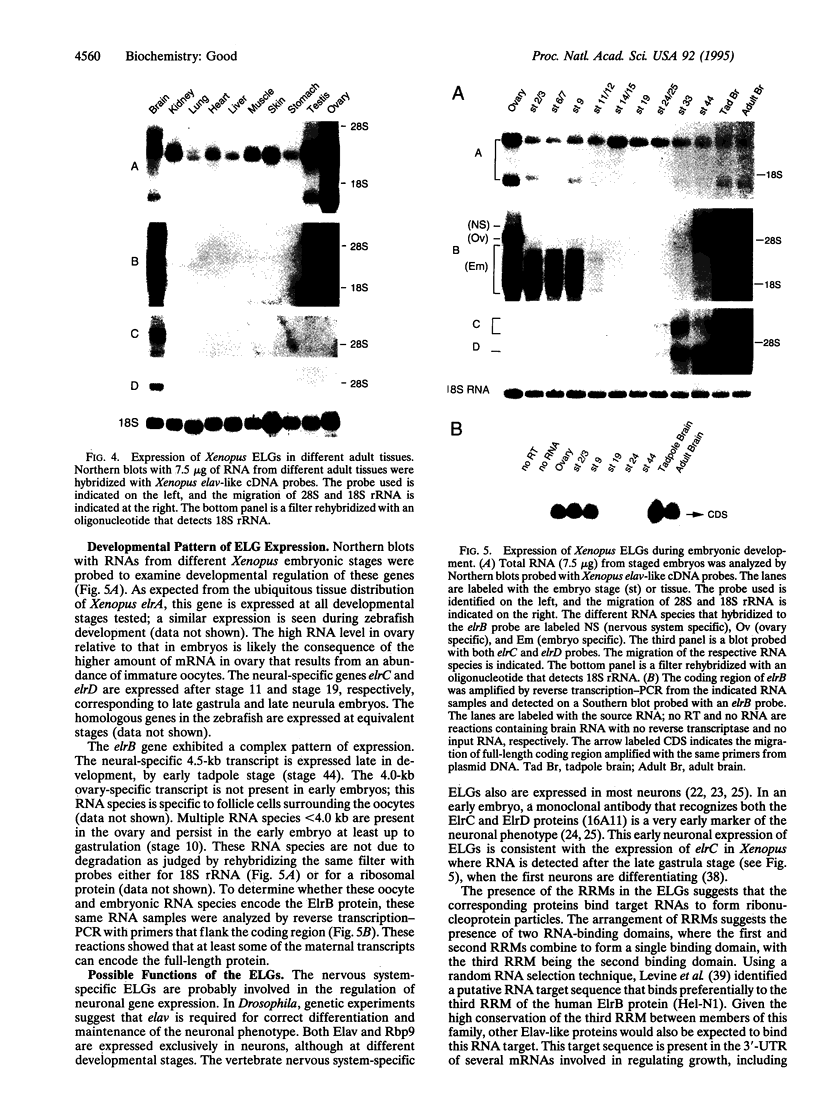

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandziulis R. J., Swanson M. S., Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. doi: 10.1101/gad.3.4.431. [DOI] [PubMed] [Google Scholar]
- Bellen H. J., Kooyer S., D'Evelyn D., Pearlman J. The Drosophila couch potato protein is expressed in nuclei of peripheral neuronal precursors and shows homology to RNA-binding proteins. Genes Dev. 1992 Nov;6(11):2125–2136. doi: 10.1101/gad.6.11.2125. [DOI] [PubMed] [Google Scholar]
- Bier E., Ackerman L., Barbel S., Jan L., Jan Y. N. Identification and characterization of a neuron-specific nuclear antigen in Drosophila. Science. 1988 May 13;240(4854):913–916. doi: 10.1126/science.3129785. [DOI] [PubMed] [Google Scholar]
- Birney E., Kumar S., Krainer A. R. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993 Dec 25;21(25):5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C. G., Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994 Jul 29;265(5172):615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- Campos A. R., Grossman D., White K. Mutant alleles at the locus elav in Drosophila melanogaster lead to nervous system defects. A developmental-genetic analysis. J Neurogenet. 1985 Jun;2(3):197–218. doi: 10.3109/01677068509100150. [DOI] [PubMed] [Google Scholar]
- Dalmau J., Furneaux H. M., Cordon-Cardo C., Posner J. B. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992 Oct;141(4):881–886. [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret L., Dorkeld F., Gautier C. Strong conservation of non-coding sequences during vertebrates evolution: potential involvement in post-transcriptional regulation of gene expression. Nucleic Acids Res. 1993 May 25;21(10):2315–2322. doi: 10.1093/nar/21.10.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. B., Dworkin-Rastl E. Functions of maternal mRNA in early development. Mol Reprod Dev. 1990 Jul;26(3):261–297. doi: 10.1002/mrd.1080260310. [DOI] [PubMed] [Google Scholar]
- Good P. J., Rebbert M. L., Dawid I. B. Three new members of the RNP protein family in Xenopus. Nucleic Acids Res. 1993 Feb 25;21(4):999–1006. doi: 10.1093/nar/21.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good P. J., Richter K., Dawid I. B. A nervous system-specific isotype of the beta subunit of Na+,K(+)-ATPase expressed during early development of Xenopus laevis. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9088–9092. doi: 10.1073/pnas.87.23.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J. D., Kobel H. R. Genetics of Xenopus laevis. Methods Cell Biol. 1991;36:19–34. doi: 10.1016/s0091-679x(08)60270-8. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., de la Torre J. R., Holt C., Harland R. M. Cephalic expression and molecular characterization of Xenopus En-2. Development. 1991 Mar;111(3):715–724. doi: 10.1242/dev.111.3.715. [DOI] [PubMed] [Google Scholar]
- Homyk T., Jr, Isono K., Pak W. L. Developmental and physiological analysis of a conditional mutation affecting photoreceptor and optic lobe development in Drosophila melanogaster. J Neurogenet. 1985 Nov;2(5):309–324. doi: 10.3109/01677068509102326. [DOI] [PubMed] [Google Scholar]
- Homyk T., Jr, Szidonya J., Suzuki D. T. Behavioral mutants of Drosophila melanogaster. III. Isolation and mapping of mutations by direct visual observations of behavioral phenotypes. Mol Gen Genet. 1980;177(4):553–565. doi: 10.1007/BF00272663. [DOI] [PubMed] [Google Scholar]
- Jiménez F., Campos-Ortega J. A. Genes in subdivision 1B of the Drosophila melanogaster X-chromosome and their influence on neural development. J Neurogenet. 1987 Jun;4(4):179–200. [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Baker B. S. Isolation of RRM-type RNA-binding protein genes and the analysis of their relatedness by using a numerical approach. Mol Cell Biol. 1993 Jan;13(1):174–183. doi: 10.1128/mcb.13.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. J., Baker B. S. The Drosophila gene rbp9 encodes a protein that is a member of a conserved group of putative RNA binding proteins that are nervous system-specific in both flies and humans. J Neurosci. 1993 Mar;13(3):1045–1056. doi: 10.1523/JNEUROSCI.13-03-01045.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. H., Levine T. D., Fremeau R. T., Jr, Keene J. D. Mammalian homologs of Drosophila ELAV localized to a neuronal subset can bind in vitro to the 3' UTR of mRNA encoding the Id transcriptional repressor. J Neurosci. 1994 Apr;14(4):1943–1952. doi: 10.1523/JNEUROSCI.14-04-01943.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamborghini J. E. Rohon-beard cells and other large neurons in Xenopus embryos originate during gastrulation. J Comp Neurol. 1980 Jan 15;189(2):323–333. doi: 10.1002/cne.901890208. [DOI] [PubMed] [Google Scholar]
- Landsman D. RNP-1, an RNA-binding motif is conserved in the DNA-binding cold shock domain. Nucleic Acids Res. 1992 Jun 11;20(11):2861–2864. doi: 10.1093/nar/20.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine T. D., Gao F., King P. H., Andrews L. G., Keene J. D. Hel-N1: an autoimmune RNA-binding protein with specificity for 3' uridylate-rich untranslated regions of growth factor mRNAs. Mol Cell Biol. 1993 Jun;13(6):3494–3504. doi: 10.1128/mcb.13.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich M. F., Furneaux H. M., Henion P. D., Weston J. A. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994 Feb;25(2):143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- Marusich M. F., Weston J. A. Identification of early neurogenic cells in the neural crest lineage. Dev Biol. 1992 Feb;149(2):295–306. doi: 10.1016/0012-1606(92)90285-o. [DOI] [PubMed] [Google Scholar]
- Mattox W., Ryner L., Baker B. S. Autoregulation and multifunctionality among trans-acting factors that regulate alternative pre-mRNA processing. J Biol Chem. 1992 Sep 25;267(27):19023–19026. [PubMed] [Google Scholar]
- Richter K., Good P. J., Dawid I. B. A developmentally regulated, nervous system-specific gene in Xenopus encodes a putative RNA-binding protein. New Biol. 1990 Jun;2(6):556–565. [PubMed] [Google Scholar]
- Richter K., Grunz H., Dawid I. B. Gene expression in the embryonic nervous system of Xenopus laevis. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8086–8090. doi: 10.1073/pnas.85.21.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinow S., Campos A. R., Yao K. M., White K. The elav gene product of Drosophila, required in neurons, has three RNP consensus motifs. Science. 1988 Dec 16;242(4885):1570–1572. doi: 10.1126/science.3144044. [DOI] [PubMed] [Google Scholar]
- Robinow S., White K. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J Neurobiol. 1991 Jul;22(5):443–461. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- Robinow S., White K. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol. 1988 Apr;126(2):294–303. doi: 10.1016/0012-1606(88)90139-x. [DOI] [PubMed] [Google Scholar]
- Sachs A. B. Messenger RNA degradation in eukaryotes. Cell. 1993 Aug 13;74(3):413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- Sakai K., Gofuku M., Kitagawa Y., Ogasawara T., Hirose G., Yamazaki M., Koh C. S., Yanagisawa N., Steinman L. A hippocampal protein associated with paraneoplastic neurologic syndrome and small cell lung carcinoma. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1200–1208. doi: 10.1006/bbrc.1994.1358. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. mRNA translation: influence of the 5' and 3' untranslated regions. Curr Opin Genet Dev. 1994 Apr;4(2):310–315. doi: 10.1016/s0959-437x(05)80059-0. [DOI] [PubMed] [Google Scholar]
- Szabo A., Dalmau J., Manley G., Rosenfeld M., Wong E., Henson J., Posner J. B., Furneaux H. M. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell. 1991 Oct 18;67(2):325–333. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- Taira M., Jamrich M., Good P. J., Dawid I. B. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992 Mar;6(3):356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- Wolfner M. F. Sex-specific gene expression in somatic tissues of Drosophila melanogaster. Trends Genet. 1988 Dec;4(12):333–337. doi: 10.1016/0168-9525(88)90052-2. [DOI] [PubMed] [Google Scholar]
- Yao K. M., Samson M. L., Reeves R., White K. Gene elav of Drosophila melanogaster: a prototype for neuronal-specific RNA binding protein gene family that is conserved in flies and humans. J Neurobiol. 1993 Jun;24(6):723–739. doi: 10.1002/neu.480240604. [DOI] [PubMed] [Google Scholar]
- Yao K. M., Samson M. L., Reeves R., White K. Gene elav of Drosophila melanogaster: a prototype for neuronal-specific RNA binding protein gene family that is conserved in flies and humans. J Neurobiol. 1993 Jun;24(6):723–739. doi: 10.1002/neu.480240604. [DOI] [PubMed] [Google Scholar]