Abstract
Objective
Telomere shortening in arteries could lead to telomere uncapping and cellular senescence, which in turn could promote the development of hypertension.
Methods and results
To assess the novel role of arterial telomere dysfunction in hypertension, we compared mean telomere length (qPCR), telomere uncapping (serine 139 phosphorylated histone γ-H2A.X (γ-H2) localized to telomeres: ChIP), and tumor suppressor protein p53 (P53)/cyclin-dependent kinase inhibitor 1A (P21)-induced senescence (P53 bound to P21 gene promoter: ChIP) in arteries from 55 age-matched hypertensive and nonhypertensive individuals. Arterial mean telomere length was not different in hypertensive patients compared with nonhypertensive individuals (P = 0.29). Arterial telomere uncapping and P53/P21- induced senescence were two-fold greater in hypertensive patients compared with nonhypertensive individuals (P = 0.04 and P = 0.02, respectively). Arterial mean telomere length was not associated with telomere uncapping or P53/P21-induced senescence (r=– 0.02, P = 0.44 and r = 0.01, P = 0.50, respectively), but telomere uncapping was a highly influential covariate for the hypertension group difference in P53/P21-induced senescence (r = 0.62, P < 0.001, ηp2 = 0.35). Finally, telomere uncapping was a significant predictor of hypertension status (P = 0.03), whereas mean telomere length was not (P = 0.68).
Conclusion
Collectively, these findings demonstrate that arterial telomere uncapping and P53/P21-induced senescence are linked to hypertension independently of mean telomere length, and telomere uncapping influences hypertension status more than mean telomere length.
Keywords: arteries, cellular senescence, hypertension, telomere
INTRODUCTION
Arterial telomere dysfunction may contribute to the pathogenesis of hypertension by inducing cellular senescence. Telomeres are terminal sequences of TTAGGG repeats that make up the natural ends of chromosomes [1,2]. Telomeres form specialized structures that protect chromosome ends from being recognized as dsDNA breaks and initiating a dsDNA break response [3–5], which can induce cellular senescence through tumor suppressor protein p53 (P53)-dependent expression of cyclin-dependent kinase inhibitor 1A (P21) [5,6]. In-vitro studies in various human cell types have shown that breakdown of telomere structure, referred to as telomere uncapping, leads to P53 activation and P53/P21-induced senescence [5,6]. Replication and genotoxic stress-mediated telomere shortening beyond a critical telomere length may lead to uncapping in human cells [5–8]. Following in-vitro telomere uncapping in human cells, phosphorylation of histone γ-H2A.X at serine 139 (γ-H2) occurs at telomeric chromatin to aid in the dsDNA break response and initiation of P53/P21-induced senescence [5,6]. Importantly, cellular senescence has been implicated in the etiology of chronic diseases [9,10], including cardiovascular diseases (CVDs) [11,12].
Although the role of arterial telomere dysfunction in hypertension has not been assessed, telomere shortening in white blood cells (WBCs) has been linked to hyper-tension [13,14], and telomere shortening in arteries has been associated with abdominal aortic aneurysm [15] and atherosclerotic plaque development [16,17]. Telomere shortening in arteries could lead to telomere uncapping and P53/P21-induced senescence, which in turn could promote the development of hypertension. Interestingly, some studies have suggested that arterial telomere uncap-ping is more closely linked to P53/P21-induced senescence than telomere shortening in human cells [18,19], including arterial tissues [20]. Insight into the relative contributions of arterial telomere shortening and telomere uncapping to the etiology of hypertension will lead to a more complete understanding of the potential role of telomere dysfunction in hypertension. This in turn could lead to novel therapeutic strategies that target telomere dysfunction to delay onset, attenuate severity, or even reverse hypertension.
Therefore, an important unexplored hypothesis is that arterial telomere dysfunction and P53/P21-induced senescence are associated with hypertension. To test this hypothesis, we compared mean telomere length, telomere uncapping (γ-H2 localized to telomeres), and P53/P21-induced senescence (P53 bound to P21 gene promoter) in arteries from an age-matched sample of hypertensive and nonhypertensive individuals. Next, we used logistic regression to compare the influence of arterial mean telomere length and telomere uncapping on hypertension status in these individuals.
METHODS
Arterial biopsy collection and general sample processing
Arterial biopsies were excised from patients undergoing a prophylactic procedure for melanoma-associated sentinel lymph node biopsy at the Huntsman Cancer Hospital, University of Utah. A heterogeneous sample (n) of 55 age-matched individuals (34 men and 21 women) consented to donate arterial biopsies for the study. A comprehensive outline of biometric, physiological, and medical characteristics for all participants enrolled in the study was collected (Table 1). Participants were classified as hypertensive or nonhypertensive according to prior hypertension diagnosis reported in medical history. Both medical histories and prescription medication use were noted, and participants with prior diagnosis of CVD other than hypertension or metastatic melanoma were excluded. Participant blood pressures were measured and recorded during physician consultations according to standard clinical blood pressure measurement guidelines [21]. Participants with high lactate dehydrogenase (LDH) blood values were excluded from the study, as blood LDH levels are considered a strong indicator of melanoma metastasis when outside the normal range [22,23]. Thus, all participants were within our institutionally specified normal range of 313–618 U/l, and LDH levels were not different between groups (P = 0.32) nor correlated with any outcomes (all P ≥ 0.08). No participants included in this study had received chemotherapy, as this criterion was a contraindication for surgery. The Institutional Review Boards of the University of Utah and the Salt Lake City Veteran's Affairs Medical Center approved all protocols, and written informed consent was obtained from all participants prior to biopsy collection.
TABLE 1.
Age-matched nonhypertensive and hypertensive participant characteristics
| Characteristic | Nonhypertensive (n = 26) | Hypertensive (n = 29) | P |
|---|---|---|---|
| Age (years) | 63.7 ± 1.5 | 63.8 ± 2.4 | 0.48 |
| Sex (M/F) | 16/10 | 18/11 | 0.48 |
| BMI (kg/m2) | 27.2 ± 0.9 | 30.1 ± 1.1 | 0.01* |
| SBP (mmHg) | 135.8 ± 3.8 | 140.8 ± 3.5 | 0.17 |
| DBP (mmHg) | 78.4 ± 2.4 | 78.5 ± 2.4 | 0.48 |
| PP (mmHg) | 57.5 ± 2.5 | 62.3 ± 2.5 | 0.09 |
| Prescription medications | |||
| Calcium channel blockers | 0.0% (0) | 24.1% (7) | N/A |
| β-Blockers | 0.0% (0) | 17.2% (5) | N/A |
| ACE inhibitors | 0.0% (0) | 37.9% (11) | N/A |
| Angiotensin blockers | 0.0% (0) | 17.2% (5) | N/A |
| Diuretics | 0.0% (0) | 34.5% (10) | N/A |
Data presented are mean ± SEM, % (n), and n across groups (P < 0.05).
ACE, angiotensin-converting enzyme; PP, pulse pressure.
The arterial biopsies consisted of skeletal muscle feed arteries excised from the inguinal (e.g. hip adductors or quadriceps femoris) or axillary regions (e.g. serratus anterior or latissimus dorsi) and were free of melanoma cells [24]. Arterial biopsies were identified as skeletal muscle feed arteries by entry into muscle bed, gross anatomy, coloration, and pulsatile bleed pattern [24]. There were no differences in our outcomes between arteries from inguinal and axillary regions (all P < 0.10), and no interactions between the effects of biopsy ource and group were found in any outcomes (all P < 0.16). Arterial biopsies were cleaned of adipose and connective tissue, and washed to remove residual blood cells. The average size of each artery was 2 mm in length, approximately 0.5 mm in luminal diameter, and approximately 10–20 mg in mass. Cleaned arteries were then snap frozen in liquid nitrogen and stored at −08°C prior to performing the following outcomes. All samples were assayed in triplicate, and replicate means were used for analysis.
Mean telomere length
A sequence-independent multiplex qPCR technique using a SYBR Green master mix with 0.625U AmpliTaq Gold 360 DNA polymerase (Life Technologies Corporation, Grand Island, New York, USA) was utilized to determine mean telomere length as described by Cawthon [25]. Telomeric DNA (T) SQs and albumin SQs, used as single copy gene (S) to control tissue concentration in samples, were generated by standard curve and mean telomere length was expressed as the T/S ratio. Mean telomere lengths and telomere ranges were generated by converting T/S ratios to bp of DNA using the formula: bp = 3330 (T/S) þ 3730, derived by Cawthon [25].
Telomere uncapping
ChIP was used to determine the amount of γ-H2 (Santa Cruz Biotechnology Inc.) localized to telomeres. ChIPs were performed as previously described [20] and analyzed via qPCR for telomere content as described by Cawthon [25]. Final values were expressed as the ratio of background corrected starting quantity (SQ) of telomeric DNA enriched by ChIP to telomeric DNA SQ in INPUT fraction. INPUTs represented 50% of telomeric DNA present in corresponding ChIP and were used to control for tissue concentration in samples [ex: (γ-H2 — SQ background SQ)/INPUT SQ = final value].
P53/P21-induced senescence
ChIPs were performed to assess P53 bound to P21 gene promoter (EMD Millipore Corporation) as previously described [20], using a sequence-independent qPCR assay with FastStart SYBR Green Master (Roche Diagnostics Corporation, Roche Applied Science).
Data analysis
Outcome measures were defined as mean telomere length, γ-H2 localized to telomeres, P53 bound to P21 gene promoter, and hypertension status. Independent samples t-tests were performed to assess group differences in all outcomes. Analysis of variance tests were performed with least significance difference post-hoc tests to assess tertile differences in all outcomes. The Pearson correlation coefficient (r) was used to assess correlations between each outcome and between outcomes and participant characteristics. To assess group differences in participant characteristics, independent samples t-tests or χ2 tests were performed. Analysis of covariance tests with least significance difference post-hoc tests were performed with all continuous variable participant characteristics with correlated outcomes to determine the influence of covariates on any group differences in outcomes. All covariates were tested for homogeneity across groups within outcomes (P = 0.67) and covariate effect size was assessed using partial eta squared (ηP2).
Logistic regression
Forward and backward likelihood ratio logistic regression analyses were conducted to assess the influence of mean telomere length, γ-H2 localized to telomeres, and BMI on hypertension status (test statistic: Δ in 2 log likelihood). Logistic regression model goodness-of-fit was determined by comparing the significant predictors of hypertension status identified by forward and backward likelihood ratio logistic regression analyses (good model of data: same predictors). Additionally, the Hosmer–Lemeshow (good model of data: significance value ≥ 0.05) and Nagelkerke's R2 (pseudo R2) statistics were used to assess logistic regression model goodness of fit. Significance was set at P < 0.05.
RESULTS
Participant characteristics
Approximately, 83% of hypertensive patients used one or more prescription medications to control blood pressure (n = 24; Table 1). By definition, no nonhypertensive individuals used prescription blood pressure medications. BMI was greater in hypertensive patients compared with nonhypertensive individuals (P = 0.01; Table 1), whereas sex, SBP, DBP, and pulse pressure were not different between groups (all P ≥ 0.09; Table 1).
Arterial telomere dysfunction, P53/P21-induced senescence, and hypertension
Arterial mean telomere length was not different between hypertensive and nonhypertensive individuals (P = 0.29; Fig. 1). Arterial γ-H2 localized to telomeres was =nearly two-fold greater in hypertensive patients compared with nonhypertensive individuals (P = 0.04; Fig. 1). Correspondingly, arterial P53 bound to P21 gene promoter was over two-fold greater in hypertensive patients compared with nonhypertensive individuals (P = 0.02; Fig. 1). No participant characteristics were correlated with γ-H2 localized to telomeres or P53 bound to P21 gene promoter (all P ≥ 0.09).
FIGURE 1.
Arterial telomere dysfunction, P53/P21-induced senescence, and hyper-tension. (a) Mean telomere length, (b) γ-H2 localized to telomeres, and (c) P53 bound to P21 gene promoter across groups (both P < 0.04). Data presented are mean ± SEM normalized to control group. γ-H2, p-histone γ-H2A.X (ser139); P53, tumor suppressor protein p53; P21, cyclin-dependent kinase inhibitor 1A.
Influence of telomere dysfunction on P53/P21-induced senescence
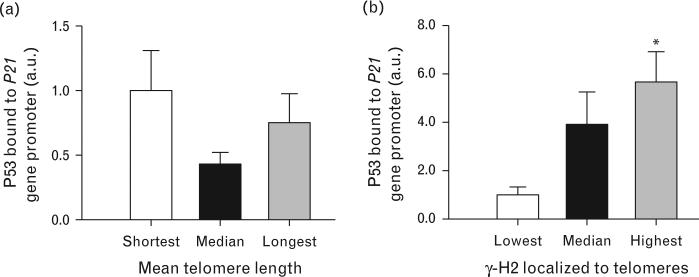
Mean telomere length was not correlated with γ-H2 localized to telomeres or P53 bound to P21 gene promoter (r=− 0.02, P = 0.44 and r=0.01, P, respectively). Additionally, were there no differences in P53 bound to P21 gene promoter between participants in the shortest (7.8–9.9 kb), median (10.6–12.4 kb), and longest (12.5– 17.2 kb) tertiles of mean telomere length (all P ≥ 0.08; Fig. 2). γ-H2 localized to telomeres demonstrated a strong positive correlation with P53 bound to P21 gene promoter (r = 0.62, P < 0.001). Likewise, there was almost six-fold greater P53 bound to P21 gene promoter among individuals in the highest tertile of γ-H2 localized to telomeres compared with those from the lowest tertile (P < 0.01; Fig. 2). Analysis of covariance results indicated that γ-H2 localized to telomeres had a large effect on the P53 bound to P21 gene promoter group difference (ηP2 = 0.35), accounting for 35% of the total hypertension-related variance in this P53/P21-induced senescence marker. Controlling for the influence of γ-H2 localized to telomeres, the adjusted group difference in P53 bound to P21 gene promoter was no longer significant (ΔP 0.04–0.24).
FIGURE 2.
Influence of telomere dysfunction on P53/P21-induced senescence. (a) P53 bound to P21 gene promoter across tertiles of mean telomere length and (b) P53 bound to P21 gene promoter across tertiles of γ-H2 localized to telomeres (P < 0.01 compared with lowest tertile). Data presented are mean ± SEM normalized to control group. γ-H2, p-histone γ-H2A.X (ser139); P53, tumor suppressor protein p53; P21, cyclin-dependent kinase inhibitor 1A.
Influence of telomere dysfunction and BMI on hypertension status
Logistic regression results indicated that γ-H2 localized to telomeres and BMI were both significant predictors of hypertension status (P = 0.03 and 0.01, respectively; Table 2), whereas mean telomere length was not (P = 0.68; Table 2). Together, γ-H2 localized to telomeres and BMI explained 25% of the variance in hypertension status (pseudo R2 = 0.25; Table 2) and correctly predicted hypertension status in over 67% of hypertensive patients (Supplemental Digital Content; Table S1, http://links.lww.com/HJH/A345. Logistic regression group classification table). Backward and forward likelihood ratio logistic regression analyses produced the same results (Table 2 and S1, http://links.lww.com/HJH/A345), and generated identical Hosmer–Lemeshow test results (both P = 0.78; Table 2).
TABLE 2.
Logistic regression results
| Backward likelihood ratio | ||
| Step 1 | Hosmer–Lemeshow (P = 0.47) | Pseudo R2 = 0.26 |
| Predictor | Δ in –2 log likelihood | P |
| γ-H2 | 4.77 | 0.03* |
| mTL | 0.18 | 0.68 |
| BMI | 6.49 | 0.01* |
| Step 2 | Hosmer–Lemeshow (P = 0.78) | Pseudo R2 = 0.25 |
| Predictor | Δ in –2 log likelihood | P |
| γ-H2 | 4.76 | 0.03* |
| BMI | 6.83 | 0.01* |
| Forward likelihood ratio | ||
| Step 1 | Hosmer–Lemeshow (P = 0.51) | Pseudo R2 = 0.14 |
| Predictor | Δ in –2 log likelihood | P |
| BMI | 5.48 | 0.02* |
| Step 2 | Hosmer–Lemeshow (P = 0.78) | Pseudo R2 = 0.25 |
| Predictor | Δ in –2 log likelihood | P |
| BMI | 6.83 | 0.01* |
| γ-H2 | 4.76 | 0.03* |
γ-H2, p-histone γ-H2A.X (ser139) localized to telomeres; mTL, mean telomere length
P < 0.05.
DISCUSSION
The key novel findings of the current study are as follows. Arterial mean telomere length was not different in hypertensive patients compared with nonhypertensive individuals. Arterial telomere uncapping and P53/P21-induced senescence were greater in hypertensive patients compared with nonhypertensive individuals. Arterial mean telomere length was not associated with telomere un-capping or P53/P21-induced senescence, but telomere uncapping was a highly influential covariate for the group difference in P53/P21-induced senescence. Finally, telo-mere uncapping was a significant predictor of hypertension status, whereas mean telomere length was not. Collectively, these findings demonstrate that arterial telomere uncapping and P53/P21-induced senescence are linked to hyper-tension independently of mean telomere length, and telo-mere uncapping influences hypertension status more than mean telomere length.
Arterial mean telomere length and hypertension
Arterial telomere shortening could play a role in hyper-tension by leading to telomere uncapping and P53/P21-induced senescence. Here we showed no difference in mean telomere length in arteries from hypertensive patients compared with those from nonhypertensive individuals. Furthermore, mean telomere length was not correlated with telomere uncapping or P53/P21-induced senescence. Damage to telomeric DNA caused by mechanical stress [26] on arterial cells from elevations in SBP or reactive oxygen species [7,8] that accumulate in the context of hypertension [27,28] could lead to telomere uncapping independent of telomere shortening. This might explain the lack of associations between mean telomere length and telomere uncapping or P53/P21 senescence. Although its role in hypertension has not previously been assessed, arterial telomere shortening has been associated with chronic obstructive pulmonary disease [12], abdominal aortic aneurysm [15], and atherosclerosis [16,17]. These data demonstrate that arterial mean telomere length is not associated with hypertension, telomere uncapping, or P53/P21-induced senescence, which suggests that arterial telomere shortening likely does not contribute to the pathogenesis of hypertension.
Telomere shortening in WBCs has been correlated with hypertension in medicated individuals [13,14], increased pulse pressure in men [29], and pulmonary hypertension severity [12]. Arterial mean telomere length should be more relevant to the etiology of hypertension than that of WBCs, but the accessibility of blood makes WBCs a preferred source of tissue for researchers interested in the role of telomere dysfunction in CVD. Tissue-specific differences in mean telomere length and rates of telomere shortening may account for the difference in our findings from those in studies with WBCs. Therefore, these results cast doubt on the biological relevance of telomere shortening in WBCs to hypertension.
Arterial telomere uncapping and hypertension
Arterial telomere uncapping could play a role in hyper-tension by leading to P53/P21-induced senescence. We showed that telomere uncapping and P53/P21 senescence were greater in arteries from hypertensive patients compared with those from nonhypertensive individuals, and these differences were independent of mean telomere length. We also reported that telomere uncapping demonstrated a strong positive correlation with P53/P21 senescence. Interestingly, telomere uncapping was a highly influential covariate for the group difference in P53/P21 senescence, accounting for about 35% of the variance in this outcome. Prior to these findings, the role of telomere uncapping in hypertension was entirely unknown. The only previous study to measure arterial telomere uncapping reported greater γ-H2 localized to telomeres with advancing age, which was positively correlated with P53/P21 senescence independent of telomere shortening [20]. Arterial P53/P21-induced senescence has been linked to atherosclerosis [11] and chronic obstructive pulmonary disease [12], but its association with hypertension was also previously unexplored. These results demonstrate that arterial telomere uncapping and P53/P21-induced senescence are associated with hypertension and establish the link between telomere uncapping and P53/P21-induced senescence. Thus, arterial telomere uncapping may contribute to the etiology of hypertension by leading to P53/P21-induced senescence.
Influence of telomere dysfunction on hypertension status
If arterial telomere dysfunction indeed plays a role in hypertension, then mean telomere length or telomere uncapping should influence hypertension status. Utilizing logistic regression analyses, we showed that telomere uncapping and BMI were significant predictors of hyper-tension status, whereas mean telomere length was not. Both telomere uncapping and BMI correctly predicted hypertension status in over two thirds of hypertensive patients and explained one-quarter of the variance in hypertension status. BMI was included in the logistic regression models because it was higher in hypertensive patients compared with nonhypertensive individuals and a well established CVD risk factor. Thus, we felt it was necessary to account for the influence of BMI on hypertension status in our logistic regression model. These findings demonstrate that telomere uncapping influences hyper-tension status more than mean telomere length, which suggests that in arteries, telomere uncapping may contribute more to the development of hypertension than mean telomere length.
Influence of participant characteristics on outcomes
Participant characteristics that include conventional CVD risk factors or prior CVD diagnoses could influence our hypertension-associated outcomes and hypertension status. To control for these potential confounds, we matched our participants for age and excluded individuals with a prior diagnosis of CVDs other than hypertension. Nonetheless, our hypertensive patients had higher BMI than nonhypertensive individuals. BMI was included in the logistic regression models to account for this group difference, as described above. Importantly, no participant characteristics were correlated with telomere uncapping or P53/P21-induced senescence. Thus, groupdifferences in these outcomes were independent of participant characteristics.
Most hypertensive patients used one or more prescription medications to control their blood pressure, and none of our nonhypertensive individuals took prescription blood pressure medications. Whereas our hypertensive patients’ mean SBP was clinically hypertensive, SBP was not different between hypertensive and nonhypertensive individuals. This was not unexpected, as most of our hypertensive patients used blood pressure medications, and the mean age of both hypertensive and nonhypertensive individuals was nearly 64 years of age. To avoid misidentification of hypertensive individuals as nonhypertensive, we based our group classification on prior diagnosis of hypertension rather than SBP measured during physician consultations. Thus, the lack of group difference in SBP did not affect our outcomes. Collectively, these experimental controls and analyses account for the influence of CVD risk factors and CVDs on our hypertension-associated outcomes and hypertension status.
In conclusion, the goal of this study was to elucidate the potential role of arterial telomere dysfunction in hyper-tension. Our findings demonstrate that arterial telomere uncapping and P53/P21-induced senescence were linked to hypertension independent of mean telomere length. We also reported that telomere uncapping had greater influence on hypertension status than mean telomere length. These results establish the framework for more mechanistic studies aimed at determining the causal role of arterial telomere dysfunction in hypertension. Furthermore, these results provide insight into the relative contributions of arterial telomere shortening and telomere uncapping to the etiology of hypertension and a more complete understanding of the role of telomere dysfunction in CVD.
Supplementary Material
Reviewers’ Summary Evaluations.
Reviewer 1 In this study the authors compared mean telomere length, telomere uncapping and senescence of 55 age-matched hypertensives to nonhypertensives. Telomere shortening is thought to lead to cellular senescence via telomere uncapping and may play a role in promoting the development of hypertension. The authors found a significant link between telomere uncapping, p53 induced senescence and hypertension. Surprisingly, mean telomere length was not associated with telomere uncapping, senescence, or hypertension. So far, association of telomere shortening with cardiovascular disease has been assessed in white blood cells only. This study casts some doubt on the biological relevance of these previous findings.
Reviewer 2 Compliments to the authors for this very interesting paper assessing a novel role of arterial telomere dysfunction in hypertension. The paper is very technical and probably addressed to more than specialist readers. The main finding is that telomere uncapping is a significant predictor of hypertension status, while mean telomere length is not. Arterial telomere uncapping is therefore linked to hyper-tension independent of mean telomere length. The paper represents a very strong and significant link between pre-clinical and clinical hypertension, what is going to represent one of the scientific challenges of the next decades.
ACKNOWLEDGEMENTS
All experiments were performed in the Translational Vascular Physiology Laboratory at the University of Utah and Veteran's Affairs Medical Center-Salt Lake City, Geriatric Research Education and Clinical Center. G.M. and A.J.D. contributed to all aspects of the study, including the conception and design of the experiments, collection, analysis, and interpretation of data, and drafting and revision of the article. S.J.I., L.A.L., R.M.C., R.H.I.A., R.D.N., A.E.W., and R.S.R. contributed to the collection and analysis of data as well as revision of the article. Additionally, R.M.C. contributed reagents and analytical tools that were essential to completing this study.
Sources of funding: awards from the NIH: National Institute of Aginγ-AG040297, National Institute of Aginγ-AG029337, National Institute of Aginγ-AG033196, National Institute of Aginγ-AG033755, NIH Heart, Lung, and Blood Institute-HL09183, The Department of Veterans Affairs Merit Grant-E6910R, and a University of Utah Center on Aging Grant financially supported this work.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- CVDs
cardiovascular diseases
- LDH
lactate dehydrogenase
- LSD
least significance difference
- P21
cyclin-dependent kinase inhibitor 1A
- P53
tumor suppressor protein p53
- pseudo R2
Nagelkerke's R2
- r
Pearson correlation coefficient
- S
single copy gene
- T
telomeric DNA
- WBCs
white blood cells
- γ-H2
phosphorylated histone γ-H2A.X at serine 139
- ηP2
partial eta squared
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 2.de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, et al. Structure and variability of human chromosome ends. Mol Cell Biol. 1990;10:518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stansel RM, de Lange T, Griffith JD. T-loop assembly in vitro involves binding of TRF2 near the 3’ telomeric overhang. EMBO J. 2001;20:5532–5540. doi: 10.1093/emboj/20.19.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 5.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 6.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 7.Sitte N, Saretzki G, von Zglinicki T. Accelerated telomere shortening in fibroblasts after extended periods of confluency. Free Radic Biol Med. 1998;24:885–893. doi: 10.1016/s0891-5849(97)00363-8. [DOI] [PubMed] [Google Scholar]
- 8.Oikawa S, Tada-Oikawa S, Kawanishi S. Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry. 2001;40:4763–4768. doi: 10.1021/bi002721g. [DOI] [PubMed] [Google Scholar]
- 9.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 11.Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, et al. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 12.Noureddine H, Gary-Bobo G, Alifano M, Marcos E, Saker M, Vienney N, et al. Pulmonary artery smooth muscle cell senescence is a pathogenic mechanism for pulmonary hypertension in chronic lung disease. Circ Res. 2011;109:543–553. doi: 10.1161/CIRCRESAHA.111.241299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Huang X, Jiang H, Zhang Y, Liu H, Qin C, et al. Short telomeres and prognosis of hypertension in a Chinese population. Hypertension. 2009;53:639–645. doi: 10.1161/HYPERTENSIONAHA.108.123752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhupatiraju C, Saini D, Patkar S, Deepak P, Das B, Padma T. Association of shorter telomere length with essential hypertension in Indian population. Am J Hum Biol. 2012;24:573–578. doi: 10.1002/ajhb.22264. [DOI] [PubMed] [Google Scholar]
- 15.Cafueri G, Parodi F, Pistorio A, Bertolotto M, Ventura F, Gambini C, et al. Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PLoS One. 2012;7:e35312. doi: 10.1371/journal.pone.0035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuda K, Khan MY, Skurnick J, Kimura M, Aviv H, Aviv A. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis. 2000;152:391–398. doi: 10.1016/s0021-9150(99)00482-7. [DOI] [PubMed] [Google Scholar]
- 17.Nzietchueng R, Elfarra M, Nloga J, Labat C, Carteaux JP, Maureira P, et al. Telomere length in vascular tissues from patients with atherosclerotic disease. J Nutr Health Aging. 2011;15:153–156. doi: 10.1007/s12603-011-0029-1. [DOI] [PubMed] [Google Scholar]
- 18.Kaul Z, Cesare AJ, Huschtscha LI, Neumann AA, Reddel RR. Five dysfunctional telomeres predict onset of senescence in human cells. EMBO Rep. 2012;13:52–59. doi: 10.1038/embor.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cesare AJ, Kaul Z, Cohen SB, Napier CE, Pickett HA, Neumann AA, et al. Spontaneous occurrence of telomeric DNA damage response in the absence of chromosome fusions. Nat Struct Mol Biol. 2009;16:1244–1251. doi: 10.1038/nsmb.1725. [DOI] [PubMed] [Google Scholar]
- 20.Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, et al. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol. 2013;305:H251–H258. doi: 10.1152/ajpheart.00197.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alpert B, McCrindle B, Daniels S, Dennison B, Hayman L, Jacobson M, et al. Recommendations for blood pressure measurement in human and experimental animals. Part 1: blood pressure measurement in humans. Hypertension. 2006;48:e3. doi: 10.1161/01.HYP.0000229661.06235.08. author reply e5. [DOI] [PubMed] [Google Scholar]
- 22.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009. 27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer SR, Erickson LA, Ichetovkin I, Knauer DJ, Markovic SN. Circulating serologic and molecular biomarkers in malignant melanoma. Mayo Clin Proc. 2011;86:981–990. doi: 10.4065/mcp.2011.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ives SJ, Andtbacka RH, Noyes RD, Morgan RG, Gifford JR, Park SY, et al. alpha1-Adrenergic responsiveness in human skeletal muscle feed arteries: the impact of reducing extracellular pH. Exp Physiol. 2013;98:256–267. doi: 10.1113/expphysiol.2012.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayr M, Hu Y, Hainaut H, Xu Q. Mechanical stress-induced DNA damage and rac-p38MAPK signal pathways mediate p53-dependent apoptosis in vascular smooth muscle cells. FASEB J. 2002;16:1423–1425. doi: 10.1096/fj.02-0042fje. [DOI] [PubMed] [Google Scholar]
- 27.Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, et al. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of thioredoxin 2. Hypertension. 2009;54:338–344. doi: 10.1161/HYPERTENSIONAHA.108.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vecchione C, Carnevale D, Di Pardo A, Gentile MT, Damato A, Cocozza G, et al. Pressure-induced vascular oxidative stress is mediated through activation of integrin-linked kinase 1/betaPIX/Rac-1 pathway. Hypertension. 2009;54:1028–1034. doi: 10.1161/HYPERTENSIONAHA.109.136572. [DOI] [PubMed] [Google Scholar]
- 29.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, et al. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.