Abstract
Given the presence of odor identification impairment in individuals with schizophrenia and recent evidence of aberrant odor hedonic processing, the aim of this investigation was to examine the influence of valence and intensity on odor identification in schizophrenia patients, their first-degree family members, and young persons at clinical risk for psychosis. Participants completed the 16-item Sniffin’ Stick Odor Identification Test. A logistic regression was conducted to assess the influence of valence and intensity on odor identification accuracy. Identification performance in the schizophrenia patients and youths at clinical risk for psychosis was significantly influenced by odor valence, but not intensity. Identification accuracy in first-degree family members was not influenced by valence or intensity. These data suggest that abnormalities in odor valence perception may represent an environmentally-mediated marker for hedonic disturbance that could have predictive utility in future conversion to psychosis. Further research examining the utility of odor valence measures as markers for psychosis risk are warranted.
Keywords: olfaction, anhedonia, emotion, schizophrenia prodrome, smell identification
Introduction
Prior studies have indicated that the ability to assign valence ratings to pleasant, but not unpleasant, odors is aberrant in schizophrenia (Crespo-Facorro, Paradiso et al., 2001). Notably, deficit syndrome patients under-rated the pleasantness of pleasant odors relative to non-deficit patients and controls (Strauss, Allen et al., 2010). We previously examined how schizophrenia patients rate the pleasantness of amyl acetate, a banana-like odor, at varying concentrations (Kamath, Moberg et al., 2013; Moberg, Arnold et al., 2003). Patients under-appreciated the pleasantness of amyl acetate at concentrations judged as pleasant by controls and over-rated its pleasantness at the concentration judged by controls, as relatively unpleasant (Kamath, et al., 2013). In contrast, first-degree family members of schizophrenia patients showed normal odor hedonic ratings (Kamath, et al., 2013; Schneider, Habel et al., 2007).
Given that odor hedonic processing and odor identification performance are disrupted in schizophrenia (Moberg, Kamath et al., 2013), recent studies have examined whether odor identification is influenced by valence. In one prior study, we found that patients were less accurate when identifying pleasant and neutral odors, but were not impaired in their ability to identify unpleasant odors (Kamath, Turetsky et al., 2011c). Similarly, schizophrenia inpatients showed a selective deficit for identifying pleasant, but not unpleasant, odors on a brief measure of odor identification (Kamath, Bedwell et al., 2011a). However, a third study, which used only a limited subset of these odorants, found no influence of valence on odor identification ability (Strauss, et al., 2010). Results are thus somewhat inconsistent. It is also unclear if this pattern of deficits extends to first-degree relatives of schizophrenia patients or to at-risk samples, two cohorts in which attenuated odor identification performance has been repeatedly observed (Brewer, Wood et al., 2003; Kopala, Good et al., 2001). The aim of the current study was to examine the influence of valence and intensity on odor identification performance in a larger cohort of schizophrenia patients and separate cohorts of non-ill first-degree relatives and youths at risk for psychosis. One limitation of all prior studies was the use of a categorical classification of odors as either pleasant or unpleasant. We therefore examined the influence of valence and intensity using continuous, rather than categorical, normative ratings of both odor attributes.
Method
Participants
Adult Cohort
The sample included sixty-four individuals meeting DSM-IV criteria for schizophrenia, 27 first-degree relatives of schizophrenia patients, and 54 healthy individuals drawn from the available subject pool at the University of Pennsylvania Schizophrenia Research Center (SRC). All subjects who participate in SRC studies are screened for any history of neurological disorder, head trauma with loss of consciousness, substance abuse within the preceding six months, positive urine drug screen, or medical conditions affecting cerebral functioning. Subjects who present with any of these conditions are not enrolled. On average 22.6% of potential subjects are excluded. None of the participants in this study had any obvious craniofacial abnormality (e.g., septal deviation) or acute respiratory condition. All study procedures were approved by the University of Pennsylvania Institutional Review Board (IRB), in compliance with the Declaration of Helsinki’s ethical standards in the treatment of human research participants. Participants provided written informed consent following a full explanation of the study procedures. Data from these subjects were included in a previous publication (Kamath, Turetsky et al., 2011b).
Consensus best-estimate DSM-IV diagnoses for schizophrenia were established using a semi-structured diagnostic interview (Structured Clinical Interview for DSM-IV - Patient Edition; First, Spitzer et al., 1996), medical record review, and available information from family and care providers. Patients were administered Scales for the Assessment of Negative Symptoms (SANS; Andreasen, 1984a) and Positive Symptoms (SAPS; Andreasen, 1984b). Controls and family members were assessed for current or past DSM-IV Axis I or Axis II disorders (First, Spitzer et al., 1995) and excluded for any current Axis I disorder, psychotropic medication use, or history of substance abuse or dependence in the preceding 6 months. Controls were also excluded if they had an Axis II cluster A disorder or a first-degree relative with a psychotic illness. A prior history of depression (major depressive disorder or depression not otherwise specified) was not exclusionary, provided there was no current clinical symptomatology or pharmacologic treatment. Three controls and 3 family members had diagnoses of past depression. The family member cohort was comprised of 5 parents, 18 siblings, and 4 offspring of schizophrenia patients. Four subjects from our family member cohort were biological relatives of our schizophrenia cohort.
Groups did not differ in age [F(2,142) = 1.52, p = .22], sex composition [χ2 = 4.50, df = 2, p = .11], or race [χ2 = 9.64, df = 6, p = .14]. Groups differed in educational attainment [F(2,142) = 8.64, p < .01]. Controls had more education than patients [F(1,142) = 17.27, p < .01]. Family members had an intermediate level of education, but did not differ significantly from either controls [F(1,142) = 3.04, p = .08] or patients [F(1,142) = 2.42, p = .12]. The three groups did not differ in parental education [Wilks’ Lambda = 0.95, F(4,228) = 1.35, p = 0.25], an estimate of potential that minimizes the confound of illness (Resnick, 1992). Group differences in smoking (packs/day) were statistically significant [F(2,142) = 6.02, p < .01]. Patients reported a higher smoking burden than controls [F(1,142) = 10.96, p < .01] or family members [F(1,142) = 4.77, p = .03; see Table 1]. Schizophrenia patients were either unmedicated (n = 4), taking atypical antipsychotic medication (n = 8), typical antipsychotic medication (n = 39), combination of both typical and atypical antipsychotic medications (n = 2), or other psychotropic medications at the time of testing (n = 10). Medication dosages were converted to chlorpromazine equivalents using published reference tables (Kroken, Johnsen et al., 2009). Medication data were unavailable for one individual and medication dosages were unknown for three individuals.
Table 1.
Demographic and Clinical Characteristics for schizophrenia patients, first-degree family members, and healthy comparison subjects.
| Schizophrenia Probands (n = 64) |
Family Members (n = 27) |
Healthy Controls (n = 54) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Characteristic | Mean | SD | n | Mean | SD | n | Mean | SD | n |
| Age (years) | 36.97 | 10.82 | 36.30 | 16.33 | 33.20 | 10.87 | |||
| Sex (Males | Females) | 36 | 28 | 9 | 18 | 30 | 24 | ||||||
| Education level* (years) | 12.67 | 2.28 | 13.44 | 1.93 | 14.33 | 2.14 | |||
| Mother’s Education (years) |
13.02 | 2.91 | 12.33 | 2.73 | 14.16 | 2.42 | |||
| Father’s Education (years) |
13.04 | 3.90 | 13.14 | 3.72 | 13.69 | 3.06 | |||
| Packs/day* | 0.47 | 0.65 | 0.21 | 0.37 | 0.15 | 0.37 | |||
| Illness Duration (years) | 15.51 | 10.19 | |||||||
| Age of Onset (years) | 21.47 | 6.70 | |||||||
| SANS† Total | 27.70 | 17.05 | |||||||
| SAPS‡ Total | 18.25 | 16.66 | |||||||
Significant difference (P < 0.05)
SANS = Scale for the Assessment of Negative Symptoms (Andreasen, 1984a)
SAPS = Scale for the Assessment of Positive Symptoms (Andreasen, 1984b)
Adolescent and Young Adult Cohort
Individuals who exhibited prodromal symptoms but did not meet criteria for a DSM-IV axis I psychotic disorder (Clinical Risk; CR, n = 15), and symptom-free comparison subjects (Low Risk; LR, n = 14) were recruited to the Neurodevelopment in Adolescence and Young Adulthood (NAYA) research program at the University of Pennsylvania. Informed consent was obtained from all young adult participants; parental consent and child assent were obtained for subjects under the age of 18. Exclusion criteria noted for the adult cohort above were applied, except that DSM-IV substance use and mood or anxiety disorders were not exclusionary for CR subjects. Five CR subjects had a depressive disorder and 2 had substance use disorders. Two CR subjects had a prior history of depression. Individuals were administered the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001) to obtain an estimate of verbal intellectual functioning. Participants with a standard score below 70 were excluded.
Trained diagnosticians administered the Diagnostic Interview for Genetic Studies (DIGS; Nurnberger, Blehar et al., 1994), the Structured Clinical Interview for DSM-IV-F (Anxiety) Module (SCID; First, et al., 1996), and the Family Interview for Genetic Studies (FIGS; NIMH Genetics Initiative, 1992) to each participant. Participants were assessed for current or history of a DSM-IV axis II cluster A disorder (First, Gibbon et al., 1997). Prodromal symptomatology was assessed using the Structured Interview for Prodromal Syndromes (SIPS; McGlashan, Miller et al., 2003) and the Scale of Prodromal Symptoms (SOPS; McGlashan, Miller et al., 2001; McGlashan, et al., 2003; Miller, McGlashan et al., 1999). Prodromal criteria included at least one positive symptom rated 3-5 or at least two negative and/or disorganized symptoms rated 3-6 on the SOPS. Symptoms had to be present during the 6 months prior to testing. Prodromal symptoms in the CR subjects were as follows: 9 showed a mixture of positive, negative and disorganized symptoms, 4 exhibited only positive symptoms, and 2 had only disorganized symptoms. Medical, developmental, psychiatric, and social history was collected for each individual and consensus diagnoses were achieved via case review by two or more doctoral level clinicians (MEC, CGK, REG, KBW, BIT). Two CR subjects (2 offspring from 2 families) were also first-degree biological relatives of schizophrenia probands.
Groups did not differ in age [F(1,27) = 3.08, p = .09], sex [χ2 = 1.66, df = 1, p = .20], or race [χ2 = 7.54, df = 3, p = .06]. Controls had more education than CR subjects [F(1,27) = 8.64, p = .03]; however, groups did not differ in parental education [Wilks’ Lambda = 0.93, F(2,22) = 0.79, p = 0.47]. CR subjects had significantly lower scores on the WTAR [F(1,27) = 12.61, p = .001]. Group difference in smoking (packs/day) was not statistically significant [F(1,24) = 0.86, p = .36; see Table 2]. CR subjects were either unmedicated (n = 12), taking atypical antipsychotic medication (n = 1), taking a stimulant medication (n = 1), or taking other psychotropic medications at the time of testing (n = 1). With the exception of 5 CR subjects, data from these subjects were included in a previous publication (Kamath, et al., 2011b).
Table 2.
Demographic and Clinical Characteristics for clinical-risk and low-risk control groups.
| Clinical Risk (n = 15) |
Low Risk (n = 14) |
|||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristic | Mean | SD | n | Mean | SD | n |
| Age (years) | 18.80 | 3.63 | 20.93 | 2.81 | ||
| Sex (Males | Females) | 10 | 5 | 6 | 8 | ||||
| Education level* (years) | 11.47 | 2.90 | 13.93 | 2.73 | ||
| Mother’s Education (years) |
14.23 | 2.83 | 15.00 | 2.48 | ||
| Father’s Education (years) |
13.62 | 3.07 | 15.31 | 2.93 | ||
| Packs/day | 0.12 | 0.21 | 0.06 | 0.15 | ||
| SOPS† Total Score | 31.27 | 13.19 | 3.14 | 3.57 | ||
Significant group difference (P < 0.05)
Scale of Prodromal Symptoms (SOPS; McGlashan, et al., 2001; McGlashan, et al., 2003; Miller, et al., 1999)
Psychophysical Olfactory Assessment
Each participant was administered the Sniffin’ Sticks Odor Identification test (Hummel, Sekinger et al., 1997; Kobal, Hummel et al., 1996). Sixteen odor-impregnated markers were presented sequentially to the participant’s nares birhinally by a trained technician. The subject was asked to identify each odor, using a four-alternative multiple-choice format and received one point for each correct response. Normative values for the valence and intensity of each odor were established, previously, by the inventors of the Sniffin’ Sticks (Hummel, et al., 1997). Subjects in their normative sample of 63 healthy subjects (32 male, 31 female) rated each of the 16 odors using a visual analog scale that ranged from −50 (absolutely unpleasant) to 50 units (absolutely pleasant) for valence and from 0 (no odor perceived) to 100 (highest intensity possible) for intensity. For example, the odor ‘orange’ was coded with an intensity value of 67 and a hedonics value of 24. Normative mean hedonic rating was +9.5 ± 22.7 SD; normative mean intensity rating was 77.2 ± 8.4 SD.
Statistical Analyses
The influence of valence and intensity on identification accuracy was assessed using the logistic regression model in the Generalized Linear Latent and Mixed Models (GLLAMM) algorithm implemented in Stata 9.0 (StataCorp; College Station, TX, USA), to allow adjustment for the nonindependence of within-subject odor scores.
In the initial logistic regression model, odor identification accuracy (coded as 1 or 0 for each odor) was the dependent measure. Odor valence, odor intensity, group (patient/relative/control), group-by-valence and group-by-intensity interactions were fixed-effect predictors of response, with subject included as a random-effects factor. Non-significant interactions (i.e., group-by-intensity interaction) were dropped from the final model. The significance levels of individual model parameters were assessed using the Wald test statistic with χ2 distribution. Significant main effects and interactions were further parsed by post-hoc computation of χ2 statistics for appropriate linear combinations of the model coefficients, along with their associated z-statistic and p-value, and odds ratios for significant predictors were determined.
Results
Odor Identification in Schizophrenia Patients and Non-ill Family Members
Effects of Odor Valence and Intensity
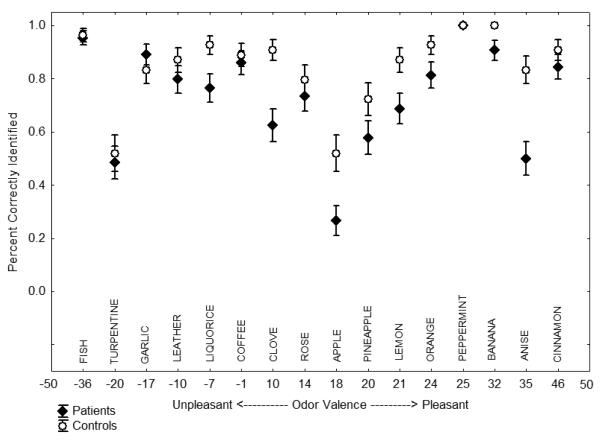
There were statistically significant main effects of diagnosis [χ2 = 17.45, df = 2, p = 0.0002] and odor intensity [χ2 = 11.25, df = 1, p = 0.0008], and a diagnosis-by-valence interaction [χ2 = 10.03, df = 2, p = 0.007]. The diagnosis-by-intensity interaction was not significant [χ2 = 2.14, df = 2, p = 0.343]. Patients were 57% as likely [OR = 0.5723092, χ2 = 16.07, df = 1, p = 0.0001] and family members were 60% as likely [OR = 0.6009236, χ2 = 8.76, df = 1, p = 0.003] as controls to correctly identify each odor. A 10-point increase in odor intensity increased the likelihood of correct odor identification by 22% across all groups (OR = 1.224347; see Figure 1). Odor identification performance was influenced by valence in schizophrenia patients [χ2 = 6.30, df = 1, p = 0.012], but not in family members [χ2 = 0.18, df = 1, p = 0.675]. Every 10-point increase in odor pleasantness reduced the likelihood of correct patient identification by 7% (OR = 0.9331055, p = .040). Sex, age and smoking (packs/day) were not significant independent predictors of odor identification accuracy and inclusion of these as covariates did not alter the observed effects.
Figure 1.
Percent accuracy (mean±SE) by normative odor valence rating for schizophrenia patients and healthy comparison subjects.
Relationships with Demographic and Clinical Indices
In schizophrenia patients, negative symptoms (SANS total score) were inversely associated with identification accuracy [χ2 = 8.42, df = 1, p = 0.004], but the negative symptoms-by-hedonic valence interaction was not significant [χ2 = 0.37, df = 1, p = 0.54]. Positive symptomatology, age, sex, medication dosage, age of onset and illness duration were all unrelated to performance and none of these measures interacted significantly with odor valence [all p’s > .10].
Odor Identification in At-Risk Youth
Effects of Odor Valence and Intensity
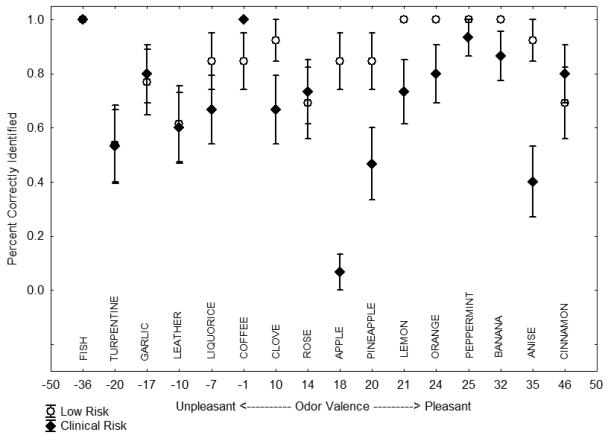
There were statistically significant main effects of diagnosis [χ2 = 7.41, df = 1, p = 0.007] and intensity [χ2 = 6.47, df = 1, p = 0.01]. Clinical risk youths were 50% as likely as low risk controls to correctly identify each odor. A 10-point increase in odor intensity increased the likelihood of correct odor identification by 41% across both groups. The diagnosis-by-valence interaction for odor identification accuracy was also statistically significant [χ2 = 4.71, df = 1, p = 0.03]. Performance of CR subjects, like that of schizophrenia patients, was adversely affected by increasing odor pleasantness. A 10-point increase in pleasantness reduced the likelihood of correct odor identification in CR subjects by 10% (see Figure 2). The diagnosis-by-intensity interaction was not significant [p = 0.17]. Inclusion of age, education, sex, and WTAR score as covariates did not alter the findings. Similarly, the presence or absence of other psychiatric comorbidity had no effect on performance.
Figure 2.
Percent accuracy (mean±SE) by normative odor valence rating for clinical-risk and low-risk control groups.
Relationships with Demographic and Clinical Indices
Within the CR group, the relationship between prodromal symptoms and overall performance was not statistically significant [p = .82], and prodromal symptoms did not interact with odor valence to modulate identification accuracy [p = .38].
Discussion
In this study, we observed that odor identification accuracy was influenced by valence in schizophrenia patients and youths at clinical risk for psychosis, but not in individuals with an isolated genetic vulnerability. Importantly, odor intensity was not a significant predictor of identification accuracy. Therefore, the effects of odor valence on identification performance were not secondary to impaired odor strength perception. These findings replicate prior findings in schizophrenia (Kamath, et al., 2011a; Kamath, et al., 2011c) and extend them to individuals at clinical risk for psychosis, using a more sophisticated quantitative analysis of individual odor attributes. The significant effect of valence on identification accuracy in patients and clinical-risk youth stands in contrast to the first-degree family members. Notably, family members had reduced identification accuracy for odors that was not impacted by the odors’ attributes. Though there is an abundance of literature on neurobehavioral impairments in unaffected first-degree family members (for a review, see: Snitz, Macdonald et al., 2006), these impairments are of relatively limited utility for predicting either schizophrenia onset or prognosis, as conversion to schizophrenia among family members is only about 10% (Seidman et al., 2010). Rather, measures that clinical-risk subjects share with schizophrenia patients, but which distinguish them from symptom-free family members, may be most informative regarding prospective or incipient conversion to psychosis. A prior study by Seidman et al. (2010) found that clinical-risk subjects had patterns of neurocognitive impairment that were distinct from those of family members with isolated genetic risk. Furthermore, domains of cognitive impairment were not predictive of transition to schizophrenia in genetic-risk youths (Whyte, Brett et al., 2006), suggesting relatively stable deficits. Thus, there is a need to ascertain what specific impairments function as genetically mediated markers of neurodevelopmental vulnerability, predictors of the emergence of psychotic symptoms, or both. The findings of the current study suggest that, while patients, non-ill relatives, and at-risk youth all have impairments in odor identification, they can be distinguished by the modulating effect of odor valence on performance. It remains to be determined, of course, whether this odor hedonic disturbance is, in fact, informative regarding conversion to psychosis or prognosis.
Studies of both schizophrenia patients and at-risk youths have noted that anhedonia and emotional disturbance are trait-related deficits that are evident before the onset of illness and may have predictive utility in differentiating converters from non-converters (Velthorst, Nieman et al., 2009). In addition, altered sulcogyral patterns in the orbitofrontal cortex (OFC), a key brain region involved in odor hedonic processing and sensory integration, were found to differentiate schizophrenia patients and individuals who convert to schizophrenia from non-converters and controls (Chakirova, Welch et al., 2010; Nakamura, Nestor et al., 2007). Therefore, odor valence measures, reflective of both hedonic disturbance and orbitofrontal impairment, could have predictive utility in identifying those at risk for the development of schizophrenia. Also, there is growing evidence that pleasant and unpleasant odors are processed, at least in part, in separate neural substrates (Grabenhorst, Rolls et al., 2007; Kim & Watanuki, 2003; Rolls, Kringelbach et al., 2003). The differential effect of positive vs. negative hedonic valence on odor identification performance may, therefore, facilitate a more precise understanding of the neuropathology underlying patient olfactory impairments.
Though odor hedonic processing has not received much attention in first-degree relatives of schizophrenia patients, one study by our group found that relatives were not impaired in odor pleasantness perception (Kamath, et al., 2013). Schneider et al. (2007) also found that siblings of schizophrenia patients did not differ from controls in subjective valence perception of either an unpleasant (yeast) or a pleasant (vanillin) odor, although they did observe reduced frontal cortical activity in both patients and their siblings during the processing of yeast but not vanillin. Thus, it is possible that relatives have abnormalities in odor hedonic processing, but these subtle deficits do not translate to impairment at the behavioral level. The evidence from this study, however, suggests that they have moderate impairments in odor identification (~0.5 standard deviations below healthy comparison subjects) that are not modulated by odor valence or intensity.
Given that olfactory deficits have also been observed in other psychiatric conditions, it remains to be determined if the influence of valence on odor identification performance is specific to schizophrenia or is also seen in other psychiatric disorders. Three prior studies examined odor identification deficits in clinical high-risk subjects relative to low-risk controls (Brewer et al., 2003; Kamath et al., 2012; Woodberry et al., 2010). These studies showed a large composite effect size of −0.77 for odor identification deficits in these subjects (Turetsky, Kamath et al., 2012) and in those individuals who subsequently developed schizophrenia (−1.12). Notably, a small effect was observed in those who subsequently developed another psychotic illness (−0.24). These data provide initial evidence that odor identification tests may be particularly sensitive to conversion to schizophrenia. Future studies examining the effect of valence and intensity across psychiatric diagnoses may provide additional utility in prediction of conversion to schizophrenia. In the current study, we did not find that smoking or antipsychotic medication dose significantly influenced olfactory performance. Both of these factors have been previously shown to have a negligible impact on study effect sizes of olfactory performance in schizophrenia. However, a recent meta-analysis by our group (Moberg, et al., 2013) found that while overall medication status (medicated vs. unmedicated) and chlorpromazine equivalents did not moderate study effect size, patients on a regimen of typical antipsychotic medication showed significantly greater olfactory deficits than those on a regimen of atypical antipsychotics. Our meta-analysis also found an overall beneficial effect of smoking on olfactory function in patients which corresponds with a prior finding in which patients with psychotic disorders who smoked showed enhanced olfactory scores (McLean, Feron et al., 2004). Indeed, nicotine has been shown to facilitate cognition in several prior studies as well (Jacobsen, D’Souza et al., 2004; Smith, Singh et al., 2002; Smith, Warner-Cohen et al., 2006). Nevertheless, it is unclear how smoking and antipsychotic medication use impacts the hedonic appreciation of odors. Thus, further work is needed to address the impact of smoking and medication on odor valence processing.
The current study was limited by the lack of subjective participant intensity and valence ratings, which would be useful in analyzing underlying differences in pleasantness and intensity perception between groups. Instead, published normative data were used to capture the dimensionality of odor valence and intensity ratings. Though we found that odor identification performance in patients was influenced by normative differences in odor valence, future studies are needed to determine if an individual participant’s perceptual rating of hedonic quality would alter the observed results. Nevertheless, the results of the current analysis suggest that valence affects odor identification in schizophrenia patients and clinical risk youths, but not in first-degree relatives, reflecting an environmentally-mediated disturbance associated with overt or subclinical illness.
Acknowledgements
We thank Dana Gatto and Dana Marchetto for assistance with subject recruitment, task administration and data entry.
Role of the Funding Source This study was funded in part by National Institutes of Health Grants MH63381 (PJM), MH59852 (BIT), K08MH79364 (MEC), and K23MH079498 (KBW), Independent Investigator Awards from the Brain Behavioral Research Foundation to Dr. Moberg and Dr. Borgmann-Winter, and unrestricted funds from the Children’s Hospital of Philadelphia to Dr. Borgmann-Winter. The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors Dr. Kamath conducted literature review, statistical analyses, and wrote the first draft of the manuscript. Dr. Moberg contributed to the study design, protocol, and manuscript writing. Dr. Calkins, Dr. Borgmann-Winter, Ms. Conroy, Dr. Kohler, and Dr. Gur oversaw all aspects of participant recruitment, screening, diagnostic assessment, and case conference of the participants. Mr. Frishberg contributed to literature review, diagnostic assessment, and participant assessment. Dr. Bilker contributed to the statistical analysis. Dr. Turetsky contributed to the study design, protocol, literature review, statistical analyses, and manuscript writing. All authors contributed to and have approved the final manuscript.
Conflicts of Interest VK, MEC, CGK, PJM, KBM, CGC, WBB and NF report no competing interests. BIT and REG report investigator-initiated research support from AstraZeneca Pharmaceuticals and Pfizer Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City, IA: 1984a. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City, IA: 1984b. [Google Scholar]
- Brewer WJ, Wood SJ, McGorry PD, Francey SM, Phillips LJ, Yung AR, et al. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. American Journal of Psychiatry. 2003;160(10):1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- Chakirova G, Welch KA, Moorhead TW, Stanfield AC, Hall J, Skehel P, et al. Orbitofrontal morphology in people at high risk of developing schizophrenia. European Psychiatry. 2010;25(6):366–372. doi: 10.1016/j.eurpsy.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, et al. Neural mechanisms of anhedonia in schizophrenia: A PET study of response to unpleasant and pleasant odors. Journal of Americal of Medical Association. 2001;286(4):427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. American Psychiatry Press; Washington, D.C.: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Stuctured Clinical Interview for DSM-IV - Nonpatient Edition (SCID-NP, Version 2.0) New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV - Patient Edition (SCID-P, Version 2.0) New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Grabenhorst F, Rolls ET, Margot C, da Silva MA, Velazco MI. How pleasant and unpleasant stimuli combine in different brain regions: odor mixtures. Journal of Neuroscience. 2007;27(49):13532–13540. doi: 10.1523/JNEUROSCI.3337-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chemical Senses. 1997;22(1):39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biological Psychiatry. 2004;55(8):850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Kamath V, Bedwell JS, Compton MT. Is the odour identification deficit in schizophrenia influenced by odour hedonics? Cogitive Neuropsychiatry. 2011a;16(5):448–460. doi: 10.1080/13546805.2011.552561. [DOI] [PubMed] [Google Scholar]
- Kamath V, Moberg PJ, Kohler CG, Gur RE, Turetsky BI. Odor hedonic capacity and anhedonia in schizophrenia and unaffected first-degree relatives of schizophrenia patients. Schizophrenia Bulletin. 2013;39(1):59–67. doi: 10.1093/schbul/sbr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Turetsky BI, Calkins ME, Kohler CG, Conroy CG, Borgmann-Winter K, et al. Olfactory processing in schizophrenia, non-ill first-degree family members, and young people at-risk for psychosis. World Journal of Biological Psychiatry. 2011b doi: 10.3109/15622975.2011.615862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Turetsky BI, Moberg PJ. Identification of pleasant, neutral, and unpleasant odors in schizophrenia. Psychiatry Research. 2011c;187(1-2):30–35. doi: 10.1016/j.psychres.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Watanuki S. Characteristics of electroencephalographic responses induced by a pleasant and an unpleasant odor. Journal of Physiological Anthropology and Applied Human Science. 2003;22(6):285–291. doi: 10.2114/jpa.22.285. [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. ‘Sniffin’ sticks’: screening of olfactory performance. Rhinology. 1996;34(4):222–226. [PubMed] [Google Scholar]
- Kopala L, Good K, Morrison K, Bassett A, Alda M, Honer W. Impaired olfactory identification in relatives of patients with familial schizophrenia. American Journal of Psychiatry. 2001;158(8):1286–1290. doi: 10.1176/appi.ajp.158.8.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroken RA, Johnsen E, Ruud T, Wentzel-Larsen T, Jørgensen HA. Treatment of schizophrenia with antipsychotics in Norwegian emergency wards, a cross-sectional national study. BMC Psychiatry. 2009;9:24. doi: 10.1186/1471-244X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson L. Instrument for the assessment of prodromal symptoms and states. In: Miller TJ, editor. Early intervention in psychotic disorders. Kluwer Academic Publishers; Dordrecht: 2001. pp. 135–150. [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW, Rosen JL, Hoffman RE, Davidson L. Structured Interview for Prodromal Syndromes, Version 4.0. Prime Clinic Yale School of Medicine; New Haven, CT: 2003. [Google Scholar]
- McLean D, Feron F, Mackay-Sim A, McCurdy R, Hirning M, Chant D, et al. Paradoxical association between smoking and olfactory identification in psychosis versus controls. Australian and New Zealand Journal of Psychiatry. 2004;38(1-2):81–83. doi: 10.1111/j.1440-1614.2004.01301.x. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, et al. Symptom assessment in schizophrenic prodromal states. Psychiatric Quarterly. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Arnold SE, Doty RL, Kohler C, Kanes S, Seigel S, et al. Impairment of odor hedonics in men with schizophrenia. American Journal of Psychiatry. 2003;160(10):1784–1789. doi: 10.1176/appi.ajp.160.10.1784. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Kamath V, Marchetto DM, Calkins ME, Doty RL, Hahn CG, et al. Meta-Analysis of Olfactory Function in Schizophrenia, First-Degree Family Members, and Youths At-Risk for Psychosis. Schizophrenia Bulletin. 2013 doi: 10.1093/schbul/sbt049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, McCarley RW, Levitt JJ, Hsu L, Kawashima T, et al. Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain. 2007;130(Pt 3):693–707. doi: 10.1093/brain/awm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMH Genetics Initiative . Family Interview for Genetic Studies (FIGS) National Institute of Mental Health; Rockville, MD: 1992. [Google Scholar]
- Nurnberger JI, Jr., Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-844. [DOI] [PubMed] [Google Scholar]
- Resnick SM. Matching for education in studies of schizophrenia. Archives of General Psychiatry. 1992;49(3):246. doi: 10.1001/archpsyc.1992.01820030078011. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, de Araujo IE. Different representations of pleasant and unpleasant odours in the human brain. European Journal of Neuroscience. 2003;18(3):695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Reske M, Toni I, Falkai P, Shah NJ. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry Research. 2007;155(2):103–112. doi: 10.1016/j.pscychresns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Archives of General Psychiatry. 2010;67(6):578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27(3):479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, et al. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006;31(3):637–643. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32(1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Ross SA, Duke LA, Schwartz J. Olfactory hedonic judgment in patients with deficit syndrome schizophrenia. Schizophrenia Bulletin. 2010;36(4):860–868. doi: 10.1093/schbul/sbn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Kamath V, Calkins ME, Brewer WJ, Wood SJ, Pantelis C, et al. Olfaction and schizophrenia clinical risk status: just the facts. Schizophrenia Research. 2012;139(1-3):260–261. doi: 10.1016/j.schres.2012.04.016. author reply 262-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthorst E, Nieman DH, Becker HE, van de Fliert R, Dingemans PM, Klaassen R, et al. Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophrenia Research. 2009;109(1-3):60–65. doi: 10.1016/j.schres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler test of adult reading. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Whyte MC, Brett C, Harrison LK, Byrne M, Miller P, Lawrie SM, et al. Neuropsychological performance over time in people at high risk of developing schizophrenia and controls. Biological Psychiatry. 2006;59(8):730–739. doi: 10.1016/j.biopsych.2005.08.028. [DOI] [PubMed] [Google Scholar]